Abstract
The survival of patients with high risk neuroblastoma has improved significantly with the use of intensive multimodality treatment regimens including chemotherapy, surgery, radiation therapy, myeloablative chemotherapy followed by stem cell rescue, and immunotherapy. This report summarizes the current treatment strategies used in the COG and SIOP for children with neuroblastoma. The improved global collaboration and the adoption of a uniform International Neuroblastoma Risk Group Staging System will help facilitate comparison of homogeneous pre-treatment cohorts across clinical trials. Future research strategies regarding the indications for and dosages of radiation therapy to the primay and metastatic sites, and the integration of MIBG therapy into the multimodal treatment program are discussed.
Keywords: Radiation therapy, surgery, chemotherapy, neuroblastoma, autologous stem cell rescue, immunotherapy, MIBG
INTRODUCTION:
Neuroblastoma is a cancer that arises in cells derived from the neural crest, and tumors can develop at any site in the sympathetic nervous system.1 It is the most common extracranial solid tumor in children, with approximately 700 cases diagnosed per year in the United States alone.2 Most children are diagnosed under the age of 5 years, with a median age at diagnosis of 17 months.3 Clinical symptoms vary depending on the location of the primary tumor, and may include an abdominal mass, abdominal pain, respiratory distress, or neurological symptoms from spinal cord involvement.4,5 Children with metastatic disease often appear ill at diagnosis, with fever, bone pain, and weight loss. While in some cases of neuroblastoma, lesions may regress spontaneously, in others, the disease may behave aggressively, with many patients succumbing to recurrent/ refractory metastatic disease. Therapy is stage- and risk-stratified, and some elements of radiotherapy may be response-adapted.
Modern protocols, including induction chemotherapy, surgical resection, high-dose chemotherapy with autologous stem cell rescue (ASCR), external beam radiotherapy (EBRT), and immunotherapy or differentiating agents, have improved outcomes, with 3-year survival rates now exceeding 60%.6 Past treatments that used less intensive chemotherapy resulted in 4-year survival rates of 10–15%. This review will focus on current treatment approaches in high-risk neuroblastoma in North America and Europe, particularly as they pertain to external beam radiotherapy.
DIAGNOSIS AND STAGING:
Biopsy of the primary neuroblastoma tumor or a metastatic soft tissue lesion is generally required to establish the diagnosis, although the diagnosis can be made based on bone marrow involvement and elevated urinary catecholamines if the risk of tumor biopsy is deemed unacceptable. Anatomic imaging of the primary site is required, as well as bone marrow aspirate and biopsies and an iodine-123 meta-iodobenzylguanidine (MIBG) scan. A FDG-PET CT can be used if the primary tumor is MIBG non-avid.
The staging system currently used worldwide is the International Neuroblastoma Risk Group Staging System (INRGSS).7,8 The INRGSS was developed to facilitate comparison across clinical trials by defining homogeneous pre-treatment patient cohorts. Staging is based on the presence or absence of imaging-defined risk factors (IDRFs), which are defined by the relationship between the tumor and adjacent structures. IDRFs were originally intended to indicate surgical risk, but are now considered proxies for biologic variables that have not yet been identified (Table 1).
Table 1.
Image-defined risk factors (IDRFs) in neuroblastic tumors
| Ipsilateral tumour extension within two body compartments |
|---|
|
Neck
|
| Tumour encasing carotid and/or vertebral artery and/or internal jugular vein |
| Tumour extending to base of the skull |
| Tumour compressing the trachea |
|
Cervico-thoracic junction |
| Tumour encasing brachial plexus roots |
| Tumour encasing subclavian vessels and/or vertebral and/or carotid artery |
| Tumour compressing the trachea |
|
Thorax |
| Tumour encasing the aorta and/or major branches |
| Tumour compressing the trachea and/or principal bronchi |
| Lower mediastinal tumour, infiltrating the costo-vertebral junction between T9 and T12 |
|
Thoraco-abdominal |
| Tumour encasing the aorta and/or the vena cava |
|
Abdomen/pelvis |
| Tumour infiltrating the porta hepatis and/or the hepatoduodenal ligament |
| Tumour encasing branches of the superior mesenteric artery and the mesenteric root |
| Tumour encasing the origin of celiac axis and/or of the superior mesenteric artery |
| Tumour invading one or both renal pedicles |
| Tumour encasing the aorta and/or the vena cava |
| Tumour encasing the iliac vessels |
| Pelvic tumour across the sciatic notch |
|
Intraspinal tumour |
| Extension, regardless of location, provided that more than one-third of the spinal canal in the axial plane is invaded and/or the perimedullary leptomeningeal spaces are not visible and/or the spinal cord signal is abnormal |
|
Infiltration of adjacent organs/structures |
| Pericardium, diaphragm, kidney, liver, duodeno-pancreatic block and mesentery |
|
Conditions to be recorded, but not considered as IDRFs |
| Multifocal primary tumours |
| Pleural effusion, with or without malignant cells |
| Ascites, with or without malignant cells |
The INRGSS recognizes four stages: localized disease without (L1) and with (L2) imaging-defined risk factors, metastatic disease (M) and metastatic disease in very young children that is limited to specific sites (MS) (Table 2). The INRG Task Force also developed the INRG Consensus Pre-treatment Classification Schema for pre-treatment risk stratification. An analysis of thirteen variables in an 8800-patient cohort revealed 16 combinations of known prognostic factors, including age (<12 months, 12–18 months, ≥18 months), stage, histology (category, grade), and molecular characteristics (MYCN amplification status, chromosome 11q status, tumor cell ploidy) (Table 3). These factors allowed for allocation of patients into four main prognostic groups (very-low, low, intermediate, and high risk) (Table 4).7
Table 2.
