We compared country-level health care resource use and outcomes among children with AGE treated in pediatric EDs in the United States and Canada.
Abstract
BACKGROUND:
Between-country variation in health care resource use and its impact on outcomes in acute care settings have been challenging to disentangle from illness severity by using administrative data.
METHODS:
We conducted a preplanned analysis employing patient-level emergency department (ED) data from children enrolled in 2 previously conducted clinical trials. Participants aged 3 to <48 months with <72 hours of gastroenteritis were recruited in pediatric EDs in the United States (N = 10 sites; 588 participants) and Canada (N = 6 sites; 827 participants). The primary outcome was an unscheduled health care provider visit within 7 days; the secondary outcomes were intravenous fluid administration and hospitalization at or within 7 days of the index visit.
RESULTS:
In adjusted analysis, unscheduled revisits within 7 days did not differ (adjusted odds ratio [aOR]: 0.72; 95% confidence interval (CI): 0.50 to 1.02). At the index ED visit, although participants in Canada were assessed as being more dehydrated, intravenous fluids were administered more frequently in the United States (aOR: 4.6; 95% CI: 2.9 to 7.1). Intravenous fluid administration rates did not differ after enrollment (aOR: 1.4; 95% CI: 0.7 to 2.8; US cohort with Canadian as referent). Overall, intravenous rehydration was higher in the United States (aOR: 3.8; 95% CI: 2.5 to 5.7). Although hospitalization rates during the 7 days after enrollment (aOR: 1.1; 95% CI: 0.4 to 2.6) did not differ, hospitalization at the index visit was more common in the United States (3.9% vs 2.3%; aOR: 3.2; 95% CI: 1.6 to 6.8).
CONCLUSIONS:
Among children with gastroenteritis and similar disease severity, revisit rates were similar in our 2 study cohorts, despite lower rates of intravenous rehydration and hospitalization in Canadian-based EDs.
What’s Known on This Subject:
Although the United States has the world’s highest per capita medical costs, these expenditures are not associated with improved outcomes. However, evaluations have rarely been focused on disease-specific outcomes, employing detailed clinical patient-level data to untangle the role of illness severity.
What This Study Adds:
In this analysis of children with diarrhea, after adjustment for severity characteristics, intravenous rehydration use was higher in the United States. Thus, a deeper understanding of nonclinical influences on decision-making is needed to reduce unnecessary intravenous rehydration use.
Although the United States has the world’s highest per capita medical costs,1 these expenditures are not associated with improved outcomes.2,3 In analyses, researchers employing administrative data have documented disparities for key outcomes, such as life expectancy and infant mortality. The aforementioned analyses, however, lack patient-level, illness severity data2 and are focused on process variables, such as access, use, and experience measures related to chronic diseases or overall metrics.2 With a disease-specific approach, focused on an acute illness, researchers, employing detailed clinical patient-level data, have the potential to clarify the effect of a system (ie, country) of care on outcomes.
With ∼30 million emergency department (ED) visits by children annually in the United States4 and known variation across countries,5,6 understanding the impact that the country where care is provided has on outcomes is crucial to optimizing care. In previous studies, researchers have employed retrospective designs, and the studies lacked test result data and comprehensive, harmonized, outcome data and thus are at risk of misclassification bias.5–7 Concerns related to heterogeneity between populations studied, the effects of early disease identification and treatment, and limited patient-level clinical data impair our ability to draw conclusions about the influence of health care systems on disease-specific outcomes.
Acute gastroenteritis (AGE) is one of the most common illnesses prompting ED visits by children.4 The recommended treatment of the majority of affected children is the provision of oral rehydration therapy (ORT),8 with the selective use of ondansetron in those with dehydration and frequent vomiting.9,10 This approach optimizes resource use while avoiding the pain associated with intravenous insertion.11 However, practice variation is common within12 and between13 countries, and numerous institutions have focused on promoting ORT.11,14
To overcome the limitations of previous between-country comparisons, we took advantage of the simultaneous conduct of 2 probiotic clinical trials15,16 in children with AGE in Canada and the United States. In the trials, the researchers followed nearly identical protocols and data collection plans,17,18 permitting a comparison of resource use, while providing detailed disease severity data. We sought to determine if the country of care (ie, United States versus Canada) is independently associated with subsequent health care visits, intravenous hydration, and hospitalization after adjustment for patient-level characteristics.
Methods
Study Design and Population
This is a preplanned secondary analysis of 2 parallel, multicenter, pediatric ED-based studies conducted in the United States (Pediatric Emergency Care Applied Research Network [PECARN] probiotic study)16,17 and Canada (Pediatric Emergency Research Canada [PERC] Probiotic Regimen for Outpatient Gastroenteritis Utility of Treatment study).15,18 Children enrolled in the PECARN study presented for care in 1 of 10 US-based EDs between July 2014 and June 2017. The PERC study was performed at 6 Canadian EDs between November 2013 and April 2017. These prospective, randomized, trials enrolled 971 and 886 children, respectively, with ethical review board approval.
For the studies, researchers recruited children aged 3 to <48 months with ≥3 watery stools in a 24-hour period who were diagnosed as having AGE and whose caregivers provided informed consent. The maximal duration of symptoms at the time of screening was 72 hours and 7 days in the Canadian and US studies, respectively. To enhance the similarity of the 2 study populations, children recruited into the PECARN study with duration of symptoms >72 hours were excluded from this analysis.
Children in both studies were excluded if they or their direct caregivers had risk factors for bacteremia or a chronic gastrointestinal disorder,17 pancreatitis, bilious emesis, hematochezia, a known allergy to investigational products, or the inability to complete study follow-up. The PECARN study excluded children with allergies to erythromycin, clindamycin, and β-lactam antibiotics. Participants were randomly assigned to receive either a 5-day course of Lactobacillus GG (United States), combined Lactobacillus rhamnosus R0011 and Lactobacillus helveticus R0052 product (Canada), or placebo. Children lost to follow-up were excluded.
