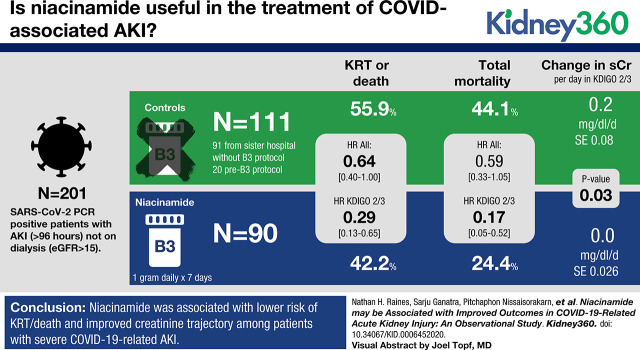
Visual Abstract
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, acute renal failure, clinical trial, COVID-19, NAD+, niacinamide, outcomes, SARS-CoV-2, vitamin B3
Abstract
Background
AKI is a significant complication of coronavirus disease 2019 (COVID-19), with no effective therapy. Niacinamide, a vitamin B3 analogue, has some evidence of efficacy in non-COVID-19-related AKI. The objective of this study is to evaluate the association between niacinamide therapy and outcomes in patients with COVID-19-related AKI.
Methods
We implemented a quasi-experimental design with nonrandom, prospective allocation of niacinamide in 201 hospitalized adult patients, excluding those with baseline eGFR <15 ml/min per 1.73 m2 on or off dialysis, with COVID-19-related AKI by Kidney Disease Improving Global Outcomes (KDIGO) criteria, in two hospitals with identical COVID-19 care algorithms, one of which additionally implemented treatment with niacinamide for COVID-19-related AKI. Patients on the niacinamide protocol (B3 patients) were compared against patients at the same institution before protocol commencement and contemporaneous patients at the non-niacinamide hospital (collectively, non-B3 patients). The primary outcome was a composite of death or RRT.
Results
A total of 38 out of 90 B3 patients and 62 out of 111 non-B3 patients died or received RRT. Using multivariable Cox proportional hazard modeling, niacinamide was associated with a lower risk of RRT or death (HR, 0.64; 95% CI, 0.40 to 1.00; P=0.05), an association driven by patients with KDIGO stage-2/3 AKI (HR, 0.29; 95% CI, 0.13 to 0.65; P=0.03; P interaction with KDIGO stage=0.03). Total mortality also followed this pattern (HR, 0.17; 95% CI, 0.05 to 0.52; in patients with KDIGO stage-2/3 AKI, P=0.002). Serum creatinine after AKI increased by 0.20 (SEM, 0.08) mg/dl per day among non-B3 patients with KDIGO stage-2/3 AKI, but was stable among comparable B3 patients (+0.01 [SEM, 0.06] mg/dl per day; P interaction=0.03).
Conclusions
Niacinamide was associated with lower risk of RRT/death and improved creatinine trajectory among patients with severe COVID-19-related AKI. Larger randomized studies are necessary to establish a causal relationship.
Introduction
Coronavirus disease 2019 (COVID-19) has led to >1,250,000 deaths worldwide and affects a host of organs, including the kidneys. The etiology of COVID-19-related AKI is likely multifactorial, but most often exhibits an acute tubular injury pattern (1). Acute tubular injury arises from inflammatory, ischemic, or nephrotoxic stressors. Consequences of COVID-19-related AKI include severe shortages of dialysis machines and fluids, strained nursing resources, and increased mortality (2,3).
Previous work links non-COVID-19 AKI arising in the context of renal ischemia and acute tubular necrosis to an acquired renal deficiency of the energy metabolism intermediate NAD+, a rate-limiting substrate for oxidative metabolism in mitochondria, through accelerated hydrolysis and decreased biosynthesis (4,5). In the mitochondria-rich renal tubule, a stress-induced NAD+ shortage curtails ATP production, which, if unchecked, can culminate in tubular dysfunction and cell death (5). A pilot, randomized, placebo-controlled trial suggested that oral niacinamide could safely increase NAD+ and potentially prevent AKI among patients who were perioperative, but it has not been tested in COVID-19-related AKI (4). There are no therapies for AKI prevention approved by the US Food and Drug Administration (FDA).
Niacinamide, the base form of vitamin B3, is a supplement (considered by the FDA as “generally recognized as safe”) whose safety at chronic high doses has been established in trials for other indications (4,5). Given its safety, immediate availability, and limited, but encouraging, evidence of efficacy in non-COVID-19 AKI, we implemented a niacinamide protocol for patients with AKI, at a single hospital in Boston, designed to prevent progression of AKI and development of related complications.
In this report, we compare rates of progression to RRT and death and trends in serum creatinine among patients with COVID-19-related AKI on the niacinamide protocol with patients both admitted at the niacinamide hospital before implementation and contemporaneously admitted at a nonintervention hospital. We hypothesized that administration of oral niacinamide would prevent progression and reduce complications associated with COVID-19-related AKI.
