Abstract
EUS has become a substantial diagnostic and therapeutic modality for many anatomical regions. The extent of endosonographic assessment is wide, and among others, allows for the evaluation of the mediastinal anatomy and related pathologies such as mediastinal lymphadenopathy and staging of central malignant lung lesions. Moreover, EUS assessment has proved more accurate in detecting small lesions missed by standard imaging examinations such as computed tomography or magnetic resonance. Endosonographically, various mediastinal anatomical landmarks and stations can be visualized by transesophageal scanning, thus providing arranged systematic examination of the mediastinum. In addition, the correct position during the examination is crucial for EUS-guided procedures such as tissue sampling and drainage of mediastinal abscesses. The evolution of EUS-guided diagnostic and interventional procedures has contributed to the increasing importance of understanding the mediastinal anatomy during the EUS examination.
Keywords: anatomy, EUS, linear, mediastinal stations
INTRODUCTION
By definition, the mediastinum is an anatomic region located between the lungs and contains various vascular structures, organs, and lymph nodes (LNs). The evaluation of mediastinum is very valuable, especially for the assessment of the primary and secondary lesions, including pathological LNs.
Endosonographic evaluation of the mediastinum seems to be very challenging. The examination technique consists of fundamental manual skills, including the echoendoscope manipulations and effective rotation and knowledge of the anatomical landmarks to achieve the optimal position. The linear echoendoscope is as effective as the radial one, but the narrow field of view makes the evaluation more challenging. Undoubtedly, EUS has found a wide application in upper and lower digestive tract pathologies. However, the mediastinum examination is still not performed routinely.
Combined imaging and tissue sampling by EUS and endobronchial ultrasound (EBUS) are the techniques of choice in case of suspicious mediastinal LNs invasion, so EUS-FNA/EBUS-FNA is the mainstay for the diagnosis and staging of central lung cancers and metastasis from other malignancies. Furthermore, EUS and EBUS are crucial for the evaluation of other mediastinal lesions.[1] CT and positron emission tomography (PET) scan have limited sensitivity and specificity for mediastinal invasion, so additional tissue acquisition is recommended. Both radial and linear EUS can give similar diagnostic information, but the great advantage of linear EUS is its capability of performing fine-needle aspiration from masses and LNs. EUS-FNA/EBUS-FNA are minimally invasive techniques with a very good safety profile.
This review will focus on the role of mediastinum assessment by the linear EUS, its clinical implications, and advantages over other imaging modalities. The described techniques of linear EUS examination include mapping of the mediastinal LNs as defined by the International Association for the Study of Lung Cancer (IASLC) classification.[2]
ANATOMY OF THE MAIN STATIONS
The esophagus begins at the level of the cricoid cartilage at approximately 15 cm distance from the central incisor teeth. For the purposes of EUS examination of the mediastinum, the esophagus is subdivided into cervical, from cricoid to 18 cm; upper thoracic, from 18 to 25 cm till approximately the tracheal bifurcation; mid-thoracic, from 25 to 32 cm till approximately below the subcarinal area; and lower thoracic, from 32 to 38 cm. The abdominal part of the esophagus is approximately 2 cm long (38–40 cm).[2] The examination of the mediastinum by linear EUS is done by 360° rotation of the scope from five positions in the esophagus.
The IASLC classification defines a total LN stations numbered from 1 to 14. Station 1 is within the root of the neck, Stations 2–9 are within the mediastinum, and Stations 10–14 are within the hila and along the bronchi in the lung parenchyma[2,3] LNs that lie adjacent to the esophagus or centrally located vessels can be visualized by EUS. LNs seen by EUS include:
Low cervical and supraclavicular LNs (LN Station 1)
Upper paratracheal LNs on the left (LN Station 2 L)
Prevascular LNs (LN Station 3A)
Low paratracheal LNs on the left (LN Station 4 L)
Subaortic LNs (LN Station 5)
Para-aortic LNs (LN Station 6)
Subcarinal LNs (LN Station 7)
Lower paraesophageal LNs (LN Station 8)
Pulmonary ligament LNs (LN Station 9)
Hilar LNs (LN Station 10 L and R)
Lower paratracheal LNs on the right (LN Station 4 R).
LN stations seen by either EUS or EBUS or both are demonstrated in Table 1 and Figure 1.
Table 1.
