Using national immunization data spanning 2008 to 2018, we describe disparities in and suboptimal uptake of HPV vaccination before age 13 years.
Abstract
BACKGROUND AND OBJECTIVES:
Routine human papillomavirus (HPV) vaccination is recommended at ages 11 to 12 years and may be initiated as early as 9 years of age.
METHODS:
Data were derived from the National Immunization Survey-Teen, spanning 2008–2018. Using health care provider–verified vaccination histories, we examined trends in human papillomavirus vaccination up-to-date (HPV-UTD) rates within ages 9 to 12 years. Furthermore, we assessed vaccination status by sociodemographic factors and US state of residence.
RESULTS:
Overall, amid evidence of recent stagnation, HPV vaccination between ages 9 to 12 increased over the years. Initiation rates rose from 17.3% in 2008 to 62.8% in 2018, and HPV-UTD rates rose from 13.5% in 2011 to 32.8% in 2018. After the inception of gender-neutral HPV vaccination, HPV-UTD rates between 2011 and 2018 rose by 31.9% among boys and only 6.6% among girls. For most of the study period, non-Hispanic Black and Hispanic individuals had higher rates of initiation and HPV-UTD than non-Hispanic white individuals. In 2018, vaccination initiation rates exceeded 70% in several states; however, HPV-UTD rates in most US states were <50%, excluding Rhode Island (61.6%), Colorado (58.7%), Hawaii (53.5%), District of Columbia (53.2%), and Ohio (50%).
CONCLUSIONS:
HPV vaccination within ages 9 to 12 years is suboptimal. To leverage the substantial benefits of HPV vaccination within this age range, it is imperative that conscious efforts are taken at the national and state levels to promote HPV vaccination for this age group.
What’s Known on This Subject:
The United States failed to achieve the Healthy People 2020 target of attaining 80% human papillomavirus (HPV) vaccination coverage. Early HPV vaccination within ages 9 to 12 years is associated with higher efficacy and higher rates of uptake and completion of the vaccination series.
What This Study Adds:
In this study, we investigate disparities and HPV vaccination trends in US children within ages 9 to 12 years over a 10-year period after the inception of the national HPV vaccination program.
Although human papillomavirus (HPV)–associated cancers are vaccine preventable, their overall incidence in the United States is on the rise,1,2 with >40 000 new cases occurring every year. The Healthy People 2020 target was to attain 80% HPV vaccination coverage by year 20203; however, this goal has not been achieved, because uptake rates remain significantly suboptimal. In 2018, human papillomavirus vaccination up-to-date (HPV-UTD) rates among US teenagers (13–17 years) was 51.1%,4 significantly lower than rates in other developed nations with similar national vaccination program initiation points.5 For example, in 2017, coverage rates in Australia for the same age group exceeded 75% in girls and 69% in boys.6 Identifying this as a major public health concern, in June 2018, all National Cancer Institute–designated comprehensive cancer centers in the United States came together to fully endorse a goal of eliminating HPV-associated cancers through widespread gender-neutral HPV vaccination.7
The Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) recommends routine HPV vaccination at ages 11 to 12 years but also states that the vaccination series may be initiated as early as 9 years of age.8 However, the American Academy of Pediatrics has stated its support for categorically expanding this recommended age period for routine HPV vaccination to ages 9 to 12 years.9
Vaccination between ages 9 to 12 years is important for several reasons. First, HPV vaccination administered within this age range is associated with higher levels of immunogenicity and efficacy compared with vaccination administered outside this age range. Studies conducted in Scotland revealed that HPV vaccination within the recommended age range had higher rates of vaccine effectiveness in preventing infection with carcinogenic strains of HPV10 and development of cervical intraepithelial neoplasia11 than did fully immunized women who received the first dose of HPV vaccine at 17 years of age. Second, HPV vaccines are most effective when administered before HPV infection. A study revealed that 28.5% of female individuals will get infected with HPV within a year of their sexual debut.12 Given that sexual activity is rare by age 12 years13 and the prevalence of sexual encounters and the attendant risk of HPV