Key Points
Relaxin attenuates tissue fibrosis in an organ- and age-specific manner.
The antifibrotic actions of relaxin are mediated via an angiotensin type 2 receptor mechanism.
Relaxin replacement therapy is a potential antifibrotic for cardiovascular and kidney disease in postmenopausal women.
Keywords: hypertension, aging, angiotensin type 2 receptor, basic science, cardiovascular system, females, fibrosis, hypertension, rats, relaxin
Visual Abstract
Abstract
Background
The antifibrotic effects of recombinant human relaxin (RLX) in the kidney are dependent on an interaction between its cognate receptor (RXFP1) and the angiotensin type 2 receptor (AT2R) in male models of disease. Whether RLX has therapeutic effects, which are also mediated via AT2R, in hypertensive adult and aged/reproductively senescent females is unknown. Thus, we determined whether treatment with RLX provides cardiorenal protection via an AT2R-dependent mechanism in adult and aged female stroke-prone spontaneously hypertensive rats (SHRSPs).
Methods
In 6-month-old (6MO) and 15-month-old ([15MO]; reproductively senescent) female SHRSP, systolic BP (SBP), GFR, and proteinuria were measured before and after 4 weeks of treatment with vehicle (Veh), RLX (0.5 mg/kg per day s.c.), or RLX+PD123319 (AT2R antagonist; 3 mg/kg per day s.c.). Aortic endothelium–dependent relaxation and fibrosis of the kidney, heart, and aorta were assessed.
Results
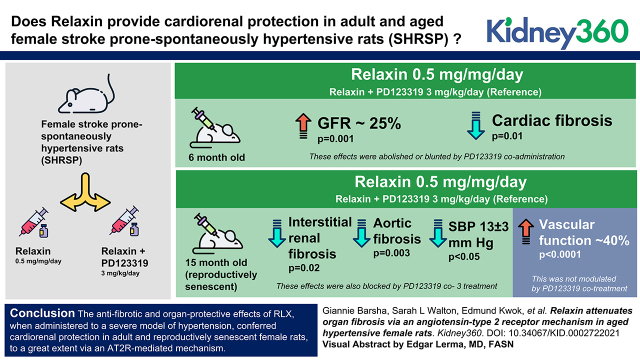
In 6MO SHRSP, RLX significantly enhanced GFR by approximately 25% (P=0.001) and reduced cardiac fibrosis (P=0.01) as compared with vehicle-treated counterparts. These effects were abolished or blunted by PD123319 coadministration. In 15MO females, RLX reduced interstitial renal (P=0.02) and aortic (P=0.003) fibrosis and lowered SBP (13±3 mm Hg; P=0.04) relative to controls. These effects were also blocked by PD123319 cotreatment (all P=0.05 versus RLX treatment alone). RLX also markedly improved vascular function by approximately 40% (P<0.001) in 15MO SHRSP, but this was not modulated by PD123319 cotreatment.
Conclusions
The antifibrotic and organ-protective effects of RLX, when administered to a severe model of hypertension, conferred cardiorenal protection in adult and reproductively senescent female rats to a great extent via an AT2R-mediated mechanism.
Introduction
Premenopausal women have a lower incidence of cardiorenal disease compared with age-matched men and postmenopausal women, although the underlying mechanisms are incompletely understood (1). Accumulating evidence points to a sex-specific role for the angiotensin type 2 receptor (AT2R) (2). AT2R suppresses angiotensin type 1 receptor (AT1R) expression and function, and it counterbalances the AT1R-mediated prohypertensive and profibrotic effects of angiotensin II (1,2). Importantly, the renoprotective effects of AT2R stimulation in rodents (2) and humans (3–5) are enhanced in adult females in association with a greater AT2R:AT1R ratio. However, expression of the AT2R and its effects in female preclinical models are decreased with age (6–8). As such, upregulating AT2R function could be a valuable therapeutic approach to target cardiorenal injury.
Renal fibrosis, one of the main drivers of CKD, is associated with increased risk of cardiovascular disease and is a major public health concern. Despite the pathophysiologic significance of renal fibrosis, there are no approved clinical treatments for delaying or reversing its progression (9). Evidence indicates that human gene-2 (H2) relaxin (RLX), the major form of human RLX, is protective against age- and injury-related renal fibrosis (10,11). Recently, the vasodilatory and antifibrotic effects of RLX in the kidney, owing to its nitric oxide–promoting and TGF-β1–inhibiting actions, were shown to require crosstalk between its cognate G protein–coupled receptor, relaxin family peptide receptor 1 (RXFP1), and the AT2R (12,13). This RXFP1-AT2R crosstalk was shown to be integral in slowing or reversing tissue fibrosis in male preclinical models of kidney disease, such that RLX indirectly mediated its therapeutic effects via AT2R signal transduction (12–14). However, whether the antifibrotic effects of RLX require the AT2R in aged females is not known. Given that the renoprotective effects of AT2R activation are downregulated in aged females (6–8) and that RLX levels decline with age in females (15), exogenous RLX administration to aged females may act via the AT2R to attenuate renal fibrosis and reinstate cardiorenal protection.
Here, we examined the therapeutic effects of recombinant H2 RLX in adult (6-month-old [6MO]) and aged (15-month-old [15MO]; reproductively senescent) female stroke-prone spontaneously hypertensive rats (SHRSPs). The adult cohort served as a control group to demonstrate the effect of age. The SHRSPs are stroke prone if fed a high-salt diet, but in this study, the stroke phenotype was not examined (16). The SHRSP on a normal sodium intake is a model of severe progressive hypertension in which renal damage is evident at approximately 6 months of age, approximately 3 months earlier than in its parental strain of spontaneously hypertensive rats (17). Moreover, female SHRSPs enter reproductive senescence at approximately 13–15 months of age. Therefore, the SHRSP is a model in which the effects of aging and reproductive senescence can be examined over a shortened time frame. We hypothesized that (1) chronic RLX treatment confers renoprotective effects with benefits for end organ fibrosis and renal function in aged reproductively senescent females and that (2) these RLX-mediated effects are negated by AT2R blockade.
Materials and Methods
Materials
RLX was provided by Corthera Inc. (San Mateo, CA; a subsidiary of Novartis International AG, Basel, Switzerland) and is bioactive in rats (18). PD123319 (AT2R antagonist) was obtained from Sigma-Aldrich (St. Louis, MO).
Animals
Experiments were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and approved by the Monash University School of Biomedical Sciences Animal Ethics Committee (MARP/2015/166). Adult (6MO) and aged (15MO) female SHRSPs were obtained from the Monash Animal Research Platform, Monash University (Clayton, VIC, Australia) and individually housed with temperature maintained at 21°C–22°C and a 12-hour light-dark cycle. Rats had ad libitum access to a normal chow (0.5% NaCl) rodent maintenance diet (Barastoc Stockfeeds, Pakenham, VIC, Australia) and water. On this sodium diet, no female SHRSPs exhibited signs of stroke throughout the study.
Study Design
Vaginal smears were taken from 15MO female SHRSPs to confirm that they were reproductively senescent. Measurements in 6MO (n=24) and 15MO (n=26) cohorts were made at baseline and 4 weeks after treatment. These included systolic BP (SBP) recorded via tail-cuff plethysmography (Hatteras Instruments), 24-hour urine collection, and GFR via a transdermal decay method using FITC-sinistrin. The rats were treated with vehicle (20 mM sodium acetate buffer subcutaneously [s.c.]), an RLX (0.5 mg/kg per day s.c.) dose that causes antifibrotic effects in male rats (19), or RLX + PD123319 (AT2R antagonist; 3 mg/kg per day s.c.) delivered via osmotic minipump (ALZET model 2ML4) for 28 days. Following 4 weeks of treatment, aorta, heart, and kidney were collected for histologic quantification of fibrosis, and blood samples were collected for determination of plasma RLX levels. Aortic endothelial vasodilator function in response to acetylcholine (ACh) and renal gene expression of the angiotensin receptors (AT1aR and AT2R) and RXFP1 were also determined.
Measurement of SBP
SBP was measured using a noninvasive tail-cuff BP analysis system (Hatteras Instruments) as described previously (20).
Measurement of GFR
GFR was measured in conscious rats via the transcutaneous clearance of FITC‐sinistrin (Fresenius Kabi Austria GmbH, Linz, Austria), a fluorescent‐labeled exogenous marker of GFR (21). The fluorescence signal intensity of FITC-sinistrin was determined using a miniaturized animal imager (noninvasive clearance kidney device; Mannheim Pharma & Diagnostics GmbH, Mannheim, Germany). FITC‐sinistrin was dissolved in 0.9% wt/vol sodium chloride solution and administered at a dose of 3–5 mg/100 g body weight.