Lymph node stations seen by either EUS or endobronchial ultrasound or both
| LN Stations | Anatomical site | Visualized by |
|---|---|---|
| LN station 1 | Low cervical, supraclavicular, and sternal notch LNs | EUS and EBUS |
| LN station 2L | Upper paratracheal LNs on the left | EUS and EBUS |
| LN station 2R | Upper paratracheal LNs on the right | EBUS |
| LN station 3A | Prevascular LNs | EUS |
| LN station 3P | Prevertebral (retro-tracheal) LNs | EBUS |
| LN station 4L | Low paratracheal LNs on the left | EUS and EBUS |
| LN station 4R | Lower paratracheal LNs on the right; above the arch of the azygos vein | EUS and EBUS |
| LN station 5 | Subaortic LNs | EUS and EBUS |
| LN station 6 | Para-aortic LNs | EUS and EBUS |
| LN station 7 | Subcarinal LNs | EUS and EBUS |
| LN station 8 | Lower paraesophageal LNs | EUS |
| LN station 9 | Pulmonary ligament LNs | EUS |
| LN station 10L | Hilar LNs on the left | EUS and EBUS |
| LN station 10R | Hilar LNs on the right; below the arch of the azygos vein | EUS and EBUS |
| LNs 11-14 | Interlobar, lobar, segmental, and sub-segmental LNs | EBUS |
EBUS: Endobronchial ultrasound; LN: Lymph nodes
Figure 1.
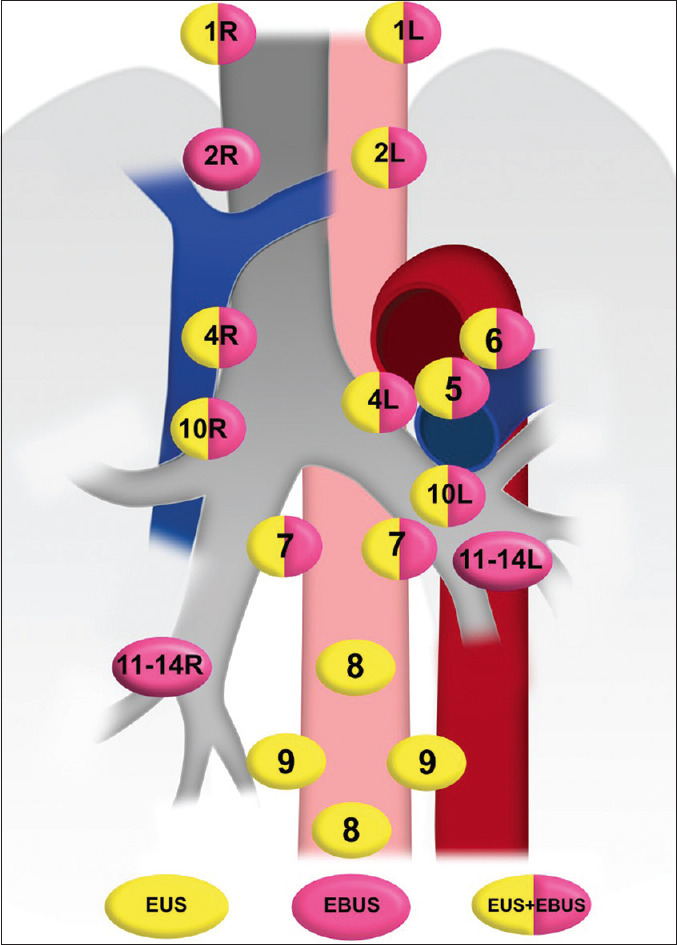
Anatomical stations of the mediastinum seen by EUS, EBUS or both. R: Right, L: Left; EBUS: Endobronchial Ultrasound
MEDIASTINUM EXAMINATION
The particular steps of the mediastinal examination may vary. However, considering the most critical areas and structures is essential for performing a competent examination.
The optimal position for the first step of assessment of the mediastinum is the cardia, where the liver and the uppermost part of inferior vena cava (IVC) can be observed. We can follow the insertion of IVC into the right atrium by withdrawing the echoendoscope about few centimeters into the chest with mild counterclockwise rotation where Station 9 is located [Figure 2].
Figure 2.
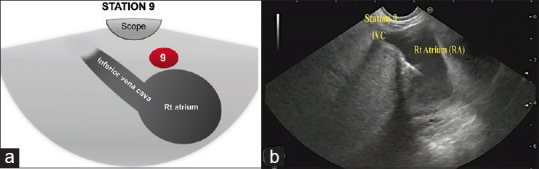
(a) Diagram of the anatomical location of Station 9 (b) Station 9 as seen by linear EUS. Rt: Right; IVC: Inferior vena cava
Gradual withdrawal of the scope with further counterclockwise rotation will bring the left atrium (LA) and then the right pulmonary artery (RPA) into the left and right parts of the screen, respectively, this is the subcarinal area and Station 7 is located in-between LA and RPA, on the depth of 27–32 cm from the incisors [Figure 3]. In this region, we can observe LA, left ventricle (LV) with the outgoing ascending aorta, and just above and below, RPA and pulmonary trunk, respectively [Figure 3]. When the ascending aorta is visualized coming from LV, clockwise rotation will locate the superior vena cava (SVC) to the right side of the screen going to RA on the left part of the screen. Station 10R could be visualized in the right side of the screen in-between the scope and SVC, about 25–27 cm from the incisors [Figure 4]. The subcarinal area requires more time for a complete assessment, due to a possible location of lung, pancreatic, and stomach cancer metastases.
