Abstract
Background and Objectives:
EUS-guided gallbladder drainage (GBD) has emerged as an alternative GBD technique, particularly for high-risk surgical patients. To prevent stent migration or to facilitate stent deployment, the lumen-apposing metal stent (LAMS) was recently developed for EUS-GBD. However, LAMS remains unavailable in several countries and is expensive compared with conventional fully covered self-expandable metal stent (FCSEMS). Although several studies have shown the clinical benefits of EUS-GBD using novel FCSEMS or LAMS compared with endoscopic transpapillary GBD (ETGBD), the choice between ETGBD and EUS-GBD using conventional FCSEMS and ETGBD remains controversial. The aim of this study was to evaluate EUS-GBD using conventional FCSEMS compared with ETGBD.
Materials and Methods:
This comparative retrospective study included consecutive symptomatic AC patients who underwent gallbladder drainage by either EUS-GBD or ETGBD between January 2015 and December 2018. The main outcome measures were technical success, clinical success, procedure-related and stent-related adverse events, and recurrence of AC during follow-up.
Results:
Fifty-four patients (44.4% female, 55.6% male) who underwent EUS-GBD (n = 25) or ETGBD (n = 29) were enrolled. Initial technical success rates were 100% with EUS-GBD and 82.7% (24/29) with ETGBD. The median procedure time was significantly shorter for the EUS-GBD group than for the ETGBD group (11.0 vs. 24.0 min, P < 0.05). Procedure-related adverse events did not differ significantly between groups (P = 0.283). During follow-up (median 522 days, range 43 – 1892 days), recurrent acute cholecystitis (AC) was only observed in 4 patients from the ETGBD group. Overall survival did not differ significantly between the EUS-GBD group (mean 1070 days) and ETGBD group (mean 1470 days) (P = 0.292).
Conclusion:
The technical success rate for EUS-GBD using FCSEMS with plastic stent insertion was significantly higher with a shorter procedure time and resulted in a lower rate of recurrent AC.
Keywords: acute cholecystitis, ERCP, endoscopic retrograde gallbladder drainage, EUS, EUS-guided gallbladder drainage
INTRODUCTION
Although the gold standard in the treatment of acute cholecystitis (AC) is laparoscopic cholecystectomy (LC) according to the 2018 update of the Tokyo guidelines (TG18),[1] alternative treatment methods are being investigated clinically due to various factors associated with patient conditions, such as complicating advanced malignancy or other severe comorbidities. For drainage of the gallbladder, percutaneous transhepatic gallbladder drainage (PTGBD) has traditionally been selected.[2,3] With recent improvements in endoscopic devices and techniques, gallbladder drainage (GBD) under ERCP is also effective. The technical success rate of endoscopic transpapillary gallbladder drainage (ETGBD) is reportedly high,[4,5] but this method shows disadvantages such as technical complexity and a risk of post-ERCP pancreatitis. Moreover, if cystic duct obstruction is present as a complication due to huge stones or carcinoma, ETGBD itself might be not indicated.
Recently, EUS-guided GBD has emerged as an alternative GBD technique, especially for high-risk surgical patients. Conventionally, EUS-GBD is attempted using an external drainage device with an internal plastic stent. However, external drainage exhibits other disadvantages, such as the risks of self-tube removal and cosmetic issues. In addition, because the diameter of the plastic stent is small, stent patency may be limited. Given this background, several authors have described the utility of EUS-GBD using a fully covered self-expandable metal stent (FCSEMS),[6,7] although FCSEMS has still not been approved for use EUS-GBD in Japan. A novel FCSEMS has recently been described, mostly by Korean groups.[8,9] More recently, to facilitate stent deployment and prevent stent migration, the lumen-apposing metal stent (LAMS) was developed not only for pancreatic fluid collection drainage, but also for EUS-guided biliary drainage including EUS-GBD.[10,11,12,13,14,15] However, LAMS and novel FCSEMS remain unavailable in several countries for use as EUS-GBD stent. In addition, LAMS is expensive compared with conventional FCSEMS. Conventional FCSEMS thus remain important as EUS-GBD stent in some countries. Although several studies have shown the clinical benefits of EUS-GBD using novel FCSEMS or LAMS compared with ETGBD, the choice between EUS-GBD using a conventional FCSEMS and ETGBD remains controversial, and long-term results associated with recurrence of AC are also unclear.
