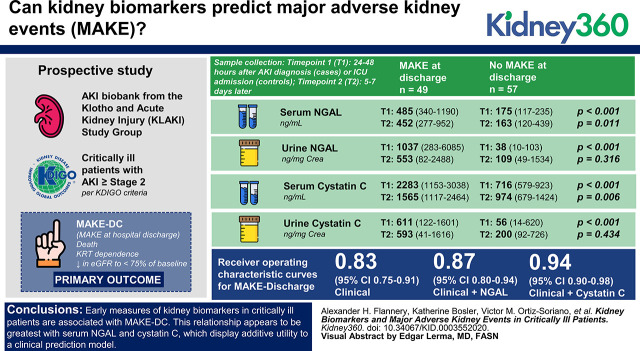
Visual Abstract
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, biomarker, critical care, critical illness, intensive care, major adverse kidney event, outcome
Abstract
Background
Several biomarkers of AKI have been examined for their ability to predict AKI before serum creatinine. Few studies have focused on using kidney biomarkers to better predict major adverse kidney events (MAKE), an increasingly used composite outcome in critical care nephrology research.
Methods
Single-center prospective study collecting blood and urine samples from critically ill patients with AKI Kidney Disease Improving Global Outcomes stage 2 or above, and matched controls from a single, tertiary care intensive care unit (ICU). Samples were collected at 24–48 hours after AKI diagnosis (patients) or ICU admission (controls), 5–7 days later, and 4–6 weeks after discharge for patients with AKI. The primary outcome of interest was MAKE at hospital discharge (MAKE-DC), consisting of the composite end point of death, RRT dependence, or a decrease in estimated glomerular filtration to <75% of baseline.
Results
Serum/urinary neutrophil gelatinase-associated lipocalin (NGAL), serum/urinary cystatin C, and urinary kidney injury molecule-1 early in the AKI or ICU course were all significantly higher in patients with MAKE-DC compared with those not experiencing MAKE-DC. Additionally, serum/urinary NGAL and serum cystatin C measurements at the first time point remained significantly associated with MAKE events at 3, 6, and 12 months. Serum cystatin C, and to a lesser extent serum NGAL, significantly improved upon a logistic regression clinical prediction model of MAKE-DC (AUROC 0.94 and 0.87 versus 0.83; P=0.001 and P=0.02, respectively). Patients without MAKE-DC experienced a greater decline in serum NGAL from first to second measurement than those patients experiencing MAKE-DC.
Conclusions
Early measures of kidney biomarkers in patients who are critically ill are associated with MAKE-DC. This relationship appears to be greatest with serum NGAL and cystatin C, which display additive utility to a clinical prediction model. Trending serum NGAL may also have utility in predicting MAKE-DC.
Introduction
The last decade has seen an explosion of research on biomarkers of AKI. Over 15 different biomarkers have been investigated, with a combination of markers receiving US Food and Drug Administration approval in 2014 for detecting AKI within the ensuing 12 hours: the product of tissue inhibitor of metalloproteinase 2 and IGF binding protein 7 (1,2). The primary focus of these studies was the search for a biomarker that would detect AKI sooner than serum creatinine, allowing earlier recognition to improve care (3,4). A secondary aim of this line of research has been to improve risk stratification and longer-term prognostication with kidney biomarkers. This is particularly relevant for patients in the intensive care unit (ICU), where the incidence of AKI is high and conversations with family and decisions regarding supportive therapies such as RRT can be facilitated by improved prognostication of AKI that extends beyond the period of the acute illness.
Major adverse kidney events (MAKE)—the composite of death, need of RRT, or worsened kidney function—is increasingly recognized as an important, patient-centered outcome for studies of AKI, particularly in patients who are critically ill (5). Previous studies of kidney biomarkers have primarily limited evaluation of MAKE to 30 days (MAKE-30), thus we sought to assess the relationship between early kidney biomarker measurements with MAKE at further time intervals from AKI onset. Further, limited literature has been published to predict MAKE (in the short- or long-term), specifically from clinical models of ICU patients. Preliminary data suggest that simple models considering severity of illness provide good performance predicting MAKE out to 30 days (6). Accordingly, we sought to assess kidney biomarker relationships with MAKE at various time points, from discharge out to 1 year of follow-up, and test the utility of adding biomarker data to a clinical prediction model for MAKE by hospital discharge (MAKE-DC).