Figure 3.
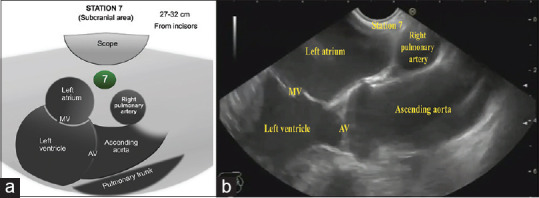
(a) Diagram of the anatomical location of the subcarinal area and station 7. (b) Subcarinal area and station 7 as seen by linear EUS. MV: Mitral Valve; AV: Aortic Valve
Figure 4.
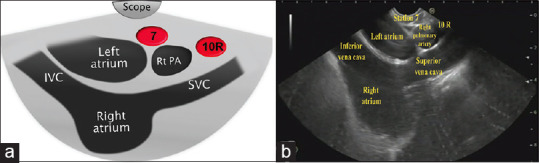
(a) Diagram of the anatomical location of station 10R (b) Station 10R as seen by linear EUS. IVC: Inferior vena cava; SVC: Superior vena cava; Rt PA: Right Pulmonary artery; 10R: 10 Right
Then slow 90° clockwise rotation allows visualizing the azygos vein, and further, 90° clockwise rotation will visualize the lower part of the descending thoracic aorta where Station 8 is located [Figure 5]. After going back to the subcarinal area by 180° counterclockwise rotation, gradual withdrawal of the scope with counterclockwise rotation, approximately 23–27 centimeters form incisors, allows achieving the position of the aorto-pulmonary window. This area includes left pulmonary artery (LPA) and aortic arch [Figure 6]. Both structures can be also visible as a “mickey mouse” sign. Stations 4 L and 5 are located in-between LPA and aortic arch, near and far from the scope, respectively. Station 6 is located deep to the aortic arch, whereas Station 10 L is located deep to LPA on the left part of the screen [Figure 6].
Figure 5.
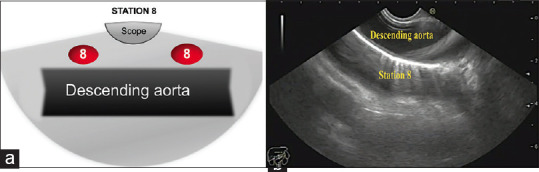
(a) Diagram of the anatomical location of station 8. (b) Station 8 as seen by linear EUS.
Figure 6.
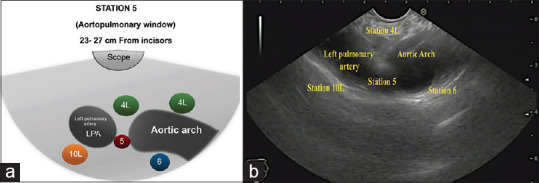
(a) Diagram of the anatomical location of the aorto-pulmonary window (A-P window) and Stations 4L, 5, 6, and 10L (b) The A-P window and Stations 4L, 5, 6, and 10L as seen by linear EUS. LPA: Left pulmonary artery; 4L: 4 Left; 10L: 10 Left
By gradual withdrawal of the scope, the origin of the left subclavian artery from the aortic arch can be visualized, Station 2 L is located between LSA and the scope, about 20–23 cm from the incisors [Figure 7].
Figure 7.
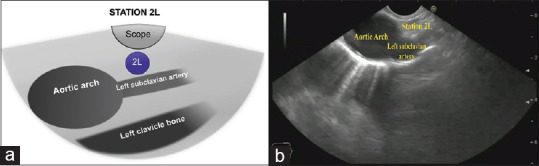
(a) Diagram of the anatomical location of station 2L. (b) Station 2L as seen by linear EUS. 2L: 2 Left
Clockwise rotation of the scope with gradual withdrawal of the scope will locate the left common carotid artery and the confluence of the left internal jugular, the left subclavian and the left brachiocephalic veins. Station 3A is located anterior to the venous confluence in the most distant part of the screen [Figure 8].
Figure 8.
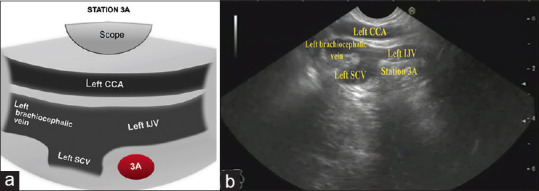
(a) Diagram of the anatomical location of Station 3A (b) Station 3A as seen by linear EUS. SCV: Subclavian vein; IJV: Internal jugular vein; CCA: Common carotid artery; 3A: 3 Anterior
Further withdrawing the echoendoscope toward the neck guides to Station 1, where the left and right lobes of the thyroid glands are visible with the right and left common carotid artery and internal jugular vein [Figure 9].
