Abstract
Up to 93% of the human immunodeficiency virus (HIV) latent reservoir comprised defective proviruses, suggesting that a functional cure is possible through the elimination of a small population of cells containing intact virus, instead of the entire reservoir. Cyclophosphamide (Cy) is an established chemotherapeutic agent for immune cell cancers. In high doses, Cy is a nonselective cytoreductor, used in allogeneic stem-cell transplantation, while in a low dose, metronomic schedule, Cy selectively depletes regulatory T cells (Tregs). We administered low and high doses to simian immunodeficiency virus (SIV)-infected rhesus macaques (RM) to assess their effects on the SIV reservoirs. As a Treg-depleting agent, Cy unselectively depleted Treg and total lymphocytes, resulting in minimal immune activation and no viral reactivation. As a cytoreductive agent, Cy induced massive viral reactivation in elite controller RMs without ART. However, when administered with antiretroviral therapy (ART), Cy had substantial adverse effects, including mortality. Our study thus dissuades further investigation of Cy as an HIV cure agent.
Keywords: cyclophosphamide, human immunodeficiency virus, HIV latency, regulatory T cells, simian immunodeficiency virus, Treg depletion
Short Communication
Testing novel strategies for human immunodeficiency virus (HIV) eradication is a priority of AIDS research. In this study, we assessed the utility of cyclophosphamide (Cy) for different cure strategies, by using two different regimens, with two different goals: Treg depletion and nonmyeloablative cytoreduction. We hypothesized that (1) low doses of Cy (LDC) administered metronomically over one month would selectively deplete regulatory T cells (Tregs), as in humans,1 allowing for increased immune activation and viral reactivation with the added benefit of bolstering the cell-mediated immune response by reducing suppressive signaling from Tregs2,3; and (2) extensive nonselective depletion of lymphocytes with high doses of Cy (HDC) may be a valid HIV cure intervention through direct elimination of reservoir cells4–6; and subsequent restriction of reservoir diversity (through depletion, followed by the clonal expansion of the integrated genomes during cell restoration).
Both strategies were tested in simian immunodeficiency virus (SIV)-infected rhesus macaques (RMs) housed and handled at the University of Pittsburgh following the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Animal Welfare Act. The University of Pittsburgh approved these studies in the Institutional Animal Care and Use Committee (IACUC) protocol 16027641.
Whole blood was serially collected and tested for complete blood counts and chemistries (Marshfield Laboratories, Cleveland, OH), and for staining immune cells, as described.3,7 Plasma viral loads (pVLs) were quantified by real-time PCR.8–10
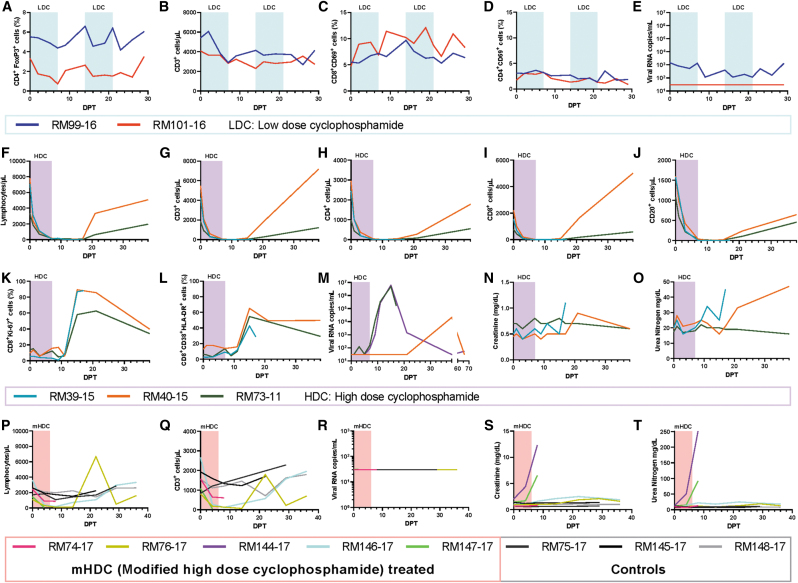
To selectively deplete Tregs, we administered a low-dose Cy (25 mg/day for 7 days, every 2 weeks for 1 month) to two SIV-infected, antiretroviral therapy (ART)-naive RMs at 337 days postinfection (dpi) and monitored the lymphocyte populations. LDC doses achieved a mild Treg depletion as demonstrated by a 42% reduction of the CD4+ FoxP3+ T cell fraction (Fig. 1A). However, Treg depletion was not specific, as 40% of the overall CD3+ T cell population was also depleted by 7 days posttreatment (dpt) (Fig. 1B). Similar trends were also observed for CD4+ and CD8+ T cells and for CD20+ lymphocytes (data not shown). Treg depletion was short-lived, which contrasts with the overall CD3+ cell depletion, which did not recover between treatments. Both LDC treatments decreased the baseline levels of Tregs with a near full recovery occurring within a week (Fig. 1A, B).
