Abstract
Point-of-care ultrasonography (POCUS) has evolved as a valuable adjunct to physical examination in the recent past and various medical specialties have embraced it. However, POCUS training and scope of practice remain relatively undefined in nephrology. The utility of diagnostic POCUS beyond kidney and vascular access is under-recognized. Assessment of fluid status is a frequent dilemma faced by nephrologists in day-to-day practice where multiorgan POCUS can enhance the sensitivity of conventional physical examination. POCUS also reduces fragmentation of care, facilitates timely diagnosis, and expedites management. Although the need for further imaging studies is obviated in selected patients, POCUS is not meant to serve as an alternative to consultative imaging. In addition, the utility of POCUS depends on the skills and experience of the operator, which in turn depend on the quality of training. In this review, we discuss the rationale behind nephrologists performing POCUS, discuss patient examples to illustrate the basic principles of focused ultrasonography, and share our experience-based opinion about developing a POCUS training program at the institutional level.
Keywords: clinical nephrology, educational personnel, nephrologists, nephrology, physical examination, POCUS, point of care ultrasound, sonography, training
Background
Point-of-care ultrasonography (POCUS) consists of limited ultrasound examinations performed by the clinician at the patient’s bedside to answer focused questions to confirm a suspected diagnosis, narrow the differential, or guide a procedure. These are often binary yes or no questions, such as, “does this patient with AKI have hydronephrosis?,” “is there a pleural effusion?,” “is this location of arteriovenous access suitable for cannulation?” etc. In contrast, comprehensive referral ultrasound studies performed by the radiology department involve complete assessment of an anatomic region and documenting predefined parameters and measurements. Over the past several years, POCUS has evolved as a component of physical examination and gained recognition as a fifth pillar of bedside assessment, joining the canonical inspection, palpation, percussion, and auscultation (1). In specialties such as emergency medicine, POCUS training is considered a core requirement by the Accreditation Council for Graduate Medical Education (2). In the recent past, uptake of POCUS has rapidly expanded in general internal medicine and subspecialties, particularly with recent technological advances resulting in miniaturization, enhanced portability, and reduced cost of the ultrasound equipment (3). Interestingly, more than half of medical schools in the United States are implementing a formal ultrasound curriculum, during both preclinical and clinical years (4,5). Although this is an innovative advancement in medical education, several subspecialties including nephrology will soon face a situation where incoming trainees are more skilled in POCUS than the supervising physicians, potentially leading to role reversal in the clinical decision-making process and confusion in medical documentation. In nephrology, ultrasound-guidance for bedside procedures such as dialysis catheter placement is well established, but the use of diagnostic POCUS remains sparse. Lack of trained faculty and formal guidelines from professional societies remain major barriers to embracing this skill and integrating with fellowship training curricula. In this article, we provide the rationale behind incorporating POCUS in day-to-day nephrology practice and suggestions for training program development, on the basis of the available literature and our experience.
Is POCUS Better than Conventional Physical Examination?
Often, conventional physical examination detects a disease after substantial tissue damage has occurred. This is because the classic diagnostic signs were described when the late-stage presentations were common. Having a more sensitive bedside tool potentially aids in detecting pathology before the onset of irreversible organ damage and alters management. For example, in one study including 50 patients with systolic heart failure (mean ejection fraction 18%), rales, edema, and elevated jugular venous pressure were absent in 18 of 43 patients with pulmonary capillary wedge pressures ≥22 mm Hg (6). Of note, this study was published >30 years ago and, since then, declining physical examination skills among physician trainees as reported by several studies has added another dimension to the problem (7,8). In contrast, POCUS-assisted assessment has shown to be superior compared with conventional physical examination for the detection of left ventricular dysfunction (sensitivity 84% versus 43%) and gross valvular disease (71% versus 46%) (9). In addition, the role of clinician-performed bedside cardiac ultrasound is well established in the evaluation of undifferentiated hypotension, which is not uncommon in nephrology practice (10). For example, when pericardial effusion is suspected in a patient on hemodialysis in the outpatient setting, obtaining a consultative echocardiogram takes at least a few days, whereas nephrologist-performed POCUS gives the answer within minutes and guides management (Figure 1).
Figure 1.
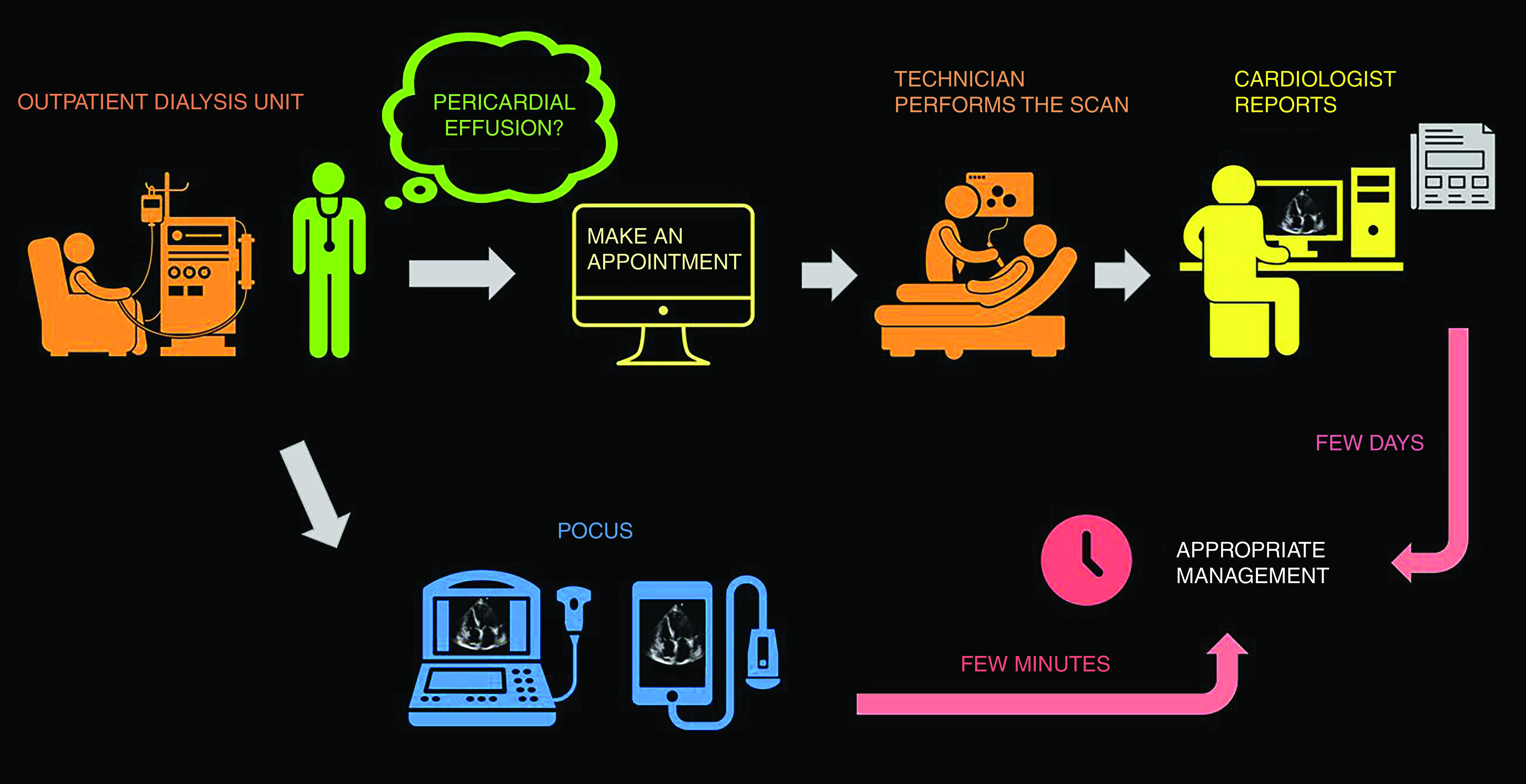
Expedited patient care with point-of-care ultrasonography (POCUS). This infographic illustrates how POCUS can provide answers to focused clinical questions (pericardial effusion in this case) within minutes as opposed to consultative imaging.