Figure 9.
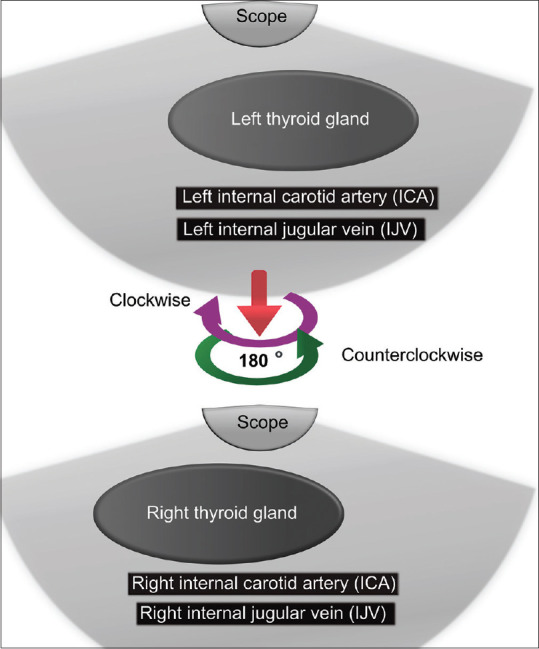
Diagram of the anatomical location of station 1 in the neck.
Table 2 demonstrates the landmarks of different LNs stations in the neck and the mediastinum.
Table 2.
EUS examination of lymph nodes stations in the mediastinum and the neck starting from the cardia up to the upper part of the esophagus
| Number of station | The distance from incisors, location | Landmark (s) |
|---|---|---|
| Station 9 [Figure 2] | 35-40 cm from the incisors Just above the cardia |
IVC Right atrium |
| Station 7 [Figure 3] | 27-32 cm from the incisors The subcarinal area |
Left atrium Left ventricle Ascending aorta Right pulmonary artery Pulmonary trunk |
| Station 10R [Figure 4] | 25-27 cm from the incisors Clockwise rotation from station 7 |
Superior vena cava |
| Station 8 [Figure 5] | 180° clockwise rotation from station 7 | Descending aorta |
| Station 4L, 5, 6, and 10L [Figure 6] | 23-27 cm form incisors Aorto-pulmonary window Upward withdrawal of the scope with counterclockwise rotation from station 7 |
Left pulmonary artery Aortic arch |
| Station 2L [Figure 7] | 20-23 cm from the incisors Upward withdrawal of the scope from station 5 |
Left subclavian artery |
| Station 3A [Figure 8] | 20-23 cm from the incisors, clockwise and slight upward withdrawal from station 2L | Common carotid artery, Venous confluence of left IJV, SCV and BCV |
| Station 1 [Figure 9] | Upward withdrawal of the scope towards the neck from station 3A | Thyroid gland Common carotid artery IJV |
IVC: Inferior vena cava; IJV: Internal jugular vein; SCV: Subclavian vein; BCV: Brachiocephalic vein
CLINICAL IMPLICATIONS OF EUS MEDIASTINAL EXAMINATION
Role of EUS in the diagnosis of different mediastinal pathologies
EUS for the diagnosis of lung cancer
Histopathological diagnosis of lung cancer is an indispensable part of the treatment. Flexible bronchoscopy remains the recommended method when it comes to centrally located lung lesions.[4] However, a substantial number of patients do not reveal endobronchial abnormalities and are out of reach of this method.[4] EBUS with real time-guided transbronchial needle aspiration (EBUS-TBNA) can be used for the diagnosis of intrapulmonary tumors located near the major airways; however, distance to the lesion is a crucial factor conditioning success.[5] In those cases, when the lesion is not close to airways, CT-guided transthoracic needle aspiration remains an option. However, it is accompanied by a significant risk of pneumothorax and hemoptysis.[6] A systematic review by Korevaar et al. evaluated the performance of EUS-FNA in the diagnosis of intrapulmonary tumors located near or adjacent to the esophagus.[7] The authors included 313 patients coming from 11 studies and reported an average yield of 0.90 (95% confidence interval 0.82–0.95) and an average sensitivity of 0.92 (0.83–0.96). In the subgroup of prospective studies (n = 3), the average yield was 0.80 (0.56–0.93) and the average sensitivity was 0.83 (0.58–0.95). Together with relatively low adverse effect rates (2%), these findings suggest that EUS-FNA is safe and has a high yield for diagnosing intrapulmonary tumors.
