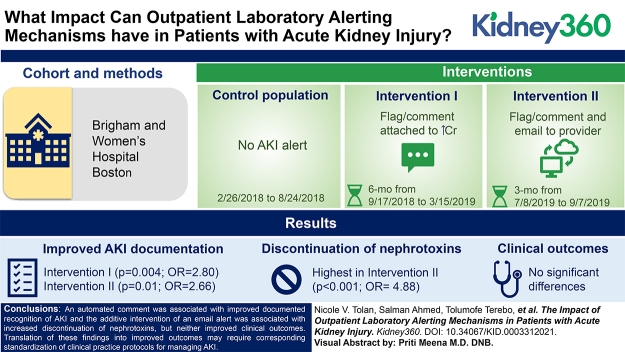
Key Points
An AKI alert attached to increasing creatinine results improved recognition of AKI and reduced the time to obtain a follow-up creatinine.
The additive intervention of an email alert to the ordering provider increased the discontinuation of nephrotoxins.
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, creatinine, documentation, laboratory alerts, nephrotoxins, outcomes, outpatients
Visual Abstract
Abstract
Background
AKI is an abrupt decrease in kidney function associated with significant morbidity and mortality. Electronic notifications of AKI have been utilized in patients who are hospitalized, but their efficacy in the outpatient setting is unclear.
Methods
We evaluated the effect of two outpatient interventions: an automated comment on increasing creatinine results (intervention I; 6 months; n=159) along with an email to the provider (intervention II; 3 months; n=105), compared with a control (baseline; 6 months; n=176). A comment was generated if a patient’s creatinine increased by >0.5 mg/dl (previous creatinine ≤2.0 mg/dl) or by 50% (previous creatinine >2.0 mg/dl) within 180 days. Process measures included documentation of AKI and clinical actions. Clinical outcomes were defined as recovery from AKI within 7 days, prolonged AKI from 8 to 89 days, and progression to CKD with in 120 days.
Results
Providers were more likely to document AKI in interventions I (P=0.004; OR, 2.80; 95% CI, 1.38 to 5.67) and II (P=0.01; OR, 2.66; 95% CI, 1.21 to 5.81). Providers were also more likely to discontinue nephrotoxins in intervention II (P<0.001; OR, 4.88; 95% CI, 2.27 to 10.50). The median time to follow-up creatinine trended shorter among patients with AKI documented (21 versus 42 days; P=0.11). There were no significant differences in clinical outcomes.
Conclusions
An automated comment was associated with improved documented recognition of AKI and the additive intervention of an email alert was associated with increased discontinuation of nephrotoxins, but neither improved clinical outcomes. Translation of these findings into improved outcomes may require corresponding standardization of clinical practice protocols for managing AKI.
Introduction
AKI is an abrupt decrease in kidney function that may be due to a diverse array of etiologies (1). The incidence of AKI varies from 22% to 57%, depending on the care setting (e.g., outpatient, intensive care unit) and the criteria applied for patient definition (2,3). AKI in the outpatient setting is common, occurring up to 32% of patients in one observational cohort (4). Possible factors associated with increasing prevalence of AKI include an aging population, rise in comorbidities that predispose to AKI (e.g., diabetes mellitus, hypertension, congestive heart failure, CKD), increased use of agents that may result in nephrotoxicity (e.g., chemotherapy, antimicrobials), and increasing frequency of invasive and surgical procedures (5,6). Each episode of AKI is consequential, and associated with outcomes including mortality, cardiovascular events, CKD, and ESKD (7,8).
Identification of AKI allows clinicians to follow consensus guideline recommendations for the appropriate management of patients with AKI, including assessment of medication dosing, avoiding nephrotoxic exposures, and managing fluid and electrolytes (8). However, there is considerable variability in clinician and health care system adherence to these guidelines to prevent, diagnose, and treat AKI, which may account for the high rates of adverse outcomes (8).
Electronic health records (EHR) can enable effective population surveillance across many fields of medicine. Related to AKI, EHR-based alert systems may allow timely recognition of AKI, guide appropriate clinical action, and improve efforts to prevent AKI (9,10). Most reports of electronic notification of AKI have focused on in-hospital AKI events (11–13). However, early identification and management of AKI in the outpatient setting may prevent worsening AKI and the associated consequences of severe and/or prolonged AKI. Kidney function in the 72-hour period immediately after AKI in patients who are hospitalized is associated with specific clinical outcomes: the development or progression of CKD, initiation of long-term dialysis, or death from any cause within a median follow-up of approximately 4.7 years (14). In this study, we implemented sequential AKI alert interventions for outpatient providers and determined if the interventions were associated with increased documentation of AKI and related clinical actions that could improve clinical outcomes.
Materials and Methods
Study Setting
Brigham and Women’s Hospital is a 793-bed tertiary care hospital located in Boston, MA. The clinical laboratories process approximately 3 million specimens/year and perform approximately 275 outpatient plasma creatinine tests per day; with a baseline rate of outpatient AKI of 1%–2%. eGFR has been calculated using the CKD Epidemiology Collaboration equation since March 6, 2018. Before that, the Modification of Diet in Renal Disease equation was utilized. The laboratory information system (LIS) is Sunquest (Sunquest Information Systems, Inc., Tucson, AZ) and EHR is Epic (Epic Healthcare Systems, Verona, WI). The project was conducted under Mass General Brigham Institutional Review Board exemption as meeting the requirements of quality improvement research.