INRG staging system
| INRG Stage | Definition |
|---|---|
| L1 | Localised disease, without imaging defined risk factors (for example encasement of major blood vessels or encroachment into the spinal canal). |
| L2 | Localised disease with imaging defined risk factors. |
| M | Metastatic disease which does not fall into the MS category (for example those aged less than 18 months with bone metastases, and all older patients with distant metastases). |
| MS | Age less than 18 months with metastatic disease limited to liver, skin or bone marrow. |
Table 3.
International Neuroblastoma Treatment Risk Groups
| INRG Stage | Age (months) | Histological classification | Grade of tumor differentiation | MYCN | 11q Aberration | Ploidy | Pretreatment risk group |
|---|---|---|---|---|---|---|---|
| L1/L2 | Any | GN maturing | A Very Low | ||||
| GNB intermixed | |||||||
| L1 | Any | Any except | NA | B Very Low | |||
| GN maturing or | Amp | K High | |||||
| GNB intermixed | |||||||
| L2 | <18 | Any except | NA | No | D Low | ||
| GN maturing or | Yes | G | |||||
| GNB intermixed | Intermediate | ||||||
| ≥18 | GNB nodular; | Differentiating | NA | No | E Low | ||
| neuroblastoma | Poorly differentiating or undifferentiating | NA | Yes | ||||
| H Intermediate | |||||||
| M | <18 | NA | Hyperdiploid | F Low | |||
| <12 | NA | Diploid | I Intermediate | ||||
| 12 to <18 | NA | Diploid | J Intermediate | ||||
| <18 | Amp | O High | |||||
| ≥18 | P High | ||||||
| MS | <18 | NA | No | C Very Low | |||
| Yes | Q High | ||||||
| Amp | R High |
Table 4.
INRG prognostic groups
| Pretreatment Risk Group | 5-year EFS (%) | Proportion of patients (%) |
|---|---|---|
| Very Low | >85 | 28.2 |
| Low | >75 to ≤85 | 26.8 |
| Intermediate | ≥50 to ≤75 | 9.0 |
| High | <50 | 36.1 |
High-risk neuroblastoma is defined as metastatic disease in a child ≥ 18 months or a patient of any age with L2, M or MS disease with amplification of the MYCN oncogene. Subsets of patients with other combinations of risk factors may benefit from high risk therapy when outcomes are expected to be suboptimal with intermediate risk therapy; these include some patients <18 months of age with MYCN non-amplified disease but an 11q abberation.
LOCAL CONTROL:
Use of Surgery and External Beam Radiotherapy to Control Primary Tumor Site and Persistent Sites of Metastatic Disease
North America
i. Surgery:
Surgical resection remains a mainstay of curative therapy for high-risk neuroblastoma. In North American paradigms, definitive surgical resection of the primary tumor is generally undertaken following 4 cycles of chemotherapy. Surgical resection has been demonstrated to improve overall survival relative to biopsy alone, 9 however the importance of gross total resection compared to sub-total resection remains unclear. Two recent studies suggested decreased mortality risk and improved event-free survival and freedom from local recurrence when at least 90% of tumor was resected.10,11 As a result, the American Pediatric Surgical Association concluded that resection at an experienced center of > 90% of the primary tumor with preservation of adjacent organs and neurovascular structures is the preferred approach in patients with high risk disease.12
ii. Primary Site Radiotherapy
Radiotherapy to the primary site and persistent MIBG-avid metastases is recommended following induction chemotherapy, surgical resection, and high dose chemotherapy and Autologous Stem Rescue (ASCR).13 Analyses from CCG 3891 suggested that patients receiving a combination of 10 Gy TBI and an additional boost of 10 Gy to the primary site with focal external beam radiotherapy had improved local control,14 setting the COG standard of focal primary site radiotherapy to 21.6 Gy following ASCR. In COG A3973, 21.6 Gy was given to the post-induction chemotherapy, pre-operative primary tumor volume. Retrospective analysis showed no benefit from radiation of uninvolved lymph nodes (elective lymph node radiation).15 Modern protocols thus use the post-chemotherapy, pre-operative tumor volume to comprise the radiation target volume. A uniform expansion is added to this volume to create the clinical target volume (CTV). Prior COG protocols used 1.5–2cm margins from the post-operative bed to create the CTV; the current COG High Risk Neuroblastoma study, ANBL1531, uses 1 cm treatment margins. CTV margins may be tailored further at tissue interfaces where invasion or infiltration is unlikely, such as the kidney, liver, and bone.16
Considerable uncertainty exists surrounding the use of higher radiation doses for patients with residual disease after surgical resection. On the COG protocol ANBL 0532, patients with incompletely resected primary neuroblastoma received an additional 14.4 Gy boost to areas of gross residual disease, for a total dose of 36 Gy; however, analysis of local control rates compared to historical controls suggested no benefit of this escalated dose, resulting in removal of the “boost” from current COG protocols.17 Some have argued for both higher18 and lower doses19 for the cone-down/boost phase of therapy when residual disease is present.