Materials and Methods
Setting
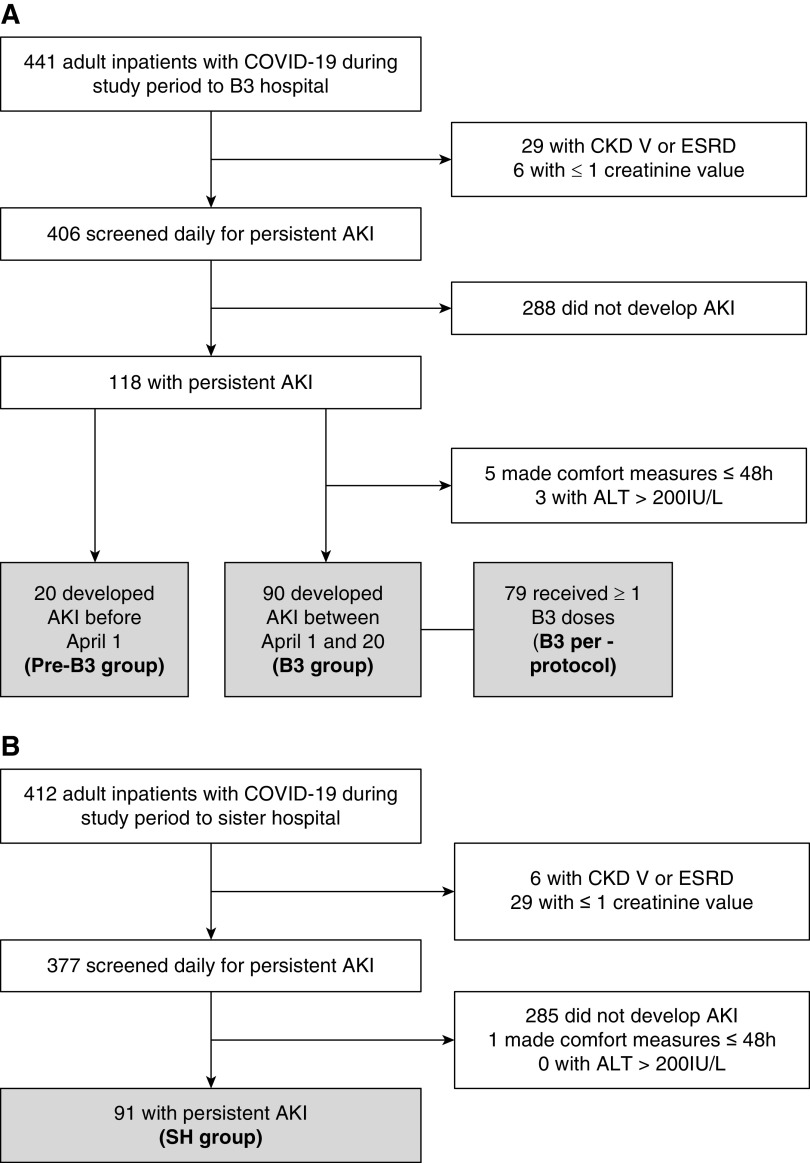
We conducted this study at two large tertiary acute-care teaching hospitals within the Beth Israel Lahey Health System (BILH) that admitted comparable numbers of patients with COVID-19 during the study period (Figure 1). One hospital implemented the niacinamide protocol as a routine part of clinical care on the basis of data in postoperative AKI (4). Both hospitals otherwise used a single COVID-19 care algorithm common to BILH.
Figure 1.
Patient flow at niacinamide protocol hospital (top) and sister hospital (bottom). ALT, alanine aminotransferase; B3, niacinamide protocol; COVID-19, coronavirus disease 2019; SH, sister hospital not implementing niacinamide protocol.
Study Design and Oversight
We used a quasi-experimental design, chosen for its ability to be rapidly implemented using existing resources and to preserve eligibility for other potential life-saving therapies available only through clinical trials (6). Oversight is described in Supplemental Materials 1 and 2.
Eligibility Criteria
We included hospitalized patients who developed persistent AKI, were at least 18 years old, had a laboratory-confirmed PCR test positive for severe acute respiratory syndrome coronavirus 2 from a nasopharyngeal swab, and were admitted to either hospital between March 15 and April 20, 2020. “Persistent AKI” was defined as a ≥0.3 mg/dl increase in serum creatinine over baseline, excluding individuals with AKI on admission that resolved to baseline with routine supportive care within 96 hours. These AKI criteria were used to enable implementation in a pragmatic fashion, with a 96-hour cutoff chosen to decrease the likelihood of including patients with prerenal azotemia. Individuals with baseline eGFR <15 ml/min per 1.73 m2 both on and off dialysis, or those who elected to receive comfort measures only up to 48 hours after development of AKI were excluded. Given the hepatic metabolism of niacinamide, individuals with alanine aminotransferase (ALT) more than five times the upper limit of normal (200 IU/L) were excluded.
Protocol Implementation
The dose of niacinamide was informed by trials with long-term exposure in patients without kidney disease (7–9) and in patients with advanced kidney disease (10,11) that affirmed its safety. Beginning April 2, 2020, nephrologists at the niacinamide protocol hospital performed daily chart review of all inpatients positive for COVID-19 to determine whether they met eligibility criteria. Once a patient became eligible, care teams were contacted via a templated note and by pager with the elective recommendation to begin oral niacinamide, 1 g once daily, for a 7-day course; patients could receive doses through orogastric or nasogastric feeding tubes if needed. A total of 79 out of 90 eligible patients (88%) received one or more niacinamide doses. Niacinamide was stopped in patients whose ALT rose more than three times their respective values on the day of eligibility. Care teams could also discontinue niacinamide at their discretion.
Data Sources and Variables Assessed
Data were obtained by review of the electronic medical record. Baseline creatinine was defined as the most recent serum creatinine in the 7–365 days before admission; when none was available, the lowest creatinine value within the first 96 hours of hospitalization was used. Severity of AKI was defined according to Kidney Disease Improving Global Outcomes (KDIGO) creatinine-based criteria (12). Further details are provided in Supplemental Materials 1 and 2 (Supplemental References).
Exposure and Comparison Group Designation
Eligible patients developing AKI on or after April 1, 2020 in the niacinamide protocol hospital were designated the “B3” group; those who developed AKI before April 1 were designated the “pre-B3” group. Eligible patients at the sister hospital not implementing the niacinamide protocol were designated the “SH” group. The pooled pre-B3 group and SH group was designated the “non-B3” group.
