Abstract
Complete remission from recurrent EBV-positive lymphoma is not mandatory before HSCT to achieve long-term cure in a patient suffering from a recently described immunodeficiency affecting the T-cell coactivation molecule 4-1BB.
Keywords: Epstein Barr virus, Recurrent lymphoproliferation, 4-1BB, HSCT
In healthy individuals, Epstein Barr virus (EBV)-induced B-cell proliferation is effectively controlled by T- and NK-cells. The primary infection in childhood is often asymptomatic, older patients suffer from self-limiting infectious mononucleosis. EBV-positive malignancies develop in a small minority of infected individuals. More than twenty monogenic diseases have been defined, which put affected individuals at risk of potentially fatal courses of foudroyant infectious mononucleosis, hemophagocytic lymphohistiocytosis (HLH) or recurrent lymphoma due to their inability to control EBV-induced lymphoproliferation [1]. We report on the clinical course of a patient with recurrent EBV-positive lymphoma due to a homozygous mutation in TNFRSF9 encoding for 4-1BB recently described [2].
The male patient originating from Saudi-Arabia was born as 2nd child to consanguineous parents. Aged 7 years he was first diagnosed with EBV-positive classical Hodgkin’s lymphoma Stage IV, nodular sclerosis subtype, after having developed multiple subcutaneous lesions as well as generalized lymph node swelling. Immunohistology was positive for CD20 and lambda light chain restriction. He received five cycles of polychemotherapy (Doxorubicin, Bleomycin, Vincristine, Etoposide, Prednisolone, Cyclophosphamide), and Rituximab leading to complete remission. About one year later he relapsed with new cutaneous and lymph node lesions, biopsy revealed progression into EBV-positive Non-Hodgkin’s lymphoma with a change in immunohistological patterns (CD20-negative, kappa light chain restriction). Again, he received three cycles of polychemotherapy (Ifosfamide, Carboplatin, Etoposide and PEG-Asparaginase) and Rituximab. He showed no clinical response and laboratory examination showed increasing EBV levels as determined by PCR. Under the assumption of a primary immunodeficiency (PID) as underlying cause of recurrent EBV-positive lymphoma he was sent to our institution.
Upon arrival in our center at nine years of age he presented with a generalized (Fig. 1) EBV-positive diffuse large B-cell lymphoma (DLBCL, CD20- and CD19-negative, CD38- positive, lambda light chain restriction, 50% of cells positive for Ki-67). Cerebrospinal fluid as well as bone marrow were not affected. Blood tests revealed elevated levels of serum IgA (7.65 g/l) and ongoing EBV-viremia. A whole exome sequencing approach had been performed and revealed a homozygous missense mutation in TNFRSF9 encoding for 4-1BB (c.325G > A). 4-1BB is a costimulatory molecule expressed on activated T cells. Its ligand (4-1BBL) is an inducible molecule expressed on antigen presenting cells and upon ligation a signaling cascade is enhanced resulting in uncontrolled proliferation of cytotoxic T-cells with increased cytokine secretion. The functional relevance of the patient’s mutation for the phenotype was demonstrated for this and one other patient from a different family with the same founder mutation [2]. Subsequently, two groups have reported five additional patients from unrelated families, with distinct homozygous mutations in TNFRSF9 and different phenotypes, demonstrating the clinical heterogeneity of this disorder [3,4]. Unfortunately, no immunophenotype prior to chemotherapy is available for this patient. Further details are given in Alosaimi et al.[2]
Fig. 1.
