Abstract
Background:
Live attenuated influenza vaccine (LAIV) and inactivated influenza vaccine (IIV) are both licensed for administration to nursing mothers. Little is known about the potential for transmission of LAIV viruses from the mother to the infant and the comparative breast milk antibody responses to LAIV and IIV.
Methods:
We performed a randomized, double-blind study comparing the immunogenicity of LAIV to IIV when administered to nursing mothers. The safety of LAIV to IIV in women and their infants was also compared. Women received LAIV + intramuscular placebo, or IIV + intranasal placebo on Day 0. Breast milk and nasal swabs (from women and infants) were collected on Days 0, 2, and 8 for detection of LAIV. Breast milk and serum antibody responses were measured at Days 0 and 28. The primary hypothesis was that LAIV would provide superior induction of breast milk IgA responses to influenza as compared to IIV when administered to nursing mothers.
Results:
Breast milk IgG, breast milk IgA (H1N1 only), serum hemagglutination inhibition (HAI), and serum IgG responses were significantly higher following administration of IIV compared to LAIV. Receipt of either LAIV or IIV was safe in women and their infants. One (1%) LAIV recipient transmitted vaccine virus to her infant who remained well. No influenza virus was detected in breast milk.
Conclusions:
Breast milk and serum antibody responses were higher for IIV compared to LAIV. LAIV and IIV were safe for nursing women but there was one (1%) possible transmission of LAIV to an infant. This study suggests that IIV may be the preferred vaccine for nursing mothers.
Keywords: Live attenuated influenza vaccine, Inactivated influenza vaccine, Breastfeeding, Infants, Safety, Immunogenicity
1. Introduction
Influenza is an important cause of respiratory illness among young infants. Among infants < 3 months old in the United States, influenza has been associated with an annual average of 3000 hospitalizations [1]. A recent prospective surveillance study reported average annual rates of hospitalization attributable to influenza to be 0.27% for those <6 months of age [2]. Influenza vaccines are not licensed for administration to infants <6 months [3]. Maternal influenza vaccination has the potential to protect the young infant from influenza by placental transport of maternal antibodies and by preventing serious influenza in the mother [4]. Therefore, pregnant women are recommended to receive inactivated influenza vaccine (IIV) during any trimester of pregnancy.
Either IIV or live attenuated influenza vaccine (LAIV) is licensed to be administered postpartum to breastfeeding women [5,6]. Little information is available to guide decisions regarding this immunization choice [7]. In addition to maternal serum antibody transferred through the umbilical cord, there may be a potential protective effect from the oral transfer of maternal antibodies through breast milk when women are vaccinated during pregnancy and their infants consume milk from immunized women [8,9]. However, the amount of vaccine-specific antibodies present in breast milk when women are immunized postpartum with LAIV versus IIV is not known [7], nor is the amount of maternal nasal shedding and the potential for transmission of vaccine virus to the infant [10,11]. Further, it is not known if vaccine virus is excreted in breast milk after LAIV administration. Experience with other live virus vaccines with respect to virus excretion in human milk is variable. While there are no data on excretion of either varicella [12] or measles vaccine viruses [10], rubella vaccine virus [13–15] may be excreted in human milk and cause infection without clinical disease in the infant. It is assumed that if infection with a live vaccine occurs, it will be well-tolerated because the vaccine virus is attenuated [16]. On the other hand, both yellow fever virus [17–19] and smallpox vaccines [16] should be avoided during breastfeeding because of the risks for transmission from mother to infant and the potential for vaccine-associated complications in the infant. To address these questions, we conducted a randomized, double-blind clinical trial comparing LAIV versus IIV administration in breastfeeding women. The primary hypothesis was that LAIV would provide superior induction of breast milk IgA antibody responses to influenza as compared to IIV when administered to nursing women.
2. Materials and methods
2.1. Subjects
Healthy lactating women 18–49 years who had not previously received current season influenza vaccine, and who delivered a healthy infant at ≥36 weeks gestation, within 28–120 days before enrollment were recruited at 5 US sites before the 2011–12 or 2012–13 influenza season. No women were enrolled in both seasons. Women were excluded if they were not eligible to receive seasonal influenza immunization, had any chronic medical conditions, or had any known immunocompromised family member/household contact. Women must also have successfully provided breast milk for at least the two days prior to enrollment. Infants were excluded if they had any chronic medical conditions or were not receiving at least half of their feeding from breast milk.
The protocol and consent forms were approved by the institutional review board at each participating site. Women provided written, informed consent for themselves and their infants.
2.2. Study design
We conducted a randomized, double-blind clinical trial. Women were randomized 1:1 to receive either LAIV + intramuscular placebo, or IIV + intranasal placebo. On the day of immunization, women were given memory aids to record solicited and unsolicited adverse events (AEs) for themselves and their infants.
Women recorded the maximum intensity of solicited and unsolicited AEs for themselves and their infants and scored the AEs on a scale of 0–3 (0 = absent, 1 = easily tolerated, 2 = interferes with normal activity, and 3 = prevents normal activity).
Women recorded injection site symptoms [pain, tenderness, erythema, and induration], and the presence of nasal congestion, runny nose, cough, sore throat, and nasal bleeding for 7 days post-immunization. Erythema and induration were scored on a scale of 0–3 (0 = absent, 1 = <20 mm, 2 = 2–50 mm, and 3 > 50 mm). They also recorded solicited systemic AEs, including fever, feverishness, fatigue, myalgia, headache, nausea, weakness, and chills for 7 days after immunization. Fever was scored on a scale of 0–3 (0 = not present, 1 = ≥37.8 °C to <38 °C, 2 = >38 °C to < 39 °C, and 3 = ≥39 °C).
During the 10-day post-immunization period, women recorded solicited AEs experienced by their infants, including fever, difficulty breathing, nasal congestion, runny nose, cough, irritability/fussiness, drowsiness, and loss of appetite. Fever was scored on the same scale used for maternal fever. Unsolicited AEs occurring in women and infants from baseline until 28 days after immunization also were recorded. Women were contacted by telephone 6 weeks and 6 months after immunization to inquire about the occurrence of serious adverse events (SAEs) for themselves and their infants.
The mother and infant were requested to return for evaluation within 72 h of symptom onset if either experienced an influenza-like illness (ILI) [20] between baseline and 28 days after maternal immunization. Examination(s) were performed on the ill individual(s) and nasal swabs were collected from both to test for the presence of influenza viruses.
Women submitted expressed milk and serum specimens, both for antibody assays, at baseline and 28 days post-immunization. Expressed milk (women) and nasal swabs (women and infants) were collected at baseline, 2 and 8 days after influenza immunization to assess influenza virus presence by polymerase chain reaction (PCR) and cell culture. Days 2 and 8 were chosen because the highest proportion of healthy adult LAIV vaccinees shed one or more vaccine strains on days 2–3 post-vaccination and few shed past day 7 [11].
2.3. Immunizations
Licensed IIV (Fluzone®, Sanofi Pasteur, Swiftwater, PA) and LAIV (FluMist®, MedImmune, Gaithersburg, MD) were used as recommended each year. For the 2011–12 season [21], IIV contained 15 μg hemagglutinin (HA) of the A/California/07/2009 (H1N1)-like virus, A/Perth/16/2009 (H3N2)-like virus, and B/Brisbane/60/2008-like virus. FluMist® [11,21] contained 106.5−7.5 FFU (fluorescent focus units) of live attenuated influenza virus reassortants of each of the three strains found in IIV.