The aim of this study was to evaluate EUS-GBD using conventional FCSEMS compared with ETGBD.
PATIENTS AND METHODS
Patients
This comparative retrospective study included consecutive symptomatic AC patients who underwent GBD by either EUS-GBD or ETGBD between January 2015 and December 2018. Inclusion criteria were as follows: (a) moderate or severe AC patients according to TG18;[16] (b) high-risk of requiring surgery due to severe comorbidities; and (c) age ≥20 years. Exclusion criteria were: (a) prior GBD using PTGBD; (b) combination with external drainage such as endoscopic retrograde nasal biliary drainage; (c) performance of surgery after ETGBD or EUS-GBD; or (d) pregnancy. All included patients provided written informed consent to participate in all procedures associated with the study. This study was approved by the Institutional Review Board at Osaka Medical College (IRB-no. 2801).
ETGBD was firstly considered as the drainage method. However, if the patients were complicated with ascites around the hepatic parenchyma, or cystic duct obstruction due to stones or tumor was suggested on to computed tomography (CT), EUS-GBD was considered as the alternative drainage method.
Technical tips for ETGBD
A duodenoscope (JF 260V; Olympus Medical Systems, Tokyo, Japan) was advanced into the duodenum, and biliary cannulation was also attempted using an ERCP catheter (MTW, Endoskopie, Wesel, Germany). After successful biliary cannulation, contrast medium was injected. The 0.025-inch guidewire was then inserted into the common bile duct, and guidewire insertion through the cystic duct was attempted, to access the gallbladder [Figure 1a]. A flexible guidewire (VisiGlide 2; Olympus Medical Systems) was selected. If guidewire insertion failed, we first changed to a more flexible guidewire (Radifocus; Terumo, Tokyo, Japan). On the other hand, if the cystic duct orifice could not be identified on cholangiography, a digital single-operator cholangioscope (SPY DS; Boston Scientific, Tokyo, Japan) was inserted into the common bile duct [Figure 1b]. After the cystic duct orifice was identified under direct visualization, guidewire insertion was attempted [Figure 1c]. After successful guidewire insertion into the gallbladder, a plastic stent (7-Fr × 12 or 15 cm, double pig-tail type; Gadelius Medical, Tokyo, Japan) was performed from the gallbladder into the duodenum [Figure 1d]. In this study, initial technical success of ETGBD was defined as successful stent deployment without SPY DS guidance, because of its high cost. Overall technical success was defined as successful stent deployment, including those patients who underwent ETGBD using SPY DS.
Figure 1.
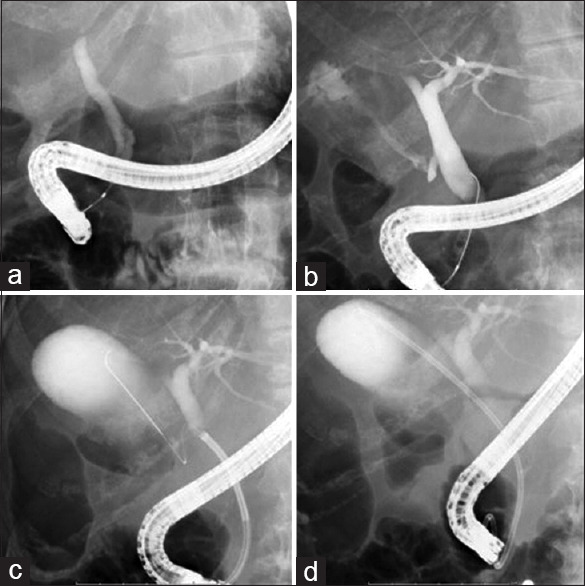
(a) After successful biliary cannulation, the 0.025-inch guidewire is inserted into the common bile duct. (b) If the cystic duct orifice cannot be identified on cholangiography, a digital single-operator cholangioscope (SPY DS; Boston Scientific, Tokyo, Japan) is inserted into the common bile duct. (c) The guidewire insertion is inserted into the gallbladder. (d) Plastic stent deployment is performed from the gallbladder into the duodenum
Technical tips for EUS-GBD
The echoendoscope (GF-UCT 260; Olympus Medical Systems) was inserted into the duodenum. The gallbladder was then identified and punctured using a 19-G fine needle aspiration (FNA) needle (SonoTip Pro Control; Medi-Globe, Rosenheim, Germany) with color Doppler imaging to avoid puncturing intervening blood vessels [Figure 2a]. After aspirating bile juice, contrast medium was injected. The 0.025-inch guidewire (VisiGlide; Olympus Medical Systems) was also inserted into the gallbladder through the FNA needle [Figure 2b]. The gallbladder and intestinal wall were then dilated using an ERCP catheter or 4-mm biliary dilation balloon catheter (REN; Kaneka Corporation, Osaka, Japan). After fistula dilation, a FCSEMS (10 mm × 6 cm, BONA biliary stent; Standard Sci Tech, Seoul, Korea) was deployed from the gallbladder to the intestine via intra-scope channel release, as described previously [Figure 2c].[16] Finally, a 7-Fr double-pig plastic stent was inserted into the FCSEMS to prevent food impaction and to prevent the FCSEMS from becoming stuck to the gallbladder wall [Figure 2d].