End Points
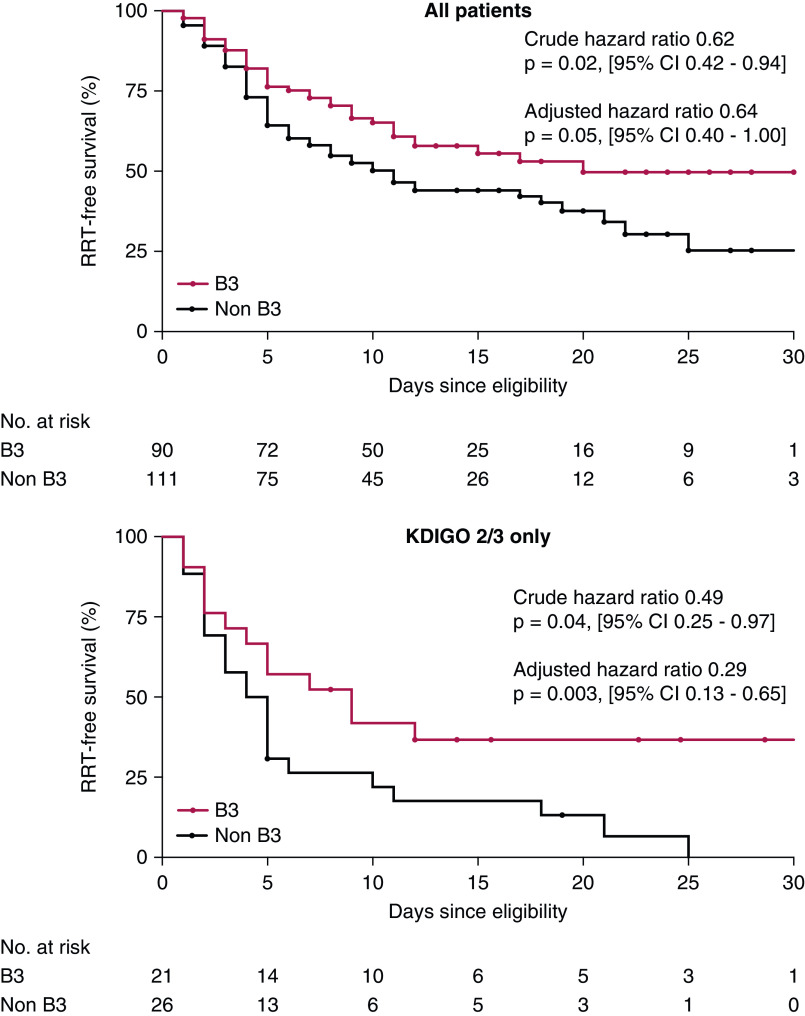
The primary end point was the time in days from date of eligibility to RRT or death, whichever occurred first, during the hospitalization (13). Data collection was censored at time of hospital discharge or on May 1, 2020; censoring events are reported in Figure 2. Secondary end points were time in days from date of eligibility to death alone and RRT alone and change in serum creatinine over the 10 days after the date of eligibility, with censoring at time of RRT, death, or discharge.
Figure 2.
Freedom from composite end point of RRT or death. Niacinamide was associated with lower risk of RRT or death over the 30-day period following development of AKI, an association driven by patients with KDIGO stage-2/3 AKI.
Statistical Analyses
Multivariable Cox proportional-hazards regression models were used to estimate the adjusted association between B3 group and the primary composite end point, and secondary end points of death alone and RRT alone. Models included age, sex, comorbid conditions, medications taken before admission, laboratory values on day of eligibility, and whether individuals received intensive care unit (ICU)–level care. Variables included in the multivariate model were selected on the basis of biologic plausibility and clinical relevance. Laboratory measurements were taken from distinct samples. Analyses were performed on an intention-to-treat basis. Two patients missing one or more covariates were excluded from multivariate analyses.
We designed one a priori subgroup analysis, comparing individuals with KDIGO stage-1 versus KDIGO stage-2 or -3 AKI using a multiplicative interaction term. One additional patient with missing baseline creatinine was excluded from these analyses.
Our secondary analysis used a generalized estimating equation model with an exchangeable correlation matrix to evaluate the secular trend in serum creatinine in the 10 days after the day of eligibility. Similar to the primary analysis, we evaluated patients in KDIGO stages 2 and 3 separately from patients with stage 1 AKI. Additional details, including sensitivity analyses, are reported in Supplemental Material 1. Statistical analyses were performed with SAS software, version 9.4.
Results
Characteristics of Cohorts
A total of 441 patients with COVID-19 were admitted to the intervention hospital and 412 patients were admitted to the nonintervention hospital between March 15 and April 19, 2020 (Figure 1). Of these, approximately a quarter developed persistent AKI at each hospital. Following exclusions, 90 patients were included in the B3 group and 111 were included in the non-B3 group, of which 20 were from the pre-B3 group and 91 were from the SH group.
Baseline creatinine and the proportion of patients with a baseline eGFR <45 ml/min per 1.73 m2 were similar between the B3 and non-B3 groups (Table 1). The frequency of AKI and distribution of AKI severity at the time of eligibility were also similar. Groups differed according to age, race, chronic obstructive pulmonary disease/asthma, smoking history, and administration of other COVID-19-targeted therapies during the same hospitalization. On the date of meeting eligibility, creatinine and AKI stage were similar across groups. Groups differed by hemoglobin, platelet count, potassium level, requirement for ICU-level care, and vasopressor medication support. Supplemental Table 1 shows the baseline characteristics with the pre-B3 and SH groups separated.
Table 1.