FIG. 1.
Comparison of different regimens of cyclophosphamide administration in SIV infection for regulatory T cell depletion and cytoreduction. Parameters of low-dose cyclophosphamide administration for Treg depletion are shown in (A–E). The percentage of CD4+ T cells expressing FoxP3 (Tregs) (A) decreased at each treatment, while CD3+ T cells (B) remained depleted through both treatments. LDC was marked with minimal immune activation as shown with CD69 expression on CD8+ T cells (C) and CD4+ T cells (D), with a reduction in plasma viral load in one animal and no reactivation in the other (E). Figures (F–O) show the parameters of high-dose cyclophosphamide administration to elite controllers for cytoreduction. Total lymphocytes (F), CD3+ T cells (G), CD4+ T cells (H), CD8+ T cells (I), and CD20+ cells (J) were all massively depleted with treatment. Recovery was accompanied by reciprocal immune activation as demonstrated by expression of Ki-67 (K) and HLA-DR/CD38 coexpression (L) on CD8+ T cells. Complimentary viral reactivation (M) occurred during the immune activation, resulting in pVLs reaching the levels of acute infection. Slight increases in kidney biomarkers creatinine (N) and urea nitrogen (O) were observed. Figures (P–T) show the parameters of modified high-dose cyclophosphamide administration in RMs infected with the pathogenic SIVmac239 treated with antiretroviral therapy (ART). Total lymphocytes (P) and CD3+ T cells (Q) were depleted to a lesser extent with the modified regimen and did not result in an observable increase in pVLs (R). However, cyclophosphamide with ART became highly toxic as demonstrated by creatinine (S) and urea nitrogen (T). Treatment durations are marked by highlighted areas on graphs. ART; dpt, day posttreatment; HDC, high-dose cyclophosphamide; LDC, low-dose cyclophosphamide; mHDC, modified high-dose cyclophosphamide; pVLs, plasma viral loads; RMs, rhesus macaques; SIV, simian immunodeficiency virus; Tregs, regulatory T cells.
As expected with Treg depletion, LDC administration was associated with increased CD69 expression on CD8+ T cells, which occurred gradually through 14 dpt, reaching a level nearly twofold over pretreatment (Fig. 1C). Immune activation did not significantly change with the second treatment, remaining elevated through 29 dpt. Conversely, CD69 expression on CD4+ T cells remained level across both treatment phases (Fig. 1D). These results support a failure to specifically deplete Treg cells during LDC administration.
Since the primary endpoint of Treg depletion in these ART-naive animals was viral reactivation,2,3 we also monitored pVLs (Fig. 1E). Before LDC administration, the two SIV-infected, ART-naive RMs receiving LDC were either viremic (RM99, at 1,457 vRNA copies/mL) or below the limit of quantification (RM101, <30 vRNA copies/mL).
Upon LDC treatment, pVLs decreased by an order of magnitude in RM99, were maintained low throughout the follow-up (up to 26 dpt), and returned to baseline by 29 dpt. In RM101, however, no change in pVL was observed throughout the follow-up (Fig. 1E). Because these animals are elite controllers11,12 and RM99 was viremic, it is possible that Treg depletion resulted in a reduction of the suppressive signaling on already active SIV-specific CD8+ T cells, bolstering the anti-SIV response without drastic increases in immune activation. However, since LDC lacked Treg specificity, it is possible that the overall loss of peripheral lymphocytes drove the decreases in pVLs. Overall, these results are lackluster and do not support the use of LDC as a Treg depleting agent for latency reversal.
Three additional SIV-infected, ART-naive RMs were utilized for initial testing of HDC as a cytoreductive agent. Without ART, 7 days of HDC (50 mg/kg/day) beginning at 456 dpi resulted in a pan depletion of peripheral blood cells (Fig. 1F), with CD3+ T cells being depleted from a baseline level of 3,852 to 9 cells/μL at 11 dpt (Fig. 1G). Complete recovery did not occur before 38 dpt, when CD3+ T cell counts reached an average of 4,195 cells/μL. CD4+, CD8+, and CD20+ lymphocytes and even NK cells decreased and recovered in a similar manner to CD3+ T cells (Figs. 1H–J).
