Summary
Currently licensed COVID-19 vaccines are all designed for intramuscular (IM) immunization. However, vaccination today failed to prevent the virus infection through the upper respiratory tract, which is partially due to the absence of mucosal immunity activation. Despite the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants, the next generation of COVID-19 vaccine is in demand and intranasal (IN) vaccination method has been demonstrated to be potent in inducing both mucosal and systemic immune responses. Presently, although not licensed, various IN vaccines against SARS-CoV-2 are under intensive investigation, with 12 candidates reaching clinical trials at different phases. In this review, we give a detailed description about current status of IN COVID-19 vaccines, including virus-vectored vaccines, recombinant subunit vaccines and live attenuated vaccines. The ongoing clinical trials for IN vaccines are highlighted. Additionally, the underlying mechanisms of mucosal immunity and potential mucosal adjuvants and nasal delivery devices are also summarized.
Keywords: COVID-19, SARS-CoV-2, Intranasal vaccine, Mucosal, Adjuvant
Introduction
COVID-19 pandemic caused by SARS-CoV-2 is detrimental to global health owing to its quick spread and high mutation rate, highlighting the importance of effective vaccines to prevent further morbidity and mortality. Currently, over 500 vaccines are under development, with over 150 candidates under clinical evaluation and 24 vaccines authorized for emergency use in humans.1, 2, 3 SARS-CoV-2 is a typical mucosal pathogen that spreads from person to person via respiratory droplets.4 It infects human epithelial cells in the respiratory tract by binding to angiotensin-converting enzyme 2 (ACE2) receptors through viral spike (S) receptor-binding domain (RBD).5 Mucosal immunity is essential for adequate and long-term protection against viral infection.6 All the licensed COVID-19 vaccines are administered via intramuscular (IM) injection, which are ineffective in inducing mucosal immunity. Alternatively, mucosal immunization has the potential to induce both mucosal and systemic immune responses. Nine mucosal vaccines have been approved for humans against various mucosal pathogens, including eight orally and one intranasally (IN) administered vaccines, all of which belong to whole virus vaccines.4,7 IN vaccines are highly attractive without the requirement of needles. The significant advances of subunit protein vaccines and virus-vectored vaccines have been translated into IN vaccines of SARS-CoV-2 in preclinical and clinical settings.
Some reviews have covered the development of IN COVID-19 vaccines,4,8,9 but most of the information was outdated and incomplete considering current momentum in vaccine development. The latest review by Chavda et al. only introduced some representative IN vaccines under clinical evaluation.9 Since most clinical trial data are yet to be released, preclinical studies are essential for us to explore about the immunogenicity and safety of IN COVID-19 vaccines. This review gives a comprehensive and state-of-the-art summary of recent advances in the development of IN COVID-19 vaccines, including vaccines in research stage and under clinical trials. We also introduced mucosal immunity to help readers understand how IN vaccines work. Finally, to facilitate clinical translation, we discussed IN vaccines' potential adjuvants and delivery systems. We hope this review will provide researchers with a comprehensive understanding of this field and inspire the future development of novel IN vaccines.
Mucosal immunity
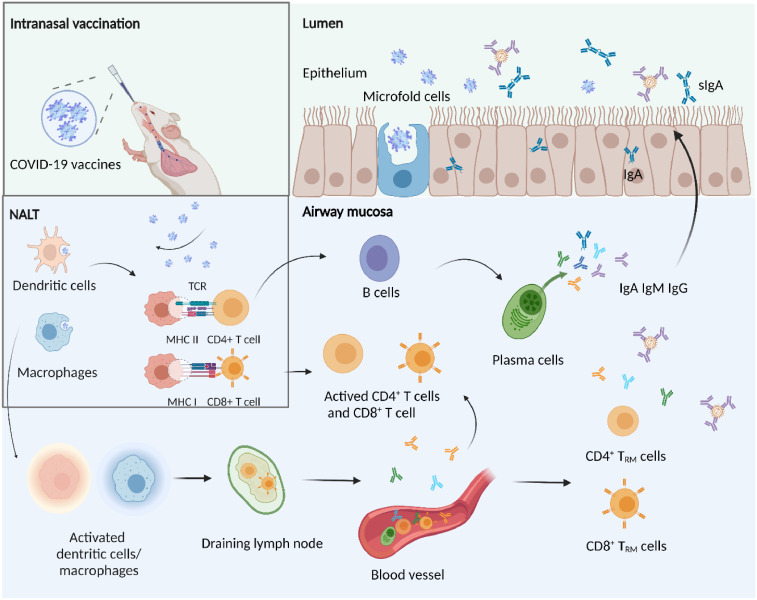
Mucosal immunity is a complex network composed of tissues, non-lymphoid cells, lymphocytes, and effector molecules (e.g., cytokines, chemokines, and antibodies).10 Generally, conventional injection vaccines are poor inducers of mucosal immunity, whereas IN immunization can induce strong mucosal immunity to avoid the entry and development of mucosal pathogens (Figure 1). When SARS-CoV-2 enters the nasal cavity, the respiratory epithelial layer becomes the first barrier against viral infection.11 Then, the innate immunity components in the upper airway mucosa become the first line of defense, which consist of diverse immune cells, including phagocytic neutrophils, macrophages, dendritic cells (DCs), natural killer cells, and mast cells.12 These immune cells can form an integrated system to directly fight against pathogens or induce adaptive mucosal immunity via multiple mechanisms.13 If the viruses reach deeper airways or lungs, pathogens-associated molecular patterns (PAMPs) expressed on viruses will be recognized by pattern recognition receptors (PRRs) expressed in respiratory epithelial cells, DCs, and alveolar macrophages.14,15 Viral particles are degraded by endosomal enzymes and release single-stranded RNA that can be recognized by toll-like receptor (TLR) 7 or TLR8.16 Activation of the PRRs leads to increased expression of proinflammatory cytokines such as type I interferon (IFN-I).17 IFN-I and IFN-III play significant roles in fighting against viral infection at early stages. However, SARS-CoV-2 infection induces a lower level of IFN-I and IFN-III than other respiratory viruses.18 Additionally, the complement system is a crucial component of innate immunity against SARS-CoV-2,19 which should be considered when designing antibody-based therapeutics and vaccines against SARS-CoV-2.
Figure 1.
Mucosal immune responses induced by IN vaccination. SARS-CoV-2 antigens uptake by M cells occurs in NALT and leads to the local induction of immune responses. DCs and macrophages rapidly absorb the antigens and present them to CD4+ or CD8+ T cells in mucosal lymphoid tissues. CD4+T cells then induce immunoglobulin-committed B cells to plasm cells that secrete immunoglobulins in blood. The produced IgA will diffuse into the lumen whereas IgG and IgM would neutralize viruses in the blood. Activated DCs/macrophages can also migrate to draining lymph nodes to prime antigen-specific CD4+ and CD8+T cells. Stimulated lymphocytes proliferate and migrate from lymph nodes, distribute to peripheral blood and finally localize at mucosal effector sites to develop a tissue-resident phenotype. Created with BioRender.com.
Mucosal immunization can induce extensive adaptive immune responses, characterized by mucosal secretory IgA (sIgA) antibodies and resident memory T (TRM) cells. Mucosal sIgA can be divided into dimeric, trimeric, tetrameric, and larger polymeric IgA (pIgA), in contrast to monomeric IgA in serum. Dimeric sIgA is the primary effective form of mucosal sIgA.20 The identified sIgA inductive sites include nasopharynx-associated lymphoid tissue (NALT), isolated lymphoid follicles, and mesenteric lymph nodes.20 NALT serves as the key site in inducing mucosal immune responses, where Th1- and Th2-polarized lymphocytes, and IgA-secreting B cells are generated.21,22 sIgA antibodies neutralize toxins or pathogens in the mucosa through three pathways: immune exclusion, antigen excretion, and intracellular neutralization.22, 23, 24 Intriguingly, mucosal sIgA levels increase promptly in infants and reach adult levels early in childhood, while serum IgA matures much slower and may not reach adult levels until puberty.25 Given the apparent differences in the susceptibility of children and adults to COVID-19,26 this difference should be taken into consideration in vaccine development.
TRM cells in the airways and lungs are vital for preventing respiratory virus infection. TRM cells confer rapid response upon restimulation with viral antigens, which induce the production of inflammatory cytokines to mediate tissue antiviral resistance and chemokines to recruit auxiliary immune cells.27 It is demonstrated that lung TRM cells provide stronger protective immunity than circulating T cells.28 IN vaccination effectively induces the generation of TRM cells, and persistent antigen present in the lungs can promote long-term maintenance of TRM cells.29 TRM cells in the lungs can further migrate to mediastinal lymph nodes through ‘retrograde migration’ process to maintain TRM cell phenotype and offer long-term immunity.30 CD8+ TRM cells are vital for direct protection against respiratory viral infection. It is indicated that a combination of mucosal prime and systemic booster vaccinations can enhance the longevity of lung CD8+TRM cells.7 CD4+ TRM cells are required to help guide the formation of protective CD8+ TRM cells and B cells.31,32 The efficacy of IN vaccines is significantly elevated owing to their capacity of inducing TRM cells in the respiratory system.
