Abstract
Background and Objectives:
There is no clear evidence of a negative impact of biliary stents on the diagnostic yield of EUS-guided fine-needle biopsy (EUS-FNB) for diagnosing pancreatic head lesions. We aimed to evaluate the association between the presence of biliary stents and the diagnostic accuracy of EUS-FNB.
Materials and Methods:
A multicenter retrospective study including all jaundiced patients secondary to pancreatic head masses was performed. Patients were divided into two groups according to the presence of a biliary stent placed before EUS-FNB. Pathological results were classified according to the Papanicolaou classification and compared against the final diagnosis. Diagnostic measures in the two groups were compared. Multivariate logistic regression analyses including potential factors affecting EUS-FNB accuracy were performed.
Results:
Overall, 842 patients were included, 495 (58.8%) without and 347 (41.2%) with biliary stent. A plastic or a metal stent was placed in 217 (62.5%) and 130 (37.5%) cases, respectively. Diagnostic sensitivity and accuracy were significantly higher in patients without biliary stent than in those with stent (91.9% and 92.1% vs. 85.9% and 86.4%, P = 0.010 At multivariate analyses, lesion size (odds ratio [OR]: 1.05, 95% confidence interval [CI]: 1.02–1.09, P = 0.01) and presence of biliary stent (OR: 0.51, 95% CI: 0.32–0.89, P = 0.01) were independently associated with diagnostic accuracy. In the subgroup of patients with biliary stent, the type of stent (plastic vs. metal) did not impact EUS-FNB yield, whereas the use of larger bore needles enhanced diagnostic accuracy (OR: 2.29, 95% CI: 1.28–4.12, P = 0.005).
Conclusions:
In this large retrospective study, an indwelling biliary stent negatively impacted the diagnostic accuracy of EUS-FNB. Preferably, EUS-FNB should precede endoscopic retrograde cholangiopancreatography, especially in the case of small tumors.
Keywords: biliary drainage, endoscopic retrograde cholangiopancreatography, fine-needle aspiration, fine-needle biopsy, pancreatic cancer
INTRODUCTION
EUS-guided tissue acquisition (EUS-TA) represents the procedure of choice for the characterization of solid pancreatic lesions (SPLs).[1] Several factors can impact the diagnostic accuracy of EUS-TA, such as needle type[2,3] and caliber,[4,5] lesion size,[6,7] use of EUS enhancement techniques,[8] sampling technique,[5,9] specimen processing,[10,11,12,13] number of passes,[14,15] and availability of rapid on-site evaluation (ROSE).[16,17]
Tumors located in the pancreatic head/uncinate process are often responsible for jaundice, which is usually the first clinical symptom. At the time of diagnosis, the majority of patients are unresectable due to locally advanced stage, with both biliary drainage and cyto/histological confirmation required before chemotherapy. Commonly, biliary decompression is perceived as a more urgent procedure than obtaining cyto/histological diagnosis and, despite combining EUS and ERCP into a single endoscopic session is technically feasible,[18,19] in a real-life world, biliary stenting sometimes precedes EUS-TA. The presence of biliary stents could impair the diagnostic yield of EUS-TA by hindering the visualization of the lesion with acoustic shadows or reverberation [Figure 1] or making challenging the cytological evaluation due to the presence of surrounding inflammation.[20] However, plastic or self-expandable metal stents (SEMS) could have different effects because of their difference in material and diameter (plastic stents are usually 10-Fr whereas SEMS are generally 10 mm).
Figure 1.
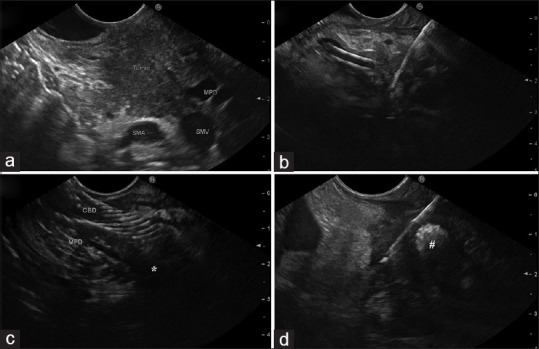
(a) A pancreatic head mass is clearly visualized on endoscopic ultrasound in the absence of a biliary stent. (b) Endoscopic ultrasound tissue acquisition of a small pancreatic head lesion in the presence of a plastic biliary stent. (c) A small pancreatic head tumor (*) is hidden by a biliary metal stent. (d) The most marginal part of a pancreatic head solid lesion is sampled beside a biliary metal stent (#)
To date, conflicting results have been published about the impact of an indwelling biliary stent on the diagnostic yield of EUS-TA. Three studies showed no difference in diagnostic yield of EUS-guided fine-needle aspiration (EUS-FNA) and fine-needle biopsy (EUS-FNB) for pancreatic head lesions in patients with a biliary plastic stent or SEMS.[21,22,23] In contrast, Kim et al. observed a lower yield of EUS-FNA in the presence of a stent, regardless of its type.[24] In a recent large retrospective study including either EUS-FNA or EUS-FNB, Bekkali et al. demonstrated that the presence of SEMS was associated with a higher rate of inconclusive procedures.[25] Another study investigating the yield of EUS-FNA in patients with predominantly plastic stents found that stents had a negative impact if placed less than 1 day before EUS.[26]
Nowadays, EUS-guided tissue sampling is moving from EUS-FNA to EUS-FNB.[27] However, only one study has evaluated the impact of plastic stents on EUS-FNB.[23] Evidence of negative impact of biliary stents on EUS-FNB could influence the choice of procedures sequence (i.e., EUS before ERCP or vice versa) or type of stent (i.e., plastic or SEMS) to be placed during ERCP if cyto/histological confirmation has not been achieved.