Materials and Methods
Study Design and Participants
This was a secondary analysis of the AKI biobank from the Klotho and AKI Study Group (7). In brief, this single center, prospective study enrolled adult patients ≥18 years old over a 1-year period admitted to the ICU with stage ≥2 AKI per Kidney Disease Improving Global Outcomes criteria and matched controls on the basis of age (10-year intervals), sex, and baseline eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation (60–89 and ≥90 ml/min per 1.73 m2) (8,9). Patients must have had a baseline eGFR ≥60 ml/min per 1.73 m2, which was determined using the most recent serum creatinine within the 6-month period before ICU admission. Patients were excluded if they had ESKD, a kidney or any other solid organ transplant, evidence of AKI before ICU admission, or uroepithelial tumors (7). This study was approved by the Institutional Review Board at University of Texas Southwestern Medical Center and all participants or their legally authorized representatives provided written, informed consent.
Data Collection and Laboratory Analysis
Blood and urine samples were taken from all patients at an initial time point (T1), 24–48 hours after AKI diagnosis (patients) or ICU admission (controls). A second set of samples (T2) was obtained 5–7 days after this initial collection. For patients with AKI, a third set (T3) was collected 4–6 weeks after discharge if patients survived and followed up with a clinic visit. These time points were chosen for the original study for their relevance to prognostication and detection of subclinical AKI. Blood and urine samples were centrifuged at 1000× g at 4°C for 10 minutes, supernatant aliquoted into cryovials, and stored at −80°C until biomarker measurement. Serum neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C, and urinary NGAL, cystatin C, and kidney injury molecule-1 (KIM-1) were measured using ELISA (BioPorto Diagnostics, Needham, MA; R&D Systems, Minneapolis, MN). Capillary electrophoresis was used to measure urinary creatinine (Ucr) and serum creatinine was obtained from routine clinical care (10). Baseline demographics, comorbidities, and clinical data associated with ICU admission were collected from electronic medical records using medical history and admission/discharge diagnoses, including the Acute Physiology and Chronic Health Evaluation (APACHE) II as a measure of illness severity within the first 24–48 hours of ICU admission and the Charlson Comorbidity Index (CCI) as a measure of comorbidity burden (11,12).
Outcomes
The primary study objective was to compare biomarker measurements between patients with and without the outcome of MAKE at the following time points: DC, 90 days (90), 6 months (6 months), and 1 year (1 year). At the specified time point, MAKE was defined as any of the following: death, RRT dependence, or decrease in eGFR to <75% of baseline (5). Secondarily, we sought to assess whether biomarker measurements at time T1 improved upon a clinical prediction model for MAKE-DC. In an exploratory analysis, we examined the trajectory of the biomarkers between times T1 and T2 (the Δ change in biomarker = value at time T2 – value at time T1) in those patients with and without MAKE-DC.
Statistical Analyses
Urinary biomarkers were normalized to Ucr in reporting. Categorical data are presented as proportions and continuous data as medians with interquartile ranges or means ± SD depending on the distribution. Categorial variables were compared using a chi-squared or Fisher’s exact test, as appropriate. Continuous variables were compared using an independent samples t test or Mann–Whitney U test. A logistic regression model for MAKE-DC was generated using a simple clinical prediction model with two variables, APACHE II and CCI, on the basis of the number of MAKE-DC events in the dataset. APACHE II was calculated within the first 24–48 hours of ICU admission, which corresponds to the first biomarker time point (median time from ICU admission to sample collection of 2 days), includes up to eight numeric points for kidney function, and also allowed for adjustment of other variables associated with mortality in the ICU (12). Collinearity was assessed with the use of variance inflation factors. The added value of individual biomarkers from T1 was assessed with their addition to the model with the use of the area under the receiver operating characteristic curve (AUROC). Statistical significance was considered a two-sided P value <0.05. Patients with missing data (e.g., anuric for urinary biomarkers or lost to follow-up) were excluded from the respective analysis. Analyses were performed in Stata (Version 16.1; Stata Corp, College Station, TX) and R (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study included a total of 106 patients: 54 with AKI and 52 matched controls. Patient characteristics are shown in Table 1. Per the study design, AKI and controls were not different in age, sex, or baseline eGFR. A greater proportion of patients with AKI were in the medical ICU compared with a greater proportion of control patients that were admitted to the surgical ICU. Patients with AKI tended to be more acutely ill overall, as evidenced by higher APACHE II and a greater need for vasopressors and mechanical ventilation. Patients with AKI also had more liver disease and higher CCIs compared with controls.
Table 1.