By the same token, in a cohort of patients on hemodialysis, the sensitivity of lung crackles and pedal edema was found to be only 9% and 3%, respectively, to detect severe lung congestion detected on POCUS (defined as >30 B lines, which are vertical artifacts seen on lung ultrasound indicative of interstitial fluid) (11). Detection of subclinical congestion is of significance in these patients because lung B lines have shown to be associated with a higher risk of cardiac events and death irrespective of the symptoms (12). Encouraging data have emerged in the recent past demonstrating favorable effect on ambulatory BP and echocardiographic parameters, when lung POCUS is used to guide dry weight reduction in patients undergoing maintenance hemodialysis (13,14).
Conventional physical examination relies on the indirect signs because it cannot visualize the internal anatomy. Conversely, POCUS allows visualization of anatomy in real time and abets confident clinical decision making. For instance, diagnosis of obstructive uropathy, systemic venous congestion etc., cannot be made without imaging, and POCUS provides the opportunity to establish diagnosis in an expedited, yet efficient, manner at the bedside (15,16). In one study, initial ultrasonography in patients with suspected nephrolithiasis was associated with a lower 6-month cumulative radiation exposure compared with abdominal computed tomography scan, without increase in complications that could be related to missed or delayed diagnosis (17). Additionally, POCUS avoids the need for additional imaging in some patients, reduces fragmentation of care and enhances patient satisfaction (18,19). Notably, in a large cohort (n=1962) comprising of both outpatients and patients who are hospitalized, further imaging was deemed unnecessary in 63% after clinician-performed POCUS examination using pocket ultrasound devices (20). Having said that, avoiding formal or consultative imaging is not the purpose of POCUS. For example, a patient with hydronephrosis without an obvious source of obstruction still needs further studies to evaluate for stones, masses, stenotic lesions, etc. Similarly, POCUS may suggest a regurgitant or stenotic valvular lesion responsible for a patient’s symptoms, but is not intended to replace a comprehensive echocardiogram performed by the cardiology department.
What Sonographic Applications Can Nephrologists Perform?
Currently, there is no consensus on the scope of nephrologist-performed POCUS. However, as the physical examination performed by a nephrologist is not very different from that of a general internist, it is conceivable we should be able to perform most of the sonographic applications relevant to internal medicine, tailored to the clinical conditions we see and procedures we perform (21,22). In general, basic POCUS involves greyscale (B-mode) ultrasound and color Doppler, whereas advanced POCUS includes spectral Doppler applications used to evaluate hemodynamics requiring a higher skill level and additional training (23). Figure 2 illustrates the common focused questions routinely asked by nephrologists that can be addressed by either basic and/or advanced POCUS. It is a common misconception that ultrasonography is solely the realm of radiologists and cardiologists. Radiologists interpret comprehensive studies that are usually performed by a diagnostic medical sonographer. In contrast, POCUS consists of limited ultrasound studies performed and interpreted by a clinician as an adjunct to history and physical examination. Similarly, cardiologists are trained in echocardiography but not necessarily skilled in multiorgan POCUS. In fact, it saves these consultants’ time if an internist or nephrologist can obtain answers to simple clinical questions at the patient’s bedside. Interestingly, the American Medical Association House of Delegates passed a resolution in 1999 (Res. 802, I-99, reaffirmed 2020) that affirms, “ultrasound imaging is within the scope of practice of appropriately trained physicians,” and recommends that hospital medical staff should grant privileges on the basis of the physicians’ training and specialty-specific guidelines (24). Therefore, the emphasis should be on appropriate training and certification, but not physician specialty.
Figure 2.
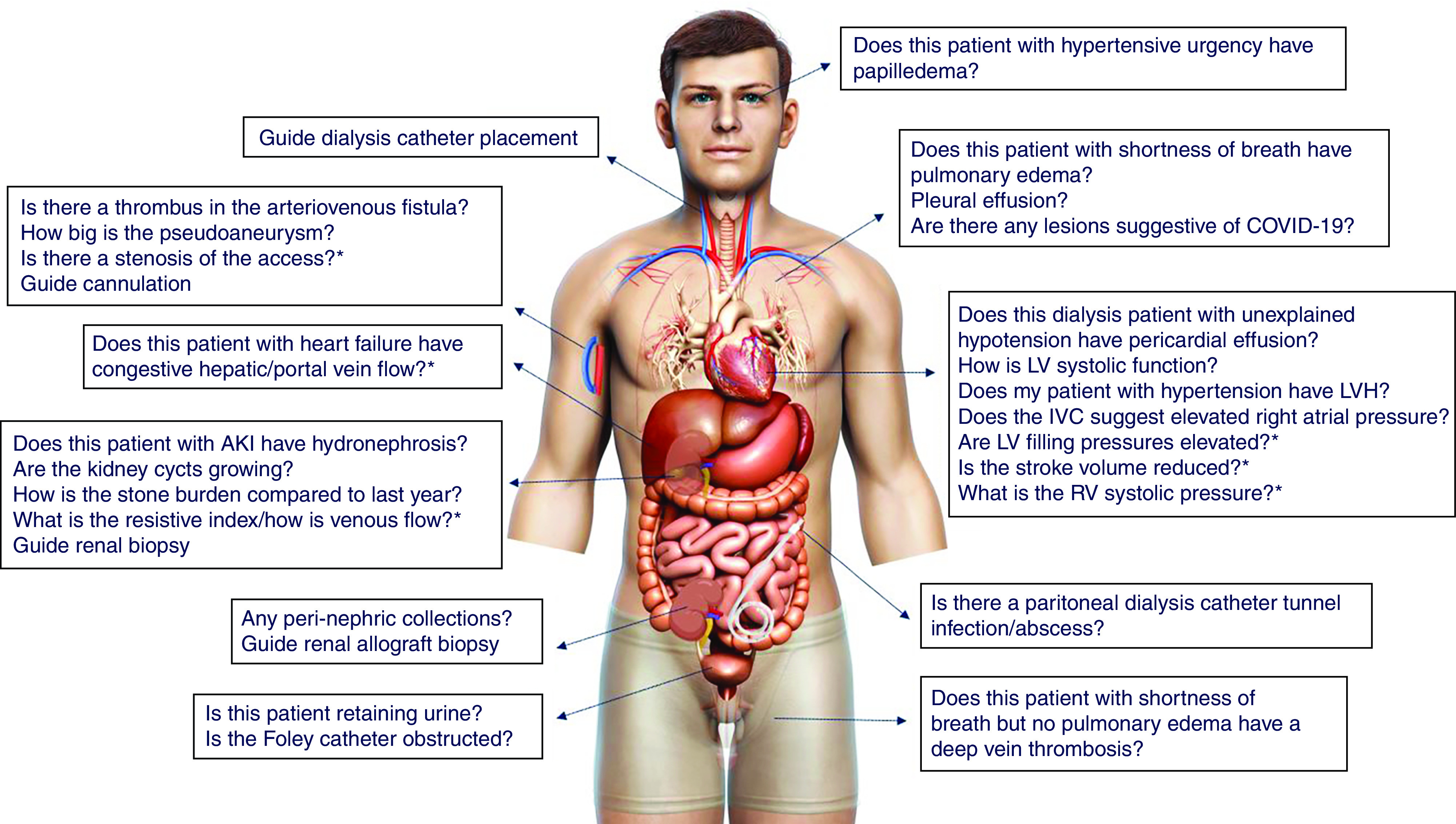
Scope of nephrology-related POCUS: Organ-specific focused questions that can be answered by bedside ultrasonography. Those marked with asterisk (*) indicate advanced sonographic applications requiring a higher operator skill level/additional training. Human body illustration licensed from Shutterstock. COVID-19, coronavirus disease 2019.