Study Outcomes
The primary outcome was an unscheduled health care provider visit for AGE symptoms within 7 days of recruitment. Visits booked before ED discharge or previously scheduled were not classified as outcomes. Unscheduled visits were subanalyzed on the basis of location: (1) ED and (2) primary care provider. Secondary outcomes analyzed at the index visit, within 7 days of the index visit, and overall included intravenous insertion and hospitalization.
Data Collection
Research assistants collected demographic and clinical data during the index ED visit. Clinical dehydration scale (CDS) scores were assigned by trained research nurses.19,20 Caregivers completed follow-up surveys every 24 hours for a minimum of 5 days and until both vomiting and diarrhea had ceased for >24 hours. All participants reported outcomes and health care use on day 14 after the index ED visit. A chart review was performed to confirm caregiver report regarding revisits.
Enteropathogen Identification
Before specific pathogens are associated with disease severity,21 rectal swabs (FecalSwab; Copan Diagnostics, Murrieta, CA), stool specimens, or both were obtained during the enrollment visit. A bacterial culture was performed locally. A multiplex nucleic acid panel that detects 15 enteric viruses, bacteria, and parasites (xTAG Gastrointestinal Pathogen Panel; Luminex, Austin, TX)22 was performed at St Louis Children’s Hospital’s research laboratory (PECARN cohort) and the Provincial Laboratory for Public Health at the Alberta Precision Laboratories, in Edmonton, Alberta, Canada (PERC cohort).
Analysis
Categorical data are presented as counts and percentages, and continuous data are presented as medians and interquartile ranges (IQRs). The CDS score is reported with means and SDs. We calculated 95% confidence intervals (CIs) for differences in proportions, medians, and means using χ2, inverted rank score tests, and ordinary least square methods, respectively. Both data sets performed multiple imputation to account for missing data, as previously described.17,18 Imputation models were stratified by country, assumed data were missing at random, and included key baseline characteristics, trial group, and efficacy outcomes.
For the primary and secondary outcomes, we used bivariable and multivariable logistic regression to calculate unadjusted and adjusted odds ratios (aORs), respectively. For exploratory outcomes, negative binomial regression was used to calculate unadjusted and adjusted rate ratios. Potential predictor variables for the model were predetermined on the basis of biological plausibility. These included age, sex, country (United States or Canada), the number of diarrheal stools and vomiting episodes in the 24 hours preceding enrollment, the duration of diarrhea and vomiting before enrollment, and CDS score.20 In a review of baseline variables (Table 1), researchers identified 3 additional important characteristics, which differed between countries and were added to the models: previous ED visits during the current illness and ED ondansetron and intravenous fluid administration. Colinearity was assessed by estimating the variance inflation factors. The site was not included as a random effect because the number of sites is small, and it is a subclassification of the country. Probiotic treatment allocation was not included in the models because previous analyses revealed no association with the outcomes of interest.17,18
TABLE 1.
Cohort Descriptions
| Country | Difference (95% CI) | ||
|---|---|---|---|
| Canada PERC Study (N = 827) | US PECARN Study (N = 588) | ||
| Age in mo, median (IQR) | 16.0 (10.0–24.0) | 17.4 (10.6–28.3) | 1.4 (−0.6 to 3.3) |
| Male, n (%) | 472 (57.1) | 309 (52.6) | −4.5% (−9.8 to 0.7) |
| Race and/or ethnicity, n (%)a | |||
| White non-Hispanic and non-Latino | — | 115 (20.2) | — |
| Hispanic or Latino | — | 202 (35.6) | — |
| Black non-Hispanic and non-Latino | — | 207 (36.4) | |
| Other non-Hispanic and non-Latino | — | 44 (7.7) | — |
| Distance to hospital, km,b median (IQR) | 10.2 (5.3–15.9) | 7.8 (3.9–14.4) | −2.4 (−3.5 to −1.3) |
| Child has a primary medical doctor, n (%) | 757 (91.5) | 557 (94.9) | 3.4 (0.8 to 6) |
| Previous ED visit for the current illness, n (%) | 83 (10.0) | 21 (3.6) | −6.4 (−9 to −3.9) |
| CDS score, continuous, mean (SD)c | 1.1 (1.34) | 0.6 (1.25) | −0.5 (−0.6 to −0.3) |
| Baseline MVS score, median (IQR) | 12 (10–13) | 11 (9–13) | −1 (−1.5 to −0.5) |
| Maximum temperature (Celsius), median (IQR) | 38.0 (37.0–39.4) | 37.8 (37.0–39.4) | −0.2 (−0.7 to 0.3) |
| Received antibiotics past 14 d, n (%) | 110 (13.3) | 46 (7.9) | −5.4 (−8.6 to −2.2) |
| Received rotavirus vaccine, n (%) | 396 (63.5) | 259 (66.6) | 3.1 (−2.9 to 9.1) |
| Diarrhea and vomiting severity | |||
| No. diarrheal episodes previous 24 h, median (IQR) | 5 (3–8) | 6 (4–9) | 1 (0.5 to 1.5) |
| Duration of diarrhea at presentation, median (IQR), h | 32.4 (22.5–51.8) | 35.6 (21.5–52.0) | 3.0 (−1.2 to 7.1) |
| Presence of vomiting at presentation, n (%) | 635 (76.8) | 448 (76.3) | −0.5% (−4.9 to 4.0) |
| No. vomiting episodes in previous 24 h,d median (IQR) | 4 (2–7) | 4 (2–6) | 0 (−0.6 to 0.6) |
| Duration of vomiting at presentation, median (IQR), hd | 36.4 (17.5–52.0) | 29.2 (16.2–49.6) | −7.1 (−10.9 to −3.2) |
| Index ED visit interventions, n (%) | |||
| IV fluids administered during index visit | 68 (8.2) | 100 (17.0) | 8.8 (5.2 to 12.4) |
| IV fluids administered at index visit or within 7 d | 79 (9.6) | 107 (18.2) | 8.6 (4.9 to 12.4) |
| Ondansetron given in the ED, n (%) | 183 (22.2) | 292 (49.7) | 27.5 (22.6 to 32.4) |
| Oral | 176 (21.3) | 264 (44.9) | 23.6 (18.7 to 28.5) |
| Intravenous | 7 (0.8) | 28 (4.8) | 3.9 (2.1 to 5.7) |
| Patient was admitted to the hospital from the ED, n (%) | 19 (2.3) | 23 (3.9) | 1.6 (−0.3 to 3.5) |
| Ondansetron administered at home, postindex ED visit, n (%) | 13 (1.8) | 59 (10.1) | 8.3 (5.7 to 10.9) |
Medians (IQR) and differences in medians with 95% CIs estimated by using the inverted rank score test are shown for continuous characteristics, with the exception of the CDS score, which displays mean (SD) with difference in means and 95% ordinary least square CI; n (%) and differences in proportions with 95% Wald CI are shown for categorical characteristics. Missing values are as follows (Canada and United States): distance to hospital (1 and 3), primary medical doctor (0 and 1), previous ED visit (0 and 5), dehydration score (0 and 4), number of diarrheal episodes (1 and 1), diarrhea duration (195 and 14), number of vomit episodes (0 and 1), ED intravenous fluids (1 and 0), ED ondansetron (1 and 0), and admitted (0 and 1). IV, intravenous; MVS, modified Vesikari scale; —, not applicable.