We performed a multicenter retrospective study on a large cohort of jaundiced patients with pancreatic head or uncinate process mass with the aim to assess the impact of the presence of biliary stents on the diagnostic accuracy of EUS-FNB.
MATERIALS AND METHODS
This study was a multicenter retrospective study involving five third-referral Italian centers, following the STrengthening the Reporting of OBservational studies in Epidemiology statement.[28] All consecutive patients who underwent EUS-FNB between January 2017 and December 2019 for the diagnosis of SPLs located in the head/uncinate process leading to obstructive jaundice (distal biliary stenosis associated with bilirubin level ≥2.5 mg/dL) were included in the study. Lesions located in other pancreatic regions or predominantly cystic lesions (more than 50% of the volume) or patients who underwent EUS-FNA were excluded from the study.
Data about sex, age, lesion site and size, type and caliber of needle used, number of passes, sampling technique, availability of ROSE, presence and type of biliary stent, time (days) between ERCP and EUS-FNB, sample processing, EUS-FNB, and patients’ outcome were collected by the study investigators at each participating center. In the absence of ROSE, macroscopic on-site evaluation of the sample was performed by the endosonographers.
The study was approved by the local Ethics Committee (Prog. 2572CESC, 2020.03.16) and was conducted according to the principles and the recommendations of the 2013 Declaration of Helsinki.
Study endpoints
Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and accuracy of EUS-FNB were calculated against the final diagnosis. Pathological diagnosis on surgical specimens was used as the gold standard whenever available. In nonresected patients, the definitive diagnosis was established based on a composite of outcomes, including imaging work-up, additional biopsy samples, and clinical disease course of at least 12 months.[29] To ensure the most accurate and most extended follow-up, information collected from electronic charts and EUS reports review were corroborated by telephone contacts with all patients performed at the time of data collection. Disease progression, distant metastases, or cancer-related death defined a lesion as malignant. A stable/reducing mass on imaging, no metastases appearance, and patient well-being after a minimum follow-up of 12 months were criteria to define the lesion as benign. Pathological interpretations were classified according to the Papanicolaou classification.[30]
To calculate diagnostic performance, both “strict” and “not strict” criteria were used. In particular, EUS-FNB pathological interpretations “suspicious for malignancy” (Papanicolaou category 5) were considered negatives or positives following “strict” and “not strict” criteria, respectively. Differently, “atypical cells” results (Papanicolaou category 3) were always considered as negative.
Statistical analysis
Continuous variables were summarized as mean ± standard deviation or median with interquartile range and were compared using the Student t-test or the Mann–Whitney test, when appropriate (i.e., if data were not normally distributed). Categorical variables were reported as percentages and compared using the Chi-square or the Fisher's exact test for cases with small expected frequencies (<5). All tests were two-tailed.
The outcomes of EUS-TA in the two groups of patients (with and without stent) were compared. The effect of the main determinants (i.e., age, sex, lesion site, lesion size, needle type and caliber, number of passes, availability of ROSE, biliary stent placement) on diagnostic accuracy was evaluated using a 2-level mixed-effects logistic regression model considering the variable center as random.[31]
Subgroup analysis, including patients with biliary stent, was also performed, including specific covariates (i.e., type of biliary stent and interval time between ERCP and EUS). Covariates with P value < 0.05 at univariate analysis were subsequently included in the multivariate analysis. The significance level was set for P < 0.05. Data were presented with odds ratios (OR) and their respective 95% confidence intervals (CI).
All the analyses were performed, including the intention-to-diagnose population (i.e., technical failures and inadequate samples were counted as false negative) and for both strict and not strict criteria. Statistical analysis was conducted using MedCalc Statistical Software Version 12.5.0 (MedCalc Software LTD, Ostend, Belgium).
RESULTS
Study population
Table 1 shows the main characteristics of 842 consecutive patients who underwent EUS-FNB that were included in the main analysis. Biliary stent before EUS-FNB was placed in 347 (41.2%) patients. In 62.5% (n = 217), the stent was plastic and in 37.5% (n = 130) SEMS. In one (0.1%) patient, lesion visualization failed due to the presence of the biliary SEMS and malignancy was ascertained during follow-up. EUS-FNB was performed using an end-cutting needle (SharkCore™, Medtronic, Dublin, Ireland, or Acquire™, Boston Scientific, Marlborough, Massachusetts, USA) in 507 (60.2%) cases or a side-fenestrated one (ProCore™, Cook Medical, Limerick, Ireland) in 335 (39.8%). ROSE was performed in 111 (13.2%) of all procedures. In eight cases (0.9%), the specimen was not sufficient to assess a pathological diagnosis. Surgical resection was performed in 169 (20.1%) patients. Most of the patients (92.0%) had a final diagnosis of pancreatic ductal adenocarcinoma, and 26 (3.1%) had a final diagnosis of benign disease (20 chronic pancreatitis and six autoimmune pancreatitis). Patients with stent were significantly different compared with those without stent in age (68 vs. 70 years, P < 0.001), lesion size (29.3 ± 8.9 vs. 31.7 ± 8.7, P < 0.001), number of needle passes (1.01 ± 0.76 vs. 2.84 ± 0.65, P < 0.001), and use of end-cutting needle (67.1% vs. 55.4%, P < 0.001).
Table 1.
