Key Points
Donor race should not be used in models to predict allograft and patient survival.
Removing donor race from the Kidney Donor Risk Index may reduce kidney discard by reclassifying approximately 50% of high kidney donor profile index kidneys.
Future prediction models should focus on using relevant biologic factors rather than social constructs when trying to predict outcomes.
Keywords: transplantation, allograft survival, allografts, donor race, kidney discard, kidney donor risk index, kidney transplantation, patient survival
Introduction
A heated debate in creatinine-based eGFR calculation is the inclusion of race, a social construct, alongside biologic factors, such as age and sex (1–3). The controversy lies with using the race variable (Black versus non-Black) as a proxy for muscle mass—a concept that is not well validated in the literature (2–4). Similarly, the race variable, specifically Black versus non-Black, has been included in the calculation of the Kidney Donor Risk Index (KDRI) because deceased donor kidneys from Black donors have historically been shown to be associated with lower allograft or patient survival (5,6). Recent evidence suggests it is not race, but likely biologic factors such as the presence of the APOL1 gene G1 and G2 coding variants, which are virtually absent in individuals lacking recent African ancestry, that actually confers a worse allograft outcome (7). Given the current climate of uncertainty with the use of race in nephrology, we sought to answer the question of whether removing donor race from the KDRI would alter its validity to assess allograft and patient survival.
Materials and Methods
Using the Scientific Registry of Transplant Recipients data from 2000 to 2017, we included adult patients with deceased donor kidney transplant only, including both en bloc/dual kidney transplant recipients in the analysis. We excluded patients with previous kidney or multiorgan transplant and donors with missing variables required in the KDRI calculation (n=2299) and blood group incompatible transplant recipients (n=729). We first compared baseline characteristics of Black versus non-Black donors using Kruskal–Wallis and chi-squared tests. We then evaluated two proportional hazard survival models for combined outcome of allograft failure or patient death, which was the outcome assessed by Rao et al. (8) in the original KDRI manuscript. The first was a replication of the original KDRI model, in which the original KDRI score was a covariate in hazard ratio (HR) with unity coefficient. In the second model, we removed the donor race variable, refit the remaining components of the HR, assessed goodness of fit by using the Akaike information criterion, and derived a modified KDRI score without race. We then compared these two models using the area under the curve (AUC) of the receiver operating characteristic curve. A receiver operating characteristic curve in this survival model shows the relationship between true positive prediction (sensitivity) and false positive (1 minus specificity) using a hazard-based predictor at each time point where there is an observed outcome. An AUC measures the overall model performance when using all possible thresholds for prediction at a given time point. We estimated AUCs using R package survAUC (9,10). We first randomly selected 40,000 records as the training sample to fit each of the two models, then used the fitted model to predict outcomes for the remaining (testing) sample (n=68,000) to report AUC monthly over the 17-year study period. We replicated this process ten times and calculated the average AUC of the replications. To assess if removing donor race differentially affect kidneys of different quality, we first converted KDRI to kidney donor profile index (KDPI) by mapping all donor KDRI before 2011 using the 2010 mapping table (the earliest mapping table available), and subsequently, donors from 2012 to 2017 using mapping tables from the year prior (2011–2016). We calculated the percentages of donors with original KDPI ≤20%, 21%–85%, and >85% and showed the percentage that were and were not reclassified to a different category using the new model, and compared survivorship between the reclassified donors with those who remained in the original KDPI category. This study was approved by the institution review board at the University of New Mexico.
Results
Table 1 depicts the baseline characteristics and transplant outcomes of Black versus non-Black donors.
Table 1.