Sample Selection, Interventions, and Exclusion Criteria
The study was divided into three intervals (Figure 1): a baseline or control population without any AKI alert (a 6-month period from February 26, 2018 to August 24, 2018), an automated result comment attached to increasing creatinine results (intervention I; a 6-month period from September 17, 2019 to March 15, 2019) and a manual email to the ordering provider regarding the result comment in addition to the automated AKI comment (intervention II; a 3-month period from July 8, 2019 to September 7, 2019). Intervention II was limited to 3 months because of the effort required by the laboratory directors to perform daily patient review and statistical power had been achieved in this timeframe.
Figure 1.
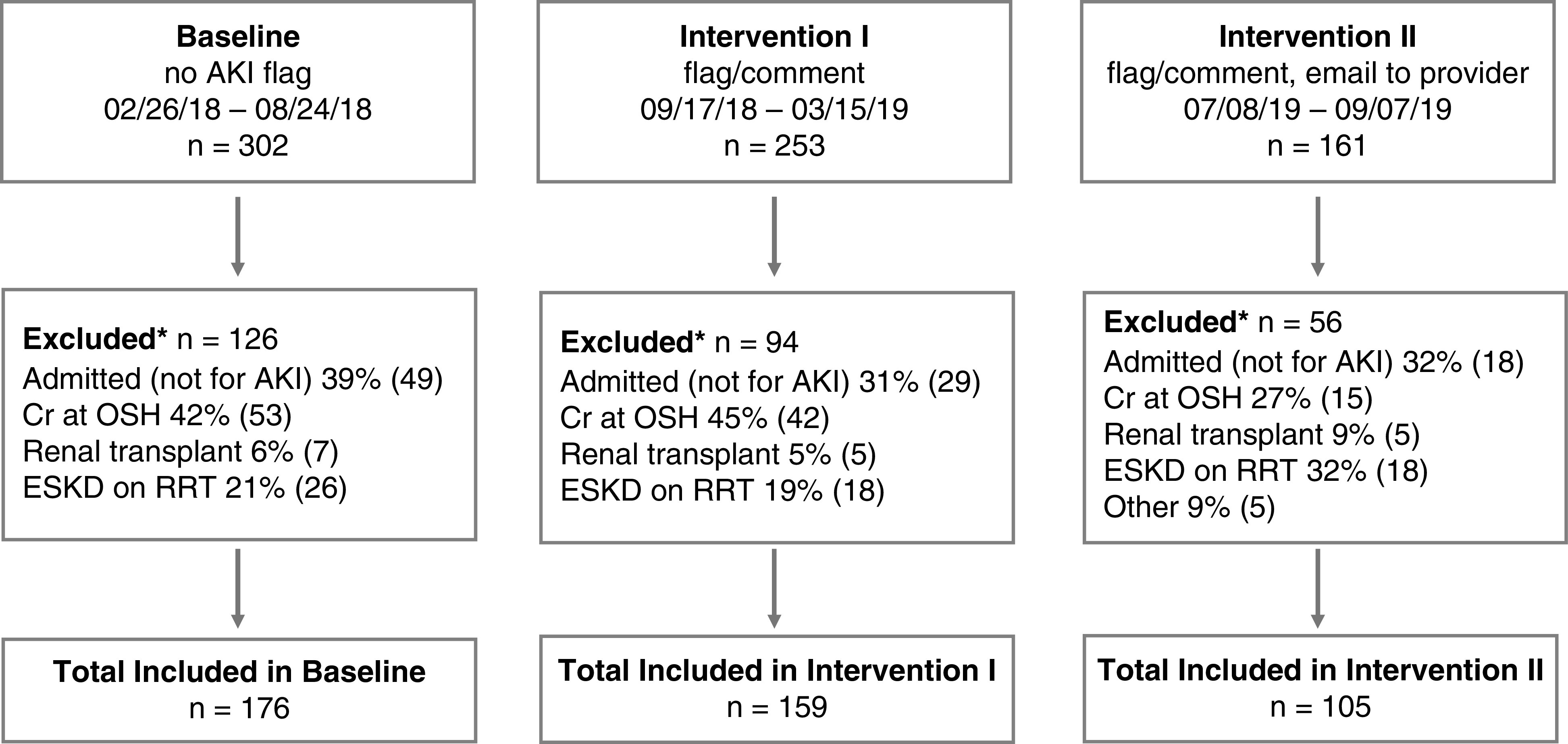
Study design. The three intervals of the study, associated interventions, number of and reason for exclusion and final patient count are depicted. *Patients may have more than one reason for exclusion. Cr, creatinine; OSH, outside hospital.
Utilizing a calculation built in our LIS, an automated creatinine result comment was generated if a patient’s creatinine increased by >0.5 mg/dl if the previous creatinine was ≤2.0 mg/dl, or increased by 50% if the previous creatinine was >2.0 mg/dl (Figure 2A). The most recent creatinine within the past 180 days was used as the baseline creatinine from which to calculate the delta. In both intervention I and II, result comments (Figure 2B) were appended to creatinine results for outpatients when these criteria were met. The result comment was not applied to patient results from dialysis locations or if no creatinine was available in the prior 180 days. In intervention II, an automated crystal report was built to identify these patients with potential AKI and was generated three times per day at approximately 2am, 10am, and 6pm. One of two laboratory directors (N.T., S.M.) manually reviewed each patient and emailed the ordering provider within 24 hours of the automated result comment, to alert them of the potential risk of AKI and included information regarding the previous and current creatinine results (Figure 2C).
Figure 2.