Urinary Protein Analyses
Rats were housed in metabolic cages for 24 hours to determine urine flow and collect a urine sample. Urinary protein was determined using a Coomassie blue assay (Pierce Biotechnology, Rockford, IL), and protein excretion rates (milligrams per hour) were expressed relative to body weight.
Vascular Function
Aortic rings (approximately 5-mm long) were obtained from the thoracic aorta, and each ring was suspended between two stainless steel wires connected to an isometric force transducer (FT-03; Grass Instruments). To examine endothelium-dependent relaxation to ACh, the vessels were preconstricted using U46619 [the thromboxane A2 receptor analog 1,5,5-hydroxy-11α, 9α-(epoxyme α-thano) prosta-5Z, 13E-dienoic acid; 100 µM] to 60%–70% of the maximal response to potassium physiologic salt solution (22). At the end of the study, a single dose of the endothelium-independent vasodilator, sodium nitroprusside (10 µM), was then added to the bath to induce maximal vasodilation and confirm vascular smooth muscle integrity.
Measurement of Plasma RLX
The Quantikine Human H2 Relaxin ELISA kit (catalog no. DRL200; R&D Systems, Inc., Minneapolis, MN) was used to measure circulating levels of RLX in vehicle-, RLX-, and RLX + PD123319–treated SHRSPs. Rat plasma was diluted 1:50, and the assay was performed according to the manufacturer’s instructions.
Quantifying Renal, Cardiac, and Aortic Fibrosis
Paraformaldehyde-fixed heart, kidney, and aorta samples were processed to paraffin, sectioned at 5 µm, and mounted on Superfrost Plus slides (Gerhard-Menzel; ThermoScientific). Heart and aorta sections were stained with 0.05% wt/vol picrosirius red solution, and kidney sections were stained with Masson trichrome (one slide per organ per animal). The Aperio Scanscope AT Turbo scanner (Leica Microsystems, Sydney, Australia) was then used to generate digital images of stained sections (at 40× magnification). Each slide was assessed by two independent researchers blinded to treatment groups and subjectively graded by assigning a score (on a scale of zero to four) on the basis of pathology severity. The extent of cortical tubulointerstitial fibrosis was graded as described previously (23). Glomerulosclerosis was defined as glomerular basement membrane thickening, mesangial hypertrophy, and capillary occlusion, and it was assigned a score from zero to one on the basis of pathology severity. Glomerular area was quantified by tracing glomerular borders when the vascular pole was evident. Thirty glomeruli from each animal were examined in Masson trichome–stained kidneys, and measurements were averaged. Cardiac ventricular fibrosis was scored on the basis of fibrotic area, and aortic fibrosis was scored on the basis of media collagen content: grade 0 (normal): 0%–10%; grade 1 (minimal): 10%–25%; grade 2 (moderate): 25%–50%; grade 3 (moderately severe): 50%–75%; and grade 4 (severe): 75%–100%. Intima media thickness was measured in the aorta (micrometers) with treatment groups blinded, and an average of four measurements per tissue was used.
Renal Gene Expression
RNA was extracted from kidneys of 6MO and 15MO SHRSPs following the 28-day infusion of vehicle, RLX, or RLX + PD123319 using the RNeasy Mini kit as outlined by the manufacturer’s instructions (Qiagen, Doncaster, VIC, Australia). One microgram of extracted RNA was converted to cDNA (5× iSCRIPT Supermix; BioRad; Life Sciences). Samples were run in triplicate using TaqMan gene expression assays for AT1aR (Agtr1a: Rn02758772_s1), AT2R (Agt2r: Rn00560677_s1), and RXFP1 (Rxfp1: Rn01495351_m1; Applied Biosystems; Life Technologies) and duplexed with 18S rRNA (Hs99999901_s1) as the internal housekeeping gene. Reactions were set up on a 384-well PCR plate using an automated liquid handler (Qiagility; Qiagen) and run using the Applied Biosystems 7900HT Fast RT-PCR system. Each 10-µl reaction contained a PCR reaction mix (2× TaqMan universal PCR master mix and TaqMan gene expression assays; Applied Biosystems; Life Technologies) and 50 ng of cDNA. Relative gene expression was determined via the comparative cycle of threshold fluorescence (2−ΔΔCT) method using real-time quantitative RT-PCR Realplex software (Applied Biosystems).
Statistical Analyses
Data are presented as mean ± SEM. Basal values for SBP, GFR, urine flow, and protein excretion were pooled for 6MO and 15MO groups, with the effect of age determined using a two-tailed, unpaired t test. The absolute change from baseline for SBP, GFR, urine flow, and protein excretion was measured, and differences between groups were analyzed using a two-way ANOVA with factors group, treatment, and their interaction followed by post hoc analysis with a Sidak correction to reduce risk of type 1 error. Body and organ weights, plasma RLX levels, and renal mRNA expression (expressed relative to the 6MO vehicle-treated group) were analyzed using a two-way ANOVA with factors age, treatment, and their interaction, followed by the Sidak multiple comparisons test. Histopathology scores as well as aortic media thicknesses were analyzed using a Mann–Whitney test. Dose-response relaxation curves to ACh were analyzed using nonlinear regression to obtain the molar concentration of agonist producing 50% of the maximum response presented as −10log EC50 and the maximal effect generated by the agonist (Emax). Values for the molar concentration of agonist producing 50% of the maximum response presented as −10log EC50 and Emax were analyzed using a one-way ANOVA with the Sidak multiple comparisons test. P=0.05 was accepted as the level of statistical significance.
Results
Body and Organ Weights
Body weight as well as total heart and kidney weights were greater with age (Page<0.001) (Supplemental Table 1) but unaffected by RLX alone or in combination with PD123319 treatment (Supplemental Table 1).
Plasma RLX Levels
Plasma RLX levels were not detectable in vehicle-treated 6MO or 15MO SHRSPs after 28 days of vehicle infusion. In comparison, levels were between approximately 15 and 30 ng/ml in RLX-treated rats, with levels in the 6MO group significantly greater than those in the 15MO group (P=0.04) (Figure 1). These plasma RLX levels were similar to the circulating RLX concentrations detected in rats at gestational days 12–14 (24,25). Cotreatment with PD123319 did not significantly affect plasma RLX levels.
Figure 1.
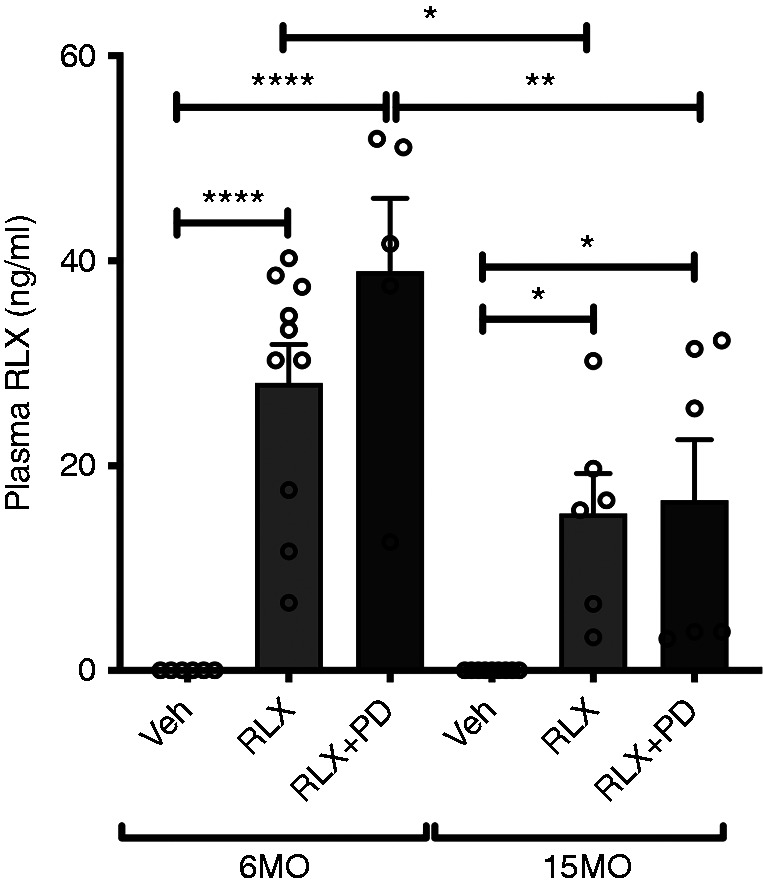
Plasma relaxin (RLX) levels. Plasma RLX levels (nanograms per milliliter) in 6-month-old (6MO) and 15-month-old (15MO) female stroke-prone spontaneously hypertensive rats (n=6–10 per group) after 4 weeks of vehicle (Veh; 20 mM sodium acetate subcutaneously), RLX (0.5 mg/kg per day subcutaneously), or RLX+PD123319 (angiotensin type 2 receptor antagonist; 3 mg/kg per day subcutaneously) treatment. Data are presented as mean±SEM and were analyzed using a two-way ANOVA with factors age, treatment, and their interaction, followed by the Sidak multiple comparisons test. *P=0.05 versus the respective group indicated; **P=0.01 versus the respective group indicated; ****P<0.001 versus the respective group indicated.