Characteristics of patients in the B3 group and non-B3 group
| Characteristics | Non-B3 (n=111) | B3 (n=90) |
| Age (yr), mean (SD) | 73.1 (12.4) | 65.6 (15.2) |
| Female, n (%) | 44 (40) | 40 (44) |
| Race, n (%) | ||
| White non-Hispanic | 91 (82) | 27 (30) |
| Black non-Hispanic | 11 (10) | 31 (34) |
| Hispanic | 5 (5) | 13 (14) |
| Asian | 2 (2) | 3 (3) |
| Other | 2 (2) | 16 (18) |
| BMI (kg/m2), mean (SD) | 30.4 (7.8) | 32.2 (7.9) |
| Baseline creatinine (mg/dl), mean (SD) | 1.17 (0.47) | 1.20 (0.55) |
| eGFR <45 ml/min per 1.73 m2 at baseline, n (%) | 24 (22) | 22 (25) |
| Past diagnoses, n (%) | ||
| COPD/asthma | 30 (27) | 13 (14) |
| Diabetes mellitus | 49 (44) | 50 (56) |
| Hypertension | 94 (85) | 70 (78) |
| HF with reduced EF | 10 (9) | 5 (6) |
| Malignancy | 17 (15) | 20 (22) |
| Current or former tobacco use | 62 (56) | 34 (39) |
| Baseline medications, n (%) | ||
| Statin | 76 (69) | 62 (69) |
| ACEi/ARB | 48 (43) | 33 (37) |
| In-hospital medications, n (%) | ||
| Hydroxychloroquine | 80 (72) | 40 (44) |
| Azithromycin | 73 (66) | 51 (57) |
| Remdesivira | 2 (2) | 11 (12) |
| Sarilumaba | 0 (0) | 18 (20) |
| Tocilizumab | 13 (12) | 10 (11) |
| Characteristics at day of eligibility | ||
| Creatinine (mg/dl), mean (SD) | 2.02 (1.35) | 2.10 (1.32) |
| KDIGO AKI stage, n (%) | ||
| Stage 1 | 85 (77) | 68 (76) |
| Stage 2 | 16 (14) | 13 (15) |
| Stage 3 | 10 (9) | 8 (9) |
| Hemoglobin (g/dl), mean (SD) | 11.4 (1.90) | 10.7 (2.19) |
| WBC count (K/μl), mean (SD) | 8.62 (4.16) | 9.41 (5.20) |
| Platelet count (K/μl), mean (SD) | 216 (108) | 255 (130) |
| Bicarbonate (mEq/L), mean (SD) | 22.6 (4.93) | 23.5 (4.97) |
| Potassium (mEq/L), mean (SD) | 4.22 (0.61) | 4.38 (0.56) |
| On vasopressors, n (%) | 34 (31) | 52 (58) |
| On mechanical ventilation, n (%) | 46 (41) | 56 (62) |
| In ICU, n (%) | 53 (48) | 63 (70) |
Non-B3, individuals not receiving niacinamide; B3, individuals receiving niacinamide; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HF, heart failure; EF, ejection fraction; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; KDIGO, Kidney Disease Improving Global Outcomes; WBC, white blood cell; ICU, intensive care unit.
Patients were enrolled in a clinical trial of remdesivir or sarilumab, and it is unknown if they were receiving study drug or placebo.
Primary End Point
Over a median follow-up of 11 days after the date of eligibility (13 days in the B3 group, 9 days in the non-B3 group), 71 of 201 patients (35%) died and 46 of 201 (23%) received RRT across all groups. Table 2 reports the proportion of patients meeting the primary end point, along with each component separately in each group. Unadjusted time-to-event analysis demonstrated a significantly lower hazard for the composite end point of RRT or death in the B3 group compared with the non-B3 group, with particularly lower risk in the subset of patients with KDIGO AKI stage 2 or 3 (among all patients, hazard ratio [HR], 0.62; 95% CI, 0.42 to 0.94; among patients with KDIGO stage-2/3 AKI, HR, 0.29; 95% CI, 0.13 to 0.65; Figure 2, Table 2). The corresponding multivariable HR for all patients was 0.64 (95% CI, 0.40 to 1.00; Table 2).
Table 2.
Association between niacinamide use group and primary and secondary end points in the entire cohort and among KDIGO subgroups
| End Point | Non-B3 (n=111) | B3 (n=90) | P |
| RRT or death, n (%) | 62 (56) | 38 (42) | |
| Unadjusted HR (95% CI) | Ref | 0.62 (0.42 to 0.94) | 0.02 |
| Adjusted HR (95% CI)a | Ref | 0.64 (0.40 to 1.00) | 0.05 |
| Adjusted HR (95% CI) | |||
| In patients with KDIGO stage-1 AKI b | Ref | 0.88 (0.51 to 1.53) | 0.66 |
| In patients with KDIGO stage-2/3 AKI c | Ref | 0.29 (0.13 to 0.65) | 0.003 |
| KDIGO interaction | 0.03 | ||
| Death alone, n (%) | 49 (44.1) | 22 (24.4) | |
| Unadjusted HR (95% CI) | Ref | 0.45 (0.27 to 0.74) | 0.002 |
| Adjusted HR (95% CI)a | Ref | 0.59 (0.33 to 1.05) | 0.07 |
| Adjusted HR (95% CI) | |||
| In patients with KDIGO stage-1 AKI b | Ref | 1.06 (0.53 to 2.11) | 0.87 |
| In patients with KDIGO stage-2/3 AKI c | Ref | 0.17 (0.05 to 0.52) | 0.002 |
| KDIGO interaction | 0.008 | ||
| RRT alone, n (%) | 23 (21) | 23 (26) | |
| Unadjusted HR (95% CI) | Ref | 1.11 (0.62 to 1.99) | 0.72 |
| Adjusted HR (95% CI)a | Ref | 1.02 (0.52 to 2.02) | 0.95 |
| Adjusted HR (95% CI) | Ref | ||
| In patients with KDIGO stage-1 AKI b | Ref | 1.09 (0.46 to 2.78) | 0.84 |
| In patients with KDIGO stage-2/3 AKI c | Ref | 0.73 (0.25 to 2.16) | 0.57 |
| KDIGO interaction | 0.56 |
Non-B3, individuals not receiving niacinamide; B3, individuals receiving niacinamide; HR, hazard ratio; KDIGO, Kidney Disease Improving Global Outcomes.