Recent progress in the development of intranasal COVID-19 vaccines
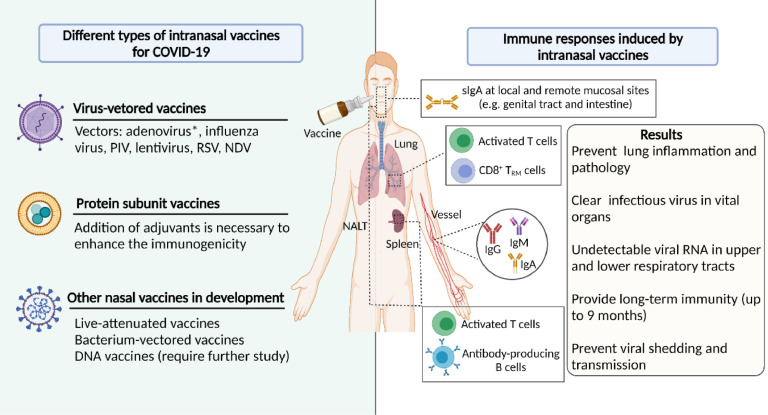
Recent studies indicate that IM vaccines are poor in controlling viral replication and nasal shedding in the upper respiratory tract, leading to asymptomatic or milder symptomatic infection that can still transmit virus to others. In contrast, IN vaccines have the potential to induce sterilizing immunity against mucosal pathogens (Figure 2).33 Antigens are exposed at the initial site of viral attack to induce potent immune responses at local or distant mucosa.34 Meanwhile, the systemic humoral and cellular immunity triggered by IN administration is comparable to or stronger than that induced by IM injection, suggesting a lower dose required to increase vaccine safety. For instance, IN immunization with a chimpanzee adenovirus (Ad)-vectored SARS-CoV-2 vaccine (ChAd-SARS-CoV-2-S) induced higher levels of S-specific neutralizing antibodies in hamsters compared to IM route.35 In addition, IN immunization can induce pan-reactive antibodies,36 which is particularly attractive owing to the increase in COVID-19 variants.
Figure 2.
Current progress in the development of IN vaccines for COVID-19. Different types of IN vaccines have been developed for COVID-19, including virus-vectored vaccines, protein subunit vaccines and others. Virus-vectored IN vaccines are under most intensive investigations, especially adenovirus-vectored vaccines. IN vaccines can induce strong immune responses against SARS-CoV-2, including mucosal (sIgA), humoral (IgM, and IgG), and cellular immune responses, which can protect immunized animals or humans against SARS-CoV-2 infection and transmission. Created with BioRender.com.
The development of IN vaccines also brings huge socio-economic value. In a global scale pandemic, such as COVID-19, immunizing whole populations as quickly as possible can be achieved through IN vaccines as they do not require to be administered by healthcare professionals, leading to higher patient compliance. The fact that these are often delivered through nasal devices makes them a better alternative for individuals who are not keen on needle injections, particularly children. Another advantage of IN vaccines is that they do not require a sterile environment for administration which benefits vaccination programs in countries with fewer resources. Moreover, IN delivery can avoid risks associated with improper use of needles, such as transmitting blood-borne diseases like hepatitis B (HBV) and human immunodeficiency virus (HIV). For these reasons, scientists are making great efforts in developing IN COVID-19 vaccines.
The major challenge in developing IN vaccines is to deliver the antigens to antigen-presenting cells (APCs) presented in the respiratory tract while overcoming nasal clearing. As described in earlier sections, the epithelial layer traps viral particles and removes them from the body. Hence, the antigen used in the vaccine must remain in the respiratory tract for an adequate amount of time to allow antigen absorption, which elicits immune responses for a long time. Besides, the antigen must be stable enough to withstand the enzymatic degradation in the mucosal lining, such as the azoreductase-mediated degradation.37 A common approach to overcome such obstacles is to turn into vaccine formulations, such as the use of adjuvants and delivery systems, to prolong the time in which the antigen remains in the mucosal lining while enhancing its stability.
Although the first IN vaccine is yet to be approved for human use at the time of writing, 12 nasal vaccines have entered different stages of clinical trials. Here we gave a comprehensive introduction about current preclinical (Table 1) and clinical (Table 2) studies of IN COVID-19 vaccines.
Table 1.
Preclinical studies of IN COVID-19 vaccines.
| Type | Vaccine | Target | Route (no. of doses) | Animal used |
|---|---|---|---|---|
| Ad-vectored vaccine | ChAd-SARS-CoV-2-S | S protein | IN (1) | hACE2 mice33,43 and rhesus macaques42 |
| ChAd-SARS-CoV-2-S | S protein | IM or IN, (1) | Golden Syrian hamsters35 | |
| AdCOVID | RBD protein | IN (1) | Mice44 | |
| Ad5.SARS-CoV2-S1 | S1 protein | IN or SC, (1) | Mice45 | |
| ChAdOx1 | S protein | IM or IN, (1 or 2) | Ferrets47, hamsters and rhesus macaques46 | |
| Ad5-nCoV | Full-length S protein | IM or IN, (1); or oral + IN, simultaneously, (1) | Mice and ferrets38 | |
| Ad5-S-nb2 | S protein | IM or IN, (1) | Mice and rhesus macaques39 | |
| Ad5‐N | N protein | IN (2) | Mice52 | |
| AdC7-S, AdC7-RBD and AdC7-RBD-tr2 | S, RBD or tandem-repeat dimeric RBD | IM or IN, (2) | hACE2 mice50 | |
| – | S and N proteins | SC or IN, (1) | Mice51 | |
| Lentivirus-vectored vaccine | – | S protein | IP + IN (2); or IM + IN (2) | Mice and golden hamsters57 |
| – | S protein | IM + IN, (2) | hACE2 mice58 | |
| Influenza virus-vectored vaccine | ∆NA(RBD)-Flu virus | RBD protein | IN (1) | Mice53 |
| Influenza A virus-vectored vaccine | scPR8-RBD-M2 | SARS-CoV-2 RBD and influenza matrix 2 protein | IN +IN or IN + IM, (2) | Mice54 |
| PIV5-vectored vaccine | CVXGA1 | S protein | IN (1) | K18-hACE2 mice and ferrets55 |
| hPIV2-vectored vaccine | BCPIV/S-2PM | S protein | IN (1or 2) | Mice and golden hamsters56 |
| NDV-vectored vaccine | AVX/COVID-12-HEXAPRO | S protein | IN +IN, IM + IM, or IN + IM, (2) | Mice, hamsters60, and pigs59 |
| NDV-FLS | S protein | IN (1 or 2) | Hamsters61 | |
| rNDV-S | S protein | IN (2) | Mice and hamsters62 | |
| VSV-vectored vaccine | rVSVSARS-CoV-2 | S protein | IN or IM, (1) | Normal/hACE2 mice, and macaques63 |
| VSV-SARS2-EBOV | SARS-CoV-2 S protein and/or the EBOV glycoprotein | IM or IN, (1) | Hamsters64 and rhesus macaques65 | |
| Virus like particles | RBD protein | IM alone or IM + IN, (3) | Ferrets72 | |
| Live-attenuated vaccine | SARS-CoV-2/ human/ Korea/ CNUHV03-CA22 °C /2020 | IN spray (1) | hACE2 transgenic mice76 | |
| COVI-VAC | IN (1) | Syrian golden hamsters77 | ||
| Bacterium-vectored vaccine | M and N proteins | ID or IN, (2) | Hamsters78 | |
| LP18:RBD | RBD | IN (2) | Mice79 | |
| Protein subunit vaccine | RBD protein | IN, IM or ID, (3) | Mice34 | |
| S1 protein | IM (3) or IN (4) | Rhesus macaques73 | ||
| RBD protein | IN or IM, (3) | Mice69 | ||
| S1 protein | IN (3) | Mice70 | ||
| Trimeric or monomeric S protein | IN (1) | Mice71 | ||
| DNA vaccine | pQAC—CoV; MVA-CoV | S and N proteins | IN or IM, (3), or IN (2) | Mice81 |
| S protein | IN | Mice80 |
PIV: parainfluenza virus; NDV: Newcastle disease virus; VSV: vesicular stomatitis virus; EBOV: Ebola virus; MVA: Modified Vaccinia Ankara.
Table 2.
Clinical trials of IN COVID-19 vaccines.
| Type | Vaccine | Developer/manufacturer | Nasal delivery device | Phase | Status | Enrollment | Clinical trial No. | Route |
|---|---|---|---|---|---|---|---|---|
| Ad-vectored vaccine | Ad5-nCoV | CanSino/Beijing Institute of Biotechnology | Aerogen Ultra Device | I | Active, not recruiting | 149 | NCT04552366 | IN, IM or IN+IM |
| I/Ⅱ | Recruiting | 840 | NCT04840992 | IM or IN | ||||
| ChAdOx1 | AstraZeneca/University of Oxford | MAD Nasal™ Intranasal Mucosal Atomization Device | I | Enrolling by invitation | 54 | NCT04816019 | IN | |
| BBV154 | Bharat Biotech International Limited | N/A | I | Active, not recruiting | 175 | NCT04751682 | IN | |
| SC-Ad6–1 | Tetherex Pharmaceuticals Corporation | N/A | I | Recruiting | 80 | NCT04839042 | IM or IN | |
| AdCOVID | Altimmune, Inc. | Pipette droppers | I | Not processing | 180 | NCT04679909 | IN | |
| NDV-vectored vaccine | AVX/COVID-12-HEXAPRO | Laboratorio Avi-Mex, S.A. de C.V. | An automatic syringe (Prima mist sprayer) | I | Recruiting | 90 | NCT04871737 | IN, IM or IN+IM |
| LAIV-vectored vaccine | DelNS1–2019-nCoV-RBD-OPT1 | University of Hong Kong, Xiamen University and Beijing Wantai Biological Pharmacy | Spray devices | I | Complete | 60 | ChiCTR2000037782 | IN |
| Ⅱ | Complete | 720 | ChiCTR2000039715 | IN | ||||
| III | – | 40,000 | ChiCTR2100051391 | IN | ||||
| PIV5-vectored vaccine | CVXGA1 | CyanVac LLC | Spray devices | I | Not recruiting | 80 | NCT04954287 | IN |
| RSV-vectored vaccine | MV-014–212 | Meissa Vaccines, Inc. | Droppers or spray devices | I | Recruiting | 130 | NCT04798001 | IN |
| Protein subunit vaccine | CIGB-669 | CIGB | Syringe-based spray devices | I/Ⅱ | Pending | 88 | RPCEC00000345 | IN alone or IN + IM |
| Razi Cov Pars | Razi Vaccine and Serum Research Institute | Spray devices | I | Complete | 133 | IRCT20201214049709N1 | IM + IN | |
| Ⅱ | Complete | 500 | IRCT20201214049709N2 | IM + IN | ||||
| III | – | 41,128 | IRCT20210206050259N3 | IM + IN | ||||
| Live attenuated vaccine | COVI-VAC | Codagenix, Inc. | Droppers | I | Active, not recruiting | 48 | NCT04619628 | IN |
NDV: Newcastle disease virus; LAIV: live attenuated influenza virus; PIV: parainfluenza virus; RSV: respiratory syncytial virus; N/A: Not available. Data from https://clinicaltrials.gov/, https://www.chictr.org.cn/index.aspx and https://covid-19.cochrane.org/.