infection rises significantly as age increases through adolescence, the 9 to 12 years age bracket is a crucial window for effective vaccination against HPV.14 Third, HPV vaccination within the 9 to 12 years age bracket improves population-level HPV vaccination coverage. Evidence from countries with successful HPV vaccination programs and high vaccination coverage rates, such as Australia and Scotland, reveals that the highest uptake occurs before age 13 years and uptake rates decline steadily as age increases beyond this point.15,16 In the same light, a study revealed that initiating the HPV vaccination series at younger ages was associated with higher rates of on-time vaccination completion.17 Another study revealed that initiating HPV vaccination after age 12 (13 years and older) doubled the odds of not completing the vaccination series, compared with those who initiated vaccination before age 13 years.18
Geographic and sociodemographic differences have been described in the uptake of HPV vaccination.19–21 Yet few studies in the United States have examined, at the national level, disparities in vaccination uptake within the crucial age bracket of 9 to 12 years. Furthermore, several studies, including a yearly surveillance report by the CDC,22 have examined HPV vaccination uptake trends among teenagers 13 to 17 years old; however, investigations of similar national and subnational trends have not been conducted specifically for HPV vaccination before age 13 years, for both boys and girls. Given the centrality of HPV vaccination in early adolescence to vaccine efficacy10,11 and population-level vaccination coverage,15–18 the purpose with this study is to bridge existing gaps in the literature by examining trends in HPV vaccination among US adolescents before their 13th birthday, by using national-level immunization data.
Methods
Data Acquisition
Data for this study were obtained from the National Immunization Survey-Teen (NIS-Teen), spanning 2008–2018. The NIS-Teen is a population-based survey that collects teenager vaccination data in 2 forms: (1) a household telephone survey answered by eligible respondents (parents or guardians to a 13–17-year-old child in their household) who are selected and contacted through random digit dialing and (2) an immunization history questionnaire mailed to health care providers. The methodology used for the NIS-Teen has been described in detail previously.23 The NIS-Teen was approved by the National Center for Health Statistics research ethics review board. Ethical approval for our analysis was not required because all data were fully anonymized and publicly available.
Study Outcomes
Using health care provider–verified vaccination histories, the index study assessed HPV vaccination initiation and HPV-UTD rates within ages 9 to 12 years. To achieve this, we examined HPV vaccination status of 13-year-old cohorts in every study year since the inception of the national HPV vaccination program in the United States, with the expectation that compliance with ACIP recommendations is indicated by HPV-UTD status by adolescents’ 13th birthday. Initiation of HPV vaccination was defined as receiving at least 1 shot of HPV vaccine. HPV-UTD was defined in accordance to the 2016 ACIP guidelines as (1) receipt of 3 or more doses or (2) receipt of 2 doses of the HPV vaccine, when the first shot was administered before age 15 years and the time between the first and second dose was at least 5 months minus 4 days.
Data Analysis
Data were weighted to be representative of the US population. Specifically, the provider-phase weights were used to estimate the vaccination coverages as recommended by the NIS. These weights were created by using adjustments for (1) nonresolution of released telephone numbers, (2) nonresponse to age-eligibility screener questions, (3) subsampling of 1 age-eligible child per household, (4) interview nonresponse, and (5) presence of multiple telephone lines in the household.
Given that NIS-Teen samples include teenagers 13 to 17 years old, we used NIS-Teen variables that accounted for age (in days, months, and years) at receipt of HPV shots to assess study participants’ vaccination status between the ages of 9 and 12 years. These data were not available for years 2008–2010; therefore, our estimation of HPV-UTD status starts at year 2011. Similarly, gender-neutral HPV vaccination in the United States began in 2010; hence, our sex-stratified estimation of vaccination uptake (ie, initiation and HPV-UTD) among boys begins at year 2011.