Below are a few real-life examples, where nephrologist-performed POCUS assisted in the diagnostic process and patient management.
Patient One
A patient with gastrointestinal tract malignancy developed oliguric AKI during hospitalization, which the nephrology service followed. A bedside urinary bladder scan performed by the nurse using an automated scanner revealed approximately 800 ml of urine. However, there was no return of urine on bladder catheterization. POCUS performed by the nephrologist revealed pelvic ascites mimicking urine. Although the anechoic (black) structure looked very similar to the urinary bladder in the transverse axis, a long axis scan revealed the irregular nature of the fluid collection and its continuity with the peritoneal cavity (Figure 3, A and B).
Figure 3.
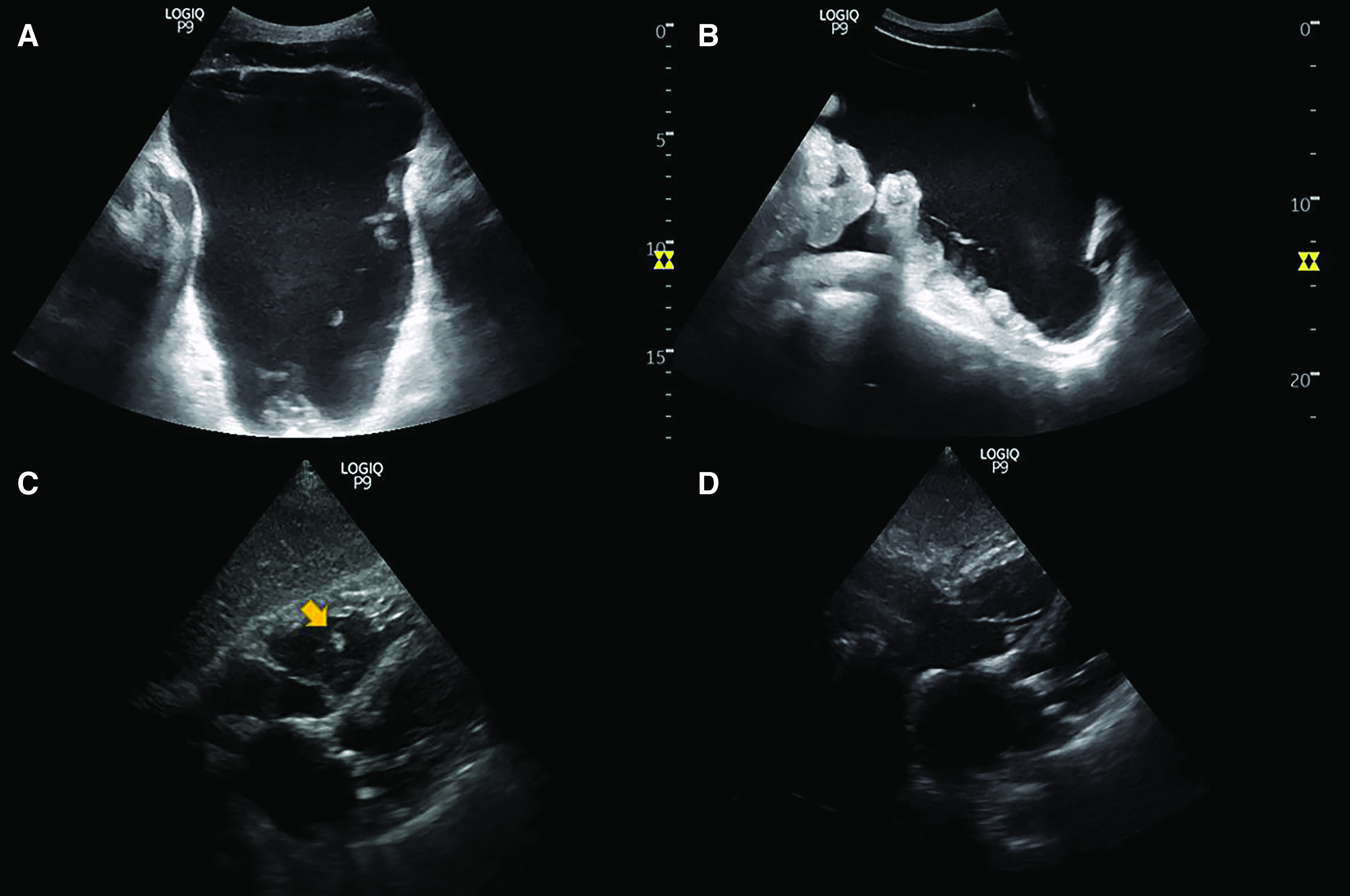
Sonographic images from patients one and two. (A) Transverse and (B) longitudinal images of the suprapubic area demonstrating pelvic ascites. Note the irregular border with anechoic free fluid interdigitating among loops of bowel, better seen in long axis. (C) Subxiphoid four-chamber view of the heart showing an echogenic structure indicated by arrow in the right ventricle, suggestive of a thrombus. (D) Follow-up scan demonstrating resolution of the thrombus after anticoagulation therapy.
POCUS Pearls
Automated bladder scanners cannot differentiate urinary bladder from other pelvic fluid collections (25). In contrast, POCUS helps to visualize the local anatomy and often aids in identifying the source of bladder obstruction, such as a misplaced or obstructed indwelling urinary catheter.
Patient Two
An elderly woman with congestive heart failure and sepsis secondary to pneumonia was admitted to the intensive care unit. Continuous RRT was started for AKI and hypervolemia. During follow-up, multiorgan POCUS was performed by the nephrologist to assess volume status and titrate ultrafiltration (16). Although there was a significant improvement in the venous congestion, cardiac ultrasound incidentally showed a mobile, echogenic structure in the right ventricle adherent to subvalvular apparatus of the tricuspid. A consultative echocardiogram performed 1 week prior did not show this abnormality. Moreover, at the time of the POCUS exam, the patient’s clinical status had improved and there was no fever. Therefore, the thrombus was thought to be more likely than a vegetation. Findings were confirmed on a comprehensive echocardiogram performed by the cardiology department and the patient was started on intravenous heparin therapy. POCUS did not reveal lower-extremity deep vein thrombosis and computed tomography of the chest was not performed. Repeat POCUS exam (Figure 3, C and D) and a transesophageal echocardiogram performed 5 days later confirmed resolution of the clot.
