Abstract
A human herpesvirus 7 (HHV-7) indirect immunofluorescence antibody avidity test was developed and used with an existing human herpesvirus 6 (HHV-6) antibody avidity test to detect and distinguish low-avidity antibodies to HHV-6 and HHV-7 and hence the respective primary infections. With sera from 269 British children aged 0 to 179 weeks, the tests showed that most (10 of 98 serum samples [13%]) HHV-6 low-avidity antibody was found in the first year of life, whereas for HHV-7, most (18 of 101 serum samples [20%]) HHV-7 low-avidity antibody was found in the second year of life. Five children had low-avidity antibodies to both viruses. Of nine Japanese children with previously serologically proven primary HHV-6 or HHV-7 infections, eight had low-avidity antibody only to the relevant virus, but one child had low-avidity antibodies to HHV-6 and HHV-7. The avidity tests were applied to five British children and further proof of viral infection was sought by the detection of specific DNA in serum or plasma, and saliva or cerebrospinal fluid. In two children who had low-avidity antibody to HHV-7 but who were seronegative for HHV-6, only HHV-7 was found. Both viruses were detected in one child with low-avidity HHV-7 antibody and high-avidity HHV-6 antibody. In two children with low-avidity antibodies to both viruses, HHV-6 and HHV-7 DNAs were found, confirming dual primary infections and excluding antibody cross-reactivity.
The discovery of human herpesvirus 6 (HHV-6) in 1986 (22) was soon followed by the identification of the closely related virus HHV-7 (10). Both viruses belong to the Roseolovirus genus of the betaherpesvirus subfamily of herpesviruses and show very similar biological behaviors: (i) after primary infection they are shed in saliva throughout life (14, 16, 18, 26, 33); (ii) primary infection with either virus causes exanthem subitum (roseola infantum) (25, 35), a classical exanthematous disease of childhood; and (iii) primary infection with either virus has been associated with childhood neurological illness, particularly febrile convulsions (12, 27, 28, 32), and the DNAs of both HHV-6 (12) and HHV-7 (20) have been detected in cerebrospinal fluid. Any study of the relationship between the two viruses and disease must therefore use diagnostic methods able to distinguish between primary HHV-6 and primary HHV-7 antibody responses.
Primary infections may be diagnosed by the use of antibody avidity tests since antibody avidity increases progressively with time after exposure to an immunogen (8). Tests for antibody avidity have been applied successfully to sera for the diagnosis of many different human virus infections (for reviews, see references 11 and 13) and rely on the fact that an agent which denatures protein will disrupt the antigen-antibody reaction preferentially, affecting low-avidity antibody but not high-avidity antibody (15, 21). If antibody avidity is low, this confirms recent primary infection, but if the avidity is high, primary infection must have occurred in the more distant past.
In this context we had previously successfully developed an HHV-6 antibody avidity test (30) for the diagnosis of primary HHV-6 infection in children with rashes (24). This test has also been proven to differentiate between a primary HHV-6 antibody response and a secondary HHV-6 antibody response in immunocompromised solid-organ graft recipients (31). The present paper describes the development and validation of an HHV-7 antibody avidity test for use in conjunction with the HHV-6 antibody avidity test. The specificities of both these tests were confirmed by the parallel detection of viral DNA in saliva and in some cases serum and/or cerebrospinal fluid.
MATERIALS AND METHODS
Samples for antibody testing.
Sera or plasma (from blood collected by venipuncture or on filter paper after finger prick) from the following patients were stored at −20°C.
(i) British children under 4 years old.
Sera from children had been submitted for routine virus investigation between 1987 and 1990 to the Clinical Virology Laboratory, Addenbrooke's Hospital, Cambridge, United Kingdom. Three hundred twenty-one serum samples from children aged 0 to 180 weeks were selected and tested previously for HHV-6 immunoglobulin G (IgG) antibody and avidity (30). Of these, 269 serum samples, each of which was from a different individual (112 females, 156 males, and 1 of unknown sex) remained in sufficient volume to test for HHV-7 IgG antibody and avidity.
(ii) British adults.
