Abstract
Background
Posttraumatic stress disorder (PTSD) is associated with markers of accelerated aging. Estimates of brain age, compared to chronological age, may clarify the effects of PTSD on the brain and may inform treatment approaches targeting the neurobiology of aging in the context of PTSD.
Method
Adult subjects (N = 2229; 56.2% male) aged 18–69 years (mean = 35.6, SD = 11.0) from 21 ENIGMA‐PGC PTSD sites underwent T1‐weighted brain structural magnetic resonance imaging, and PTSD assessment (PTSD+, n = 884). Previously trained voxel‐wise (brainageR) and region‐of‐interest (BARACUS and PHOTON) machine learning pipelines were compared in a subset of control subjects (n = 386). Linear mixed effects models were conducted in the full sample (those with and without PTSD) to examine the effect of PTSD on brain predicted age difference (brain PAD; brain age − chronological age) controlling for chronological age, sex, and scan site.
Results
BrainageR most accurately predicted brain age in a subset (n = 386) of controls (brainageR: ICC = 0.71, R = 0.72, MAE = 5.68; PHOTON: ICC = 0.61, R = 0.62, MAE = 6.37; BARACUS: ICC = 0.47, R = 0.64, MAE = 8.80). Using brainageR, a three‐way interaction revealed that young males with PTSD exhibited higher brain PAD relative to male controls in young and old age groups; old males with PTSD exhibited lower brain PAD compared to male controls of all ages.
Discussion
Differential impact of PTSD on brain PAD in younger versus older males may indicate a critical window when PTSD impacts brain aging, followed by age‐related brain changes that are consonant with individuals without PTSD. Future longitudinal research is warranted to understand how PTSD impacts brain aging across the lifespan.
Keywords: aging, machine learning, mega‐analysis, neuroimaging, posttraumatic stress disorder, trauma
Posttraumatic stress disorder (PTSD) is associated with markers of accelerated aging. We explored estimates of brain age, as compared to chronological age, as a possible marker of accelerated aging in PTSD populations leveraging a large multi‐site sample. We identified an interaction of PTSD, age, and sex which showed young males with PTSD had a greater brain predicted age difference [PAD] than young male controls, young females with PTSD, and young females without PTSD. A similar pattern was present in middle‐aged males, but the effect of PTSD was weaker than in young adults. Male controls in the old subgroup exhibited higher brain‐PAD than old males with PTSD, old females with PTSD, and old females without PTSD.

1. INTRODUCTION
Exposure to stress plays an important role in the development of medical conditions (Cohen & Manuck, 1995). A prime example is the relationship between posttraumatic stress disorder (PTSD) and increased risk for developing cardiovascular (Edmondson & von Känel, 2017) and cardiometabolic (Edmondson & von Känel, 2017) disease. Critically, PTSD has been linked with increased mortality (Edmondson & von Känel, 2017), underscoring the relationships between mental and physical health. A number of behavioral and biological mediators have been examined including sleep dysregulation, cigarette use, decreased physical activity, autonomic reactivity, and inflammation, which may all contribute to accelerated cellular aging and subsequently increased rates of medical morbidity (Wolf & Schnurr, 2016). Identification of accelerated aging processes may aid in detecting elevated risk for developing disease and provide opportunities for a personalized medicine approach to treatment.
Specific to PTSD, biomarkers of accelerated aging have been identified in the epigenome, and immune and inflammatory systems (Wolf & Morrison, 2017). Telomere length and DNA “methylation age” are two biomarkers that have shown mixed results (Wolf & Morrison, 2017), and may be more or less sensitive depending on the type of outcome measure (e.g., current vs. lifetime PTSD or specific symptom clusters). In a recent meta‐analysis, lifetime PTSD symptom severity and childhood trauma were associated with accelerated DNA methylation age (Katrinli et al., 2020; Wolf et al., 2018; Yang et al., 2020). PTSD has also been linked with diminished immune response (Aiello et al., 2016), and a heightened inflammatory response based on C‐reactive protein (Spitzer et al., 2010), TNF‐alpha (von Kanel et al., 2007), IL‐6, and IL‐1β (Newton et al., 2014), which may impact aging processes. While peripheral biomarkers provide initial evidence for a link between PTSD and accelerated aging, identification of neural biomarkers is critical to understanding the complex relationships between PTSD, aging, and the brain. Given the known deleterious effects of aging on the brain (Schmitz et al., 2018), coupled with functional and structural brain changes associated with PTSD (Clausen et al., 2020; Logue et al., 2018; Morey et al., 2020), neural markers of accelerated aging in PTSD may help to stratify disease risk, clarify the pathology of psychiatric disease on the brain, and aid in the development of tailored treatments for PTSD.
Machine learning algorithms have been applied to metrics derived from T1‐weighted structural MRI to predict biological brain age (Cole & Franke, 2017), which can be compared with chronological age to calculate a difference score, referred to as brain predicted age difference (PAD). Brain PAD has been optimized by training algorithms using chronological age in large samples of healthy individuals (Cole & Franke, 2017) and has been tested in aging (J. H. Cole et al., 2018), schizophrenia, bipolar disorder, and depression (Besteher et al., 2019; Han et al., 2021; Schnack et al., 2016). In aging, higher brain PAD, which is indicative of an "older appearing" brain, has been linked with weaker grip strength, diminished lung function, slower walking speed, lower fluid intelligence, higher allostatic load, and critically, increased mortality risk (Cole et al., 2018). Patients with schizophrenia and bipolar disorder exhibit, on average, higher brain PAD, suggesting that these disorders may be associated with accelerated brain aging (Hajek et al., 2019; Schnack et al., 2016). By contrast, findings with brain PAD in patients with depression have been inconsistent (Besteher et al., 2019; Han et al., 2021), but suggest a small effect of depression on brain PAD. Research examining brain PAD in PTSD is in its infancy, but initial evidence suggests PTSD is associated with accelerated brain aging. In a relatively large sample (N = 804) of individuals aged 8–21, subjects with PTSD exhibited higher brain PAD relative to controls (Liang et al., 2019). However, the study was conducted in children, adolescents, and very young adults, and group sample sizes were highly imbalanced (PTSD, n = 70; control, n = 734); the control group also lacked trauma exposure.
The primary aim of the present study was to examine the relationship between PTSD and brain PAD in adults with and without PTSD who were aggregated from 21 sites in the ENIGMA‐PTSD Consortium. As PTSD has been linked with other markers of accelerated aging (Li et al., 2017; Wolf et al., 2018), we hypothesized that the PTSD group would show, on average, higher brain PAD. A wide range of different brain age prediction techniques are available (Cole et al., 2019). Therefore, prior to embarking on our primary goal of investigating brain aging in PTSD, a test set of controls was used to evaluate the performance of three machine learning‐based pipelines including one voxel‐based algorithm and two algorithms that rely on region‐of‐interest (ROI) cortical and subcortical measures from the FreeSurfer 5.3 segmentation. We hypothesized the voxel‐based method, which uses greater structural detail than the ROI approaches, would provide more accurate estimates of brain age.
2. METHOD
2.1. Subjects
Subjects included 2315 adults from 21 sites participating in the ENIGMA‐PGC PTSD Consortium (see Table 1 for demographic and clinical characteristics). Each site had unique inclusion and exclusion criteria. Common exclusion criteria included meeting current diagnostic criteria for a substance use disorder, neurological disorder, active psychosis, and moderate‐to‐severe head injuries. Subjects were included in the present analysis if they completed a 3D T1‐weighted brain scan with magnetic resonance imaging (MRI), and assessment of PTSD, as well as reported age and sex. Eighty‐six subjects were removed from the analysis due to missing data (see Supporting Information for subject exclusion). Therefore, a total of 2229 subjects with (n = 882) and without (n = 1347) PTSD were included in the final analysis. Control subjects included trauma‐exposed (n = 1226) and unexposed (n = 118) individuals. Three control subjects were missing trauma‐exposure data. Analyses were conducted with and without unexposed controls and those missing trauma exposure data. No significant differences were identified in the main or supplemental analyses; thus, unexposed controls, and those missing trauma‐exposure data, were included in the analyses.
TABLE 1.