For the 2012–13 season [22], IIV contained 15 mg HA of the A/California/07/2009 NYMC X-179A (H1N1)-like virus, A/Victoria/361/2011 IVR-165 (H3N2)-like virus, and B/Texas/6/2011 (a B/Wisconsin/1/2010-like virus). FluMist® [11,22] contained 106.5−7.5 FFU of live attenuated influenza virus reassortants of each of the strains: A/California/07/2009 (H1N1)-like virus, A/Victoria/361/2011 (H3N2)-like virus, and B/Wisconsin/1/2010-like virus. The intranasal placebo was sucrose phosphate buffer and the intramuscular placebo was sterile saline.
2.4. Antibody assays
Breast milk and serum were stored at ≤ −65 °C until time of performance of the antibody assays at the Laboratory for Specialized Clinical Studies (LSCS) at Cincinnati Children’s Hospital Medical Center (CCHMC). The pre- and post-vaccination samples were run on the same day in the same assay and on the same plate to allow direct comparisons. Enzyme-Linked Immunosorbent Assay (ELISA) to detect hemagglutinin (HA)-specific Immunoglobulin A and G (IgA and IgG) in human milk and serum samples was performed as described by Schlaudecker, et al. [8]. A reference standard was used for each assay and assigned an arbitrary value. The amount of HA-specific antibody in the samples was derived by extrapolation from a standard curve assayed simultaneously using the reference standard and expressed as units per mL. The lower limit of detection was 5.82 units/mL for IgA and 2.56 units/mL for IgG. Titers below the limit of detection were reported as one-half the limit of detection.
The hemagglutination inhibition (HAI) assay was performed in duplicate as previously described [8,23]. Primary analyses evaluated the antibody responses to the influenza strains in the vaccine received. Exploratory analyses evaluated the cross reactivity to strains not present in the vaccine received (e.g., the A/Victoria/361/2011 virus and B/Wisconsin/1/2010-like virus among 2011–12 vaccine recipients).
2.5. Polymerase chain reaction (PCR) and cell culture assays
Breast milk and nasal swabs were stored at ‒65 °C or colder and tested at the LSCS laboratory. Breast milk whey [24] was tested by cell culture and PCR to detect LAIV. Samples from every woman who received LAIV, and her infant, and approximately 25% of women who received IIV, and their infants, were assayed by PCR and cell culture for the presence of vaccine strain or circulating influenza virus. Subjects in the IIV group were included as controls for the virologic assays. Only samples that produced positive results by PCR for the influenza A strain were tested to subtype for the H1N1 and H3N2 strains and were tested by PCR at MedImmune Laboratory to distinguish vaccine and wild-type virus.
2.6. Statistical considerations
The primary hypothesis was that LAIV would provide superior induction of breast milk IgA antibody responses to influenza as compared to IIV when administered to nursing women. A previous study [8] suggested a standard deviation of HA-specific IgA, estimated to be 13.25 ELISA units/mL (EU/mL) for vaccinated women for the H3N2 HA. Based on this estimate, a sample size of 120 per group would have 80% power to detect a statistically significant difference in the mean HA-specific IgA responses using a two-tailed t-test when the difference in means was greater than or equal to 4.82 EU/mL. This difference was not adjusted for the three comparisons required to test all three strains.
Breast milk and sera HA-specific IgA and IgG measured by ELISA were compared in the two groups by calculating the geometric mean titer (GMT) and 95% confidence intervals (CI) for each group. Hemagglutination inhibition (HAI) antibodies in sera were compared in the two groups by calculating the GMT and 95% CI, along with determining the seroprotection rate (HAI titer ≥ 40) and seroconversion rate (four-fold rise from pre-vaccination to post-vaccination) and 95% CI. Seroprotection and seroconversion rates were both tested for differences between treatment groups with Fisher’s exact test. T-tests were conducted, for both HA-specific antibodies and HAI antibodies, to test whether the GMT was significantly higher in one treatment group compared to the other (ie, the ratios of the GMTs in the two groups was significantly different from 1.0). The geometric mean fold rise from pre-vaccination to post-vaccination within each group, and the GMT ratio of the two groups at each visit was determined.
There was no primary safety hypothesis. The primary safety objective was to compare the AE profiles of LAIV and IIV when administered to nursing mothers. The proportion of women and their infants who experienced AEs of any severity were compared between groups and influenza seasons using Pearson’s chi-square or Fisher’s exact test. The significance level for all hypothesis tests was α = 0.05. Additional safety objectives were to assess women for the excretion of LAIV in nasal secretions and the breast milk and to assess infants for infection by LAIV virus strains in nasal secretions.
3. Results
3.1. Subjects
A total of 248 women (and 249 infants) were enrolled over the 2011–12 and 2012–13 influenza seasons. A consort diagram of participant disposition and the analysis populations is in the Supplemental Materials. Tables S1 and S2 display demographic data for participants. Most (79%) women were white and non-Hispanic (97%). At enrollment, the median ages of infants and women were 76 days and 31.4 years, respectively. No significant differences in ethnicity (in women p = 0.12 between groups and p = 0.44 between seasons; in infants p = 0.32 between groups and p = 0.79 between seasons), race (in women p = 0.93 between groups and p = 0.86 between seasons; in infants p = 0.99 between groups and p = 0.65 between seasons), or age (in women p = 0.64 between groups and p = 0.15 between seasons; in infants p = 0.38 between groups and p = 0.90 between seasons) were noted between the two groups of women and their infants or across vaccine seasons.
3.2. Vaccine safety and reactogenicity in women
Solicited AEs of any severity were reported in similar proportions of IIV and LAIV recipients across both influenza seasons. Nasal congestion was the most frequently occurring local AE in LAIV recipients with 45% reporting this AE vs. 21% of IIV recipients (p < 0.0001). Pain at the injection site was the most frequently occurring local AE reported in IIV recipients with 56% reporting this AE vs. only 7% of LAIV recipients (p < 0.0001). No grade 3 events were reported by LAIV recipients. Three grade 3 events (two induration and one erythema at the injection site) were reported by three IIV recipients.
There were no statistically significant differences for solicited systemic AEs between IIV and LAIV recipients. For LAIV recipients, 59% reported a solicited systemic AE of any severity compared to 56% for the IIV recipients (p = 0.80). Headache was the most frequently occurring solicited systemic AE in vaccine recipients (43% for the LAIV group vs. 39% for the IIV group, p = 0.61). No grade 3 events were reported by LAIV recipients. One grade 3 event of fever on Day 1 was reported by one IIV recipient.
The proportions of women reporting unsolicited non-serious AEs were similar in LAIV and IIV recipients (35.5% vs. 38.7%, p = 0.69). Upper respiratory infections were the most frequently reported unsolicited non-serious AEs in both groups (10.5% of both LAIV and IIV recipients). Most of these infections occurred 8–28 days after vaccination. Overall, 6 (4.8%) LAIV recipients and 3 (2.4%) IIV recipients experienced unsolicited AEs deemed related to vaccination (p = 0.5).
3.3. Safety in infants
Four unrelated SAEs were reported in three infants: one congenital syphilis, two urinary tract infections in one infant, and one RSV bronchiolitis. The only statistically significant difference for solicited AEs among the two groups of infants was for irritability/fussiness (59.7% of those whose mothers received LAIV vs. 44.8% of those whose mothers received IIV, p = 0.02).
3.4. Detection of LAIV virus strains
No vaccine or wild-type influenza virus was detected in breast milk. No influenza virus was detected in the nasal secretions collected from women who received IIV. Table 1 displays the influenza A and B results by PCR and cell culture at scheduled visits for all LAIV recipients. The nasal swab for one women who received LAIV (1%) tested positive for influenza A vaccine strain by PCR at baseline, suggesting either previous transmission from a contact who had received LAIV or that the nasal swab specimen was inadvertently collected after LAIV administration.
Table 1.