Baseline characteristics of 842 patients who underwent EUS-guided fine-needle biopsy
| Overall (n=842) | Without stent (n=495) | With stent (n=347) | P | |
|---|---|---|---|---|
| Female sex, n (%) | 349 (41.4) | 204 (41.2) | 145 (41.8) | 0.92 |
| Age, median (IQR) | 70 (61-76) | 70 (64-78) | 68 (57.5-76) | <0.001 |
| Lesion site, n (%) | 0 | |||
| Head | 757 (89.9) | 447 (90.3) | 310 (89.3) | 0.73 |
| Uncinate process | 85 (10.1) | 48 (9.7) | 37 (10.7) | |
| Lesion size (mm), mean±SD | 30.7±8.9 | 31.7±8.7 | 29.3±8.9 | <0.001 |
| FNB needle type*, n (%) | ||||
| Side-fenestrated | 335 (39.8) | 221 (44.6) | 114 (32.9) | <0.001 |
| End-cutting | 507 (60.2) | 274 (55.4) | 233 (67.1) | |
| Needle caliber, n (%) | ||||
| 25G | 230 (27.3) | 129 (26.1) | 101 (29.1) | 0.37† |
| 22G | 511 (60.7) | 294 (59.4) | 217 (62.5) | |
| 20G | 101 (12.0) | 72 (14.5) | 29 (8.4) | |
| Number of needle passes, mean±SD | 2.88±0.7 | 2.81±0.68 | 2.98±0.79 | <0.001 |
| ROSE, n (%) | 111 (13.2) | 70 (14.1) | 41 (11.8) | 0.38 |
| Type of stent, n (%) | - | - | ||
| Plastic | 217 (62.5) | - | ||
| Metallic | 130 (37.5) | |||
| Time (days) between ERCP and EUS, median (IQR) | - | - | 11.0 (3-30) | - |
| Final diagnosis | ||||
| PDAC | 775 (92.0) | 453 (91.5) | 322 (92.8) | |
| pNET | 17 (2.0) | 13 (2.6) | 4 (1.2) | |
| Chronic pancreatitis | 20 (2.0) | 13 (2.6) | 7 (2.0) | |
| Autoimmune pancreatitis | 6 (0.7) | 3 (0.6) | 3 (0.9) | |
| Acinar carcinoma | 3 (0.4) | 2 (0.4) | 1 (0.3) | |
| Metastasis | 12 (1.4) | 8 (1.6) | 4 (1.2) | |
| Others‡ | 7 (0.8) | 3 (0.6) | 4 (1.2) | |
| Centers, n (%) | ||||
| Verona | 365 (43.3) | 142 (28.7) | 223 (64.3) | |
| Milano | 120 (14.3) | 71 (14.3) | 49 (14.1) | |
| Fermo | 224 (26.6) | 163 (32.9) | 61 (17.6) | |
| Forlì | 41 (4.9) | 37 (7.5) | 4 (1.2) | |
| Palermo | 97 (10.9) | 82 (16.6) | 10 (2.9) |
*Side-fenestrated (Procore™); end-cutting (SharkCore™ or Acquire™); †25G vs. others; ‡Others include: Lymphoma (n=4); Neuroendocrine carcinoma (n=2); Cholangiocarcinoma (n=1); Continuous data are expressed as mean±SD or median and IQR. Categorical data are expressed as number (percentage). IQR: Interquartile range; SD: Standard deviation; FNB: Fine-needle biopsy; ROSE: Rapid on-site evaluation; PDAC: Pancreatic ductal adenocarcinoma; pNET: Pancreatic neuroendocrine tumor
Main study outcome
Pathological results of EUS samples according to reference standard are shown in Supplementary Table 1.
Table S1.
Pathological findings of samples collected in patients with solid pancreatic head lesions who underwent EUS fine-needle biopsy stratified according to the papanicolaou classification and presented with results of the reference standard, using strict and not strict criteria and according to the presence and type of biliary stent
| EUS-FNB diagnosis | Reference standard, strict criteria | Reference standard, not strict criteria | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Overall population (n=842) | Positive findings (814) | Negative findings (28) | Positive findings (814) | Negative findings (28) | ||||
|
|
|
|
|
|||||
| TP (728) | FN (86) | TN (28) | FP (0) | TP (771) | FN (43) | TN (27) | FP (1) | |
| Malignant | 713 (87.6) | 0 | 0 | 0 | 713 (87.6) | 0 | 0 | 0 |
| Suspicious for malignancy | 0 | 43 (5.3) | 1 (3.6) | 0 | 43 (5.3) | 0 | 0 | 1 (3.6) |
| Neoplastic benign/other | 15 (1.8) | 0 | 0 | 0 | 15 (1.8) | 0 | 0 | 0 |
| Atypical NOS | 0 | 22 (2.7) | 0 | 0 | 0 | 22 (2.7) | 0 (0) | 0 |
| Benign (negative for malignancy) | 0 | 12 (1.5) | 27 (96.4) | 0 | 0 | 12 (1.5) | 27 (96.4) | 0 |