POCUS Pearls
Focused cardiac ultrasound or cardiac POCUS is an integral component of fluid status assessment and availability of a recent comprehensive echocardiogram should not preclude it. Incidental abnormal findings on POCUS should be documented and promptly conveyed to appropriate consultants/teams.
Patient Three
A patient with ESKD on hemodialysis presented after a missed dialysis session. He reported that he had a recent pericardiocentesis at an outside facility and scheduled to see his cardiologist the next week. He was told he still has some fluid around the heart. Because he also complained of mild shortness of breath, a bedside cardiac ultrasound was performed to evaluate for the effusion. It revealed mild to moderate pericardial effusion (anechoic fluid separating the pericardial layers in diastole by approximately 1.2 cm). In general, a separation of <1 cm is considered mild pericardial effusion, whereas 1–2 cm is considered moderate, and >2 cm severe (26). There was no chamber collapse or a significant variation in mitral inflow Doppler to suggest tamponade physiology (Figure 4, A and B). The patient was discharged in a stable condition and reinforced to follow-up with cardiology.
Figure 4.
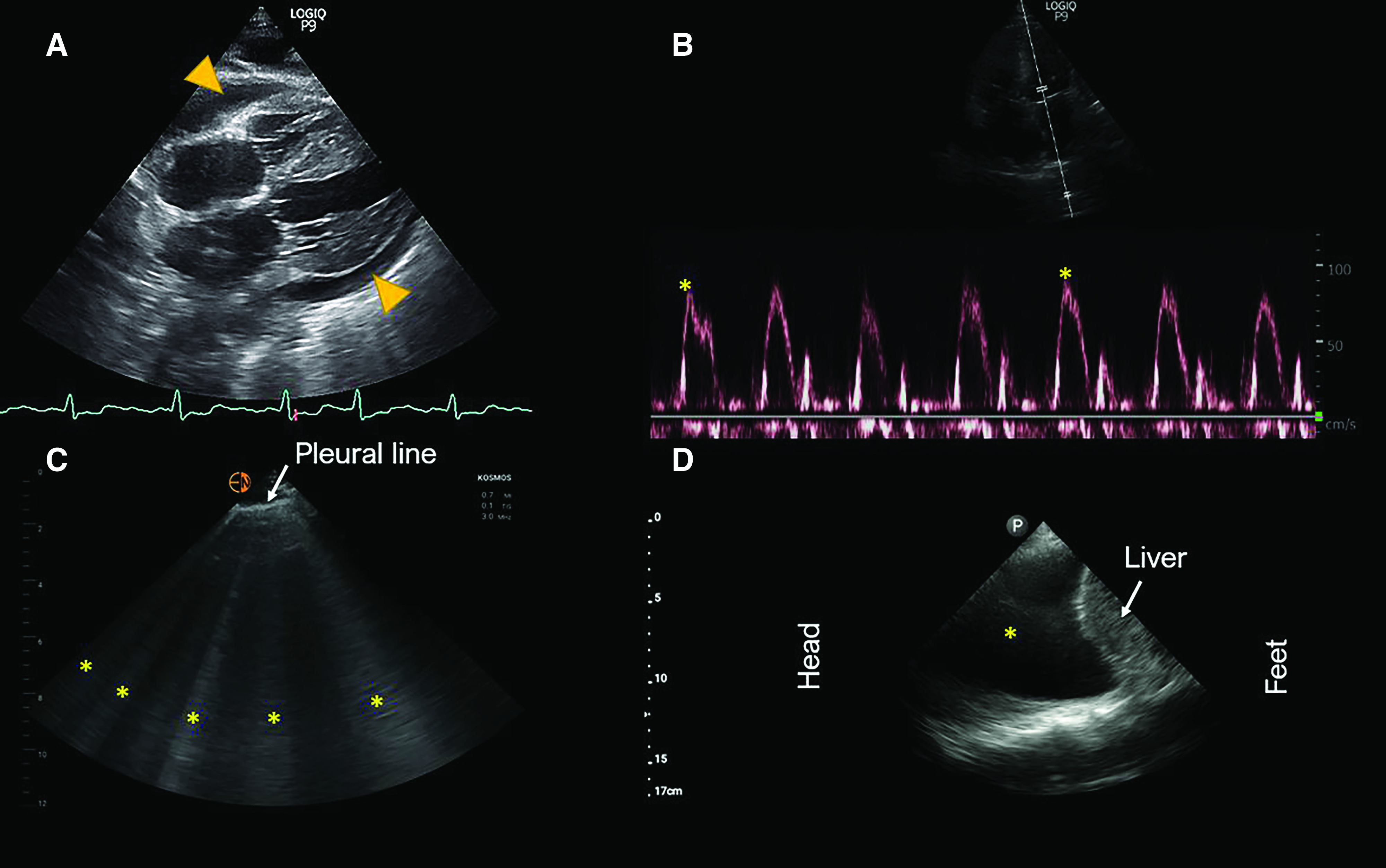
Sonographic images from patients three, four and five. (A) Subxiphoid four-chamber view of the heart demonstrating pericardial effusion (arrowheads). (B) Mitral inflow Doppler in the apical four-chamber view demonstrating minimal variation in the flow velocity with respiration. Inspiratory reduction of peak velocity by approximately 25% is suggestive of tamponade physiology. (C) Lung ultrasound demonstrating vertical hyperechoic artifacts, that is B lines indicated by asterisks. (D) Right pleural effusion: anechoic area indicated by asterisk.
POCUS Pearls
Cardiac POCUS allows rapid diagnosis of pericardial effusion and grading of its severity. Physicians trained in Doppler ultrasound can assess additional parameters to gauge hemodynamic effect of the effusion. In selected patients, POCUS avoids unnecessary consultative care or imaging.
Patient Four
A patient with alcoholic liver cirrhosis was seen for presumed hepatorenal syndrome and was receiving intravenous albumin at the time of evaluation by nephrologist. Auscultation findings were documented as normal. However, lung ultrasound revealed a diffuse B-line pattern, indicating significantly elevated extravascular lung water (Figure 4C). A recommendation was made to stop albumin immediately and administer an intravenous diuretic. Subsequently, urine microscopy demonstrated bilirubin-stained tubular epithelial cell casts, suggestive of cholemic nephropathy.
POCUS Pearls
Although intravenous albumin is frequently used concomitantly with vasoconstrictor therapy in the treatment of suspected hepatorenal syndrome, this should be guided by careful assessment of fluid volume status (27). Lung POCUS has shown to be more sensitive than auscultation and even chest radiography in various clinical scenarios (11,28,29), and hence it would be prudent to routinely utilize it in patients who are at high risk for hypervolemia.
Patient Five
In the outpatient dialysis unit, a patient complained of worsening dyspnea. The dry weight had been challenged during the preceding 2–3 dialysis sessions, but the patient developed hypotension. Nephrologist-performed POCUS revealed a large right pleural effusion (Figure 4D) and an urgent referral to pulmonology was made for further management. Unfortunately, the patient was found to have a malignant pleural effusion related to metastases.