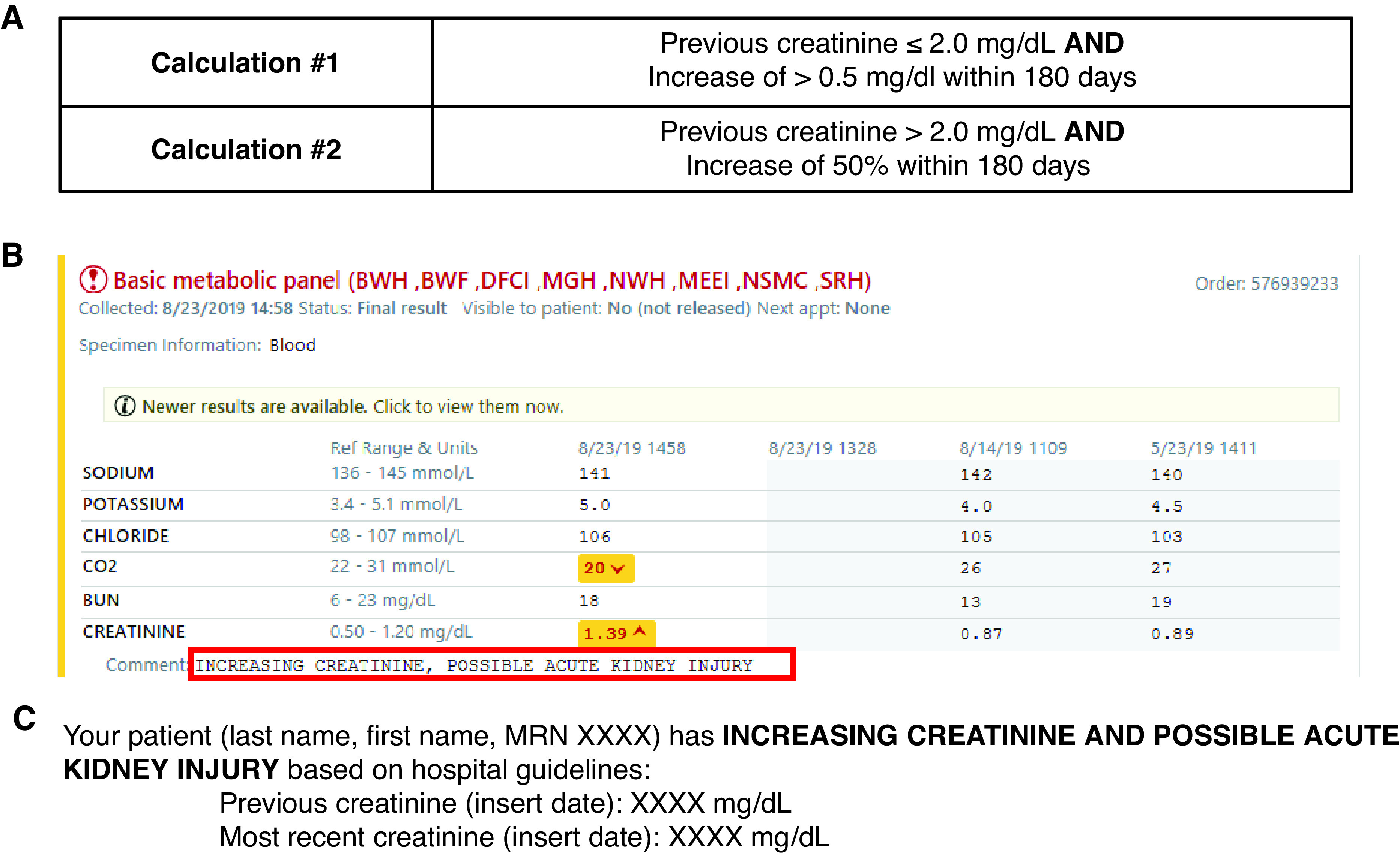
Study interventions. (A) The laboratory information system (LIS) calculations to flag outpatient creatinine in patients with possible acute kidney injury are shown. (B) The comment that displayed in our electronic health records (EHR) in intervention I and intervention II is depicted. (C) The email template sent to the ordering provider in intervention II is shown.
Before analysis, a manual review was conducted by one of two laboratorians (N.T, S.M.) to exclude patients from each interval if (1) the patient had the diagnosis of ESKD or was on RRT, (2) the patient had a creatinine resulted at an outside hospital between the flagged and previous creatinine at BWH and the patient no longer met the criteria for AKI (Figure 2A), or (3) the patient was hospitalized for reasons other than AKI the day the flagged creatinine was measured (Figure 1). If the patient had more than one flagged creatinine within a 2-week period, only the earliest flagged creatinine was included. In intervention II, the laboratory director did not email the provider if there was a note in the chart that the provider was already aware of the results at the time of review.
Process Measures
Process measures included documentation of AKI and clinical action taken. Clinical actions were only analyzed if the provider documented AKI. We also determined if the time to obtain a follow-up creatinine differed between providers that did and did not document AKI. Furthermore, we investigated whether documentation and clinical actions differed between ordering provider services and whether the interventions changed provider behavior related to these process measures.
Outcome Measures
Given the outpatient population studied, we defined our clinical outcomes as (1) recovery from AKI within 7 days, (2) prolonged AKI between 8 and 89 days, and (3) progression to CKD from 90 to 120 days after the AKI alert. Recovery was defined as a decrease in creatinine of ≥0.3 mg/dl or 25% from peak creatinine. Prolonged AKI was defined as a creatinine that did not meet the recovery criteria. Progression to CKD was defined as two eGFR <60 ml/min per 1.73 m2 separated by 90–120 days.