Virus-vectored vaccines
Preclinical studies
Adenovirus-vectored vaccines
Wu et al. firstly demonstrated that IN vaccination with a replication-defective Ad5-vectored vaccine encoding the full-length S protein (Ad5-nCoV) induced both systemic and local mucosal immune responses against SARS-CoV-2.38 Ferrets receiving a single mucosal (simultaneous oral and IN delivery), or IM dose of Ad5-nCoV generated high levels of serum neutralizing IgG antibodies. However, mice studies indicated that only IN vaccination can increase the generation of S-specific IgA antibodies in the trachea-lung washes. Upon challenge with SARS-CoV-2, mucosal vaccinated ferrets were immune from viral infection in the upper and lower respiratory tracts, as measured by the infectious virus and viral loads in lung and nasal turbinates, respectively. Contrarily, IM immunization showed limited efficacy in reducing the viral loads in the upper respiratory tract.
Soon after, Feng et al. demonstrated that a single IN immunization with Ad5-S-nb2 induced both systemic and local mucosal protective immunity in mice and rhesus macaques.39 Ad5-S-nb2 is a replication-incompetent Ad5-vectored vaccine encoding S1 protein of SARS-CoV-2. Notably, the preexisting anti-Ad5 immunity in humans from natural exposure or previous vaccination may compromise the efficacy of Ad5-vectored vaccines. It is reported that the existence of vector-specific serum antibodies severely hampers the seroconversion (an alteration from seronegative to seropositive state) of neutralizing antibodies against SARS-CoV-2 in IM vaccinated volunteers.40 Consistent with previous results, IN administration of Ad5-S-nb2 in macaques showed delayed increase and lower serum antibody titers against Ad5 vectors than IM dosing,39,41 indicating that IN vaccination may be devoid of the undesired influence caused by anti-vector immunity.
ChAd-SARS-CoV-2-S vaccine encodes a prefusion stabilized S protein of SARS-CoV-2.33,42,43 Prefusion stabilization enhances antigenicity by increasing the expression of viral fusion glycoproteins that are the targets of neutralizing antibodies.5 One IN dose of ChAd-SARS-CoV-2-S induced neutralizing antibodies and T cell responses, preventing or limiting viral infection in nasal swabs, bronchoalveolar lavage fluid (BALF), and lungs of rhesus macaques.42 IM immunization of mice expressing human ACE2 (hACE2), on the other hand, only trigged a minimal mucosal immune response and scarce antigen-specific IgA in the serum, resulting in detectable viral RNA in the lungs. Therefore, IM vaccination could only reduce, but not abrogate, lung infection. IN immunization also increased the percentage of IFN γ-secreting CD8+ TRM cells in the lungs, providing a first response against reinfection.33 Then, the durability and protectivity of ChAd-SARS-CoV-2-S vaccine was also evaluated.43 A single IN dose induced long-term immunity that lasted for at least nine months, with an increase in the numbers of antibody-secreting long-lived plasma cells in the bone marrow. More importantly, IN vaccination elicited excellent protectivity against mutated strains of SARS-CoV-2 (B.1.351, B.1.1.28 and B.1.617.1). Altimmune, Inc. developed a replication-deficient Ad5-vectored vaccine candidate encoding the RBD of SARS-CoV-2, called AdCOVID.44 A single IN vaccination with AdCOVID was reported to be highly effective in mice. Another team from the University of Pittsburgh developed an Ad5-vectored vaccine that encodes SARS-CoV-2 S1 protein.45 A single subcutaneous or IN administration of this vaccine induced robust and durable antiviral immune responses that lasted for at least 24 weeks.
Bricker et al. found that nasal shedding can only be reduced in IN immunized hamsters and rhesus macaques other than IM vaccinated ones using ChAdOx1 vaccine (an Ad-based vaccine encoding S protein of SARS-CoV-2).35,46,47 In a transmission study model, Syrian hamsters IN or IM immunized with ChAdOx1 vaccine were co-housed with unvaccinated donor animals that were pre-infected with SARS-CoV-2.46 In the transmission model, only partial protection of the lower respiratory tract was observed in IM vaccinated hamsters, whereas IN vaccination showed complete control of viral infection. It is possible that in challenge studies, a high dose of live viruses is directly sent to the lower respiratory tract by swift intratracheally or IN route, where activated systemic immunity could combat virus in the lungs. However, the transmission model simulates an actual state of natural infection, where viral infection is low-dose, persistent, and attacks the upper respiratory tract at first. In SARS-CoV-2 infected patients, it is reported that neutralizing IgA antibodies are often detectable earlier than the appearance of IgG, while mucosal IgA remains detectable for a long time.48 The neutralizing potency of IgA dimers in nasopharynx is 7.5-fold greater than IgG equivalents in the serum.49 High level of sIgA in nasal mucosa induced by IN immunization prevents SARS-CoV-2 infection and stops viral transmission, which is absent in IM immunized animals. Therefore, we can conclude that most IM administered vaccines are unable to clear subgenomic and genomic viral RNA in the upper respiratory tract, suggesting viral replication and the possibility of viral transmission.
Ad-vectored vaccines can also express S antigens with advanced structures or encode other potential antigens to enhance the magnitude and breadth of immune responses. Xu et al. developed several chimpanzee Ad-vectored COVID-19 vaccines expressing S protein (Ad-S), RBD (Ad-RBD), or tanderm-repeat dimeric RBD (Ad-RBD-tr2), respectively. Compared with Ad-S or Ad-RBD, Ad-RBD-tr2 induced the highest level of antibody responses that could neutralize both wild-type and variant strains of SARS-CoV-2, especially the Delta variant.50 N protein alone, or combined with S protein, can also be the target of Ad vector-based IN vaccines, which could induce strong N- and S-specific immune responses in mice.51,52 Further studies are necessary to strengthen the potential of N protein as an ideal vaccine target for SARS-CoV-2.
Vaccines based on other viral vectors
Loes et al. incorporated the coding sequence of RBD into the genome of attenuated influenza virus.53 A single IN dose induced RBD-specific neutralizing antibodies, with titers up to 1:200 in mouse sera. However, high levels of influenza-specific neutralizing antibodies were also detected, halting the development of this vaccine. A single-cycle influenza virus-based vaccine (scPR8-RBD-M2) was also developed to encode SARS-CoV-2 RBD and influenza matrix 2 protein.54 Mice that received two doses of scPR8-RBD-M2 (IN + IN or IN + IM) elicited robust mucosal and systemic immune responses. A parainfluenza virus (PIV) 5-based COVID-19 vaccine, named CVXGA1, prevented virus infection and blocked contact transmission in K18-hACE2 mice and ferrets after a single IN dose.55 Non-propagative human PIV2-based IN COVID-19 vaccine (BC-PIV/S-2PM) protected the lungs from viral infection after one dose and conferred complete protection of the nasal turbinates after two doses.56
A single intraperitoneal injection of a lentivirus-vectored COVID-19 vaccine decreased viral replication in the lungs of golden hamsters. Further boosting via IM route failed to improve the protection rate, whereas IN booster induced high levels of neutralizing IgG and IgA antibodies in the serum, resulting in >3 log10 decreases in lung viral loads.57 Therefore, an IP + IN or IM + IN prime-boost strategy may provide high prophylactic efficacy and allow sterilizing protection against SARS-CoV-2 infection. Another non-integrate lentivirus-vectored IN vaccine induced sterilizing protection of lungs and central nervous system against both the ancestral SARS-CoV-2 and its P.1 variant of concern.58 This vaccine is attractive owing to its protection against the mutated strains, which is the major challenge of the persistent pandemic.
Laboratorio Avi-Mex, S.A. de C.V. developed a live Newcastle disease virus (NDV)-vectored vaccine (AVX/COVID-12-HEXAPRO; Patria) that encodes prefusion stabilized S protein. It induced strong immunity against the wild-type SARS-CoV-2 and some common variants (B.1.1.7, B.1.351, and P.1) in pigs via IN and IM alone, or IN + IM immunization methods.59 No adverse reactions were observed at any dose level or immunization models, indicating a desirable safety profile. Similar results were also observed in mice and hamster models.60 It is suggested that two-dose IN immunization with NDV-vectored vaccines may induce more robust protection than single-dose.61 Two doses of IN rNDV-S (a recombinant NDV-based vaccine expressing S protein of SARS-CoV-2) elicited strong protective immunity and reduced viral shedding in nasal turbinate and lungs of hamsters.62
A vesicular stomatitis virus (VSV)-based COVID-19 vaccine induced eight-fold higher titers of neutralizing antibodies after one single IN dose than single IM dose in macaques.63 Another VSV-based vaccine, which encodes SARS-CoV-2 S protein alone or with Ebola virus glycoprotein (VSV-SARS2-EBOV), induced early CD8+ T cell response after IN immunization while IM immunization tended to cause CD4+ T and NK cell responses.64 These results indicated the potential of VSV as an ideal IN vaccine carrier. Nevertheless, Furuyama et al.’s VSV-based IN vaccine showed no protection of nonhuman primates from viral challenge.65 Therefore, to enhance the efficacy, antigens should be well-designed, and the VSV vectors need to be further optimized.