Additional poststratification adjustments were made on the basis of race and ethnicity, education of mother, age category, sex, US state of residence, telephone status, and missing provider data to ensure national representativeness.24
The weighted prevalence and associated 95% confidence intervals of initiation and HPV-UTD were calculated for the overall study sample. Vaccination status (initiation and HPV-UTD) was stratified in relation to sociodemographic variables, including sex (male or female), race (white or Black), ethnicity (Hispanic or non-Hispanic), and region (US state of residence). Sociodemographic categorizations were based on self-identification by study respondents. The survey year was included in a logistic model to test for trends in HPV vaccination uptake. The R package ggmap was used to create the graphs for the region estimates of vaccination coverage over the years.25 All analyses were conducted by using R version 4.0.3.
Results
The age-restricted (13-year-old cohorts) sample sizes (unweighted [n] and weighted [N]) for the survey years included in this study were 2008 (n = 3455, N = 4 067 653), 2009 (n = 3915, N = 3 948 056), 2010 (n = 3914, N = 4 041 896), 2011 (n = 4763, N = 4 079 539), 2012 (n = 3937, N = 4 194 188), 2013 (n = 3735, N = 4 120 085), 2014 (n = 4292, N = 4 196 860), 2015 (n = 4476, N = 4 108 938), 2016 (n = 4209, N = 4 054 732), 2017 (n = 4283, N = 4 176 574), and 2018 (n = 3852, N = 4 250 369).
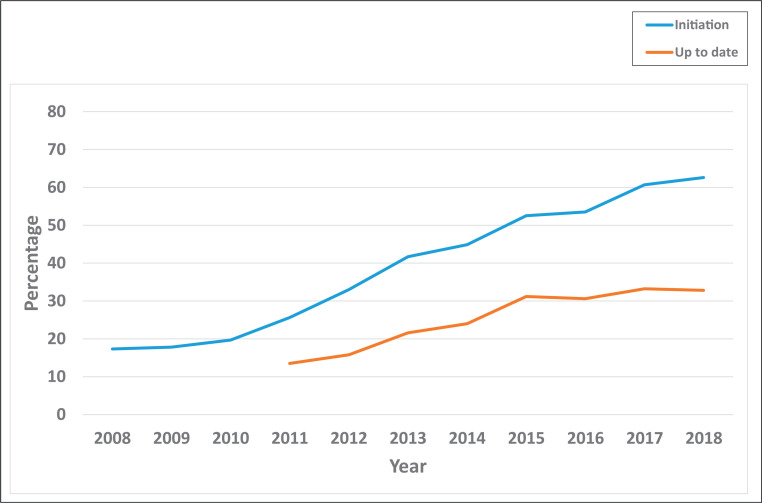
Overall, initiation of HPV vaccination within ages 9 to 12 years increased over the years, from 17.3% in 2008 to 62.6% in 2018 (Fig 1) (trend P < .0001). HPV-UTD rates also rose steadily, albeit at a slower pace, from 13.5% in 2011 to 32.8% in 2018 (trend P < .0001). Over the study period, the rates of noncompletion of HPV vaccination by age 13 years increased. This increase in noncompletion during this period was depicted by the widening gap between HPV vaccination initiation and HPV-UTD over the study years, from 12.1% in 2011 to 29.8% in 2018.
FIGURE 1.
Trends in routine HPV vaccination within ages 9 to 12 years, NIS-Teen, 2008–2018. HPV-UTD was defined as receiving at least 2 shots of HPV vaccine. Vaccination status between ages 9 to 12 years was derived by using NIS-Teen variables that accounted for age (in days, months, and years) at receipt of HPV shots. These data were not available for years 2008–2010.