Medical Record Review for Process Measures and Clinical Outcomes
Medical record review to obtain process measures of AKI and clinical actions was completed independently by one of two clinicians (S.A., Z.V.) using a standardized review instrument. To assess reliability of the medical record review, 10% of patients were reviewed by the other clinician using the same standardized template and analyzed for concordance.
The following parameters were obtained during medical record review (Supplemental Table 1): documented recognition of AKI and date, documented clinical action and date, type of clinical action (i.e., repeat Cr, encourage hydration, discontinuation of nephrotoxins, change dosing, nephrology consult, hospitalization), presence of underlying CKD, and cause of AKI. Each parameter, except dates and cause of AKI, was captured as binary data, having occurred or not. Only documentation and clinical action occurring within the 2 weeks after the AKI alert was included.
AKI cause was further categorized as hypovolemia (including overdiuresis), cardiorenal syndrome, drugs (including contrast), or other, to allow for binary analysis. Patients with more than one documented AKI cause were also categorized as multifactorial. If a patient was hospitalized, the date of admission and length of stay were recorded. The time (in days) between the creatinine result comment and the documentation of each recognition of AKI and clinical action, was determined.
LIS and EHR Data
Patient demographics (i.e., age, sex, race) and follow-up creatinine measurement(s) from 0 to 120 days after AKI alert were obtained from our LIS. The time to first follow-up creatinine was also calculated. The ordering provider and their clinical service was obtained electronically from the specialty description field in our EHR. Provider services were grouped into five categories: internal medicine (study total n=187; average across intervals n=62), cardiology (total n=83; average n=28), pulmonary (total n=71; average n=24), nephrology (total n=50; average n=17), and surgical services/transplant (total n=49; average n=16). Dermatology, endocrinology, gastroenterology, infectious disease, primary care, and family medicine were grouped with internal medicine, and anesthesiology, urology, oncology, cardiac transplant, and pulmonary transplant were considered surgical services/transplant.
Statistical Methods
Patient demographics and characteristics were compared between intervention categories using Pearson’s chi-squared test for categorical outcomes, Fisher’s exact test for categorical outcomes when cell counts were <5, and Wilcoxon rank-sum test for non-normally distributed continuous variables. A Cohen’s kappa statistical test was performed to assess agreement between two reviewers for all process measures and AKI causes. Logistic regression adjusted for age, sex, race, flagged creatinine, and AKI causes (drugs including contrast, multifactorial, and no documented cause) was used to assess differences in the likelihood of each process measure between intervention categories. P values are reported both for the comparison of each intervention category to the baseline, and for the trend. On the basis of the sample size of this investigation, the minimum % increase in the dichotomous outcomes that could be detected as statistically significant—in any comparison—was 15%, assuming a power of 0.80, and an α level of 5%. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and STATA version SE15 (StataCorp., 2015, Stata Statistical Software: Release 14, College Station, TX: StataCorp LP).
Results
Study Characteristics and Patient Demographics
The overall dataset curation, including the number of excluded patients and reasons for exclusions, are shown in Figure 1. There was no difference in excluded patients in each interval (P=0.29). The majority of creatinine result comments were on the basis of calculation 1 (Figure 2A) with only 3%, 0.7%, and 4% flagged on the basis of calculation 2 in baseline, intervention I, and intervention II, respectively. The rates of recurrent AKI were low and similar in each interval, ranging from 2 to 7% (P=0.14). Table 1 lists the patient demographics and characteristics in each interval of the study. There was a difference in Hispanic race (P=0.01), median creatinine results with automated comments (i.e., median flagged creatinine) (P=0.02), and the AKI causes of drugs including contrast (P<0.001), multifactorial (P<0.001), and no documented cause (P=0.001) between the intervals, requiring normalization in subsequent analyses.
Table 1.
Patient demographics and characteristics
| Demographics and Characteristics | Baseline | Intervention I | Intervention II | P Value |
|---|---|---|---|---|
| (n=176) | (n=159) | (n=105) | ||
| Sex, M, % (n) | 55 (97) | 49 (78) | 53 (56) | 0.53 |
| Age, y, mean±SD | 65±14.0 | 65±13.3 | 66±15.9 | 0.27 |
| Racial and ethnicity, % (n) | ||||
| White, % (n) | 76 (133) | 66 (105) | 72 (76) | 0.15 |
| Black, % (n) | 11 (20) | 18 (29) | 18 (19) | 0.15 |
| Hispanic, % (n) | 11 (20) | 5 (8) | 3 (3) | 0.01 |
| Asian, % (n) | 2 (3) | 1 (2) | 1 (1) | 0.86 |
| Flagged Cr, median, (IQR) | 2.13 (0.68) | 1.94 (0.63) | 2.08 (0.67) | 0.02 |
| Underlying CKD, % (n) | 68 (119) | 67 (107) | 66 (69) | 0.94 |
| Hypovolemia, % (n) | 53 (94) | 50 (80) | 51 (54) | 0.92 |
| Cardiorenal syndrome, % (n) | 9 (15) | 3 (4) | 8 (8) | 0.06 |
| Drugs (incl. contrast), % (n) | 30 (53) | 16 (25) | 9 (9) | <0.001 |
| Multifactorial, % (n) | 19 (33) | 18 (13) | 4 (4) | <0.001 |
| No documented cause, % (n) | 14 (25) | 31 (49) | 26 (27) | 0.001 |
| Other, % (n) | 12 (21) | 8 (13) | 11 (11) | 0.52 |
Cr, creatinine; IQR, interquartile range; incl., Including.