| Inadequate/technical failures | 0 | 9 (1.1) | 0 | 0 | 0 | 9 (1.1) | 0 | 0 |
| All diagnoses | 728 (89.4) | 87 (10.6) | 28 (100) | 0 | 771 (94.7) | 43 (5.3) | 27 (96.4) | 1 (3.6) |
|
| ||||||||
| Without stent (n=495) | Positive findings (479) | Negative findings (16) | Positive findings (479) | Negative findings (16) | ||||
|
|
|
|
|
|||||
| TP (440) | FN (39) | TN (16) | FP (0) | TP (461) | FN (18) | TN (14) | FP (0) | |
|
| ||||||||
| Malignant | 428 (89.4) | 0 | 0 | 0 | 428 (89.4) | 0 | 0 | 0 |
| Suspicious for malignancy | 0 | 21 (4.4) | 0 | 0 | 21 (4.4) | 0 | 0 | 0 |
| Neoplastic benign/other | 12 (2.5) | 0 | 0 | 0 | 12 (2.5) | 0 | 0 | 0 |
| Atypical NOS | 0 | 9 (1.9) | 0 | 0 | 0 | 9 (1.9) | 0 | 0 |
| Benign (negative for malignancy) | 0 | 6 (1.2) | 16 (100) | 0 | 0 | 6 (1.2) | 16 (100) | 0 |
| Inadequate | 0 | 3 (0.6) | 0 | 0 | 0 | 3 (0.6) | 0 | 0 |
| All diagnoses | 440 (91.9) | 39 (8.1) | 16 (100) | 0 | 461 (96.2) | 18 (3.8) | 16 (100) | 0 |
|
| ||||||||
| Plastic stent (n=217) | Positive findings (205) | Negative findings (12) | Positive findings (205) | Negative findings (12) | ||||
|
|
|
|
|
|||||
| TP (174) | FN (31) | TN (12) | FP (0) | TP (187) | FN (18) | TN (11) | FP (1) | |
|
| ||||||||
| Malignant | 172 (83.9) | 0 | 0 | 0 | 172 (83.9) | 0 | 0 | 0 |
| Suspicious for malignancy | 0 | 13 (6.3) | 1 (8.3) | 0 | 13 (6.3) | 0 | 0 | 1 (8.3) |
| Neoplastic benign/other | 2 (1.0) | 0 | 0 | 0 | 2 (1.0) | 0 | 0 | 0 |
| Atypical NOS | 0 | 9 (4.4) | 0 | 0 | 0 | 9 (4.4) | 0 | 0 |
| Benign (negative for malignancy) | 0 | 4 (1.9) | 11 (91.7) | 0 | 0 | 4 (1.9) | 11 (91.7) | 0 |
| Inadequate | 0 | 5 (2.4) | 0 | 0 | 0 | 5 (2.4) | 0 | 0 |
| All diagnoses | 174 (84.9) | 31 (15.1) | 12 (100) | 0 | 187 (90.0) | 18 (10.0) | 11 (91.7) | 1 (8.3) |
|
| ||||||||
| Metal stent (n=130) | Positive findings (130) | Negative findings (0) | Positive findings (130) | Negative findings (0) | ||||
|
|
|
|
|
|||||
| TP (113) | FN (16) | TN (0) | FP (0) | TP (123) | FN (7) | TN (0) | FP (0) | |
|
| ||||||||
| Malignant | 113 (87.6) | 0 | 0 | 0 | 113 (87.6) | 0 | 0 | 0 |
| Suspicious for malignancy | 0 | 9 (7.0) | 0 | 0 | 9 (7.0) | 0 | 0 | 0 |
| Neoplastic benign/other | 1 (0.8) | 0 | 0 | 0 | 1 (0.8) | 0 | 0 | 0 |
| Atypical NOS | 0 | 4 (2.3) | 0 | 0 | 0 | 4 (2.3) | 0 | 0 |
| Benign (negative for malignancy) | 0 | 2 (1.6) | 0 | 0 | 0 | 2 (1.6) | 0 | 0 |
| Technical failures | 0 | 1 (0.8) | 0 | 0 | 0 | 1 (0.8) | 0 | 0 |
| All diagnoses | 114 (87.7) | 16 (12.3) | 0 | 0 | 123 (94.6) | 7 (5.4) | 0 | 0 |
TP: True positives; FN: False negatives; TN: True negatives. FP: False positives; EUS-FNB: EUS-guided fine-needle biopsy; NOS: Not otherwise specified
Table 2 reports diagnostic yield (sensitivity, specificity, NPV, PPV, and accuracy) of EUS-TA in patients with and without biliary stent using strict and not strict criteria. Using strict criteria, overall sensitivity, specificity, NPV, PPV, and accuracy were, respectively, 89.4% (95% CI: 87.1–91.5), 100% (95% CI: 87.6–100), 24.6% (95% CI: 21.1–28.5), 100%, and 89.8% (95% CI: 87.5–91.8). Sensitivity and accuracy were significantly higher in patients without biliary stent than in those who underwent stent placement (91.9%, 95% CI: 89.0–94.2 and 92.1%, 95% CI: 89.4–94.3 vs. 85.9%, 95% CI: 81.7–89.5 and 86.4%, 95% CI: 82.4–89.9, P = 0.010 and P = 0.014, respectively). Similar results were obtained by using not strict criteria. Sensitivity and accuracy were similar according to the type of stent (i.e., plastic or SEMS) with both strict and not strict criteria.
Table 2.