Donor characteristics and transplantation outcomes by donor race
| Covariates | Black (n=16,292) |
Non-Black (n=102,686) |
P Value |
|---|---|---|---|
| Donor characteristics | |||
| Sex, M, n (%) | 9743 (59.80) | 61,263 (59.66) | 0.73 |
| Cause of death: CVA, n (%) | 6921 (42.48) | 37,606 (36.62) | <0.001 |
| Diabetic, n (%) | 1435 (8.81) | 7150 (6.96) | <0.001 |
| Hypertensive, n (%) | 6137 (37.67) | 28,274 (27.53) | <0.001 |
| Donation after cardiac death, n (%) | 1242 (7.62) | 16,270 (15.84) | <0.001 |
| Positive for hepatitis C virus, n (%) | 370 (2.27) | 3018 (2.94) | <0.001 |
| Age, yr, mean (SD) | 36.05 (17.03) | 39.26 (16.47) | <0.001 |
| Height, cm, mean (SD) | 167.12 (22.24) | 168.68 (18.19) | 0.35 |
| Weight, kg, mean (SD) | 79.17 (27.89) | 79.29 (24.26) | 0.14 |
| Serum creatinine, mean (SD) | 1.33 (1.05) | 1.14 (0.96) | <0.001 |
| Transplant factors | |||
| En bloc/dual transplant, n (%) | 740 (4.54) | 2986 (2.91) | <0.001 |
| Transplant outcome | |||
| Graft failure, n (%) | 2972 (18.3) | 15,007 (14.7) | <0.001 |
| Median survival, yr, (IQR) | 2.69 (0.83, 5.22) | 3.03 (0.82, 5.88) | <0.001 |
| Death, n (%) | 2514 (15.5) | 17,946 (17.6) | <0.001 |
| Median survival, yr, (IQR) | 3.51 (1.29, 6.43) | 4.42 (1.75, 7.32) | <0.001 |
| Censoring, n (%) | 10,753 (66.2) | 69,295 (67.8) | <0.001 |
| Median survival, yr, (IQR) | 3.78 (1.11, 6.61) | 3.92 (1.51, 6.99) | <0.001 |
Censoring, patient who did not have allograft failure or die at the last date of reporting. M, male; CVA, cerebrovascular accident; IQR, interquartile range.
When we removed donor race from KDRI, the AUC differed negligibly between the two models, with minimum, maximum, and mean difference of 0.001, 0.006, and 0.002, respectively, over the 17-year follow-up period. Under the convention that an AUC 0.7–0.8 is acceptable (11), the AUC (Figure 1) indicated both models had a moderate level of predictive accuracy. With the removal of donor race, Akaike information criterion value changed from 107,902 to 107,880; five donor-related regression coefficients resulted in <1% change in HR; three donor variables (cerebrovascular accident, death, donation after cardiac death [DCD], hypertension) changed between 1% and 5%, whereas donor creatinine, hepatitis C virus (HCV) status, and diabetes resulted in HR changes between 5% and 11% (Table 2). Most HR changes in association with the coefficients of transplant and recipient-related factors were <1% with the exceptions of en bloc/dual transplant (7%) and recipient HCV positivity (4%). Donor factors with >1% HR changes were factors that were significantly different between Black and non-Black donors (Table 1). We also conducted a stratified analysis with separate models for Black and non-Black donor races to verify the role of donor race. For example, HR associated with diabetes is smaller in the Black-donor model than in the non-Black model (1.13 versus 1.28; 95% confidence interval [95% CI], 0.74 to 1.06 for the ratio). This result disputes with the original KDRI model, which suggests diabetes-associated HR is greater for Black donors than for non-Black donors (1.36 versus 1.14; 95% CI, 1.13 to 1.15 for the ratio). HR changes after removing donor race are comparable between non-Black donors and Black donors, with a minimum, median, and maximum fraction of change −0.64, 0.03, and 0.97, and −0.63, −0.14, and 0.52 for the two groups, respectively.
Figure 1.
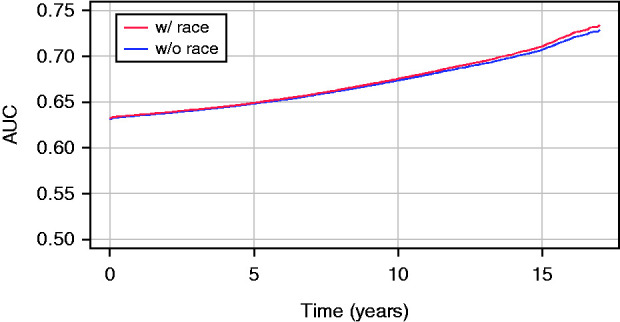
Time-dependent area under the curve of allograft/patient survival models with or without donor race. AUC, area under the curve; w, with; w/o, without.