Process Measures
Providers were more likely to document AKI in both intervention I (P=0.004; odds ratio [OR], 2.80; 95% CI, 1.38 to 5.67) and intervention II (P=0.01; OR, 2.66; 95% CI, 1.21 to 5.81), compared with baseline (Tables 2 and 3). The trend P value for documentation of AKI was also significant (P=0.006; OR, 1.74; 95% CI, 1.17 to 2.50). Providers were also more likely to discontinue nephrotoxins in intervention II compared with baseline (P<0.001; OR, 4.88; 95% CI, 2.27 to 10.50), and there was a significant trend across intervals (P<0.001; OR, 2.22; 95% CI, 1.51 to 3.29) (Table 3). However, there was no significant difference in the type of nephrotoxins discontinued across the intervals (Table 4). The AKI alert interventions did not significantly affect any other clinical action (repeat Cr, encourage hydration, changing dosing, nephrology consult, or hospitalization) (Tables 2 and 3).
Table 2.
Clinical actions taken during each interval
| Clinical Action, % (n) | Baseline | Intervention I | Intervention II |
|---|---|---|---|
| (n=123) | (n=109) | (n=76) | |
| Repeat Cr | 81 (99) | 83 (90) | 83 (63) |
| Follow up Cr at 0–7 days | 35 (61) | 42 (66) | 28 (29) |
| Follow up Cr at 8–89 days | 79 (139) | 71 (113) | 70 (73) |
| Follow up Cr at 90–120 days | 30 (87) | 41 (65) | 36 (38) |
| Encourage hydration | 42 (51) | 48 (52) | 49 (37) |
| Discontinuation of nephrotoxins | 18 (22) | 19 (21) | 38 (29) |
| Change dosing | 35 (43) | 36 (39) | 25 (19) |
| Nephrology consult | 7 (9) | 2 (2) | 0 (0) |
| Hospitalization | 15 (18) | 12 (13) | 15 (11) |
Cr, creatinine.
Table 3.
Process measures and clinical outcomes
| Outcomes | Intervention I, n, Odds Ratio (95% Confidence Interval) |
Intervention II, n, Odds Ratio (95% Confidence Interval) | Trend P Value |
|---|---|---|---|
| (n=159) | (n=105) | ||
| Documented AKI | 111 2.80 (1.38 to 5.67) | 77 2.66 (1.21 to 5.81) | 0.006 |
| Clinical action | 2.54 (0.50 to 12.83) | 2.01 (0.35 to 11.73) | 0.37 |
| Repeat Cr | 1.60 (0.77 to 3.30) | 1.69 (0.77 to 3.73) | 0.17 |
| Encourage hydration | 1.57 (0.87 to 2.83) | 1.82 (0.95 to 3.48) | 0.06 |
| Discontinuation of nephrotoxins | 1.60 (0.76 to 3.35) | 4.88 (2.27 to 10.50) | <0.001 |
| Change dosing | 1.02 (0.57 to 1.81) | 0.60 (0.31 to 1.17) | 0.17 |
| Nephrology consult | 0.33 (0.07 to 1.70) | — | 0.05 |
| Hospitalization | 0.95 (0.42 to 2.17) | 1.20 (0.50 to 2.90) | 0.71 |
| Recovery within 7 days | 34 0.60 (0.29 to 1.2) | 18 0.92 (0.37 to 2.30) | 0.61 |
| Prolonged AKI | 33 0.92 (0.54 to 1.58) | 19 0.79 (0.42 to 1.48) | 0.46 |
| Progression to CKD | 49 0.91 (0.43 to 1.94) | 33 1.97 (0.680 to 5.72) | 0.31 |
AKI, acute kidney injury.
Table 4.
Discontinuation of nephrotoxins in each interval
| Class of Nephrotoxin Discontinued, % (n) | Baseline, % (n) | Intervention I, % (n) | Intervention II, % (n) |
|---|---|---|---|
| (n=22) | (n=21) | (n=29) | |
| Diuretic | 50 (11) | 52 (11) | 72 (21) |
| ACE inhibitor/ARB | 32 (7) | 19 (4) | 24 (7) |
| Immunosuppressant | 18 (4) | 5 (1) | 7 (2) |
| Antibiotics | 14 (3) | 10 (2) | 0 (0) |
| NSAID | 9 (2) | 19 (4) | 3 (1) |
| Antidiabetic | 0 (0) | 10 (2) | 7 (2) |
| Antigout | 0 (0) | 10 (2) | 0 (0) |
| Antihypertensive | 0 (0) | 5 (1) | 3 (1) |
| Antiarrthythmic | 0 (0) | 5 (1) | 0 (0) |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; NSAID, nonsteroidal anti-inflammatory.
Although not statistically significant, the median time to obtain a follow-up creatinine across all intervals by providers who documented AKI and did not document AKI was 21 days (interquartile range, 68) and 42 days (interquartile range, 63), respectively (P=0.11).
Clinical Outcomes
The AKI alert interventions did not significantly affect the clinical outcomes of AKI recovery, prolonged AKI, or progression to CKD when compared with baseline (Table 3). The number of follow-up creatinine results at 0–7 days, 8–89 days, and 90–120 days was similar across the interventions (P=0.07, P=0.13, P=0.07; respectively) (Table 2). Similarly, the presence of underlying CKD in patients with CKD progression was 87% in baseline, 84% in intervention I, and 85% in intervention II (P=0.91).