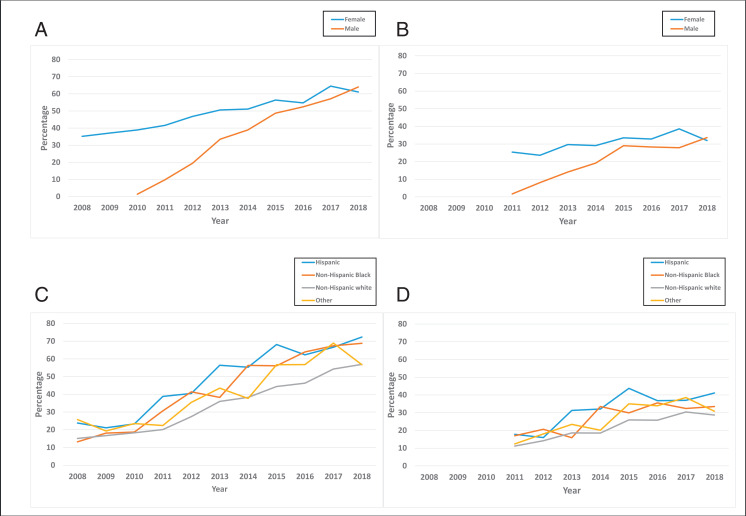
After the inception of gender-neutral HPV vaccination in 2010, the rate of HPV-UTD among boys increased by 31.9% from 2011 to 2018, whereas HPV-UTD among girls rose by only 6.6% during the same period (Fig 2B). Overall, Hispanic populations had higher HPV vaccination uptake rates than non-Hispanic Black or non-Hispanic white populations or other racio-ethnic groups throughout the study period (Fig 2 C and D). In 2011, the HPV-UTD rate among Hispanic populations was 17.8%, which was 6.6% and 0.8% higher than those of non-Hispanic white and non-Hispanic Black populations, respectively. In 2018, the HPV-UTD rate among Hispanic populations was 41.1%, which was higher by 12.4% and 7.6% than those of non-Hispanic white and non-Hispanic Black populations, respectively. Similarly, the overall HPV-UTD rate was higher among non-Hispanic Black populations compared with non-Hispanic white populations (17.0% vs 11.2% in 2011 to 33.5% vs 28.7% in 2018). Specific weighted prevalence rates and associated confidence intervals are provided in Supplemental Table 1.
FIGURE 2.
Trends in routine HPV vaccination within ages 9 to 12 years by sociodemographic factors, NIS-Teen, 2008–2018. A, HPV vaccination initiation by sex. HPV vaccination surveys for boys were introduced in 2010, corresponding with the introduction of gender-neutral HPV vaccines, hence data are unavailable for boys in 2008 and 2010. B, HPV-UTD by sex. HPV vaccination surveys for boys were introduced in 2010, corresponding with the introduction of gender-neutral HPV vaccines, hence data are unavailable for boys in 2008 and 2010. HPV-UTD was defined as receiving at least 2 shots of HPV vaccine. Vaccination status between ages 9 to 12 years was derived by using NIS-Teen variables that accounted for age (in days, months, and years) at receipt of HPV