Even modern primary site radiotherapy following ASCR is associated with increased risk for acute and late complications. Acute complications may include nausea, vomiting, weight loss, anorexia, radiation dermatitis, diarrhea, esophagitis, and pneumonitis. Late complications may include renal insufficiency, pancreatic endocrine and exocrine insufficiency, tissue hypoplasia and fibrosis, risk for second cancers, and bone deformities. Non-adrenal primary site radiotherapy can lead to hypogonadism, pulmonary fibrosis, and focal nodular hyperplasia of the liver20–24.
Attempts at mitigating the radiation-associated complications have included dose reduction and risk stratification, as well as reduction in radiation exposure using proton radiotherapy, CTV volume reduction, and organ avoidance strategies.19, 20, 25–27 Early reports on dose reduction suggest that doses as low as 18 Gy might control the primary site when MYCN amplification and residual disease are absent28. The use of IMRT or proton therapy may reduce bowel and vertebral body dose, lowering the risk of skeletal deformities and bony hypoplasia25,29.
iii. Metastatic Site Radiotherapy
Historically, treatment of metastatic sites has been a topic of relative uncertainty because of the paucity of clinical data. The COG protocol ANBL1531 mandates focal radiation to ≤ 5 metastatic sites that do not completely respond to induction chemotherapy.30 A few retrospective cohort studies have evaluated focal metastatic site radiotherapy. Bradfield et al reported that one of 21 patients who received RT to initial metastatic sites had local failure at the primary site and an irradiated bony metastatic site. Four of 17 patients (24%) developed recurrences at metastatic sites, but not within the treatment field.31 Casey et al. analyzed 159 patients with radiation of 244 metastatic sites. Metastatic sites that cleared with induction chemotherapy had improved local control (LC) compared with sites with persistent uptake on MIBG scans (LC rate, 92% vs 67%; P < .0001). Patients who had local control at irradiated metastatic sites had improved overall survival compared with those who did not (71% vs 50%; p<0.0001).32 Gatcombe et al reported that only one of 6 patients had in-field relapse after radiation to persistent metastatic disease on postinduction scans.33 Similarly, Polishchuk et al also found a higher risk of relapses in metastatic sites with no RT: 3 of 19 (15.8%) after RT, compared with 128 of 506 (25.3%) after no-RT to metastatic sites.34 These data, while retrospective, support continued investigation of the use of RT to control metastatic disease in patients with high-risk neuroblastoma. In a retrospective analysis of three large high-risk neuroblastoma trials, Li et al found that patients who received total body irradiation (TBI) as part of an older consolidation model had significantly fewer metastatic relapses in previously MIBG-avid sites compared with patients that did not receive TBI. These authors suggest that I-131 MIBG (systemic radiotherapy) may supplant the use of TBI and improve metastatic site control.13 Direct comparisons of I-131 MIBG to consolidative metastatic site-directed external beam radiotherapy do not exist, although supplanting external beam radiotherapy with use of I-131 MIBG in the setting of diffuse metastatic disease with an incomplete response is an attractive approach due to practical and toxicity concerns associated with delivery. Secondary analyses of RT response in COG ANBL 0532 should provide additional valuable data regarding benefit of treating metastatic sites. Future studies investigating the role of I-131 MIBG compared to EBRT are needed.
Europe:
i. Surgery:
The current strategy in most high-risk neuroblastoma treatment protocols in Europe involves resection of the primary tumor after completion of all induction chemotherapy, rather than following only 4–5 cycles of systemic treatment as in North American regimens. Thus, surgical intervention occurs at a relatively later point in the treatment course in the European protocols. Several details regarding the role of aggressive surgery in high-risk neuroblastoma remain controversial. Inconsistent documentation of surgical approaches and lack of immediate post-operative imaging should be addressed in upcoming trials as in the European International Collaboration for Neuroblastoma Research (SIOPEN) HR-NBL2 trial.
ii. Primary Site Radiotherapy
External beam RT to the primary site has an established role in treatment of patients with high-risk neuroblastoma based on substantial clinical evidence, despite the absence of randomized data. Currently, different international groups use varying RT strategies. The recently closed SIOPEN High Risk Neuroblastoma 1 study prescribed a dose of 21 Gy in 14 fractions to the pre-surgical primary tumor volume, including regional lymph nodes if involved, for all patients. A compromise in dose or volume was allowed in cases with very large tumors to meet normal tissue tolerances. This dose was given regardless of extent of the disease or surgery. Preliminary retrospective results presented at ASCO 2018 showed that the 5-year EFS for patients with complete macroscopic excision who received RT was 44 ± 2%, but 31 ± 6% without RT (p = 0.013).35 The use of radiation was not randomized on this trial, however RT was omitted in some very young patients or those with very large primary tumors.