Nasal Swab Influenza A and B Results by PCR and Cell Culture at Scheduled Visits for All LAIV Recipients.
| Study day | N Tested | Any PCR+ or Culture + | Influenza A PCR‒Culture + | Influenza A PCR+ Culture‒ | Influenza A PCR+ Culture + | Influenza A H1N1 PCR+ and/or Culture+a | Influenza A H3N2 PCR+ and/or Culture+a | Influenza B PCR‒Culture + | Influenza B PCR+ Culture‒ | Influenza B PCR+ Culture+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | 120 | 1 (1%) | 0 | 1 (1%) | 0 | 0/1 | 0/1 | 0 | 0 | 0 |
| Day 2 | 120 | 58 (48%) | 1 (1%) | 22 (18%) | 5 (4%) | 13/27 (48%) | 18/27 (67%) | 0 | 34 (28%) | 17 (14%) |
| Day 8 | 119 | 12 (10%) | 0 | 1 (1%) | 0 | 0/1 | 0/1 | 0 | 8 (7%) | 3 (3%) |
Samples that produced positive results by PCR for the Influenza A strain were tested to determine if they were positive for the H3N2 and H1N1 strains. Samples that produced negative or indeterminate results for Influenza A were not tested for H3N2 or H1N1.
One infant (1%) whose mother received the 2011–12 LAIV had an influenza A vaccine strain detected from a nasal swab by PCR and cell culture at Day 2. The sample was positive for H1N1 by cell culture, positive for H3N2 by PCR and cell culture, and negative for influenza B. This infant’s nasal secretions were negative for all influenza viruses by Day 8. The mother of this infant had negative nasal testing for all influenza viruses at Day 2, but influenza A vaccine strain was detected by PCR at Day 8. This infant did not experience any AEs that were considered to be associated with maternal LAIV receipt.
3.5. Influenza-like illness evaluations in women and their infants
None of the women who received IIV or their infants had a supplemental research visit for ILI symptoms. One women who received 2011–12 LAIV had vaccine strain influenza A and B viruses detected by PCR at an ILI visit on Day 3. No infants whose mothers received LAIV had influenza virus detected at ILI visits. No wild-type influenza virus strains were detected among women or their infants.
3.6. Antibody responses
3.6.1. Breast milk
Fig. 1 summarizes the primary immunogenicity endpoint: comparison of breast milk HA-specific IgA and IgG at Day 28 for all women in both seasons. The breast milk IgA response to H1N1 was higher in IIV recipients compared to LAIV recipients over both seasons (p = 0.003), but was not significantly different for any other test antigen. Breast milk IgG titers were higher in IIV recipients compared to LAIV recipients for all five strains over both seasons (p ≤ 0.0002).
Fig. 1.
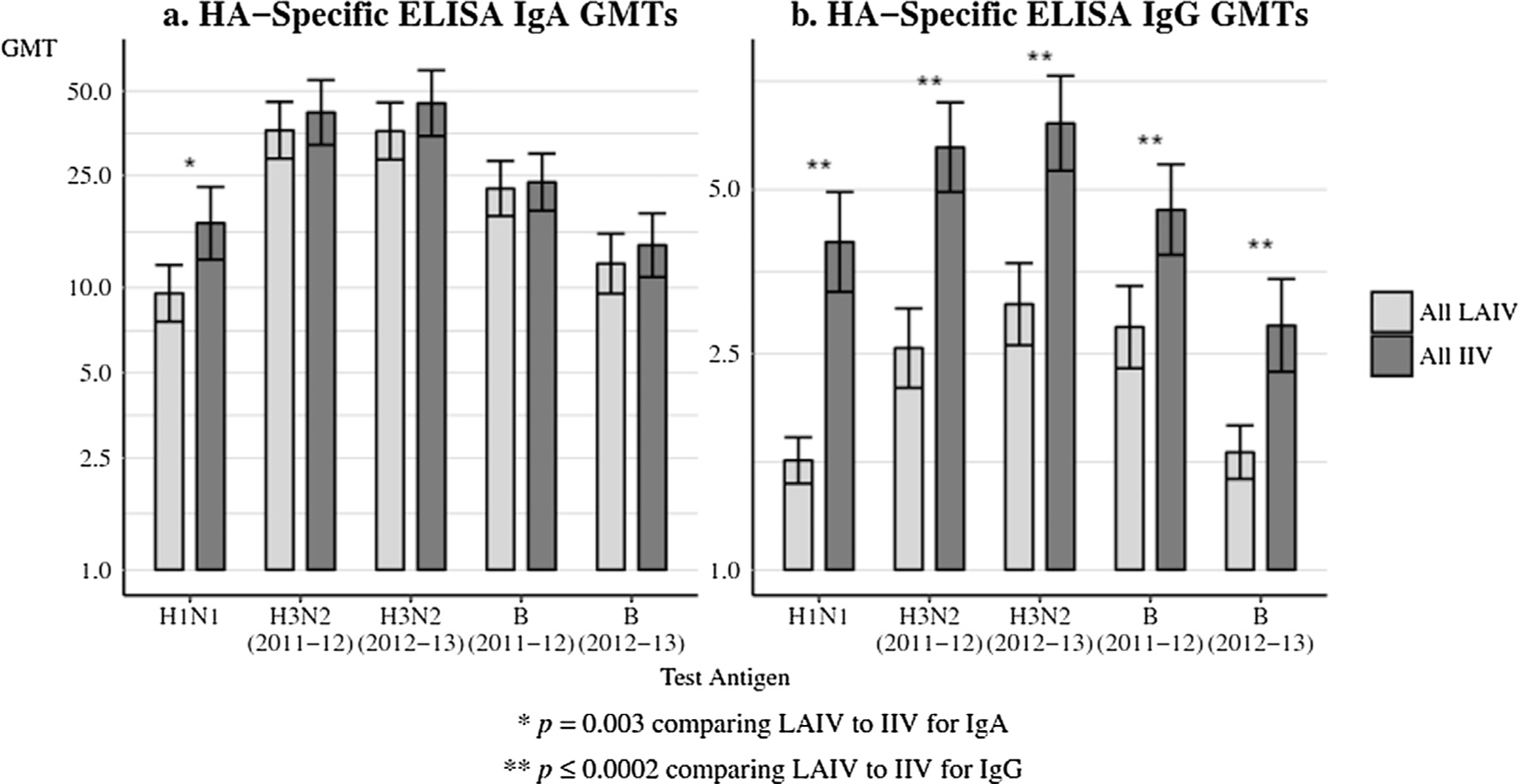
(a and b) Breast milk antibody GMTs to vaccine received at day 28, comparing all LAIV to all IIV. Panel 1a: HA-specific ELISA IgA GMTs and Panel 1b: HA-specific ELISA IgG GMTs. HA = hemagglutinin; GMTs = geometric mean titers; LAIV = live attenuated influenza vaccine; IIV = inactivated influenza vaccine. The error bars indicate the two-sided 95% confidence intervals (CIs).
Figs. 2 and 3 display data for each season’s vaccine groups. Breast milk IgA GMTs were significantly higher in 2011 vaccine recipients for H1N1, and H3N2 (2012–13) (p < 0.05) (Fig. 2). Breast milk IgG GMTs were significantly higher in 2011 vaccine recipients for H1N1, H3N2 (2011–12), H3N2 (2012–13) and B (2011–12) (p ≤ 0.001) (Fig. 3, Table S3). Breast milk IgG GMTs were significantly higher in 2012 vaccine recipients for H1N1, H3N2 (2011–12), H3N2 (2012–13) and B (2012–13) (p ≤ 0.0005) (Fig. 3, Table S3).
Fig. 2.