SBP
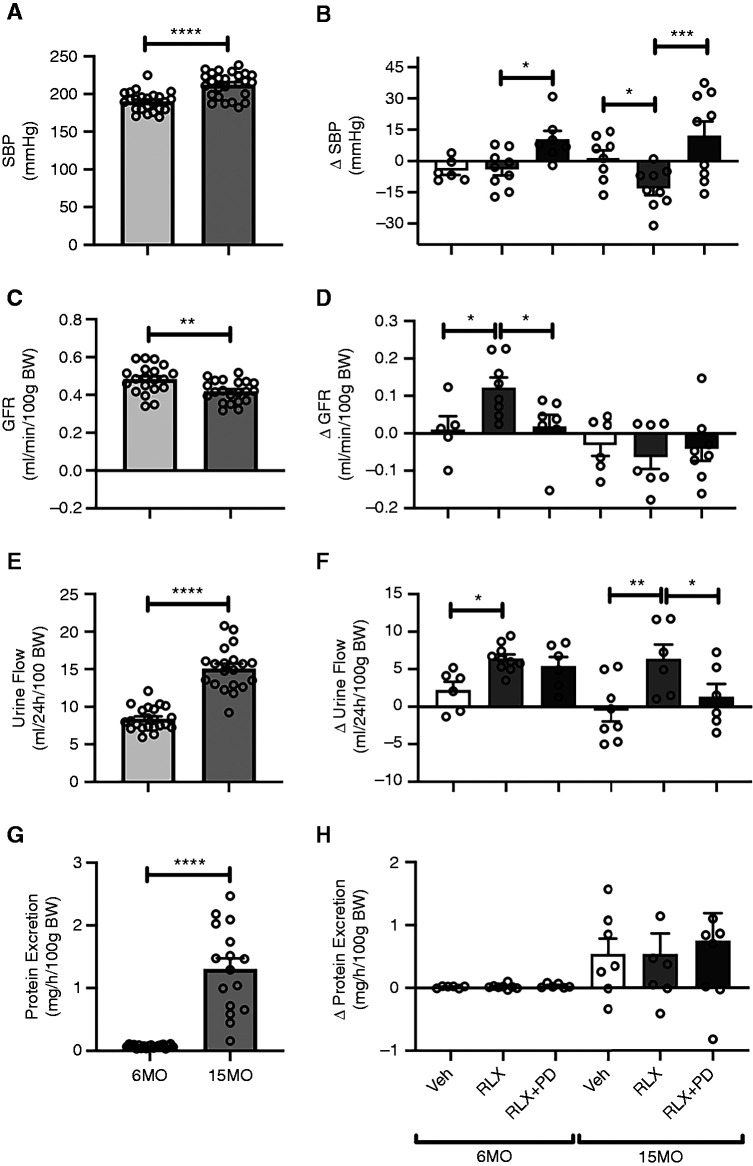
SBP was similar to baseline levels following 4 weeks of vehicle or RLX treatment in the 6MO group. However, in response to RLX+PD123319 treatment, SBP increased by 10±4 mm Hg from baseline in the 6MO group (P=0.05 versus the RLX-treated group) (Figure 2B). SBP was approximately 20 mm Hg higher in the 15MO as compared with the 6MO females (P<0.001) (Figure 2A), but it was decreased by approximately 13±3 mm Hg following RLX as compared with vehicle infusion (P=0.04) (Figure 2B). This RLX-induced response was reversed by coinfusion of RLX+PD123319 (P<0.001 versus the 15MO RLX group) (Figure 2B).
Figure 2.
Systolic BP (SBP) and renal function. Baseline and absolute changes (Δ) in (A and B) SBP, (C and D) GFR, (E and F) urine flow, and (G and H) total protein excretion were measured in 6MO and 15MO female stroke-prone spontaneously hypertensive rats (n=5–10 per group) after 4 weeks of Veh (20 mM sodium acetate subcutaneously), RLX (0.5 mg/kg per day subcutaneously), or RLX+PD123319 (angiotensin type 2 receptor antagonist; 3 mg/kg per day subcutaneously) treatment. Data are presented as mean±SEM. Baseline data were analyzed using a two-tailed t test, and absolute change from baseline was analyzed using a two-way ANOVA with factors group, treatment, and their interaction, followed by the Sidak multiple comparisons test. BW, body weight. *P=0.05; **P=0.01; ***P<0.001; ****P<0.001.
GFR, Urine Flow, and Proteinuria
In 6MO SHRSPs, GFR was approximately 25% greater after RLX treatment (0.57±0.03 ml/min per 100 g body weight after treatment versus 0.45±0.03 ml/min per 100 g body weight before treatment; P=0.001) (Figure 2D). This effect of RLX in 6MO animals was abolished by concomitant infusion of RLX+PD123319 (P=0.04) (Figure 2D). In the 6MO group, the change in urine flow from baseline was 190% greater in response to chronic RLX infusion as compared with vehicle treatment (P=0.05) (Figure 2F). Coinfusion of RLX+PD123319, however, did not alter the urine flow response as compared with RLX treatment alone (P=0.80) (Figure 2F). Administration of RLX and RLX+PD123319 to 6MO females did not significantly affect urinary protein excretion (Figure 2H).
Basal GFR was lower (P=0.004) (Figure 2C), whereas basal urine flow and proteinuria were greater in 15MO females as compared with their 6MO counterparts (both Page<0.001) (Figure 2, E and G, respectively). GFR did not change significantly in response to any treatment in 15MO SHRSPs (Figure 2D). Urine flows in 15MO animals were approximately 40% and approximately 33% greater in the RLX-treated group relative to the vehicle-treated (P=0.001) and RLX+PD123319–treated (P=0.03) groups, respectively (Figure 2F). No significant difference in protein excretion was detected between RLX or RLX+PD123319 in the 15MO groups (Figure 2H).
Vascular Function
Stimulation of the endothelium with ACh evoked concentration-dependent relaxation in the abdominal aorta of all groups (Figure 3, Table 1). In 6MO SHRSPs, relaxation to ACh was similar in vehicle- and RLX-treated groups (P=0.20) (Figure 3A, Table 1). However, the vasodilatory response to ACh was less in the RLX+PD123319 group compared with RLX treatment alone (64%±4% versus 91%±3%; P=0.003) (Figure 3A, Table 1). A significant age-induced vascular dysfunction was observed in female SHRSPs (Emax: 57%±8% in the 15MO vehicle group versus 78%±5% in the 6MO vehicle group; P=0.02) (Figure 3B, Table 1). RLX treatment promoted aortic relaxation in 15MO SHRSPs (Emax: 95%±3% versus 57%±8%; P<0.001) (Figure 3B, Table 1), but this was unchanged by RLX+PD123319 treatment (P=0.70) (Figure 3B, Table 1).
Figure 3.