Race and ethnicity data were unavailable for the Canada (PERC) cohort.
Distance was calculated as the geodetic distance between a patient’s residence and the hospital zip (postal) code.
Dehydration was assessed by using the CDS score.20,23 A score of 0 indicates no dehydration, scores of 1 to 4 indicate some dehydration, and scores of 5 to 8 indicate moderate-to-severe dehydration.
Out of those experiencing vomiting.
The two-sided α level was set at .05. Analyses were performed by using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Participants
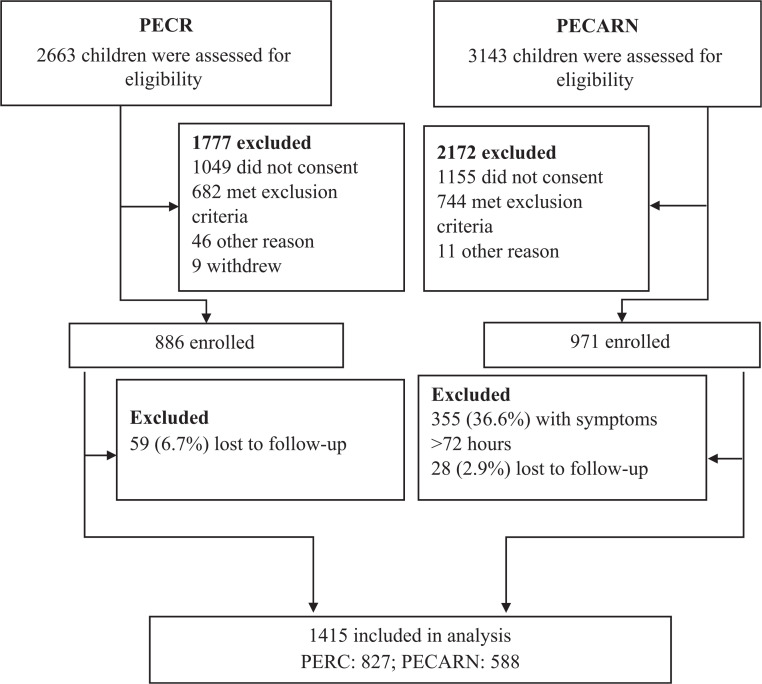
A total of 1415 eligible participants are included (Fig 1); 588 (41.6%) and 827 (58.5%) were enrolled in US and Canadian sites, respectively. US participants were less likely to have a previous ED visit during the current illness (3.6% vs 10.0%; difference: −6.4%; 95% CI of the difference: −9.0% to −3.9%) and had more diarrheal stools in the 24 hours preceding randomization (median: 6 [IQR: 4–9] vs 5 [IQR: 3–8]; difference: 1; 95% CI of the difference: 0.5 to 1.5 episodes) but were less dehydrated (mean CDS scores of 0.6 ± 1.3 vs 1.1 ± 1.3; difference: −0.5; 95% CI of the difference: −0.6 to −0.3 points; Table 1). Children in the United States that were excluded because of ≥72 hours of symptoms at the time of enrollment were similar to those that were included, with the exception of symptom duration, vomiting frequency in the preceding 24 hours, and ondansetron administration (Supplemental Table 4).
FIGURE 1.
Study participants.
Viral pathogens were identified in 43.7% and 51.7% of study participants in the United States and Canada, respectively (Supplemental Table 5). Although investigations were infrequently performed in both countries, blood tests and diagnostic imaging were more commonly performed in the Canadian and US cohorts, respectively (Table 2).
TABLE 2.
Index ED Visit Clinically Ordered (ie, Not Part of Research) Laboratory Investigations and Diagnostic Imaging
| Country | Difference, %a (95% Wald CIs) | ||
|---|---|---|---|
| Canada PERC Study; n (%) (N = 827) | US PECARN Study; n (%) (N = 588) | ||
| Urine dipstick or urinalysis | 78 (9.4) | 56 (9.5) | 0.1 (−3 to 3.2) |
| Urine culture | 51 (6.2) | 41 (7.0) | 0.8 (−1.8 to 3.4) |
| Serum electrolytes | 84 (10.2) | 75 (12.8) | 2.6 (−0.8 to 6) |
| Blood gas | 51 (6.2) | 31 (5.3) | −0.9 (−3.3 to 1.5) |
| Complete blood count | 81 (9.8) | 37 (6.3) | −3.5 (−6.3 to −0.7) |
| Blood culture | 31 (3.7) | 10 (1.7) | −2 (−3.7 to −0.4) |
| Stool viral studiesa | Not collected | 16 (2.7) | — |
| Stool bacterial culturea | 81 (9.9) | 45 (7.7) | −2.2 (−5.2 to 0.7) |
| Chest radiograph | 24 (2.9) | 15 (2.6) | −0.4 (−2.1 to 1.4) |
| Abdominal radiograph | 1 (0.1) | 17 (2.9) | 2.8 (1.4 to 4.1) |
| Abdominal ultrasound | 4 (0.5) | 19 (3.2) | 2.7 (1.2 to 4.3) |
Missing values are as follows (Canada and US): stool culture (7 and 0). —, not applicable.