Forty serum samples were chosen randomly from 40 women who had been tested for rubella antibodies at the Clinical Virology Laboratory, Addenbrooke's Hospital.
(iii) Japanese children with serologically proven primary HHV-6 and HHV-7 infection.
Acute- and convalescent-phase plasma samples were kindly provided by Y. Asano, Department of Pediatrics, Fujita Health University School of Medicine, Aichi, Japan. Samples from six children with primary HHV-6 infection (patients 1 to 6) had been tested for HHV-6 antibody by a neutralization assay in Japan (23), and seroconversion for HHV-6 IgG together with the presence of low-avidity antibody was confirmed in our laboratory (for full details on patients 1 to 5, see patients 3 to 7, respectively, in reference 30). Samples from three children with primary HHV-7 infection (patients 7 to 9) had been tested in Japan for HHV-6 and HHV-7 IgG antibody by an indirect immunofluorescence assay (37). The ages of the children and the timing of sample collection (the number of days after the onset of symptoms) were as follows: 12 months, days 3 and 19; 19 months, days 7 and 60, and 21 months, days 4 and 60, respectively.
(iv) British children referred for diagnosis of HHV-6 and -7 infections.
Plasma or serum from patients 10 to 14 had been referred to the Diagnostic Virology Laboratory, University College Hospital, London, United Kingdom, for diagnosis of HHV-6 and HHV-7 infections.
Indirect immunofluorescence tests. (i) HHV-6 and HHV-7 antibodies.
HHV-6 IgG antibody was detected by immunofluorescence (29). The same method was used to detect HHV-7 IgG antibody but using the MK strain of HHV-7 (kindly provided by D. A. Clark, Department of Virology, Royal Free and University College School of Medicine, London, United Kingdom) passaged in Sup-T1 cells. Serum or plasma samples were tested in doubling dilutions, starting at a 1 in 10 dilution except for those from patients 10 to 14, for whom the starting dilution was either 1 in 8 or 1 in 16 if blood had been collected on filter paper and serum had been extracted (9). Seroconversion was defined as a change from undetectable antibody to antibody detectable at eight times or more than the minimum level. An eightfold or greater increase in antibody titer was considered a significant rise.
(ii) Avidities of HHV-6 and HHV-7 IgG antibodies.
The IgG tests described above were modified to elute low-avidity antibody with urea as described previously for HHV-6 (30). Those samples whose antibody titers were reduced eightfold or greater by urea were defined as having low avidity, and conversely, sera whose titers were reduced fourfold or less were defined as having high avidity.
PCR for HHV-6 and HHV-7.
The samples tested had been taken for diagnostic purposes from the British children referred for diagnosis of HHV-6 and HHV-7 infections.
Saliva was collected with a sponge swab (19), extracted from the swab (5), and stored at −20°C. At the time of testing the saliva extract was thawed and then centrifuged at 15,000 × g for 5 min. Fifty microliters of supernatant was removed, boiled for 10 min, cooled immediately on ice, and held briefly at 4°C until required.
Nucleic acid (both RNA and DNA) was prepared from 140 μl of serum, plasma, or cerebrospinal fluid with the QIAmp Viral RNA Mini kit (Qiagen Ltd., Crawley, United Kingdom) according to the manufacturer's instructions. Ten microliters of boiled saliva or nucleic acid from serum, plasma, or cerebrospinal fluid was tested for HHV-6 and -7 DNAs by PCR (17). All positive results were confirmed by a repeat test; for this, a further boiled aliquot of saliva was used. If sufficient amounts of the other samples remained, a second extract of nucleic acid was prepared; if not, an aliquot of the previously prepared nucleic acid was used.
RESULTS
HHV-6 and HHV-7 IgG antibodies and avidities in British children under 4 years old and adults.