Difference Among Clinical Services
Regardless of the interval, surgical services/transplant were less likely than any other service to document both AKI (P<0.001; OR, 0.26; 95% confidence interval [95% CI], 0.12 to 0.57) and clinical action (P<0.001; OR, 0.20; 95% CI, 0.11 to 0.37). In contrast, the pulmonary service was more likely to both document AKI (P=0.01; OR, 3.18; 95% CI, 1.29 to 7.85) and clinical action (P=0.03, OR, 2.12; 95% CI, 1.09 to 4.09).
Internal medicine providers were more likely to document AKI in both intervention I (P=0.02, OR, 3.97; 95% CI, 1.27 to 11.85) and intervention II (P=0.008, OR, 6.07; 95% CI, 1.61 to 22.90) compared with baseline, with a trend P=0.004 (Table 5). There were no significant changes in documentation of AKI in other services. Internal medicine providers were also more likely to document clinical action. There was difference between baseline and intervention II (P=0.02, OR, 5.76; 95% CI, 1.30 to 25.69) and a significant trend (P=0.02, OR, 2.41; 95% CI, 1.15 to 5.03) (Table 5). The interventions did not significantly affect clinical action in the other services.
Table 5.
Documentation of acute kidney injury and clinical action by clinical service
| Service | Intervention I, Odds Ratio (95% Confidence Interval) | Intervention II, Odds Ratio (95% Confidence Interval) | Trend P Value |
|---|---|---|---|
| (n=159) | (n=105) | ||
| Documented recognition of AKI | |||
| Internal medicine (n=189) | 3.90 (1.27 to 11.85) | 6.07 (1.61 to 22.90) | 0.004 |
| Cardiology (n=83) | 2.35 (0.43 to 12.75) | 1.11 (0.16 to 7.83) | 0.72 |
| Nephrology (n=49) | 1.52 (0.15 to 15.28) | 0.07 (0.002 to 2.045) | 0.13 |
| Documented clinical action | |||
| Internal medicine (n=189) | 2.42 (0.72 to 8.13) | 5.76 (1.30 to 25.69) | 0.02 |
| Cardiology (n=83) | 2.02 (0.38 to 10.83) | 1.00 (0.16 to 6.02) | 0.91 |
| Nephrology (n=49) | 2.91 (0.035 to 23.91) | 0.52 (0.49 to 5.56) | 0.74 |
Surgical and pulmonary services are not shown due to low numbers.
Reviewer Agreement
Reviewers achieved almost perfect agreement for hospitalization (K=0.81) and cardiorenal syndrome (K=0.85). Reviewers achieved substantial agreement on documented recognition of AKI (K=0.63), encourage hydration (K=0.68), discontinuation of nephrotoxins (K=0.74), hypovolemia (K=0.76), and no documented cause of AKI (K=0.61). Moderate agreement was achieved for documented clinical action (K=0.50) and other as a cause for AKI (K=0.45). Reviewers achieved fair agreement for repeat labs (K=0.40), change dosing (K=0.38), nephrology consult (K=0.37), and drugs (including contrast) (K=0.36). All components of the medical record review scored a K value ≥0.36 corresponding to fair or better and ≥75% agreement.
Discussion
In this intervention-based cohort study, we found that an EHR-based automated comment displaying with increasing creatinine results was associated with increased documented recognition of AKI, particularly by internal medicine providers, and a decreased the time to obtain follow-up creatinine measurements. The additive intervention of an email notification sent by a laboratory director to the ordering provider was associated with an increase in the discontinuation of nephrotoxins. To our knowledge, this is the first study to examine the effect of AKI alerts and define clinical outcomes specific for the outpatient setting.
Previous studies on the effect of AKI alerts were performed on hospitalized patients and reported variable effects on clinical outcomes (8–13,15–17). Aiyegbusi et al. performed a study of patients who were hospitalized, and showed an AKI alert was associated with a dramatic reduction in the repeat-testing interval for creatinine (15). However, Wilson et al. in a single-center, randomized controlled trial of >23,000 hospitalized adult patients found that an AKI alert had no effect on the clinical outcomes of peak creatinine concentration, initiation of RRT, or death during the current hospital admission, or within 30 days (11). In that study, the intervention group received a text page alert indicating potential AKI, following the 2012 Kidney Disease: Improving Global Outcomes consensus definition for AKI (8). Another randomized clinical trial also showed no effect on clinical outcomes of a pop-up alert for AKI in patients who were hospitalized (13).
Laboratories usually have well-defined processes for communicating immediately life-threatening or critical results, such as hyperkalemia, but processes and algorithms for communicating significant changes in results, such as creatinine, are not routinely implemented or standardized across institutions. The effectiveness of electronic notifications is variable and reportedly more successful if alerts are simplistic, evidence based, and only triggered at the appropriate time (18,19). An effective AKI alert should include both an accurate baseline creatinine, the definition of which is a subject of debate (8,20–22), and a change in creatinine that is sensitive, yet specific for AKI (8). According to Siew et al., the mean outpatient creatinine from the past 7–365 days is the best approximation of the baseline creatinine concentration (23). However, not all laboratories have the time, resources, and technologic infrastructure to implement AKI alerts using running mean creatinine values. The 2012 Kidney Disease: Improving Global Outcomes consensus definition for AKI is described as a change of ≥0.3 mg/dl in the prior 48 hours (8), but a high number of false-positive AKI alerts have been seen using these guidelines, particularly in patients with underlying CKD and higher baseline creatinine concentrations (24,25).