Furthermore, the confirmation of mediastinal LN metastases in cases of potentially curable non-small cell lung cancer (NSCLC) is crucial. The combination of EBUS-TBNA and EUS-FNA is preferred over either test alone.[8]
Lymphadenopathy of unknown origin
According to the guidelines of the European Society of Gastrointestinal Endoscopy EUS-guided (or EBUS) sampling is recommended for lymphadenopathy of unknown origin if the pathological result is likely to affect patient management and no easily accessible superficial lymphadenopathy.[9] In available large observations, EUS-guided sampling was shown to have high sensitivity (88%–95.3%), specificity (96%–100%), negative predictive value (70%–80%), and positive predictive value (100%) for diagnosing the cause of LNs enlargement[10,11,12] In contrary to CT-guided biopsy and mediastinoscopy, EUS-guided sampling is a less expensive and a less invasive option with a low adverse incidence ratio[13,14] Sample processing (flow cytometry in suspicion of lymphoma or sarcoidosis, polymerase chain reaction-assay in suspected mycobacterial tuberculosis [TB]) plays a crucial role in evaluating LNs enlargement of unknown origin.[15,16,17]
Metastatic mediastinal lymph nodes
EUS is considered a valuable tool for detecting metastatic LNs in patients in whom this could affect cancer staging and further management.[18] Many neoplasms metastasize to mediastinal LNs. The most commonly encountered in everyday practice are lung (especially NSCLCs), esophagus, breast, thyroid, and renal cell cancer.[19] There are some high-suspicion stigmata on EUS-imaging which suggest the malignant-metastatic origin of LNs. These imaging features are as follows: Diameter >1 cm, round shape, sharp border, loss of hyperechoic hilum, and homogeneous echopoor pattern. Nevertheless, neither the morphology of LNs on conventional imaging modalities or EUS nor physiological imaging modalities such as PET and single-photon emission computed tomography (CT) was effectively differentiated malignant from nonmalignant etiologies of LNs.[20] Therefore, tissue acquisition is considered the gold standard for the diagnosis of the mediastinal LNs.[21]
Station 5 and 7 nodes sampling by EUS are accessible and safe while EUS-guided FNA of Station 6 lymphadenopathy through transaortic approach has been reported, but further studies are needed to assess the maneuver's safety.[22]
Lung cancer with ipsilateral or subcarinal LN involvement (N2 disease) is associated with a 5-year survival of 7%–13% and usually precludes resection but may identify a subset of patients who might benefit from neoadjuvant therapy. Contralateral nodal disease (N3) is surgically incurable with fewer than 5% of patients surviving 5 years.[23]
In esophageal cancer patients, more advanced disease is frequently encountered, and the presence and number of LNs are the most significant prognostic factor for staging and prediction of the long-term survival of the patients. There are several factors that determine the outcome significance of the LN location. Among these factors include histology, primary tumor location, T-stage, and neo-adjuvant therapy.[24] The complexity of lymphatic network surrounding the vessels in the mediastinum contributes to multidirectional spread in this area as well as the abdomen and neck. In addition, “skip metastasis” which do not follow the normal order of LN array by skipping the first LN and spread to the 2nd level LN are frequently encountered in both histological subtypes of esophageal cancer, adenocarcinoma, and squamous cell carcinoma. Consequently, unexpected distant site of LN metastasis can complicate the way of standardized resection and lymphadenectomy, hence making treatment of esophageal cancer more difficult.[25,26]
Mediastinal cystic lesions
Cystic masses of the mediastinum are heterogeneous groups of lesions, including congenital abnormalities, infections, and neoplasms and account for 15%–20% of all mediastinal masses.[27] The diagnosis of mediastinal cystic lesions is difficult as benign lesions can mimic cancer manifestations in CT or magnetic resonance imaging (MRI) evaluation.[28] Although available data are scarce, EUS-guided sampling seems to have tremendous diagnostic potential in the differentiation of those lesions. A recent retrospective study by Zhao et al. described 37 patients who underwent EUS-FNA of mediastinal cystic lesions.[27] Cyst fluid was collected and further examined using cytological and biochemical techniques. Interestingly, the authors revealed that prior CT or MRI mistakenly distinguished eight cases as solid masses (27.03%), but EUS revealed cystic characteristics. Moreover, they suggested that elevated LDH value from cyst fluid chemical analysis could be used as an auxiliary indicator for diagnosing malignancy.