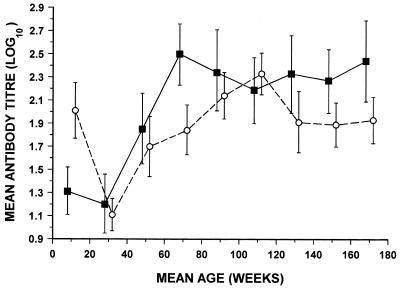
The 269 serum samples from British children were investigated to confirm the known age of acquisition of antibodies to the two viruses (26, 30, 36, 37) and to see whether the distribution of low-avidity antibody coincided with the expected times of primary infection (6). Figure 1 shows the geometric mean titers of IgG antibodies to HHV-6 and HHV-7 at 20-week intervals. Titers of antibody to both viruses reached their lowest point at about 30 weeks and rose to a maximum at 70 weeks for HHV-6 and at 110 weeks for HHV-7. The sera from 40 adults, expected to have been infected as children, were included to determine whether the assays would discriminate between recent and past infections. All the adult sera contained IgG antibody to HHV-6 (geometric mean titer [log10], 2.2; 95% confidence limits, 2.03 to 2.37) and HHV-7 (geometric mean titer [log10], 2.35; 95% confidence limits, 2.25 to 2.45). For both groups in whom the antibody titer was 80 or greater, avidity was also measured. Low-avidity antibody to HHV-6 and/or HHV-7 was found in the children (54 of 269; 20%) but not in the adults (0 of 40; 0%). Low-avidity antibody to HHV-6 (13 of 98; 13%) occurred in the highest proportion of children at 0 to 59 weeks, whereas it occurred in smaller proportions of children at 60 to 119 weeks (10 of 101; 10%) and 120 to 179 weeks (5 of 70; 7%). In contrast, for HHV-7 the time that the highest proportion of children had low-avidity antibody was seen later (incidences, 8 of 98 [8%], 20 of 101 [20%], and 3 of 70 [4%] for the three time periods, respectively). Five children had low-avidity antibody to both viruses. Of the 23 children with low-avidity antibody to HHV-6 but not HHV-7, 1 had no HHV-7 IgG, 15 had HHV-7 IgG of high avidity, and 7 had HHV-7 IgG at a low titer of 10 or 20. Of the 26 children with low-avidity antibody to HHV-7 but not to HHV-6, 5 had no HHV-6 IgG, 20 had HHV-6 IgG of high avidity, and 1 had HHV-6 IgG at a low titer of 20.
FIG. 1.
Comparison of the change with age of the geometric mean titers of IgG antibodies to HHV-6 and HHV-7 in children. Geometric mean titers for HHV-6 (■) and HHV-7 (○) were calculated for 20-week age intervals from birth to 179 weeks. When the titer was <10 it was given a nominal value of 5. Bars represent the mean ± twice the standard error of the mean. The numbers of serum samples tested in each group were as follows: 0 to 19 weeks, 26; 20 to 39 weeks, 36; 40 to 59 weeks, 36; 60 to 79 weeks, 35; 80 to 99 weeks, 31; 100 to 119 weeks, 35; 120 to 139 weeks, 27; 140 to 159 weeks, 24; 160 to 179 weeks, 19.
HHV-6 and HHV-7 IgG antibodies and avidities in Japanese children with serologically proven primary HHV-6 infection.
Seroconversion to HHV-6 IgG was found in patients 1 to 5 (see patients 3 to 7, respectively, in reference 30), and patient 6 showed a 32-fold rise in titer from 10 to 320. Every convalescent-phase sample showed low-avidity antibody. In contrast, seroconversion to HHV-7 IgG with low-avidity antibody was found only in patient 1 (HHV-6 and HHV-7 IgG titers for the acute-phase sample, <10; titers for a convalescent-phase sample, 2,560 for HHV-6 but it was reduced to 40 by urea, and 80 for HHV-7, but it was reduced to <10 by urea). Patient 2 showed a rising titer of HHV-7 IgG, but it was of high avidity (the IgG antibody titers in the acute-phase sample were <10 for HHV-6 and 10 for HHV-7; those for the convalescent-phase sample were 1,280 for HHV-6, which was reduced to 20 by urea, and 80 for HHV-7, which was reduced to 20 by urea). Patient 4 was HHV-7 IgG seronegative, and patients 3, 5, and 6 had very low levels of HHV-7 IgG of 10 or 20.
HHV-6 and HHV-7 IgG antibodies and avidities in Japanese children with serologically proven primary HHV-7 infection.