Diagnostic measures of EUS-guided tissue acquisition in patients with jaundice and pancreatic head lesions with or without previously placed biliary stent
| Strict criteria | |||||
|---|---|---|---|---|---|
|
| |||||
| Overall population | Without biliary stent | With biliary stent | |||
|
| |||||
| Overall | Plastic | Metallic | |||
| Sensitivity, percentage (95% CI) | 89.4 (87.1-91.5) | 91.9 (89.0-94.2) | 85.9 (81.7-89.5) | 84.9 (79.2-89.5) | 89.0 (82.8-93.6) |
| Specificity, percentage (95% CI) | 100.0 (87.6-100) | 100 (79.4-100) | 100 (73.5-100) | 100 (73.5-100) | - |
| NPV, percentage (95% CI) | 24.6 (21.1-28.5) | 29.1 (23.3-35.7) | 20.3 (16.4-25.0) | 27.9 (21.9-34.9) | 0 |
| PPV, percentage (95% CI) | 100.0 | 100 | 100 | 100 | 100 |
| Accuracy, percentage (95% CI) | 89.8 (87.5-91.8) | 92.1 (89.4-94.3) | 86.4 (82.4-89.9) | 85.7 (80.3-90.1) | - |
|
| |||||
| Not strict criteria | |||||
|
| |||||
| Overall population | Without biliary stent | With biliary stent | |||
|
| |||||
| Overall | Plastic | Metallic | |||
|
| |||||
| Sensitivity, percentage (95% CI) | 94.7 (92.9-96.1) | 96.2 (94.1-97.8) | 92.5 (89.2-95.1) | 91.2 (86.5-94.7) | 94.6 (89.2-97.8) |
| Specificity, percentage (95% CI) | 96.4 (81.6-99.9) | 100 (76.8-100) | 91.7 (61.5-99.8) | 91.7 (61.5-99.8) | - |
| NPV, percentage (95% CI) | 38.6 (31.8-45.9) | 43.8 (33.1-55.0) | 30.6 (22.5-40.0) | 37.9 (27.6-49.5) | 0 |
| PPV, percentage (95% CI) | 99.9 (99.1-100) | 100 | 99.7 (97.9-99.9) | 99.5 (96.6-99.9) | 100 |
| Accuracy, percentage (95% CI) | 94.8 (93.1-96.2) | 96.3 (94.3-97.8) | 92.5 (89.2-95.1) | 91.2 (86.7-94.7) | - |
NPV: Negative predictive value; PPV: Positive predictive value; CI: Confidence interval
Univariate and multivariate logistic regression analyses for diagnostic accuracy using strict criteria in the overall population are shown in Table 3. By multivariate analysis, increasing lesion size (OR: 1.05, 95% CI: 1.02–1.09, P = 0.01) and use of large bore needles (OR: 1.70, 95% CI: 1.09–2.66, P = 0.02) were independently associated with higher diagnostic accuracy. In contrast, the presence of biliary stent was independently associated with lower diagnostic accuracy (OR: 0.51, 95% CI: 0.32–0.89, P = 0.01). When not strict criteria were used [Supplementary Table 2], increasing lesion size (OR: 1.07, 95% CI: 1.01–1.13, P = 0.01) and presence of biliary stent (OR: 0.57, 95% CI: 0.29–0.91, P = 0.02) were associated with higher and lower accuracy, respectively.
Table 3.
Univariate and multivariate logistic regression of factors associated with diagnostic accuracy in 842 patients who underwent EUS-guided fine-needle biopsy, by using strict criteria
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 0.98 | 0.99-1.00 | 0.09 | |||
| Sex (male vs. female) | 0.57 | 0.35-1.03 | 0.08 | |||
| Lesion site (head vs. uncinate process) | 0.89 | 0.41-1.91 | 0.77 | |||
| Lesion size (mm) | 1.07 | 1.03-1.10 | <0.0001 | 1.05 | 1.02-1.09 | 0.01 |
| FNB needle type (side-fenestrated vs. end-cutting*) | 0.83 | 0.52-1.32 | 0.45 | |||
| Needle caliber (20G vs. 22G and 25G) | 3.05 | 1.10-8.53 | 0.03 | 1.70 | 1.09-2.66 | 0.02 |
| Number of passes | 0.99 | 0.72-1.35 | 0.94 | |||
| ROSE (yes vs. no) | 0.94 | 0.49-1.80 | 0.83 | |||
| Biliary stent (yes vs. no) | 0.54 | 0.34-0.85 | 0.008 | 0.51 | 0.32-0.89 | 0.01 |
*ProCore™ vs. SharkCore™ or Acquire™. OR: Odds ratio; CI: Confidence interval; FNB: Fine-needle biopsy; ROSE: Rapid on-site evaluation
Table S2.
Univariate and multivariate logistic regression of factors associated with diagnostic accuracy in 842 patients who underwent EUS-guided fine-needle biopsy, by using not strict criteria
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 0.99 | 0.96-1.01 | 0.43 | |||
| Sex (male vs. female) | 0.54 | 0.26-1.04 | 0.07 | |||
| Lesion site (head vs. uncinate process) | 0.78 | 0.29-2.03 | 0.61 | |||
| Lesion size (mm) | 1.08 | 1.03-1.23 | <0.0001 | 1.07 | 1.01-1.13 | 0.01 |
| FNB needle type (side-fenestrated vs. end-cutting*) | 0.85 | 0.44-1.59 | 0.63 | |||
| Needle caliber (20G vs. 22G and 25G) | 1.64 | 0.96-2.81 | 0.07 | |||
| Number of passes | 0.79 | 0.51-1.24 | 0.31 | |||
| ROSE: (yes vs. no) | 1.92 | 0.58-6.33 | 0.28 | |||
| Biliary stent (yes vs. no) | 0.46 | 0.24-0.85 | 0.008 | 0.57 | 0.29-0.91 | 0.02 |
*ProCore™ vs. SharkCore™ or Acquire™. CI: Confidence interval; FNB: Fine-needle biopsy; ROSE: Rapid on-site evaluation
Subgroup of patient with biliary stent
In the group of patients with biliary stent, multivariate analysis using strict criteria revealed that the use of large bore needles (OR: 2.29, 95% CI: 1.28–4.12, P = 0.005) were associated with higher accuracy [Table 4].
Table 4.