Demographic characteristics
| Full group (N = 2229) | Controls (n = 1347) | PTSD (n = 884) | Comparison between groups | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | t‐value (p‐value) | |
| Age | 35.6 (11.0) | 35.4 (11.0) | 35.9 (10.9) | −0.90 (.370) |
| Predicted age | 35.1 (10.4) | 34.7 (10.6) | 35.6 (10.1) | −1.91 (.056) |
| Brain PAD | −0.5 (8.7) | −0.7 (8.4) | −0.3 (9.0) | −1.17 (.242) |
| Childhood trauma severity | N = 1352, 1.3 (0.86) | n = 846, 1.1 (0.9) | n = 506, 1.6 (0.7) | −12.44 (< .001) |
| % with (n) | % with (n) | % with (n) | X2 or F‐statistic (p‐value) | |
| Males | 56.2% (1252) | 57.8% (778) | 53.6% (474) | 3.33 (.068) |
| % Caucasian | 54.6% (1217) | 55.8% (752) | 52.6% (465) | 14.61 (.001) |
| MDD diagnosis | N = 1878, 32.6% (613) | n = 1142, 11.8% (135) | n = 736, 64.9% (478) | 572.07 (<.001) |
| Military status | N = 2102, 42.1% (884) | n = 1238, 45.7% (566) | n = 864, 36.8% (318) | 16.23 (<.001) |
Abbreviations: PAD, predicted age difference; MDD, major depressive disorder; PTSD, posttraumatic stress disorder; SD, standard deviation.
2.2. Clinical assessment
A breakdown of clinical assessments by site is included in Tables S1 and S2. Clinical assessments were conducted for PTSD, depression, and childhood trauma using either self‐report measures or clinician‐administered interviews. When available, severity scores were used to estimate PTSD and depression diagnosis using previously established cut‐off scores (Table S1).
2.2.1. PTSD
Current PTSD diagnosis was assessed with one of the following instruments: Clinician Administered PTSD Scale for DSM‐IV (Pfohl et al., 1997) or DSM‐5 (Weathers et al., 2018), the PTSD Symptom Checklist for DSM‐IV Civilian (Weathers et al., 2015), Military (Yarvis et al., 2012) versions, or for DSM‐5 (Weathers et al., 2015), Davidson Trauma Scale (Davidson, 1996), the structured clinical interview for DSM‐IV (Spitzer et al., 1992) or DSM‐5 (First et al., 2014) disorders (SCID), or the Mini International Neuropsychiatric Inventory (MINI) 6.0 (Sheehan & Lecrubier, 2010) or 7.0 (Sheehan et al., 2014).
2.2.2. Childhood trauma
Thirteen of the 21 participating sites collected information on childhood trauma. The majority of sites (n = 11) used the Childhood Trauma Questionnaire (Bernstein et al., 2003). One site used the Early Trauma Inventory (Bremner et al., 2000), and one site used the Stressful Life Events Screening Questionnaire—Revised (Green et al., 2006). Childhood trauma measures were harmonized to yield a score of 0 (no trauma exposure), 1 (exposure to one type [e.g., physical vs. emotional abuse] of trauma), or 2 (exposure to two or more types of traumas).
2.2.3. Depression
Twenty of the 21 participating sites diagnosed depression and/or its severity with one of the following instruments: the Beck Depression Inventory–II (Beck et al., 1996) and 1A (Steer et al., 1999), the Hamilton Rating Scale for Depression (Bremner et al., 2000), the Hospital Anxiety and Depression Scale (Snaith & Zigmond, 2000), the Physical Health Questionnaire−9 (Kroenke & Spitzer, 2002), the Center for Epidemiology Studies – Depression (Orme et al., 1986), the Depression and Anxiety Stress Scale – depression subscale (Lovibond & Lovibond, 1995), the MINI, or the SCID.
2.3. MRI acquisition and preprocessing
A high resolution T1‐weighted brain MRI scan, optimized for tissue contrast, was acquired at each site and was analyzed locally (Table S2). Raw T1‐weighted images were then sent to Duke University for preprocessing and quality assurance using standardized ENIGMA protocols to harmonize image analyses across multiple sites. Raw and preprocessed images (for FreeSurfer 5.3) were visually inspected for pathology, image quality, and quality of automated segmentation (http://enigma.ini.usc.edu/protocols/imaging‐protocols/). All scans were preprocessed using the same version of FreeSurfer to minimize the variability between segmentation results. Version 5.3 was chosen based on previously established preprocessing procedures for a previously trained brain age algorithm.
2.4. Machine learning pipelines
We examined three pre‐trained machine learning pipelines to estimate brain age in a subset of controls (n = 386). One algorithm used voxel‐based data, referred to as brainageR and two algorithms, PHOTON Brain Age (PHOTON‐BA; https://www.photon‐ai.com/enigma_brainage) and Brain‐Age Regression Analysis and Computation Utility Software (BARACUS), and used cortical and subcortical segmentation results from FreeSurfer 5.3. Descriptions of each model are included in the Supporting Information. Briefly, brainageR applies a Gaussian process regression model to T1‐weighted scans, parcellating gray and white matter, to predict chronological age (Cole et al., 2018) (https://github.com/james‐cole/brainageR; https://doi.org/10.5281/zenodo.3476365 ). While both PHOTON‐BA and BARACUS rely on FreeSurfer segmented data, PHOTON‐BA estimates brain age by applying a ridge regression model (Schmaal et al., 2020) whereas BARACUS uses a linear support vector regression model (Liem et al., 2017).
2.5. Statistical analysis
All analyses were conducted in R Statistical Software Package (freely available at https://cran.r‐project.org). To assess model performance, we calculated intraclass correlations (ICC) between chronological and predicted age, proportion of the variance explained by the model (R 2), Pearson's R between predicted brain age and chronological age, mean absolute error (MAE), and root mean square error (RMSE) for each machine learning algorithm in a subset of control subjects. This subset was a convenience sample from three participating sites which had previously processed MRI data and calculated estimates for brain age using the three machine learning models (Minneapolis VA, Duke University, University of South Dakota). The algorithm with the highest R, R 2, and ICC values, and lowest RMSE and MAE for control subjects, was applied to the full sample from 21 participating sites to examine relationships between brain age estimates and PTSD.
Brain PAD (predicted brain age − chronological age) was calculated for each subject, using the algorithm with the best fit, and was used as the primary outcome measure. Brain PAD is typically overestimated for children and young adults and underestimated in older individuals, which is explained by the well‐known statistical phenomenon of regression to the mean (Le et al., 2018; Liang et al., 2019). Two methods have been proposed to adjust for this phenomenon, including incorporating age as a covariate in the main analysis (Le et al., 2018; Liang et al., 2019) and developing residualized brain PAD scores by regressing chronological age onto brain PAD (Le et al., 2018). Because we aimed to examine main and interaction effects of age, we incorporated chronological age as a covariate, as opposed to developing a residualized brain PAD score. To ensure age did not significantly differ between groups with and without PTSD, we examined differences in age between control and PTSD groups. To mega‐analyze differences in brain PAD between subjects with and without PTSD, we used linear mixed effects (LME) modeling (R Library lme4; R command lmer). PTSD diagnosis, age, age2, and sex were then entered into the LME as fixed effects. Age and age2 were centered prior to performing the analysis. As subjects were nested within each site, the site was included as a random effect in all models to adjust for differences in scanners and systematic differences between sites. We explored main and interaction effects of all covariates and PTSD diagnosis.
Model 1 Main Effects: brain PAD ∼ age + age2 + sex + PTSD diagnosis + (1 | site)
Model 2 Interaction Effects: brain PAD ∼ age * age2 * sex * PTSD diagnosis + (1 | site)
We conducted supplemental analyses to explore the effects of race, childhood trauma, MDD diagnosis, and military status by adding covariates that related to brain PAD at or below the trend level (p < .10) to LME Model 2 as a fixed main effect. In a subsample of subjects with PTSD who completed the CAPS‐IV (n = 515), LME modeling analogous to the main analysis was conducted to explore effects of PTSD severity in relation to brain PAD. Findings were considered significant with a critical p‐value < .05. AIC and BIC indices were used to assess model fit.