Figure 2.
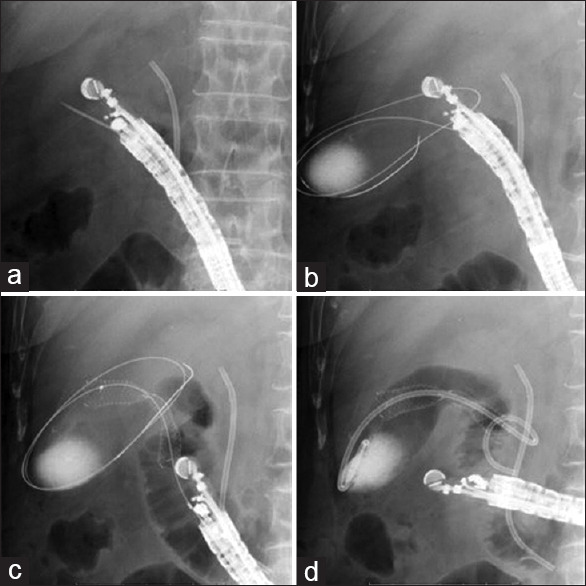
(a) The gallbladder is punctured using a 19-G fine needle aspiration (FNA) needle. (b) The 0.025-inch guidewire is inserted into the gallbladder through the FNA needle. (c) A fully covered self-expandable metal stent (FCSEMS) is deployed from the gallbladder to the intestine. (d) A 7-Fr double-pig plastic stent is inserted into the FCSEMS to prevent food impaction and stacking to the gallbladder wall
Follow-up after each procedure
CT was performed 1 day after EUS-GBD for all patients to detect early adverse events such as stent migration or dislocation. Laboratory examinations were performed in all patients 1 day after each procedure. If clinical symptoms had resolved and levels of inflammatory marker were decreased, oral intake was resumed. In this study, stent removal was performed after obtaining resolution of AC in both groups. If the patient showed severe complications (American Society of Anesthesiologists (ASA) Physical Status Classification IV or higher), the scheduled stent removal was not attempted. However, if the condition of the patient was improved, stent removal was attempted. After the patient was discharged, patient follow-up was performed based on outpatient examinations every 3–6 months or whenever adverse events occurred.
Definitions and statistical analysis
The physical condition of patients before EUS-GBD or ETGBD was evaluated according to the ASA system.[17] AC was diagnosed and graded in severity based on TG18.[18] The main outcome measures were technical success, clinical success, procedure-related and stent-related adverse events, and recurrence of AC during follow-up. Technical success of EUS-GBD was defined as successful deployment of the FCSEMS from the gallbladder to the duodenum. Technical success of ETGBD was defined as successful deployment of the plastic stent from the gallbladder to the duodenum. Clinical success was defined as resolution of symptoms along with evidence of objective improvements in biochemical and radiographic findings of cholecystitis within 3 days after intervention. AC was considered to have recurred if typical symptoms such as abdominal pain were observed or imaging modalities confirmed AC Procedure time was measured from scope insertion to successful stent deployment. Descriptive statistics are presented as median (interquartile range [IQR]) and frequency for continuous and categorical variables, respectively. Overall survival (OS) was measured from the completion of procedures to death or last follow-up of the patient, and survival curves were estimated using Kaplan-Meier methods. Differences of P < 0.05 were considered statistically significant. All data were statistically analyzed mostly using SPSS version 13.0 statistical software (SPSS Inc., Chicago, IL, USA). Adverse events associated with procedures including ERGBD and PTGBD were evaluated according to the severity grading system of the American Society for Gastrointestinal Endoscopy lexicon.[19]
RESULTS
A total 54 patients (44.4% female, 55.6% male) who underwent EUS-GBD or ETGBD were enrolled. Table 1 shows demographic and patient characteristics for the entire cohort. Median age was 76 years (IQR, 48–98 years). The median number of comorbidities was 2 (IQR: 1.0–6.0). Malignant tumor was the most common comorbidity (n = 19; 35.2%) followed by cardiovascular disease (n = 18; 33.3%), chronic renal failure (n = 13: 24.1%), cerebrovascular disease (n = 10; 18.5%), and diabetes (n = 7; 13.0%). Among the entire cohort, anticoagulation treatment was given to 31.5% (n = 28). Causes of AC were calculous in 48.1% (n = 26) and acalculous in 51.9% (n = 28). AC was severe in 59.3% of patients (n = 32), and moderate in the remaining 40.7% (n = 22). Reasons for high-risk surgery were ASA IV (35.2%, n = 19), ASA III (29.6%, n = 16), complicating advanced malignant tumor (25.9%, n = 14) and age >90 years (9.3%, n = 5). ETGBD was attempted in 53.7% of patients (n = 29), and EUS-GBD in 46.3% (n = 25). Mean follow-up was 552 days (range, 43–1892 days). During follow-up, recurrent AC was observed 4 patients (7.4%).