Patient characteristics
| Characteristics | AKI (n=54) | No AKI (n=52) | P Value |
| Age (yr) | 55.8±16.7 | 58.0±14.5 | 0.47 |
| Sex (% men) | 31 (57.4) | 29 (55.8) | 0.87 |
| Race (%) | |||
| White | 34 (63.0) | 40 (76.9) | 0.18 |
| Black | 12 (22.2) | 5 (9.6) | |
| Over | 8 (14.8) | 7 (13.5) | |
| ICU type, n (%) | |||
| SICU | 9 (16.7) | 18 (34.6) | 0.001 |
| MICU | 26 (48.2) | 6 (11.5) | |
| CCU | 10 (18.5) | 15 (28.9) | |
| Other ICU | 9 (16.7) | 13 (25) | |
| Diabetes (%) | 17 (31.5) | 10 (19.2) | 0.15 |
| Hypertension (%) | 27 (50.0) | 29 (55.8) | 0.55 |
| Heart failure (%) | 15 (27.8) | 17 (32.7) | 0.59 |
| Liver disease (%) | 18 (33.3) | 6 (11.5) | 0.007 |
| Cancer (%) | 23 (42.6) | 23 (45.1) | 0.80 |
| Baseline serum creatinine (mg/dl) | 0.86±0.26 | 0.82±0.20 | 0.32 |
| Baseline eGFR (ml/min) | 88 (69.5–104) | 92 (78.3–103) | 0.40 |
| APACHE II | 20 (16–26) | 12 (9–19) | <0.001 |
| Number pressors/inotropes | 1.5 (0–3) | 0 (0–1) | 0.001 |
| Mechanical ventilation (%) | 34 (63.0) | 20 (38.5) | 0.01 |
| Charlson Comorbidity Index | 4 (2.8–7) | 2 (2–4) | <0.001 |
ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; SICU, Surgical ICU; MICU, Medical ICU; CCU, Coronary Care Unit.
Biomarker values at time points T1, T2, and T3 are compared between patients with AKI and controls in Table 2. Seven patients with AKI were anuric and thus unable to contribute urine specimens. Death, discharge, or loss to follow-up were other reasons for absences of repeated measurements at the later time points of T2 and T3. Serum and urine biomarkers at all time points were higher, often by several orders of magnitude, in patients with AKI compared with controls, with the exception of urinary KIM-1/Ucr at time point T2. Of note, patients with AKI with measurements at time point T3, several weeks after AKI, demonstrated sustained elevations in biomarkers, particularly serum NGAL and cystatin C, that were as high as or higher than values in the control group at time T1.
Table 2.
Kidney biomarker measurements stratified by presence or absence of AKI
| Biomarker | n | AKI (n=54) | n | No AKI (n=52) | P Value |
| Serum NGAL (ng/ml) | |||||
| Time 1 | 54 | 487 (342–1317) | 52 | 163 (115–213) | <0.001 |
| Time 2 | 29 | 441 (290–705) | 7 | 153 (118–185) | 0.001 |
| Time 3 | 22 | 233 (178–360) | |||
| Urinary NGAL/Ucr (ng/mg Cr) | |||||
| Time 1 | 47 | 1305 (304–6068) | 52 | 32 (9–85) | <0.001 |
| Time 2 | 26 | 791 (301–2436) | 7 | 62 (29–82) | 0.001 |
| Time 3 | 20 | 72 (30–498) | |||
| Serum cystatin C (ng/ml) | |||||
| Time 1 | 54 | 2122 (1189–2978) | 52 | 712 (576–842) | <0.001 |
| Time 2 | 29 | 1506 (1111–2258) | 7 | 833 (623–974) | 0.001 |
| Time 3 | 21 | 1299 (908–2387) | |||
| Urinary cystatin C/Ucr (ng/mg Cr) | |||||
| Time 1 | 47 | 785 (125–1561) | 52 | 48 (9–166) | <0.001 |
| Time 2 | 26 | 652 (182–1462) | 7 | 63 (17–128) | 0.003 |
| Time 3 | 19 | 68 (44–325) | |||
| Urinary KIM-1/Ucr (ng/mg Cr) | |||||
| Time 1 | 47 | 5.7 (3.3–13.9) | 52 | 2.3 (1.2–4.4) | <0.001 |
| Time 2 | 47 | 5.2 (3.1–8.1) | 7 | 4.1 (0.9–5.4) | 0.31 |
| Time 3 | 20 | 2.3 (1.7–4.3) |
Time 1: 24–48 h after AKI diagnosis (patients) or intensive care unit admission (controls). Time 2: 5–7 d after time 1 collection. Time 3: 4–6 wk after discharge if patient survived and followed up with clinic visit. NGAL, neutrophil gelatinase-associated lipocalin; Ucr, urinary creatinine; Cr, creatinine; KIM-1, kidney injury molecule-1.