Due to this massive cytoreduction, the immune activation levels could not be properly assessed at the nadir of lymphocyte depletion. Yet, with the lymphocyte restoration, high levels of cells expressing Ki-67 (Fig. 1K) and HLA-DR/CD38 (Fig. 1L) were expressed in CD8+ T cells.
Before HDC treatment, in all these ART-naive SIV-infected RM controllers, the virus was undetectable. HDC administration resulted in massive viral reactivation in two of three RMs (Fig. 1M), with detectable pVLs at 3 and 7 dpt in RM39 and RM73, respectively. In both these RMs, viral replication peaked at 15 dpt at 4.9 × 106 (RM39) and 6.2 × 106 (RM73) vRNA copies/mL. Plasma viremia again was undetectable at 38 dpt in RM73 and partially controlled (at 164,490 copies/mL) by 17 dpt in RM39, which then required euthanasia due to treatment-induced severe anemia. In the remaining RM, virus reactivation occurred late and was transient (21,257 copies/mL at 38 dpt), followed by undetectable levels in the next time point.
Because the immune activation was decreasing leading up to 38 dpt and other immune populations had returned to baseline, it is unlikely that the belated reactivation was due to HDC. Interestingly, after HDC administration, two of the RMs demonstrated slightly increased serum creatinine (Fig. 1N) and urea nitrogen (Fig. 1O), beyond the normal range in RMs,13,14 indicating some nephrotoxicity.
With the promising observation of massive viral reactivation and significant cytoreduction observed after HDC administration to SIV-infected ART naive RMs, we next designed a study aimed at reducing/eliminating the viral reservoir in HIV-infected individuals who were chronically infected and on ART at the time of interventions. Eight RMs were infected with barcoded SIVmac239M15 intravenously and received daily subcutaneous injections of a coformulated ART regimen (tenofovir, emtricitabine, and dolutegravir)16 starting from 12 dpi for a duration of 8 months to achieve a durable viral suppression. At 252 dpi, while still on ART, a modified HDC treatment (50 mg/kg, 4 days and 25 mg/kg, 2 days) was administered to five RMs with the remaining three RMs used as controls (RM75, RM145, and RM148) and did not receive HDC.
We hypothesized that this modified dose of HDC would be less toxic to SIV-infected, ART-treated RMs. The new regimen was less effective in inducing cytoreduction than the original one, with 15% residual lymphocytes persisting at the nadir (Fig. 1P) versus <0.1% remaining in the first HDC group. The same was true for the CD3+ T cells (Fig. 1Q). Remarkably, no virus reactivation was observed in these SIV-infected, ART-treated RMs after the modified HDC administration (Fig. 1R), suggesting that the high pVLs observed in the ART-naive RMs likely stemmed from a small reactivation event that was highly exacerbated by de novo infections in the absence of ART.
Unfortunately, after Cy administration, 2 SIV-infected, ART-treated RMs required euthanasia at 8 dpt due to subcutaneous hemorrhaging on limbs, chest, and oral mucosa; these RMs also presented with diarrhea, lethargy, and RM144 developed hemorrhagic cystitis. At necropsy, we observed additional intestinal hemorrhages. Serum creatinine (Fig. 1S) and blood urea nitrogen (Fig. 1T) demonstrated severe nephrotoxicity in these two RMs. These very severe adverse effects prompted study discontinuation.
In conclusion, our study assessing the usefulness of two Cy dosing regimens as different strategies toward an HIV cure did not yield acceptable results. When used for Treg depletion, LDC was not specific, failed to induce viral reactivation, and only increased immune activation by a small amount relative to other Treg depletion agents.2,3 When the HDC was used, a substantial cytoreduction was achieved, but when given with ART, as necessary if used as an HIV therapeutic, generated unacceptable toxicity and morbidity with virtually no viral reactivation. Therefore, further studies of Cy in HIV-infected, ART-treated patients are not advised based upon these findings.
Acknowledgments
We thank Drs. Theresa Whiteside, John Mellors, and Bernard J. Macatangay for helpful discussions and suggestions. We thank Drs. Romas Geleziunas, Joseph Hesselgesser (Gilead Sciences, Foster City, CA), and Katie Kitrinos (ViiV Healthcare, Research Triangle Park, NC) for the help with the antiretrovirals and advice related to the preparation of the coformulation. We also thank Drs. Brigitte Sanders and Peter Perrin of the NIH for their continuous support of our work. Finally, we thank all our laboratory members who helped with this study and this publication.