Clinical trials
A total of nine IN vaccines based on viral vectors are under clinical studies. Bharat Biotech International Limited developed a replication-deficient chimpanzee Ad-vectored vaccine that expresses stabilized S protein of SARS-CoV-2 (BBV154). BBV154 is now under a phase I randomized multicenter clinical trial involving 175 participants, who receive one or two IN doses of vaccination (NCT04751682). CanSino Biologics Inc./Beijing Institute of Biotechnology is evaluating Ad5-nCoV's safety and immunogenicity in an open-label phase I clinical trial involving 149 participants. Participants were allocated into six groups to receive different doses of vaccination via only IN or IM routes, or combining IM prime with IN booster (NCT04552366). The Ad5-nCoV inhalation vaccine is also registered in another phase I/II clinical trial (NCT04840992) involving 840 volunteers. SC-Ad6–1, another Ad-vectored vaccine, is being assessed in a phase I study operated by Tetherex Pharmaceuticals Corporation (NCT04839042). 80 Volunteers were administered with SC-Ad6–1 via IN or IM route to evaluate the safety, reactogenicity, and immunogenicity. The University of Oxford is evaluating the potency of IN-administered ChAdOx1 in 54 healthy volunteers (NCT04816019), which has already been approved for human use via IM injection. Altimmune, Inc. registered a clinical trial to evaluate the safety and immunogenicity of AdCOVID (NCT04679909). However, clinical trial data indicated that AdCOVID did not stimulate adequate antiviral immunity.66 Thus, Altimmune, Inc. declared that further development of AdCOVID will be discontinued, removing AdCOVID from the potential vaccine candidate list.
Four IN vaccines based on other viral vectors are also under clinical evaluation. The NDV-based AVX/COVID-12-HEXAPRO vaccine is administered to 90 healthy volunteers in a phase I clinical trial. Two doses of this vaccine were evaluated with three schemes of vaccination (IM and IN alone, or IN + IM) (NCT04871737). DelNS1–2019-nCoV-RBD-OPT1, a live attenuated influenza virus-vectored vaccine targeting RBD, has completed phase I (ChiCTR2000037782) and phase II (chiCTR2000039715) clinical evaluation in China. Results indicated that the DelNS1–2019-nCoV-RBD-OPT1 vaccine is very tolerated and immunogenic in inducing the generation of mucosal IgA, systemic IgG, and T cell responses.67 The promising results supported the initiation of a phase III study (ChiCTR2100051391). The PIV5-based vaccine (CVXGA1) is also under phase I clinical investigation (NCT04954287).55 Meissa Vaccines, Inc. developed MV-014–212 vaccine based on a live-attenuated respiratory syncytial virus (RSV), which expresses the S protein of SARS-CoV-2 (NCT04798001). 130 participants are recruited to receive single or two doses of MV-014–212 via IN drops or spray.
Protein subunit vaccines
Preclinical studies
While more tolerable, subunit vaccines are always less immunogenic due to the swift enzymatic degradation and the deficiency in stimulating APCs.68 One commonly accepted method to increase their immunogenicity at mucosa is to use vaccine adjuvants and delivery systems. Our team investigated the potential of different cationic nanocarriers as IN adjuvants for a recombinant RBD vaccine of SARS-CoV-2. We found out that polyethyleneimine (PEI), N-[1-(2,3-Dioleoyloxy) propyl]-N,N,N-trimethylammonium chloride (DOTAP), and chitosan are all promising mucosal adjuvants, whereas PEI-based vaccine induced the strongest immune responses.69 PEI can also increase antigen uptake by APCs and promote the maturation of APCs in vitro. Jangra et al. generated an IN nanoemulsion-based adjuvant for a recombinant SARS-CoV-2 S protein, which assisted in evoking strong antiviral immune responses that offered protection against both homologous SARS-CoV-2 and an N501Y mutated strain in mice.70
An et al. developed an IN subunit vaccine of SARS-CoV-2 composed of trimeric or monomeric S protein which was adjuvanted with a liposomal agonist of cyclic GMP-AMP receptor stimulator interferon genes (STING).71 A single dose IN administration effectively induced antiviral immune responses. Moreover, the trimeric antigen was much more effective than the monomeric antigen by inducing significantly higher neutralizing IgG and sIgA titers. Single-cell RNA-sequencing confirmed the activation of T and B cells within the NALT, which is the inductive site to elicit potent and long-lasting immunity locally and systemically.71 Kim et al. utilized self-assembling Helicobacter pylori-bullfrog ferritin nanoparticles to deliver a RBD protein vaccine of SARS-CoV-2.72 In the presence of AddaVax adjuvant (a squalene-based oil-in-water nanoemulsion), IN vaccination with the RBD-ferritin nanoparticle completely protected immunized ferrets from SARS-CoV-2 challenge.
An IM prime followed by an IN booster combines the advantages of the two immunization routes. Sui et al. compared the efficacy of an S1 protein-based vaccine using IM +IM or IM + IN prime-booster vaccination regimes in rhesus macaques.73 The mucosal vaccine was incorporated into DOTAP or poly(lactic-co-glycolic acid) (PLGA) nanoparticles containing CpG, poly I:C, and IL-15 adjuvants. IM boosting strategy induced strong and persistent humoral and cellular immunity, whilst IN boosting induced significantly higher dimeric IgA and IFN-α responses. After SARS-CoV-2 challenge, genomic copies in nasal swabs of the IN boosted animals were much lower than IM boosted ones, indicating the higher efficiency in the clearance of invading virus. Therefore, compared with the conventional IM-only strategy, IN booster may be a complementary reinforcement to better and complete protection against viral infection.
The mucosal immunity triggered by IN vaccines is not limited to local respiratory sites. Du et al. reported that mice IN immunized with a recombinant RBD vaccine in the presence of aluminum oxyhydroxide gel adjuvant exhibited increased RBD-specific sIgA in not only nasal mucosa, but also genital and intestine tracts.34 Since SARS-CoV-2 infection may result in viral shedding from feces74 and disturb female reproductive function,75 high levels of sIgA in the vagina and intestine may provide broad-spectrum protection and completely abrogate viral transmission.
Clinical trials
Center for Genetic Engineering and Biotechnology (CIGB) developed a protein subunit vaccine (CIGB-669) comprising of RBD protein and HBV nucleocapsid (N) antigen (AgnHB, an immunopotentiator). In a phase I/Ⅱ clinical trial launched in Cuba, CIGB-669 is administered to 88 participants via IN spray alone without adjuvant or in combination with IM injection with alum adjuvant (RPCEC00000345). Razi Vaccine and Serum Research Institute also developed a recombinant S protein vaccine (Razi Cov Pars), which has completed phase I (IRCT20201214049709N1) and phase Ⅱ (IRCT20201214049709N2) clinical trials and is now under phase III study (IRCT20210206050259N3) in Iran.
Other types of IN vaccines
Preclinical studies
Other COVID-19 vaccines have also been designed for IN immunization, including live-attenuated vaccines, bacterium-vectored vaccines, and nucleic acid-based vaccines. Seo and Jang developed a cold-adapted live-attenuated COVID-19 vaccine, a single IN spray of which induced potent humoral, cellular, and mucosal IgA immune responses in K18-hACE2 mice.76 Immunized mice were completely protected from viral challenge without detectable viral titers in nasal turbinates and vital organs. Codagenix Inc also developed a scalable live-attenuated SARS-CoV-2 vaccine candidate (COVI-VAC) that was proven safe and effective.77 COVI-VAC was developed by recoding a segment of S protein and deleting the furin cleavage site within S protein for enhanced safety. Syrian golden hamsters IN immunized with COVI-VAC generated high levels of neutralizing antibodies comparable to SARS-CoV-2 infected animals.
A replicating bacterium-vectored COVID-19 vaccine was produced using live-attenuated Francisella tularensis subsp. holarctica vector, LVS ΔcapB vector to express the membrane and N proteins of SARS-CoV-2. Golden Syrian hamsters intradermally or IN immunized with this vaccine were protected from live viral infection, correlated with serum anti-N antibody titers.78 The bacterium-vectored vaccine is safe, inexpensive to generate, easy to be stored and distributed. Another Lactobacillus plantarum-based vaccine that expresses surface-displayed RBD of SARS-CoV-2 induced strong mucosal IgA (in respiratory and intestinal tracts) after IN immunization.79 Further investigation is desired to evaluate bacterium-based vaccines’ immunogenicity and safety in bigger animal models to promote their clinical translation.
DNA vaccines possess several advantages including stability, durability, and ease of manufacture. Kumar et al. designed a DNA vaccine targeting the S protein of SARS-CoV-2 based on gold-nanostar-chitosan carrier.80 IN immunization with this vaccine induced strong mucosal IgA, lung TRM cell, as well as neutralizing IgG responses in mice. However, another IN plasmid vaccine seems to be poor in inducing antiviral immunity via neither IN nor IM route.81 Only a prime with the plasmid vaccine followed by an IN booster with a virus-vectored vaccine triggered effective immune responses, which can purely attribute to the virus-vectored vaccine. Presently, major challenges in developing mucosal DNA vaccines include the low transfection efficiency and poor immunogenicity due to the weak ability to activate local APCs.82 Therefore, more studies are required to explore proper DNA delivery and adjuvant systems for the development of mucosal DNA vaccines.