EUS-guided access to the heart and pericardial area
Because of anatomical conditions, access to the heart and heart-surrounding area is feasible through the esophagus under EUS-guided techniques. The first reported EUS-guided experimental heart interventions were successfully performed by Fritscher-Ravens et al. in a porcine model.[29] There are only few reports considering advanced EUS-guided cardiac tissue acquisition or therapy in humans. Furthermore, EUS-FNA was performed in cases of right-arterial masses and primary pericardial neoplasms. There is also a report about a successful EUS-guided therapeutic drainage of a pericardial cyst.[30,31,32]
Systemic and infectious diseases
Sarcoidosis
Is a systemic granulomatous disease of unknown cause that results in inflammatory noncaseating granuloma. It might be asymptomatic with indolent course or it can be presented by bilateral hilar adenopathy, shortness of breath, fever, and fatigue. The role of EUS in diagnosing sarcoidosis is not defined as EUS-FNA is substantially made to exclude malignancy.[33,34]
Lymphoma
Mediastinal adenopathy due to lymphoma represents a major clinical challenge in the diagnosis as well as when obtaining tissue biopsy is being decided. EUS-FNA may be helpful in diagnosing mediastinal lymphoma; however, the sensitivity is reported at 73%–80% 39–42 and excisional biopsy should be performed when FNA is negative.[35,36] Increasingly, the cytology may not provide prognostic information about the certain types of lymphoma in comparison to that can be obtained when tissue biopsy is made. A 19G needle can provide tissue acquisition for pathological examination.[37,38,39]
Further studies are required to provide information about the significance of EUS-FNA and FNB in the evaluation of mediastinal adenopathy ascribed to lymphoma.
Infections
TB and other systemic infection can cause mediastinal lymphadenopathy and its diagnosis using EUS-FNA had been reported.[40,41,42] If systemic infection has been suspected, the specimen obtained by EUS-FNA should be rendered for acid-fast stain, culture for TB and fungal culture as well. Complications as mediastinal-esophageal fistula have been reported after EUS-FNA.[43] Further studies are needed to evaluate the role of EUS-FNA to diagnose mediastinal TB.
In their study, Junare et al. evaluated the efficacy of combined EUS FNA/FNB of mediastinal lymphadenopathy. The sensitivity and specificity were 100% for reactive lymphadenitis, sarcoidosis, lymphoma, and metastatic LNs, whereas the sensitivity and specificity for TB lymphadenitis reached 93.75% and 100%, respectively.[44] This denotes the importance of EUS-guided FNA/FNB in the diagnosis of mediastinal lymphadenopathy and that it can be a safe alternative to surgical biopsy.
EUS-guided mediastinal paraesophageal abscess drainage
Esophageal perforations and intrathoracic anastmotic dehiscence carry high risk of morbidity and mortality reaching 10%–35%.[45] Contained intrathoracic esophageal transmural disruptions and leakage can be treated in a conservative manner.[46] However, morbidity and mortality rates increase when mediastinal abscess has formed; hence, a complicated course and prolonged hospital stay will ensue. Wehrmann et al. in their single-center case series demonstrated the feasibility and safety of EUS-guided drainage of paraesophgeal and mediastinal abscess.[47] Furthermore, Fritscher-Ravens et al. in their case report have described a successful EUS-guided mediastinal abscess drainage as a sequel of percutaneous dilatational tracheotomy.[48] In addition, Kahaleh et al. illustrated the safety of EUS-guided retrocarinal collection drainage at the site of gastroesophageal anastomosis after Ivory-Lewis esophagectomy for severe esophageal stricture not responding to conventional endoscopic therapy.[49] In whole, EUS-guided drainage of mediastinal abscess can be a safe and feasible alternative to surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
Hussein Hassan Okasha is an Editorial Board Member of the journal. The article was subject to the journal's standard procedures, with peer review handled independently of this editor and his research groups.
REFERENCES
- 1.Jenssen C, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part 2: Mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis. 2015;7:E439–58. doi: 10.3978/j.issn.2072-1439.2015.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanoue LT. Staging of non-small cell lung cancer. Semin Respir Crit Care Med. 2008;29:248–60. doi: 10.1055/s-2008-1076745. [DOI] [PubMed] [Google Scholar]