Table 1.
Demographic and patient characteristics in the entire cohort
| Variable | n (%) |
|---|---|
| Total number of patients | 54 |
| Age (years), median (IQR) | 76.00 (48.00-98.00) |
| <75 | 57.4 (31) |
| ≥75 | 22.6 (23) |
| Gender | |
| Female | 44.4 (24) |
| Male | 55.6 (30) |
| Number of comorbidity, median (IQR) | 2 (1.00-6.00) |
| Kinds of main comorbidity | |
| Malignant tumor | 35.2 (19) |
| Cardiovascular disease | 33.3 (18) |
| Chronic renal failure | 24.1 (13) |
| Cerebrovascular disease | 18.5 (10) |
| Diabetes | 13.0 (7) |
| Anticoagulation treatment | 31.5 (17) |
| Pathology of AC | |
| Calculous | 48.1 (26) |
| Acalculous | 51.9 (28) |
| Severity of AC | |
| Severe | 59.3 (32) |
| Moderate | 40.7 (22) |
| Causes underlying high surgical risk | |
| ASA IV | 35.2 (19) |
| ASA III | 29.6 (16) |
| Advanced cancer | 25.9 (14) |
| High age (>90 years old) | 9.3 (5) |
| Kinds of gallbladder drainage | |
| ETGBD | 53.7 (29) |
| EUS-GBD | 46.3 (25) |
| Mean follow-up period, days (range) | 522 (43-1892) |
| Recurrence of AC | 7.4 (4) |
AC: Acute cholecystitis; IQR: Interquartile range; EUS-GBD: EUS-guided gallbladder drainage; ETGBD: Endoscopic transpapillary guided gallbladder drainage; ASA: American society of anesthesiologists
Table 2 shows a comparison between the EUS-GBD (n = 25) and ETGBD (n = 29) groups. No significant differences between groups were seen in age, sex, severity of AC, clinical success, or time to resumption of oral intake. The initial technical success rate was 100% with EUS-GBD and 82.7% (24/29) with ETGBD. Among the 5 patients with failed ETGBD, the cystic duct could not be identified in 2 patients, necessitating guidewire insertion under SPY-DS guidance; ETGBD was successfully performed. In the remaining 3 patients, guidewire insertion under SPY-DS guidance was attempted, but guidewire insertion failed. These 3 patients underwent PTGBD. The overall technical success rate for ETGBD was thus 89.7% (26/29). The median procedure time was significantly shorter for the EUS-GBD group (11.0 min) than for the ETGBD group (24.0 min, P < 0.05). The frequency of procedure-related adverse events did not differ significantly between groups (P = 0.283). Stent removal was attempted in 40% of patients in the EUS-GBD group, and in 38% of patients in the ETGBD group (P = 0.876). Stent-related adverse events such as stent migration or dislocation were not seen in any patients in either group. Recurrent AC was observed in 4 patients from the ETGBD group. Two patients with recurrent AC showed complicating stent occlusion, which might have been associated with the recurrence (at 48 days and 205 days after procedures). These stent occlusions were due to sludge. In the remaining 2 patients, recurrence of AC was due to gallbladder stones at 48 days in one patient and 205 days in the other patient after discharge. Among patients with recurrent AC, ETGBD was performed in 3 patients, and conservative treatment was applied in 1 patient. These treatments were successful in all 4 patients. Finally, OS did not differ significantly between the EUS-GBD group (mean 1070 days; 95% confidence interval (CI) 725.39–1416.08 days) and ETGBD group (mean 1470 days; 95% CI 1170.72–1769.71 days; P = 0.292) [Figure 3].