Of 106 patients enrolled in the study, 49 (46.2%) experienced MAKE at discharge. Patients experiencing MAKE-DC had longer ICU [10 (3–18) versus 2 (2–5) days] and hospital lengths of stay [17 (9–31) versus 8 (4–15) days] compared with patients without MAKE-DC, respectively (P<0.001 for both). Of the 49 patients experiencing MAKE at discharge, 24 (49.0%) were categorized by death, 5 (10.2%) by RRT dependence at discharge, and 20 (40.8%) by decrease in eGFR to <75% of baseline.
All biomarkers assessed at time T1 were significantly more elevated in patients experiencing MAKE-DC than those that did not (Table 3). Serum NGAL and cystatin C were the only biomarkers at time T2 that were significantly higher in patients experiencing MAKE-DC compared with those that did not. The biomarker values at T1 and T2 between patients experiencing MAKE-DC and those that did not can be visualized in Figure 1. Serum/urinary NGAL and serum cystatin C measurements at time T1 were significantly higher in those patients with MAKE-DC, MAKE-90 days, MAKE-6 months, and MAKE-1 year. Urinary cystatin C/Ucr and urinary KIM-1/Ucr measurements at time point T1 were significantly higher in MAKE patients than non-MAKE patients out to 6 months, but not at 1 year (Supplemental Material).
Table 3.
Kidney biomarkers and major adverse kidney events at discharge
| Kidney Biomarker | Major Adverse Kidney Events at Discharge (n=49) | No Major Adverse Kidney Events at Discharge (n=57) | P Value |
| Serum NGAL (ng/ml) | |||
| Time 1 | 485 (340–1190) | 175 (117–235) | <0.001 |
| Time 2 | 452 (277–952) (n=23) | 163 (120–439) (n=13) | 0.01 |
| Urinary NGAL/Ucr (ng/mg Cr) | |||
| Time 1 | 1037 (283–6085) (n=42) | 38 (10–103) | <0.001 |
| Time 2 | 553 (82–2488) (n=20) | 109 (49–1534) (n=13) | 0.32 |
| Serum cystatin C (ng/ml) | |||
| Time 1 | 2283 (1153–3038) | 716 (579–923) | <0.001 |
| Time 2 | 1565 (1117–2464) (n=23) | 974 (679–1424) (n=13) | 0.006 |
| Urinary cystatin C/Ucr (ng/mg Cr) | |||
| Time 1 | 611 (122–1601) (n=42) | 56 (14–620) | <0.001 |
| Time 2 | 593 (41–1616) (n=20) | 200 (92–726) (n=13) | 0.43 |
| Urinary KIM-1/Ucr (ng/mg Cr) | |||
| Time 1 | 5.6 (3.0–13.4) (n=42) | 2.4 (1.2–6.1) | <0.001 |
| Time 2 | 5.2 (3.0–7.9) (n=20) | 4.1 (2.2–7.5) (n=13) | 0.65 |
NGAL, neutrophil gelatinase-associated lipocalin; Ucr, urinary creatinine; Cr, creatinine; KIM-1, kidney injury molecule-1.
Figure 1.