Table 2.
Proportional hazard survival model with and without donor race: Results of donor and transplant related factors
| Covariates | Original Kidney Donor Profile Index Model | Kidney Donor Profile Index Without Donor Race Model | Relative Hazard Ratio Changea | ||||
|---|---|---|---|---|---|---|---|
| Akaike Information Criterion 107,902 | Akaike Information Criterion 107,880 | ||||||
| Concordance 0.6125 | Concordance 0.6131 | ||||||
| Coefficient | Hazard Ratio (95% Confidence Interval) | P Value | Coefficient | Hazard Ratio (95% Confidence Interval) | P Value | ||
| Donor factors (KDRI) | |||||||
| Age, y | |||||||
| all | 0.01 | 1.013 (1.011 to 1.015) | <0.001 | 0.01 | 1.010 (1.008 to 1.011) | <0.001 | 0.3% |
| <18 | −0.02 | 0.98 (0.97 to 0.99) | 0.003 | −0.02 | 0.985 (0.976 to 0.994) | 0.002 | −0.5% |
| >50 | 0.01 | 1.011 (1.005 to 1.016) | <0.001 | 0.009 | 1.009 (1.005 to 1.013) | <0.001 | 0.2% |
| Race | |||||||
| Other | ref | ref | |||||
| Black | 0.18 | 1.20 (1.13 to 1.27) | <0.001 | – | – | – | – |
| Height, 170 cm per 10 cm increase | −0.05 | 0.96 (0.94 to 0.97) | <0.001 | −0.04 | 0.96 (0.95 to 0.98) | <0.001 | −0.3% |
| Weight, ≤80 kg per 5 kg increase | −0.02 | 0.98 (0.97 to 0.99) | <0.001 | −0.01 | 0.986 (0.978 to 0.994) | <0.001 | −0.6% |
| Cause of death | |||||||
| Other | ref | ref | |||||
| Cerebrovascular accident | 0.09 | 1.09 (1.04 to 1.14) | <0.001 | 0.06 | 1.06 (1.03 to 1.09) | <0.001 | 2.6% |
| Donation after cardiac death | |||||||
| No | ref | ref | |||||
| Yes | 0.13 | 1.14 (1.02 to 1.28) | 0.02 | 0.11 | 1.12 (1.07 to 1.17) | <0.001 | 1.7% |
| Creatinine –1: all | 0.22 | 1.25 (1.17 to 1.33) | <0.001 | 0.16 | 1.17 (1.12 to 1.22) | <0.001 | 6.3% |
| Creatinine –1.5: creatinine >1.5 | −0.21 | 0.81 (0.74 to 0.89) | <0.001 | −0.16 | 0.86 (0.81 to 0.90) | <0.001 | −5.6% |
| Diabetes | |||||||
| No | ref | ref | |||||
| Yes | 0.13 | 1.14 (1.04 to 1.24) | 0.004 | 0.24 | 1.27 (1.21 to 1.33) | <0.001 | −11.4% |
| Hypertension | |||||||
| No | ref | ref | |||||
| Yes | 0.13 | 1.13 (1.08 to 1.19) | <0.001 | 0.15 | 1.16 (1.12 to 1.20) | <0.001 | −2.7% |
| Hepatitis C virus | |||||||
| Negative | ref | ref | |||||
| Positive | 0.24 | 1.27 (1.13 to 1.43) | <0.001 | 0.31 | 1.36 (1.25 to 1.48) | <0.001 | −7.2% |
| Transplant factors | |||||||
| Shared organ | |||||||
| No | ref | ref | |||||
| Yes | 0.05 | 1.05 (1.01 to 1.09) | 0.005 | 0.04 | 1.04 (1.01 to 1.08) | 0.02 | 0.8% |
| Human leukocyte antigen-B | |||||||
| 2 | ref | ref | |||||
| 0 | −0.09 | 0.91 (0.87 to 0.96) | <0.001 | −0.11 | 0.90 (0.85 to 0.95) | <0.001 | 1.6% |
| 1 | −0.02 | 0.98 (0.95 to 1.01) | 0.12 | −0.02 | 0.98 (0.95 to 1.01) | 0.11 | 0.1% |
| Human leukocyte antigen-DR | |||||||
| 1 | ref | ref | |||||
| 0 | −0.08 | 0.93 (0.89 to 0.97) | <0.001 | −0.08 | 0.92 (0.89 to 0.96) | <0.001 | 0.6% |
| 2 | 0.02 | 1.02 (1.00 to 1.06) | 0.01 | 0.03 | 1.03 (1.00 to 1.06) | 0.03 | −0.8% |
| Year of transplant | −0.05 | 0.952 (0.947 to 0.957) | <0.001 | −0.05 | 0.952 (0.946 to 0.957) | <0.001 | 0.0% |
| En bloc/dual transplant | |||||||
| No | ref | ref | |||||
| Yes | −0.30 | 0.74 (0.69 to 0.80) | <0.001 | −0.24 | 0.79 (0.73 to 0.86) | <0.001 | −6.7% |