POCUS Pearls
Besides pulmonary congestion, patients undergoing dialysis can have shortness of breath from several causes including pleural and pericardial effusions, pneumonia, pulmonary embolism, etc. POCUS helps to narrow the differential and facilitates timely intervention. Of note, as we described previously, POCUS is a valuable tool in the evaluation of patients on dialysis with coronavirus disease 2019 pneumonia (30).
POCUS Training and Program Development
Currently, there is no uniform standard for POCUS training in nephrology and very few fellowship programs have a structured curriculum. Further, there are limited training opportunities for practicing nephrologists. Workshops and short courses organized in conjunction with professional society conferences serve as a good introduction to the technique and image interpretation (31). However, without continued practice and longitudinal hands-on experience, the knowledge and skills decay quickly (32). Learning POCUS involves multiple components: the operator must be able to formulate the appropriate focused questions, identify suitable acoustic windows and acquire images, possess knowledge of alternative imaging windows, recognize artifacts, interpret images in the right clinical context, effectively integrate the information to guide patient management, and recognize limitations and seek expert consultation when indicated. As such, it is not realistic to expect that anyone would attain proficiency after a short training session. Dedicated 1-year POCUS fellowships, once confined to emergency medicine, are now available for internal medicine graduates at some institutions (33) but are not limited to teaching nephrology-related sonographic applications. Moreover, some fellowships lay more emphasis on procedures, such as thoracentesis, lumbar puncture, etc., which are typically not performed by nephrologists. Nevertheless, having a POCUS fellowship–trained nephrology faculty is an advantage because they are familiar with practical aspects of program administration, including equipment and personnel management, image archiving, billing, and coding.
We suggest the following framework for nephrology fellowship programs contemplating incorporation of POCUS training. First, the division should consider identifying faculty champion(s) motivated to learn and teach POCUS and provide them with time and resources to do so. Protected time for the POCUS director is typically around 0.15–0.25 full-time equivalent (FTE), depending on the number of learners and tasks involved (21). Among internal medicine programs, the combined faculty salary FTE for administering POCUS program ranged from 0.05 to 2.5 FTE, according to a recent survey (Professor Nilam J. Soni, personal communication, May 2021). At the Medical College of Wisconsin, nephrology POCUS director (AK) is supported with an effort of 0.3 FTE for program administration. The responsibilities of a POCUS director broadly include, but are not limited to, development of curricular content, lectures, facilitated learning activities, longitudinal mentoring, supervised scholarship, oversight of documentation/billing, fostering multidisciplinary collaborations, quality assessment, and improvement. The proportion of time spent for each of these activities is expected to change as the program grows and more faculty are trained in POCUS. For example, an increase in the number of users adds to the number of scans needing quality assessment and the time for general oversight. In contrast, preparation time for lectures and educational material eventually decreases.
A good starting point would be to encourage these faculty to pursue a structured, multistep POCUS certification program, such as the one offered by the American College of Chest Physicians and the Society of Hospital Medicine (34). Having such certification facilitates the hospital credentialing process. Seeking advice and possibly shadowing institutional experts such as emergency medicine POCUS faculty helps to understand the local workflow and quality assurance process.
Procuring dedicated ultrasound equipment for the use of faculty and nephrology fellows is the next step. We suggest the equipment should consist of both a cart-based portable ultrasound machine and a few handheld devices, depending on the size of the fellowship program. Although purchasing only the handheld devices is an attractive option due to enhanced portability and low cost, faculty need to be aware of their limitations. These devices generally have simplified transducer technology, lower resolution, and limited ability to adjust image quality, making it difficult for the novice users to appreciate sonographic anatomy. In addition, most such devices do not have spectral Doppler capability to quantify blood flow (although newer, relatively expensive, devices do offer this). With respect to kidney ultrasound, it is often difficult to appreciate relative cortical echogenicity and identify small lesions such as stones when using handhelds. Having a machine with a bigger screen also helps in an educational environment where multiple learners (or even patients and families) are watching the images. Nonetheless, if the primary intent is to teach basic applications, such as lung ultrasound and excluding obstructive uropathy/large pericardial effusion, handheld devices alone are sufficient. Figure 5 compares the images obtained using a portal ultrasound machine and two of the handheld devices we used.
Figure 5.
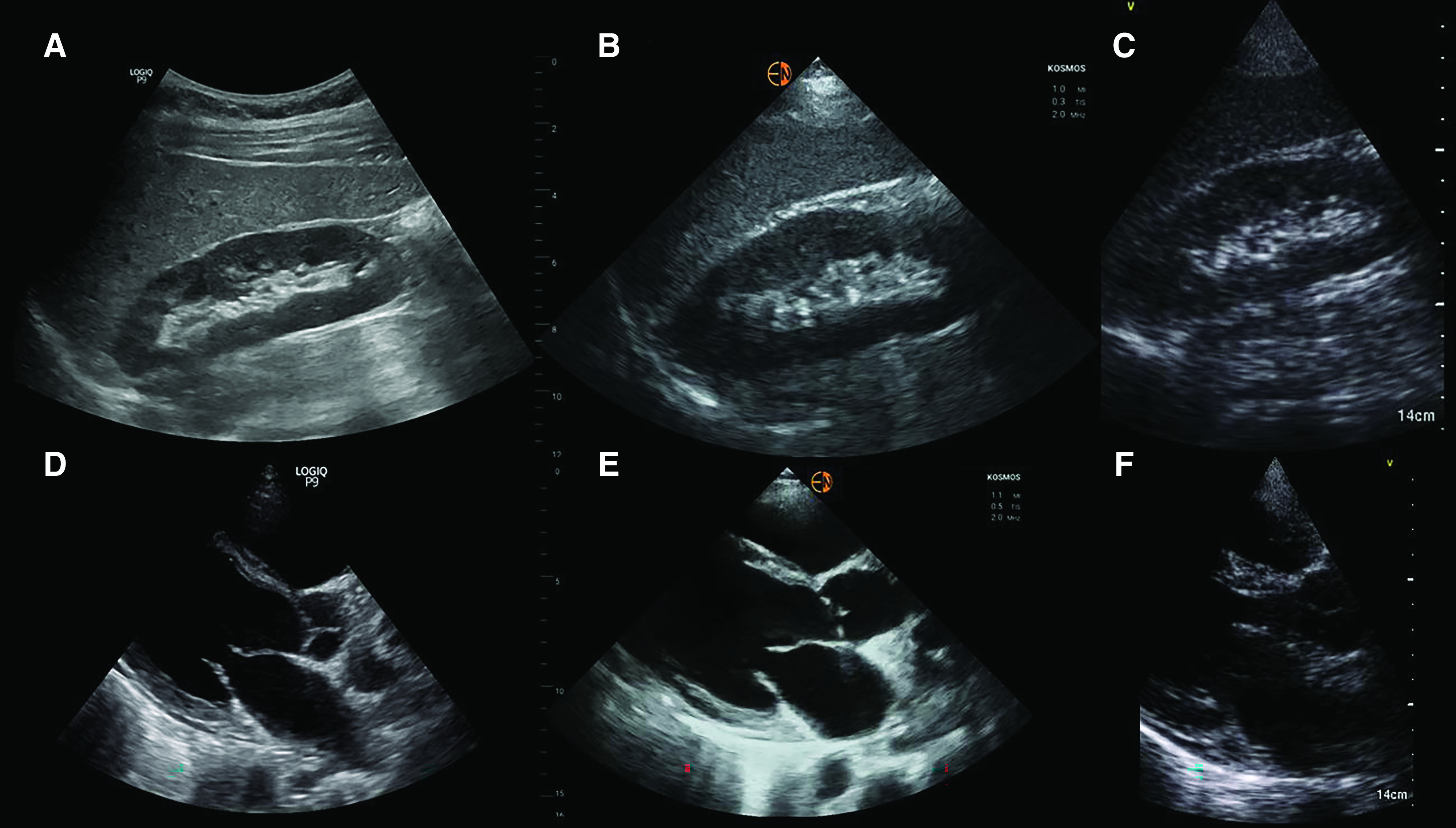
Comparison of sonographic images from a cart-based machine and handheld devices. (A) Right kidney image obtained using a cart-based portable ultrasound system, GE logiq P9. The same kidney imaged using a (B) high-resolution (relatively expensive) handheld ultrasound device, Kosmos, and (C) low-resolution (less expensive) handheld ultrasound device, GE Vscan. (D) Parasternal long-axis view of the heart obtained using GE logiq P9, (E) Kosmos, and (F) GE Vscan systems. Note how the image quality changes from well defined to grainy when using a low-resolution system. As mentioned in the article text, adequacy of image quality essentially depends on the focused questions being asked.