Table 2.
Comparison between EUS-guided gallbladder drainage and endoscopic transpapillary guided gallbladder drainage groups
| EUS-GBD | ETGBD | P | |
|---|---|---|---|
| Total patients | 25 | 29 | - |
| Age (years), median (range) | 78 (48-98) | 75 (51-88) | 0.117 |
| Gender (male:female) | 14:11 | 22:7 | 0.123 |
| Severe AC, n (%) | 68.0 (17) | 51.7 (15) | 0.175 |
| Initial technical success, n (%) | 100.0 (25/25) | 82.7 (24/29) | <0.05 |
| Clinical success | 96.0 (24/25) | 79.3 (23/29) | 0.069 |
| Procedure time (min), median (IQR) | 11.00 (6.00-21.00) | 24.00 (8.00-52.00) | <0.05 |
| Procedure-related adverse event (n) | |||
| Pneumoperitoneum | 1 | 0 | 0.283 |
| Cholangitis | 0 | 1 | |
| Acute pancreatitis | 0 | 2 | |
| Stent-related adverse event (n) | |||
| Stent occlusion | 0 | 2 | 0.283 |
| Time to oral intake (days), median (IQR) | 3.00 (1.00-17.00) | 6.00 (2.00-20.00) | 0.375 |
| Number of stent removal, n (%) | 40 (10) | 38 (11) | 0.876 |
| Time to stent removal (days), median (IQR) | 13.00 (7.00-48.00) | 55.00 (12.00-193.00) | <0.05 |
| Recurrence of AC, n (%) | 0 (0/25) | 4/26 | 0.059 |
AC: Acute cholecystitis; IQR: Interquartile range; EUS-GBD: EUS-guided gallbladder drainage; ETGBD: Endoscopic transpapillary guided gallbladder drainage
Figure 3.
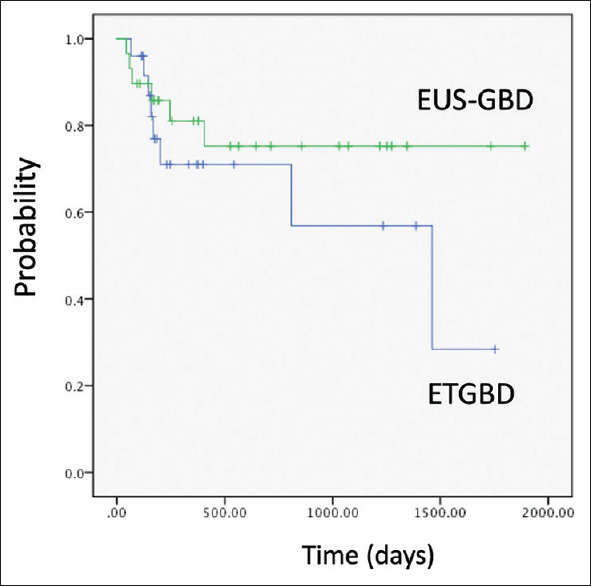
Comparison of overall survival between the EUS-gallbladder drainage group (mean 1070 days; 95% CI 725.39–1416.08 days) and endoscopic transpapillary gallbladder drainage group (mean 1470 days; 95% CI 1170.72–1769.71 days; P = 0.292)
DISCUSSION
If patients are considered unsuitable for surgery, severe AC warrants drainage. Among the various drainage techniques, EUS-GBD has recently emerged as a feasible alternative, although PTGBD is considered first according to TG18.[1] However, PTGBD shows several disadvantages, such as external drainage and cosmetic issues. According to recent comparison studies regarding EUS-GBD and ETGBD, lower postprocedural pain scores with a trend toward lower adverse event rates are seen with EUS-GBD.[20] On the other hand, ETGBD can be considered as a drainage option if an expert endoscopist is available. To date, several studies comparing EUS-GBD with ETGBD have been published.