The German Pediatric Oncology and Hematology Group (GPOH) utilized intensified local therapy with RT in metastatic neuroblastoma patients with unresectable MIBG-avid residual primary tumors on the GPOH NB97 trial :36 A retrospective analysis of 110 patients showed that 13 patients who received RT for local residual disease had a similar outcome to 74 unirradiated patients without any MIBG-positive residual (3-year EFS 85% with RT vs 61%, 3-year OS 92% with RT vs 75%). The outcome was worse in 23 children without EBRT to the residual primary (3-year EFS 25%, 3-year OS 51%). These data support the use of EBRT to address residual primary disease in patients with HR-NB, although the question of benefit of radiotherapy for completely resected disease remains in this population, with findings limited by small patient numbers and retrospective nature. Currently, the GPOH uses primary site EBRT only in patients > 1 year old with MIBG-positive residual disease after induction chemotherapy. In these patients, a total dose of 36 Gy is delivered to the residual tumor volume following high dose chemotherapy and stem cell transplantation. Based on the GPOH experience and previous SIOPEN experience, the current SIOPEN High-Risk Neuroblastoma Study 2 (SIOPEN/HR-NBL2) will investigate whether dose escalation beyond 21 Gy will improve local control and survival for patients with residual disease. Patients with macroscopic residual disease after induction chemotherapy, surgery, high dose chemotherapy, and autologous stem cell rescue will be randomized to receive 21 Gy to the pre-operative tumor volume versus 21 Gy to the pre-operative tumor bed + 15 Gy boost to the residual tumor.
iii. Metastatic Site Radiotherapy
Radiation to a limited number of metastatic sites, in addition to the primary site, is still controversial. Unlike COG trials, the SIOPEN strategy is to omit RT for metastatic disease because of lack of clear supporting data. In a retrospective analysis of SIOPEN data, patients with more than 3 distinct spots or diffuse disease on MIBG scan had a significantly poorer outcome than patients with ≤ 3 distinct spots after induction chemotherapy.37 These findings bolstered the argument for adding radiotherapy to persistent metastatic sites to improve outcomes. Randomized trials are needed to more accurately define the role of radiotherapy for metastatic sites in neuroblastoma.
Total body irradiation (TBI) has been abandoned for the treatment of neuroblastoma in North America and Europe. Significant long-term side effects after TBI for neuroblastoma included cataracts, hypothyroidism, growth delay, and the risk of secondary tumors.38
SYSTEMIC THERAPY:
North America:
The general treatment paradigm in North America includes 5 cycles of six-drug induction therapy utilizing topotecan, cyclophosphamide, cisplatin, etoposide, doxorubicin, and vincristine. Maximal safe resection of the primary tumor is undertaken after 4 cycles of chemotherapy. Induction chemotherapy is followed by tandem cycles of high-dose chemotherapy and autologous stem cell rescue, and finally, immunotherapy, historically consisting of alternating cycles of dinutuximab/granulocyte macrophage colony stimulating factor (GM-CSF) and dinutuximab/Interleukin-2 (IL-2). This paradigm results in 5-year event-free survival rates of approximately 60%, and has been developed based on results of a series of clinical trials led by the Children’s Oncology Group.6 Of particular interest is the importance of immunotherapy in improving prognosis – neuroblastoma is one of the few cancers for which immunotherapy has been demonstrated to provide significant benefit over conventional chemotherapy alone, and has thus been incorporated as part of up front treatment for the past decade. Permutations to the above paradigm currently under investigation include addition of therapeutic 131I-MIBG and targeted small molecules based on individual disease, patient, and tumor characteristics (Fig 1).