Model 3 Additive Effects: brain PAD ∼ age * age2 * sex * PTSD diagnosis + covariate + (1 | site)
3. RESULTS
3.1. Brain age prediction performance
We examined the predictive ability of the brainageR, PHOTON‐BA, and BARACUS machine learning pipelines to estimate brain age in a subsample of control subjects (n = 386) from three sites. Results of pipeline comparisons are presented in the Supporting Information and Figures S1–S3. Briefly, brainageR demonstrated a stronger relationship between chronological and predicted age (R = 0.72) and lower error (MAE = 5.68) compared to BARACUS (R = 0.64; MAE = 8.8) and PHOTON‐BA (R = 0.62; MAE = 6.38) models. Model validation for the brainageR pipeline is displayed in Figure 1.
FIGURE 1.
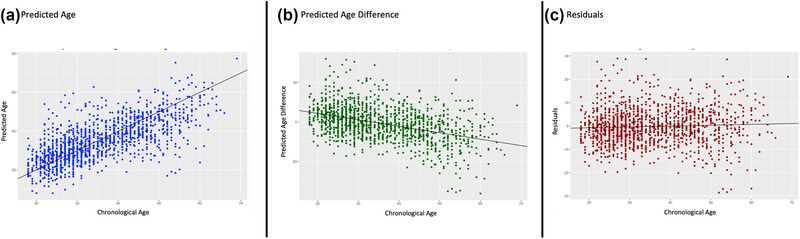
Model validation in control subjects. (a) A significant, positive relationship was identified between chronological age and predicated age in control subjects (R = 0.70, R 2 = 0.49, p > .0001). (b) A significant negative relationship was observed between chronological age and predicated age difference (predicted age − chronological age) in control subjects (R = −0.33, R 2 = 0.18, p > .0001). (c) While the effect was small, we continued to observe a significant relationship when plotting the residual effects of the linear relationship between predicted age difference and chronological age (shown in Figure 1b) with chronological age in controls (R = 0.04, R 2 = 0.002, p = .05)
3.2. Covariates
For the main analysis, we examined linear and nonlinear effects of age, and sex, with brainageR brain PAD. Age (r(2227) = −0.45, p < .001), age2 (r(2227) = −0.45, p < .001), and sex (t(2227) = −1.84, p = .06), when examined separately, were related (p < .1) to brain PAD. For the supplementary analyses, we examined other potentially contributing factors including race, military status, depression, and childhood trauma. Both race (F[1,2227] = 4.768, p = .029), and military status (t(2104) = −3.0858, p = .002) when examined separately, significantly related to brain PAD. Neither childhood trauma (F(1,1350) = 1.78, p = .182) nor depression (t(1876) = −0.99, p = .321) related to brain PAD. See Figure 2 for visualization of covariates. We also examined the relationship between age and PTSD diagnosis. Age did not significantly differ between groups (t(2227) = −0.897, p = .370), and there was a high degree of overlap across distributions in age for the PTSD and control groups (Figure 3).
FIGURE 2.
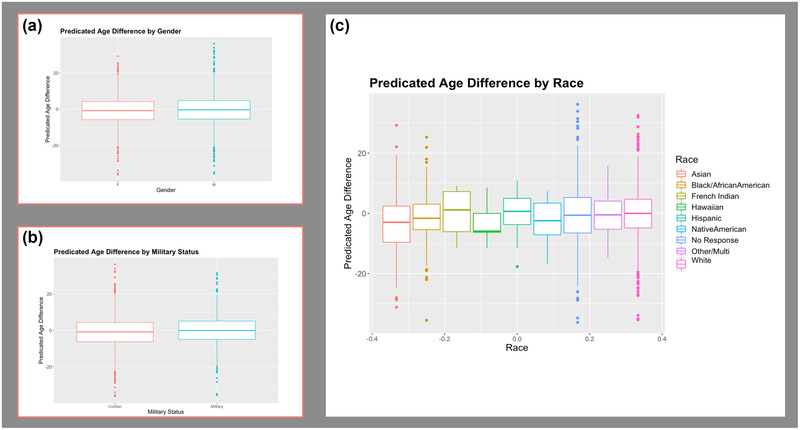
Potential covariates. (a) Predicted age difference by sex. (b) Predicted age difference by military status. (c) Predicted age difference by each racial or ethnic group (driven solely by differences in the Beijing data). Sex was included as a covariate in the main analysis. Both military status and race were examined in supplementary analyses
FIGURE 3.
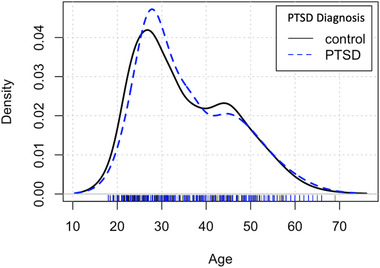
Age density for diagnostic groups. The probability density as a function of subjects’ age in the PTSD and control groups
3.3. Impact of PTSD diagnosis on brain age
Results for each LME model are presented in Table 2. Model 1 examined the main effects of age, age2, sex, and PTSD on brain PAD. Age (β = −0.38, p < .001) and sex (β = 0.84, p = .027) significantly related to brain PAD. There was no main effect of age2 (β < 0.001, p = .520) or PTSD diagnosis (β = −0.280, p = .420).
TABLE 2.
Linear mixed effects (LME) results for PTSD diagnosis
| Beta | CI | t‐value | p | ||
|---|---|---|---|---|---|
| Model 1 AIC = 15,344.3, BIC = 15,384.3, N = 2229, Marginal R2 / Conditional R2 = 0.220 / 0.266 | |||||
| Intercept | —1.13 | −2.18 to −0.08 | −2.12 | .040 | |
| PTSD | 0.28 | −0.40 – 0.96 | 0.81 | .420 | |
| Age | −0.38 | −0.41 to −0.34 | −22.38 | <.001* | |
| Age2 | 0.00 | −0.00 – 0.00 | 0.644 | .520 | |
| Sex (M) | 0.84 | 0.10 – 1.58 | 2.21 | .027* | |
| ** Model 2 AIC = 15,336.9, BIC = 15,399.7, N = 2229, Marginal R2 / Conditional R2 = 0.226 / 0.270 | |||||
| Intercept | −1.36 | −2.45 to −0.28 | −2.46 | .017 | |
| PTSD | 0.75 | −0.29 – 1.80 | 1.43 | .153 | |
| Age | −0.40 | −0.45 to −0.34 | −13.94 | <.001* | |
| Age2 | 0−0 | −0.00 – 0.00 | 0.69 | .493 | |
| Sex (M) | 1.15 | 0.26 – 2.04 | 2.53 | .017* | |
| PTSD*Age | −0.00 | −0.09 – 0.08 | −0.14 | .890 | |
| PTSD*Sex (M) | −0.76 | −2.09 – 0.57 | −1.11 | .265 | |
| Age*Sex (M) | 0.09 | 0.02 – 0.16 | 2.39 | .017* | |
| PTSD*Age*Sex (M) | −0.14 | −0.26 to −0.02 | −2.34 | .019* | |
| Model 3 AIC = 15,338.7, BIC = 15,407.3, N = 2229, Marginal R2 / Conditional R2 = 0.227 / 0.270 | |||||
| Intercept | −1.23 | −2.46 to −0.01 | −1.97 | .053 | |
| PTSD | 0.76 | −0.28 – 1.80 | 1.43 | .150 | |
| Age | −0.40 | −0.45 to −0.34 | −13.93 | <.001* | |
| Age2 | 0.00 | −0.00 – 0.00 | 0.69 | .488 | |
| Sex (M) | 1.14 | 0.25 – 2.04 | 2.50 | .012* | |
| Race | −0.03 | −0.18 – 0.11 | −0.44 | .664 | |
| PTSD*Age | −0.01 | −0.09 – 0.08 | −0.15 | .883 | |
| PTSD*Sex (M) | −0.76 | −2.09 – 0.58 | −1.11 | .267 | |
| Age*Sex (M) | 0.09 | 0.02 – 0.16 | 2.38 | .018* | |
| PTSD*Age*Sex (M) | −0.14 | −0.26 to −0.02 | −2.33 | .020* | |
| Model 4 AIC = 14,472.6, BIC = 14,540.4, N = 2102, Marginal R2 / Conditional R2 = 0.247 / 0.292 | |||||
| Intercept | –1.75 | −2.93 to −0.57 | −2.91 | .005 | |
| PTSD | 0.79 | −0.26 – 1.85 | 1.48 | .140 | |
| Age | −0.42 | −0.48 to −0.37 | −14.00 | <.001* | |
| Age2 | 0.00 | −0.00 – 0.00 | 0.64 | .524 | |
| Sex (M) | 1.11 | 0.14 – 2.08 | 2.25 | .025 | |
| Military status | 1.06 | −0.25 – 2.36 | 1.59 | .114 | |
| PTSD*Age | 0.02 | −0.07 – 0.10 | 0.40 | .689 | |
| PTSD*Sex (M) | −0.88 | −2.25 – 0.48 | −1.27 | .206 | |
| Age*Sex (M) | 0.09 | 0.01 – 0.16 | 2.17 | .030 | |
| PTSD*Age*Sex (M) | −0.15 | −0.27 to −0.03 | −2.4 | .017 | |
Abbreviations: LME, linear mixed effects; PTSD, posttraumatic stress disorder; AIC, Akaike's information criteria; BIC, Bayesian information criteria.