| Total cold ischemic time (ref: 20 h) | 0.004 | 1.004 (1.002 to 1.006) | <0.001 | 0.004 | 1.004 (1.002 to 1.006) | <0.001 | 0.0% |
Model comparison: Survival model with 118,978 recipients and 38,695 events. Missing data: donor factors: race (n=32); height (n=5); cerebrovascular accident (n=3); creatinine (n=605); diabetes (n=713); hypertension (n=941). Transplant factors: donor-recipient (D-R) B mismatch (n=10); D-R DR mismatch (n=28); cold ischemic time (n=8638). Candidate factors: pretransplant transfusion (n=41,370, labeled as unknown); height (n=2088); weight (n=1367); peripheral vascular disease (n=5277, labeled as unknown); COPD (n=14,880, labeled as unknown); hepatitis C status (n=9990, labeled as unknown); angina (n=101,728, labeled as unknown); PRA (n=18,013). Candidate factors are not reported in this table. KDRI, kidney donor risk index; COPD, chronic obstructive pulmonary disease; PRA, panel reactive antibody; ref, reference; w, with; w/o, without.
Relative HR change = (HR w/race−HR w/o race)/HR w/race.
After donor race was removed, 45,484 donors had increased in KDRI whereas 73,494 donors had decreased in KDRI. We showed in Figure 2A that the removal of donor race preferentially affected donors with KDPI >85%. Among the 11,865 donors with KDPI >85% in the original model, 53% (n=6251) would have been reclassified as KDPI ≤85% using the new model without race. Using Kaplan–Meier estimate (Figure 2B), we showed a median allograft/patient survival of 6.55 (95% CI, 6.27 to 6.81) years for donor kidneys with reclassified KDPI ≤85%, compared with 5.81 (95% CI, 5.58 to 6.08) years for donor kidneys that remained KDPI >85% (P<0.001). The new model reclassified 73% (2700 out of 3692) of Black donors with KDPI >85% to ≤85%, with a median allograft/patient survival of 6.54 years (95% CI, 6.20 to 6.88) compared with 5.39 years (95% CI, 4.67 to 6.01) for those with KDPI remaining >85%. Removal of donor race reclassified 18% (3955 out of 22,608) of donors with KDPI ≤20% to a KDPI >20%. Median allograft/patient survival of recipients whose kidney were reclassified to KDPI >20% was 0.1 years shorter compared with those whose kidney had remained KDPI ≤20% (P=0.87).
Figure 2.
Effect of removing donor race from kidney donor profile index. (A) Original and reclassified KDPI. Removing donor race preferentially affected kidneys with KDPI >85. (B) Kaplan-Meier survival curves with 95% confidence interval (95% CI; shaded) of donors with KDPI >85. Median allograft/patient survival is 6.55 (95% CI, 6.27 to 6.81) years for donors with reclassified KDPI ≤85 compared with 5.81 (95% CI, 5.58 to 6.08) years for donors that remained KDPI >85 (P<0.001). KDPI, kidney donor profile index.
Discussion
Our modeling and analyses showed that removing donor race from the original KDRI did not alter the overall model predictability of allograft or patient survival. We showed that donor factors included in the KDRI have largely accounted for differential risk between Black and non-Black donors. Removal of donor race as a proxy allows these clinical factors to account more directly for their effects on the outcome. Including race in KDRI appears to have biased risk estimate and is unwarranted.