All three Japanese children (patients 7 to 9) showed rising titers of HHV-7 IgG. In patients 7 and 8 both samples had low-avidity antibody (the HHV-7 IgG antibody titers in the acute-phase samples were 80 and 160, respectively, but both were reduced to <10 by urea; those in the convalescent-phase samples were 1,280 for both patients, but they were reduced to 20 and 80 by urea, respectively), whereas in patient 9 the acute-phase plasma sample showed low-avidity antibody (HHV-7 IgG antibody titer, 320, but it was reduced to 20 by urea), but the convalescent-phase plasma sample taken 2 months after disease onset contained high-avidity antibody (HHV-7 IgG antibody titer, 2,560, but it was reduced to 320 by urea). In contrast, HHV-6 IgG was of high titer and high avidity in both acute- and convalescent-phase samples from all three patients (for the three patients, the HHV-6 IgG antibody titers in acute-phase samples were 1,280 reduced to 320, 640 reduced to 320, and 5,120 unchanged at 5,120 by urea, respectively; those for the convalescent-phase samples were 5,120 reduced to 2,560, 640 reduced to 320, and ≥20,480 reduced to 10,240 by urea, respectively).
Primary HHV-7 Infection in British children: antibody response and detection of viral DNA.
Table 1 shows the results of HHV-6 and HHV-7 testing of samples from patients 10, 11, and 12. Patients 10 and 11 showed seroconversion to HHV-7 IgG with low antibody avidity, and HHV-7 DNA was detected in saliva. HHV-7 DNA was also detected in the acute-phase serum of patient 10. In patient 11, the antibody response had matured to high avidity 5 months after seroconversion was first detected. There was no evidence of HHV-6 infection in either patient. In contrast, patient 12 showed rising titers of both HHV-6 and HHV-7 IgG antibodies; the HHV-6 antibody was of high titer and high avidity, whereas the HHV-7 antibody was of low avidity initially but had matured to high avidity by 4 months. Both HHV-6 and HHV-7 DNAs were detected in saliva; HHV-7 DNA was also detected in the acute-phase plasma.
TABLE 1.
Primary HHV-7 infection in British children: antibody responses and detection of viral DNA
| Patient no. | Age (mo) | Day after onset of symptoms | Serum or plasma
|
|||||
|---|---|---|---|---|---|---|---|---|
| HHV-6 IgG titera
|
HHV-6 DNA | HHV-7 IgG titera
|
HHV-7 DNA | |||||
| Without urea | With urea | Without urea | With urea | |||||
| 10 | 17 | 4 | <8 | <8 | − | <8 | <8 | + |
| 11 | 16 | NTb | − | 512 | 8 | − | ||
| 41c | <16 | <16 | NT | 512 | 16 | NT | ||
| 168 | 8 | <8 | − | 512 | 128 | − | ||
| 11 | 21 | 10 | <8 | <8 | − | <8 | <8 | − |
| 172cd | <16 | <16 | NT | 2,048 | 64 | NT | ||
| 206 | <8 | <8 | − | 1,024 | 32 | − | ||
| 334 | <8 | <8 | − | 256 | 64 | − | ||
| 340c | <16 | <16 | NT | 512 | 256 | NT | ||
| 12 | 13 | 3 | 512 | 256 | − | 64 | 8 | + |
| 15c | 8,192 | 4,096 | NT | 8,192 | 16 | NT | ||
| 96 | 1,024 | 2,048 | − | 4,096 | 32 | − | ||
| 119e | 512 | NT | NT | 256 | 64 | NT | ||
Those sera whose antibody titers were reduced eightfold or greater by urea are defined as having low-avidity antibody and the results are highlighted in bold. Those sera whose titers were reduced fourfold or less by urea are defined as having high-avidity antibody.
NT, not tested.
Saliva contained HHV-7 DNA but not HHV-6 DNA.
Patient 11 presented with an encephalopathic Illness. HHV-7 seroconversion was picked up by chance in a convalescent-phase specimen.
Saliva contained HHV-7 DNA and HHV-6 DNA.
Dual primary HHV-6 and HHV-7 infections in British children: antibody response and detection of viral DNA.