shots. These data were unavailable for years 2008–2010. C, HPV vaccination initiation by race and ethnicity. D, HPV-UTD by race and ethnicity. HPV-UTD was defined as receiving at least 2 shots of HPV vaccine. Vaccination status between ages 9 to 12 years was derived by using NIS-Teen variables that accounted for age (in days, months, and years) at receipt of HPV shots. These data were unavailable for years 2008–2010.
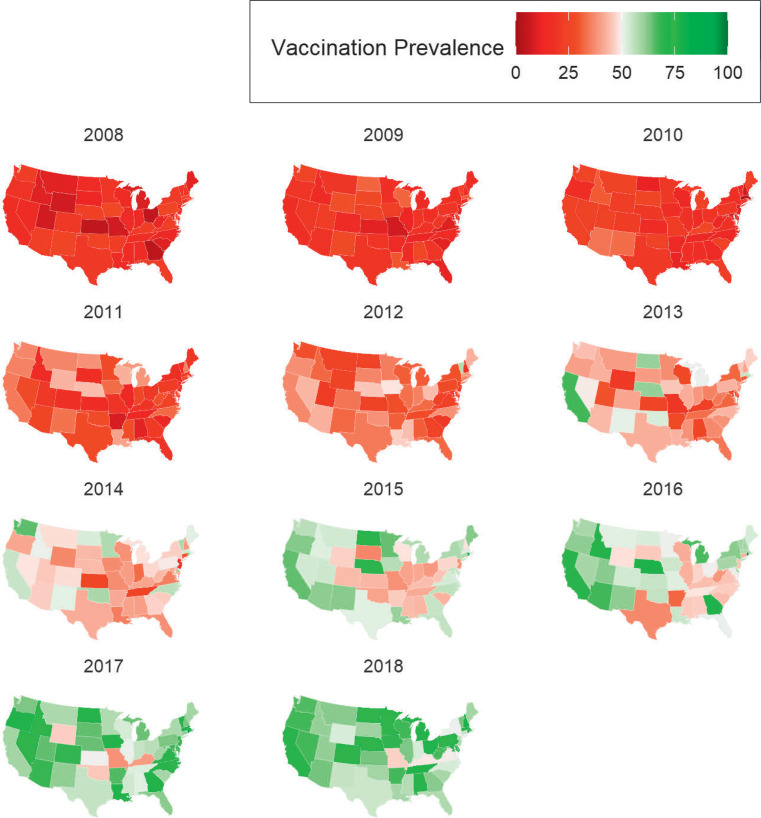
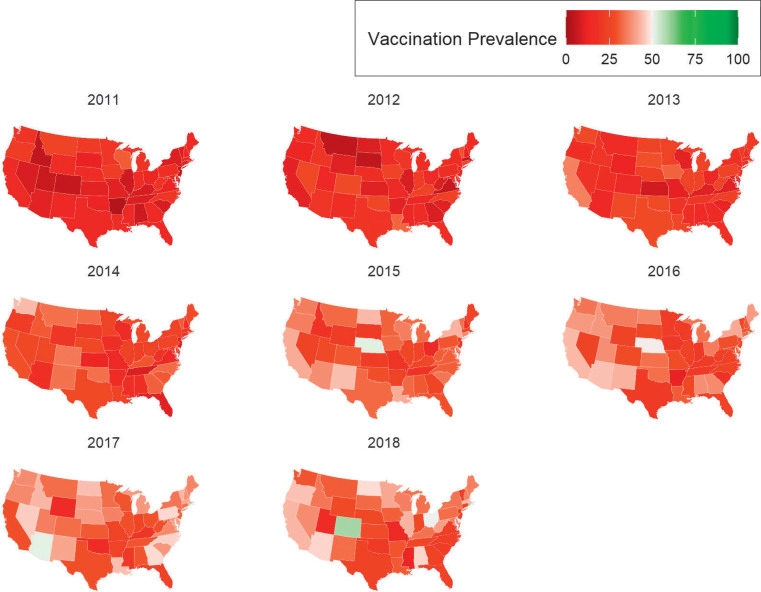
In 2018, rates of HPV vaccination initiation exceeded 60% in 32 US states and exceeded 70% in 16 states, and only 1 state exceeded the 80% threshold (Rhode Island, 85.6%) (Fig 3). Most states had HPV-UTD rates <50%, excluding only Rhode Island (61.6%), Colorado (58.7%), Hawaii (53.5%), District of Columbia (53.2%), and Ohio (50%) (Fig 4). The states with the lowest HPV-UTD rates were Mississippi (12.5%), Missouri (14.2%), and Utah (15.7%).
FIGURE 3.
National maps depicting trends in HPV vaccination initiation within ages 9 to 12 years, by state, NIS-Teen 2008–2018.
FIGURE 4.
National maps depicting trends in HPV-UTD within ages 9 to 12 years, by state, NIS-Teen 2008–2018.
Discussion
Overall, although uptake of HPV vaccination rose steadily over the years, completion of the vaccination series by age 13 years was low, with a large proportion of adolescents getting their full series as catch-up shots. In 2018, 51.1% of teenagers aged 13 to 17 years were HPV-UTD4; however, per the index study, 32.8% of the US adolescent population in 2018 were HPV-UTD by their 13th birthday. These data imply that compliance with the ACIP recommendation is poor, and HPV vaccination is being deferred by a significant proportion of the US general population to the catch-up phase: a period during which HPV vaccination is less effective.10,11 This is highly concerning.