Our study contradicts results from previous studies showing that Black deceased donor kidneys have worse allograft and patient outcomes (5,6,12,13), but our findings that Black donors were more likely to have clinical risk factors associated with worse allograft outcomes such as diabetes, hypertension, death from cerebrovascular accident, etc. may provide an explanation for why Black donors appear to be a proxy of worse outcomes. Previous studies have not accounted for these factors. Locke et al. (14) showed that DCD kidneys from Black donors have better outcomes because the donors in that study sample tended to be younger and were less likely to have diabetes and hypertension. As noted, the recurrent theme is that it is the underlying risk factor associated with the donor race that confers differential outcome rather than donor race itself.
In this study, we have also shown the removal of donor race would reclassify 50% of donor kidneys with KDPI >85% to ≤85%. These reclassified kidneys have better allograft survival compared with kidneys that were not reclassified. As we strive to increase the number of kidney transplants by reducing kidney discards, removing donor race may reduce the risk of kidney discard.
In conclusion, we have shown that removing donor race from KDRI has little effect on allograft or patient survival and may help reduce the risk of kidney discard by reclassifying 50% of the high KDPI kidneys. Precision medicine research offers the future promise to identify genetic, environmental, and lifestyle factors that will guide decision making in areas such as kidney transplantation. Even if donor race were a proxy for any heretofore unknown but relevant biologic factors, such as the APOL1 gene variants, it is statistically more valid and scientifically more rigorous to incorporate such biologic factors, rather than race, in the calculation of risk for allograft failure (15). Finally, social determinants of health gleaned from precision medicine has the potential to replace race when accounting for the effects of disparities on transplant outcomes.
Disclosures
C. Argyropoulos reports having consultancy agreements with Alkahest and Momenta Pharma; reports receiving research funding from Dialysis Clinic Inc. and the University of Pennsylvania; reports being a scientific advisor or member of Baxter Healthcare, Bayer, and Health Services Advisory Group; and reports other interests/relationships with AbbVie as Sub-I in a phase 3 study of an experimental agent in diabetic nephropathy, Akebia principal investigator in two phase 3 trials of an investigational product for the correction and maintenance of anemia in patients with nondialysis-dependent CKD and one phase 3 study of the same agent in dialysis, Dialysis Clinic Inc. as Medical Director of the Outpatient Dialysis Unit in Cuba, New Mexico, and the Dialysis Outcomes and Practice Patterns Program, principal investigator for CKD-DOPP. Y. Zhu reports receiving research funding from Dialysis Clinic Inc., Patient-Centered Outcomes Research Institute, the National Institutes of Health, and US Department of State. All remaining authors have nothing to disclose.
Funding
This work is supported by the Dialysis Clinic Inc. grant 4130.
Acknowledgments
Portions of this research have been posted on medRxiv as https://doi.org/10.1101/2021.04.01.21254772.
Author Contributions
C. Argyropoulos and Y.-H. Ng conceptualized the study; C. Argyropoulos, I. Litvinovich, Y.-H. Ng, and Y. Zhu were responsible for the formal analysis; C. Argyropoulos and Y.-H. Ng were responsible for the funding acquisition; C. Argyropoulos, I. Litvinovich, Y.-H. Ng, and Y. Zhu were responsible for the methodology; Y.-H. Ng and Y. Zhu provided supervision; K. Chong was responsible for the visualization; C. Argyropoulos, S.S. Chen, K. Chong, Y.-H. Ng, and Y. Zhu wrote the original draft; C. Argyropoulos, S.S. Chen, K. Chong, I. Litvinovich, Y.-H. Ng, and Y. Zhu reviewed and edited the manuscript. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Footnotes
This article contains a podcast at https://www.asn-online.org/media/podcast/K360/2021_11_24_KID0002932021.mp3
See related editorial, “What to Do with Race: Social Factors and Evaluating Clinical Risk in Kidney Transplantation,” on pages 1691–1692.