Table 2 shows the results of HHV-6 and HHV-7 IgG antibody and avidity testing of samples from patients 13 and 14; in each patient there was seroconversion to both viruses with low-avidity antibody. In patient 13, HHV-6 DNA was detected in cerebrospinal fluid, and HHV-7 DNA was detected in saliva. In patient 14, both HHV-6 and HHV-7 DNAs were detected in saliva and HHV-6 DNA was also detected in cerebrospinal fluid and acute-phase serum.
TABLE 2.
HHV-6 and HHV-7 dual primary infection in British children: antibody responses and detection of DNA from both viruses
| Patient no. | Age (mo) | Day after onset of symptoms | Serum or plasma
|
|||||
|---|---|---|---|---|---|---|---|---|
| HHV-6 IgG titera
|
HHV-6 DNA | HHV-7 IgG titera
|
HHV-7 DNA | |||||
| Without urea | With urea | Without urea | With urea | |||||
| 13 | 10 | 1c | 16 | NTb | − | <8 | NT | − |
| 14d | 2,048 | 32 | NT | 128 | <16 | NT | ||
| 14 | 12 | 3c | NT | NT | + | <8 | <8 | − |
| 4e | <16 | <16 | NT | NT | NT | NT | ||
| 19 | 2,048 | 64 | NT | 256 | <16 | NT | ||
Those sera whose antibody titers were reduced eightfold or greater by urea are defined as having low-avidity antibody and the results are highlighted in bold. Those sera whose titers were reduced fourfold or less by urea are defined as having high-avidity antibody.
NT, not tested.
Cerebrospinal fluid contained HHV-6 DNA but not HHV-7 DNA.
Saliva contained HHV-7 DNA but not HHV-6 DNA.
Saliva contained HHV-6 DNA and HHV-7 DNA.
DISCUSSION
Diagnosis of primary HHV-6 and/or HHV-7 infection by antibody responses is challenging since the two viruses share common antigenic epitopes (for reviews, see references 1 and 4) and there is limited cross-reactivity between naturally induced human antibodies against the two viruses (3). Primary HHV-6 infection may be easily diagnosed in an HHV-7-seronegative individual by comparing the titers of acute- and convalescent-phase sera and demonstrating HHV-6 antibody seroconversion and vice versa. However, difficulty occurs when there are rising titers of antibodies to both viruses (34). Such rises might be the result of dual primary infection, a dual rise in preexisting antibodies to each virus, or a primary infection with one virus that elicits a secondary antibody response to the other. In this paper we describe a solution to this problem by using indirect immunofluorescence tests for antibody avidity to differentiate the HHV-6 and HHV-7 antibody titers and avidity and, hence, primary and secondary responses.
In the first tests, HHV-6 and HHV-7 IgG antibody titers and avidities were measured by immunofluorescence with a panel of children's sera taken from birth up to age 3 years. The results (Fig. 1) confirm those of earlier seroepidemiological investigations, which have shown that HHV-6 infects almost all individuals at between 7 and 13 months of age, most commonly at about 8 or 9 months (30, 36), whereas HHV-7 infection occurs later (26, 37). As expected, low-avidity antibodies were found only in the sera from the children and not in the sera from the adults, and the age distribution in the children with low-avidity antibodies agrees with that from a recent study of the age at which primary HHV-6 and HHV-7 infections are found (6). In most patients the low-avidity antibody was detected for one virus only and the individual either was seronegative or, more usually, had high-avidity antibody to the other virus, but in a few patients, low-avidity antibody to one virus was accompanied by a low level of antibody (titers, <40) of indeterminate avidity to the other virus, suggesting limited cross-reactivity. In the remaining five patients, dual low-avidity antibody (titers in all patients, ≥80) was found, raising the possibility of an antigenic cross-reaction or concurrent primary HHV-6 and HHV-7 infections. Overall, however, the results supported the concept that HHV-6 low-avidity antibody occurs only after primary HHV-6 infection and vice versa. Nevertheless, there was clearly a need to validate further the combined use of the two antibody avidity tests with paired sera from patients with well-defined primary HHV-6 and HHV-7 infections.