Does not include stool testing performed as part of the research protocols.
Primary Outcome
ED or primary care provider revisits within 7 days of enrollment occurred in 14.0% of the study cohort (11.6% in United States; 17.3% in Canada); in the adjusted analysis, they did not differ between countries (aOR: 0.72; 95% CI: 0.50 to 1.02; Table 3). In subanalysis of the primary outcome based on the location of the revisit, researchers identified that, although primary care revisits did not differ (aOR: 0.76; 95% CI: 0.45 to 1.28) between groups, ED revisits were less likely in the US cohort (aOR: 0.61; 95% CI: 0.39 to 0.95).
TABLE 3.
Primary and Secondary Outcome Measures
| Canada PERC Study (N = 827) | US PECARN Study (N = 588) | Unadjusted Odds Ratio or Rate Ratio (95% CI)a | Unadjusted P | aOR or Rate Ratio (95% CI)b | Adjusted P | |
|---|---|---|---|---|---|---|
| Primary outcomes | ||||||
| ED or primary care visit within 7 d of discharge | 130 (17.3%) | 68 (11.6%) | 0.72 (0.52 to 0.98) | .04 | 0.72 (0.5 to 1.02) | .07 |
| ED revisit within 7 d of dischargec | 87 (11.7%) | 40 (6.8%) | 0.63 (0.43 to 0.93) | .02 | 0.61 (0.39 to 0.95) | .03 |
| Primary care visit within 7 d of dischargec | 54 (6.5%) | 30 (5.1%) | 0.79 (0.5 to 1.25) | .32 | 0.76 (0.45 to 1.28) | .30 |
| Secondary outcomes | ||||||
| IV fluids administered during index visit | 68 (8.2%) | 100 (17.0%) | 2.29 (1.65 to 3.19) | <.001 | 4.56 (2.93 to 7.11) | <.001 |
| IV rehydration within 7 d of discharge | 22 (3.0%) | 16 (2.7%) | 1.08 (0.56 to 2.06) | .82 | 1.38 (0.67 to 2.84) | .38 |
| IV fluids administered during index visit or within 7 d of discharge | 79 (9.6%) | 107 (18.2%) | 2.13 (1.55 to 2.91) | <.001 | 3.76 (2.49 to 5.67) | <.001 |
| Patient was admitted to the hospital from the ED | 19 (2.3%) | 23 (3.9%) | 1.75 (0.94 to 3.24) | .076 | 3.24 (1.55 to 6.77) | .002 |
| Hospitalization within 7 d of discharge | 18 (2.5%) | 9 (1.5%) | 0.77 (0.35 to 1.74) | .54 | 1.08 (0.44 to 2.63) | .87 |
| Hospitalization within 7 d (including index visit) | 34 (4.1%) | 31 (5.3%) | 1.33 (0.81 to 2.19) | .27 | 2.19 (1.22 to 3.95) | .009 |
| Exploratory outcomes | ||||||
| Duration of diarrhea postenrollment, h | 46.2 (0.1 to 85.0) | 51.1 (21.6 to 87.1) | 0.85 (0.74 to 0.98) | .02 | 0.91 (0.79 to 1.06) | .22 |
| Maximal No. diarrheal episodes per 24 h period postenrollment | 3.0 (1.0 to 5.0) | 3.0 (2.0 to 4.0) | 0.83 (0.75 to 0.92) | <.001 | 0.84 (0.76 to 0.92) | <.001 |
| Duration of vomiting postenrollment, h | 0.0 (0.0 to 38.6) | 0.0 (0.0 to 12.5) | 0.55 (0.39 to 0.77) | <.001 | 0.51 (0.35 to 0.75) | <.001 |
| Maximal number of vomiting episodes per 24 h period postenrollment | 0.0 (0.0 to 2.0) | 0.0 (0.0 to 1.0) | 0.66 (0.53 to 0.82) | <.001 | 0.68 (0.54 to 0.86) | .001 |
All outcomes include health care use through 7 d after enrollment consistent with gastroenteritis. Canada is used as reference category. Odds ratios are shown for primary and secondary outcomes, and rate ratios are shown for the exploratory outcomes.
Overall rates from the United States and Canadian studies, including both placebo and probiotic-treated patients. Unadjusted odds ratios compare these groups without adjusting for other factors.
All models are adjusted for country, the number of diarrheal stools and vomiting episodes in the 24 h before enrollment, the duration of vomiting and diarrhea before enrollment, dehydration scale, age, sex, previous ED visit during current illness, and ED ondansetron administration. Primary outcomes are additionally adjusted for intravenous fluid administration at the index visit.
Post hoc analysis.
Secondary Outcomes
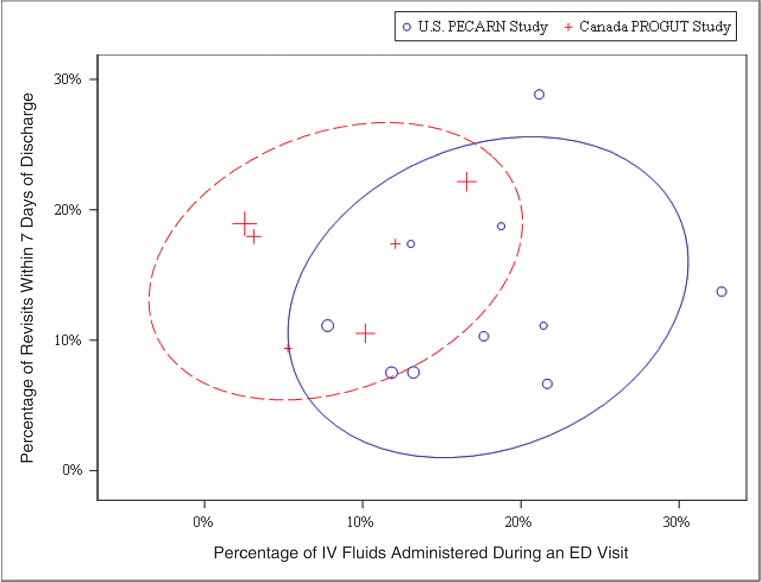
Intravenous fluid administration at the index visit was more common in the US cohort (17.0% vs 8.2%; difference: 8.8%; 95% CI: of the difference 5.2% to 12.4%), even after covariate adjustment (OR: 4.6; 95% CI: 2.9 to 7.1; P < .001). There was no difference in the proportions of children receiving intravenous rehydration during the 7 days after the index ED visit (aOR: 1.4; 95% CI: 0.7 to 2.8; P = .38, with the Canadian cohort serving as the referent group). Overall, intravenous fluid administration was more common in the US cohort (18.2% vs 9.6%; difference: 8.6%; 95% CI: of the difference 4.9% to 12.4%). This finding persisted after adjustment (aOR: 3.8; 95% CI: 2.5 to 5.7; P < .001). In Figure 2, we illustrate that, relative to the US sites, intravenous fluids were administered less often at the Canadian sites (ie, are grouped closer to the y-axis), without a higher probability of a revisit (similar height on y-axis).