Indicates best model fit.
Next, we explored interaction effects of age, sex, and PTSD diagnosis on brain PAD (Model 2, Figure 4). We identified a three‐way interaction between age, sex, and PTSD diagnosis (β = −0.14, p = .019). This model exhibited lower AIC, but higher BIC relative to Model 1 Revised (Model 1: AIC = 15,344.3, BIC = 15,384.3; Model 2: AIC = 15,336.9, BIC = 15,399.7). BIC penalizes model complexity more heavily than AIC. Thus, it is expected that the interaction model has a higher BIC relative to examination of only main effects. Model 2 was selected as the model of best fit.
FIGURE 4.
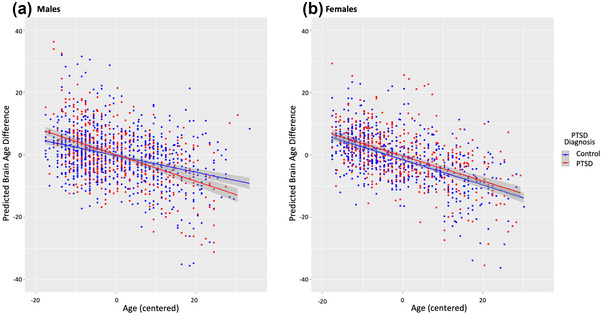
Relationships between PTSD, chronological age, age2 and sex with predicted brain age difference (PAD). Brain PAD scores were calculated using the brainageR pipeline. Main and interaction effects of PTSD, age (centered), and sex with main fixed effects of age2 (centered) and site included as a random effect are displayed. (a) Linear plot of age by PTSD diagnosis interaction in male subjects. Males with PTSD exhibited a steeper, negative relationship between age and brain PAD relative to males without PTSD. (b) Linear plot of age by PTSD diagnosis in female subjects. No interaction was observed between PTSD diagnosis and age in female subjects. Females with PTSD exhibited qualitatively higher brain PAD relative to females without PTSD
To visually characterize the three‐way interaction, we divided the sample by sex (Figure 4) and plotted the relationship between age and predicted brain age by PTSD status. Males with PTSD exhibited a steeper relationship between chronological age and brain PAD compared to males without PTSD. Males with PTSD exhibited higher brain PAD at a younger age, and lower brain PAD at an older age compared to males without PTSD. Whereas females with PTSD tended to have higher brain PAD relative to females without PTSD with the exception of younger control subjects and old subjects with PTSD.
In order to further explore the three‐way interaction, we conducted pairwise comparisons of the relationship between chronological age and brain PAD in males and females with and without PTSD using the package cocor in R (Diedenhofen & Musch, 2015). Females with (Fischer's z = 3.54, p < .001) and without PTSD (Fischer's z = 5.15, p < .001) exhibited a stronger negative correlation between chronological age and brain PAD relative to males without PTSD. No differences were observed in the relationship of chronological age and brain PAD between males with and without PTSD (Fischer's z = 1.43, p = .154), females without PTSD and males with PTSD (Fischer's z = −1.79, p = .074), females with and without PTSD (Fischer's z = −1.04, p = .300), nor males and females with PTSD (Fischer's z = 0.65, p = .517).
Next, we conducted supplementary analyses to examine the potential effects of race and military status. Race and military status were added as fixed effects to Model 2 separately. When added as a fixed effect to Model 2, race did not significantly contribute to brain PAD (Model 3; race β = −0.03, p = .664). Compared to Model 2 AIC and BIC, the inclusion of race as a main effect resulted in higher AIC and BIC (Model 3 AIC = 15,338.7, BIC = 15,407.3). Classification of military status was available for 2102 subjects. When added to Model 2, there was no main effect of military status (Model 4; military status β = 1.06, p = .114) on brain PAD. As Model 4 was completed in a subset of subjects, we are unable to directly compare AIC and BIC to previous models.
3.4. Influence of PTSD symptom severity on brain age
We repeated the above analyses in a subsample of subjects with a PTSD diagnosis based on the CAPS‐IV (N = 515) to explore PTSD symptom severity. We chose to restrict our analyses to subjects with a diagnosis of PTSD because PTSD severity was not consistently assessed across sites in trauma‐exposed controls. We used the CAPS‐IV as it was used most widely across sites to assess PTSD severity. Age, age2, sex, depression, childhood trauma, race, and military status were tested as covariates in this subsample.
Age (R(515) = −0.50, p < .001), and age2 (R(515) = −0.49, p < .001) were significantly related to brain PAD in these PTSD subjects. Sex (t(513) = −1.10, p = .270), depression (t(374) = −0.09, p = .925), childhood trauma (F(1,267) = 0.25, p = .612), race (F(1,513) = 0.09, p = .765) and military status (t(513) = −0.69, p = .490) were not related to brain PAD. Age, age2, and PTSD severity were entered as fixed effects. Site was again included as a random effect.
Age significantly predicted brain PAD (β = −0.47, p < .001); however, neither PTSD severity (β = −0.01, p = .504) nor age2 (β = 0.004, p = .183) was significantly related to brain PAD. The interaction between age and PTSD was also not significant (β < 0.001, p = .125). See Table S3 for PTSD severity LME model results.
4. DISCUSSION
In this study, we investigated the difference between chronological age and MRI‐predicted brain age. The role of other potentially relevant contributors to brain age such as sex, race, childhood trauma exposure, military service, depression, and chronological age were also investigated. To apply the most accurate method for estimating brain age from structural MRI, we first compared three leading machine learning models. We determined that the voxel‐based machine learning method based on Gaussian Process Regression (brainageR) (Cole et al., 2018) outperformed two competing ROI methods (PHOTON‐BA and BARACUS) that rely on regional mean cortical thickness, surface area, and volume generated by FreeSurfer automated segmentation. Leveraging a large, multi‐site mega‐analysis of 2229 PTSD cases and controls, we identified that brain PAD was negatively related with chronological age in control subjects, such that increasing age significantly related to lower brain PAD. Furthermore, an interaction between sex and age demonstrated females had a stronger negative correlation between brain PAD and chronological age than males. In other words, males showed greater brain aging than females and this sex difference increased with age (Figure 4). We also found an interaction of PTSD, age, and sex which showed young males with PTSD had a greater brain PAD than young male controls, young females with PTSD, and young females without PTSD. A similar pattern was present in middle‐aged males, but the effect of PTSD was weaker than in the younger subgroup. Male controls in the older subgroup exhibited higher brain PAD than older males with PTSD, older females with PTSD, and old females without PTSD.