Model adjusted for age; sex; history of diabetes, hypertension, malignancy, and heart failure with reduced ejection fraction; hemoglobin, leukocyte count, platelet count, serum creatinine, potassium, and bicarbonate on the day of eligibility; preadmission use of hepatic hydroxymethyl glutaryl–CoA reductase inhibitors, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers; and requirement of the intensive care unit on day of eligibility. N=109 in the non-B3 group; N=90 in the B3 group.
Model adjusted for the same variables as above. N=83 in the non-B3 group; N=68 in the B3 group.
Model adjusted for the same variables as above. N=26 in the non-B3 group; N=21 in the B3 group.
The adjusted association between risk of RRT or death and niacinamide implementation was significantly modified by KDIGO stage at the time of eligibility (P interaction=0.03). Among patients with KDIGO stage-1 AKI, we observed no reduced hazard for death or RRT. In contrast, niacinamide was associated with lower risk of the primary outcome among patients with more severe AKI (Table 2).
Secondary End Points
When components of the composite end point were analyzed separately, the association with death was similar to that for the combined end point. As with the primary outcome, the association of niacinamide with mortality was significantly modified by KDIGO stage (P interaction=0.008), with no significant association among patients with KDIGO stage-1 AKI, but approximately 80% lower hazard among patients with KDIGO stage-2/3 AKI in the B3 group compared with the non-B3 group. The corresponding estimates for RRT alone were all nonsignificant.
Creatinine on the day of eligibility was similar between the B3 and non-B3 groups (Table 1). Over the 10 days after eligibility, we did not observe an overall significant difference in creatinine trends between the B3 and non-B3 groups (P=0.54). However, like the primary composite end point and mortality, there were apparent differences on the basis of AKI severity.
Among patients with KDIGO stage-2/3 AKI, creatinine values were similar in both groups on the day of eligibility (3.16 mg/dl in B3; 3.17 in non-B3; P=0.99). These values increased significantly over the ensuing 10 days in the non-B3 group by an average of 0.20 (SEM, 0.08) mg/dl per day, whereas they remained flat in the B3 group (change of 0.01 [SEM, 0.06] mg/dl per day; P=0.89). The adjusted difference in these trends was statistically significant (P=0.03). In contrast, creatinine demonstrated no significant linear trend over 10 days among patients with KDIGO stage-1 AKI, regardless of treatment group (change of 0.003 [SEM, 0.04] mg/dl per day in the non-B3 group; 0.007 [SEM, 0.02] mg/dl in the B3 group; P=0.93).
Safety
Nine of 79 (11%; 95% CI, 5% to 21%) individuals receiving niacinamide in the B3 group had the medication stopped because of a three-fold increase in ALT compared with the first day they received niacinamide. Transaminitis (ALT >200 IU/L) occurred in 13 out of 88 individuals (15%; 95% CI, 7% to 22%) in the B3 group who had at least one ALT value measured post-AKI. No other adverse events were reported in the B3 group. Whereas ALT was measured within 24 hours of the day of eligibility and at least once in the 10 days afterward in 88 of 90 (98%) patients in the B3 group, these data were available in only 49 out of 91 (54%) patients in the SH group.
Sensitivity Analyses
Supplemental Table 2 repeats the time-to-event analyses in Table 2, but with the pre-B3 and SH groups analyzed separately. We observed no significant difference between the pre-B3 and SH groups for any outcome.
We examined the association of calendar date with outcome in each treatment group and found no confounding by ongoing secular trends (Supplemental Table 3).
Analysis of the 79 patients within the B3 group who actually received niacinamide showed a similarly reduced hazard for the primary end point in the adjusted time-to-event analysis when compared with the non-B3 group (Supplemental Table 4).
Repeated primary analyses with additional adjustment or exclusions to ensure robustness yielded highly concordant results (Supplemental Table 5), as did analyses using a single prognostic score covariate in place of separated covariates in the model (Supplemental Table 6). Baseline characteristics of the B3 and non-B3 groups in the subset of individuals with KDIGO stage-2/3 AKI are shown in Supplemental Table 7.
Discussion
In this quasi-experimental study conducted in two large hospitals, implementation of a niacinamide protocol among patients with COVID-19-related AKI was associated with lower risk for the composite of RRT or death, driven primarily by a lower risk among the subset of patients with KDIGO stage-2 or -3 AKI. Secondary end point analysis showed that the difference in composite outcome was driven by mortality and not RRT. Patients with KDIGO stage-2/3 AKI in the B3 group demonstrated stable creatinine in the 10 days after diagnosis, whereas those in the non-B3 group had increasing creatinine by an average of 0.2 mg/dl per day.
COVID-19 AKI affected 27% of patients hospitalized with COVID-19 across the two institutions in this study. This proportion is between the 22% and 36% described among hospitalized patients in reports from New York City (14,15). Given the substantial increase in mortality reported with AKI in patients hospitalized with COVID-19, the high frequency of AKI represents a profound risk among patients who are already vulnerable.
The renal tubule is a known target of non-COVID-19 AKI, requiring constant energy from mitochondria for active solute transport. Ischemic, inflammatory, and toxic stressors reduce renal NAD+, compromising transport function (5,16). Part of this shortfall may result from a local decrease of niacinamide and other biosynthetic precursors (4,5,17). If this energetic deficit persists, acute tubular injury arises. Our results demonstrate that niacinamide supplementation had no significant association with measured outcomes in patients with KDIGO stage-1 AKI. On average, these individuals had no net increase in serum creatinine after meeting eligibility regardless of niacinamide administration, suggesting they were likely to recover on their own regardless of therapy. Although speculative, individuals with mild AKI may not be sufficiently NAD+ deficient to benefit from NAD+ boosting. Consistently, experimental AKI models exhibit a proportional relationship between renal NAD+ reduction and AKI severity (5). Of note, results from the RECOVERY Trial demonstrate a similar trend for dexamethasone and mortality, with benefit seen only among patients who are sicker (18).