Figure 1:
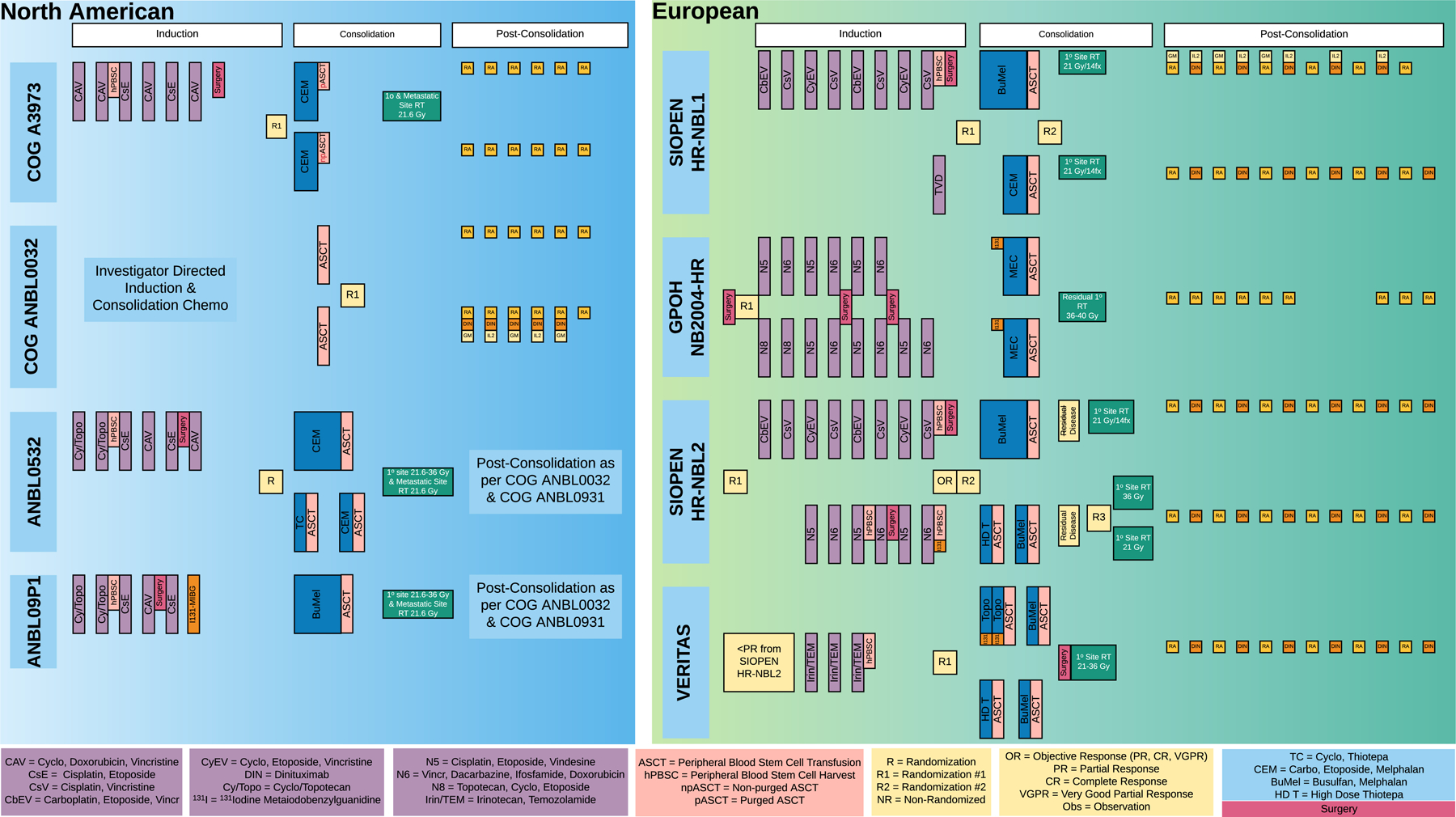
Multidisciplinary treatments and high dose chemotherapy and stem cell transplant strategies adopted in recent COG and European Protocols.
A historic approach is essential to understanding the rationale behind the current complex, multi-modality treatment of high-risk neuroblastoma. In the 1990s, the Children’s Cancer Group (CCG) trial 3891 investigated 1) the combination of high dose chemotherapy (HDC)/ total body irradiation/ ASCR compared to intensive conventional chemotherapy and 2) the addition of 13-cis-retinoic acid after completion of chemotherapy. This trial of 539 patients showed improvement in EFS with the HDC regimens and the addition of 13-cis-retinoic acid as continuation therapy; the group that received both interventions achieved 3-year EFS of 55% compared to 18% for those that received neither.39 Subsequently, COG ANBL0032 demonstrated improvement in 2-year EFS from 46% to 66% with the addition of anti-GD2 antibody dinutuximab and cytokines combined with cis-retinoic acid compared to cis-retinoic acid alone. COG ANBL0532 demonstrated that use of tandem cycles of high-dose chemotherapy with ASCR compared to a single cycle resulted in improved 3-year EFS of 63% vs 49%; high-dose regimens in this study included carboplatin/etoposide/melphalan (CEM) with or without addition of thiotepa/cyclophosphamide (TC).6 (Fig.1)
Single agent 131I-MIBG at doses greater than 12mCi/kg may result in disease response for over 30% of patients with relapsed and refractory neuroblastoma, and 131I-MIBG has been used in the setting of relapsed/refractory disease for several decades.39,40 Kraal et al showed that upfront therapy with 131I-MIBG before systemic therapy as per the German NB2004-HR protocol was feasible, tolerable and effective in newly diagnosed high risk NBL patients.41
More recently, 131I-MIBG has been incorporated into pilot trials after induction therapy both in Europe and the United States: COG ANBL09P1 was a pilot study designating 15mCi/kg as the appropriate dose of this agent when given after completion of induction chemotherapy and prior to ASCR. Earlier administration of 131I-MIBG is being studied in the randomized portion of the COG phase III trial, ANBL1531 (currently open to enrollment). Patients on this study whose tumors are found to have aberrations in the ALK gene (expected to be approximately 15% of newly diagnosed patients with high risk disease)42 will receive standard multi-modality therapy with the addition of the small molecule ALK inhibitor, crizotinib, using a dose previously evaluated in ADVL0912.43 The ANBL1531 study is also evaluating different high-dose chemotherapy regimens used during consolidation therapy. This study will randomize patients to receive the TC/CEM tandem regimen (that improved outcomes in the ANBL0532 study compared to CEM alone) versus a single cycle of HDC busulfan/melphalan (BuMel). The BuMel conditioning regimen was found to be superior to a single CEM transplant in European trials44 and found to be safe when used with United States regimens in the ANBL12P1 trial.45 Overall, the goals of the open ANBL1531 study are to 1) understand potential for improved outcomes based on addition of novel agents to induction regimens, and 2) determine the optimal HDC conditioning regimen preceding ASCR.