- 3.Sharma M, Pathak A, Shoukat A, et al. Imaging of spaces of neck and mediastinum by endoscopic ultrasound. Lung India. 2016;33:292–305. doi: 10.4103/0970-2113.180866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;143(5 Suppl):e142S–65S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert C, Akulian J, Ortiz R, et al. Novel bronchoscopic strategies for the diagnosis of peripheral lung lesions: Present techniques and future directions. Respirology. 2014;19:636–44. doi: 10.1111/resp.12301. [DOI] [PubMed] [Google Scholar]
- 6.Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis. 2014;6(Suppl 1):S99–107. doi: 10.3978/j.issn.2072-1439.2013.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korevaar DA, Colella S, Spijker R, et al. Esophageal endosonography for the diagnosis of intrapulmonary tumors: A systematic review and meta-analysis. Respiration. 2017;93:126–37. doi: 10.1159/000452958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilmann P, Clementsen P, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) Endoscopy. 2015;47:545–59. doi: 10.1055/s-0034-1392040. [DOI] [PubMed] [Google Scholar]
- 9.Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline-Updated January 2017. Endoscopy. 2017;49:695–714. doi: 10.1055/s-0043-109021. [DOI] [PubMed] [Google Scholar]
- 10.Hirdes MM, Schwartz MP, Tytgat KM, et al. Performance of EUS-FNA for mediastinal lymphadenopathy: Impact on patient management and costs in low-volume EUS centers. Surg Endosc. 2010;24:2260–7. doi: 10.1007/s00464-010-0946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanisaka Y, Ryozawa S, Kobayashi M, et al. Usefulness of endoscopic ultrasound-guided fine needle aspiration for lymphadenopathy. Oncol Lett. 2018;15:4759–66. doi: 10.3892/ol.2018.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. Endoscopic ultrasound: It's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–37. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehmood S, Loya A, Yusuf MA. Clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of mediastinal and intra-abdominal lymphadenopathy. Acta Cytol. 2013;57:436–42. doi: 10.1159/000351474. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Chen Q, Wu X, et al. Role of endoscopic ultrasound-guided fine-needle aspiration in evaluating mediastinal and intra-abdominal lymphadenopathies of unknown origin. Oncol Lett. 2018;15:6991–9. doi: 10.3892/ol.2018.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: The GRANULOMA randomized clinical trial. JAMA. 2013;309:2457–64. doi: 10.1001/jama.2013.5823. [DOI] [PubMed] [Google Scholar]
- 16.Jin M, Wakely PE., Jr Endoscopic/endobronchial ultrasound-guided fine needle aspiration and ancillary techniques, particularly flow cytometry, in diagnosing deep-seated lymphomas. Acta Cytol. 2016;60:326–35. doi: 10.1159/000447253. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwoudt M, Lameris R, Corcoran C, et al. Polymerase chain reaction amplifying mycobacterial DNA from aspirates obtained by endoscopic ultrasound allows accurate diagnosis of mycobacterial disease in HIV-positive patients with abdominal lymphadenopathy. Ultrasound Med Biol. 2014;40:2031–8. doi: 10.1016/j.ultrasmedbio.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Jiang C. Endoscopic ultrasound in the diagnosis of mediastinal diseases. Open Med (Wars) 2015;10:560–5. doi: 10.1515/med-2015-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai A, Gress F. Clinical Gastrointestinal Endoscopy. 3rd ed. Philadelphia: Elsevier; 2019. Extraintestinal endosonography. [Google Scholar]
- 20.Jamil LH, Kashani A, Scimeca D, et al. Can endoscopic ultrasound distinguish between mediastinal benign lymph nodes and those involved by sarcoidosis, lymphoma, or metastasis? Dig Dis Sci. 2014;59:2191–8. doi: 10.1007/s10620-014-3164-9. [DOI] [PubMed] [Google Scholar]
- 21.Fritscher-Ravens A, Sriram PV, Bobrowski C, et al. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol. 2000;95:2278–84. doi: 10.1111/j.1572-0241.2000.02243.x. [DOI] [PubMed] [Google Scholar]
- 22.von Bartheld MB, Rabe KF, Annema JT. Transaortic EUS-guided FNA in the diagnosis of lung tumors and lymph nodes. Gastrointest Endosc. 2009;69:345–9. doi: 10.1016/j.gie.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1711–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 24.Castoro C, Scarpa M, Cagol M, et al. Nodal metastasis from locally advanced esophageal cancer: How neoadjuvant therapy modifies their frequency and distribution. Ann Surg Oncol. 2011;18:3743–54. doi: 10.1245/s10434-011-1753-9. [DOI] [PubMed] [Google Scholar]
- 25.Akutsu Y, Matsubara H. Lymph node dissection for esophageal cancer. Gen Thorac Cardiovasc Surg. 2013;61:397–401. doi: 10.1007/s11748-013-0237-1. [DOI] [PubMed] [Google Scholar]