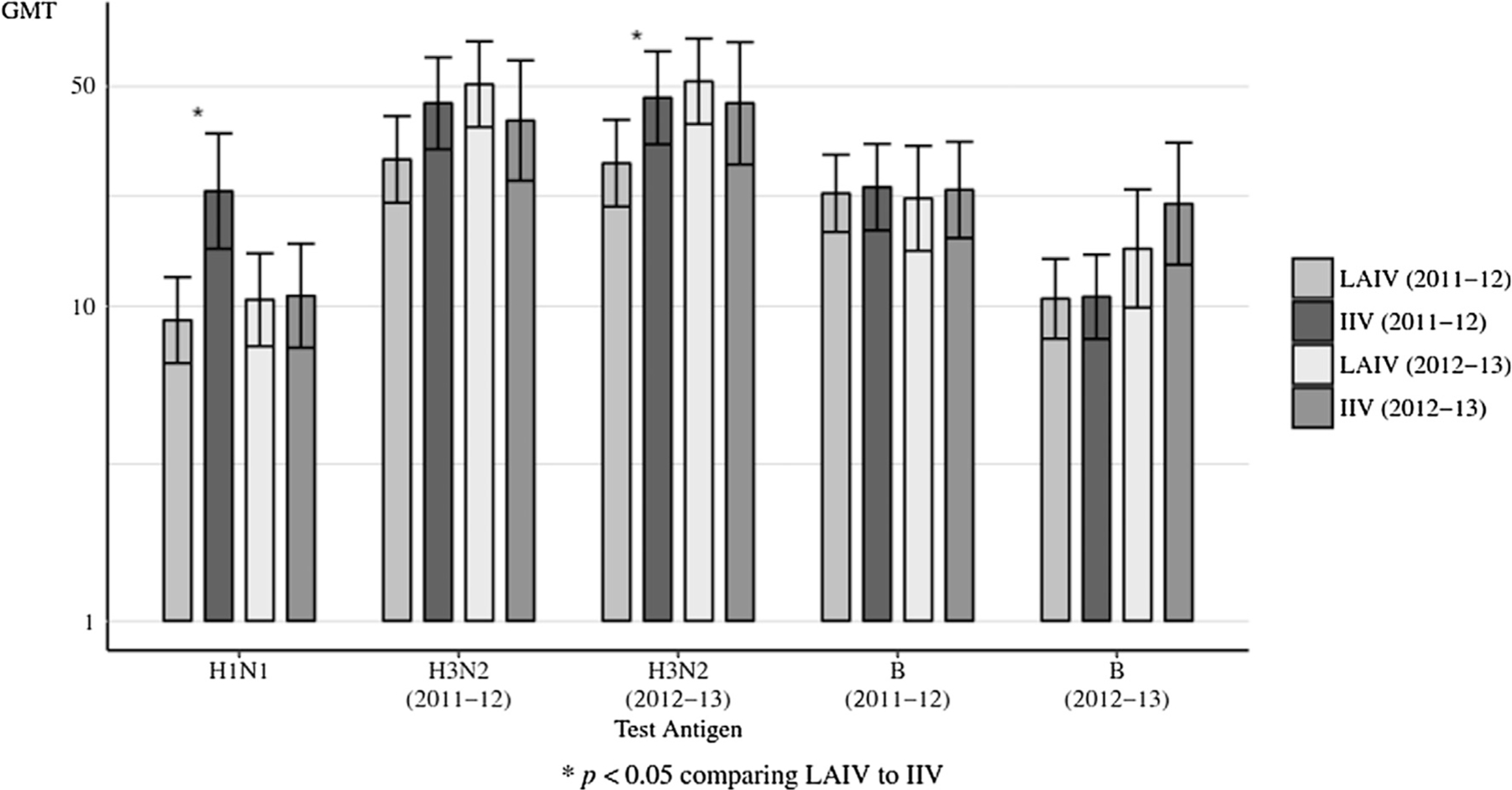
Breast milk HA-specific ELISA IgA GMTs to vaccine received at Day 28, comparing LAIV to IIV by influenza season. HA = hemagglutinin. GMTs = geometric mean titers; LAIV = live attenuated influenza vaccine; IIV = inactivated influenza vaccine. The error bars indicate the two-sided 95% confidence intervals (CIs).
Fig. 3.
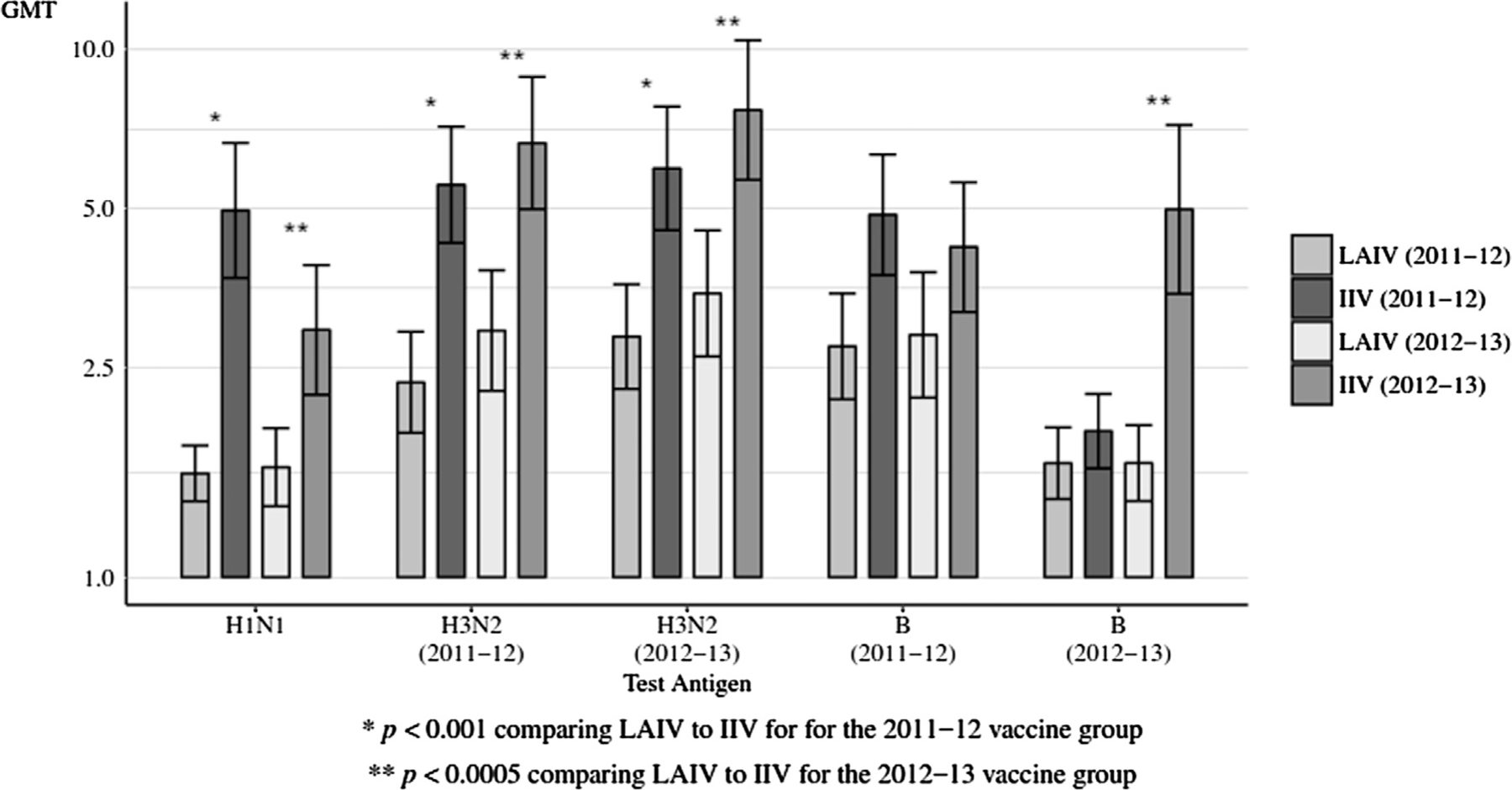
Breast milk HA-specific ELISA IgG GMTs to vaccine received at Day 28, comparing LAIV to IIV by influenza season. HA = hemagglutinin. GMTs = geometric mean titers; LAIV = live attenuated influenza vaccine; IIV = inactivated influenza vaccine. The error bars indicate the two-sided 95% confidence intervals (CIs).