Performing educational scans and comparing findings with consultative imaging is a good way to gain confidence. Organizing 1- to 2-day hands-on workshops locally in collaboration with experts from other departments or those invited from outside institutions helps to improve the image acquisition technique and interpretation. As opposed to external courses, such workshops allow discussion of sonographic applications tailored to local practice and provide an opportunity to practice using equipment that learners are familiar with. Further short training sessions with nephrology fellows and other faculty in the division can be organized in the medical school’s or hospital’s simulation laboratory. Smaller nephrology divisions may choose to set up their own training room in the outpatient clinic or other available office space to practice on volunteers (35). Once comfortable with the basic applications, ultrasound faculty should consider enhancing their knowledge and skills by attending POCUS-related continuing medical education programs. Those interested in advanced POCUS may pursue certifications such as National Board of Echocardiography’s special competency in Critical Care Echocardiography and Registered Physician in Vascular Interpretation (36,37).
It is imperative to establish a workflow for image archiving and retrieval for the purposes of billing, comparison with consultative imaging, quality assurance, and provide learner feedback. At our respective institutions, we use a separate workflow for clinically indicated, billable scans and educational/practice scans performed by the trainees. Clinical scans are transmitted to picture archiving and communication system and subsequently retrievable from the patient’s electronic medical record. Deidentified educational scans are stored separately. This helps the learners to maintain a portfolio of studies required for hospital credentialing and/or certification. Some institutions use middleware/data management software to organize clinical and educational images, run digital reports etc., which are associated with recuring operational costs. These costs can be potentially shared with other POCUS-performing specialties. In contrast, some places have onsite personnel to facilitate this process. For example, Froedtert hospital, the primary teaching affiliate of Medical College of Wisconsin, has a dedicated team of experienced sonographers who perform initial quality checks on all of the POCUS studies, before uploading them in the patient’s chart. Billing for POCUS studies helps to offset the costs of ultrasound equipment over time.
With the advent of free, open-access medical education, the need to create didactic material from scratch is obviated. For example, NephroPOCUS.com, recognized by the American Society of Nephrology (ASN) as an innovative teaching tool consists of curricular lectures, POCUS-related short cases/videos, and self-assessment quizzes specifically designed for nephrologists (38). Similarly, the “Focus on POCUN” series hosted by the Renal Fellow Network, a blog that partners with the ASN, is another good resource (39). In addition, fellowship programs can use our previously published curriculum as a framework to design their own, adapted to local practices and needs (40). Finally, a robust quality assurance and learner feedback system must be established, which is better done in collaboration with multidisciplinary experts within the institution, including radiologists, cardiologists, emergency medicine physicians, and internal medicine proceduralists. Figure 6 summarizes the above-mentioned components of POCUS program development.
Figure 6.
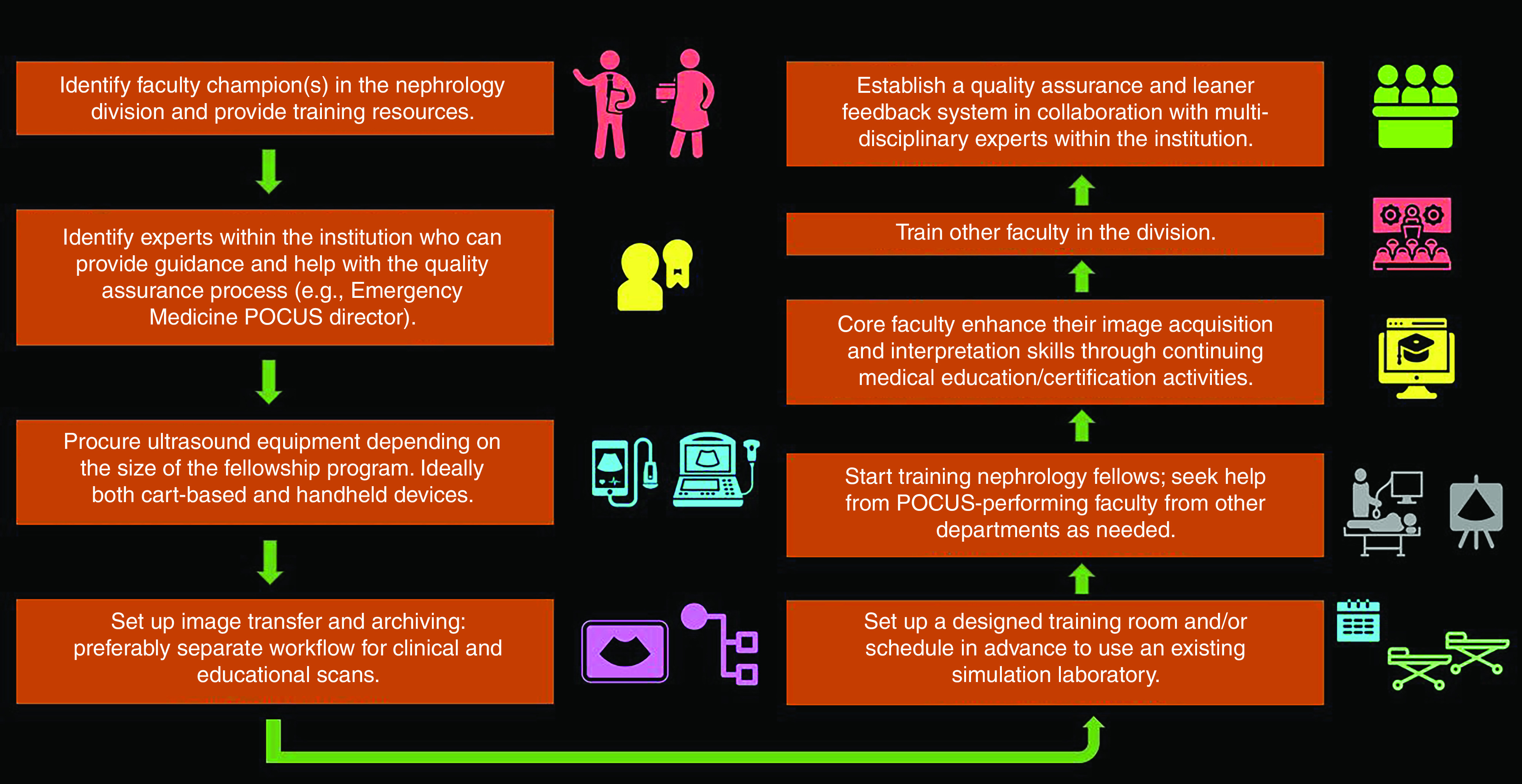
Flow chart demonstrating the key elements of setting up a POCUS program.