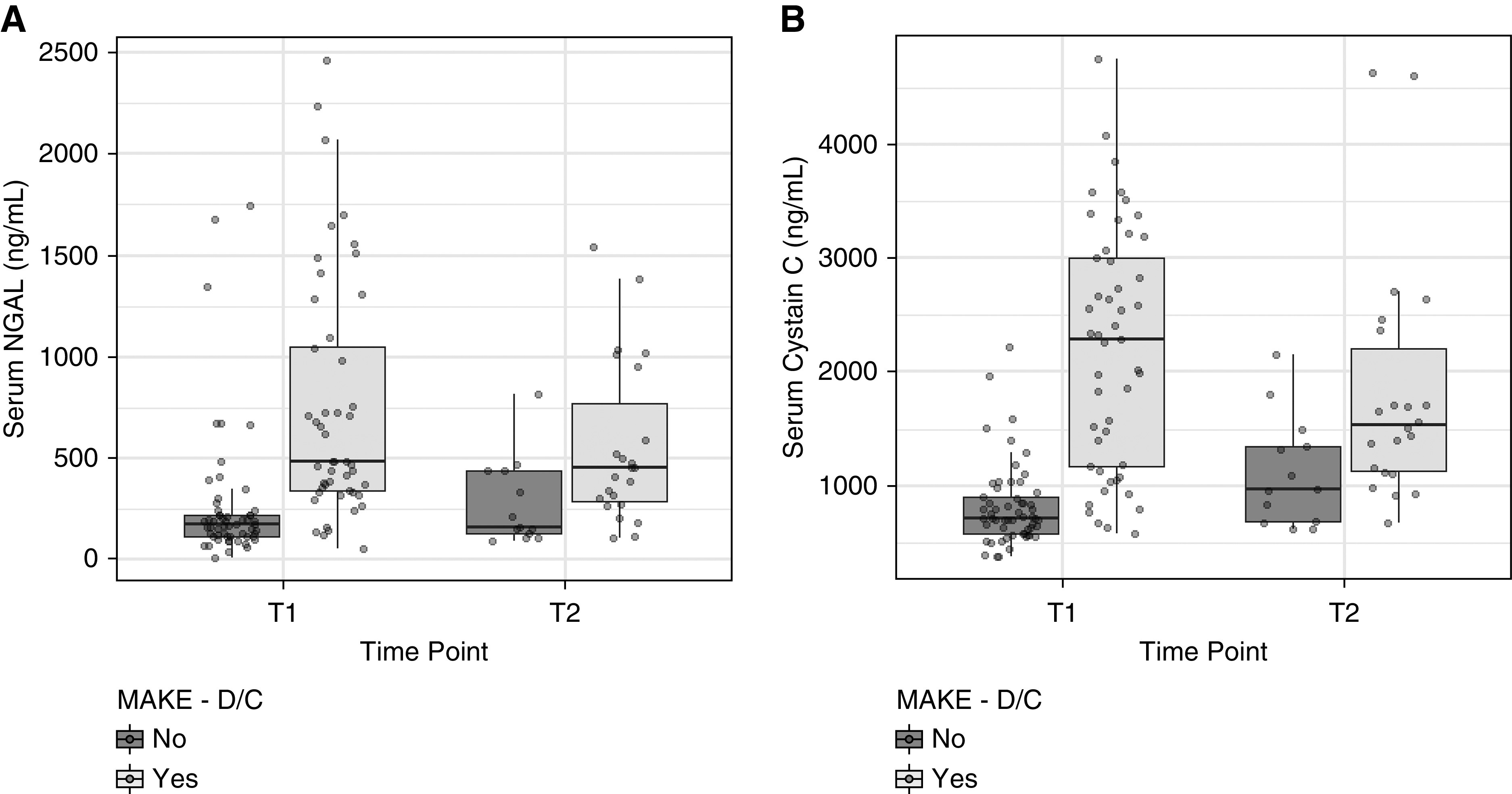
Kidney biomarker trends from time 1 (24–48 hours) to time 2 (5–7 days). DC, hospital discharge; MAKE, major adverse kidney event; NGAL, neutrophil gelatinase-associated lipocalin.
The simple clinical model for MAKE-DC including APACHE II and CCI produced an AUROC of 0.827, with both APACHE II [odds ratio (OR) 1.13; 95% confidence interval (CI), 1.06 to 1.21] and CCI (OR 1.41; 95% CI, 1.15 to 1.74) independent predictors of MAKE-DC. Both serum NGAL (AUROC=0.873) and serum cystatin C (AUROC=0.941) when independently added to the simple clinical model improved the AUROC (P=0.02 and P=0.001, respectively) as shown in Figure 2. None of the urinary biomarkers improved upon the simple clinical model for the prediction of MAKE-DC.
Figure 2.