Oh et al. compared the outcomes of EUS-GBD and ETGBD.[9] In that retrospective study, 76 patients underwent EUS-GBD and 96 patients underwent ETGBD. A modified FCSEMS with anti-migrating flare (BONA-AL Stent; Standard Sci-Tech, Seoul, Korea) was used as the EUS-GBD stent. According to procedural outcomes, technical success rates were significantly higher in the EUS-GBD group (98.8%) than in the ETGBD group (83.3%; P < 0.01), although procedural time (EUS-GBD 18.3 min vs. ETGBD 19.5 min; P = 0.31) and adverse event rate (EUS-GBD 7.2% vs. ETGBD 9.4%; P = 0.31) did not differ significantly between groups. However, regarding long-term outcomes (to around 20 months), recurrence rates for AC or cholangitis were higher with EUS-GBD (12.4%) than with ETGBD (3.2%). Oh et al. therefore concluded that EUS-GBD may be more suitable in patients with AC who were unfit for surgery, compared with ETGBD.[9] Higa et al. evaluated EUS-GBD using LAMS with ETGBD for AC.[21] In that study, electrocautery-enhanced and non-electrocautery-enhanced LAMS were used. Among a total of 478 patients with AC, initial GBD was successfully performed in 71 patients (EUS-GBD n = 39 vs. ETGBD n = 32). The technical success rate tended to be higher for EUS-GBD (97.5%, 39/40) than for ETGBD (84.2%, 32/38), although the difference was not significant (adjusted odds ratio, 9.83; 95% CI 0.93–103.86; P = 0.058). On the other hand, the clinical success rate was significantly higher for EUS-GBD (95%, 38/40) than for ETGBD (76.3%, 29/38) (adjusted odds ratio, 7.14; 95% CI 1.32–38.52; P = 0.02). The rate of recurrence of AC was lower with EUS-GBD (2.6%) than with ETGBD (18.8%; P = 0.023), but multiple regression modeling revealed no significant difference.
As noted above, EUS-GBD might show several advantages compared with ETGBD. We also compared EUS-GBD using conventional FCSEMS and ETGBD using a plastic stent in a clinical setting. Our study found no significant differences regarding characteristics such as age, sex, or severity of AC, but technical success rate and procedure time were significantly superior in the EUS-GBD group than in the ETGBD group. Recurrent AC tended to be more frequent in the ETGBD group, and clinical success tended to be more favorable in the EUS-GBD group, although these two variables did not differ significantly between groups. In addition, compared with previous studies,[9,21] our study was conducted in a setting of poor conditions such as severe ASA grade and including severe AC. In addition, we were able to evaluate the rate of AC recurrence with long-term follow-up. We thus believe that EUS-GBD might prove useful even in patients complicated by severe conditions.
Compared with previous reports, our study shows two advantages. First, stent dysfunction (such as stent occlusion due to food impaction) was not seen in any patients in our study. This might be explained by transduodneal stenting and additional plastic stent insertion into the FCSEMS. The latter might have prevented food impaction or prevented the FCSEMS from getting stuck to the gallbladder wall after drainage. Our strategies for EUS-GBD might thus be useful, although studies comparing conventional FCSEMS with and without plastic stent insertion are needed. Second, our study used conventional FCSEMS. FCSEMS is available in all countries, so the technique for FCSEMS deployment might be familiar to all endoscopists. On the other hand, electrocautery-enhanced LAMS has recently been able to be deployed without any fistula dilation. This fact might lead to shorter procedure times and a lower risk of bile leak from the fistula. EUS-BD using electrocautery-enhanced LAMS might thus offer clinical advantages over non-electrocautery-enhanced metal stents although LAMS is expensive compared with conventional FCSEMS. However, Cho et al. evaluated EUS-GBD using LAMS and anti-migration FSEMS (ATSEMS).[10] Non-electrocautery LAMS was used in that study, so fistula dilation was attempted using a needle knife or balloon catheter before stent deployment. Among the 71 patients (LAMS group, n = 36; ATSEMS group, n = 35), procedure time was significantly greater for the LAMS group (15.5 min) than for the ATSEMS group (11 min; P = 0.017), but technical success (LAMS 94% vs. ATSEMS 100%; P = 0.49), clinical success (LAMS 94% vs. ATSEMS 100%; P = 0.49), procedure-related adverse events (LAMS 0% vs. ATSEMS 2.9%; P = 0.99), and stent-related late adverse events (LAMS 11.8% vs. ATSEMS 5.8%; P = 0.99) did not differ significantly between groups. In addition, rates of AC recurrence were similar at 6 months (LAMS 3.4% vs. ATSEMS 3.1%; P = 0.49) and 12 months (LAMS 8.3% vs. ATSEMS 3.1%; P = 0.56). According to that study, LAMS and ATSEMS showed comparable results for EUS-GBD. Those results suggest that EUS-GBD using FCSEMS with plastic stent insertion might be more useful than LAMS based on cost-benefit analyses and familiarity of the technique. Indeed, our results are not inferior in terms of technical or clinical success rate, frequency of adverse events, or recurrence of AC [Table 3]. We recognize several limitations to the present study. First, this was a retrospective study with a relatively small cohort. Second, although ETGBD and EUS-GBD were performed by experienced endoscopists, technical proficiency can affect the technical success rate of these procedures. Third, no cost comparison could be performed. Therefore, further prospective comparison studies including cost effectiveness are needed to confirm our results.