Clinical trials
COVI-VAC has been launched into a phase I clinical trial, where 40 participants are enrolled to receive one or two IN doses of vaccination (NCT04619628). Symptoms including oral body temperature and adverse events will be recorded, and some basic examinations like chest X-rays and physical exams will be performed after immunization.
Adjuvants for intranasal vaccines
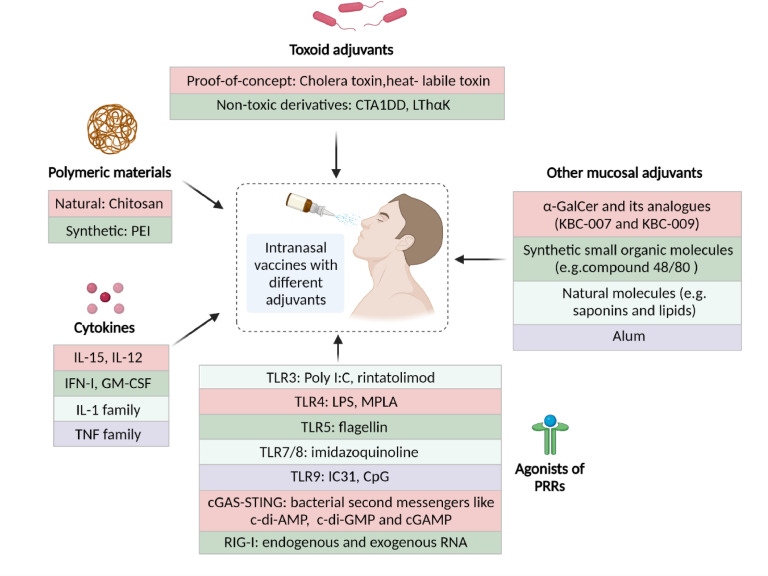
Due to the low affinity of most antigens with epithelium and the tight junctions between epithelial cells, antigens tend to be cleared rapidly by mucociliary removal.83 Therefore, vaccine adjuvants are commonly added for enhanced immunogenicity in the mucosa, especially for subunit vaccines. Adjuvants can increase the magnitude and durability of antiviral immunity and exert a dose-sparing effect.84 Here we briefly introduced current advances in nasal adjuvants (Figure 3).
Figure 3.
A schematic illustration of mucosal adjuvants for IN vaccines. Created with BioRender.com.
Toxoid adjuvants
Two enterotoxin-derived adjuvants, Cholera toxin (CT, with unit A and unit B) and Escherichia coli heat- labile toxin (LT), are used as the proof-of-concept mucosal adjuvants. They can regulate immune responses via binding to GM1 ganglioside receptors presented on the membranes of most APCs.85 However, their application is severely hampered by safety concerns, including Bell's palsy.86 Alternatively, detoxified derivatives were developed. CTA1DD is a nontoxic derivative of CT whose unit B is removed and replaced with a dimer of fragment D of protein A from Staphylococcus aureus.87 CTA1DD adjuvanted vaccines effectively induced antibody responses and prevented influenza virus infection and transmission after IN immunization.88 LThαK is a detoxified derivative of LT, which exhibits inhibited ADP-ribosylating enzyme activity. Nasal delivery of LThαK did not result in redirection to central nerve system, suggesting its safety profiles.89 An LThαK-adjuvanted influenza vaccine was reported to be remarkably effective and tolerable after two IN vaccinations in phase Ⅰ (NCT03293732) and phase II (NCT03784885) clinical trials.
Polymers
Polymers are promising mucosal adjuvants given their vaccine delivery capacity and immunostimulatory effects. Chitosan, a natural derivative of α-chitin, is an attractive mucosal adjuvant due to its mucoadhesive and immune-stimulatory properties.90,91 Chitosan-based nanoparticles can prolong local retention, enhance mucosal antigen absorption, and protect them from degradation in the mucosa.91 Chitosan can also trigger innate and adaptive immune responses via DNA sensor cGAS-STING dependent activation of APCs.84,92 Mice IN immunized with chitosan-adjuvanted influenza vaccines induced strong antiviral immunity and conferred complete protectivity against homologous and heterologous influenza virus challenge.93,94 Chitosan-adjuvanted inactivated mutant diphtheria toxoid CRM197 vaccine also generated high levels of neutralizing antibodies in humans after IN immunization.95 Volunteers that received two doses of norovirus vaccine, which was adjuvanted by monophosphoryl lipid A (MPL) and chitosan, showed no signs of vaccine-related serious adverse events (NCT00806962) and induced strong antigen-specific humoral responses (NCT00973284).
PEI, a synthetic cationic polymer widely used as a gene transduction agent, is also an excellent adjuvant for IN vaccines. Current application of PEI as mucosal adjuvant is limited to experimental researches owing to its safety concerns in humans.69,96 IN administration of mice with PEI-adjuvanted RBD vaccine induced high titers of neutralizing antibodies against SARS-CoV-2.69 A single IN dose of subunit glycoprotein antigens with PEI evoked robust antibody-mediated protection from lethal challenge of influenza or herpes simplex virus type-2 (HSV-2), superior to conventional CT adjuvant.96 PEI could form a nanoscale complex with protein antigens which can be quickly taken up by APCs. The adjuvanticity of PEI relies on the release of host double-stranded DNA and Irf3 signal pathway.96
Agonists of pattern recognition receptors
Ligands for PRRs are the most frequently used molecular adjuvants that are considered as conserved PAMPs.97 PRRs are located on the cell surface or in the cytoplasm, including TLR, STING, nucleotide-binding and oligomerization domain- (NOD-) like receptors (NLR), retinoic acid inducible gene I (RIG-I) etc. TLR agonists constitute a major category of nasal adjuvants. For instance, the commercial synthetic bacterial DNA, IC31, and other CpG oligonucleotides are common mucosal adjuvants that act through the TLR9 signaling pathway.6,98 Poly I:C is a synthetic double-stranded viral RNA that activates TLR3 in macrophages and DCs to induce durable and protective T cell responses.99 Rintatolimod, a modified form of poly I:C, was reported to be well-tolerated as the adjuvant for a trivalent influenza vaccine in a phase Ⅰ clinical trial.100 A mixture of CpG and Poly I:C was proven to be a safe and effective nasal adjuvant for a COVID-19 subunit vaccine in rhesus macaques since no vaccine-induced immune pathology was observed even after 3–4 doses.73 Lipopolysaccharide (LPS) is a classic agonist of TLR4 and an effective mucosal adjuvant.101 But the potential toxicity of LPS significantly limited its application in humans. Thus, a low-toxicity derivative of LPS was produced: MPL.102 Clinical trials have verified the safety and efficacy of MPL as an IN adjuvant for a norovirus vaccine (NCT00806962, NCT00973284). Flagellin is a protein-based vaccine carrier and adjuvant which could activate TLR5. IN immunization with flagellin-adjuvanted influenza virus subunit vaccine induced significantly stronger antiviral immunity than naked antigen in chickens.103 Imidazoquinolines-based TLR7/TLR8 agonists are also attractive nasal adjuvants owing to their high safety profile and effectivity.104
Bacterial second messengers, including cyclic di-nucleotides (e.g., c-di-AMP and c-di-GMP) are representatives of novel mucosal adjuvants.6 They activate the STING pathway to produce IFN-I and further induce adaptive immune response.105 IN vaccination with recombinant Tc52 or its amino-terminal domain combined with c-di-AMP adjuvant triggered Th17+ Th1-specific immunity and protected mice against Trypanosoma cruzi infection.106 A COVID-19 vaccine successfully incorporated S protein antigen and a commercial agonist of STING, 2′−3′’cyclic guanosine monophosphate adenosine monophosphate (cGAMP), into liposomes for IN immunization.71 A mixed adjuvant composed of various TLR agonists (CpG (TLR9), LP1569 (TLR2)) and c-di-GMP induced IL-17-secreting TRM cells after IN immunization, which were responsible for sustained protective immunity against Bordetella pertussis nasal colonization.107 RIG-I can detect endogenous and exogenous RNA to initiate innate antiviral immunity.108 Jangra et al. successfully integrated an RNA agonist of RIG-I (IVT DI) with a nanoemulsion (an activator of TLRs and NLRP3) as the adjuvant for an S protein antigen of SARS-CoV-2.70 Nevertheless, it must be noted that PRR-based adjuvants may cause severe effects on developing fetuses.109 Thus, PRR agonist adjuvant-based vaccines should be used with careful evaluation in special populations like pregnant women.
Cytokines
Numerous cytokines have been added to enhance the immune responses of IN vaccines, owing to their intrinsic characteristics of activating and regulating adaptive immunity. For instance, IL-15 was used as the adjuvant in developing an IN subunit vaccine against SARS-CoV-2 infection in rhesus macaques.73 Others cytokines like IL-12, IFN-I, IL-1 family, TNF family, and granulocyte-macrophage colony-stimulating factors are also frequently used as IN adjuvants.110 Another review concerning cytokine adjuvants is recommended for readers for more information.110 However, it is worth noting that adverse effects may occur after high-dose and repeated administration of cytokine-containing vaccines.
Others
α-GalCer is an activator of the invariant natural killer T cells and a promising IN adjuvant. The presentation of α-GalCer is dependent on CD1d molecule expressed on APCs.111 It is most frequently used in the generation of IN vaccines of influenza virus to induce strong and long-lasting protective immunity.111,112 Of note, two analogs of α-GalCer with improved solubility, KBC-007 and KBC-009, were developed as safe and effective IN adjuvants for inactivated influenza virus vaccine.113 Recent studies showed that the first licensed aluminum salts are potentially tolerable nasal adjuvants and can help elicit antiviral immunity systemically and locally at multiple mucosal sites.34,114 Apart from the adjuvants mentioned above, some other synthetic small organic molecules (e.g., compound 48/80, vitamin E TPGS) and natural molecules (e.g., saponins and lipids) have been successfully used as IN adjuvants in experimental settings. Readers are recommended to refer to another review of this section6 if interested.