Univariate and multivariate logistic regression of factors associated with diagnostic accuracy in 347 patients who underwent EUS-guided fine-needle biopsy after biliary stent placement, by using strict criteria
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 0.99 | 0.96-1.01 | 0.42 | |||
| Sex (male vs. female) | 2.24 | 0.99-3.50 | 0.06 | |||
| Lesion site (head vs. uncinate process) | 0.99 | 0.37-2.70 | 0.99 | |||
| Lesion size (mm) | 1.04 | 0.99-1.07 | 0.08 | |||
| FNB needle type (side-fenestrated vs. end-cutting*) | 0.88 | 0.45-1.72 | 0.71 | |||
| Needle caliber (20G vs. 22G and 25G) | 3.26 | 1.17-9.07 | 0.02 | 2.29 | 1.28-4.12 | 0.005 |
| Number of passes | 1.40 | 0.92-2.11 | 0.11 | |||
| ROSE (yes vs. no) | 0.90 | 0.36-2.28 | 0.83 | |||
| Type of biliary stent (metallic vs. plastic) | 0.75 | 0.39-1.45 | 0.40 | |||
| Interval time between ERCP and EUS (days) | 0.99 | 0.99-1.00 | 0.15 | |||
*ProCore™ vs. SharkCore™ or Acquire™. OR: Odds ratio; CI: Confidence interval; FNB: Fine-needle biopsy; ROSE: Rapid on-site evaluation
Using not strict criteria, multivariate logistic regression analysis showed the use of large-bore needles (OR: 2.55, 95% CI: 1.14–5.66, P = 0.02) and longer interval time between ERCP and EUS (OR: 0.99, 95% CI: 0.986–0.998, P = 0.02) independently associated with higher diagnostic accuracy [Supplementary Table 3]. The type of stent (i.e., plastic vs. SEMS) was not associated with diagnostic accuracy, both with strict and not strict criteria.
Table S3.
Univariate and multivariate logistic regression of factors associated with diagnostic accuracy in 347 patients who underwent EUS-guided fine-needle biopsy after biliary stent placement, by using not strict criteria
| Covariate | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (years) | 0.99 | 0.96-1.03 | 0.74 | |||
| Sex (male vs. female) | 0.52 | 0.23-1.14 | 0.09 | |||
| Lesion site (head vs. uncinate process) | 1.27 | 0.28-5.65 | 0.75 | |||
| Lesion size (mm) | 1.04 | 0.99-1.10 | 0.14 | |||
| FNB needle type (side-fenestrated vs. end-cutting*) | 0.71 | 0.27-1.84 | 0.47 | |||
| Needle caliber (20G vs. 22G and 25G) | 2.12 | 1.01-4.55 | 0.05 | 2.55 | 1.14-5.66 | 0.02 |
| Number of passes | 1.21 | 0.69-2.12 | 0.51 | |||
| ROSE (yes vs. no) | 1.44 | 0.32-6.37 | 0.63 | |||
| Type of biliary stent (metallic vs. plastic) | 0.57 | 0.22-1.48 | 0.25 | |||
| Interval time between ERCP and EUS (days) | 0.99 | 0.989-0.999 | 0.03 | 0.99 | 0.986-0.998 | 0.02 |
*ProCore™ vs. SharkCore™ or Acquire™. CI: Confidence interval; FNB: Fine-needle biopsy; ROSE: Rapid on-site evaluation
DISCUSSION
There is no clear evidence of association between the presence of biliary stents and diagnostic yield of EUS-FNB for diagnosing pancreatic head lesions, and conflicting indications have been suggested in two different consensuses. The Canadian Society for EUS stated that EUS-TA should precede ERCP.[32] However, the international consensus for endoscopic management of distal biliary stricture concluded that biliary stent does not adversely affect the diagnostic yield of EUS-TA, and ERCP is an appropriate first-line procedure for both diagnostic and therapeutic purposes.[33] Most of previous studies[21,22,24,26] have investigated this topic in patients who underwent EUS-FNA, whereas results of EUS-FNB are lacking.
To better clarify this issue, we performed a large multicenter retrospective study including 842 jaundiced patients with a pancreatic head lesion who underwent EUS-FNB with or without a previously placed biliary stent, and outcomes of the two groups were compared. Uni- and multivariate analyses were performed in the whole population and in the subgroup of patients with biliary stent, both using strict criteria (classifying samples suspicious for malignancy as negatives) and not strict criteria (classifying samples suspicious for malignancy as positives). The use of strict criteria is relevant because suspicious samples are not conventionally considered sufficient by oncologists to start chemotherapy.
Diagnostic measures were better in the nonstent group, with accuracy decreasing approximately from 92% to 86% and from 96% to 92% in patients with a stent, using strict and not strict criteria, respectively. The robustness of this finding was also confirmed at multivariate analysis which revealed that the absence of biliary stent was independently associated with higher diagnostic accuracy. Evaluating the subgroup of patients with biliary stent, the type of stent did not impact on accuracy, both using strict and not strict criteria. The current study agrees with Kim et al., who reported a decreased yield of EUS-FNA with a biliary indwelling stent, regardless of its type.[24]
In contrast, Bekkali et al. concluded that, by using strict criteria, SEMS significantly impacted the diagnostic accuracy of EUS-TA.[25] However, in the same study, diagnostic accuracy in the plastic stents group was significantly lower than that of the nonstent group (68.9% vs. 78.0%), thus suggesting that plastic stents had some impact on EUS-TA outcome too.[25] However, no relation between the presence of biliary stent and EUS-TA was reported in other studies. Ranney et al. found similar accuracy of EUS-FNA comparing 150 patients with and 64 without biliary stent, regardless of the type of stent.[22] In another study comparing 98 patients with plastic stents and 170 without, similar outcomes in the two groups were reported.[26] However, in the above-mentioned studies,[22,26] suspicious for malignancy results were considered positive, whereas in Bekkali et al.,[25] as in the current study, two criteria were used, and two different analyses were done, documenting that the impact of biliary stent is demonstrated when strict criteria are applied.
Bekkali et al. also identified tumor size, the number of passes, and the use of fork-tip needles as additional factors associated with accuracy.[25] The relation between lesion size and diagnostic yield has been previously reported for the diagnosis of pancreatic lesions.[6,7] In patients with biliary stent, the acoustic shadow/reverberation artifact could completely mask small lesions, making its sampling difficult. Conceivably, it is likely that a portion of larger masses remains visible beside the placement of a stent. In the present study, using both strict and not strict criteria, larger tumor size was associated with better accuracy, supporting previous findings.