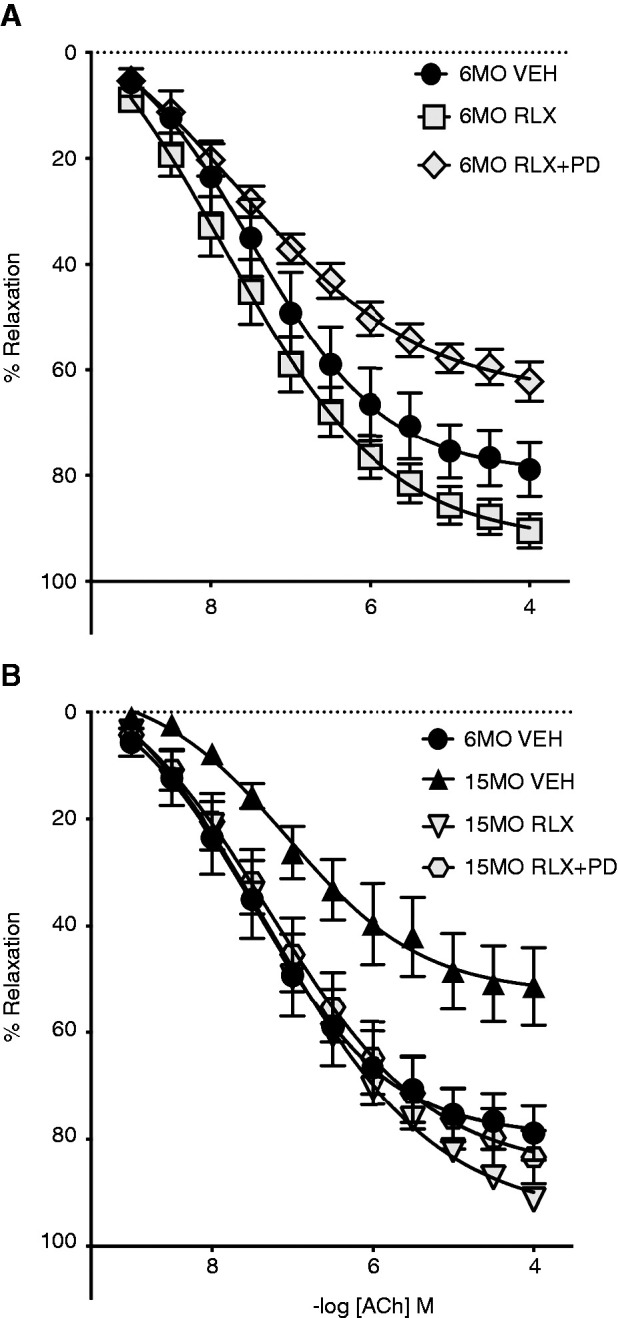
Vascular function. Vasorelaxation in response to acetylcholine (ACh) in isolated aortic rings was obtained from (A) 6MO and (B) 15MO female stroke-prone spontaneously hypertensive rats (n=6–10 per group) after 4 weeks of Veh (20 mM sodium acetate subcutaneously), RLX (0.5 mg/kg per day subcutaneously), or RLX+PD123319 (angiotensin type 2 receptor antagonist; 3 mg/kg per day subcutaneously) treatment. Data are presented as mean±SEM (n = 4–7 per group) and are expressed as a percentage of the preconstriction response to U46619 (Table 1). The same 6MO Veh data have been presented in (A) and (B) as comparators.
Table 1.
Aortic vascular function
| Treatment Group | pEC50 | Emax |
|---|---|---|
| 6MO SHR | ||
| Veh | 7.5±0.3 | 78±5 |
| RLX | 7.7±0.2 | 91±3 |
| RLX+PD | 7.6±0.3 | 64±4a |
| 15MO SHR | ||
| Veh | 6.9±0.4 | 57±8b |
| RLX | 7.2±0.2 | 95±3c |
| RLX + PD | 7.2±0.3 | 86±4 |
The molar concentration of agonist producing 50% of the maximum response presented as −10log EC50 (pEC50) and the maximal effect generated by the agonist (Emax) values for acetylcholine in isolated aortic arteries of 6-month-old (6MO) and 15-month-old (15MO) female stroke-prone spontaneously hypertensive rat (SHR) following 4 weeks of treatment with vehicle (Veh; 20 mM sodium acetate subcutaneously), relaxin ([RLX]; 0.5 mg/kg per day subcutaneously), or RLX+PD123319 (RLX+PD; angiotensin type 2 receptor antagonist; 3 mg/kg per day subcutaneously) are shown. Data are presented as mean±SEM (n=4–7 per group). Acetylcholine relaxation responses were analyzed using nonlinear regression to obtain pEC50 and Emax. Data were analyzed using an ANOVA with selected post hoc comparisons with Bonferroni post hoc tests. P=0.05 was considered statistically significant.
P=0.01 relative to 6MO RLX.
P=0.05 relative to 6MO Veh.
P<0.001 relative to 15MO Veh.
Organ Fibrosis
Renal interstitial fibrosis in the 6MO cohort was modest in the vehicle- and RLX-treated groups (0.3±0.1 and 0.4±0.1, respectively; P=0.50). In 6MO females, renal interstitial fibrosis was greater in the RLX+PD123319 group as compared with RLX treatment alone (0.7±0.1 versus 0.4±0.1; P=0.003) (Figure 4, A and B). Renal interstitial fibrosis (P<0.001), glomerulosclerosis (P<0.001), and glomerular hypertrophy (P=0.02) were greater in 15MO compared with 6MO females (Figure 4, Supplemental Figure 1). In 15MO females, renal interstitial fibrosis was significantly less in the RLX-treated group as compared with the vehicle-treated group (1.5±0.2 versus 2.1±0.2; P=0.02) (Figure 4, A and B). However, this effect was abolished by RLX+PD123319 treatment (2.1±0.1; P=0.009) (Figure 4, A and B).
Figure 4.
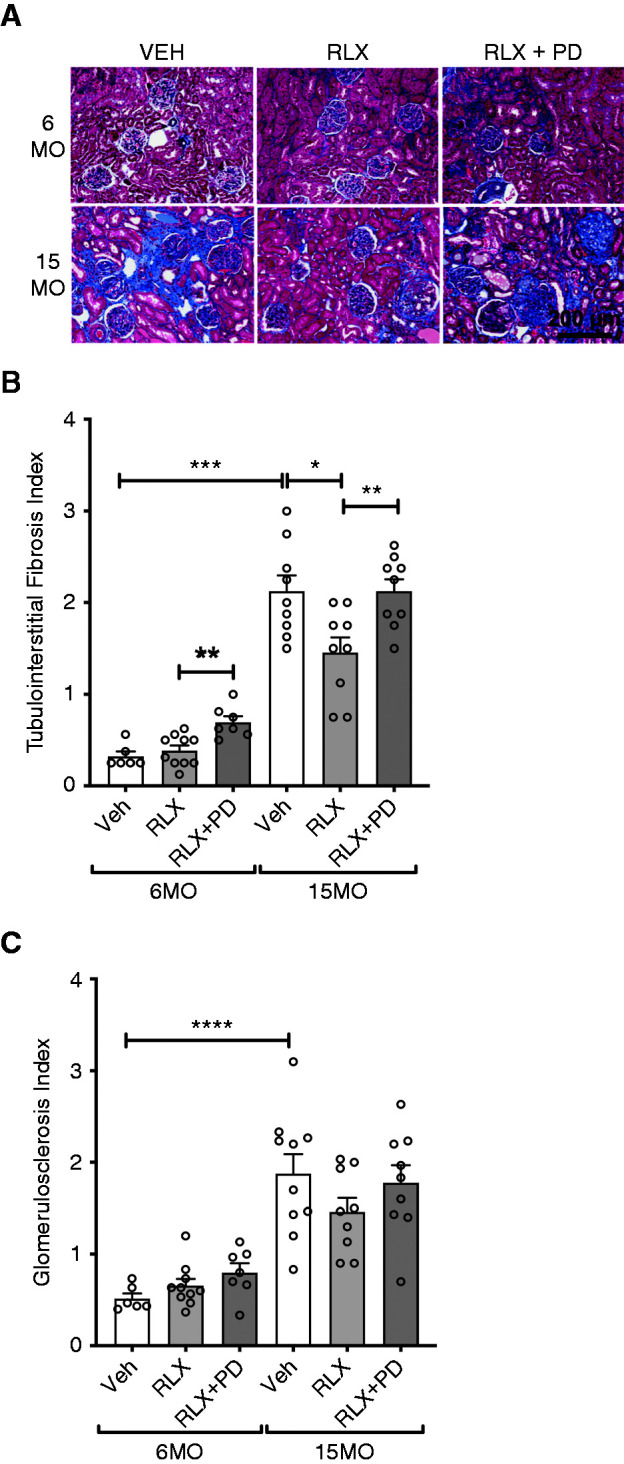
Renal fibrosis. Tubulointerstitial fibrosis and glomerulosclerosis index measured in 6MO and 15MO female stroke-prone spontaneously hypertensive rats (n=6–10 per group) after 4 weeks of Veh (20 mM sodium acetate subcutaneously), RLX (0.5 mg/kg per day subcutaneously), or RLX+PD123319 (angiotensin type 2 receptor antagonist; 3 mg/kg per day subcutaneously) treatment. (A) Representative images of the Masson trichrome staining of renal cortex in female stroke-prone spontaneously hypertensive rats demonstrate the extent of extracellular matrix (ECM) deposition (fibrosis; blue staining) in each of the groups studied. Scale bar: 200 µm. (B and C) Data are presented as mean±SEM (n=6–10 per group) and were analyzed using a Mann–Whitney test. *P=0.05; **P=0.01; ***P<0.001; ****P<0.001.