Table 3.
Summary of previous studies regarding endoscopic transpapillary guided gallbladder drainage versus EUS-guided gallbladder drainage
| Author/year | Type of drainage | Kinds of stent (n) | Total patients (n) | Technical success (%) | Clinical success (%) | Adverse events (n) | Recurrence of AC (n) |
|---|---|---|---|---|---|---|---|
| Siddiqui et al./2019 | ETGBD | PS | 124 | 88 | 80 | 9 | 4 |
| EUS-GBD | LAMS | 102 | 94 | 90 | 12 | 1 | |
| Higa et al./2019[20] | ETGBD | PS | 38 | 84.2 | 76.3 | 3 | 6 |
| EUS-GBD | LAMS | 40 | 97.5 | 95 | 7 | 1 | |
| Oh et al./2019[9] | ETGBD | PS | 96 | 86.6 | 86 | 9 | 10 |
| EUS-GBD | AT-SEMS | 82 | 99.3 | 99.3 | 6 | 3 | |
| Present study/2021 | ETGBD | PS | 29 | 82.7 | 79.3 | 3 | 4 |
| EUS-GBD | SEMS with PS | 25 | 100 | 96 | 1 | 0 |
PS: Plastic stent; LAMS: Lumen-apposing metal stent; SEMS: Self-expandable metal stent; AT-SEMS: Anti-migration SEMS; AC: Acute cholecystitis; EUS-GBD: EUS-guided gallbladder drainage; ETGBD: Endoscopic transpapillary guided gallbladder drainage
CONCLUSION
The technical success rate for EUS-GBD using FCSEMS with plastic stent insertion was significantly higher with a shorter procedure time and resulted in a lower rate of AC recurrence. Further prospective randomized trials are needed to verify our results.
Financial support and sponsorship
Nil.
Conflicts of interest
Takeshi Ogura is an Editorial Board Member of the journal. The article was subject to the journal's standard procedures, with peer review handled independently of this editor and his research groups.
REFERENCES
- 1.Okamoto K, Suzuki K, Takada T, et al. Tokyo Guidelines 2018: Flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:55–72. doi: 10.1002/jhbp.516. [DOI] [PubMed] [Google Scholar]
- 2.Ito K, Fujita N, Noda Y, et al. Percutaneous cholecystostomy versus gallbladder aspiration for acute cholecystitis: A prospective randomized controlled trial. AJR Am J Roentgenol. 2004;183:193–6. doi: 10.2214/ajr.183.1.1830193. [DOI] [PubMed] [Google Scholar]
- 3.Van Steenbergen W, Ponette E, Marchal G, et al. Percutaneous transhepatic cholecystostomy for acute complicated cholecystitis in elderly patients. Am J Gastroenterol. 1990;85:1363–9. [PubMed] [Google Scholar]
- 4.Itoi T, Sofuni A, Itokawa F, et al. Endoscopic transpapillary gallbladder drainage in patients with acute cholecystitis in whom percutaneous transhepatic approach is contraindicated or anatomically impossible (with video) Gastrointest Endosc. 2008;68:455–60. doi: 10.1016/j.gie.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 5.Lee TH, Park DH, Lee SS, et al. Outcomes of endoscopic transpapillary gallbladder stenting for symptomatic gallbladder diseases: A multicenter prospective follow-up study. Endoscopy. 2011;43:702–8. doi: 10.1055/s-0030-1256226. [DOI] [PubMed] [Google Scholar]
- 6.Manta R, Zulli C, Zullo A, et al. Endoscopic ultrasound-guided gallbladder drainage for acute cholecystitis with a silicone-covered nitinol short bilaterally flared stent: A case series. Endosc Int Open. 2017;5:E1111–5. doi: 10.1055/s-0043-118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamata K, Takenaka M, Kitano M, et al. Endoscopic ultrasound-guided gallbladder drainage for acute cholecystitis: Long-term outcomes after removal of a self-expandable metal stent. World J Gastroenterol. 2017;23:661–7. doi: 10.3748/wjg.v23.i4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang JW, Lee SS, Park DH, et al. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest Endosc. 2011;74:176–81. doi: 10.1016/j.gie.2011.03.1120. [DOI] [PubMed] [Google Scholar]