Findings of this study also point to a slowing in recent years of HPV vaccination uptake within ages 9 to 12 years. After an initial momentum, HPV-UTD rates mostly stagnated among boys between 2015 and 2018, whereas uptake rates slowed even further among girls over the study period, with male HPV-UTD rates surpassing female rates for the first time in 2018. Researchers in a study conducted in Europe also points to declining HPV vaccination uptake rates.26 Although researchers of this study of Danish girls attributed vaccination declines to HPV-related media coverage, further investigation would be needed to gain insight to the underlying causes of the stagnation observed in HPV vaccination uptake in the United States. However, low levels of knowledge and awareness of HPV vaccination within the general US population27–29 could be a potent contributor.
For most of the study period, Hispanic and non-Hispanic Black individuals had higher rates of HPV vaccination initiation and completion before age 13 years than non-Hispanic individuals. This finding deviates from those of previous studies largely focused on other vaccination age ranges, in which researchers have shown higher HPV vaccination uptake among non-Hispanic white individuals.30,31 However, the results of a systematic review in which the data were restricted to provider-verified vaccination data corroborate our finding of higher HPV vaccination uptake among racial minorities compared with non-Hispanic white individuals.32
Our analysis of vaccination uptake over time in each US state revealed marked regional differences in HPV vaccination within ages 9 to 12 years. Although all US states continue to underperform, Rhode Island has consistently achieved significantly higher HPV vaccination rates within the 9 to 12 years age bracket than other US states. Several factors have contributed to this standing, including highly successful public health programs and the fact that Rhode Island is a universal vaccine purchase state,33 with the state covering costs and logistics of procuring HPV vaccines, thereby helping avert cost-related barriers to HPV vaccination. Rhode Island’s Vaccinate Before You Graduate program, a school-based immunization program targeting adolescents entering seventh grade, provides a valid explanation for our finding of a relatively high HPV vaccination completion by age 13 years in this state.33 Another impactful program implemented in Rhode Island is the Assessment, Feedback, Incentive, Exchange program, a CDC-funded quality improvement program that targets practices in Rhode Island that have low HPV vaccine administration rates. This program employs a physician consultant who visits each practice, conducts on-site assessments, and provides postassessment recommendations of strategies to boost vaccination uptake.33
Achieving high HPV vaccination coverage in girls and boys is essential for the elimination of carcinogenic strains of HPV,34 yet the United States has failed to achieve the Healthy People 2020 target of attaining 80% HPV vaccination coverage and remains significantly far from reaching this goal. Given the advantages of vaccination within ages 9 to 12 years, which include immunologic benefits,10,11 higher rates of on-time vaccination completion,17,18 and significant boosts to overall HPV vaccination coverage,15,16 aggressive efforts must be taken to implement established interventions (eg, standing orders, administering vaccines in a standard “bundle”)35 aimed at boosting HPV vaccination within ages 9 to 12 years. Moreover, findings of the current study support American Academy of Pediatrics’ recommendation to expand the recommended period for HPV vaccination to ages 9 to 12 years9 with additional pertinent advantages. Our finding of higher HPV vaccination among racial minorities during this period compared with their non-Hispanic white counterparts is unique. This may suggest that HPV vaccination within this age range is favored by minority populations, probably because of the added benefit of receiving the vaccine along with others bundled and given at this time. Thus, vaccination within the 9 to 12 years age bracket presents an important opportunity to narrow the existing disparities in HPV vaccination. Nevertheless, vaccination in the catch-up phase must also be encouraged for individuals who could not receive the vaccine within this ideal period.
Limitations of this study are mainly related to the low response rates associated with some study years of the NIS-Teen. Nonetheless, this study has several strengths. First, we used a nationally representative data set that has been consistently used to conduct national surveillance on HPV vaccination uptake among US teenagers. Second, data used in this study were obtained from health records by using provider-verified vaccination histories.
Conclusions
It is important to investigate the underlying factors responsible for the disparities and stagnating rates of HPV vaccination observed in this study, such as the stagnating uptake rates among girls and the low uptake rates among non-Hispanic white populations compared with other racio-ethnic groups. Following models of other high-income countries with high HPV vaccination coverage, it is imperative that the United States expand its efforts centered on vaccination within ages 9 to 12 years, particularly through school-based programs. Rhode Island serves as an important case study because it presents key evidence that high rates of HPV vaccination are achievable in the United States with the aid of effective public health interventions. Hence, interventions employed in this state should be assessed for national scalability. Evaluation of the efficacy of single-dose vaccinations are under way36 and may be a useful intervention to bridge marked gaps between HPV vaccination initiation and completion.