3.6.2. Serum
Fig. 4 summarizes the comparison of serum HA-specific IgA and IgG GMTs at day 28 post-vaccination for all women in both seasons. Figs. S1 and S2 and Table S4 display data for each season’s vaccine groups. Serum IgA titers were significantly higher in 2011 IIV recipients against H1N1, and H3N2 (2011–12) (both p < 0.03) and in 2012 IIV recipients for B (2012–13) (p < 0.03). For serum IgG, all comparisons were statistically significant with IIV having greater GMTs than LAIV.
Fig. 4.
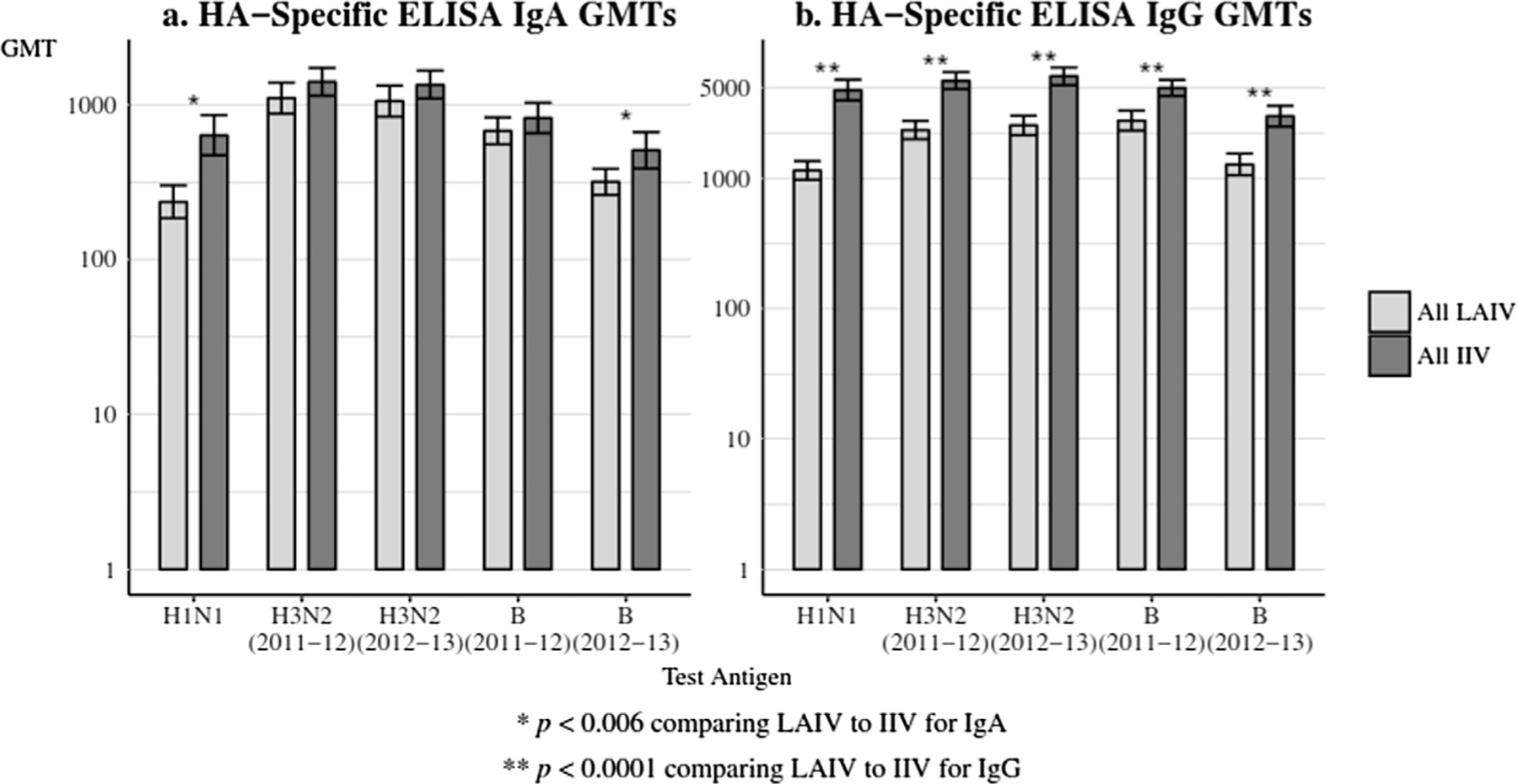
(a and b) Serum antibody GMTs to vaccine received at Day 28, comparing all LAIV to all IIV. Panel 4a: HA-specific ELISA IgA GMTs and Panel 4b: HA-specific ELISA IgG GMTs. HA = hemagglutinin; GMTs = geometric mean titers; LAIV = live attenuated influenza vaccine; IIV = inactivated influenza vaccine. The error bars indicate the two-sided 95% confidence intervals (CIs).
The HAI titers in the LAIV and IIV groups were similar at baseline. On Day 28, the GMTs in the IIV groups were statistically significantly higher than for LAIV for all strains in the 2011–12 vaccine group, the 2012–13 vaccine group, and in all women aggregated in both seasons (Table 2). Table S5 shows the serum immune responses to vaccine antigens by proportions with HAI titers ≥40. IIV recipients compared to LAIV recipients had statistically significantly higher seroconversion (Table 2) and seroprotection (Table S5) rates for all groups and antigens except for the seroprotection rate for the B (2012–13) antigen in the 2011–12 vaccine group [p ≤ 0.01 for all comparisons except for the seroprotection rate for the B (2012–13) antigen].
Table 2.
Serum hemagglutination inhibition (HAI) antibody responses according to vaccine group and antigen.