- 9.Oh D, Song TJ, Cho DH, et al. EUS-guided cholecystostomy versus endoscopic transpapillary cholecystostomy for acute cholecystitis in high-risk surgical patients. Gastrointest Endosc. 2019;89:289–98. doi: 10.1016/j.gie.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Cho SH, Oh D, Song TJ, et al. Comparison of the effectiveness and safety of lumen-apposing metal stents and anti-migrating tubular self-expandable metal stents for EUS-guided gallbladder drainage in high surgical risk patients with acute cholecystitis. Gastrointest Endosc. 2020;91:543–50. doi: 10.1016/j.gie.2019.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Teoh AY, Kitano M, Itoi T, et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: An international randomised multicentre controlled superiority trial (DRAC 1) Gut. 2020;69:1085–91. doi: 10.1136/gutjnl-2019-319996. [DOI] [PubMed] [Google Scholar]
- 12.Cho DH, Jo SJ, Lee JH, et al. Feasibility and safety of endoscopic ultrasound-guided gallbladder drainage using a newly designed lumen-apposing metal stent. Surg Endosc. 2019;33:2135–41. doi: 10.1007/s00464-018-6485-5. [DOI] [PubMed] [Google Scholar]
- 13.Irani S, Ngamruengphong S, Teoh A, et al. Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin Gastroenterol Hepatol. 2017;15:738–45. doi: 10.1016/j.cgh.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Irani S, Baron TH, Grimm IS, et al. EUS-guided gallbladder drainage with a lumen-apposing metal stent (with video) Gastrointest Endosc. 2015;82:1110–5. doi: 10.1016/j.gie.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Dollhopf M, Larghi A, Will U, et al. EUS-guided gallbladder drainage in patients with acute cholecystitis and high surgical risk using an electrocautery-enhanced lumen-apposing metal stent device. Gastrointest Endosc. 2017;86:636–43. doi: 10.1016/j.gie.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Miyano A, Ogura T, Yamamoto K, et al. Clinical Impact of the intra-scope channel stent release technique in preventing stent migration during EUS-guided hepaticogastrostomy. J Gastrointest Surg. 2018;22:1312–8. doi: 10.1007/s11605-018-3758-1. [DOI] [PubMed] [Google Scholar]
- 17. [Last accessed on 2015 Jan 10];American Society of Anesthesologists. Standards for Basic Anesthetic Monitoring American Society of Anesthesologists. 2011 Available from: https://www.asahq.org/~/media/Sites/ASAHQ/Files/Public/Resources/standards-guidelines/standards-for-basic-anesthetic-monitoring.pdf . [Google Scholar]
- 18.Yokoe M, Hata J, Takada T, et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos) J Hepatobiliary Pancreat Sci. 2018;25:41–54. doi: 10.1002/jhbp.515. [DOI] [PubMed] [Google Scholar]
- 19.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui A, Kunda R, Tyberg A, et al. Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: Clinical outcomes and success in an International, Multicenter Study. Surg Endosc. 2019;33:1260–70. doi: 10.1007/s00464-018-6406-7. [DOI] [PubMed] [Google Scholar]
- 21.Higa JT, Sahar N, Kozarek RA, et al. EUS-guided gallbladder drainage with a lumen-apposing metal stent versus endoscopic transpapillary gallbladder drainage for the treatment of acute cholecystitis (with videos) Gastrointest Endosc. 2019;90:483–92. doi: 10.1016/j.gie.2019.04.238. [DOI] [PubMed] [Google Scholar]