As known, increasing the number of passes improves the diagnostic accuracy of EUS-FNA.[14,15] Differently, in the current study, we found number of passes not associated with accuracy, in contrast with Bekkali et al.[24] However, we included only patients who underwent EUS-FNB and it is known that the use of EUS-FNB reduces the number of passes needed to achieve diagnosis, as reported in current guidelines.[1]
Another important factor impacting accuracy raised in Bekkali et al. was the use of the fork-tip needle.[25] However, we didn’t find any difference in accuracy comparing the side-fenestrated with the end-cutting needle. This is consistent with a previous randomized trial where, despite a higher rate of histological specimens collected with fork-tip needles, the accuracy was similar to that obtained using side-fenestrated ones.[34] As a difference, in the current study, using strict criteria, EUS-FNB performed with large bore needles (20G) increased diagnostic accuracy as compared with smaller needles, as previously reported.[5] However, in the group of thinner needles, we included both first and second-generation FNB needles (i.e., reverse-beveled side-fenestrated and end-cutting ones). This reason could explain different results reported in other studies where 22G forward-acquiring needles outperformed 20G forward-bevel side-fenestrated ones.[17,35]
We also evaluated the interval time between ERCP and EUS-FNB in patients with biliary stent. Using not strict criteria, we found a significant correlation with a shorter interval time and lower diagnostic accuracy. Similarly, Fisher et al. found reduced accuracy rate in the group of patients receiving biliary stent <1 day before EUS-FNA.[26] As a reason, we postulated that some pancreatic phlogistic cytological changes related to the close stent implantation could impair pathological interpretations leading to a reduced rate of correct diagnoses.[20]
Considering all the above-mentioned points, together with the reduced EUS capability of correct tumor staging in the presence of biliary stent,[36] we believe that EUS-TA should be strongly recommended before ERCP, especially in patients with small tumors. If biliary stent placement was already performed at the time of EUS-FNB, it could be reasonable to use new generation needles and, if possible, perform EUS-FNB after a few days from ERCP.
The retrospective design represents the main limitation of this study and could have led to selection biases. A statistically significant difference in tumor size was found between the stent and nonstent groups. However, we believe this difference unlikely to have impacted the study's findings because the mean gap was only 2.5 mm, a measure not relevant in clinical practice. On the other hand, a higher number of passes and a higher rate of use of new generation end-cutting FNB needles in the stent group were observed, potentially influencing results in favor of the stent group. Nevertheless, the accuracy in the stent group was inferior. Moreover, a large number of patients and the multivariate analyses, including all covariates previously reported to impact diagnostic accuracy, should reduce the possibility of residual confounding.
In conclusion, in this large retrospective study, a biliary stent negatively impacted the diagnostic accuracy of EUS-FNB for the diagnosis of pancreatic head lesions. In jaundiced patients, preferably, EUS-FNB should precede ERCP, especially in the case of small tumors. When a biliary stent has already been placed, the use of new generation needles could optimize diagnostic yield. Larger prospective and randomized studies are needed to validate these results.
Financial support and sponsorship
Nil.
Conflicts of interest
Alberto Larghi is an Editorial Board Member of the journal. The article was subject to the journal's standard procedures, with peer review handled independently of the editor and his research groups.
REFERENCES
- 1.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline-March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 2.Oppong KW, Bekkali NL, Leeds JS, et al. Fork-tip needle biopsy versus fine-needle aspiration in endoscopic ultrasound-guided sampling of solid pancreatic masses: A randomized crossover study. Endoscopy. 2020;52:454–61. doi: 10.1055/a-1114-5903. [DOI] [PubMed] [Google Scholar]
- 3.Di Leo M, Crinò SF, Bernardoni L, et al. EUS-guided core biopsies of pancreatic solid masses using a new fork-tip needle: A multicenter prospective study. Dig Liver Dis. 2019;51:1275–80. doi: 10.1016/j.dld.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Facciorusso A, Wani S, Triantafyllou K, et al. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest Endosc. 2019;90:893–903.e7. doi: 10.1016/j.gie.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Armellini E, Manfrin E, Trisolini E, et al. Histologic retrieval rate of a newly designed side-bevelled 20G needle for EUS-guided tissue acquisition of solid pancreatic lesions. United Eur Gastroenterol J. 2019;7:96–104. doi: 10.1177/2050640618804443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqui AA, Brown LJ, Hong SK, et al. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig Dis Sci. 2011;56:3370–5. doi: 10.1007/s10620-011-1782-z. [DOI] [PubMed] [Google Scholar]
- 7.Crinò SF, Bellocchi MC, Bernardoni L, et al. Diagnostic yield of EUS-FNA of small (≤15 mm) solid pancreatic lesions using a 25-gauge needle. Hepatobiliary Pancreat Dis Int. 2018;17:70–4. doi: 10.1016/j.hbpd.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Facciorusso A, Mohan BP, Crinò SF, et al. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15:821–8. doi: 10.1080/17474124.2021.1880893. [DOI] [PubMed] [Google Scholar]
- 9.Bang JY, Magee SH, Ramesh J, et al. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–50. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crinò SF, Larghi A, Bernardoni L, et al. Touch imprint cytology on endoscopic ultrasound fine-needle biopsy provides comparable sample quality and diagnostic yield to standard endoscopic ultrasound fine-needle aspiration specimens in the evaluation of solid pancreatic lesions. Cytopathology. 2019;30:179–86. doi: 10.1111/cyt.12662. [DOI] [PubMed] [Google Scholar]