Limitations and Future Directions
As mentioned, POCUS is not intended to replace consultative imaging, although it may reduce the need for further diagnostic testing in selected patients (20,41). Moreover, the additional information provided by POCUS is futile if the user fails to interpret it in the clinical context and appropriately integrate it into medical decision making. Lack of attention to detail, improper technique, misidentification of the structures, or failure to recognize abnormal findings may result in diagnostic and management errors. For instance, mistaking the aorta for a dilated inferior vena cava in a patient who is volume depleted and administering diuretics without paying attention to the clinical scenario may lead to patient harm. Attaining competency in POCUS is a gradual process; as such, clinicians should elude overconfidence and seek expert opinion in a timely manner when unsure about the sonographic findings. Proper documentation and image archiving practices will facilitate this. Concerns about missed findings and resultant legal implications are a barrier to the wide adoption of POCUS (42). However, there is no evidence suggesting that missed findings on focused ultrasound examinations led to an adverse legal action against physicians. In contrast, failure to perform POCUS when indicated has resulted in lawsuits (43–46). Although POCUS is often criticized for being operator dependent, it is an inherent limitation of ultrasonography compared with other modalities, such as computed tomography with standardized image acquisition, and not specific to POCUS. In fact, essentially every element of physician-patient interaction is operator dependent, including history-taking, physical examination, interpretation of the laboratory data, and so forth. Therefore, proper training of the operator should be emphasized rather than blaming the imaging modality.
Having formal support and guidelines from the professional societies governing nephrologists would facilitate the development of universal standards in POCUS training. In the later part of 2021, the American Society of Diagnostic and Interventional Nephrology will release certification requirements for POCUS in nephrology. Also, currently, data regarding POCUS training methods and learner competency are largely extrapolated from the emergency medicine literature. As such, future research should focus on establishing nephrology-specific correlates and studying the effect of POCUS-guided care on patient outcomes. In addition, the use of novel educational modalities and exposure to the breadth of the nephrology practice have been proposed to increase the interest in nephrology among medical students (47). It would be interesting to study if adding POCUS to nephrology rounds enhances the students’ and trainees’ understanding of the structural and pathophysiological aspects of the clinical decision-making process, thus boosting the fellowship recruitment.
Disclosures
All authors have nothing to disclose.
Funding
None.
Author Contributions
A. Koratala was responsible for the data curation and project administration, and wrote the original draft; A. Koratala and N. Reisinger conceptualized the study and were responsible for the methodology; and N. Reisinger reviewed and edited the manuscript.
References
- 1.Narula J, Chandrashekhar Y, Braunwald E: Time to add a fifth pillar to bedside physical examination: Inspection, palpation, percussion, auscultation, and insonation. JAMA Cardiol 3: 346–350, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Accreditation Council for Graduate Medical Education: ACGME Program Requirements for Graduate Medical Education in Emergency Medicine. 2020. Available at: https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/110_EmergencyMedicine_2020.pdf?ver=2020-06-26-125701-320. Accessed May 4, 2021
- 3.LoPresti CM, Jensen TP, Dversdal RK, Astiz DJ: Point-of-care ultrasound for internal medicine residency training: A position statement from the Alliance of Academic Internal Medicine. Am J Med 132: 1356–1360, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Sohaey R, Di Salvo DN, Bluth EI, Lockhart ME, Cohen HL, Pellerito JS, Baltarowich OH, Nisenbaum HL, Coleman BG: Medical student ultrasound education: The radiology chair weighs in. Ultrasound Q 37: 3–9, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Bahner DP, Goldman E, Way D, Royall NA, Liu YT: The state of ultrasound education in U.S. medical schools: Results of a national survey. Acad Med 89: 1681–1686, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 261: 884–888, 1989 [PubMed] [Google Scholar]
- 7.Mangione S, Nieman LZ, Gracely E, Kaye D: The teaching and practice of cardiac auscultation during internal medicine and cardiology training. A nationwide survey. Ann Intern Med 119: 47–54, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Mangione S, Nieman LZ: Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency. JAMA 278: 717–722, 1997 [PubMed] [Google Scholar]
- 9.Marbach JA, Almufleh A, Di Santo P, Jung R, Simard T, McInnes M, Salameh JP, McGrath TA, Millington SJ, Diemer G, West FM, Domecq MC, Hibbert B: Comparative accuracy of focused cardiac ultrasonography and clinical examination for left ventricular dysfunction and valvular heart disease: A systematic review and meta-analysis. Ann Intern Med 171: 264–272, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Spencer KT, Flachskampf FA: Focused cardiac ultrasonography. JACC Cardiovasc Imaging 12: 1243–1253, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Torino C, Gargani L, Sicari R, Letachowicz K, Ekart R, Fliser D, Covic A, Siamopoulos K, Stavroulopoulos A, Massy ZA, Fiaccadori E, Caiazza A, Bachelet T, Slotki I, Martinez-Castelao A, Coudert-Krier MJ, Rossignol P, Gueler F, Hannedouche T, Panichi V, Wiecek A, Pontoriero G, Sarafidis P, Klinger M, Hojs R, Seiler-Mussler S, Lizzi F, Siriopol D, Balafa O, Shavit L, Tripepi R, Mallamaci F, Tripepi G, Picano E, London GM, Zoccali C: The agreement between auscultation and lung ultrasound in hemodialysis patients: The LUST study. Clin J Am Soc Nephrol 11: 2005–2011, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoccali C, Torino C, Tripepi R, Tripepi G, D’Arrigo G, Postorino M, Gargani L, Sicari R, Picano E, Mallamaci F; Lung US in CKD Working Group: Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol 24: 639–646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loutradis C, Sarafidis PA, Ekart R, Papadopoulos C, Sachpekidis V, Alexandrou ME, Papadopoulou D, Efstratiadis G, Papagianni A, London G, Zoccali C: The effect of dry-weight reduction guided by lung ultrasound on ambulatory blood pressure in hemodialysis patients: A randomized controlled trial. Kidney Int 95: 1505–1513, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Loutradis C, Papadopoulos CE, Sachpekidis V, Ekart R, Krunic B, Karpetas A, Bikos A, Tsouchnikas I, Mitsopoulos E, Papagianni A, Zoccali C, Sarafidis P: Lung ultrasound-guided dry weight assessment and echocardiographic measures in hypertensive hemodialysis patients: A randomized controlled study. Am J Kidney Dis 75: 11–20, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Riddell J, Case A, Wopat R, Beckham S, Lucas M, McClung CD, Swadron S: Sensitivity of emergency bedside ultrasound to detect hydronephrosis in patients with computed tomography-proven stones. West