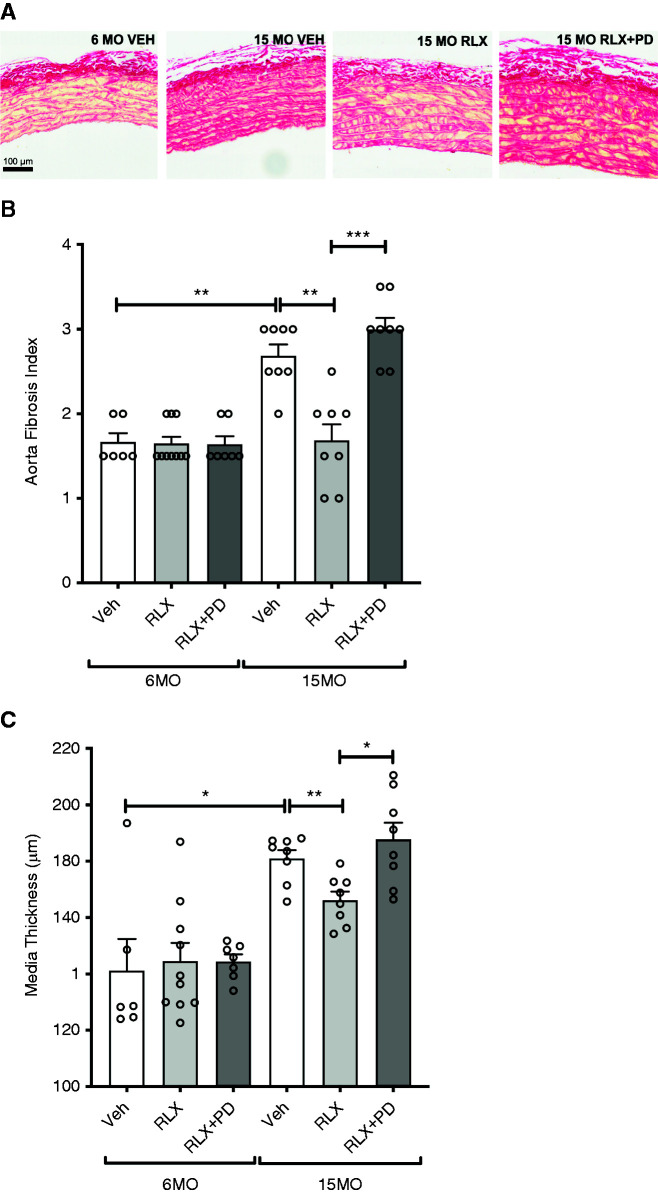
In the 6MO cohort, cardiac fibrosis was less following RLX treatment compared with vehicle-treated SHRSPs (1.8±0.1 versus 2.3±0.1; P=0.01) (Figure 5). RLX+PD123319 infusion did not significantly reverse the effect of RLX treatment alone (2.1±0.3; P=0.20), but there was no significant difference in cardiac fibrosis between vehicle- versus RLX+RD123319–treated SHRSPs (Figure 5). Chronic RLX infusion alone or in combination with PD123319 did not modulate aortic fibrosis or media thickness in 6MO females (Figure 6). Cardiac fibrosis (P=0.01) (Figure 5) and aortic fibrosis (P=0.002) (Figure 6, A and B) were greater in 15MO female SHRSPs as compared with their 6MO counterparts. The age-induced aortic fibrosis coincided with a greater media thickness in 15MO relative to 6MO females (P=0.04) (Figure 6, A and C). Neither RLX alone nor RLX+PD123319 treatment modified the level of cardiac fibrosis in vehicle-treated 15MO SHRSPs (Figure 5). However, aortic fibrosis (1.7±0.2 versus 2.7 ± 0.1; P = 0.003) and intima media thickness (166±3 versus 181±5 µm; P=0.007) were significantly attenuated in RLX-treated 15MO SHRSPs compared with vehicle (Figure 6). Coinfusion of RLX+PD123319 in 15MO animals blocked the RLX-mediated attenuation of both aortic fibrosis (P<0.001) and media thickness (P=0.01) (Figure 6).
Figure 5.
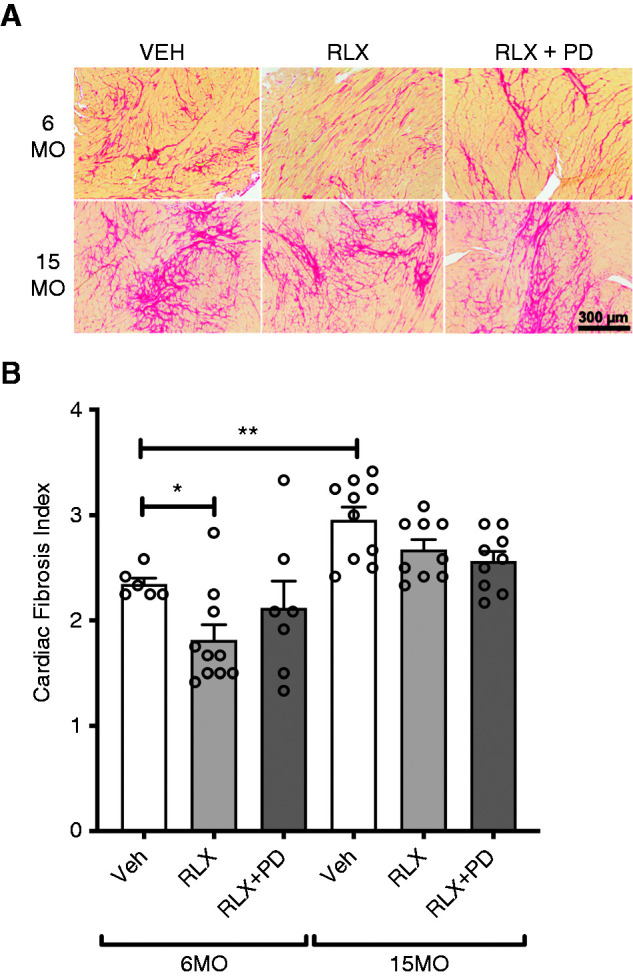
Cardiac fibrosis. Cardiac fibrosis measured in 6MO and 15MO female stroke-prone spontaneously hypertensive rats (n=6–10 per group) after 4 weeks of Veh (20 mM sodium acetate subcutaneously), RLX (0.5 mg/kg per day subcutaneously), or RLX+PD123319 (angiotensin type 2 receptor antagonist; 3 mg/kg per day subcutaneously) treatment. (A) Representative images of Picrosirius red staining show the extent of interstitial fibrosis within the left ventricle (LV) of each of the groups studied. Scale bar: 300 µm. (B) LV histopathology was expressed as a score on the basis of pathology severity as quantified from Picrosirius red staining: zero, normal; one, minimal; two, moderate; three, moderately severe; and four, severe. Data are presented as mean±SEM (n=6–10 per group) and were analyzed using a Mann–Whitney test. *P=0.05; **P=0.01.
Figure 6.
Aortic fibrosis and media thickness. Aortic fibrosis and media thickness of aorta measured in 6MO and 15MO female stroke-prone spontaneously hypertensive rats (n=6–10 per group) after 4 weeks of Veh (20 mM sodium acetate subcutaneously), RLX (0.5 mg/kg per day subcutaneously), or RLX+PD123319 (angiotensin type 2 receptor antagonist; 3 mg/kg per day subcutaneously) treatment. (A) Representative images of Picrosirius red staining of aorta sections in female stroke-prone spontaneously hypertensive rats show the extent of fibrosis in each of the groups studied. Scale bar: 100 µm. (B) Aortic histopathology was expressed as a score on the basis of pathology severity as quantified by Picrosirius red staining: zero, normal; one, minimal; two, moderate; three, moderately severe; and four, severe. (C) Media thickness (micrometers) of the aorta. Data in (B) and (C) are presented as mean±SEM (n=6–10 per group) and were analyzed using a Mann–Whitney test. *P=0.05; **P=0.01; ***P<0.001.
Renal Gene Expression
RXFP1, AT1aR, and AT2R gene expression was identified in the kidneys of 6MO and 15MO SHRSPs (Supplemental Figure 2). However, there was no significant effect of age or any treatment investigated on the gene expression of these receptors in the kidney.
Discussion
This study demonstrated that the antifibrotic and cardiorenal-protective effects of RLX in female SHRSPs are mediated to a large extent via the AT2R and that RLX treatment in aged reproductively senescent females reduced cardiorenal injury. Chronic RLX infusion improved GFR and reduced cardiac fibrosis in 6MO female SHRSPs. In 15MO female SHRSPs, RLX treatment reduced SBP and renal and aortic fibrosis and attenuated endothelial dysfunction. The RLX-mediated improvement of GFR and the reduction of SBP and renal fibrosis in female SHRSPs occurred via an AT2R-dependent mechanism because AT2R blockade prevented these effects. Importantly, our data suggested that endogenous RLX via the AT2R in female 6MO SHRSPs may be exerting protective actions because renal fibrosis was greater during AT2R blockade. Our data, therefore, provide evidence for a significant role of the AT2R in the antifibrotic, vasodilatory, and antihypertensive effects of RLX in adult and aged reproductively senescent females.