| IIV |
LAIV |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Season | Visit | Statistic | Antigen | N | Value (95% CI) | N | Value (95% CI) | p-value | Adjusted p-value |
| 2011–12 | Day 0 (Pre Vaccination) | GMT | H1N1 | 74 | 42.9 (30.1, 61.1) | 75 | 44.7 (31.9, 62.6) | 0.8684 | – |
| . | H3N2 (2011–12) | 74 | 32.9 (23.5, 46.0) | 75 | 45.5 (32.6, 63.5) | 0.1712 | – | ||
| . | H3N2 (2012–13) | 74 | 38.0 (26.6, 54.2) | 75 | 47.0 (33.2, 66.6) | 0.3946 | – | ||
| . | B (2011–12) | 74 | 15.7 (12.8, 19.2) | 75 | 20.2 (16.2, 25.1) | 0.0924 | – | ||
| . | B (2012–13) | 74 | 9.1 (7.6, 11.0) | 75 | 10.3 (8.4, 12.7) | 0.3894 | – | ||
| . | Day 28 After Vaccination | GMT | H1N1 | 74 | 382.3 (284.3, 514.2) | 75 | 46.8 (33.6, 65.1) | <.0001 | – |
| . | H3N2 (2011–12) | 74 | 119.7 (88.2, 162.4) | 75 | 51.6 (37.2, 71.5) | 0.0003 | – | ||
| . | H3N2 (2012–13) | 74 | 207.0 (161.1, 265.9) | 75 | 56.3 (39.7, 79.8) | <.0001 | – | ||
| . | B (2011–12) | 74 | 42.3 (33.1, 54.1) | 75 | 21.6 (17.4, 26.9) | <.0001 | – | ||
| . | B (2012–13) | 74 | 15.8 (12.6, 19.9) | 75 | 10.9 (8.9, 13.4) | 0.0168 | – | ||
| . | % Seroconversion | H1N1 | 74 | 0.57 (0.45, 0.68) | 75 | 0.00 (0.00, 0.05) | <.0001 | – | |
| . | H3N2 (2011–12) | 74 | 0.45 (0.33, 0.57) | 75 | 0.04 (0.01, 0.11) | <.0001 | – | ||
| . | H3N2 (2012–13) | 74 | 0.55 (0.43, 0.67) | 75 | 0.07 (0.02, 0.15) | <.0001 | – | ||
| . | B (2011–12) | 74 | 0.31 (0.21, 0.43) | 75 | 0.00 (0.00, 0.05) | <.0001 | – | ||
| . | B (2012–13) | 74 | 0.11 (0.05, 0.20) | 75 | 0.00 (0.00, 0.05) | 0.0030 | – | ||
| 2012–13 | Day 0 (Pre Vaccination) | GMT | H1N1 | 50 | 64.1 (43.1, 95.3) | 48 | 44.6 (31.6, 63.0) | 0.1700 | – |
| . | H3N2 (2011–12) | 50 | 52.1 (37.7, 71.9) | 48 | 53.0 (36.1, 77.8) | 0.9418 | – | ||
| . | H3N2 (2012–13) | 50 | 62.8 (43.7, 90.2) | 48 | 60.4 (42.5, 85.7) | 0.8769 | – | ||
| . | B (2011–12) | 50 | 17.5 (14.1, 21.8) | 48 | 18.2 (14.2, 23.4) | 0.8178 | – | ||
| . | B (2012–13) | 50 | 10.6 (8.6, 13.1) | 48 | 8.7 (7.0, 10.7) | 0.1799 | – | ||
| . | Day 28 After Vaccination | GMT | H1N1 | 50 | 197.0 (146.1, 265.7) | 48 | 44.9 (31.8, 63.4) | <.0001 | – |
| . | H3N2 (2011–12) | 50 | 133.6 (103.7, 172.2) | 48 | 59.9 (41.6, 86.2) | 0.0004 | – | ||
| . | H3N2 (2012–13) | 50 | 247.6 (194.2, 315.6) | 48 | 81.2 (60.1, 109.6) | <.0001 | – | ||
| . | B (2011–12) | 50 | 31.0 (25.2, 38.0) | 48 | 18.5 (14.3, 23.8) | 0.0019 | – | ||
| . | B (2012–13) | 50 | 41.1 (30.2, 56.1) | 48 | 9.0 (7.4, 10.9) | <.0001 | – | ||
| . | % Seroconversion | H1N1 | 50 | 0.34 (0.21, 0.49) | 48 | 0.00 (0.00, 0.07) | <.0001 | – | |
| . | H3N2 (2011–12) | 50 | 0.34 (0.21, 0.49) | 48 | 0.04 (0.01, 0.14) | 0.0002 | – | ||
| . | H3N2 (2012–13) | 50 | 0.52 (0.37, 0.66) | 48 | 0.10 (0.03, 0.23) | <.0001 | – | ||
| . | B (2011–12) | 50 | 0.14 (0.06, 0.27) | 48 | 0.00 (0.00, 0.07) | 0.0125 | – | ||
| . | B (2012–13) | 50 | 0.42 (0.28, 0.57) | 48 | 0.00 (0.00, 0.07) | <.0001 | – | ||
| All | Day 0 (Pre Vaccination) | GMT | H1N1 | 124 | 50.4 (38.7, 65.7) | 123 | 44.6 (35.0, 56.9) | 0.5006 | 0.5097 |
| . | H3N2 (2011–12) | 124 | 39.6 (31.1, 50.3) | 123 | 48.3 (37.7, 62.0) | 0.2528 | 0.2416 | ||
| . | H3N2 (2012–13) | 124 | 46.5 (35.9, 60.2) | 123 | 51.8 (40.4, 66.6) | 0.5516 | 0.5311 | ||
| . | B (2011–12) | 124 | 16.4 (14.2, 19.0) | 123 | 19.4 (16.5, 22.8) | 0.1320 | 0.1327 | ||
| . | B (2012–13) | 124 | 9.7 (8.4, 11.1) | 123 | 9.6 (8.3, 11.2) | 0.9546 | 0.9532 | ||
| . | Day 28 After Vaccination | GMT | H1N1 | 124 | 292.6 (235.0, 364.3) | 123 | 46.1 (36.3, 58.5) | <.0001 | <.0001 |
| . | H3N2 (2011–12) | 124 | 125.1 (101.8, 153.8) | 123 | 54.7 (43.0, 69.6) | <.0001 | <.0001 | ||
| . | H3N2 (2012–13) | 124 | 222.5 (186.4, 265.6) | 123 | 64.9 (51.0, 82.7) | <.0001 | <.0001 | ||
| . | B (2011–12) | 124 | 37.3 (31.5, 44.1) | 123 | 20.3 (17.3, 23.9) | <.0001 | <.0001 | ||
| . | B (2012–13) | 124 | 23.3 (19.0, 28.4) | 123 | 10.1 (8.8, 11.7) | <.0001 | <.0001 | ||
| . | % Seroconversion | H1N1 | 124 | 0.48 (0.39, 0.57) | 123 | 0.00 (0.00, 0.03) | <.0001 | <.0001 | |
| . | H3N2 (2011–12) | 124 | 0.40 (0.32, 0.50) | 123 | 0.04 (0.01, 0.09) | <.0001 | <.0001 | ||
| . | H3N2 (2012–13) | 124 | 0.54 (0.45, 0.63) | 123 | 0.08 (0.04, 0.14) | <.0001 | <.0001 | ||
| . | B (2011–12) | 124 | 0.24 (0.17, 0.33) | 123 | 0.00 (0.00, 0.03) | <.0001 | <.0001 | ||
| . | B (2012–13) | 124 | 0.23 (0.16, 0.32) | 123 | 0.00 (0.00, 0.03) | <.0001 | <.0001 | ||
At 28 days after vaccination, the IIV group had better responses to an H3N2 variant not contained in the vaccine as measured by GMTs, attainment of a titer ≥ 40 or a four-fold rise for each season and for the two seasons in aggregate (p ≤ 0.01), except for the proportion of subjects with four-fold rise in the 2012–13 vaccine group (Table S6).
Of the 248 maternal participants, 178 had received seasonal IIV in the preceding season. Of the participants enrolled in the 2011–12 season, 48 participants in the IIV group and 50 participants in the LAIV group received 2010–11 seasonal IIV. Of the participants enrolled in the 2012–13 season, 44 participants in the IIV group and 36 participants in the LAIV group received 2011–12 IIV. Receipt of seasonal IIV in the preceding season was associated in both groups with sustained higher serum HAI titers at baseline against the H1N1, H3N2 (2011–12), and H3N2 (2012–13) antigens (p ≤0.0001). Participants who received IIV and had not received seasonal influenza vaccine in the preceding season were more frequently noted to have a ≥ four-fold rise in serum HAI titer at Day 28 for all strains except for the B (2012–13) antigen (p<=0.003). Receipt of seasonal IIV was also associated with sustained higher serum HAI titers at Day 28 against the H3N2 (2012–13) antigen (p = 0.006) (data not shown).