FIGURE 2.
Overlapping prediction ellipses (1 SD)44 demonstrating the country-specific tendencies of the study sites to administer intravenous fluids and have patients with a revisit within 7 days. The center of the ellipse represents the sample mean. The size of the individual symbols are directly correlated with the number of patients recruited at a given site. IV, intravenous; PROGUT, Probiotic Regimen for Outpatient Gastroenteritis Utility of Treatment.
We found no difference in the proportions of children in the United States, relative to the Canadian cohort, who required hospitalization during the 7 days after randomization (aOR: 1.1; 95% CI: 0.4 to 2.6; P = .87). Although hospitalization at the index ED visit and overall did not vary by country (Table 1), adjustment for a priori identified covariates revealed higher overall (aOR: 2.2; 95% CI: 1.2 to 4.0; P = .009) and index visit (aOR: 3.2; 95% CI: 1.6 to 6.8; P = .002) hospitalization rates in the US cohort.
Exploratory Outcomes
Children in the US cohort experienced a lower maximal number of diarrheal and vomiting episodes in a 24-hour period and shorter duration of vomiting after an index ED visit; Table 3.
Discussion
In this study, we determined that children with AGE managed in the United States experienced greater rates of intravenous rehydration and hospitalization at the index visit, compared with those managed in Canadian-based EDs. This more interventional approach did not lead to a reduction in overall unscheduled AGE-related health care visits or intravenous rehydration during the subsequent 7 days, after adjustment for patient-level characteristics. With these findings, it is suggested that more judicious use of health care resources could be achieved in the ED without leading to adverse outcomes or undesirable consequences.
In children with AGE, intravenous rehydration use is associated with dysnatremia, acidosis, fluid overload, and, in severe cases, seizures and death.24,25 However, perhaps more importantly, intravenous cannula insertion is almost universally associated with pain.26 Although both pharmacologic and nonpharmacologic techniques are available to reduce children’s acute pain and distress during venipuncture and intravenous catheter insertion,27 avoiding unnecessary procedures is a fundamental priority. Additionally, unnecessary intravenous rehydration has financial implications: on the basis of data provided by 4 of our US-based study sites, 2 hours of intravenous fluid therapy is associated with a mean charge of $892 (range: $603–$1136).
Because both countries have similar AGE management guidelines that promote the use of ORT in the absence of severe dehydration, the differences identified are unlikely related to variations in standards of care.8,28 Although we found that the higher intravenous rehydration rate in the United States may reduce the likelihood of 7-day ED revisits, this finding was only detected in subgroup analysis, and the overall a priori identified primary outcome, any revisit, did not differ between groups. Moreover, with an upper bound of the 95% CI of 0.95, 192 children would need to be treated in the US model of care to prevent 1 unscheduled ED revisit. Given our estimate of an 8.8% absolute difference in index ED visit intravenous hydration rates (ie, 1 in every 11 children), nearly 17 additional children would need to be administered intravenous fluids to prevent 1 ED revisit. Supportive evidence that targeted quality improvement efforts to reduce intravenous rehydration use can do so without increasing ED revisits was provided by researchers of a study from Seattle, which revealed that a 22% absolute reduction in the intravenous rehydration rate had no effect on the 72-hour revisit rate.14
Although, in our study, we do not discern to what extent the intravenous rehydration rates are attributable to systematic differences in health care systems, health seeking behaviors, or practice patterns, we did determine that intravenous rehydration use could be curtailed without incurring adverse outcomes.29,30 Two decades ago, the use of ORT in lieu of intravenous rehydration was promoted as a “reverse-transfer” of technology from low- to high-income countries.31 Nonetheless, the deimplementation of intravenous rehydration remains a challenge, in part because of a possible reluctance of physicians to abandon practices that provide limited benefits.32
ED revisits are important metric of ED care because they are common and costly,33 occurring within 72 hours in 3% to 5% of all pediatric ED visits,34–37 and nearly 20% of all children with ED revisits are hospitalized.34,35,38 Variables associated with revisits in both countries include younger age, illness acuity and progression, and social factors.34,36,37,39 Although previous work has revealed similar ED visit rates and patterns in the 2 countries,40 the drivers appear to differ; although long wait times to see primary care physicians hinder non-ED–based follow-up in Canada, the high cost of care is the culprit in the United States.41 In our study, we did note that a greater proportion of all unscheduled follow-up visits occurred in EDs in the Canadian cohort.
AGE is associated with a disproportionately high revisit rate, especially when based on patient-reported revisit data. In our study, ED revisit rates (7% and 12% in the United States and Canada, respectively) were higher than those suggested by aforementioned database studies (3% to 5%),34–37 in which researchers often fail to include revisits to EDs other than those in which the patient initially presented. As it relates to understanding baseline AGE revisits, there is significant variability on the basis of the study population. In a tertiary-care–center Canadian study of 174 children who received intravenous fluids, 18% of participants had an ED revisit within 7 days.42 At the same institution, in a database study that included 3346 children, researchers reported a 16% 7-day ED revisit rate.43 However, in a provincial database study that included 55 520 discharged children, the 72-hour ED revisit rate was only 4.3%.44 Interestingly, in a US-based 21-institution study, the median ED revisit rate among children diagnosed with AGE was only 2.2%.45 These between-country differences warrant further exploration but may be partially explained by differences in the ability of databases to completely capture ED revisits. Provincial databases available in Canada incorporate all EDs in the province, whereas the data reported in US reports generally include only site-specific ED revisits.