Potential delivery devices for intranasal vaccines
Various devices can be developed to deliver IN vaccines, including pipette droppers and spray devices with different operational mechanisms. The conventional pipette-based delivery method, in which the vaccines are dropped into the nostrils, is unprecise and unstandardized, with drug volumes easily exceeding the nasal cavity volume. A variety of spray devices have dominated the nasal delivery market due to their excellent performance in cost and clinical efficacy.115 Spray devices can be designed to achieve an optimal mean particle size. For instance, the only approved nasal vaccine FluMist® used AccuSpray Device that consists of a single-use prefilled syringe to produce large particles, leading to decreased vaccine deposition into lower airways and reduced side effects.116 Metered-dose spray pumps are most studied for vaccines, which are portable, easy to use, self-contained, and safe. They can be actuated via hand press, breath, or electrical power. It is reported that intranasal vaccination of Shigella antigens with a spray device (Dolphin™) induced significantly higher plasma and antibody-secreting cell immune responses than pipette delivery of the same dose of antigens.117 The intranasal Ad5-nCoV vaccine utilized the advanced Aerogen Ultra Device, which could produce numerous homogeneous small particles with vibrating net technology (Table 2). However, the wide use of metered-dose spray devices is limited by drug waste caused by residues in the devices and drug leakage during vaccination which is environment-unfriendly.
Safety of intranasal vaccines
IN vaccines can be developed based on multiple vaccine platforms, e.g., viral vectors and protein subunit vaccines. An essential consideration for IN vaccine development is safety. There are some concerns about whole pathogen-based vaccines due to the possibility of reversion into replicating state. Meanwhile, adjuvants are essential for strong immune responses, especially for protein subunit vaccines. But safety problems can also arise along with the addition of adjuvants owing to their immunomodulatory properties. Another alarming problem for IN vaccines is the possibility of retrograde transport to the brain via olfactory nerves, which has been observed in live attenuated adenovirus.118,119 In developing IN COVID-19 vaccines, increasing numbers of preclinical studies have proven their safety profiles. For instance, Sui et al. reported no observation of vaccine-induced immune pathology even after 3 or 4 doses in rhesus macaques.73 Other vaccine candidates, including a lentivirus-vectored vaccine, a chimpanzee adenovirus-vectored vaccine, and a PIV5-based vaccine, were all demonstrated to be highly tolerable after IN dose in different animal models.43,55,57 Nevertheless, long-term observation and more clinical data are required to prove the safety of those IN vaccines.
Conclusion and perspectives
IN vaccine is a promising preventive strategy for SARS-CoV-2 considering the remarkable protective immunity in the mucosal sites. Although all the licensed mucosal vaccines are based on whole pathogens, the investigation of IN COVID-19 vaccines predominantly focused on safer vaccine platforms, especially viral vectors and protein subunits. Nevertheless, the poor immunogenicity and stability of highly pure subunit antigens require the application of mucosal adjuvants and delivery systems to overcome the harsh mucosal barriers and enhance immune responses. Avoiding nasal clearing is one of the biggest challenges in the development of IN vaccines. Current studies have demonstrated that IN immunization with different types of COVID-19 vaccines induced robust protective immunity in animal models. The promising results tremendously promoted their evaluation in clinical trials. However, we still know little about how the mucosal and systemic immune responses are triggered by IN immunization with various vaccines. Furthermore, tremendous efforts are necessary to develop desirable mucosal adjuvants and delivery systems with high effectivity and tolerability to efficiently deliver the intact forms of antigens towards target cells. Finally, there may be ethical considerations of immunization with IN COVID-19 vaccines without IM protection. Since IN vaccines exhibit plenty of advantages over IM vaccines as discussed in the earlier section, we should popularize the public about IN vaccines' features and immune mechanisms, and offer the general public a choice of the immunization methods. In a word, IN immunization route can induce sterilizing mucosal and systemic immunity, further preventing virus infection and transmission. Application of IN vaccines will hopefully help deal with the persistent COVID-19 pandemic and potential viral contagious diseases in the future.
Outstanding questions
To further optimize the potential values of IN vaccines of SARS-CoV-2 and promote their translation into clinic, there remains a lot to be done, including:
-
1.
More precise mechanisms of how IN vaccines activate the mucosal and systemic immune responses need to be clarified, such as how different types of antigens are processed by various immune cells of the respiratory system; how diverse components of the IN vaccines, like adjuvants, affect the induction of immune responses.
-
2.
While ensuring the efficacy of IN vaccines, how to seek an adoptable scale-up strategy of the manufacturing process to make COVID-19 vaccines available for wide-scale use before the pandemic ends.
-
3.
It is necessary to develop ideal mucosal adjuvants and delivery systems that can guarantee the efficacy and safety of IN vaccine at the same time. Moreover, these adjuvants and delivery systems are best to be simple in composition, stable in activity, easy for manufacturing, and of low cost.
Search strategy and selection criteria
Data for this review were identified by searches of PubMed and Web of Science, and references from relevant articles using the search terms “SARS-CoV-2”, “COVID-19”, “vaccine”, “IN”, “mucosal”, “immunity”, “adjuvant”, and “delivery”. Only articles published in English were included up to 24 November 2021. Data from WHO (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines), COVID-19 vaccine tracker (https://covid19.trackvaccines.org/) and clinical trial.gov (https://clinicaltrials.gov/) were also included.
Contributors
Xiawei Wei contributed to the conception and design of the review. The first draft of the manuscript was written by Aqu Alu and Li Chen. Aqu Alu, Li Chen and Hong Lei created all the figures and tables. Yuquan Wei and Xiaohe Tian critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Declaration of interests
The authors declare that they have no conflicts of interests.
Acknowledgements
This work was supported by the National Natural Science Foundation Regional Innovation and Development (No. U19A2003), the National Science Foundation for Excellent Young Scholars (32122052) and Excellent Youth Foundation of the Sichuan Scientific Committee Grant in China (No. 2019JDJQ008). The funders had no role in paper design, data collection, data analysis, interpretation, and writing of the paper.
Contributor Information
Xiaohe Tian, Email: xiaohe.t@wchscu.cn.
Xiawei Wei, Email: xiaweiwei@scu.edu.cn.
References
- 1.WHO’s landscape of COVID-19 vaccine candidates. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines, 24-November 2021.
- 2.Registry data of COVID-19 vaccine candidates. https://covid19.trackvaccines.org/, 24-November 2021.
- 3.Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27(2):205–211. doi: 10.1038/s41591-021-01230-y. [DOI] [PubMed] [Google Scholar]
- 4.Tiboni M., Casettari L., Illum L. Nasal vaccination against SARS-CoV-2: synergistic or alternative to intramuscular vaccines? Int J Pharm. 2021;603 doi: 10.1016/j.ijpharm.2021.120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh C.L., Goldsmith J.A., Schaub J.M., et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369(6510):1501–1505. doi: 10.1126/science.abd0826. (New York, NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S., Liu H., Zhang X., Qian F. Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies. Protein Cell. 2015;6(7):480–503. doi: 10.1007/s13238-015-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavelle E.C., Ward R.W. Mucosal vaccines — fortifying the frontiers. Nat Rev Immunol. 2021 doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J.H., Lee H.K. Delivery routes for COVID-19 vaccines. Vaccines. 2021;9(5):524. doi: 10.3390/vaccines9050524. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavda V.P., Vora L.K., Pandya A.K., Patravale V.B. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov Today. 2021;26(11):2619–2636. doi: 10.1016/j.drudis.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyd J. Immunity against mucosal pathogens. Hum Vaccin. 2010;6(3):10016. doi: 10.4161/hv.6.3.10016. [DOI] [PubMed] [Google Scholar]