Acknowledgments
Editorial support was provided by Mr Bryan Tutt in Scientific Publications Services, Research Medical Library, University of Texas MD Anderson Cancer Center.
Glossary
- ACIP
Advisory Committee on Immunization Practices
- CDC
US Centers for Disease Control and Prevention
- HPV
human papillomavirus
- HPV-UTD
human papillomavirus vaccination up-to-date
- NCI
National Cancer Institute
- NIS-Teen
National Immunization Survey-Teen
Footnotes
Dr Chido-Amajuoyi contributed to the conceptualization and design of the study, drafted the initial manuscript, interpreted study data, and reviewed and revised the manuscript; Dr Talluri contributed to the conceptualization and design of the study, conducted all statistical analysis, interpreted study data, and reviewed and revised the manuscript; Dr Wonodi contributed to interpretation of study data and reviewed and revised the manuscript; Dr Shete contributed to the conceptualization and design of the study, interpreted study data, reviewed and revised the manuscript, obtained funding, and supervised the study; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Cancer Institute (P30CA016672 to Dr Shete), the Betty B. Marcus Chair in Cancer Prevention (to Dr Shete), the Duncan Family Institute for Cancer Prevention and Risk Assessment (Dr Shete), and the Cancer Prevention Research Institute of Texas (grant RP170259 to Dr Shete). A cancer prevention fellowship award was supported by the Mrs Harry C. Wiess Cancer Research Fund and the Laura and John Arnold Foundation (to Dr Chido‐Amajuoyi). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Wonodi is a member of Merck’s Global Vaccine Confidence Advisory Board and has no conflict of interest with respect to this study; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus-associated cancers - United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2018;67(33):918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Healthy People 2020. Immunization and infectious diseases. 2000. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed February 2, 2021
- 4. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brotherton JML, Bloem PN. Population-based HPV vaccination programmes are safe and effective: 2017 update and the impetus for achieving better global coverage. Best Pract Res Clin Obstet Gynaecol. 2018;47:42–58 [DOI] [PubMed] [Google Scholar]
- 6. National HPV Vaccination Program Register. HPV vaccination coverage by dose number (Australia) for males/females by age group in 2017. 2018. Available at: https://www.health.gov.au/resources/publications/national-hpv-vaccination-coverage-by-dose-number-for-adolescents-by-age-group. Accessed March 27, 2021
- 7. The National Cancer Institute. NCI-designated cancer centers endorse goal of eliminating HPV-related cancers. 2018. Available at: https://www.mdanderson.org/content/dam/mdanderson/documents/prevention-and-screening/HPV-Statement.pdf. Accessed February 3, 2021
- 8. Petrosky E, Bocchini JA Jr., Hariri S, et al. ; Centers for Disease Control and Prevention (CDC) . Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304 [PMC free article] [PubMed] [Google Scholar]
- 9. O’Leary ST, Nyquist AC. Why AAP recommends initiating HPV vaccination as early as age 9. 2019. Available at: https://www.aappublications.org/news/2019/10/04/hpv100419. Accessed November 10, 2020
- 10. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17(12):1293–1302 [DOI] [PubMed] [Google Scholar]
- 11. Palmer T, Wallace L, Pollock KG, et al. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12–13 in Scotland: retrospective population study. BMJ. 2019;365:l1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winer RL, Feng Q, Hughes JP, O’Reilly S, Kiviat NB, Koutsky LA. Risk of female human papillomavirus acquisition associated with first male sex partner. J Infect Dis. 2008;197(2):279–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finer LB, Philbin JM. Sexual initiation, contraceptive use, and pregnancy among young adolescents. Pediatrics. 2013;131(5):886–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Committee opinion no. 704: human papillomavirus vaccination. Obstet Gynecol. 2017;129(6):1. [DOI] [PubMed] [Google Scholar]