- 11.Chandan S, Mohan BP, Khan SR, et al. Comparison of EUS-guided conventional smear and liquid-based cytology in pancreatic lesions: A systematic review and meta-analysis. Endosc Int Open. 2020;8:E1611–22. doi: 10.1055/a-1240-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crinò SF, Bernardoni L, Manfrin E, et al. Endoscopic ultrasound features of pancreatic schwannoma. Endosc Ultrasound. 2016;5:396–8. doi: 10.4103/2303-9027.195873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ieni A, Todaro P, Crino SF, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology in pancreaticobiliary carcinomas: Diagnostic efficacy of cell-block immunocytochemistry. Hepatobiliary Pancreat Dis Int. 2015;14:305–12. doi: 10.1016/s1499-3872(15)60367-8. [DOI] [PubMed] [Google Scholar]
- 14.Mohamadnejad M, Mullady D, Early DS, et al. Increasing number of passes beyond 4 does not increase sensitivity of detection of pancreatic malignancy by endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2017;15:1071–8.e2. doi: 10.1016/j.cgh.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–81. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 16.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 17.Crinò SF, Di Mitri R, Nguyen NQ, et al. Endoscopic ultrasound-guided fine-needle biopsy with or without rapid on-site evaluation for diagnosis of solid pancreatic lesions: A randomized controlled non-inferiority trial. Gastroenterology. 2021;161:899–9019.e5. doi: 10.1053/j.gastro.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Ross WA, Wasan SM, Evans DB, et al. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc. 2008;68:461–6. doi: 10.1016/j.gie.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 19.Aslanian HR, Estrada JD, Rossi F, et al. Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for obstructing pancreas head masses: Combined or separate procedures? J Clin Gastroenterol. 2011;45:711–3. doi: 10.1097/MCG.0b013e3182045923. [DOI] [PubMed] [Google Scholar]
- 20.Kulesza P, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration: Sampling, pitfalls, and quality management. Clin Gastroenterol Hepatol. 2007;5:1248–54. doi: 10.1016/j.cgh.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui AA, Fein M, Kowalski TE, et al. Comparison of the influence of plastic and fully covered metal biliary stents on the accuracy of EUS-FNA for the diagnosis of pancreatic cancer. Dig Dis Sci. 2012;57:2438–45. doi: 10.1007/s10620-012-2170-z. [DOI] [PubMed] [Google Scholar]
- 22.Ranney N, Phadnis M, Trevino J, et al. Impact of biliary stents on EUS-guided FNA of pancreatic mass lesions. Gastrointest Endosc. 2012;76:76–83. doi: 10.1016/j.gie.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonini F, Fuccio L, Giorgini S, et al. Biliary plastic stent does not influence the accuracy of endoscopic ultrasound-guided sampling of pancreatic head masses performed with core biopsy needles. Dig Liver Dis. 2017;49:898–902. doi: 10.1016/j.dld.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, Walia S, Lee SH, et al. Lower yield of endoscopic ultrasound-guided fine-needle aspiration in patients with pancreatic head mass with a biliary stent. Dig Dis Sci. 2015;60:543–9. doi: 10.1007/s10620-014-3367-0. [DOI] [PubMed] [Google Scholar]
- 25.Bekkali NL, Nayar MK, Leeds JS, et al. Impact of metal and plastic stents on endoscopic ultrasound-guided aspiration cytology and core histology of head of pancreas masses. Endoscopy. 2019;51:1044–50. doi: 10.1055/a-0824-6982. [DOI] [PubMed] [Google Scholar]
- 26.Fisher JM, Gordon SR, Gardner TB. The impact of prior biliary stenting on the accuracy and complication rate of endoscopic ultrasound fine-needle aspiration for diagnosing pancreatic adenocarcinoma. Pancreas. 2011;40:21–4. doi: 10.1097/MPA.0b013e3181f66e64. [DOI] [PubMed] [Google Scholar]
- 27.Rimbaş M, Crino SF, Gasbarrini A, et al. EUS-guided fine-needle tissue acquisition for solid pancreatic lesions: Finally moving from fine-needle aspiration to fine-needle biopsy? Endosc Ultrasound. 2018;7:137–40. doi: 10.4103/eus.eus_23_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 29.Wani S, Muthusamy VR, McGrath CM, et al. AGA white paper: Optimizing endoscopic ultrasound-guided tissue acquisition and future directions. Clin Gastroenterol Hepatol. 2018;16:318–27. doi: 10.1016/j.cgh.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Pitman MB, Layfield L. The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology. Switzerland: Springer International Publishing; 2015. p. 6. [Google Scholar]
- 31.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. 2nd ed. College Station, TX: Stata Press; 2008. [Google Scholar]
- 32.Arya N, Wyse JM, Jayaraman S, et al. A proposal for the ideal algorithm for the diagnosis, staging, and treatment of pancreas masses suspicious for pancreatic adenocarcinoma: Results of a working group of the Canadian Society for Endoscopic Ultrasound. Endosc Ultrasound. 2020;9:154–61. doi: 10.4103/eus.eus_28_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakai Y, Isayama H, Wang HP, et al. International consensus statements for endoscopic management of distal biliary stricture. J Gastroenterol Hepatol. 2020;35:967–79. doi: 10.1111/jgh.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crinò SF, Le Grazie M, Manfrin E, et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest Endosc. 2020;92:648–58.e2. doi: 10.1016/j.gie.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Karsenti D, Palazzo L, Perrot B, et al. 22G Acquire vs. 20G Procore needle for endoscopic ultrasound-guided biopsy of pancreatic masses: A randomized study comparing histologic sample quantity and diagnostic accuracy. Endoscopy. 2020;52:747–53. doi: 10.1055/a-1160-5485. [DOI] [PubMed] [Google Scholar]
- 36.Fusaroli P, Manta R, Fedeli P, et al. The influence of endoscopic biliary stents on the accuracy of endoscopic ultrasound for pancreatic head cancer staging. Endoscopy. 2007;39:813–7. doi: 10.1055/s-2007-966590. [DOI] [PubMed] [Google Scholar]