Europe:
Strong evidence from randomized trials supports use of several systemic therapy regimens in treatment of high-risk neuroblastoma (Fig.1). Alkylating agents, platinum analogues, vinca alkaloids, epipodophyllotoxins and anthracyclines are considered standard agents. Only a few new drugs have been introduced in recent years, and include topotecan, irinotecan and temozolomide.46
The SIOPEN HR-NBL1 trial compared RAPID COJEC (vincristine, carboplatin, etoposide, cisplatin, cyclophosphamide) induction chemotherapy with the Memorial Sloan Kettering modified N7 regimen (cyclophosphamide, doxorubicin, vincristine, cisplatin,etoposide). Preliminary results showed no difference in survival and metastatic response rates.47 However, RAPID COJEC was less toxic than the modified N7 regimen, so this regimen was selected to be the SIOPEN reference induction regimen. In the induction phase of the German (GPOH) NB2004-HR trial, patients were randomized between six N5 (cisplatin, vindesine, etoposide)-N6 (vincristine, dacarbazine, ifosfamide, doxorubicin) cycles or the experimental induction chemotherapy having two additional topotecan-based cycles (N8 (topotecan, cyclophosphamide, topotecan, etoposide) -N5-N6 cycles). Final results of the trial are expected in 2020. The GPOH trials NB97 and NB2004 utilize therapy with 131I-MIBG for patients with residual MIBG-avid disease (either metastatic or at the primary site) at the end of induction therapy.46 (Fig. 1)
The benefit of HDC consolidation was demonstrated in three randomized trials.46 Recent COG data also suggested that tandem intensification was feasible and could potentially benefit certain patients.6 The VERITAS trial by SIOPEN randomizes very high-risk neuroblastoma patients to single HDC with Bu-Mel versus tandem HDC with Thiotepa and Bu-Mel, followed by ASCR, with addition of a131I-MIBG arm.26 (Fig. 1)
FUTURE RESEARCH AND DIRECTIONS:
Despite significant progress in treatment of children with high-risk neuroblastoma, many children are not cured with the intensive multi-modality therapy outlined above. In both North America and Europe, future trials will focus on improvements in induction therapy and optimization of high-dose chemotherapy regimens. In North America, ANBL1531 is examining introduction of 131I-MIBG and crizotinib into induction regimens, and recent data from the St. Jude Children’s Cancer Research Hospital NB2012 trial indicate that immunotherapy can be safely combined with induction chemotherapy.48 This approach is currently being studied in a multi-center COG pilot. The European SIOPEN HR-NBL2 trial will aim to define the most effective chemotherapy induction regimen, comparing head to head RAPID COJEC and the GPOH N5-N6 regimens. Efforts through COG and SIOPEN will also be directed towards understanding specific uses of tandem and single cycles of HDC using different agents and in the setting of specific consolidation regimens. Future trials may aim to further individualize induction therapy, potentially with inclusion of targeted antibodies with and without immune modulating cytokines. Increasing numbers of targeted small molecules are being developed and tested in preclinical and clinical settings, and may also be included in future pilot studies. Newer and potentially more effective inhibitors of ALK signalling are being evaluated in the setting of relapsed disease,49 and may soon be incorporated into frontline trials. Because studies have shown that aberrations in components of the MAPK signalling pathway are more common in tumors sampled at relapse compared with paired tumors from the same patient obtained at the time of diagnosis,50 considerable interest exists in development of inhibitors of these molecules for neuroblastoma therapy.
Many questions remain regarding optimal use of external beam radiotherapy – these include dose to primary site, particularly in the setting of residual disease after induction chemotherapy and surgical resection, and optimal treatment of persistent metastatic disease sites. As systemic therapy continues to evolve, the role of local control and focused therapies require continued evaluation, recognizing that this disease requires aggressive multimodality therapy in order to be cured, but also that optimal therapy may ultimately be highly individualized.