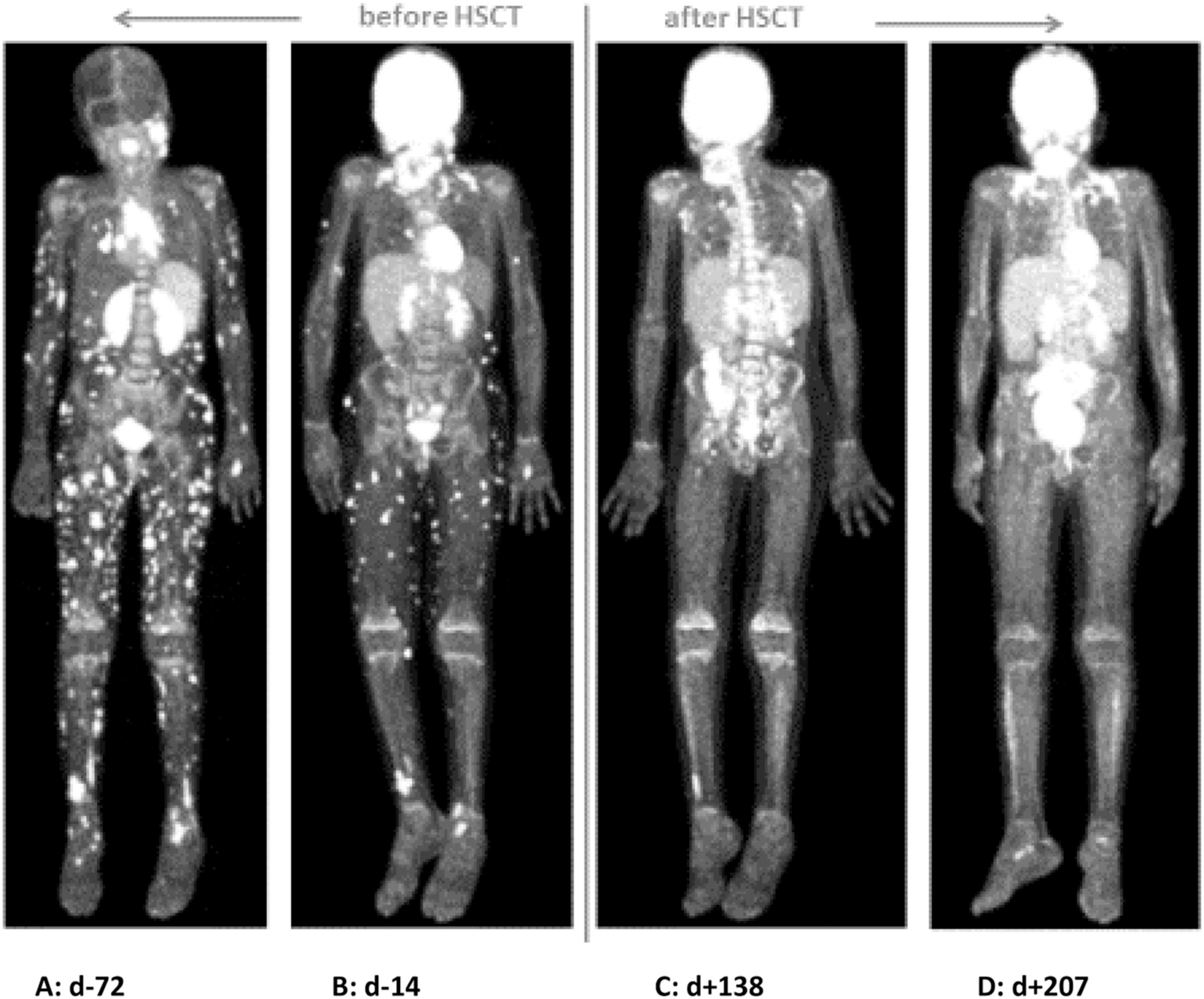
CXCR4-PET Scan (A) and FDG-PET Scans (B to D) during treatment. PET Scan A before treatment (day —72 before HSCT) shows disseminated subcutaneous lesions. PET Scan B (day —14 before HSCT) shows response to treatment with Daratumumab, Bortezomib and Rituximab. PET Scan C (day +138 after HSCT) shows remission of lymphoma but one unclear lesion of the right ankle, PET scan D (day +207 after HSCT) shows complete remission of lymphoma after HSCT.
According to the immunohistology of the DLBCL (see above) we initiated a therapy consisting of Daratumumab (anti-CD38 mAb), Bortezomib (Proteasome-inhibitor) and Dexamethasone and Rituximab (Figs. 1 and 2). This was followed by an allogenic hematopoietic stem cell transplantation (HSCT) from a 10/10 matched unrelated donor. Myeloablative conditioning included Busulfan (targeted AUC 90 mg/l*h), Fludarabine (150 mg/m2), Thiotepa (20 mg/kg), Alemtuzumab (0,7 mg/kg) and Rituximab (375 mg/m2). The bone marrow graft contained 7,08 × 106/kg body weight of CD34+ cells and 69,4 × 106/kg body weight of CD3+ cells. Following HSCT, the main complication was CMV-reactivation in combination with chronic GvHD of the skin. The palpable subcutaneous lesions steadily resolved over time (Fig. 1). Serum IgA levels normalized and EBV viremia turned negative on day +84 after HSCT (Fig. 2). Currently, after a post-transplant follow up at 13 months, he is well with complete donor chimerism in all lineages and in clinical and radiological remission of the lymphoma. Immunosuppression and oral Valganciclovir were ceased. Immunoglobulin substitution is continued with normal B-cell counts but incomplete differentiation to switched memory B-cells. B-cell development will be reevaluated in future visits.
Fig. 2.