Surprisingly, brain PAD was higher in younger subjects with PTSD than in older subjects with PTSD. Some possible interpretations are that brain age in the context of PTSD may vary across lifespan and on the basis of sex and thus may not reflect a consistent biomarker of aging in PTSD such that the younger brain is more vulnerable to aging effects of PTSD (i.e., representing a critical window for PTSD's impact on brain age) or there may be additional factors such as cardiovascular or cerebrovascular disease that may influence the relationship between age and brain PAD particularly in older individuals. It is possible the brain aging process undergoes some remediation in the chronic stage of PTSD developing greater resilience over time. This assumes that onset of PTSD in the present sample generally occurred during early or early‐mid adulthood as reported in the National Comorbidity Survey Replication, which found 23 years to be the median age of onset (Kessler et al., 2005). However, such a hypothesis can only be tested with longitudinal assessments initiated soon after initial trauma exposure. Indeed, symptoms in a subset of patients with PTSD tend to remit as the trauma becomes more distant in time (Magruder et al., 2016; Sun et al., 2018). This trajectory is consistent with the natural history of the posttraumatic period where most individuals experience acute symptoms in the hours and days following exposure to a traumatic event (Cahill & Pontoski, 2005). This acute stress response generally resolves, but may persist for more than 1 month in a smaller or larger subset of individuals, depending on the nature of the trauma; this subset is deemed to have PTSD (Kolassa et al., 2010). The relationship of brain PAD to chronological age in our results may be a methodological limitation of a one‐size‐fits‐all approach we took that may bias brain age prediction in either the youngest or oldest subgroups (Elliott et al., 2019). Other approaches may include use of classification as opposed to regression to approximate ordinal effects, or use of auxiliary loss functions in deep neural networks. An alternate, perhaps unlikely interpretation is that the usual neurobiological processes that operate in senescence are mitigated by PTSD, but this hypothesis lacks empirical support from epigenetic and inflammatory research (Katrinli et al., 2020; Miller et al., 2018). Unfortunately, there is a lack of published evidence on brain aging across the lifespan in PTSD, particularly studies on neurobiological mechanisms of action. Thus, meaningful interpretation of the present results is rather challenging without comparisons to empirical evidence.
In previous research, PTSD has been associated with higher brain PAD in relatively small sample of PTSD patients (n = 70) who exhibited higher brain PAD relative to control subjects (Liang et al., 2019). Relatedly, “negative fateful life events” such as death in the family or financial hardship were associated with higher predicted brain age relative to chronological age in Vietnam era twin veterans, but unfortunately associations with PTSD were not reported (Hatton et al., 2018). Consistent with the present results, higher brain PAD diminished with advancing chronological age, which was attributed to negative fateful life events being more commonplace in the early‐mid stage of life or before. Importantly, the former study was conducted in a PTSD sample that was mostly female (71%) and focused on children and young adults (ages 8–21), and the latter study was conducted in all older male adults (mean age = 62 years). The present analysis included both males (56%) and females (44%) ranging in age from 18 to 69 and thus demonstrates the differential relationship of age and brain age across age groups and sex. The present results also align with reports in schizophrenia and bipolar disorder that are characterized by a so‐called “early‐hit non‐progressive” phenomenon, where accelerated brain aging occurs at younger ages followed by age‐related brain changes that are consonant with unaffected individuals (Shahab et al., 2019).
PTSD and trauma impart prolonged stress which is associated with neurodegenerative cellular processes as a result of altered immune system gene expression, elevated gene expression of inflammatory mediators, diminished expression of antiviral processes, and synthesis of antibodies (Cole, 2014; Miller & Sadeh, 2014). The accumulation of damage to DNA within neurons triggers repair processes to be activated. However, impaired repair processes cascade to accumulation of DNA damage that produces cellular senescence, apoptosis, neurodegeneration, and premature brain aging (Coppedè & Migliore, 2010). The prolonged burden of lifetime stress has been shown to accelerate epigenetic aging via glucocorticoid signaling (Gassen et al., 2017). The foregoing evidence provides some possibilities for mechanisms operating in PTSD at the molecular level to impact accelerated brain aging, particularly in young males.
The methodology for predicting brain age from neuroimaging is relatively new and represents a nonspecific biomarker of brain development and brain health (Franke & Gaser, 2019). It captures information about a large number of variables from many brain locations (or regions) into a single composite metric. Thus, patients in the clinical setting may find this to be a readily accessible and interpretable indicator of brain health, which is adjusted for chronological age. It is conceivable that brain PAD screening could facilitate clinical trial recruitment. Subjects could be stratified by brain PAD for investigating various clinical phenomenon such as mild cognitive impairment (Liem et al., 2017), elevated risk for psychosis (Gaser et al., 2013; Koutsouleris et al., 2014), progression to Alzheimer disease, borderline personality disorder (Han et al., 2021; Koutsouleris et al., 2014), major depression, bipolar disorder (Shahab et al., 2019), schizophrenia (Koutsouleris et al., 2014; Shahab et al., 2019), and brain development more generally (Brown et al., 2012; Dosenbach et al., 2010; Smyser et al., 2016). Many of the strengths of brain PAD as a biomarker also pose significant shortcomings, which include the lack of direct information about the most predictive features of brain age, which could differ in each person. In principle, direct information about the relative importance of features used in making predictions can be extracted from most machine learning algorithms (Domingos, 2015). Knowledge of these critical features may be of little interest in some machine learning applications such as face recognition, whereas such a knowledge map is vital in neuroscience and research applications more broadly. A map of the most predictive brain features may guide future research on causal mechanisms of brain aging, but was outside the scope of the present study. Future studies with deep clinical phenotyping and longitudinal assessments of mental and somatic health—along with comorbidities, illness duration, inflammatory markers, health behaviors, and multi‐omics panels (e.g., genomics) (Amoroso et al., 2019)—will help further refine and evaluate the value of brain PAD estimates, or be used in conjunction with brain PAD estimates (Han et al., 2021). Recent application of deep learning methods such as convolutional neural networks hold the promise of enhanced performance (R 2 = 0.87) and lower MAE (Cole et al., 2017; Jónsson et al., 2019) while also offering more specificity about the most predictive features (Cole et al., 2017).
4.1. Limitations and conclusion
A key strength of our study is the large sample size that allowed us to test interactions with and associations within subgroups such as sex, chronological age, military status, and other variables. This large sample also constitutes a limitation, as combining data from many different sites and scanners may introduce confounds, particularly if there is a stratification of variables of interest by site/scanner. A second limitation is the use of a single imaging modality, as multi‐modal imaging inputs such diffusion imaging and resting‐state functional connectivity have been successfully employed in brain age estimation (Cole, 2020). Lastly, a number of factors, some of which covary with PTSD, that influence the aging process such as socioeconomic status, cardiometabolic function, diet, environmental pollutants, head trauma, and substance use, could not be accounted for in our study (Dowling et al., 2008; Fotenos et al., 2008; Griesbach et al., 2018; Joseph et al., 2009; Mehta et al., 2015). Future research is warranted to further understand how these factors may contribute to aging processes in the context of PTSD. Our results provide evidence of a link between PTSD diagnosis and accelerated brain aging processes, influenced by sex and chronological age. We offer a framework for exploring neurobiological markers of accelerated aging and potential underlying processes in PTSD.
CONFLICT OF INTEREST
Dr. Krystal is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol‐Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries. She is also on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer; she is a stockholder in Biohaven Pharmaceuticals, holds stock options in Mnemosyne Pharmaceuticals, Inc., holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, US Patent No. 5,447,948 (issued September 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, US Patent No. 8,778,979 (issued July 15, 2014) and filed a patent for Intranasal Administration of Ketamine to Treat Depression. US Application No. 14/197,767 (filed on March 5, 2014); US application or Patent Cooperation Treaty international application No. 14/306,382 (filed on June 17, 2014). She also filed a patent for using mTOR inhibitors to augment the effects of antidepressants (filed on August 20, 2018). Dr. Abdallah has served as a consultant, speaker and/or on advisory boards for FSV7, Lundbeck, Psilocybin Labs, Genentech and Janssen, and editor of Chronic Stress for Sage Publications, Inc.; he has filed a patent for using mTOR inhibitors to augment the effects of antidepressants (filed on August 20, 2018). Dr. Schmahl is consultant for Boehringer Ingelheim International GmbH. Dr. Davidson is the founder and president of, and serves on the board of directors for, the non‐profit organization Healthy Minds Innovations, Inc. Dr. Thompson received partial research support from Biogen, Inc. (Boston, USA) for research unrelated to the topic of this manuscript. Dr. Cole is a consultant to and shareholder in Brain Key, a medical image analysis software company.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2413
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Department of Veterans Affairs via support for the National Center for PTSD; DoD W81XWH‐10‐1‐0925; Center for Brain and Behavior Research Pilot Grant; South Dakota Governor's Research Center Grant; Vanderbilt Psychiatric Genotype/Phenotype Project, and the Vanderbilt Institute for Clinical and Translational Research (through grant 1‐UL‐1‐TR000445 from the National Center for Research Resources/NIH); CX001600 VA CDA; MRC/UKRI Innovation Fellowship; German Research Foundation grant to Judith K. Daniels (numbers DA 1222/4‐1 and WA 1539/8‐2); NIMH R01‐MH043454; NIMH T32‐MH018931; VA RR&D IK2RX002922‐01A1; MH101380; National Institute of Mental Health training grant (T32MH13043); German Federal Ministry of Education and Research (BMBF RELEASE 01KR1303A); German Research Society (Deutsche Forschungsgemeinschaft, DFG; SFB/TRR 58: C06, C07); MH098212; MH071537; M01RR00039; UL1TR000454; HD071982; HD085850; R21MH112956; R01MH096987; Anonymous Women's Health Fund; Kasparian Fund; Trauma Scholars Fund; Barlow Family Fund; K01 MH118467; ZonMw, the Netherlands organization for Health Research and Development (40‐00812‐98‐10041) and a grant from the Academic Medical Center Research Council (110614) both awarded to Miranda Olff; NIAAA via support for (P50) Center for the Translational Neuroscience of Alcohol; NCATS via support of (CTSA) Yale Center for Clinical Investigation; CIHR; CIMVHR; NIH R01 MH106574; F32MH109274; VISN6 MIRECC; BOF 2–4 year project to Sven C. Mueller (01J05415); R01MH105355; National Institute of Mental Health F31 MH113271; NIMH K23MH112873; Veterans Affairs Merit Review Program (10/01/08–09/30/13); NHMRC Career Development Fellowship (1140764); DoD W81XWH08‐2‐0159 to Murray Stein; CDMRP, W81XWH‐08‐2‐0038; VA RR&D, I01RX000622; Narsad Young Investigator; German Research Society; K01 MH118428; Brain and Behavior Research Foundation Grant 07040. ENIGMA was also supported in part by NIH U54 EB020403 from the Big Data to Knowledge (BD2K) program, R56AG058854, R01MH116147, R01MH111671, and P41 EB015922. Writing of this paper was supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Durham VA Health Care System, and the Department of Veterans Affairs Mid‐Atlantic MIRECC. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, the United States government, or any other funding sources listed here.