Inter- and intrainstitutional data have revealed wide variability in intravenous rehydration rates between sites12 and practitioners.11 However, in multidisciplinary quality improvement initiatives focused on reduced intravenous rehydration, sustained reductions in intravenous rehydration use, ED length of stay, and costs have been achieved.11,14 Key drivers of intravenous fluid administration include parental acceptance of ORT, provider knowledge of local and national guidelines, challenges with hydration assessment, and primary care provider desires.11 Although our study did not specifically explore drivers of intravenous rehydration use, one notable difference between countries is the financial underpinnings of health care delivery. Although institutions in the United States receive greater reimbursement for more complex care, with ED revenues exceeding costs by $6.1 billion nationally in 2009,46 EDs in Canada operate on fixed annual budgets provided by provincial governments, which leads them to adopt approaches to minimize expenditures. These financial implications may intersect with parental and primary care provider expectations, which, thus, may play a greater role in the delivery of health care in the United States,47 relative to Canada. By demonstrating that increased intravenous rehydration use does not lead to an overall reduction in unscheduled visits, in this study, we provide important data that can inform conversations with caregivers and primary care physicians of children with AGE.
Our study has several limitations. The original United States data set included 355 children with >72 hours of symptoms who were excluded from our analysis to permit a direct comparison of similar populations. Although we adjusted for the most important clinical characteristics influencing the decision to administer intravenous rehydration, social drivers, such as parental leave policies and preferences48 and the medicolegal environment,49 were not included in our analysis. However, many of these factors are country specific and, therefore, are integrated into the “country” variable in our models.
Although the frequency of previous ED visits differed between countries and these visits may have been associated with testing or interventions, in our adjusted models, we included this variable, and, thus, our adjusted models do not explain our findings. We also did not include prescheduled follow-up primary care or ED visits; in a previous report, the latter accounted for 17% of all pediatric ED revisits. Although primary care follow-up is recommended for children with AGE by over three-quarters of pediatric ED physicians in the United States, they are desired by less than one-third of primary care physicians and, thus, likely reflect overuse, rather than a method of preventing ED revisits.50 Moreover, because planned revisits are anecdotally more common in the United States, with our data, we likely underestimate primary care and, possibly, ED revisits in our US cohort. Patient satisfaction and costs are not included in this analysis; these outcomes are important considerations for future exploration. Although, in our study, we included a limited number of academic centers within each country, between-site variability was small (intraclass correlation coefficient = 0.04 for revisits), which supports our “country” model approach. Although, in other reports (including pediatric ED physician self-report)51 on the use of intravenous rehydration, researchers support our findings of lower usage rates in Canada (relative to the United States),14,52–54 generalizing our findings to other EDs must be done with caution.
Conclusions
Despite greater rates of intravenous rehydration and hospitalization in children with AGE in US-based EDs, when compared with Canadian-based EDs, we found no differences in overall unscheduled revisits after ED discharge. Thus, judicious use of health care resources may be possible without leading to adverse outcomes or undesirable consequences. This points to an urgent need to better understand the drivers of intravenous rehydration use and hospitalization to improve the care of children with AGE.
Glossary
- AGE
acute gastroenteritis
- aOR
adjusted odds ratio
- CDS
clinical dehydration scale
- CI
confidence interval
- ED
emergency department
- IQR
interquartile range
- ORT
oral rehydration therapy
- PECARN
Pediatric Emergency Care Applied Research Network
- PERC
Pediatric Emergency Research Canada
Footnotes
Dr Freedman conceptualized and designed the study and drafted the initial manuscript; Drs Schnadower and Tarr and Mr VanBuren and Norris conceptualized and designed the study; Drs Roskind, Schuh, Hurley, Levine, Rogers, Bhatt, Gouin, Mahajan, Vance, Powell, Farion, Sapien, O’Connell, and Poonai designed the study, and coordinated and supervised data collection; and all authors reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifiers NCT01773967 and NCT01853124).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The US-based study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R01HD071915); the Emergency Medical Services for Children Program of the Maternal and Child Health Bureau, Health Resources and Services Administration, under cooperative agreement (awards U03MC00001, U03MC00003, U03MC00006, U03MC00007, U03MC00008, U03MC22684, and U03MC22685); the Washington University Biobank Core, supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grant P30DK052574); and iHealth, which provided Lactobacillus rhamnosus GG and placebo capsules in kind. The Canadian-based study was supported by the Canadian Institutes of Health Research (grants 286384 and 325412), the Alberta Children’s Hospital Foundation Professorship in Child Health and Wellness (to Dr Freedman), a grant from the Alberta Children’s Hospital Foundation to the Pediatric Emergency Medicine Research Associates’ Program, Calgary Laboratory Services (in kind), Provincial Laboratory for Public Health in Alberta, Luminex, and Copan Italia. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. World Health Organization. Health expenditure profile. Available at: http://apps.who.int/nha/database/Country_Profile/Index/en. Accessed March 22, 2020