- 26.Mariette C, Piessen G. Oesophageal cancer: How radical should surgery be? Eur J Surg Oncol. 2012;38:210–3. doi: 10.1016/j.ejso.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Wang R, Wang Y, et al. Application of endoscopic ultrasound-guided-fine needle aspiration combined with cyst fluid analysis for the diagnosis of mediastinal cystic lesions. Thorac Cancer. 2019;10:156–62. doi: 10.1111/1759-7714.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeung MY, Gasser B, Gangi A, et al. Imaging of cystic masses of the mediastinum. Radiographics. 2002;22(Suppl 1):S79–93. doi: 10.1148/radiographics.22.suppl_1.g02oc09s79. [DOI] [PubMed] [Google Scholar]
- 29.Fritscher-Ravens A, Ganbari A, Mosse CA, et al. Transesophageal endoscopic ultrasound-guided access to the heart. Endoscopy. 2007;39:385–9. doi: 10.1055/s-2007-966440. [DOI] [PubMed] [Google Scholar]
- 30.Romero-Castro R, Rios-Martin JJ, Jimenez-Garcia VA, et al. EUS-FNA of 2 right atrial masses. VideoGIE. 2019;4:323–4. doi: 10.1016/j.vgie.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sisman G, Barbur E, Şahin D. Endoscopic ultrasonography-guided diagnosis of pericardial gastrointestinal stromal tumor: First case in the literature (with video) Dig Endosc. 2019;31:463. doi: 10.1111/den.13398. [DOI] [PubMed] [Google Scholar]
- 32.Larghi A, Stobinski M, Galasso D, et al. EUS-guided drainage of a pericardial cyst: Closer to the heart (with video) Gastrointest Endosc. 2009;70:1273–4. doi: 10.1016/j.gie.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 33.von Bartheld MB, Veselic-Charvat M, Rabe KF, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of sarcoidosis. Endoscopy. 2010;42:213–7. doi: 10.1055/s-0029-1243890. [DOI] [PubMed] [Google Scholar]
- 34.Michael H, Ho S, Pollack B, et al. Diagnosis of intra-abdominal and mediastinal sarcoidosis with EUS-guided FNA. Gastrointest Endosc. 2008;67:28–34. doi: 10.1016/j.gie.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 35.Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol. 2010;5:804–9. doi: 10.1097/jto.0b013e3181d873be. [DOI] [PubMed] [Google Scholar]
- 36.Hehn ST, Grogan TM, Miller TP. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004;22:3046–52. doi: 10.1200/JCO.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 37.Ribeiro A, Pereira D, Escalón MP, et al. EUS-guided biopsy for the diagnosis and classification of lymphoma. Gastrointest Endosc. 2010;71:851–5. doi: 10.1016/j.gie.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 38.Eloubeidi MA, Mehra M, Bean SM. EUS-guided 19-gauge trucut needle biopsy for diagnosis of lymphoma missed by EUS-guided FNA. Gastrointest Endosc. 2007;65:937–9. doi: 10.1016/j.gie.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 39.Berger LP, Scheffer RC, Weusten BL, et al. The additional value of EUS-guided Tru-cut biopsy to EUS-guided FNA in patients with mediastinal lesions. Gastrointest Endosc. 2009;69:1045–51. doi: 10.1016/j.gie.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 40.Puri R, Vilmann P, Sud R, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462–7. doi: 10.1055/s-0029-1244133. [DOI] [PubMed] [Google Scholar]
- 41.Berzosa M, Tsukayama DT, Davies SF, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14:578–84. [PubMed] [Google Scholar]
- 42.Sendino O, Pellisé M, Ghita G, et al. Aspergillus mediastinitis diagnosed by EUS-guided FNA. Gastrointest Endosc. 2008;67:153. doi: 10.1016/j.gie.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 43.von Bartheld MB, van Kralingen KW, Veenendaal RA, et al. Mediastinal-esophageal fistulae after EUS-FNA of tuberculosis of the mediastinum. Gastrointest Endosc. 2010;71:210–2. doi: 10.1016/j.gie.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Junare PR, Jain S, Rathi P, et al. Endoscopic ultrasound-guided-fine-needle aspiration/fine-needle biopsy in diagnosis of mediastinal lymphadenopathy – A boon. Lung India. 2020;37:37–44. doi: 10.4103/lungindia.lungindia_138_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zwischenberger JB, Alpard SK, Orringer MB. Esophagus. In: Townsend CM Jr, editor. Sabiston Textbook of Surgery. 16th ed. Philadelphia: WB Saunders; 2001. pp. 723–7. [Google Scholar]
- 46.Cameron JL, Kieffer RF, Hendrix TR, et al. Selective nonoperative management of contained intrathoracic esophageal disruptions. Ann Thorac Surg. 1979;27:404–8. doi: 10.1016/s0003-4975(10)63335-8. [DOI] [PubMed] [Google Scholar]
- 47.Wehrmann T, Stergiou N, Vogel B, et al. Endoscopic debridement of paraesophageal, mediastinal abscesses: A prospective case series. Gastrointest Endosc. 2005;62:344–9. doi: 10.1016/j.gie.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Fritscher-Ravens A, Sriram PV, Pothman WP, et al. Bedside endosonography and endosonography-guided fine-needle aspiration in critically ill patients: A way out of the deadlock? Endoscopy. 2000;32:425–7. doi: 10.1055/s-2000-9001. [DOI] [PubMed] [Google Scholar]
- 49.Kahaleh M, Yoshida C, Kane L, Yeaton P. EUS drainage of a mediastinal abscess. Gastrointest Endosc. 2004;60:158–60. doi: 10.1016/s0016-5107(04)01310-0. [DOI] [PubMed] [Google Scholar]