Clausen, A. N. , Fercho, K. A. , Monsour, M. , Disner, S. , Salminen, L. , Haswell, C. C. , Rubright, E. C. , Watts, A. A. , Buckley, M. N. , Maron‐Katz, A. , Sierk, A. , Manthey, A. , Suarez‐Jimenez, B. , Olatunji, B. O. , Averill, C. L. , Hofmann, D. , Veltman, D. J. , Olson, E. A. , Li, G. , … Morey, R. A. (2022). Assessment of brain age in posttraumatic stress disorder: Findings from the ENIGMA PTSD and brain age working groups. Brain and Behavior, 12, e2413. 10.1002/brb3.2413
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aiello, A. E. , Dowd, J. B. , Jayabalasingham, B. , Feinstein, L. , Uddin, M. , Simanek, A. M. , Cheng, C. K. , Galea, S. , Wildman, D. E. , Koenen, K. , & Pawelec, G. (2016). PTSD is associated with an increase in aged T cell phenotypes in adults living in Detroit. Psychoneuroendocrinology, 67, 133–141. 10.1016/j.psyneuen.2016.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso, N. , La Rocca, M. , Bellantuono, L. , Diacono, D. , Fanizzi, A. , Lella, E. , Lombardi, A. , Maggipinto, T. , Monaco, A. , Bellotti, R. , & Tangaro, S. (2019). Deep learning and multiplex networks for accurate modeling of brain age. Frontiers in Aging Neuroscience, 11, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (1996). Beck depression inventory‐II. San Antonio, 78(2), 490–498. [Google Scholar]
- Bernstein, D. P. , Stein, J. A. , Newcomb, M. D. , Walker, E. , Pogge, D. , Ahluvalia, T. , Stokes, J. , Handelsman, L. , Medrano, M. , Zule, W. , & Desmond, D. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. [DOI] [PubMed] [Google Scholar]
- Blevins, C. A. , Weathers, F. W. , Davis, M. T. , Witte, T. K. , & Domino, J. L. (2015). The Posttraumatic Stress Disorder Checklist for DSM‐5 (PCL‐5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 10(28), 489–498. [DOI] [PubMed] [Google Scholar]
- Besteher, B. , Gaser, C. , & Nenadić, I. (2019). Machine‐learning based brain age estimation in major depression showing no evidence of accelerated aging. Psychiatry Research: Neuroimaging, 290, 1–4. [DOI] [PubMed] [Google Scholar]
- Bremner, J. D. , Vermetten, E. , & Mazure, C. M. (2000). Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: The early trauma inventory. Depression and Anxiety, 12(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Brown, T. T. , Kuperman, J. M. , Chung, Y. , Erhart, M. , McCabe, C. , Hagler, D. J. Jr ., Venkatraman, V. K. , Akshoomoff, N. , Amaral, D. G. , Casey, B. J. , Chang, L. , Ernst, T. M. , Frazier, J. A. , Gruen, J. R. , Kaufmann, W. E. , Kenet, T. , Kennedy, D. N. , Murray, S. S. , Sowell, E. R. , … Bloss, C. S. (2012). Neuroanatomical assessment of biological maturity. Current Biology, 22(18), 1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, S. P. , & Pontoski, K. (2005). Post‐traumatic stress disorder and acute stress disorder I: Their nature and assessment considerations. Psychiatry, 2(4), 14. [PMC free article] [PubMed] [Google Scholar]
- Clausen, A. N. , Clarke, E. , Phillips, R. D. , Haswell, C. , & Morey, R. A. (2020). Combat exposure, posttraumatic stress disorder, and head injuries differentially relate to alterations in cortical thickness in military veterans. Neuropsychopharmacology, 45(3), 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. , & Manuck, S. B. (1995). Stress, reactivity, and disease. Psychosomatic Medicine, 57(5), 423–426. [DOI] [PubMed] [Google Scholar]
- Cole, S. W. (2014). Human social genomics. PLOS Genetics, 10(8), e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J. H. (2020). Multimodality neuroimaging brain‐age in UK biobank: Relationship to biomedical, lifestyle, and cognitive factors. Neurobiology of Aging, 92, 34–42. 10.1016/j.neurobiolaging.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J. H. , & Franke, K. (2017). Predicting age using neuroimaging: Innovative brain ageing biomarkers. Trends in Neurosciences, 40(12), 681–690. [DOI] [PubMed] [Google Scholar]
- Cole, J. H. , Franke, K. , & Cherbuin, N. (2019). Quantification of the biological age of the brain using neuroimaging. In A. Moskalev (Ed.), Biomarkers of human aging (pp. 293–328). Springer. [Google Scholar]
- Cole, J. H. , Poudel, R. P. , Tsagkrasoulis, D. , Caan, M. W. , Steves, C. , Spector, T. D. , & Montana, G. (2017). Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage, 163, 115–124. [DOI] [PubMed] [Google Scholar]
- Cole, J. H. , Ritchie, S. J. , Bastin, M. E. , Hernández, M. V. , Maniega, S. M. , Royle, N. , Corley, J. , Pattie, A. , Harris, S. E. , Wray, N. R. , Redmond, P. , Marioni, R. E. , Starr, J. M. , Cox, S. R. , Wardlaw, J. M. , Sharp, D. J. , Deary, I. J. , & Zhang, Q. (2018). Brain age predicts mortality. Molecular Psychiatry, 23(5), 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppedè, F. , & Migliore, L. (2010). DNA repair in premature aging disorders and neurodegeneration. Current Aging Science, 3(1), 3–19. [DOI] [PubMed] [Google Scholar]
- Davidson, J. R. (1996). Davidson trauma scale (DTS).