In our study, providers were significantly more likely to document recognition of AKI in both interventions. The time to obtain a follow-up creatinine was 21 days if AKI was documented, compared with 42 days if AKI was not documented across all interventions. Although not statistically significant, the difference is clinically significant and similar to the findings of Aiyegbusi et al. (15) It is notable that more participants had a follow-up creatinine at 8–89 days as opposed to the other time windows, which may have implications on recovery from AKI. However, we hypothesize that this pattern reflects outpatient AKI management involving a period of observation after a clinical action. Providers were also significantly more likely to discontinue nephrotoxins in intervention II compared with baseline. Although prerenal causes of AKI including hypovolemia are common, as seen in our study, the effect of discontinuation of nephrotoxins can be significant. A larger sample size was likely necessary to show an effect of discontinuing nephrotoxins on recovery from AKI, prolonged AKI, and progression to CKD. The effect of interventions on nephrology consults could not be assessed because there were no consults in intervention II. This may suggest the email increased the confidence of non-nephrology providers in diagnosing and managing AKI. Overall, our findings suggest a result comment alone improved the documented recognition of AKI and consequentially decreased the time to obtain follow-up creatinine. However, an email was necessary to increase the discontinuation of nephrotoxins and possibly reduce nephrology consults.
Across all intervals, surgical and transplant services were least likely to document AKI and clinical actions, whereas pulmonary was most likely to document AKI and clinical actions. This may be due to the availability of standardized protocols for documentation that have been implemented by the pulmonary team at our institution. Internal medicine providers, the largest group of providers in our study, were significantly were more likely to document recognition of AKI in both intervention I and II and more likely to document clinical action in intervention II. This suggests internal medicine, unlike other services, may be the most responsive to alerts and managing AKI in the outpatient setting, and could reflect greater clinical expertise and education about AKI as opposed to other provider groups. Furthermore, it may be more effective to alert the primary care provider of AKI in addition to, or instead of, the ordering provider, regardless of service.
We speculate that we did not see an effect of our interventions on clinical outcomes for several reasons. First, we had a limited sample size, constrained on the basis of the number of patients with outpatient creatinine obtained during the time periods outlined, which likely limited our power to observe differences in clinical outcomes. Second, we were limited to data within our EHR, which means patients may have had labs checked, indicating recovery or CKD development that we were unable to capture. Third, we did not capture data on the patient population, medical, and social complexity, which can affect the ability to mitigate the consequences of AKI. Unlike previously published studies on AKI alerts, our patients had AKI in the outpatient setting, higher baseline kidney function, and were less likely to be hospitalized as a result of AKI (<15% in each interval; Table 2). Therefore, providers may have been focused on managing other comorbidities. Fourth, we did not educate providers about strategies to manage AKI and about the alert interventions themselves. We recognize that AKI alerts alone may not be enough to improve clinical outcomes. Although the effect of our interventions on process measures including AKI documentation and discontinuation of nephrotoxins was promising, integrated education, such as standardized clinical practice guidelines, daily review of alerts by a nephrologist, and/or more robust alerts such as a text page, may be necessary to affect process measures and clinical outcomes, particularly for providers outside of internal medicine.
Our study has several limitations. First, the study was performed at a large academic medical center and results may not be generalizable. Second, our study was not randomized and interventions were sequential, which limits causality, although there were no other efforts undertaken at the same time to improve AKI. Utilizing the available functionality in our LIS, we evaluated each creatinine against the most recent creatinine measurement in the previous 180 days and our criteria for AKI were modified for our outpatient population. Therefore, the determination of baseline was not optimal, leading to both false-positive and false-negative AKI alerts (25). However, feedback from providers receiving the alerts was generally positive (e.g., “This new notification system is great and will improve patient safety”). Furthermore, no concerns were raised about the accuracy or timing of the alert. Lastly, in intervention II we did not email the ordering provider with the AKI alert if the provider noted in the EHR that they were aware of the increasing creatinine. Therefore, we may have underestimated the effect of intervention II throughout our analyses.
On the basis of our findings, the laboratory continues to append an automated comment to increasing creatinine results. We encourage nephrologists to collaborate with their laboratory colleagues and implement a calculation in their LIS that appends a comment to increasing creatinine results indicative of AKI in the outpatient setting. Additional studies are required to confirm the optimal baseline creatinine, the thresholds for AKI alert in the outpatient population, the most effective mechanism of notification (such as a text page), and which providers should send the notification. The utility of alerts to trigger a review of nephrotoxins and prevent AKI should also be explored. Finally, developing education that accompanies the alert and consensus guidelines for management of AKI for outpatients will be important.
Disclosures
M.L. Mendu reports having consultancy agreements with Bayer AG. N.V. Tol an reports receiving research funding from Abbott Diabetes Care and Biomerieux; and reports being a scientific advisor or member of the American Association for Clinical Chemistry, College of American Pathologists, and the Journal of Applied Laboratory Medicine. S. Ahmed reports having an ownership interest in The Kidney Health and Preventive Medicine Institute. All remaining authors have nothing to disclose.
Funding
None.
Footnotes
See related editorial, “Identifying Acute Kidney Injury in the Outpatient Setting: The First Step,” on pages 1549–1550.