References
- 1.Fontanarosa PB, Bauchner H: Race, ancestry, and medical research. JAMA 320: 1539–1540, 2018. 10.1001/jama.2018.14438 [DOI] [PubMed] [Google Scholar]
- 2.Eneanya ND, Yang W, Reese PP: Reconsidering the consequences of using race to estimate kidney function. JAMA 322: 113–114, 2019. 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 3.Delgado C, Baweja M, Burrows NR, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey-Mims MM, Roberts GV, St. Peter WL, Warfield C, Powe NR: Reassessing the inclusion of race in diagnosing kidney diseases: An interim report from the NKF-ASN Task Force. J Am Soc Nephrol 32: 1305–1317, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu J, Johansen KL, Hsu CY, Kaysen GA, Chertow GM: Higher serum creatinine concentrations in black patients with chronic kidney disease: Beyond nutritional status and body composition. Clin J Am Soc Nephrol 3: 992–997, 2008. 10.2215/CJN.00090108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson SJ, Hypolite IO, Agodoa LYC, Batty DS Jr, Hshieh PB, Cruess D, Kirk AD, Peters TG, Abbott KC: Effect of donor factors on early graft survival in adult cadaveric renal transplantation. Am J Transplant 2: 68–75, 2002. 10.1034/j.1600-6143.2002.020112.x [DOI] [PubMed] [Google Scholar]
- 6.Callender CO, Cherikh WS, Miles PV, Hermesch A, Maddox G, Nash J, Hernandez A, Burston B: Blacks as donors for transplantation: Suboptimal outcomes overcome by transplantation into other minorities. Transplant Proc 40: 995–1000, 2008. 10.1016/j.transproceed.2008.03.063 [DOI] [PubMed] [Google Scholar]
- 7.Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, Gautreaux MD, Hauptfeld V, Bray RA, Gebel HM, Kirk AD, Gaston RS, Rogers J, Farney AC, Orlando G, Stratta RJ, Mohan S, Ma L, Langefeld CD, Hicks PJ, Palmer ND, Adams PL, Palanisamy A, Reeves-Daniel AM, Divers J: Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant 15: 1615–1622, 2015. 10.1111/ajt.13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009. 10.1097/TP.0b013e3181ac620b [DOI] [PubMed] [Google Scholar]
- 9.Chambless LE, Diao G: Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med 25: 3474–3486, 2006. 10.1002/sim.2299 [DOI] [PubMed] [Google Scholar]
- 10.Potapov S, Adler W, Schmid M: Package ‘survAUC’, 2015. Available at: https://cran.r-project.org/web/packages/survAUC/survAUC.pdf. Accessed September 28, 2021.
- 11.Hosmer D, Lemeshow S: Assessing the Fit of the Model. In: Applied Logistic Regression, edited by Shewhart WA, Wilks SS, New York, John Wiley & Sons, Inc., 2000. p 143–202 [Google Scholar]
- 12.Molnar MZ, Kovesdy CP, Bunnapradist S, Streja E, Krishnan M, Mucsi I, Norris KC, Kalantar-Zadeh K: Donor race and outcomes in kidney transplant recipients. Clin Transplant 27: 37–51, 2013. 10.1111/j.1399-0012.2012.01686.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callender CO, Cherikh WS, Traverso P, Hernandez A, Oyetunji T, Chang D: Effect of donor ethnicity on kidney survival in different recipient pairs: An analysis of the OPTN/UNOS database. Transplant Proc 41: 4125–4130, 2009. 10.1016/j.transproceed.2009.06.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locke JE, Warren DS, Dominici F, Cameron AM, Leffell MS, McRann DA, Melancon JK, Segev DL, Simpkins CE, Singer AL, Zachary AA, Montgomery RA: Donor ethnicity influences outcomes following deceased-donor kidney transplantation in Black recipients. J Am Soc Nephrol 19: 2011–2019, 2008. 10.1681/ASN.2008010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julian BA, Gaston RS, Brown WM, Reeves-Daniel AM, Israni AK, Schladt DP, Pastan SO, Mohan S, Freedman BI, Divers J: Effect of replacing race with apolipoprotein L1 genotype in calculation of kidney donor risk index. Am J Transplant 17: 1540–1548, 2017. 10.1111/ajt.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]