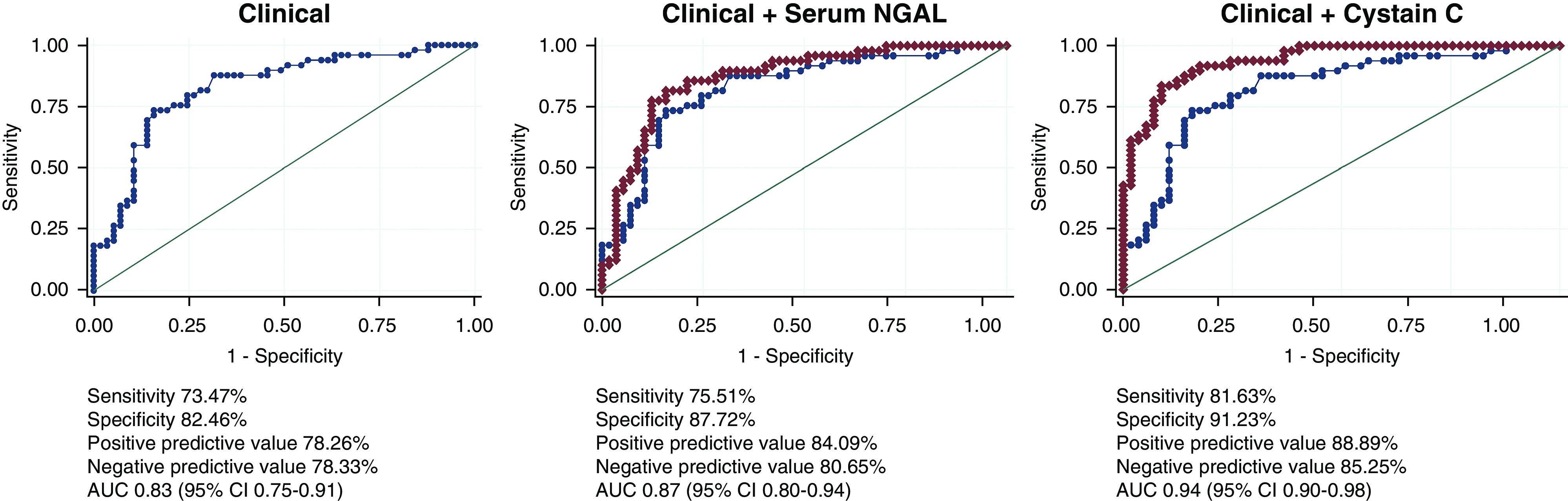
Receiver operating characteristic curves for MAKE-DC. AUC, area under the curve; 95% CI, 95% confidence interval.
In the exploratory analysis of biomarker trajectory from time point T1 to T2, only the change in serum NGAL (Δ NGAL) was significantly different in those patients experiencing MAKE-DC compared with those not. Those patients experiencing greater decreases in serum NGAL from time point T1 to T2 were less likely to experience MAKE-DC compared with those not (P=0.01) (Table 4). To account for baseline serum NGAL at time T1, both T1 and T2 serum NGAL values were assessed in a logistic regression model for MAKE-DC and the serum NGAL value at T2 remained significantly associated with MAKE-DC (OR 1.01; 95% CI, 1.00 to 1.01).
Table 4.
Change in kidney biomarkers and major adverse kidney events
| Δ Biomarker | Major Adverse Kidney Events at Hospital Discharge (n=49) | No Major Adverse Kidney Events at Hospital Discharge (n=57) | P Value |
| Δ serum NGAL (ng/ml) | 12 (−143 to 58) (n=23) | −84 (−363 to −41) (n=13) | 0.01 |
| Δ urinary NGAL (ng/mg Cr) | −41 (−2491 to 144) (n=19) | −20 (−869 to 80) (n=13) | 0.92 |
| Δ serum cystatin C (ng/ml) | −82 (−1121 to 734) (n=23) | −87 (−232 to 142) (n=13) | 0.68 |
| Δ urinary cystatin C (ng/mg Cr) | −112 (−545 to 552) (n=19) | 18 (−547 to 105) (n=13) | 0.89 |
| Δ urinary KIM-1 (ng/mg Cr) | 0 (−6 to 2) (n=19) | −1 (−8.5 to 0) (n=13) | 0.19 |
Δ, value at 5–7 d (Time 2) minus value at 24–48 h (Time 1). NGAL, neutrophil gelatinase-associated lipocalin; Cr, creatinine; KIM-1, kidney injury molecule-1. Results are shown as median (interquartile range).
Discussion
In this study of critically ill patients, serum and urinary biomarkers of AKI early in the course of AKI, or absence of AKI in the ICU stay, were significantly higher in those patients experiencing MAKE at several time periods, including DC, 90 days, 6 months, and 1 year. Of these, serum NGAL and cystatin C appeared to have the most utility when added to a simple clinical prediction model consisting of APACHE II and CCI. We also present evidence suggesting that trending these biomarkers in AKI may carry prognostic value. Overall, for the purpose of predicting MAKE events and trending biomarkers, serum NGAL and cystatin C appeared to perform most strongly from the population in our study.