- 11.Villena J., Kitazawa H. The modulation of mucosal antiviral immunity by immunobiotics: could they offer any benefit in the SARS-CoV-2 pandemic? Front Physiol. 2020;11:699. doi: 10.3389/fphys.2020.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmgren J., Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Q., Walker W.A. Innate immunity of the gut: mucosal defense in health and disease. J Pediatr Gastroenterol Nutr. 2004;38(5):463–473. doi: 10.1097/00005176-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Mudgal R., Nehul S., Tomar S. Prospects for mucosal vaccine: shutting the door on SARS-CoV-2. Hum Vaccin Immunother. 2020;16(12):2921–2931. doi: 10.1080/21645515.2020.1805992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moradi-Kalbolandi S., Majidzadeh A.K., Abdolvahab M.H., Jalili N., Farahmand L. The role of mucosal immunity and recombinant probiotics in SARS-CoV2 vaccine development. Probiotics Antimicrob Proteins. 2021;13(5):1239–1253. doi: 10.1007/s12602-021-09773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Bowie A.G., Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8(12):911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtovic L., Beeson J.G. Complement factors in COVID-19 therapeutics and vaccines. Trends Immunol. 2021;42(2):94–103. doi: 10.1016/j.it.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Jin L., Chen T. The effects of secretory IgA in the mucosal immune system. Biomed Res Int. 2020;2020 doi: 10.1155/2020/2032057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacchi L., Musharrafieh R., Larragoite E.T., et al. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat Commun. 2014;5:5205. doi: 10.1038/ncomms6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strugnell R.A., Wijburg O.L. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8(9):656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 23.Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogier E.W., Frantz A.L., Bruno M.E., Kaetzel C.S. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens. 2014;3(2):390–403. doi: 10.3390/pathogens3020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurkic J., Numanovic F., Arnautalic L., Tihic N., Halilovic D., Jahic M. Diagnostic significance of reduced IgA in children. Med Arch. 2014;68(6):381–383. doi: 10.5455/medarh.2014.68.381-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer A. Resistance of children to COVID-19. How? Mucosal Immunol. 2020;13(4):563–565. doi: 10.1038/s41385-020-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakhra K., Abraham W., Wang C., et al. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. Sci Immunol. 2021;6(57):eabd8003. doi: 10.1126/sciimmunol.abd8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slütter B., Pewe L.L., Kaech S.M., Harty J.T. Lung airway-surveilling CXCR3hi memory CD8+ T cells are critical for protection against influenza A virus. Immunity. 2013;39(5):939–948. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uddback I., Cartwright E.K., Scholler A.S., et al. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol. 2021;14(1):92–99. doi: 10.1038/s41385-020-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stolley J.M., Johnston T.S., Soerens A.G., et al. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J Exp Med. 2020;217(8):e20192197. doi: 10.1084/jem.20192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swarnalekha N., Schreiner D., Litzler L.C., et al. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 2021;6(55) doi: 10.1126/sciimmunol.abb6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Son Y.M., Cheon I.S., Wu Y., et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci. Immunol. 2021;6(55):eabb6852. doi: 10.1126/sciimmunol.abb6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan A.O., Kafai N.M., Dmitriev I.P., et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du Y., Xu Y., Feng J., et al. Intranasal administration of a recombinant RBD vaccine induced protective immunity against SARS-CoV-2 in mouse. Vaccine. 2021;39(16):2280–2287. doi: 10.1016/j.vaccine.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bricker T.L., Darling T.L., Hassan A.O., et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021;36(3) doi: 10.1016/j.celrep.2021.109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lijek R.S., Luque S.L., Liu Q., Parker D., Bae T., Weiser J.N. Protection from the acquisition of Staphylococcus aureus nasal carriage by cross-reactive antibody to a pneumococcal dehydrogenase. Proc Natl Acad Sci USA. 2012;109(34):13823–13828. doi: 10.1073/pnas.1208075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saffran M., Kumar G.S., Savariar C., Burnham J.C., Williams F., Neckers D.C. A new approach to the oral administration of insulin and other peptide drugs. Science. 1986;233(4768):1081–1084. doi: 10.1126/science.3526553. (New York, NY) [DOI] [PubMed] [Google Scholar]
- 38.Wu S., Zhong G., Zhang J., et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11(1):4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng L., Wang Q., Shan C., et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat Commun. 2020;11(1):4207. doi: 10.1038/s41467-020-18077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu F.C., Li Y.H., Guan X.H., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson J.S., Pillet S., Bello A.J., Kobinger G.P. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J Virol. 2013;87(7):3668–3677. doi: 10.1128/JVI.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan A.O., Feldmann F., Zhao H., et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep Med. 2021;2(4) doi: 10.1016/j.xcrm.2021.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassan A.O., Shrihari S., Gorman M.J., et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 2021;36(4) doi: 10.1016/j.celrep.2021.109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King R.G., Silva-Sanchez A., Peel J.N., et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects mice from lethal challenge. Vaccines. 2021;9(8):881. doi: 10.3390/vaccines9080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim E., Weisel F.J., Balmert S.C., et al. A single subcutaneous or intranasal immunization with adenovirus-based SARS-CoV-2 vaccine induces robust humoral and cellular immune responses in mice. Eur J Immunol. 2021;51(7):1774–1784. doi: 10.1002/eji.202149167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Doremalen N., Purushotham J.N., Schulz J.E., et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci Transl Med. 2021;13(607):eabh0755. doi: 10.1126/scitranslmed.abh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh G.A., McAuley A.J., Au G.G., et al. ChAdOx1 nCoV-19 (AZD1222) vaccine candidate significantly reduces SARS-CoV-2 shedding in ferrets. NPJ Vaccin. 2021;6(1):67. doi: 10.1038/s41541-021-00315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sterlin D., Mathian A., Miyara M., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577):eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z., Lorenzi J.C.C., Muecksch F., et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021;13(577):eabf1555. doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu K., An Y., Li Q., et al. Recombinant chimpanzee adenovirus AdC7 expressing dimeric tandem-repeat spike protein RBD protects mice against COVID-19. Emerg Microbes Infect. 2021;10(1):1574–1588. doi: 10.1080/22221751.2021.1959270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice A., Verma M., Shin A., et al. Intranasal plus subcutaneous prime vaccination with a dual antigen COVID-19 vaccine elicits T-cell and antibody responses in mice. Sci Rep. 2021;11(1):14917. doi: 10.1038/s41598-021-94364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J., Huang J.R., Zhang Y.L., Zhang J. SARS-CoV-2 nucleocapsid protein intranasal inoculation induces local and systemic T cell responses in mice. J Med Virol. 2021;93(4):1923–1925. doi: 10.1002/jmv.26769. [DOI] [PubMed] [Google Scholar]
- 53.Loes A.N., Gentles L.E., Greaney A.J., Crawford K.H.D., Bloom J.D. Attenuated influenza virions expressing the SARS-CoV-2 receptor-binding domain induce neutralizing antibodies in mice. Viruses. 2020;12(9):987. doi: 10.3390/v12090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koonpaew S., Kaewborisuth C., Srisutthisamphan K., et al. A single-cycle influenza a virus-based SARS-CoV-2 vaccine elicits potent immune responses in a mouse model. Vaccines. 2021;9(8):850. doi: 10.3390/vaccines9080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.An D., Li K., Rowe D.K., et al. Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci Adv. 2021;7(27):eabi5246. doi: 10.1126/sciadv.abi5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohtsuka J., Imai M., Fukumura M., et al. Non-propagative human parainfluenza virus type 2 nasal vaccine robustly protects the upper and lower airways against SARS-CoV-2. iScience. 2021;24(12) doi: 10.1016/j.isci.2021.103379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ku M.W., Bourgine M., Authié P., et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29(2):236–249.e6. doi: 10.1016/j.chom.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ku M.W., Authié P., Bourgine M., et al. Brain cross-protection against SARS-CoV-2 variants by a lentiviral vaccine in new transgenic mice. EMBO Mol Med. 2021;13(12):e14459. doi: 10.15252/emmm.202114459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lara-Puente J.H., Carreño J.M., Sun W., et al. Safety and immunogenicity of a newcastle disease virus vector-based SARS-CoV-2 vaccine candidate, AVX/COVID-12-HEXAPRO (Patria), in pigs. mBio. 2021;12(5) doi: 10.1128/mBio.01908-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun W., Liu Y., Amanat F., et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat Commun. 2021;12(1):6197. doi: 10.1038/s41467-021-26499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warner B.M., Santry L.A., Leacy A., et al. Intranasal vaccination with a Newcastle disease virus-vectored vaccine protects hamsters from SARS-CoV-2 infection and disease. iScience. 2021;24(11) doi: 10.1016/j.isci.2021.103219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J.G., Oladunni F.S., Rohaim M.A., et al. Immunogenicity and protective efficacy of an intranasal live-attenuated vaccine against SARS-CoV-2. iScience. 2021;24(9) doi: 10.1016/j.isci.2021.102941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H., Zhang Y., Li D., et al. Enhanced protective immunity against SARS-CoV-2 elicited by a VSV vector expressing a chimeric spike protein. Signal Transduct Target Ther. 2021;6(1):389. doi: 10.1038/s41392-021-00797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.OD K.L., Clancy C.S., Griffin A.J., et al. Optimization of single dose VSV-based COVID-19 vaccination in hamsters. bioRxiv. 2021 doi: 10.3389/fimmu.2021.788235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furuyama W., Shifflett K., Pinski A.N., et al. Rapid protection from COVID-19 in nonhuman primates vaccinated intramuscularly but not intranasally with a single dose of a recombinant vaccine. bioRxiv. 2021 doi: 10.1128/mbio.03379-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altimmune announces update on AdCOVID™ phase 1 clinical trial. https://www.globenewswire.com/news-release/2021/06/29/2255167/0/en/Altimmune-Announces-Update-on-AdCOVID-Phase-1-Clinical-Trial.html, 24-November 2021.