- 15. Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Ther. 2014;36(1):17–23 [DOI] [PubMed] [Google Scholar]
- 16. Sinka K, Kavanagh K, Gordon R, et al. Achieving high and equitable coverage of adolescent HPV vaccine in Scotland. J Epidemiol Community Health. 2014;68(1):57–63 [DOI] [PubMed] [Google Scholar]
- 17. St Sauver JL, Rutten LJF, Ebbert JO, Jacobson DJ, McGree ME, Jacobson RM. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beachler DC, Gonzales FA, Kobrin SC, Kreimer AR. HPV vaccination initiation after the routine-recommended ages of 11–12 in the United States. Papillomavirus Res. 2016;2:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niccolai LM, Mehta NR, Hadler JL. Racial/ethnic and poverty disparities in human papillomavirus vaccination completion. Am J Prev Med. 2011;41(4):428–433 [DOI] [PubMed] [Google Scholar]
- 20. Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(3):896–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(33):909–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention (CDC). National Immunization Survey-Teen: A User’s Guide for the 2016 Public-Use Data File. Chicago, IL: National Opinion Research Center. Available at: https://ftp.cdc.gov/pub/health_statistics/nchs/dataset_documentation/nis/nisteenpuf14_dug.pdf. Accessed February 3, 2021 [Google Scholar]
- 24. Jain N, Singleton JA, Montgomery M, Skalland B. Determining accurate vaccination coverage rates for adolescents: the National Immunization Survey–Teen 2006. Public Health Rep. 2009;124(5):642–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kahle D, Wickham H. Ggmap: spatial visualization with ggplot2. R J. 2013;5(1):144–161 [Google Scholar]
- 26. Suppli CH, Hansen ND, Rasmussen M, Valentiner-Branth P, Krause TG, Mølbak K. Decline in HPV-vaccination uptake in Denmark - the association between HPV-related media coverage and HPV-vaccination. BMC Public Health. 2018;18(1):1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Domgue JF, Chido-Amajuoyi OG, Yu RK, Shete S. Beliefs about HPV vaccine’s success at cervical cancer prevention among adult US women. JNCI Cancer Spectr. 2019;3(4):pkz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suk R, Montealegre JR, Nemutlu GS, et al. Public knowledge of human papillomavirus and receipt of vaccination recommendations. JAMA Pediatr. 2019;173(11):1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chido-Amajuoyi OG, Jackson I, Yu R, Shete S. Declining awareness of HPV and HPV vaccine within the general US population. Hum Vaccin Immunother. 2021;17(2):420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boersma P, Black LI. Human papillomavirus vaccination among adults aged 18–26, 2013–2018. NCHS Data Brief. 2020;(354):1–8 [PubMed] [Google Scholar]
- 31. Walker TY, Elam-Evans LD, Williams CL, et al. Trends in human papillomavirus (HPV) vaccination initiation among adolescents aged 13–17 by metropolitan statistical area (MSA) status, National Immunization Survey - Teen, 2013–2017. Hum Vaccin Immunother. 2020;16(3):554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spencer JC, Calo WA, Brewer NT. Disparities and reverse disparities in HPV vaccination: a systematic review and meta-analysis. Prev Med. 2019;123:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim HH, Washburn T, Marceau K, Duggan-Ball S, Raymond P. Human papillomavirus (HPV) vaccination coverage among Rhode Island adolescents, 2008–2016. R I Med J (2013). 2018;101(2):49–51 [PubMed] [Google Scholar]
- 34. Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1(1):e8–e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farmar A-LM, Love-Osborne K, Chichester K, Breslin K, Bronkan K, Hambidge SJ. Achieving high adolescent HPV vaccination coverage. Pediatrics. 2016;138(5):e20152653. [DOI] [PubMed] [Google Scholar]
- 36. Safaeian M, Sampson JN, Pan Y, et al. ; Costa Rica HPV Vaccine Trial (CVT) Group . Durability of protection afforded by fewer doses of the HPV16/18 vaccine: the CVT trial. J Natl Cancer Inst. 2018;110(2):205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]