As regards the cases of serologically proven primary HHV-7 infection in Japanese children, in all three children a rising titer of low-avidity antibody to HHV-7 was accompanied by high-titer, high-avidity HHV-6 IgG antibody, and in agreement with others (25, 27), it was found that primary HHV-7 infection normally occurs after infection with HHV-6 and is accompanied by a secondary response to HHV-6. Turning to serologically proven primary HHV-6 infection in Japanese children, in all six children we documented seroconversion with low-avidity antibody to HHV-6, but in two children there were significant titers of antibody to HHV-7. In one child the rising titer of high-avidity antibody excluded primary HHV-7 infection. However, in the other child there was a rising antibody titer of low avidity to both viruses, suggesting dual primary infection, but the possibility of an antigenic cross-reaction could not be excluded.
Therefore, it remained necessary to further confirm the specificities of the two antibody avidity tests and to confirm that dual primary HHV-6 and HHV-7 infections can be distinguished. One way to do this would be by immunoblot antibody tests for specific HHV-6 and HHV-7 proteins known not to cross-react (2, 3). Another possibility is to confirm antibody specificity by testing for the presence of virus either by culture or by detection of viral DNA; such tests for HHV-6 or HHV-7 viremia have already been used in conjunction with antibody tests to confirm primary infection (6, 7). In the event, as viremia may be transient, we decided to test not only serum or plasma but also saliva and, where available, cerebrospinal fluid from the British children for HHV-6 and HHV-7 DNAs. The results (Table 2) show that when there was low-avidity antibody to both viruses, both HHV-6 and HHV-7 DNAs were detected, confirming dual primary HHV-6 and HHV-7 infections and excluding cross-reactivity. In addition, when there was serological evidence of primary HHV-7 infection but the child was seronegative for HHV-6, only HHV-7 DNA was detected, whereas when primary HHV-7 infection occurred after prior infection with HHV-6, both viruses were detected (Table 1).
In conclusion, the presence of viral DNA thus fits exactly with the antibody avidity findings and validates the use of the antibody avidity test to distinguish between primary HHV-6 and HHV-7 infections, despite the sharing of epitopes between these two closely related agents. Thus, future studies with HHV-6 and HHV-7 antibody avidity tests will be useful for the investigation of the clinical features of infection with these two closely related viruses.
ACKNOWLEDGMENTS
We thank Shabnam Ansari for excellent technical assistance.
This work was supported in part by project grants to K.N.W. from Action Research (reference S/P/2459) and the Wellcome Trust (reference 051350/Z/97/Z).
REFERENCES
- 1.Black J B, Pellet P E. Human herpesvirus 7. Rev Med Virol. 1999;9:245–262. doi: 10.1002/(sici)1099-1654(199910/12)9:4<245::aid-rmv253>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Black J B, Durigon E, Kite-Powell K, de Souza L, Curli S P, Sardinha Afonso A M, Theobaldo M, Pellett P E. Seroconversion to human herpesvirus 6 and human herpesvirus 7 among Brazilian children with clinical diagnoses of measles or rubella. Clin Infect Dis. 1996;23:1156–1158. doi: 10.1093/clinids/23.5.1156. [DOI] [PubMed] [Google Scholar]