- 67.Chen J., Wang P., Yuan L., et al. A live attenuated influenza virus-vectored intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2 infection. bioRxiv. 2021 doi: 10.1016/j.scib.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karch C.P., Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem Pharmacol. 2016;120:1–14. doi: 10.1016/j.bcp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lei H., Alu A., Yang J., et al. Cationic nanocarriers as potent adjuvants for recombinant S-RBD vaccine of SARS-CoV-2. Signal Transd Target Ther. 2020;5(1):291. doi: 10.1038/s41392-020-00434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jangra S., Landers J.J., Rathnasinghe R., et al. A combination adjuvant for the induction of potent antiviral immune responses for a recombinant SARS-CoV-2 protein vaccine. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.729189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.An X., Martinez-Paniagua M., Rezvan A., et al. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience. 2021;24(9) doi: 10.1016/j.isci.2021.103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim Y.I., Kim D., Yu K.M., et al. Development of spike receptor-binding domain nanoparticles as a vaccine candidate against SARS-CoV-2 infection in ferrets. mBio. 2021;12(2):e00230–21. doi: 10.1128/mBio.00230-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sui Y., Li J., Zhang R., et al. Protection against SARS-CoV-2 infection by a mucosal vaccine in rhesus macaques. JCI Insight. 2021;6(10):e148494. doi: 10.1172/jci.insight.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y.I., Kim S.G., Kim S.M., et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5):704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jing Y., Run-Qian L., Hao-Ran W., et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26(6):367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seo S.H., Jang Y. Cold-adapted live attenuated SARS-Cov-2 vaccine completely protects human ACE2 transgenic mice from SARS-Cov-2 infection. Vaccines. 2020;8(4):584. doi: 10.3390/vaccines8040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Yang C., Song Y., et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc Natl Acad Sci USA. 2021;118(29) doi: 10.1073/pnas.2102775118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jia Q., Bielefeldt-Ohmann H., Maison R.M., et al. Replicating bacterium-vectored vaccine expressing SARS-CoV-2 Membrane and Nucleocapsid proteins protects against severe COVID-19-like disease in hamsters. NPJ Vaccines. 2021;6(1):47. doi: 10.1038/s41541-021-00321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li L., Wang M., Hao J., et al. Mucosal IgA response elicited by intranasal immunization of Lactobacillus plantarum expressing surface-displayed RBD protein of SARS-CoV-2. Int J Biol Macromol. 2021;190:409–416. doi: 10.1016/j.ijbiomac.2021.08.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar U.S., Afjei R., Ferrara K., Massoud T.F., Paulmurugan R. Gold-nanostar-chitosan-mediated delivery of SARS-CoV-2 DNA vaccine for respiratory mucosal immunization: development and proof-of-principle. ACS Nano. 2021 doi: 10.1021/acsnano.1c05002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chandrasekar S.S., Phanse Y., Hildebrand R.E., et al. Localized and systemic immune responses against SARS-CoV-2 following mucosal immunization. Vaccines. 2021;9(2):132. doi: 10.3390/vaccines9020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu M., Zhao H., Li M., Yue Y., Xiong S., Xu W. Intranasal vaccination with mannosylated chitosan formulated DNA vaccine enables robust IgA and cellular response induction in the lungs of mice and improves protection against pulmonary mycobacterial challenge. Front Cell Infect Microbiol. 2017;7:445. doi: 10.3389/fcimb.2017.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaman M., Chandrudu S., Toth I. Strategies for intranasal delivery of vaccines. Drug Deliv Transl Res. 2013;3(1):100–109. doi: 10.1007/s13346-012-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moran H.B.T., Turley J.L., Andersson M., Lavelle E.C. Immunomodulatory properties of chitosan polymers. Biomaterials. 2018;184:1–9. doi: 10.1016/j.biomaterials.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 85.Lycke N., Bemark M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 2010;3(6):556–566. doi: 10.1038/mi.2010.54. [DOI] [PubMed] [Google Scholar]
- 86.Mutsch M., Zhou W., Rhodes P., et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 87.Eriksson A.M., Schön K.M., Lycke N.Y. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. J Immunol. 2004;173(5):3310–3319. doi: 10.4049/jimmunol.173.5.3310. (Baltimore, Md : 1950) [DOI] [PubMed] [Google Scholar]
- 88.Bernasconi V., Norling K., Gribonika I., et al. A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection. Mucosal Immunol. 2021;14(2):523–536. doi: 10.1038/s41385-020-0334-2. [DOI] [PubMed] [Google Scholar]
- 89.Pan S.C., Hsieh S.M., Lin C.F., Hsu Y.S., Chang M., Chang S.C. A randomized, double-blind, controlled clinical trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with adjuvant LTh(αK): a phase I study. Vaccine. 2019;37(14):1994–2003. doi: 10.1016/j.vaccine.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Wen Z.S., Xu Y.L., Zou X.T., Xu Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar Drugs. 2011;9(6):1038–1055. doi: 10.3390/md9061038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shim S., Yoo H.S. The application of mucoadhesive chitosan nanoparticles in nasal drug delivery. Mar Drugs. 2020;18(12):605. doi: 10.3390/md18120605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carroll E.C., Jin L., Mori A., et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016;44(3):597–608. doi: 10.1016/j.immuni.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sawaengsak C., Mori Y., Yamanishi K., Mitrevej A., Sinchaipanid N. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech. 2014;15(2):317–325. doi: 10.1208/s12249-013-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sui Z., Chen Q., Wu R., et al. Cross-protection against influenza virus infection by intranasal administration of M2-based vaccine with chitosan as an adjuvant. Arch Virol. 2010;155(4):535–544. doi: 10.1007/s00705-010-0621-4. [DOI] [PubMed] [Google Scholar]
- 95.Mills K.H., Cosgrove C., McNeela E.A., et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infect Immun. 2003;71(2):726–732. doi: 10.1128/IAI.71.2.726-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wegmann F., Gartlan K.H., Harandi A.M., et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol. 2012;30(9):883–888. doi: 10.1038/nbt.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 98.Wizel B., Persson J., Thörn K., Nagy E., Harandi A.M. Nasal and skin delivery of IC31®-adjuvanted recombinant HSV-2 gD protein confers protection against genital herpes. Vaccine. 2012;30(29):4361–4368. doi: 10.1016/j.vaccine.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 99.Trumpfheller C., Caskey M., Nchinda G., et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105(7):2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Overton E.T., Goepfert P.A., Cunningham P., et al. Intranasal seasonal influenza vaccine and a TLR-3 agonist, rintatolimod, induced cross-reactive IgA antibody formation against avian H5N1 and H7N9 influenza HA in humans. Vaccine. 2014;32(42):5490–5495. doi: 10.1016/j.vaccine.2014.07.078. [DOI] [PubMed] [Google Scholar]
- 101.Bal S.M., Slütter B., Verheul R., Bouwstra J.A., Jiskoot W. Adjuvanted, antigen loaded N-trimethyl chitosan nanoparticles for nasal and intradermal vaccination: adjuvant- and site-dependent immunogenicity in mice. Eur J Pharm Sci. 2012;45(4):475–481. doi: 10.1016/j.ejps.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 102.Mata-Haro V., Cekic C., Martin M., Chilton P.M., Casella C.R., Mitchell T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. (New York, NY) [DOI] [PubMed] [Google Scholar]
- 103.Song L., Xiong D., Song H., et al. Mucosal and Systemic immune responses to influenza H7N9 antigen HA1-2 Co-delivered intranasally with flagellin or polyethyleneimine in mice and chickens. Front Immunol. 2017;8:326. doi: 10.3389/fimmu.2017.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Velasquez L.S., Hjelm B.E., Arntzen C.J., Herbst-Kralovetz M.M. An intranasally delivered toll-like receptor 7 agonist elicits robust systemic and mucosal responses to Norwalk virus-like particles. Clin Vaccine Immunol. 2010;17(12):1850–1858. doi: 10.1128/CVI.00230-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hopfner K.P., Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat Rev Mol Cell Biol. 2020;21(9):501–521. doi: 10.1038/s41580-020-0244-x. [DOI] [PubMed] [Google Scholar]
- 106.Matos M.N., Cazorla S.I., Schulze K., Ebensen T., Guzmán C.A., Malchiodi E.L. Immunization with Tc52 or its amino terminal domain adjuvanted with c-di-AMP induces Th17+Th1 specific immune responses and confers protection against Trypanosoma cruzi. PLoS Negl Trop Dis. 2017;11(2) doi: 10.1371/journal.pntd.0005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allen A.C., Wilk M.M., Misiak A., Borkner L., Murphy D., Mills K.H.G. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting T(RM) cells. Mucosal Immunol. 2018;11(6):1763–1776. doi: 10.1038/s41385-018-0080-x. [DOI] [PubMed] [Google Scholar]
- 108.Xu X.X., Wan H., Nie L., Shao T., Xiang L.X., Shao J.Z. RIG-I: a multifunctional protein beyond a pattern recognition receptor. Protein Cell. 2018;9(3):246–253. doi: 10.1007/s13238-017-0431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McColl E.R., Piquette-Miller M. Poly(I:C) alters placental and fetal brain amino acid transport in a rat model of maternal immune activation. Am J Reprod Immunol. 2019;81(6):e13115. doi: 10.1111/aji.13115. (New York, NY : 1989) [DOI] [PubMed] [Google Scholar]
- 110.Wang X., Meng D. Innate endogenous adjuvants prime to desirable immune responses via mucosal routes. Protein Cell. 2015;6(3):170–184. doi: 10.1007/s13238-014-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ko S.Y., Ko H.J., Chang W.S., Park S.H., Kweon M.N., Kang C.Y. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175(5):3309–3317. doi: 10.4049/jimmunol.175.5.3309. (Baltimore, Md : 1950) [DOI] [PubMed] [Google Scholar]
- 112.Youn H.J., Ko S.Y., Lee K.A., et al. A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007;25(28):5189–5198. doi: 10.1016/j.vaccine.2007.04.081. [DOI] [PubMed] [Google Scholar]
- 113.Lee Y.S., Lee K.A., Lee J.Y., et al. An α-GalCer analogue with branched acyl chain enhances protective immune responses in a nasal influenza vaccine. Vaccine. 2011;29(3):417–425. doi: 10.1016/j.vaccine.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 114.Thakkar S.G., Warnken Z.N., Alzhrani R.F., et al. Intranasal immunization with aluminum salt-adjuvanted dry powder vaccine. J Control Release. 2018;292:111–118. doi: 10.1016/j.jconrel.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chandel A., Goyal A.K., Ghosh G., Rath G. Recent advances in aerosolised drug delivery. Biomed Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108601. [DOI] [PubMed] [Google Scholar]
- 116.Xu H., Cai L., Hufnagel S., Cui Z. Intranasal vaccine: factors to consider in research and development. Int J Pharm. 2021;609 doi: 10.1016/j.ijpharm.2021.121180. [DOI] [PubMed] [Google Scholar]
- 117.Riddle M.S., Kaminski R.W., Williams C., et al. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine. 2011;29(40):7009–7019. doi: 10.1016/j.vaccine.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 118.Lemiale F., Kong W.P., Akyurek L.M., et al. Enhanced mucosal immunoglobulin a response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J Virol. 2003;77(18):10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neutra M.R., Kozlowski P.A. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]