Organ fibrosis increased with age in female SHRSPs. The antifibrotic properties of RLX have been reported previously in preclinical models of aging and disease, albeit primarily in males (10,26). A role for RLX in organ fibrosis in females has not been widely studied and has not been consistently demonstrated. Recently, chronic RLX treatment of aged (24-month-old) female F-344/Brown Norway rats was found to decrease the expression of atrial natriuretic peptide and TGF-β1 (markers associated with cardiac hypertrophy and fibrosis) to levels measured in adult (9-month-old) females (27). Similarly, adenoviral treatment with RLX attenuated left ventricular fibrosis in adult (7-month-old) female β2-adrenoreceptor transgenic mice (a model of fibrotic cardiomyopathy) (28). However, in other studies, cardiac and renal fibrosis in RLX-knockout mice was age-dependently increased in males but not in females (29,30). This suggested that endogenous RLX alone did not protect against fibrosis in females, whereas it appeared to play a greater antifibrotic role in males. In this study, we showed that chronic RLX treatment attenuated cardiac fibrosis in 6MO female SHRSPs, whereas RLX treatment of 15MO female SHRSPs attenuated renal interstitial and aortic fibrosis. The temporal and organ-specific effects of RLX in this study may be related to the fact that cardiac fibrosis is more advanced than renal fibrosis in the SHRSPs, with cardiac fibrosis present by approximately 2–3 months of age (31) versus renal fibrosis present by approximately 6–7 months of age (32). Thus, an antifibrotic effect of RLX was apparent at an earlier time point in the heart but delayed in the kidney. The inability of RLX to significantly ameliorate cardiac fibrosis at 15 months of age is in line with evidence indicating that the potency and efficacy of RLX as an antifibrotic agent are limited in severe established organ fibrosis (10). Therefore, our findings suggest that exogenous RLX has antifibrotic effects in a severe, genetic model of essential hypertension in females that are both age and organ specific. Thus, further investigation of the potential of RLX as an antifibrotic agent in females is warranted.
The AT2R counter-regulates the prohypertensive and profibrotic actions of AT1R, with the contribution being greater in young adult females compared with age-matched males or aged reproductively senescent females (33). Our findings that the antifibrotic effects of RLX in the kidney and aorta of aged female SHRSPs were abrogated by concomitant treatment with PD123319 confirmed a role for AT2R in the fibrotic pathways. Moreover, the organ-specific effects of RLX on fibrosis may relate to differences in AT2R gene expression as the heart has been shown to have lower expression than the kidney and vasculature (34). RLX does not directly bind to AT2R (13). However, an RLX-RXFP1-AT2R interaction has been shown to drive a cascade of signaling transduction events that regulate and diminish ECM deposition to ameliorate renal fibrosis in males. This included the activation of extracellular signal–related kinase 1/2 phosphorylation and a neuronal nitric oxide synthase-nitric oxide-cyclic guanosine monophosphate–dependent pathway that resulted in the inhibition of the TGF-β/Smad2/Smad3–mediated differentiation and accumulation of renal myofibroblasts and the promotion of various collagen-degrading matrix metalloproteinase levels (12). Furthermore, in young adult female SHRSPs, RLX alone did not alter organ fibrosis or improve vascular function. AT2R appeared to be limiting renal interstitial fibrosis because after PD123319 treatment, renal fibrosis was greater. A similar pattern in 6MO females was seen in BP, and vascular function in that AT2R blockade increased SBP and reduced aortic ACh-induced relaxation. These data suggest that endogenous RLX in young females may be acting via AT2R to exert cardioprotective effects, but this needs to be confirmed under conditions of RXFP1 blockade. Therefore, it is possible that RLX mediates its antifibrotic and related cardiorenal protective effects via an interaction with AT2R, and this warrants further investigation.
In this study, beneficial effects of RLX on BP and vascular and renal function were also observed. RLX caused a modest reduction in SBP in aged females, an effect that was abolished by AT2R blockade. Previous reports on the effects of chronic RLX on arterial pressure are equivocal. In young adult or aged normotensive male and female rats, chronic RLX administration had no effect on mean arterial pressure due to a decrease in peripheral resistance that was balanced by increased cardiac output (35,36). However, in the context of chronic hypertension, chronic RLX infusion lowered mean arterial pressure, as reported in the 5/6th renal mass reduction model (37), spontaneously hypertensive rats (19), and rats chronically infused with angiotensin II (38). Clearly, the effect of RLX on arterial pressure is dependent on baseline levels of cardiac function and vascular tone. Furthermore, the reduction in SBP may reflect the duration of treatment in this study (4 weeks), which was longer than that used in most previous studies (2–3 weeks). Our findings, therefore, support the several lines of evidence demonstrating that RLX lowers arterial pressure in the setting of hypertension. The fall in SBP in the aged females in response to RLX infusion was prevented by AT2R blockade. This could be due to reduced tubular sodium reabsorption as AT2R has been shown to cause a leftward shift in the pressure-natriuresis-diuresis mechanism in male and female rats (39) and mice (7). However, we also demonstrated an age-related decline (approximately 30%) in vascular function in female SHRSPs, which was markedly reversed by chronic RLX treatment (approximately 45% increase in relaxation), independent of the AT2R. This agrees with previous studies showing that RLX treatment enhances vasodilation in animal and human blood vessels, which could also contribute to the fall in BP (26).
The antifibrotic effects of RLX in the kidney may also be linked to changes in renal hemodynamic and tubular function. Acute and chronic RLX infusion in nonpregnant, intact, or ovariectomized female rats and in male rats has been shown to cause vasodilation, increase GFR and urine flow, and reduce urinary protein excretion (40–44). In the kidney, RLX is a potent vasodilator dependent on a functional nitric oxide synthase-nitric oxide system (45). Mechanistically, RLX dilates both the afferent and efferent arterioles, causing a significant increase in renal blood flow and a marked increase in GFR and urine flow (40,43,44). In addition, RLX has direct effects on the renal tubules, possibly via an AT2R-bradykinin-nitric oxide-cyclic guanosine monophosphate mechanism (46,47), to inhibit sodium reabsorption (40), which may also contribute to an increase in urine flow. An increase in renal blood flow may drive a reduction in proteinuria through increased hydrostatic pressure and shear stress/nitric oxide production within the glomerular capillary (48). In line with these findings, it was demonstrated in this study that 4 weeks of RLX treatment in adult, but not aged, female SHRSPs improved GFR. In addition, chronic RLX administration enhanced urine flow in both adult and aged SHRSPs. However, these RLX-mediated changes in renal hemodynamics were not associated with a reduction in glomerulosclerosis or urinary protein excretion. Furthermore, we now understand that AT2R plays a greater role in the regulation of renal function in adult as compared with aged reproductively senescent females (33). Given that RLX improved GFR via an AT2R-mediated mechanism in adult but not aged females, this suggests that the AT2R may be associated with the renal vasodilator effects of RLX in adult hypertensive females and that the renal vasodilatory effects of RLX may be blunted by age. This may be attributed to a reduction in AT2R gene expression and/or a reduction in nitric oxide availability in the kidney. Although renal AT2R gene expression was not altered by age in our model, deficits in the downstream signaling pathways of the AT2R with age in the renal vasculature may directly attenuate the ability of RLX to promote its vasodilatory effects (49).
There were a number of strengths and weakness in this study. A major strength was the inclusion of aged animals as successful clinical translation of potential new therapies often fail because they are not tested in aged animals with established fibrosis. A limitation of this study was that a group receiving the AT2R antagonist alone was not included in the experimental design. However, previous studies have shown that PD123319 alone does not affect cardiovascular end points in female or male rats (34,50,51). Another limitation is that the renal, vascular, and antifibrotic effects of RLX cannot be dissociated from the fall in BP. Future studies should address whether RLX treatment in aged females directly attenuates fibrosis and improves both GFR and vasorelaxation or whether such changes are secondary to the BP-lowering effect of RLX. Specifically, it will be important to confirm the BP effects via radiotelemetry recording (particularly given that the fall in SBP was not reflected by a reduction in cardiac hypertrophy) and to examine the effect of blocking the RXFP1 receptor on the response to RLX treatment.
In conclusion, this study supports a role for RLX within the kidney, heart, and aorta to reduce fibrosis via the AT2R. Importantly, RLX replacement therapy in aged reproductively senescent females attenuated renal interstitial and vascular fibrosis. Ongoing studies are required to further investigate the mechanistic pathways involved in the RLX-mediated antifibrotic effects. This study highlights the potential of RLX as an emerging antifibrotic therapeutic for cardiovascular and kidney disease in postmenopausal women.