AKI biomarker panels, including multiple markers such as tubular injury and renal stress, have been theorized to have important implications for personalized medicine of patients with AKI and the potential to improve prognostic, diagnostic, and therapeutic tools (13). Although not explored in our analysis, the combination of these biomarker panels has been shown in some settings to improve the predictive capability for AKI, particularly in the postcardiac surgery setting (14–17). The value of biomarker trajectory modeling in critical illness is gaining traction, and although AKI subphenotypes on the basis of serum creatinine have been examined, the value of repeated kidney biomarker assessments remains an area of active investigation (18). The repeated assessment of kidney biomarkers not only applies to the acute care setting, but to outpatient follow-up as well. Follow-up with a nephrologist after severe AKI has been associated with reduced all-cause mortality, and these biomarker assessments in the AKI recovery period may help to guide prognosis and therapy to reduce the development of CKD post-AKI (19). Our sample size from the post-AKI visit at 4–6 weeks after AKI was unfortunately not large enough to evaluate this utility further. We did note that serum NGAL and cystatin C values at the T3 time point in patients with AKI tended to be numerically higher than values obtained from the control group at T1 and T2, and are worthy of additional study in the post-AKI recovery setting.
This is certainly not the first study to investigate serum NGAL or cystatin C. However, the vast majority of the literature on these biomarkers is related to the prediction of AKI, for which they have generally not performed as well as anticipated (1). Given the increasing use of MAKE-DC or MAKE-30 as a primary outcome of clinical trials of AKI, particularly in the ICU setting, the ability to apply a biomarker to clinical prediction has great benefit in terms of prognostic enrichment and risk stratification in clinical trials. The association of biomarker levels with important patient-centered outcomes, including death, need of RRT, or reduced eGFR, out to 1 year is also important in the consideration of family discussions, discharge planning, and goals of care in the ICU setting.
A major strength of our study is the prospective design and follow-up of biomarkers and clinical outcomes at repeated time intervals, including out to 1 year. Although biomarkers are generally recognized to respond differently depending on the specific kidney insult (i.e., sepsis, ischemia, etc.), our study encompassed medical, surgical, and cardiac ICUs, which increases its generalizability to several different areas of practice and research. Although each type of kidney insult may produce its own specific pattern of kidney biomarker expression, often the kidney injury in the ICU is multifactorial, thus biomarkers applicable to a wide variety of clinical settings are desirable. We used well-established criteria for the diagnosis of AKI and the outcome of interest (MAKE) (5,8).
Our findings are not without their noted limitations. First, the cohort was from a single center and relatively small sample size. Because of death or loss to follow-up, missing data were more prevalent than preferred for both biomarker measurement at time T2 or T3 and MAKE events after DC, which increases the chance of bias when analyzed using complete-case analysis as we have done. These factors limited the number of covariates we could include in the clinical prediction model, and the possibility exists that a more detailed clinical prediction model for MAKE events may not benefit from the addition of biomarker measurements. Although we noted the broad population of the cohort as a strength, it could potentially represent a limitation if it diluted the effect of any one biomarker in a specific patient population, such as sepsis. Because of the number of biomarkers and time points evaluated, multiple comparisons were performed that may increase the chance of type I error. Finally, we only included AKI stage 2 and greater without CKD at baseline, thus patients with AKI stage 1 or CKD may not be adequately addressed in this study. Insufficient data were available for analyses accounting for repeated measures of data, such as a mixed effects Cox model, which would be preferable for longitudinal biomarker studies. Evaluation of AKI during critical illness is affected by death as a competing event, which was present in this study as well, as death competed with a patient’s chance of being RRT-dependent or with a reduced eGFR at discharge. Potential approaches in future studies include repeated measures time-to-event models with death as a competing event. Future research offers the opportunity to evaluate the predictive performance of these biomarkers, particularly serum NGAL and cystatin C, and more specifically their trajectories, in larger numbers of critically ill patients with AKI to further investigate the dose-response relationship and trajectory relationships between biomarker elevation and pertinent clinical outcomes.
Disclosures
J. Lambert reports honoraria from SAS Institute, outside the submitted work. A.H. Flannery is supported by KidneyCure/American Society of Nephrology and the La Jolla Pharmaceutical company, outside the submitted work. All remaining authors have nothing to disclose.
Funding
This work was supported, in part, by the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center via the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK079328-06) and the National Center for Advancing Translational Sciences (NCATS) (UL1TR001105). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the University of Texas Southwestern. J.A. Neyra is currently supported by an Early Career Pilot grant from NCATS, NIH through grant UL1 TR001998. R.D. Toto is supported by the NIH (grants R21-HL145424 and UH3-DK114870). O.W. Moe is supported by NIH grants R01-DK091392 and R01-DK0924610, the George O’Brien Kidney Research Center (P30-DK-07938), and the Charles and Jane Pak Center Endowed Professor Collaborative Research Support and Innovative Research Support.