J Emerg Med 15: 96–100, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koratala A, Kazory A: Point of care ultrasonography for objective assessment of heart failure: Integration of cardiac, vascular, and extravascular determinants of volume status. Cardiorenal Med 11: 5–17, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Smith-Bindman R, Aubin C, Bailitz J, Bengiamin RN, Camargo CA Jr, Corbo J, Dean AJ, Goldstein RB, Griffey RT, Jay GD, Kang TL, Kriesel DR, Ma OJ, Mallin M, Manson W, Melnikow J, Miglioretti DL, Miller SK, Mills LD, Miner JR, Moghadassi M, Noble VE, Press GM, Stoller ML, Valencia VE, Wang J, Wang RC, Cummings SR: Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med 371: 1100–1110, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Mathews BK, Miller PE, Olson APJ: Point-of-care ultrasound improves shared diagnostic understanding between patients and providers. South Med J 111: 395–400, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Koratala A: Point of care ultrasonography enhanced physical examination: A nephrologist’s perspective. Am J Med 133: e384–e385, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Colli A, Prati D, Fraquelli M, Segato S, Vescovi PP, Colombo F, Balduini C, Della Valle S, Casazza G: The use of a pocket-sized ultrasound device improves physical examination: Results of an in- and outpatient cohort study. PLoS One 10: e0122181, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LoPresti CM, Schnobrich DJ, Dversdal RK, Schembri F: A road map for point-of-care ultrasound training in internal medicine residency. Ultrasound J 11: 10, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambasta A, Balan M, Mayette M, Goffi A, Mulvagh S, Buchanan B, Montague S, Ruzycki S, Ma IWY; Canadian Internal Medicine Ultrasound (CIMUS) Group: Education indicators for internal medicine point-of-care ultrasound: A consensus report from the Canadian Internal Medicine Ultrasound (CIMUS) Group [published correction appears in J Gen Intern Med 35: 624, 2020 10.1007/s11606-020-05632-5]. J Gen Intern Med 34: 2123–2129, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieillard-Baron A, Millington SJ, Sanfilippo F, Chew M, Diaz-Gomez J, McLean A, Pinsky MR, Pulido J, Mayo P, Fletcher N: A decade of progress in critical care echocardiography: A narrative review [published correction appears in Intensive Care Med 45: 911, 2019 10.1007/s00134-019-05616-y]. Intensive Care Med 45: 770–788, 2019 [DOI] [PubMed] [Google Scholar]
- 24.American Medical Association Privileging for Ultrasound Imaging H-230: 960. Reaffirmed 2020. Available at: https://policysearch.ama-assn.org/policyfinder/detail/Ultrasoundimaging?uri=%2FAMADoc%2FHOD.xml-0-1591.xml Accessed May 4, 2021
- 25.Bejjanki H, Koratala A: The TIE fighter sign on point of care ultrasonography. CEN Case Rep 9: 91–93, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Casares A, Cesar S, Brunet-Garcia L, Sanchez-de-Toledo J: Echocardiographic evaluation of pericardial effusion and cardiac tamponade. Front Pediatr 5: 79, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velez JCQ, Petkovich B, Karakala N, Huggins JT: Point-of-care echocardiography unveils misclassification of acute kidney injury as hepatorenal syndrome. Am J Nephrol 50: 204–211, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ: Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 100: 9–15, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Tierney DM, Huelster JS, Overgaard JD, Plunkett MB, Boland LL, St Hill CA, Agboto VK, Smith CS, Mikel BF, Weise BE, Madigan KE, Doshi AP, Melamed RR: Comparative performance of pulmonary ultrasound, chest radiograph, and CT among patients with acute respiratory failure. Crit Care Med 48: 151–157, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Reisinger N, Koratala A: Lung ultrasound: A valuable tool for the assessment of dialysis patients with COVID-19. Clin Exp Nephrol 24: 850–852, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardio Renal University Point-of-Care Ultrasound Immersion Course. 2020. Available at: https://www.renalfellow.org/2020/01/25/point-of-care-immersion-ultrasound-course-for-nephrologists/. Accessed May 4, 2021
- 32.Hempel D, Stenger T, Campo Dell’ Orto M, Stenger D, Seibel A, Röhrig S, Heringer F, Walcher F, Breitkreutz R: Analysis of trainees’ memory after classroom presentations of didactical ultrasound courses. Crit Ultrasound J 6: 10, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Society of Clinical Ultrasound Fellowships, 2021. Available at: https://eusfellowships.com/about-us/. Accessed May 4, 2021
- 34.Point-of-Care Ultrasound Certificate of Completion Program. Available at: https://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/SHM-COC. Accessed May 4, 2021
- 35.Koratala A: Execution of the NephroPOCUS curriculum. NephroPOCUS.com. Available at: https://nephropocus.com/2020/06/14/execution-of-the-nephropocus-curriculum/. Accessed May 4, 2021
- 36.National Board of Echocardiography Application for Certification in Critical Care Echocardiography. Available at: https://echoboards.org/docs/CCEeXAM-Cert_App-2020.pdf. Accessed May 4, 2021
- 37.Alliance for Physician Certification & Advancement Registered Physician in Vascular Interpretation Certification. Available at: https://www.apca.org/certifications-examinations/Registered-Physician-in-Vascular-Interpretation/. Accessed: May 4, 2021
- 38.Koratala A. NephroPOCUS Teaching Tool. Available at: https://nephropocus.com/about/. Accessed May 4, 2021
- 39.Renal Fellow Network : Focus on POCUN Series. Available at: https://www.renalfellow.org/category/focus-on-pocun/. Accessed May 4, 2021
- 40.Koratala A, Segal MS, Kazory A: Integrating point-of-care ultrasonography into nephrology fellowship training: A model curriculum. Am J Kidney Dis 74: 1–5, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Barchiesi M, Bulgheroni M, Federici C, Casella F, Medico MD, Torzillo D, Janu VP, Tarricone R, Cogliati C: Impact of point of care ultrasound on the number of diagnostic examinations in elderly patients admitted to an internal medicine ward. Eur J Intern Med 79: 88–92, 2020 [DOI] [PubMed] [Google Scholar]
- 42.Koratala A, Bhattacharya D, Kazory A: Harnessing Twitter polls for multi-specialty collaboration in standardizing point-of-care ultrasonography in nephrology. Clin Nephrol 94: 50–52, 2020 [DOI] [PubMed] [Google Scholar]
- 43.Blaivas M, Pawl R: Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med 30: 338–341, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S: A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med 16: 1–4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reaume M, Farishta M, Costello JA, Gibb T, Melgar TA: Analysis of lawsuits related to diagnostic errors from point-of-care ultrasound in internal medicine, paediatrics, family medicine and critical care in the USA. Postgrad Med J 97: 55–58, 2021 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen J, Cascione M, Noori S: Analysis of lawsuits related to point-of-care ultrasonography in neonatology and pediatric subspecialties. J Perinatol 36: 784–786, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Sozio SM, Pivert KA, Shah HH, Chakkera HA, Asmar AR, Varma MR, Morrow BD, Patel AB, Leight K, Parker MG: Increasing medical student interest in nephrology. Am J Nephrol 50: 4–10, 2019 [DOI] [PubMed] [Google Scholar]