- Diedenhofen, B. , & Musch, J. (2015). Cocor: A comprehensive solution for the statistical comparison of correlations. PLOS One, 10(3), e0121945. 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos, P. (2015). The master algorithm: How the quest for the ultimate learning machine will remake our world. Basic Books. [Google Scholar]
- Dosenbach, N. U. , Nardos, B. , Cohen, A. L. , Fair, D. A. , Power, J. D. , Church, J. A. , Nelson, S. M. , Wig, G. S. , Vogel, A. C. , Barnes, K. A. , Dubis, J. W. , Feczko, E. , Coalson, R. S. , Pruett, J. R. , Barch, D. M. , Petersen, S. E. , Schlaggar, B. L. , & Lessov‐Schlaggar, C. N. (2010). Prediction of individual brain maturity using fMRI. Science, 329(5997), 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling, G. J. , Weiss, S. R. , & Condon, T. P. (2008). Drugs of abuse and the aging brain. Neuropsychopharmacology, 33(2), 209–218. [DOI] [PubMed] [Google Scholar]
- Edmondson, D. , & von Känel, R. (2017). Post‐traumatic stress disorder and cardiovascular disease. The Lancet Psychiatry, 4(4), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, M. L. , Belsky, D. W. , Knodt, A. R. , Ireland, D. , Melzer, T. R. , Poulton, R. , Ramrakha, S. , Caspi, A. , Moffitt, T. E. , & Hariri, A. R. (2019). Brain‐age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Molecular Psychiatry, 26, 3829–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. , Williams, J. , Karg, R. , & Spitzer, R. (2014). Structured clinical interview for DSM‐5 disorders–research version (SCID‐5‐RV). American Psychiatric Assocation. [Google Scholar]
- Fotenos, A. F. , Mintun, M. A. , Snyder, A. Z. , Morris, J. C. , & Buckner, R. L. (2008). Brain volume decline in aging: Evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Archives of Neurology, 65(1), 113–120. [DOI] [PubMed] [Google Scholar]
- Franke, K. , & Gaser, C. (2019). Ten years of BrainAGE as a neuroimaging biomarker of brain aging: What insights have we gained? Frontiers in Neurology, 10, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser, C. , Franke, K. , Klöppel, S. , Koutsouleris, N. , Sauer, H. , & Initiative, A. S. D. N. (2013). BrainAGE in mild cognitive impaired patients: Predicting the conversion to Alzheimer's disease. PLOS One, 8(6), e67346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen, N. C. , Chrousos, G. P. , Binder, E. B. , & Zannas, A. S. (2017). Life stress, glucocorticoid signaling, and the aging epigenome: Implications for aging‐related diseases. Neuroscience & Biobehavioral Reviews, 74, 356–365. [DOI] [PubMed] [Google Scholar]
- Green, B. L. , Chung, J. Y. , Daroowalla, A. , Kaltman, S. , & DeBenedictis, C. (2006). Evaluating the cultural validity of the stressful life events screening questionnaire. Violence Against Women, 12(12), 1191–1213. [DOI] [PubMed] [Google Scholar]
- Griesbach, G. S. , Masel, B. E. , Helvie, R. E. , & Ashley, M. J. (2018). The impact of traumatic brain injury on later life: Effects on normal aging and neurodegenerative diseases. Journal of Neurotrauma, 35(1), 17–24. [DOI] [PubMed] [Google Scholar]
- Hajek, T. , Franke, K. , Kolenic, M. , Capkova, J. , Matejka, M. , Propper, L. , Uher, R. , Stopkova, P. , Novak, T. , Kopecek, M. , Spaniel, F. , Alda, M. , & Paus, T. (2019). Brain age in early stages of bipolar disorders or schizophrenia. Schizophrenia Bulletin, 45(1), 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, L. K. , Dinga, R. , Hahn, T. , Ching, C. , Eyler, L. , Aftanas, L. , Aghajani, M. , Aleman, A. , Baune, B. T. , Brak, I. , Filho, G. B. , Carballedo, A. , Connolly, C. G. , Couvy‐Duchesne, B. , Cullen, K. R. , Dannlowski, U. , Davey, C. G. , Dima, D. , Duran, F. L. S. , … Berger, K. (2021). Brain aging in major depressive disorder: Results from the ENIGMA major depressive disorder working group. Molecular Psychiatry, 560623. 26, 5124–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton, S. N. , Franz, C. E. , Elman, J. A. , Panizzon, M. S. , Hagler, D. J. Jr. , Fennema‐Notestine, C. , Eyler, L. T. , Mcevoy, L. K. , Lyons, M. J. , Kremen, W. S. , & Dale, A. M. (2018). Negative fateful life events in midlife and advanced predicted brain aging. Neurobiology of Aging, 67, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jónsson, B. A. , Bjornsdottir, G. , Thorgeirsson, T. , Ellingsen, L. M. , Walters, G. B. , Gudbjartsson, D. , Stefansson, H. , Stefansson, K. , & Ulfarsson, M. (2019). Brain age prediction using deep learning uncovers associated sequence variants. Nature Communications, 10(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, J. , Cole, G. , Head, E. , & Ingram, D. (2009). Nutrition, brain aging, and neurodegeneration. Journal of Neuroscience, 29(41), 12795–12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrinli, S. , Stevens, J. , Wani, A. H. , Lori, A. , Kilaru, V. , van Rooij, S. J. H. , Hinrichs, R. , Powers, A. , Gillespie, C. F. , Michopoulos, V. , Gautam, A. , Jett, M. , Hammamieh, R. , Yang, R. , Wildman, D. , Qu, A. , Koenen, K. , Aiello, A. E. , Jovanovic, T. , … Smith, A. K. (2020). Evaluating the impact of trauma and PTSD on epigenetic prediction of lifespan and neural integrity. Neuropsychopharmacology, 45, 1609–1616. 10.1038/s41386-020-0700-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C. , Berglund, P. , Demler, O. , Jin, R. , Merikangas, K. R. , & Walters, E. E. (2005). Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kolassa, I.‐T. , Ertl, V. , Eckart, C. , Kolassa, S. , Onyut, L. P. , & Elbert, T. (2010). Spontaneous remission from PTSD depends on the number of traumatic event types experienced. Psychological Trauma: Theory, Research, Practice, and Policy, 2(3), 169. [Google Scholar]
- Koutsouleris, N. , Davatzikos, C. , Borgwardt, S. , Gaser, C. , Bottlender, R. , Frodl, T. , Falkai, P. , Riecher‐Rossler, A. , Moller, H.‐J. , Pantelis, C. , Meisenzahl, E. , & Reiser, M. (2014). Accelerated brain aging in schizophrenia and beyond: A neuroanatomical marker of psychiatric disorders. Schizophrenia Bulletin, 40(5), 1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K. , & Spitzer, R. L. (2002). The PHQ‐9: A new depression diagnostic and severity measure. Psychiatric Annals, 32(9), 509–515. [Google Scholar]
- Le, T. T. , Kuplicki, R. T. , McKinney, B. A. , Yeh, H.‐W. , Thompson, W. K. , Paulus, M. P. , & Tulsa 1000 Investigators. (2018). A nonlinear simulation framework supports adjusting for age when analyzing BrainAGE. Frontiers in Aging Neuroscience, 10, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Wang, J. , Zhou, J. , Huang, P. , & Li, J. (2017). The association between post‐traumatic stress disorder and shorter telomere length: A systematic review and meta‐analysis. Journal of Affective Disorders, 218, 322–326. [DOI] [PubMed] [Google Scholar]
- Liang, H. , Zhang, F. , & Niu, X. (2019). Investigating systematic bias in brain age estimation with application to post‐traumatic stress disorders. Human Brain Mapping, 40(11), 3143–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem, F. , Varoquaux, G. , Kynast, J. , Beyer, F. , Masouleh, S. K. , Huntenburg, J. M. , Lampe, L., Rahim, M., Abraham, A., Craddock, R. C. , Riedel‐Heller, S., Luck, T., Loeffler, M., Schroeter, M. L., Witte, A. V., Villringer, A., & Margulies, D. S. (2017). Predicting brain‐age from multimodal imaging data captures cognitive impairment. NeuroImage, 148, 179–188. [DOI] [PubMed] [Google Scholar]
- Logue, M. W. , van Rooij, S. J. , Dennis, E. L. , Davis, S. L. , Hayes, J. P. , Stevens, J. S. , Densmore, M. , Haswell, C. C. , Ipser, J. , Korgaonkar, M. , Lebois, L. A. M. , Peverill, M. , Baker, J. T. , Boedhoe, P. S. W. , Frijling, J. L. , Gruber, S. A. , Harpaz‐Rotem, I. , Jahanshad, N. , Koopowitz, S. , … Koch, S. B. (2018). Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA‐PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biological Psychiatry, 83(3), 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond, P. F. , & Lovibond, S. H. (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33(3), 335–343. [DOI] [PubMed] [Google Scholar]