- 2. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. [published correction appears in JAMA. 2018;319(17):1824]. JAMA. 2018;319(10):1024–1039 [DOI] [PubMed] [Google Scholar]
- 3. GBD 2015 Healthcare Access and Quality Collaborators. Electronic address: cjlm@uw.edu; GBD 2015 Healthcare Access and Quality Collaborators. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990-2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet. 2017;390(10091):231–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McDermott KW, Stocks C, Freeman WJ. Overview of Pediatric Emergency Department Visits, 2015: Statistical Brief #242. Rockville, MD: Agency for Healthcare Research and Quality; 2018 [PubMed] [Google Scholar]
- 5. Jamal A, Finkelstein Y, Kuppermann N, et al. ; Pediatric Emergency Research Networks . Pharmacotherapy in bronchiolitis at discharge from emergency departments within the Pediatric Emergency Research Networks: a retrospective analysis. Lancet Child Adolesc Health. 2019;3(8):539–547 [DOI] [PubMed] [Google Scholar]
- 6. Zipursky A, Kuppermann N, Finkelstein Y, et al. ; Pediatric Emergency Research Networks . International practice patterns of antibiotic therapy and laboratory testing in bronchiolitis. Pediatrics. 2020;146(2):e20193684. [DOI] [PubMed] [Google Scholar]
- 7. Cohen E, Rodean J, Diong C, et al. Low-value diagnostic imaging use in the pediatric emergency department in the United States and Canada. [published correction appears in JAMA Pediatr. 2019;173(8):801]. JAMA Pediatr. 2019;173(8):e191439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. King CK, Glass R, Bresee JS, Duggan C; Centers for Disease Control and Prevention . Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR):1–16 [PubMed] [Google Scholar]
- 9. Cheng A. Emergency department use of oral ondansetron for acute gastroenteritis-related vomiting in infants and children. Paediatr Child Health. 2011;16(3):177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schnadower D, Finkelstein Y, Freedman SB. Ondansetron and probiotics in the management of pediatric acute gastroenteritis in developed countries. Curr Opin Gastroenterol. 2015;31(1):1–6 [DOI] [PubMed] [Google Scholar]
- 11. Creedon JK, Eisenberg M, Monuteaux MC, Samnaliev M, Levy J. Reduction in resources and cost for gastroenteritis through implementation of dehydration pathway. Pediatrics. 2020;146(1):e20191553. [DOI] [PubMed] [Google Scholar]
- 12. Freedman SB, Gouin S, Bhatt M, et al. ; Pediatric Emergency Research Canada . Prospective assessment of practice pattern variations in the treatment of pediatric gastroenteritis. Pediatrics. 2011;127(2). Available at: www.pediatrics.org/cgi/content/full/127/2/e287 [DOI] [PubMed] [Google Scholar]
- 13. Pelc R, Redant S, Julliand S, et al. Pediatric gastroenteritis in the emergency department: practice evaluation in Belgium, France, The Netherlands and Switzerland. BMC Pediatr. 2014;14:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rutman L, Klein EJ, Brown JC. Clinical pathway produces sustained improvement in acute gastroenteritis care. Pediatrics. 2017;140(4):e20164310. [DOI] [PubMed] [Google Scholar]
- 15. Freedman SB, Williamson-Urquhart S, Schuh S, et al. ; Pediatric Emergency Research Canada (PERC) Gastroenteritis Study Group . Impact of emergency department probiotic treatment of pediatric gastroenteritis: study protocol for the PROGUT (Probiotic Regimen for Outpatient Gastroenteritis Utility of Treatment) randomized controlled trial. Trials. 2014;15:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schnadower D, Tarr PI, Casper TC, et al. Randomised controlled trial of Lactobacillus rhamnosus (LGG) versus placebo in children presenting to the emergency department with acute gastroenteritis: the PECARN probiotic study protocol. BMJ Open. 2017;7(9):e018115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schnadower D, Tarr PI, Casper TC, et al. Lactobacillus rhamnosus GG versus placebo for acute gastroenteritis in children. N Engl J Med. 2018;379(21):2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freedman SB, Williamson-Urquhart S, Farion KJ, et al. ; PERC PROGUT Trial Group . Multicenter trial of a combination probiotic for children with gastroenteritis. N Engl J Med. 2018;379(21):2015–2026 [DOI] [PubMed] [Google Scholar]
- 19. Freedman SB, Vandermeer B, Milne A, Hartling L; Pediatric Emergency Research Canada Gastroenteritis Study Group . Diagnosing clinically significant dehydration in children with acute gastroenteritis using noninvasive methods: a meta-analysis. J Pediatr. 2015;166(4):908–916–e6 [DOI] [PubMed] [Google Scholar]
- 20. Friedman JN, Goldman RD, Srivastava R, Parkin PC. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145(2):201–207 [DOI] [PubMed] [Google Scholar]
- 21. Xie J, Nettel-Aguirre A, Lee BE, et al. ; Pediatric Emergency Research Canada (PERC) and the Alberta Provincial Pediatric EnTeric Infection TEam (APPETITE Team) . Relationship between enteric pathogens and acute gastroenteritis disease severity: a prospective cohort study. Clin Microbiol Infect. 2019;25(4):454–461 [DOI] [PubMed] [Google Scholar]
- 22. Perry MD, Corden SA, Howe RA. Evaluation of the Luminex xTAG Gastrointestinal Pathogen Panel and the Savyon Diagnostics Gastrointestinal Infection Panel for the detection of enteric pathogens in clinical samples. J Med Microbiol. 2014;63(pt 11):1419–1426 [DOI] [PubMed] [Google Scholar]
- 23. Freedman SB, Vandermeer B, Milne A, Hartling L; Pediatric Emergency Research Canada Gastroenteritis Study Group . Diagnosing clinically significant dehydration in children with acute gastroenteritis using noninvasive methods: a meta-analysis. J Pediatr. 2015;166(4):908–916–e6 [DOI] [PubMed] [Google Scholar]
- 24. Feld LG, Neuspiel DR, Foster BA, et al. ; Subcommittee on Fluid and Electrolyte Therapy . Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6):e20183083. [DOI] [PubMed] [Google Scholar]
- 25. Fonseca BK, Holdgate A, Craig JC. Enteral vs intravenous rehydration therapy for children with gastroenteritis: a meta-analysis of randomized controlled trials. Arch Pediatr Adolesc Med. 2004;158(5):483–490 [DOI] [PubMed] [Google Scholar]