- 3.Black J B, Schwarz T F, Patton J L, Kite-Powell K, Pellet P E, Wiersbitzky S, Bruns R, Müller C, Jäger G, Stewart J A. Evaluation of immunoassays for detection of antibodies to human herpesvirus 7. Clin Diagn Lab Immunol. 1996;3:79–83. doi: 10.1128/cdli.3.1.79-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun D K, Dominguez G, Pellet P E. Human herpesvirus 6. Clin Microbiol Rev. 1997;10:1–47. doi: 10.1128/cmr.10.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D W G, Ramsay M E B, Richards A F, Miller E. Salivary diagnosis of measles: a study of measles in the United Kingdom, 1991-3. BMJ. 1994;308:1015–1017. doi: 10.1136/bmj.308.6935.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caserta M T, Breese Hall C, Schnabel K, Long C E, D'Heron N. Primary human herpesvirus 7 infection: a comparison of human herpesvirus 7 and human herpesvirus 6 infections in children. J Paediatr. 1998;133:386–389. doi: 10.1016/s0022-3476(98)70275-6. [DOI] [PubMed] [Google Scholar]
- 7.Clark D A, Kidd I M, Collingham K E, Tarlow M, Ayeni T, Riordan A, Griffiths P D, Emery V C, Pillay D. Diagnosis of primary human herpesvirus 6 and 7 infections in febrile infants by polymerase chain reaction. Arch Dis Child. 1997;77:42–45. doi: 10.1136/adc.77.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisen H N, Siskind G W. Variations in affinities of antibodies during the immune response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 9.Faraclegan H, Quinn T, Polk B F. Detecting antibodies to human immunodeficiency virus in dried blood on filter papers. J Infect Dis. 1987;157:1073–1074. doi: 10.1093/infdis/155.5.1073. [DOI] [PubMed] [Google Scholar]
- 10.Frenkel N, Schirmer E C, Wyatt L S, Katsafanas G, Roffman E, Danovich R M, June C H. Isolation of a new herpesvirus from CD4+ T cells. Proc Natl Acad Sci USA. 1990;87:748–752. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldblatt D. Simple solid phase assays of avidity. In: Johnstone A, Turner M W, editors. Immunochemistry 2: a practical approach. Oxford, United Kingdom: IRL Press at Oxford University Press; 1997. pp. 31–51. [Google Scholar]
- 12.Hall C B, Long C E, Schnabel K C, Caserta M T, Mclntyre K M, Costanzo M A, Knott A, Dewhurst S, Insel R A, Epstein L G. Human herpesvirus-6 infection in children: a prospective study of complications and reactivation. N Engl J Med. 1994;331:432–438. doi: 10.1056/NEJM199408183310703. [DOI] [PubMed] [Google Scholar]
- 13.Hedman K, Lappalainen M, Söderland M, Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol. 1993;4:123–129. [Google Scholar]
- 14.Hidaka Y, Liu Y, Yamamoto M, Mori R, Miyazaki C, Kusuhara K, Okada K, Ueda K. Frequent isolation of human herpesvirus 7 from saliva samples. J Med Virol. 1993;40:343–346. doi: 10.1002/jmv.1890400416. [DOI] [PubMed] [Google Scholar]
- 15.Inouye S, Hasegawa A, Matsuno S, Katow S. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol. 1984;20:525–529. doi: 10.1128/jcm.20.3.525-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarrett R F, Clark D A, Josephs S F, Onions D E. Detection of human herpesvirus-6 DNA in peripheral blood and saliva. J Med Virol. 1990;32:73–76. doi: 10.1002/jmv.1890320113. [DOI] [PubMed] [Google Scholar]
- 17.Kidd I M, Clark D A, Bremner J A G, Pillay D, Griffiths P D, Emery V C. A multiplex PCR assay for the simultaneous detection of human herpesvirus 6 and human herpesvirus 7, with typing of HHV-6 by enzyme cleavage of PCR products. J Virol Methods. 1998;70:29–36. doi: 10.1016/s0166-0934(97)00165-1. [DOI] [PubMed] [Google Scholar]
- 18.Kido S, Kazuhiro K, Kondo T, Morishima T, Takahashi M. Detection of human herpesvirus 6 DNA in throat swabs by polymerase chain reaction. J Med Virol. 1990;32:139–142. doi: 10.1002/jmv.1890320302. [DOI] [PubMed] [Google Scholar]
- 19.Mortimer P P, Parry J V. Non invasive virological diagnosis: are saliva and urine specimens adequate for blood? Rev Med Virol. 1991;1:73–78. [Google Scholar]
- 20.Portolani M, Leoni S, Guerra A, Cermelli C, Mantovani G, Pietrosemoli P, Meacci M, Sabbatini A M, Pecorari M. Human herpesvirus-7 DNA in cerebrospinal fluid. Minerva Paediatr. 1998;50:39–44. [PubMed] [Google Scholar]