4. Discussion
Our study demonstrated higher serum HA-specific IgG responses in postpartum IIV recipients compared to LAIV recipients. This finding is similar to past investigations [25,26] that noted IIV induced higher serum HAI and HA-specific IgG responses compared to LAIV among healthy adults.
Because secretory IgA is also the major immunoglobulin in human milk and is produced by plasma cells in the mammary tissue [27], we hypothesized that breast milk levels of IgA would be highest in LAIV recipients. However, we found low levels of breast milk influenza HA-specific IgA in both groups in contrast to a study [25] that demonstrated that LAIV induced greater levels of local secretory IgA in the nose compared to IIV. It appears that the level of mucosal LAIV replication may have been too low or too brief to induce robust levels of serum or breast milk antibodies. LAIV depends on some level of virus replication to generate an immune response [26].
Similar to a previous report [28], we noted 61(49%) LAIV recipients shed vaccine virus in the nasopharynx detected by PCR or viral culture, which peaked on the second day after vaccination. Shedding of attenuated vaccine virus is common in the first few days following vaccination with LAIV [28–30]. However, the quantity of virus shed in these adults was 100–10,000-fold lower than the median human infectious dose required for LAIV vaccination in adults [28].
One (1%) infant had vaccine strain H1N1 (by cell culture) and H3N2 (by PCR and cell culture) (by testing at MedImmune Laboratory) in nasal secretions at day 2 after maternal vaccination, suggesting transmission. This infant remained asymptomatic. This infant’s mother had negative nasal testing for influenza viruses at day 2, but influenza A virus was detected by PCR at day 8. We considered the possibility that the mother’s and infant’s day 2 samples were inadvertently switched but retrospectively, we were not able to determine which day 2 sample was collected from the mother versus the infant. It is possible that there was no transmission and the positive day 2 and day 8 samples were both from the mother.
To date, there was been only one previously documented episode of LAIV transmission, which occurred in a day care attendee as part of a placebo-controlled LAIV trial [31] where shedding of vaccine virus would be expected to be higher than in our study of adults. Further, because reversion of LAIV to wild-type virus has never been demonstrated [32], and secondary transmission from a person who recently received LAIV has never resulted in illness in the contact [11] including our case, we are not overly concerned that the infant would become ill. However, because LAIV vaccination is not recommended under 2 years of age, there could be some concern about transmission to a young infant.
No evidence of LAIV virus excretion in breast milk was noted. Although our study confirms the safety of LAIV administration in postpartum breastfeeding women [6], it is possible that under the study conditions, women were more careful with handwashing and protecting their infants from their nasal secretions and therefore, transmission to the infant was minimized.
A limitation of this study was that, in order to meet the enrollment goal determined in the sample size calculations, two years of data needed to be combined. This is problematic because the seasonal vaccines only shared the H1N1 antigen in common; the H3N2 and B antigens were different. Therefore, we provided data for each season as well as the two seasons combined. Comparisons between seasons, even for the same antigen, are difficult because the participants were different between the two seasons, and in addition to the vaccine received, they had exposures to circulating influenza viruses that likely affected their immune responses. We do not have a good explanation for the differences noted between IgG and IgA titers when comparing the two seasons.
Another limitation of this study is that only serum and breast milk antibody responses were assessed as immune correlates of protection. Strain-specific HAI antibodies induced by IIVs have been validated as a surrogate correlate of protection for adults in human challenge and infection studies [33]. In contrast, the mechanisms of protective immunity after LAIV receipt are still incompletely defined [34]. LAIV also induces nasal IgA responses and antigen-specific cytokine-secreting T cells. These T cell responses may provide heterosubtypic influenza-specific protective immunity [35].
Additionally, we did not follow the mothers throughout the influenza seasons to assess the effectiveness of vaccination. The relative effectiveness of LAIV and IIV in the seasons studied is pertinent to any conclusion that IIV is preferred. In the United States for the 18–49 year age group, the adjusted vaccine effectiveness in preventing medically attended influenza was 44% (95% CI, 21–60) [36] and 39% (95% CI, 26–50) [37] for the 2011–12 and 2012–13 seasons, respectively.
In summary, breast milk ELISA IgG, and serum ELISA IgG and HAI titers were significantly higher in IIV compared to LAIV recipients, while safety of both formulations was reaffirmed. The Advisory Committee on Immunization Practices (ACIP) recommended against LAIV administration in the 2016–17 and 2017–18 influenza seasons in the United States because it was poorly effective against A/H1N1 viruses [38,39]. For the 2018–19 influenza season, ACIP has recommended that providers may choose to administer any licensed, age-appropriate influenza vaccine [40]. Therefore, LAIV will be an option for healthy breastfeeding women and the findings of this study may be applicable.
Supplementary Material
Acknowledgements
The authors would like to thank all the women and infants who participated in this research study. Additional acknowledgements include:
Cincinnati Children’s Hospital (Stacie Wethington, RN, BSN; Michelle Dickey, APRN; and Jesse LePage, BS).
Duke University (Amanda Anderson RN, BSN; Luis Ballon; Lynn Harrington RN, BSN; Lori Hendrickson RN, BSN; Beth Patterson RN, BSN; and Bonnie Thiele RN, BSN).
Emory Children’s Center (Kurt Martinuzzi, MD; Kathy Stephens, RN, BSN).
Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (Robin Mason, MS, MBA; Soju Chang, MD, MPH).
Funding
This study was funded by National Institute of Allergy and Infectious Diseases, Division of Microbiology and Infectious Diseases, Vaccine Treatment and Evaluation Unit contracts HHSN272200800003C, HHSN272200800004C, HHSN27220080 0005C, HHSN272200800006C, HHSN272200800009C, and HHSN2722008000013C and HHSN2722008000057C (Duke was under subcontract to University of Maryland).
Footnotes
Trial registration
ClinicalTrials.gov identifier: NCT01181323 has a link to the full protocol.
Competing interests
Dr. Brady has received funding from PaxVax and Pfizer to conduct clinical research studies.
Dr. Walter has received funding from CSL, GlaxoSmithKline, Merck, Novartis, Novavax, and Pfizer to conduct clinical research studies. He has received support from Novartis as a member of a Data Safety Monitoring Board and from Merck as a consultant.
Dr. Swamy has received funding from Novavax and Novartis to conduct clinical research studies. She has received support from GlaxoSmithKline as a member of a Data Safety Monitoring Board.
All other authors have no competing interests to report.
Representatives from MedImmune reviewed and commented on the manuscript and figures and were involved in testing to distinguish wild-type versus vaccine influenza viruses.
Results of this study were presented in part ID Week 2015, Abstract #1902.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.06.036.
References
- [1].Chaves SS, Perez A, Farley MM, Miller L, Schaffner W, Lindegren ML, et al. The burden of influenza hospitalizations in infants from 2003 to 2012, United States PMID: 24577042. Pediatr Infect Dis J 2014;33(9):912–9. [DOI] [PubMed] [Google Scholar]
- [2].Poehling KA, Edwards KM, Griffin MR, Szilagyi PG, Staat MA, Iwane MK, et al. The burden of influenza in young children, 2004–2009 PMID: 23296444. Pediatrics 2013;131(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].American Academy of Pediatrics. Influenza. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: 2015 Report of the Committee on Infectious Diseases Elk Grove Village, IL: American Academy of Pediatrics; 2015. p. 476–93. [Google Scholar]
- [4].Healy CM. Vaccines in pregnant women and research initiatives PMID: 22510631. Clin Obstet Gynecol 2012;55(2):474–86. [DOI] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention. Pregnant women & influenza (flu); 2016. Available at: http://www.cdc.gov/flu/protect/vaccine/pregnant.htm [accessed November 15, 2016].