Disclosures
K.M. Denton reports scientific advisor or membership with American Journal of Physiology–Renal Physiology, Experimental Physiology, Frontiers of Science–Integrative Physiology, Hypertension, and Journal of Hypertension. C.S. Samuel reports scientific advisor or membership as a senior editor of Clinical and Experimental Pharmacology and Physiology, an associate editor of Frontiers in Pharmacology–Cardiovascular and Smooth Muscle Pharmacology, a subject editor of Nephrology, and a scientific advisory board member of Relaxera Pharmazeutische Gesellschaft GmbH & Co. KG, Bensheim, Germany. All remaining authors have nothing to disclose.
Funding
This research was supported by an Australian Government (Federal Government) research training program stipend scholarship (to G. Barsha); Department of Health, Australian Government, National Health & Medical Research Council project grant GNT1101552 (to K.M. Denton, C.S. Samuel, and R.E. Widdop); and Department of Health, Australian Government, National Health & Medical Research Council fellowships GNT1136813 (to K.M. Denton), GNT1112125 (to K.M. Mirabito Colafella), and GNT1041766 (to C.S. Samuel).
Acknowledgments
The authors acknowledge the technical assistance of the Monash Histology Platform, Department of Anatomy and Developmental Biology, Monash University.
Author Contributions
K.M. Denton, T.A. Gaspari, L.M. Hilliard Krause, C.S. Samuel, and R.E. Widdop conceptualized the study; G. Barsha, K.M. Denton, E. Kwok, and S.L. Walton were responsible for data curation; G. Barsha, K.M. Denton, T.A. Gaspari, L.M. Hilliard Krause, E. Kwok, A.A. Pinar, and S.L. Walton were responsible for investigation; G. Barsha, K.M. Denton, E. Kwok, K.M. Mirabito Colafella, and S.L. Walton were responsible for formal analysis; G. Barsha, K.M. Denton, E. Kwok, and S.L. Walton were responsible for methodology; K.M. Denton and S.L. Walton were responsible for project administration; K.M. Denton, K.M. Mirabito Colafella, C.S. Samuel, and S.L. Walton were responsible for validation; K.M. Denton and S.L. Walton were responsible for visualization; K.M. Denton and C.S. Samuel were responsible for funding acquisition; K.M. Denton, T.A. Gaspari, L M. Hilliard Krause, K.M. Mirabito Colafella, and S.L. Walton provided supervision; G. Barsha, E. Kwok, K.M. Mirabito Colafella, and S.L. Walton wrote the original draft; and G. Barsha, K.M. Denton, T.A. Gaspari, L.M. Hilliard Krause, E. Kwok, K.M. Mirabito Colafella, A.A. Pinar, C.S. Samuel, S.L. Walton, and R.E. Widdop reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0002722021/-/DCSupplemental.
Glomerular area. Download Supplemental Figure 1, PDF file, 173 KB (172.8KB, pdf)
Relative renal gene expression of RXFP1 and angiotensin receptors. Download Supplemental Figure 2, PDF file, 173 KB (172.8KB, pdf)
Effect of age and treatment on body and tissue weights recorded on day 28. Download Supplemental Table 1, PDF file, 173 KB (172.8KB, pdf)
References
- 1.Colafella KMM, Denton KM: Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 14: 185–201, 2018. 10.1038/nrneph.2017.189 [DOI] [PubMed] [Google Scholar]
- 2.Barsha G, Walton SL, Kwok E, Denton KM: Sex differences in the role of the angiotensin type 2 receptor in the regulation of blood pressure. In: Sex Differences in Cardiovascular Physiology and Pathophysiology, edited by LaMarca B, Alexander BT, London, Academic Press, 2019, pp 73–103 10.1016/B978-0-12-813197-8.00006-3 [DOI] [Google Scholar]
- 3.Anton L, Brosnihan KB: Systemic and uteroplacental renin—Angiotensin system in normal and pre-eclamptic pregnancies. Ther Adv Cardiovasc Dis 2: 349–362, 2008. 10.1177/1753944708094529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller JA, Anacta LA, Cattran DC: Impact of gender on the renal response to angiotensin II. Kidney Int 55: 278–285, 1999. 10.1046/j.1523-1755.1999.00260.x [DOI] [PubMed] [Google Scholar]
- 5.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW: Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol 17: 2554–2560, 2006. 10.1681/ASN.2005101095 [DOI] [PubMed] [Google Scholar]
- 6.Mirabito KM, Hilliard LM, Head GA, Widdop RE, Denton KM: Pressor responsiveness to angiotensin II in female mice is enhanced with age: Role of the angiotensin type 2 receptor. Biol Sex Differ 5: 13, 2014. 10.1186/s13293-014-0013-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirabito KM, Hilliard LM, Kett MM, Brown RD, Booth SC, Widdop RE, Moritz KM, Evans RG, Denton KM: Sex- and age-related differences in the chronic pressure-natriuresis relationship: Role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol 307: F901–F907, 2014. 10.1152/ajprenal.00288.2014 [DOI] [PubMed] [Google Scholar]
- 8.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D: A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004. 10.1016/j.cardiores.2004.01.020 [DOI] [PubMed] [Google Scholar]
- 9.Distler JHW, Györfi AH, Ramanujam M, Whitfield ML, Königshoff M, Lafyatis R: Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol 15: 705–730, 2019. 10.1038/s41584-019-0322-7 [DOI] [PubMed] [Google Scholar]
- 10.Samuel CS, Royce SG, Hewitson TD, Denton KM, Cooney TE, Bennett RG: Anti-fibrotic actions of relaxin. Br J Pharmacol 174: 962–976, 2017. 10.1111/bph.13529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen TY, Li X, Hung CH, Bahudhanapati H, Tan J, Kass DJ, Zhang Y: The relaxin family peptide receptor 1 (RXFP1): An emerging player in human health and disease. Mol Genet Genomic Med 8: e1194, 2020. 10.1002/mgg3.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Pinar AA, Widdop RE, Hossain MA, Bathgate RAD, Denton KM, Kemp-Harper BK, Samuel CS: The anti-fibrotic actions of relaxin are mediated through AT2R-associated protein phosphatases via RXFP1-AT2R functional crosstalk in human cardiac myofibroblasts. FASEB J 34: 8217–8233, 2020. 10.1096/fj.201902506R [DOI] [PubMed] [Google Scholar]
- 13.Chow BS, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RA, Hewitson TD, Samuel CS: Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 86: 75–85, 2014. 10.1038/ki.2013.518 [DOI] [PubMed] [Google Scholar]
- 14.Chow BSM, Kocan M, Shen M, Wang Y, Han L, Chew JY, Wang C, Bosnyak S, Mirabito-Colafella KM, Barsha G, Wigg B, Johnstone EKM, Hossain MA, Pfleger KDG, Denton KM, Widdop RE, Summers RJ, Bathgate RAD, Hewitson TD, Samuel CS: AT1R-AT2R-RXFP1 functional crosstalk in myofibroblasts: Impact on the therapeutic targeting of renal and cardiac fibrosis. J Am Soc Nephrol 30: 2191–2207, 2019. 10.1681/ASN.2019060597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bani D: Relaxin as a natural agent for vascular health. Vasc Health Risk Manag 4: 515–524, 2008. 10.2147/VHRM.S2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nabika T, Ohara H, Kato N, Isomura M: The stroke-prone spontaneously hypertensive rat: Still a useful model for post-GWAS genetic studies? Hypertens Res 35: 477–484, 2012. 10.1038/hr.2012.30 [DOI] [PubMed] [Google Scholar]
- 17.Churchill PC, Churchill MC, Griffin KA, Picken M, Webb RC, Kurtz TW, Bidani AK: Increased genetic susceptibility to renal damage in the stroke-prone spontaneously hypertensive rat. Kidney Int 61: 1794–1800, 2002. 10.1046/j.1523-1755.2002.00321.x [DOI] [PubMed] [Google Scholar]
- 18.Lekgabe ED, Kiriazis H, Zhao C, Xu Q, Moore XL, Su Y, Bathgate RA, Du XJ, Samuel CS: Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension 46: 412–418, 2005. 10.1161/01.HYP.0000171930.00697.2f [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Chakravorty A, Bathgate RA, Dart AM, Du XJ: Relaxin therapy reverses large artery remodeling and improves arterial compliance in senescent spontaneously hypertensive rats. Hypertension 55: 1260–1266, 2010. 10.1161/HYPERTENSIONAHA.109.149369 [DOI] [PubMed] [Google Scholar]
- 20.Widdop RE, Li XC: A simple versatile method for measuring tail cuff systolic blood pressure in conscious rats. Clin Sci (Lond) 93: 191–194, 1997. 10.1042/cs0930191 [DOI] [PubMed] [Google Scholar]