Author Contributions
A.H. Flannery and J.A. Neyra conceptualized and designed the study; F. Gianella, V.M. Ortiz-Soriano, V. Prado, and J.A. Neyra were responsible for sample acquisition and data capture and management; K. Bosler was responsible for biomarker measurements. A.H. Flannery and J. Lambert were responsible for statistical analysis; O.W. Moe and R.D. Toto were responsible for funding support and resources; J.A. Neyra was responsible for study supervision; A.H. Flannery and J.A. Neyra wrote the original manuscript draft; and all authors reviewed, edited and approved the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003552020/-/DCSupplemental.
Major adverse kidney events at 90 days. Download Supplemental Table 1, PDF file, 37 KB (36.2KB, pdf)
Major adverse kidney events at 6 months. Download Supplemental Table 2, PDF file, 37 KB (36.2KB, pdf)
Major adverse kidney events at 1 year. Download Supplemental Table 3, PDF file, 37 KB (36.2KB, pdf)
References
- 1.Griffin BR, Gist KM, Faubel S: Current status of novel biomarkers for the diagnosis of acute kidney injury: A historical perspective. J Intensive Care Med 35: 415–424, 2020. 10.1177/0885066618824531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, Nolin TD, Bihorac A; American Society of Nephrology Acute Kidney Injury Advisory Group: Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for Acute kidney injury risk assessment. Am J Kidney Dis 68: 19–28, 2016. 10.1053/j.ajkd.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA: Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17: R25, 2013. 10.1186/cc12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX; TRIBE-AKI Consortium: Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011. 10.1681/ASN.2010121302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billings FT 4th, Shaw AD: Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 127: 89–93, 2014. 10.1159/000363725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKown AC, Wang L, Wanderer JP, Ehrenfeld J, Rice TW, Bernard GR, Semler MW: Predicting major adverse kidney events among critically ill adults using the electronic health record. J Med Syst 41: 156, 2017. 10.1007/s10916-017-0806-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neyra JA, Li X, Mescia F, Ortiz-Soriano V, Adams-Huet B, Pastor J, Hu MC, Toto RD, Moe OW: Urine klotho is lower in critically ill patients with versus without acute kidney injury and associates with major adverse kidney events. Crit Care Explor 1: e0016, 2019. 10.1097/CCE.0000000000000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinellu A, Caria MA, Tavera C, Sotgia S, Chessa R, Deiana L, Carru C: Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem 342: 186–193, 2005. 10.1016/j.ab.2005.01.045 [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 12.Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: A severity of disease classification system. Crit Care Med 13: 818–829, 1985. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 13.Basu RK: Dynamic biomarker assessment: A diagnostic paradigm to match the AKI syndrome. Front Pediatr 7: 535, 2020. 10.3389/fped.2019.00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD; SAKInet Investigators: Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int 85: 431–438, 2014. 10.1038/ki.2013.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS, Devarajan P, Goldstein SL: Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery [published correction appears in J Am Coll Cardiol 65: 1158–1159, 2015]. J Am Coll Cardiol 64: 2753–2762, 2014. 10.1016/j.jacc.2014.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meisner A, Kerr KF, Thiessen-Philbrook H, Wilson FP, Garg AX, Shlipak MG, Kavsak P, Whitlock RP, Coca SG, Parikh CR: Development of biomarker combinations for postoperative acute kidney injury via Bayesian model selection in a multicenter cohort study. Biomark Res 6: 3, 2018. 10.1186/s40364-018-0117-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neyra JA, Hu MC, Minhajuddin A, Nelson GE, Ahsan SA, Toto RD, Jessen ME, Moe OW, Fox AA: Kidney tubular damage and functional biomarkers in acute kidney injury following cardiac surgery. Kidney Int Rep 4: 1131–1142, 2019. 10.1016/j.ekir.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatraju PK, Mukherjee P, Robinson-Cohen C, O’Keefe GE, Frank AJ, Christie JD, Meyer NJ, Liu KD, Matthay MA, Calfee CS, Christiani DC, Himmelfarb J, Wurfel MM: Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit Care 20: 372, 2016. 10.1186/s13054-016-1546-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, Ray JG, Luo J, Li P, Quinn RR, Forster A, Perl J, Bell CM: Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013. 10.1038/ki.2012.451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Major adverse kidney events at 90 days. Download Supplemental Table 1, PDF file, 37 KB (36.2KB, pdf)
Major adverse kidney events at 6 months. Download Supplemental Table 2, PDF file, 37 KB (36.2KB, pdf)
Major adverse kidney events at 1 year. Download Supplemental Table 3, PDF file, 37 KB (36.2KB, pdf)