The absence of association between niacinamide and RRT is notable. Three potential factors may have contributed to this finding. First, all patients in our study have AKI and, hence, mortality among patients with AKI is essentially a test of AKI prognosis. Second, as we discuss in our limitations below, timing of RRT may be less objective than mortality. Third, although we observed a strong association with mortality, we also observed a statistically significant association with improved creatinine trend. Creatinine trend and death may provide objective bookend indicators of the true effect, which is likely to be moderate.
Experimental results suggest that NAD+ augmentation may not only protect the kidney, but also fortify brain, heart, lung, liver, and even vasculature against disease (19). Safety analyses showed that, despite the subset of patients with AKI being sicker than the overall patient population hospitalized with COVID-19, rates of transaminitis in individuals receiving niacinamide were comparable with those reported previously, although true rates of liver injury from COVID-19 remain poorly understood (20). Although this study was focused on renal outcomes, the association of niacinamide with a reduction in mortality among patients who are severely ill supports further investigation of NAD+ augmentation in preventing and treating extrarenal complications of COVID-19.
Until an effective vaccine for COVID-19 is available, modulating host susceptibility to adverse outcomes will also remain important. Compared with pharmacologic modulation of host susceptibility—e.g., with IL-6 inhibition—niacinamide is distinguished by its unique safety profile as a nutritional supplement generally recognized as safe and its inexpensiveness. High-dose niacinamide has been safely administered for months to patients with advanced CKD (10). Compared with other NAD+ precursors, niacinamide also has the theoretic advantage of inhibiting stress-induced enzymes such as CD38 and poly-ADP ribose polymerases that hydrolyze NAD+ and may be deleterious to organ function (19).
Our study has important limitations. Niacinamide outcomes reflect a single-center experience. We made a practical decision to offer niacinamide as standard treatment, precluding placebo controls. Our comparators were, therefore, either historical from the same hospital or contemporary from another hospital. This precludes determination of causality. There was a significant age disparity between the B3 and non-B3 groups, and there was disparity in race, certain comorbidities, concurrent therapies, and rates of ICU utilization. We performed statistical adjustments to address these differences and, although age and certain comorbidities were significantly associated with outcomes, they did not substantially alter the association between B3 and our composite end point, mortality alone, or creatinine trends after AKI. Of note, the age discrepancy was mitigated in the subset of patients with KDIGO stage-2/3 AKI, among whom the associations were strongest. We also performed separate sensitivity analyses for variables of interest that could not be included in the primary multivariate model, all of which yielded similar results. Unmeasured confounding is, nonetheless, still likely to have affected our results; the majority of patients in the non-B3 group were not at the same hospital as the B3 group, introducing different processes of care despite the use of the same COVID-19 care algorithm. Availability of prior creatinine values to determine an accurate baseline was variable, and may have led to misclassification of AKI, particularly in the KDIGO stage-1 AKI group. Furthermore, whereas KDIGO guidelines support the use of a baseline creatinine against which to evaluate in-hospital changes, we acknowledge that application of a 0.3 mg/dl change could include too many individuals with modest creatinine elevation that is not consistent with AKI. However, inclusion of such individuals is balanced between the groups and would tend to bias the results toward the null. Timing of RRT initiation can be subjective, which, in the context of an open-label trial, introduces the potential for bias. However, mean serum creatinine at the time of RRT initiation was indistinguishable between the groups. Moreover, mortality was the primary driver of the composite end point rather than time to RRT initiation, which did not significantly differ between institutions. The large effect size on the composite outcome and mortality alone we report in the KDIGO stage-2/3 AKI group should not be considered definitive evidence of the magnitude of niacinamide’s effect in this group. Finally, the number of patients was relatively small, increasing the likelihood that the large differences observed here may not hold when applied to a broader population. Only a large randomized trial can establish whether niacinamide positively affects outcomes in COVID-19-related AKI.
This quasi-experimental study found that niacinamide administered for the prevention of COVID-19-related AKI progression was safe and associated with reduced estimated risk of death or the need for RRT compared with historical controls and contemporaneous patients from a sister hospital. The association was strongest in severe AKI. Creatinine levels also stabilized among patients with severe AKI receiving niacinamide. Larger randomized studies of niacinamide in patients with COVID-19 are needed to confirm these apparent benefits.