- 21.Hilliard LM, Denton KM: Transcutaneous assessment of glomerular filtration rate in unanesthetized rats using a small animal imager: Impact on arterial pressure, heart rate, and activity. Physiol Rep 4: e12723, 2016. 10.14814/phy2.12723 [DOI] [Google Scholar]
- 22.Khan SI, Shihata WA, Andrews KL, Lee MKS, Moore XL, Jefferis AM, Vinh A, Gaspari T, Dragoljevic D, Jennings GL, Murphy AJ, Chin-Dusting JPF: Effects of high- and low-dose aspirin on adaptive immunity and hypertension in the stroke-prone spontaneously hypertensive rat. FASEB J 33: 1510–1521, 2019. 10.1096/fj.201701498RR [DOI] [PubMed] [Google Scholar]
- 23.Tomat AL, Inserra F, Veiras L, Vallone MC, Balaszczuk AM, Costa MA, Arranz C: Moderate zinc restriction during fetal and postnatal growth of rats: Effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol 295: R543–R549, 2008. 10.1152/ajpregu.00050.2008 [DOI] [PubMed] [Google Scholar]
- 24.Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP: Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 147: 5126–5131, 2006. 10.1210/en.2006-0567 [DOI] [PubMed] [Google Scholar]
- 25.Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM: Relaxin has a role in establishing a renal response in pregnancy. Fertil Steril 86: 253–255, 2006. 10.1016/j.fertnstert.2005.11.070 [DOI] [PubMed] [Google Scholar]
- 26.Sarwar M, Du XJ, Dschietzig TB, Summers RJ: The actions of relaxin on the human cardiovascular system. Br J Pharmacol 174: 933–949, 2017. 10.1111/bph.13523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin B, Gabris-Weber BA, Reddy R, Romero G, Chattopadhyay A, Salama G: Relaxin reverses inflammatory and immune signals in aged hearts. PLoS One 13: e0190935, 2018. 10.1371/journal.pone.0190935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bathgate RA, Lekgabe ED, McGuane JT, Su Y, Pham T, Ferraro T, Layfield S, Hannan RD, Thomas WG, Samuel CS, Du XJ: Adenovirus-mediated delivery of relaxin reverses cardiac fibrosis. Mol Cell Endocrinol 280: 30–38, 2008. 10.1016/j.mce.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 29.Du XJ, Samuel CS, Gao XM, Zhao L, Parry LJ, Tregear GW: Increased myocardial collagen and ventricular diastolic dysfunction in relaxin deficient mice: A gender-specific phenotype. Cardiovasc Res 57: 395–404, 2003. 10.1016/S0008-6363(02)00663-6 [DOI] [PubMed] [Google Scholar]
- 30.Samuel CS, Zhao C, Bond CP, Hewitson TD, Amento EP, Summers RJ: Relaxin-1-deficient mice develop an age-related progression of renal fibrosis. Kidney Int 65: 2054–2064, 2004. 10.1111/j.1523-1755.2004.00628.x [DOI] [PubMed] [Google Scholar]
- 31.Jesmin S, Hattori Y, Togashi H, Ueno K, Yoshioka M, Sakuma I: Age-related changes in cardiac expression of VEGF and its angiogenic receptor KDR in stroke-prone spontaneously hypertensive rats. Mol Cell Biochem 272: 63–73, 2005. 10.1007/s11010-005-7635-3 [DOI] [PubMed] [Google Scholar]
- 32.Schreiber S, Bueche CZ, Garz C, Kropf S, Kuester D, Amann K, Heinze HJ, Goertler M, Reymann KG, Braun H: Kidney pathology precedes and predicts the pathological cascade of cerebrovascular lesions in stroke prone rats. PLoS One 6: e26287, 2011. 10.1371/journal.pone.0026287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colafella KM, Hilliard LM, Denton KM: Epochs in the depressor/pressor balance of the renin-angiotensin system. Clin Sci (Lond) 130: 761–771, 2016. 10.1042/CS20150939 [DOI] [PubMed] [Google Scholar]
- 34.Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM: Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 52: 666–671, 2008. 10.1161/HYPERTENSIONAHA.108.114058 [DOI] [PubMed] [Google Scholar]
- 35.Danielson LA, Welford A, Harris A: Relaxin improves renal function and histology in aging Munich Wistar rats. J Am Soc Nephrol 17: 1325–1333, 2006. 10.1681/ASN.2005121307 [DOI] [PubMed] [Google Scholar]
- 36.Debrah DO, Conrad KP, Jeyabalan A, Danielson LA, Shroff SG: Relaxin increases cardiac output and reduces systemic arterial load in hypertensive rats. Hypertension 46: 745–750, 2005. 10.1161/01.HYP.0000184230.52059.33 [DOI] [PubMed] [Google Scholar]
- 37.Garber SL, Mirochnik Y, Brecklin C, Slobodskoy L, Arruda JA, Dunea G: Effect of relaxin in two models of renal mass reduction. Am J Nephrol 23: 8–12, 2003. 10.1159/000066302 [DOI] [PubMed] [Google Scholar]
- 38.Sasser JM, Molnar M, Baylis C: Relaxin ameliorates hypertension and increases nitric oxide metabolite excretion in angiotensin II but not N(ω)-nitro-L-arginine methyl ester hypertensive rats. Hypertension 58: 197–204, 2011. 10.1161/HYPERTENSIONAHA.110.164392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, Evans RG, Denton KM: Gender differences in pressure-natriuresis and renal autoregulation: Role of the Angiotensin type 2 receptor. Hypertension 57: 275–282, 2011. 10.1161/HYPERTENSIONAHA.110.166827 [DOI] [PubMed] [Google Scholar]
- 40.Bogzil AH, Eardley R, Ashton N: Relaxin-induced changes in renal sodium excretion in the anesthetized male rat. Am J Physiol Regul Integr Comp Physiol 288: R322–R328, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Conrad KP: Renal hemodynamics during pregnancy in chronically catheterized, conscious rats. Kidney Int 26: 24–29, 1984. 10.1038/ki.1984.129 [DOI] [PubMed] [Google Scholar]
- 42.Danielson LA, Sherwood OD, Conrad KP: Relaxin is a potent renal vasodilator in conscious rats. J Clin Invest 103: 525–533, 1999. 10.1172/JCI5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng A, Conrad K, Baylis C: Relaxin-mediated renal vasodilation in the rat is associated with falls in glomerular blood pressure. Am J Physiol Regul Integr Comp Physiol 314: R147–R152, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao W, Rosales CB, Gonzalez C, Prieto MC, Navar LG: Effects of serelaxin on renal microcirculation in rats under control and high-angiotensin environments. Am J Physiol Renal Physiol 314: F70–F80, 2018. 10.1152/ajprenal.00201.2017 [DOI] [PubMed] [Google Scholar]
- 45.Jelinic M, Marshall SA, Leo CH, Parry LJ, Tare M: From pregnancy to cardiovascular disease: Lessons from relaxin-deficient animals to understand relaxin actions in the vascular system. Microcirculation 26: e12464, 2019. 10.1111/micc.12464 [DOI] [PubMed] [Google Scholar]
- 46.Hilliard LM, Jones ES, Steckelings UM, Unger T, Widdop RE, Denton KM: Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: A novel therapeutic target for hypertension. Hypertension 59: 409–414, 2012. 10.1161/HYPERTENSIONAHA.111.184986 [DOI] [PubMed] [Google Scholar]
- 47.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski MC, Roques BP, Carey RM: Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension 51: 460–465, 2008. 10.1161/HYPERTENSIONAHA.107.103242 [DOI] [PubMed] [Google Scholar]
- 48.Baylis C, Brenner BM: The physiologic determinants of glomerular ultrafiltration. Rev Physiol Biochem Pharmacol 80: 1–46, 1978 [DOI] [PubMed] [Google Scholar]
- 49.van Drongelen J, Ploemen IH, Pertijs J, Gooi JH, Sweep FC, Lotgering FK, Spaanderman ME, Smits P: Aging attenuates the vasodilator response to relaxin. Am J Physiol Heart Circ Physiol 300: H1609–H1615, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Safari T, Nematbakhsh M, Evans RG, Denton KM: High-dose estradiol-replacement therapy enhances the renal vascular response to angiotensin II VIA AN AT2-receptor dependent mechanism. Adv Pharmacol Sci 2015: 682745, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hilliard LM, Chow CL, Mirabito KM, Steckelings UM, Unger T, Widdop RE, Denton KM: Angiotensin type 2 receptor stimulation increases renal function in female, but not male, spontaneously hypertensive rats. Hypertension 64: 378–383, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glomerular area. Download Supplemental Figure 1, PDF file, 173 KB (172.8KB, pdf)
Relative renal gene expression of RXFP1 and angiotensin receptors. Download Supplemental Figure 2, PDF file, 173 KB (172.8KB, pdf)
Effect of age and treatment on body and tissue weights recorded on day 28. Download Supplemental Table 1, PDF file, 173 KB (172.8KB, pdf)