- [6].Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 influenza season PMID: 26247435. MMWR Morb Mortal Wkly Rep 2015;64:818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gorman JR, Chambers CD. Pregnant women’s attitudes toward influenza vaccination while breastfeeding PMID: 26844088. Prev Med Rep 2015;2:333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schlaudecker EP, Steinhoff MC, Omer SB, McNeal MM, Roy E, Arifeen SE, et al. IgA and neutralizing antibodies to influenza A virus in human milk: a randomized trial of antenatal influenza immunization PMID: 23967126. PLoS One 2013;8(8):e70867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maertens K, De Schutter S, Braeckman T, Baerts L, Van Damme P, De Meester I, et al. Breastfeeding after maternal immunisation during pregnancy: Providing immunological protection to the newborn: a review PMID: 24530929. Vaccine 2014;32:1786–92. [DOI] [PubMed] [Google Scholar]
- [10].Alain S, Dommergues MA, Jacquard AC, Caulin E, Launay O. State of the art: could nursing mothers be vaccinated with attenuated live virus vaccine? PMID: 22659446 Vaccine 2012;30:4921–6. [DOI] [PubMed] [Google Scholar]
- [11].United States Department of Health and Human Services, Food and Drug Administration, Silver Spring (MD): FDA. FluMist® Quadrivalent (Influenza vaccine live, intranasal) package insert; September 2016. Available at: http://www.fda.gov/downloads/biologicsbloodvaccines/approvedproducts/ucm293952.htm [accessed November 15, 2016]. [Google Scholar]
- [12].Bohlke K, Galil K, Jackson LA, Schmid DS, Starkovich P, Loparev VN, et al. Postpartum varicella vaccination: is the vaccine virus excreted in breast milk? PMID: 14672472 Obstet Gynecol 2003;102:970–7. [DOI] [PubMed] [Google Scholar]
- [13].Buimovici-Klein E, Hite RL, Byrne T, Cooper LZ. Isolation of rubella virus in milk after postpartum immunization. J Pediatr 1977;91(6):939–41. PMID: 925824. [DOI] [PubMed] [Google Scholar]
- [14].Losonsky GA, Fishaut JM, Strussenberg J, Ogra PL. Effect of immunization against rubella on lactation products. I. Development and characterization of specific immunologic reactivity in breast milk. J Infect Dis 1982;145 (5):654–60. PMID: 7077089. [DOI] [PubMed] [Google Scholar]
- [15].Losonsky GA, Fishaut JM, Strussenberg J, Ogra PL. Effect of immunization against rubella on lactation products II. Maternal-neonatal interactions. J Infect Dis 1982;145(5):661–6. PMID: 7077090. [DOI] [PubMed] [Google Scholar]
- [16].Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices PMID: 21293327. MMWR Morb Mortal Wkly Rep 2011. Jan 28;60(2):1–64. [PubMed] [Google Scholar]
- [17].Centers for Disease Control and Prevention. Transmission of yellow fever vaccine virus through breast-feeding-Brazil, 2009 PMID: 20150888. MMWR Morb Mortal Wkly Rep 2010;59(5):130–2. [PubMed] [Google Scholar]
- [18].Kuhn S, Twele-Montecinos L, MacDonald J, Webster P, Law B. Case report: probable transmission of vaccine strain of yellow fever virus to an infant via breast milk PMID: 21324845. CMAJ 2011;183(4):E243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Staples JE, Gershman M, Fischer M, Centers for Disease Control and Prevention. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010;59(RR-7):1–27. PMID: 20671663. [PubMed] [Google Scholar]
- [20].Centers for Disease Control and Prevention. Overview of influenza surveillance in the United States; 2016 Available at: http://www.cdc.gov/flu/weekly/overview.htm [accessed March 29, 2016].
- [21].Centers for Disease Control and Prevention. Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine PMID: 21637185. MMWR Morb Mortal Wkly Rep 2011;60(21):705–12. [PubMed] [Google Scholar]
- [22].Centers for Disease Control and Prevention. Update: influenza activity—United States, 2011–12 season, and composition of the 2012–13 influenza vaccine PMID: 22672977. MMWR Morb Mortal Wkly Rep 2012;61(22):414–20. [PubMed] [Google Scholar]
- [23].United States Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, Silver Spring (MD): FDA. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines; May 2007. Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm07479 [November 15, 2016]. [Google Scholar]
- [24].Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors PMID: 23178060. Pediatr Clin North Am 2013;60(1):49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Beyer WEP, Palache AM, de Jong JC, Osterhaus ADME. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis PMID: 11818152. Vaccine 2002;20:1340–53. [DOI] [PubMed] [Google Scholar]
- [26].Sealy R, Webby RJ, Crumpton JC, Hurwitz JL. Differential localization and function of antibody-forming cells responsive to inactivated or live-attenuated influenza virus vaccines PMID: 23143476. Int Immunol 2012;25(3):183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk PMID: 22254105. Nutrients 2011;3:442–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Block SL, Yogev R, Hayden FG, Ambrose CS, Zeng W, Walker RE. Shedding and immunogenicity of live attenuated influenza vaccine virus in subjects 5–49 years of age PMID: 18662737. Vaccine 2008;26(38):4940–6. [DOI] [PubMed] [Google Scholar]
- [29].Hammitt LL, Bartlett JP, Li S, Rahkola J, Lang N, Janoff EN, et al. Kinetics of viral shedding and immune responses in adults following administration of cold-adapted influenza vaccine PMID: 19800447. Vaccine 2009;27:7359–66. [DOI] [PubMed] [Google Scholar]
- [30].Talbot TR, Babcock H, Cotton D, Maragakis LL, Poland GA, Septimus EJ, et al. The use of live attenuated influenza vaccine (LAIV) in healthcare personnel (HCP): Guidance from the Society for Healthcare Epidemiology of America (SHEA) PMID: 22961016. Infect Control Hosp Epidemiol 2012;33(10):981–3. [DOI] [PubMed] [Google Scholar]
- [31].Vesikari T, Karvonen A, Korhonen T, Edelman K, Fainionpää R, Salmi A, et al. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine PMID: 16804427. Pediatr Infect Dis J 2006;25(7):590–5. [DOI] [PubMed] [Google Scholar]
- [32].Tosh PK, Boyce TG, Poland GA. Flu myths: dispelling the myths associated with live attenuated influenza vaccine PMID: 18174020. Mayo Clin Proc 2008;83 (1):77–84. [DOI] [PubMed] [Google Scholar]
- [33].Sridhar S, Brokstad KA, Cox RJ. Influenza vaccination strategies: comparing inactivated and live attenuated influenza vaccines PMID: 26343192. Vaccines (Basel) 2015;3:373–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wright PF, Hoen AG, Ilyushina NA, Brown EP, Ackerman ME, Wieland-Alter W, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016;3 (2). 10.1093/ofid/ofw108. PMID: 27419180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in children PMID: 21846636. J Infect Dis 2011;204:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates PMID: 24235265. Clin Infect Dis 2014;58(3):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type PMID: 25406334. J Infect Dis 2015;211(10):1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2016–17 influenza season PMID: 27560619. MMWR Morb Mortal Wkly Rep 2016;65 (5):1–54. [DOI] [PubMed] [Google Scholar]
- [39].Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Breese JS, Fry AM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2017–18 influenza season PMID: 28841201. MMWR Morb Mortal Wkly Rep 2017;66 (2):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season PMID: 29879095. MMWR Morb Mortal Wkly Rep 2018;67(22):643–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