Disclosures
A. Asnani reports being a scientific advisor or members of Sarnoff Cardiovascular Research Foundation, and receiving honoraria from UpToDate. R.S. Brown received royalties from Börm Bruckmeier Publishing LLC for a book and two applications, Nephrology Pocket and Acid Base and Electrolytes, on smartphones, with the second edition of Nephrology Pocket to be published by Elsevier, Inc. In R.S. Brown’s role at Harvard Medical Faculty Physicians (HMFP) at Beth Israel Deaconess Medical Center, he serves as an associate medical director of DaVita Dialysis Center (Brookline, MA). All income paid by this facility goes directly to HMFP, from which he receives a fixed salary. He also reports receiving honoraria from Harvard Medical School Department of Continuing Education, Beth Israel Deaconess Medical Center; having ownership interest in Innovative Wellness Systems, Inc.; being a member of the medical advisory board of the National Kidney Foundation of New England; and being on the board of directors and president of The Organization for Renal Care in Haiti (TORCH) Inc., a nonprofit, charitable corporation in Massachusetts. No income is received from any of these entities. K. Mukamal reports having other interests/relationships with US Highbush Blueberry Council and Wolters Kluwer. S.M. Parikh is listed as an inventor on patent filings from Beth Israel Deaconess Medical Center related to NAD+. S.M. Parikh reports having consultancy agreements with Aerpio, Alkermes, Astellas, Cytokinetics, Hope Pharmaceuticals, Janssen, Leerink Swann, Mission Therapeutics, and Mitobridge; being a scientific advisor for or member of Aerpio, JASN, Kidney360, and Raksana; receiving consulting fees in the last 3 years from Alkermes, Astellas, Cytokinetics, Daiichi Sankyo, Janssen, and Mission Therapeutics; receiving honoraria from American Society of Nephrology; receiving research funding from Baxter; and having ownership interest in Eunoia and Raksana. Work in S.M. Parikh’s laboratory is supported by National Heart, Lung, and Blood Institute grant R35-HL139424, National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK095072, and National Institute on Aging grant R01-AG027002. A. Poyan Mehr reports receiving research funding from ASN Carl W. Gottschalk Research Scholar Grant (2018) and Retrophin; being the site principle investigator for the DUPLEX Study (a phase-3 randomized trial in primary FSGS); having industry-sponsored clinical trial agreements with OMEROS, Roche, and Vertex; being the director of the GlomCon Project, an initiative to enhance education about glomerular disorders. This project is collaborating closely with the NephCure Foundation, which has provided financial and in-kind support for a continuing medical education–accredited conference series focused on clinical trials. A. Poyan Mehr has additional educational collaborations with the Renal Pathology Society and the European Renal Association. A. Poyan Mehr’s employer does not permit serving on any speaker bureau, advisory board, perform consultancy, or receive industry honoraria. N.H. Raines reports being a scientific advisor for or member of La Isla Network. J. Schlondorff reports being a scientific advisor for or member of the Alport Syndrome Foundation Medical Advisory Committee and having patents and inventions with Partners Healthcare. T.I. Steinman reports being a reviewer for CJASN and a member of the editorial board for Nephrology News and Issues; receiving research funding from Kadmon, Reata, and Retrophin; receiving honoraria from Mallinkrodt and Otsuka; being on the medical advisory board for the National Kidney Foundation of Massachusetts, Rhode Island, New Hampshire, and Vermont; and having other interests/relationships with National Kidney Foundation and Polycystic Kidney Foundation. M.L. Zeidel reports being a scientific advisor for or member of the Beth Israel Deaconess Medical Center and Hebrew Senior Life. All remaining authors have nothing to disclose.
Funding
N.H. Raines is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant T32-DK007199.
Acknowledgments
The authors would like to thank members of the Division of Nephrology at Beth Israel Deaconess Medical Center for outstanding patient care under duress, generous input, and enthusiastic support of this protocol. They also thank the Department of Pharmacy and the Pharmacy and Therapeutics Committee at Beth Israel Deaconess Medical Center.
Author Contributions
A. Asnani, R.S. Brown, S.M. Parikh, M. Sadrolashrafi, and M.L. Zeidel were responsible for resources; A. Asnani, S. Ganatra, A. Morales, P. Nissaisorakarn, A. Pandit, S.M. Parikh, N.H. Raines, and M. Sadrolashrafi were responsible for project administration; V. Bang, S. Brar, S.S. Dani, R. Maheshwari, A. Morales, P. Nissaisorakarn, A. Pandit, S. M. Parikh, R. Patel, N.H. Raines, M. Sadrolashrafi, K. Shreyder, and A. Singh were responsible for investigation; R. Bhargava, S. Ganatra, P. Nissaisorakarn, S.M. Parikh, N. H. Raines, J. Schlondorff, and T. I. Steinman provided supervision; R.S. Brown, S.M. Parikh, A. Poyan Mehr, N.H. Raines, and M.L. Zeidel conceptualized the study; S. Ganatra, K.J. Mukamal, S.M. Parikh, and N.H. Raines wrote the original draft; S. Knapp, K.J. Mukamal, S.M. Parikh, and N.H. Raines were responsible for data curation; K.J. Mukamal, S.M. Parikh, A. Poyan Mehr, and N.H. Raines were responsible for methodology; K.J. Mukamal, S.M. Parikh, and N.H. Raines reviewed and edited the manuscript; K.J. Mukamal and N.H. Raines were responsible for formal analysis; and S.M. Parikh was responsible for funding acquisition.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author S.M. Parikh, pending approval from our institutional review board. The data are not publicly available because they contain information that could compromise research participant privacy.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0006452020/-/DCSupplemental.
Baseline characteristics of the B3, Pre-B3, and SH groups Download Supplemental Table 1, PDF file, 693 KB (692.1KB, pdf)
Associations among B3 group, pre-B3 group, and sister hospital (SH) for composite endpoint of RRT or death, death alone, and RRT alone. Download Supplemental Table 2, PDF file, 693 KB (692.1KB, pdf)
Association between AKI date and primary and secondary outcomes in B3 hospital and sister hospital. Download Supplemental Table 3, PDF file, 693 KB (692.1KB, pdf)
Adjusted time-to-event analysis in subset of B3 group receiving one or more doses of niacinamide (per-protocol) compared to non-B3 group. Download Supplemental Table 4, PDF file, 693 KB (692.1KB, pdf)
Additional sensitivity analyses exploring the association between niacinamide use group and primary and secondary endpoints in the entire cohort and among KDIGO subgroups. Download Supplemental Table 5, PDF file, 693 KB (692.1KB, pdf)
Association between niacinamide use group and primary and selected secondary endpoints in the entire cohort and among KDIGO subgroups using prognostic scoring. Download Supplemental Table 6, PDF file, 693 KB (692.1KB, pdf)
Characteristics of patients with KDIGO stage 2 or 3 AKI in B3 group and non-B3 groups. Download Supplemental Table 7, PDF file, 693 KB (692.1KB, pdf)
Supplemental Methods. Download Supplemental Material 1, PDF file, 693 KB (692.1KB, pdf)
Study protocol. Download Supplemental Material 2, PDF file, 693 KB (692.1KB, pdf)
Download Supplemental References, PDF file, 693 KB (692.1KB, pdf)
References
- 1.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abelson R, Fink S, Kulish N, Thomas K: An overlooked, possibly fatal coronavirus crisis: A dire need for kidney dialysis. New York Times, 2020.