Author Contributions
Y. Kelly, S. Melanson, M. Mendu, A. Petrides, and N. Tolan conc eptualized the study; S. Ahmed, Y. Kelly, S. Melanson, J. Ransohoff, T. Terebo, N. Tolan, and Z. Virk were responsible for data curat ion; S. Ahmed, C. Demetriou, S. Melanson, A. Petrides, and N. Tolan were responsible for formal analysis; S. Ahmed, C. Demetriou, S. Melanson, M. Mendu, A. Petrides, N. Tolan, and Z. Virk were responsible for the investigation; C. Demetriou, Y. Kelly, S. Melanson, M. Mendu, A. Petrides, and N. Tolan were responsible for the methodology; J. Ransohoff, T. Terebo were responsible for project administration; Y. Kelly, S. Melanson, M. Mendu, and N. Tolan provided supervision; S. Melanson was responsible for the resources; S. Ahmed, C. Demetriou, Y. Kelly, S. Melanson, M. Mendu, J. Ransohoff, T. Terebo, N. Tolan, and Z. Virk were responsible for the validation; S. Ahmed, M. Mendu, J. Ransohoff, T. Terebo, N. Tolan, and Z. Virk were responsible for the visualization; S. Melanson and N. Tolan wrote the original draft; S. Ahmed, C. Demetriou, Y. Kelly, A. Petrides, S. Melanson, M. Mendu, J. Ransohoff, T. Terebo, N. Tolan, and Z. Virk reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003312021/-/DCSupplemental.
Definitions of parameters collected during medical record review. Download Supplemental Table 1, PDF file, 71 KB (70.6KB, pdf)
References
- 1.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS: Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14: 607–625, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen A-M, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Yeh H-C, Ting I-W, Huang H-C, Chiang H-Y, Kuo C-C: Acute kidney injury in the outpatient setting associates with risk of end-stage renal disease and death in patients with CKD. Sci Rep 9: 17658, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siew ED, Davenport A: The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int 87: 46–61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waikar SS, Liu KD, Chertow GM: The incidence and prognostic significance of acute kidney injury. Curr Opin Nephrol Hypertens 16: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Kidney Int 2: 1–141, 2012 [DOI] [PubMed] [Google Scholar]
- 9.James MT, Hobson CE, Darmon M, Mohan S, Hudson D, Goldstein SL, Ronco C, Kellum JA, Bagshaw SM; Acute Dialysis Quality Initiative (ADQI) Consensus Group : Applications for detection of acute kidney injury using electronic medical records and clinical information systems: workgroup statements from the 15th ADQI Consensus Conference. Can J Kidney Health Dis 3: 9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoste EAJ, Kashani K, Gibney N, Wilson FP, Ronco C, Goldstein SL, Kellum JA, Bagshaw SM; 15 ADQI Consensus Group : Impact of electronic-alerting of acute kidney injury: workgroup statements from the 15th ADQI Consensus Conference. Can J Kidney Health Dis 3: 10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, Feldman HI, Fernandez H, Gitelman Y, Lin J, Negoianu D, Parikh CR, Reese PP, Urbani R, Fuchs B: Automated, electronic alerts for acute kidney injury: A single-blind, parallel-group, randomised controlled trial. Lancet 385: 1966–1974, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lachance P, Villeneuve P-M, Rewa OG, Wilson FP, Selby NM, Featherstone RM, Bagshaw SM: Association between e-alert implementation for detection of acute kidney injury and outcomes: A systematic review. Nephrol Dial Transplant 32: 265–272, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson FP, Martin M, Yamamoto Y, Partridge C, Moreira E, Arora T, Biswas A, Feldman H, Garg AX, Greenberg JH, Hinchcliff M, Latham S, Li F, Lin H, Mansour SG, Moledina DG, Palevsky PM, Parikh CR, Simonov M, Testani J, Ugwuowo U: Electronic health record alerts for acute kidney injury: Multicenter, randomized clinical trial. BMJ 372: m4786, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatraju PK, Zelnick LR, Chinchilli VM, Moledina DG, Coca SG, Parikh CR, Garg AX, Hsu CY, Go AS, Liu KD, Ikizler TA, Siew ED, Kaufman JS, Kimmel PL, Himmelfarb J, Wurfel MM: Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw Open 3: e202682, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiyegbusi O, Witham MD, Lim M, Gauld G, Bell S: Impact of introducing electronic acute kidney injury alerts in primary care. Clin Kidney J 12: 253–257, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prendecki M, Blacker E, Sadeghi-Alavijeh O, Edwards R, Montgomery H, Gillis S, Harber M: Improving outcomes in patients with acute kidney injury: The impact of hospital based automated AKI alerts. Postgrad Med J 92: 9–13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sykes L, Nipah R, Kalra P, Green D: A narrative review of the impact of interventions in acute kidney injury. J Nephrol 31: 523–535, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas ME, Blaine C, Dawnay A, Devonald MAJ, Ftouh S, Laing C, Latchem S, Lewington A, Milford DV, Ostermann M: The definition of acute kidney injury and its use in practice. Kidney Int 87: 62–73, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup : Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Embi PJ, Leonard AC: Evaluating alert fatigue over time to EHR-based clinical trial alerts: Findings from a randomized controlled study. J Am Med Inform Assoc 19[e1]: e145–e148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, Spurr C, Khorasani R, Tanasijevic M, Middleton B: Ten commandments for effective clinical decision support: Making the practice of evidence-based medicine a reality. J Am Med Inform Assoc 10: 523–530, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I, Dwyer JP, Srichai M, Hung AM, Smith JP, Peterson JF: Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 7: 712–719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Fernandez H, Shashaty MGS, Negoianu D, Testani JM, Berns JS, Parikh CR, Wilson FP: False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol 10: 1723–1731, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Khoury JM, Hoenig MP, Jones GRD, Lamb EJ, Parikh CR, Tolan NV, Wilson FP: AACC guidance document on laboratory investigation of acute kidney injury. J Appl Lab Med 6: 1316–1337, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definitions of parameters collected during medical record review. Download Supplemental Table 1, PDF file, 71 KB (70.6KB, pdf)