- 26. Birnie KA, Noel M, Chambers CT, Uman LS, Parker JA. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2018;10(10):CD005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trottier ED, Doré-Bergeron M-J, Chauvin-Kimoff L, Baerg K, Ali S. Managing pain and distress in children undergoing brief diagnostic and therapeutic procedures. Paediatr Child Health. 2019;24(8):509–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leung A, Prince T; Canadian Paediatric Society; Nutrition and Gastroenterology Committee . Oral rehydration therapy and early refeeding in the management of childhood gastroenteritis. Paediatr Child Health. 2006;11(8):527–531 [Google Scholar]
- 29. Emanuel EJ, Fuchs VR. The perfect storm of overutilization. JAMA. 2008;299(23):2789–2791 [DOI] [PubMed] [Google Scholar]
- 30. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513–1516 [DOI] [PubMed] [Google Scholar]
- 31. Santosham M, Keenan EM, Tulloch J, Broun D, Glass R. Oral rehydration therapy for diarrhea: an example of reverse transfer of technology. Pediatrics. 1997;100(5). Available at: www.pediatrics.org/cgi/content/full/100/5/e10 [DOI] [PubMed] [Google Scholar]
- 32. Powers BW, Jain SH, Shrank WH. De-adopting low-value care: evidence, eminence, and economics. JAMA. 2020;324(16):1603–1604 [DOI] [PubMed] [Google Scholar]
- 33. Duseja R, Bardach NS, Lin GA, et al. Revisit rates and associated costs after an emergency department encounter: a multistate analysis. Ann Intern Med. 2015;162(11):750–756 [DOI] [PubMed] [Google Scholar]
- 34. Akenroye AT, Thurm CW, Neuman MI, et al. Prevalence and predictors of return visits to pediatric emergency departments. J Hosp Med. 2014;9(12):779–787 [DOI] [PubMed] [Google Scholar]
- 35. Marchese RF, Taylor A, Voorhis CB, Wall J, Szydlowski EG, Shaw KN. A framework for quality assurance of pediatric revisits to the emergency department [published online ahead of print February 26, 2020]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000002063 [DOI] [PubMed] [Google Scholar]
- 36. Goldman RD, Ong M, Macpherson A. Unscheduled return visits to the pediatric emergency department-one-year experience. Pediatr Emerg Care. 2006;22(8):545–549 [DOI] [PubMed] [Google Scholar]
- 37. Alessandrini EA, Lavelle JM, Grenfell SM, Jacobstein CR, Shaw KN. Return visits to a pediatric emergency department. Pediatr Emerg Care. 2004;20(3):166–171 [DOI] [PubMed] [Google Scholar]
- 38. Sabbatini AK, Kocher KE, Basu A, Hsia RY. In-hospital outcomes and costs among patients hospitalized during a return visit to the emergency department. JAMA. 2016;315(7):663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tran QK, Bayram JD, Boonyasai RT, et al. Pediatric emergency department return: a literature review of risk factors and interventions. Pediatr Emerg Care. 2016;32(8):570–577 [DOI] [PubMed] [Google Scholar]
- 40. Li G, Lau JT, McCarthy ML, Schull MJ, Vermeulen M, Kelen GD. Emergency department utilization in the United States and Ontario, Canada. Acad Emerg Med. 2007;14(6):582–584 [DOI] [PubMed] [Google Scholar]
- 41. Sanmartin C, Ng E, Blackwell D, Gentleman J, Martinez M, Simile C. Joint Canada/United States Survey of Health, 2002-03. Ottawa, Canada: Statistics Canada; 2006 [Google Scholar]
- 42. Freedman SB, DeGroot JM, Parkin PC. Successful discharge of children with gastroenteritis requiring intravenous rehydration. J Emerg Med. 2014;46(1):9–20 [DOI] [PubMed] [Google Scholar]
- 43. Freedman SB, Thull-Freedman JD, Rumantir M, Atenafu EG, Stephens D. Emergency department revisits in children with gastroenteritis. J Pediatr Gastroenterol Nutr. 2013;57(5):612–618 [DOI] [PubMed] [Google Scholar]
- 44. Bahm A, Freedman SB, Guan J, Guttmann A. Evaluating the impact of clinical decision tools in pediatric acute gastroenteritis: a population-based cohort study. Acad Emerg Med. 2016;23(5):599–609 [DOI] [PubMed] [Google Scholar]
- 45. Kharbanda AB, Hall M, Shah SS, et al. Variation in resource utilization across a national sample of pediatric emergency departments. [published correction appears in J Pediatr. 2015;167(4):943]. J Pediatr. 2013;163(1):230–236 [DOI] [PubMed] [Google Scholar]
- 46. Wilson M, Cutler D. Emergency department profits are likely to continue as the Affordable Care Act expands coverage. Health Aff (Millwood). 2014;33(5):792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spahr CD, Flugstad NA, Brousseau DC. The impact of a brief expectation survey on parental satisfaction in the pediatric emergency department. Acad Emerg Med. 2006;13(12):1280–1287 [DOI] [PubMed] [Google Scholar]
- 48. Nicholson E, McDonnell T, De Brún A, et al. Factors that influence family and parental preferences and decision making for unscheduled paediatric healthcare - systematic review. BMC Health Serv Res. 2020;20(1):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brooker JA, Hastings JW, Major-Monfried H, et al. The association between medicolegal and professional concerns and chest pain admission rates. Acad Emerg Med. 2015;22(7):883–886 [DOI] [PubMed] [Google Scholar]
- 50. Chen J, Singer E. Primary care follow-up after emergency department visits for routine complaints: what primary care physicians prefer and what emergency department physicians currently recommend. Pediatr Emerg Care. 2016;32(6):371–376 [DOI] [PubMed] [Google Scholar]
- 51. Freedman SB, Sivabalasundaram V, Bohn V, Powell EC, Johnson DW, Boutis K. The treatment of pediatric gastroenteritis: a comparative analysis of pediatric emergency physicians’ practice patterns. Acad Emerg Med. 2011;18(1):38–45 [DOI] [PubMed] [Google Scholar]
- 52. Doan Q, Chan M, Leung V, Lee E, Kissoon N. The impact of an oral rehydration clinical pathway in a paediatric emergency department. Paediatr Child Health. 2010;15(8):503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Freedman SB, Hall M, Shah SS, et al. Impact of increasing ondansetron use on clinical outcomes in children with gastroenteritis. JAMA Pediatr. 2014;168(4):321–329 [DOI] [PubMed] [Google Scholar]
- 54. Freedman SB, Tung C, Cho D, Rumantir M, Chan KJ. Time-series analysis of ondansetron use in pediatric gastroenteritis. J Pediatr Gastroenterol Nutr. 2012;54(3):381–386 [DOI] [PubMed] [Google Scholar]