- 3.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyan Mehr A, Tran MT, Ralto KM, Leaf DE, Washco V, Messmer J, Lerner A, Kher A, Kim SH, Khoury CC, Herzig SJ, Trovato ME, Simon-Tillaux N, Lynch MR, Thadhani RI, Clish CB, Khabbaz KR, Rhee EP, Waikar SS, Berg AH, Parikh SM: De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat Med 24: 1351–1359, 2018. 10.1038/s41591-018-0138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM: PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531: 528–532, 2016. 10.1038/nature17184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators: Remdesivir for 5 or 10 days in patients with severe covid-19. N Engl J Med 383: 1827–1837, 2020. 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale EA, Bingley PJ, Emmett CL, Collier T; European Nicotinamide Diabetes Intervention Trial (ENDIT) Group: European Nicotinamide Diabetes Intervention Trial (ENDIT): A randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363: 925–931, 2004. 10.1016/S0140-6736(04)15786-3 [DOI] [PubMed] [Google Scholar]
- 8.Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA; European Nicotinamide Diabetes Intervention Trial Group: Safety of high-dose nicotinamide: A review. Diabetologia 43: 1337–1345, 2000. 10.1007/s001250051536 [DOI] [PubMed] [Google Scholar]
- 9.Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, Scolyer RA, Dhillon HM, Vardy JL, Kricker A, St George G, Chinniah N, Halliday GM, Damian DL: A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med 373: 1618–1626, 2015. 10.1056/NEJMoa1506197 [DOI] [PubMed] [Google Scholar]
- 10.Ix JH, Isakova T, Larive B, Raphael KL, Raj DS, Cheung AK, Sprague SM, Fried LF, Gassman JJ, Middleton JP, Flessner MF, Block GA, Wolf M: Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: The COMBINE trial. J Am Soc Nephrol 30: 1096–1108, 2019. 10.1681/ASN.2018101058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DWA: A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol 3: 1131–1138, 2008. 10.2215/CJN.04211007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO): 2012. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury (AKI). Available at: https://kdigo.org/guidelines/acute-kidney-injury/. Accessed March 25, 2020
- 13.Palevsky PM: Endpoints for clinical trials of acute kidney injury. Nephron 140: 111–115, 2018. 10.1159/000493203 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium: Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; the Northwell COVID-19 Research Consortium: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area [published correction appears in JAMA 323: 2098, 2020 10.1001/jama.2020.7681]. JAMA 323: 2052–2059, 2020. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch MR, Tran MT, Ralto KM, Zsengeller ZK, Raman V, Bhasin SS, Sun N, Chen X, Brown D, Rovira II, Taguchi K, Brooks CR, Stillman IE, Bhasin MK, Finkel T, Parikh SM: TFEB-driven lysosomal biogenesis is pivotal for PGC1α-dependent renal stress resistance. JCI Insight 5: e126749, 2019. 10.1172/jci.insight.126749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, Ryu D, Cialabrini L, Matilainen O, Liscio P, Giacchè N, Stokar-Regenscheit N, Legouis D, de Seigneux S, Ivanisevic J, Raffaelli N, Schoonjans K, Pellicciari R, Auwerx J: De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 563: 354–359, 2018. 10.1038/s41586-018-0645-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ; RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with covid-19 – preliminary report [published online ahead of print July 17, 2020]. N Engl J Med [Google Scholar]
- 19.Katsyuba E, Auwerx J: Modulating NAD+ metabolism, from bench to bedside. EMBO J 36: 2670–2683, 2017. 10.15252/embj.201797135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Shi L, Wang FS: Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol 5: 428–430, 2020. 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of the B3, Pre-B3, and SH groups Download Supplemental Table 1, PDF file, 693 KB (692.1KB, pdf)
Associations among B3 group, pre-B3 group, and sister hospital (SH) for composite endpoint of RRT or death, death alone, and RRT alone. Download Supplemental Table 2, PDF file, 693 KB (692.1KB, pdf)
Association between AKI date and primary and secondary outcomes in B3 hospital and sister hospital. Download Supplemental Table 3, PDF file, 693 KB (692.1KB, pdf)
Adjusted time-to-event analysis in subset of B3 group receiving one or more doses of niacinamide (per-protocol) compared to non-B3 group. Download Supplemental Table 4, PDF file, 693 KB (692.1KB, pdf)
Additional sensitivity analyses exploring the association between niacinamide use group and primary and secondary endpoints in the entire cohort and among KDIGO subgroups. Download Supplemental Table 5, PDF file, 693 KB (692.1KB, pdf)
Association between niacinamide use group and primary and selected secondary endpoints in the entire cohort and among KDIGO subgroups using prognostic scoring. Download Supplemental Table 6, PDF file, 693 KB (692.1KB, pdf)
Characteristics of patients with KDIGO stage 2 or 3 AKI in B3 group and non-B3 groups. Download Supplemental Table 7, PDF file, 693 KB (692.1KB, pdf)
Supplemental Methods. Download Supplemental Material 1, PDF file, 693 KB (692.1KB, pdf)
Study protocol. Download Supplemental Material 2, PDF file, 693 KB (692.1KB, pdf)
Download Supplemental References, PDF file, 693 KB (692.1KB, pdf)