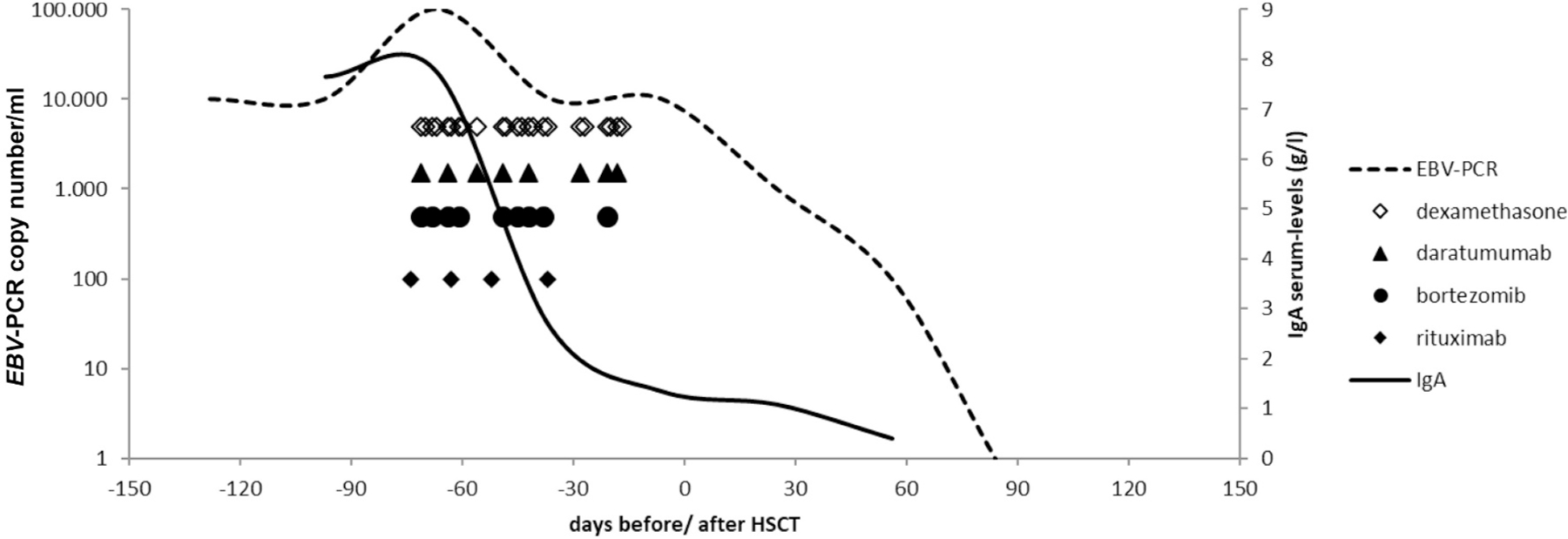
EBV- and serum IgA-levels during treatment.
This report demonstrates that the immunophenotype of patients with 4-1BB-deficiency is reversible by HSCT. Patients with 4-1BB deficiency or other primary immunodeficiencies suffering from recurrent EBV-positive lymphoma should be considered for HSCT in time to avoid accumulation of toxicity due to recurrent courses of chemotherapy. Depending on the immunohistology of the lymphoma, less toxic and more specific therapies can be considered to reduce the tumor burden before HSCT. Complete clinical and radiological remission before HSCT is not mandatory to achieve control of the lymphoma. The reconstitution of donor T-cell function to control EBV is a relevant component of the therapeutic strategy for definite cure from lymphoma and PID.
Acknowledgements
Supported by 1R01AI139633–01 (R.S.G.) and the Perkin Fund.
Abbreviations:
- AUC
Area under the curve
- CMV
Cytomegalovirus
- DLBCL
Diffuse large B-cell lymphoma
- EBV
Epstein Barr virus
- GvHD
Graft versus host disease
- HSCT
Hematopoietic stem cell transplantation
- HLH
Hemophagocytic lymphohistiocytosis
- mAb
Monoclonal antibody
- PCR
Polymerase chain reaction
- PID
Primary immunodeficiency
Footnotes
Declaration of Competing Interest
None.
References
- [1].Tangye SG, Latour S, Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection, Blood 135 (9) (2020) 644–655. [DOI] [PubMed] [Google Scholar]
- [2].Alosaimi M, Hoenig M, Jaber F, Platt CD, Jones J, Wallace J, et al. , Immunodeficiency and Epstein Barr virus induced lymphoproliferation caused by 4-1BB deficiency, J. Allergy Clin. Immunol 144 (2019), 574–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Somekh I, Thian M, Medgyesi D, Gülez N, Magg T, Duque AD, et al. , CD137 deficiency causes immune dysregulation with predisposition to lymphomagenesis, Blood 134 (18) (2019) 1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rodriguez R, Fournier B, Cordeiro DJ, Winter S, Izawa K, Martin E, et al. , Concomitant PIK3CD and TNFRSF9 deficiencies cause chronic active Epstein-Barr virus infection of T cells, J. Exp. Med 216 (12) (2019) 2800–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]