ABBREVIATIONS
- INRGSS
International Neuroblastoma Risk Group Staging System
- HR NBL
High risk neuroblastoma
- RT
Radiation Therapy
- MIBG
Meta-iodobenzyl guanidine
- PET
Positron Emission Tomography Scan
- IDRFs
Image-defined risk factors
- ASCR
Autologous Stem Rescue
- TBI
Total body Irradiation
- CTV
clinical target volume
- IMRT
Intensity Modulated Radiation Therapy
Footnotes
CONFLICT OF INTEREST: NONE
REFERENCES:
- 1.Maris JM. Recent advances in neuroblastoma. The New England journal of medicine 2010;362:2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: a cancer journal for clinicians 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 3.Goodman MTGJ, Smith MA, Olshan AF. Sympathethic Nervous System Tumors. Cancer Incidence and Survival among Children and Adolescents: : United States SEER Program, 1975–1995. Bethesda, MD: 1999. [Google Scholar]
- 4.Neuroblastic tumours of adrenal gland and sympathetic nervous system. Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC; 2000:153. [Google Scholar]
- 5.Angstman KB, Miser JS, Franz WB 3rd. Neuroblastoma. American family physician 1990;41:238–44. [PubMed] [Google Scholar]
- 6.Park JR, Kreissman SG, London WB, et al. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children’s Oncology Group (COG) study. 2016;34:LBA3-LBA. [Google Scholar]
- 7.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du L, Liu L, Zhang C, et al. Role of surgery in the treatment of patients with high-risk neuroblastoma who have a poor response to induction chemotherapy. Journal of pediatric surgery 2014;49:528–33. [DOI] [PubMed] [Google Scholar]
- 10.La Quaglia MP. State of the art in oncology: high risk neuroblastoma, alveolar rhabdomyosarcoma, desmoplastic small round cell tumor, and POST-TEXT 3 and 4 hepatoblastoma. Journal of pediatric surgery 2014;49:233–40. [DOI] [PubMed] [Google Scholar]
- 11.von Allmen D, Davidoff AM, London WB, et al. Impact of Extent of Resection on Local Control and Survival in Patients From the COG A3973 Study With High-Risk Neuroblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman EA, Abdessalam S, Aldrink JH, et al. Update on neuroblastoma. Journal of pediatric surgery 2018. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Polishchuk A, DuBois S, et al. Patterns of Relapse in High-Risk Neuroblastoma Patients Treated With and Without Total Body Irradiation. International journal of radiation oncology, biology, physics 2017;97:270–7. [DOI] [PubMed] [Google Scholar]
- 14.Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: a Children’s Cancer Group study. International journal of radiation oncology, biology, physics 2003;56:28–39. [DOI] [PubMed] [Google Scholar]
- 15.Haas-Kogan D, Douglas J, London WB, et al. Extent of Lymph Node Radiation Coverage in High-Risk Neuroblastoma Does Not Affect Clinical Outcome: A Report From the COG A3973 Study. International Journal of Radiation Oncology • Biology • Physics 2014;90:S114. [Google Scholar]
- 16.Children’s Oncology Group https://childrensoncologygroup.org/anbl1531. Accessed 5/13/20.
- 17.Liu KX, Naranjo A, Zhang FF, et al. Role of Radiotherapy Dose-Escalation for High-Risk Neuroblastoma with Post-Surgical Primary Site Gross Residual Disease: A Reports from the COG ANBL0532 Study. Int J Radiat Oncol Biol Phys 2019;105(1):S3. [Google Scholar]
- 18.Casey DL, Kushner BH, Cheung NV, Modak S, LaQuaglia MP, Wolden SL. Dose-escalation is needed for gross disease in high-risk neuroblastoma. Pediatric blood & cancer 2018;65:e27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas JT Jr., et al. Implications of Image-Defined Risk Factors and Primary Site Response on Local Control and Radiation Treatment Delivery in the Management of High Risk Neuroblastoma: Is there a Role for De-escalation of Adjuvant Primary Site Radiotherapy? International Journal of Radiation Oncology, Biology, and Physics 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utriainen P, Vatanen A, Toiviainen-Salo S, Saarinen-Pihkala U, Makitie O, Jahnukainen K. Skeletal outcome in long-term survivors of childhood high-risk neuroblastoma treated with high-dose therapy and autologous stem cell rescue. Bone marrow transplantation 2017;52:711–6. [DOI] [PubMed] [Google Scholar]
- 21.Elzembely MM, Dahlberg AE, Pinto N, et al. Late effects in high-risk neuroblastoma survivors treated with high-dose chemotherapy and stem cell rescue. Pediatr Blood Cancer 2018:e27421. [DOI] [PubMed] [Google Scholar]
- 22.Fahy AS, Roberts A, Nasr A, Irwin MS, Gerstle JT. Long term outcomes after concurrent ipsilateral nephrectomy versus kidney-sparing surgery for high-risk, intraabdominal neuroblastoma. Journal of pediatric surgery 2018. [DOI] [PubMed] [Google Scholar]
- 23.Stone A, Novetsky Friedman D, Worgall S, et al. Long-term Pulmonary Outcomes in Pediatric Survivors of High-risk Neuroblastoma. Journal of pediatric hematology/oncology 2017;39:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong AE, Danner-Koptik K, Golden S, et al. Late Effects in Pediatric High-risk Neuroblastoma Survivors After Intensive Induction Chemotherapy Followed by Myeloablative Consolidation Chemotherapy and Triple Autologous Stem Cell Transplants. Journal of pediatric hematology/oncology 2018;40:31–5. [DOI] [PubMed] [Google Scholar]
- 25.Ng LW, Wong KK, Ally Wu CL, Sposto R, Olch AJ. Dose Sculpting Intensity Modulated Radiation Therapy for Vertebral Body Sparing in Children With Neuroblastoma. International journal of radiation oncology, biology, physics 2018;101:550–7. [DOI] [PubMed] [Google Scholar]