- 21.Pullen G R, Fitzgerald M G, Hosking C S. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;106:119–125. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 22.Salahuddin S Z, Ablashi D V, Markham P D, Josephs S F, Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramorsky B, Gallo R C. Isolation of a new virus (HBLV) in patients with lymphoproliferative disorders. Science. 1986;234:596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- 23.Suga S, Yoshikawa T, Asano Y, Yazaki T, Ozaki T. Neutralizing antibody assay for human herpesvirus-6. J Med Virol. 1990;30:14–19. doi: 10.1002/jmv.1890300104. [DOI] [PubMed] [Google Scholar]
- 24.Tait D R, Ward K N, Brown D W G, Miller E. Exanthem subitum (roseola infantum) misdiagnosed as measles or rubella. BMJ. 1996;312:101–102. doi: 10.1136/bmj.312.7023.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka K, Kondo T, Torigoe S, Okada S, Mukai T, Yamanishi K. Human herpesvirus-7: another causal agent for roseola (exanthem subitum) J Pediatr. 1994;125:1–5. doi: 10.1016/s0022-3476(94)70113-x. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka-Taya K, Kondo T, Mukai T, Miyoshi H, Yamamoto Y, Okada S, Yaminishi K. Seroepidemiological study of human herpesvirus-6 and -7 in children of different ages and detection of these two viruses in throat swabs by polymerase chain reaction. J Med Virol. 1996;48:88–94. doi: 10.1002/(SICI)1096-9071(199601)48:1<88::AID-JMV14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Torigoe S, Kumamoto T, Koide W, Taya K, Yamanishi K. Clinical manifestations associated with human herpesvirus 7 infection. Arch Dis Child. 1995;72:518–519. doi: 10.1136/adc.72.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torigoe S, Koide W, Yamada M, Miyashiro E, Tanaka-Taya K, Yamanishi K. Human herpesvirus 7 infection associated with central nervous system manifestations. J Pediatr. 1996;129:301–305. doi: 10.1016/s0022-3476(96)70259-7. [DOI] [PubMed] [Google Scholar]
- 29.Ward K N, Gray J J, Efstathiou S. Brief report: primary human herpesvirus-6 infection in a patient following liver transplantation from a seropositive donor. J Med Virol. 1989;28:69–72. doi: 10.1002/jmv.1890280203. [DOI] [PubMed] [Google Scholar]
- 30.Ward K N, Gray J J, Fotheringham M W, Sheldon M J. IgG antibodies to human herpesvirus-6 in young children: changes in avidity of antibody correlate with time after infection. J Med Virol. 1993;39:131–138. doi: 10.1002/jmv.1890390209. [DOI] [PubMed] [Google Scholar]
- 31.Ward K N, Gray J J, Joslin M E, Sheldon M J. Avidity of IgG antibodies to human herpesvirus-6 distinguishes primary from recurrent infection in organ transplant recipients and excludes cross-reactivity with other herpesviruses. J Med Virol. 1993;39:44–49. doi: 10.1002/jmv.1890390109. [DOI] [PubMed] [Google Scholar]
- 32.Ward K N, Gray J J. Primary human herpesvirus-6 infection is frequently overlooked as a cause of febrile fits in young children. J Med Virol. 1994;42:119–123. doi: 10.1002/jmv.1890420204. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt L S, Frenkel N. Human herpesvirus-6 is a constitutive inhabitant of adult human saliva. J Virol. 1992;66:3206–3209. doi: 10.1128/jvi.66.5.3206-3209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyatt L S, Rodriguez W J, Balachandran N, Frenkel N. Human herpesvirus 7: antigenic properties and prevalence in children and adults. J Virol. 1991;65:6260–6265. doi: 10.1128/jvi.65.11.6260-6265.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;i:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa T, Suga S, Asano Y, Yazaki T, Kodama H, Ozaki T. Distribution of antibodies to a causative agent of exanthem subitum (human herpesvirus-6) in healthy individuals. Paediatrics. 1989;84:675–677. [PubMed] [Google Scholar]
- 37.Yoshikawa T, Asano Y, Koyabashi I, Nakashima T, Yazaki T, Suga S, Ozaki T, Wyatt L S, Frenkel N. Seroepidemiology of human herpesvirus 7 in healthy children and adults in Japan. J Med Virol. 1993;41:319–323. doi: 10.1002/jmv.1890410412. [DOI] [PubMed] [Google Scholar]