- Magruder, K. M. , Goldberg, J. , Forsberg, C. W. , Friedman, M. J. , Litz, B. T. , Vaccarino, V. , Heagerty, P. J. , Gleason, T. C. , Huang, G. D. , & Smith, N. L. (2016). Long‐term trajectories of PTSD in Vietnam‐era veterans: The course and consequences of PTSD in twins. Journal of Traumatic Stress, 29(1), 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, A. J. , Kubzansky, L. D. , Coull, B. A. , Kloog, I. , Koutrakis, P. , Sparrow, D. , Spiro, A. , Vokonas, P. , & Schwartz, J. (2015). Associations between air pollution and perceived stress: The Veterans Administration Normative Aging Study. Environmental Health, 14(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. W. , Lin, A. P. , Wolf, E. J. , & Miller, D. R. (2018). Oxidative stress, inflammation, and neuroprogression in chronic PTSD. Harvard Review of Psychiatry, 26(2), 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. W. , & Sadeh, N. (2014). Traumatic stress, oxidative stress and post‐traumatic stress disorder: Neurodegeneration and the accelerated‐aging hypothesis. Molecular Psychiatry, 19(11), 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey, R. A. , Clarke, E. K. , Haswell, C. C. , Phillips, R. D. , Clausen, A. N. , Mufford, M. S. , Saygin, Z. , Wagner, H. R. , LaBar, K. S. , & Calhoun, P. S. (2020). Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(3), 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, T. L. , Fernandez‐Botran, R. , Miller, J. J. , & Burns, V. E. (2014). Interleukin‐6 and soluble interleukin‐6 receptor levels in posttraumatic stress disorder: Associations with lifetime diagnostic status and psychological context. Biological Psychology, 99, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme, J. G. , Reis, J. , & Herz, E. J. (1986). Factorial and discriminant validity of the center for epidemiological studies depression (CES‐D) scale. Journal of Clinical Psychology, 42(1), 28–33. [DOI] [PubMed] [Google Scholar]
- Pfohl, B. , Blum, N. , & Zimmerman, M. (1997). Structured interview for DSM‐IV personality: Sidp‐IV. American Psychiatric Pub. [Google Scholar]
- Schmaal, L. , Ching, C. R. , McMahon, A. B. , Jahanshad, N. , & Thompson, P. M. (2020). Neuroimaging, genetics, and personalized psychiatry: Developments and opportunities from the ENIGMA consortium. In B. Baune (Ed.), Personalized psychiatry (pp. 483–497). Elsevier. [Google Scholar]
- Schmitz, B. , Wang, X. , Barker, P. B. , Pilatus, U. , Bronzlik, P. , Dadak, M. , Kahl, K. G. , Lanfermann, H. , & Ding, X. Q. (2018). Effects of aging on the human brain: A proton and phosphorus MR spectroscopy study at 3T. Journal of Neuroimaging, 28(4), 416–421. [DOI] [PubMed] [Google Scholar]
- Schnack, H. G. , Van Haren, N. E. , Nieuwenhuis, M. , Hulshoff Pol, H. E. , Cahn, W. , & Kahn, R. S. (2016). Accelerated brain aging in schizophrenia: A longitudinal pattern recognition study. American Journal of Psychiatry, 173(6), 607–616. [DOI] [PubMed] [Google Scholar]
- Shahab, S. , Mulsant, B. H. , Levesque, M. L. , Calarco, N. , Nazeri, A. , Wheeler, A. L. , Foussias, G. , Rajji, T. K. , & Voineskos, A. N. (2019). Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology, 44(5), 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D. V. , Giddens, J. M. , & Sheehan, I. S. (2014). Status update on the Sheehan‐suicidality tracking scale (S‐STS) 2014. Innovations in Clinical Neuroscience, 11(9‐10), 93. [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D. , & Lecrubier, Y. (2010). The mini‐international neuropsychiatric interview version 6.0 (MINI 6.0). Medical Outcomes System Inc.: Jacksonville, FL.
- Smyser, C. D. , Dosenbach, N. U. , Smyser, T. A. , Snyder, A. Z. , Rogers, C. E. , Inder, T. E. , Schlaggar, B. L. , & Neil, J. J. (2016). Prediction of brain maturity in infants using machine‐learning algorithms. NeuroImage, 136, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith, R. , & Zigmond, A. (2000). Hospital anxiety and depression scale (HADS). Handbook of psychiatric measures (pp. 547–548). American Psychiatric Association. [Google Scholar]
- Spitzer, C. , Barnow, S. , Volzke, H. , Wallaschofski, H. , John, U. , Freyberger, H. J. , Löwe, B. , & Grabe, H. J. (2010). Association of posttraumatic stress disorder with low‐grade elevation of C‐reactive protein: Evidence from the general population. Journal of Psychiatric Research, 44(1), 15–21. 10.1016/j.jpsychires.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Spitzer, R. L. , Williams, J. B. , Gibbon, M. , & First, M. B. (1992). The structured clinical interview for DSM‐III‐R (SCID): I: History, rationale, and description. Archives of General Psychiatry, 49(8), 624–629. [DOI] [PubMed] [Google Scholar]
- Steer, R. A. , Clark, D. A. , Beck, A. T. , & Ranieri, W. F. (1999). Common and specific dimensions of self‐reported anxiety and depression: The BDI‐II versus the BDI‐IA. Behaviour Research and Therapy, 37(2), 183–190. [DOI] [PubMed] [Google Scholar]
- Sun, D. , Davis, S. L. , Haswell, C. C. , Swanson, C. A. , Mid‐Atlantic MIRECC Workgroup , LaBar, K. S. , Fairbank, J. A. , Morey, R. A. , & Brancu, M. (2018). Brain structural covariance network topology in remitted posttraumatic stress disorder. Frontiers in Psychiatry, 9, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel, R. , Hepp, U. , Kraemer, B. , Traber, R. , Keel, M. , Mica, L. , & Schnyder, U. (2007). Evidence for low‐grade systemic proinflammatory activity in patients with posttraumatic stress disorder. Journal of Psychiatric Research, 41(9), 744–752. 10.1016/j.jpsychires.2006.06.009 [DOI] [PubMed] [Google Scholar]
- Weathers, F. W. , Bovin, M. J. , Lee, D. J. , Sloan, D. M. , Schnurr, P. P. , Kaloupek, D. G. , Keane, T. M. , & Marx, B. P. (2018). The clinician‐administered PTSD scale for DSM–5 (CAPS‐5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30(3), 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, E. J. , Maniates, H. , Nugent, N. , Maihofer, A. X. , Armstrong, D. , Ratanatharathorn, A. , Ashley‐Koch, A. E. , Garrett, M. , Kimbrel, N. A. , Va Mid‐Atlantic Mirecc Workgroup , Aiello, A. E. , Baker, D. G. , Beckham, J. C. , Boks, M. P. , Galea, S. , Geuze, E. , Hauser, M. A. , Kessler, R. C. , Koenen, K. C. , Miller, M. W. , … Lori, A. (2018). Traumatic stress and accelerated DNA methylation age: A meta‐analysis. Psychoneuroendocrinology, 92, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, E. J. , & Morrison, F. G. (2017). Traumatic stress and accelerated cellular aging: From epigenetics to cardiometabolic disease. Current Psychiatry Reports, 19(10), 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, E. J. , & Schnurr, P. P. (2016). Posttraumatic stress disorder‐related cardiovascular disease and accelerated cellular aging. Psychiatric Annals, 46(9), 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang , R. , Wu, G. W. Y. , Verhoeven, J. E. , Gautam, A. , Reus, V. I. , Kang, J. I. , Flory, J. D. , Abu‐Amara, D. , PTSD Systems Biology Consortium, Hood, L. , Doyle, F. J. , Yehuda, R. , Marmar, C. R. , Jett, M. , Hammamieh, R. , Mellon, S. H. , & Wolkowitz, O. M. (2020). A DNA methylation clock associated with age‐related illnesses and mortality is accelerated in men with combat PTSD. Molecular Psychiatry. 26, 4999–5009. 10.1038/s41380-020-0755-z [DOI] [PubMed] [Google Scholar]
- Yarvis, J. S. , Yoon, E. , Ameuke, M. , Simien‐Turner, S. , & Landers, G. (2012). Assessment of PTSD in older veterans: The posttraumatic stress disorder checklist: Military version (PCL‐M). Advances in Social Work, 13(1), 185–202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