- 26.Turcotte LM, Whitton JA, Friedman DL, et al. Risk of Subsequent Neoplasms During the Fifth and Sixth Decades of Life in the Childhood Cancer Survivor Study Cohort. J Clin Oncol 2015;33:3568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kralik SF, Watson GA, Shih CS, Ho CY, Finke W, Buchsbaum J. Radiation-Induced Large Vessel Cerebral Vasculopathy in Pediatric Patients With Brain Tumors Treated With Proton Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;99:817–24. [DOI] [PubMed] [Google Scholar]
- 28.Casey D, Kushner B, Cheung NK, Modak S, La Quaglia M, Wolden S. Local Control with Reduced-Dose Radiation Therapy for High-Risk Neuroblastoma: 3-Year Results from a Prospective Trial. Pediatric Blood & Cancer 2018;65:S242-S. [Google Scholar]
- 29.Hattangadi JA, Rombi B, Yock TI, et al. Proton radiotherapy for high-risk pediatric neuroblastoma: early outcomes and dose comparison. International journal of radiation oncology, biology, physics 2012;83:1015–22. [DOI] [PubMed] [Google Scholar]
- 30.DeWitt JC, Mock A, Louis DN. The 2016 WHO classification of central nervous system tumors: what neurologists need to know. Curr Opin Neurol 2017;30:643–9. [DOI] [PubMed] [Google Scholar]
- 31.Bradfield SM, Douglas JG, Hawkins DS, Sanders JE, Park JR. Fractionated low-dose radiotherapy after myeloablative stem cell transplantation for local control in patients with high-risk neuroblastoma. Cancer 2004;100:1268–75. [DOI] [PubMed] [Google Scholar]
- 32.Casey DL, Pitter KL, Kushner BH, et al. Radiation Therapy to Sites of Metastatic Disease as Part of Consolidation in High-Risk Neuroblastoma: Can Long-term Control Be Achieved? International journal of radiation oncology, biology, physics 2018;100:1204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatcombe HG, Marcus RB Jr., Katzenstein HM, Tighiouart M, Esiashvili N. Excellent local control from radiation therapy for high-risk neuroblastoma. International journal of radiation oncology, biology, physics 2009;74:1549–54. [DOI] [PubMed] [Google Scholar]
- 34.Polishchuk AL, Li R, Hill-Kayser C, et al. Likelihood of bone recurrence in prior sites of metastasis in patients with high-risk neuroblastoma. International journal of radiation oncology, biology, physics 2014;89:839–45. [DOI] [PubMed] [Google Scholar]
- 35.Holmes KPU, Sarnacki S, Monclair T, Cecchetto G, Gomez Chacon J, et al. Influence of Surgical Excision on Survival in High-Risk Neuroblastoma Revisted After introduction of ch14.18/CHO Immunotherapy in the HR-NBL1/SIOPEN Trial. ASCO2018. [Google Scholar]
- 36.Simon T, Hero B, Bongartz R, Schmidt M, Muller RP, Berthold F. Intensified external-beam radiation therapy improves the outcome of stage 4 neuroblastoma in children > 1 year with residual local disease. Strahlenther Onkol 2006;182:389–94. [DOI] [PubMed] [Google Scholar]
- 37.Ladenstein R, Lambert B, Potschger U, et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials. European journal of nuclear medicine and molecular imaging 2018;45:292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flandin I, Hartmann O, Michon J, et al. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric oncology. International journal of radiation oncology, biology, physics 2006;64:1424–31. [DOI] [PubMed] [Google Scholar]
- 39.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. The New England journal of medicine 1999;341:1165–73. [DOI] [PubMed] [Google Scholar]
- 40.DuBois SG, Matthay KK. Radiolabeled metaiodobenzylguanidine for the treatment of neuroblastoma. Nuclear medicine and biology 2008;35 Suppl 1:S35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraal KC, Bleeker GM, van Eck-Smit BL, et al. Feasibility, toxicity and response of upfront metaiodobenzylguanidine therapy therapy followed by German Pediatric Oncology Group Neuroblastoma 2004 protocol in newly diagnosed stage 4 neuroblastoma patients. European journal of cancer (Oxford, England : 1990) 2017;76:188–96. [DOI] [PubMed] [Google Scholar]
- 42.Bresler SC, Weiser DA, Huwe PJ, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer cell 2014;26:682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. The Lancet Oncology 2013;14:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ladenstein R, Potschger U, Pearson ADJ, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. The Lancet Oncology 2017;18:500–14. [DOI] [PubMed] [Google Scholar]
- 45.Granger M, Yanik GA, Naranjo A, et al. Myeloablative busulfan/melphalan (BuMel) consolidation following induction chemotherapy for patients with high-risk neuroblastoma: A Children’s Oncology Group (COG) study. 2016;34:10528-. [Google Scholar]
- 46.Amoroso L, Erminio G, Makin G, et al. Topotecan-Vincristine-Doxorubicin in Stage 4 High-Risk Neuroblastoma Patients Failing to Achieve a Complete Metastatic Response to Rapid COJEC: A SIOPEN Study. Cancer research and treatment : official journal of Korean Cancer Association 2018;50:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garaventa APU, Valteau-Couanet D, Castel V, Elliot M, Ash S, Chan G Randomised Induction for High-Risk Neuroblastoma Comparing COJEC and the N5-MSKCC Regimen. Early Results from the HR-NBL1.5/SIOPEN. Advances in Neuroblastoma Research Association; 2018; San Francisco. [Google Scholar]
- 48.Furman WL, Federico SM, McCarville MB, et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-RisK Neuroblastoma. Clin Cancer Res 2019;25(21):6320–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldsmith KC, Kayser K, Groshen SG, et al. Phase I trial of lorlatinib in patients with ALK-driven refractory or relapsed neuroblastoma: A New Approaches to Neuroblastoma Consortium Study (Abstract). J Clin Oncol 38: 2020. (suppl; abstr 10504) [Google Scholar]
- 50.Eleved TF, Oldridge DA, Bernard V, et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet 2015;47(8):864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