Authors' Contributions
A.J.K., C.A., I.P., and B.F.K. designed and oversaw the study. A.J.K., R.S., and C.M. processed samples. A.J.K. and R.S. analyzed blood chemistries. A.J.K., R.S., and E.B.-C. performed and analyzed flow cytometry experiments. A.J.K. and P.S. conducted viral quantifications. A.J.K., I.P., and C.A. wrote the article. R.S., P.S., and B.F.K. edited the article. All authors approved the submitted version.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does it mention trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by grant R01 AI119346 (C.A.) from the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) and also from grants R01DK113919 (I.P./C.A.), R01DK119936 (C.A.), RO1 HL117715 (I.P.), R01 HL123096 (I.P.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and National Heart, Lung, and Blood Institute (NHLBI). A.J.K. was supported, in part, by the NIAID and T32 grants Immunology of Infectious Diseases (IID) (AI060525) and Pitt AIDS Research Training (PART) grant (AI065380). B.F.K. is supported with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E and 75N91019D00024. Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1. Ghiringhelli F, Menard C, Puig PE, et al. : Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 2007;56:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He T, Brocca-Cofano E, Policicchio BB, et al. : Cutting edge: T regulatory cell depletion reactivates latent simian immunodeficiency virus (SIV) in controller macaques while boosting SIV-specific T lymphocytes. J Immunol (Baltimore, Md.: 1950) 2016;197:4535–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sivanandham R, Kleinman AJ, Sette P, et al. : Nonhuman primate testing of the impact of different regulatory T cell depletion strategies on reactivation and clearance of latent simian immunodeficiency virus. J Virol 2020;94:e00533–00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kersten MJ, Verduyn TJ, Reiss P, Evers LM, de Wolf F, van Oers MH: Treatment of AIDS-related non-Hodgkin's lymphoma with chemotherapy (CNOP) and r-hu-G-CSF: Clinical outcome and effect on HIV-1 viral load. Ann Oncol 1998;9:1135–1138. [DOI] [PubMed] [Google Scholar]
- 5. Bartlett JA, Miralles GD, Sevin AD, et al. : Addition of cyclophosphamide to antiretroviral therapy does not diminish the cellular reservoir in HIV-infected persons. AIDS Res Hum Retroviruses 2002;18:535–543. [DOI] [PubMed] [Google Scholar]
- 6. Alonso CM, Lozada CJ: Effects of IV cyclophosphamide on HIV viral replication in a patient with systemic lupus erythematosus. Clin Exp Rheumatol 2000;18:510–512. [PubMed] [Google Scholar]
- 7. Kleinman AJ, Xu C, Cottrell ML, et al. : Pharmacokinetics and immunological effects of romidepsin in rhesus macaques. Front Immunol 2020;11:3118–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandrea I, Apetrei C, Dufour J, et al. : Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: A new model for the study of SIV pathogenesis in natural hosts. J Virol 2006;80:4858–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandrea I, Silvestri G, Onanga R, et al. : Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: Common patterns and species-specific differences. J Med Primatol 2006;35:194–201. [DOI] [PubMed] [Google Scholar]
- 10. Policicchio BB, Cardozo EF, Sette P, et al. : Dynamics of simian immunodeficiency virus two-long-terminal-repeat circles in the presence and absence of CD8(+) cells. J Virol 2018;92:e02100–e02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandrea I, Gaufin T, Gautam R, et al. : Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog 2011;7:e1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma D, Xu C, Cillo AR, et al. : Simian immunodeficiency virus SIVsab infection of rhesus macaques as a model of complete immunological suppression with persistent reservoirs of replication-competent virus: Implications for cure research. J Virol 2015;89:6155–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y, Qin S, Ding Y, et al. : Reference values of clinical chemistry and hematology parameters in rhesus monkeys (Macaca mulatta). Xenotransplantation 2009;16:496–501. [DOI] [PubMed] [Google Scholar]
- 14. Koo B-S, Lee D-H, Kang P, et al. : Reference values of hematological and biochemical parameters in young-adult cynomolgus monkey (Macaca fascicularis) and rhesus monkey (Macaca mulatta) anesthetized with ketamine hydrochloride. Lab Anim Res 2019;35:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fennessey CM, Pinkevych M, Immonen TT, et al. : Genetically-barcoded SIV facilitates enumeration of rebound variants and estimation of reactivation rates in nonhuman primates following interruption of suppressive antiretroviral therapy. PLoS Pathog 2017;13:e1006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Del Prete GQ, Smedley J, Macallister R, et al. : Short communication: Comparative evaluation of coformulated injectable combination antiretroviral therapy regimens in simian immunodeficiency virus-infected rhesus macaques. AIDS Res Hum Retroviruses 2016;32:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]