There is strong evidence to support the efficacy of alkylating agents in the treatment of patients with membranous nephropathy. Randomized controlled trials that included patients with membranous nephropathy and nephrotic syndrome reported remission rates of 77%–93% (1), which translated in improved kidney survival in studies with long follow-up (2,3). In one study, kidney survival at 10 years was 92% in patients treated with alkylating agents and 60% in controls (2). Of note, the 60% kidney survival in the control group suggested that the treatment strategy (i.e., treating all patients with membranous nephropathy and nephrotic syndrome) exposed many patients unnecessarily to the risk of immunosuppressive therapy. Indeed, in this control group, the cumulative rate of complete and partial remission was 47%, and a third of patients were in remission at last follow-up. Subsequent studies showed that treatment can be restricted to patients at high risk of disease progression (reviewed in van de Logt et al. [1]). The largest study included 254 patients with membranous nephropathy and nephrotic syndrome. After a median (interquartile range) follow-up of 57 (32–90) months, only 124 patients (49%) had received immunosuppressive therapy. Renal survival was 86% after 10 years, and remission of proteinuria developed in 83% of patients (4). Obviously, treatment that prevents development of kidney failure and obviates the need for RRT is of great benefit, both for the individual patient and for society. On the basis of the evidence (and the calculated cost efficacy), national and international guidelines recommended treatment with cyclophosphamide and steroids for patients with membranous nephropathy, nephrotic syndrome, and high risk for disease progression (5).
Many physicians and patients are reluctant to use cyclophosphamide and steroids, because of the associated toxicity. Indeed, the use of cyclophosphamide is associated with many short-term side effects, such as infections, anemia, leukocytopenia, thrombocytopenia, and liver dysfunction. Still, most can be easily managed with dose reduction, temporary withdrawal, and/or administration of antibiotics. The most feared side effects are infertility (maximal cumulative dose, 10 g/d) and malignancy (maximal cumulative dose, 36 g).
Therefore, the recent introduction of less toxic immunosuppressive therapies has created great interest. Calcineurin inhibitors were proposed as an alternative, on the basis of the high remission rates reported in the initial studies (1). Meanwhile, enthusiasm for these agents has waned. More recently, rituximab was proposed as first-line therapy, again, primarily on the basis of short-term studies suggesting high proteinuria remission rates, and the notion that the toxicity of rituximab is low. However, rituximab has not been compared with cyclophosphamide in a randomized control trial; there is, as yet, no evidence that treatment with rituximab improves kidney survival in patients with membranous nephropathy, and there are no data to prove that rituximab can be used in a restrictive treatment strategy. In the current era of evidence-based medicine, these limitations should be discussed with our patients.
A detailed analysis of the studies that are used to claim success of rituximab points to many caveats, as listed below:
-
(1)
There are no data on long-term follow-up, or on hard renal end points. Rituximab is advocated on the basis of the remission rates. However, remission of proteinuria may not be the best predictor of kidney outcome. In this respect, it is important to bear in mind the initial “apparent” success of calcineurin inhibitors. Many studies reported high remission rates, ranging from 75%–88% (6–8). However, during follow-up, most patients had relapsed. In a study that reported 5-year follow-up data, 10% of patients had died, and 30% of patients had reached a kidney failure end point (defined as doubling of serum creatinine) (9). In multivariable analysis, renal function deterioration was associated with multiple relapses.
-
(2)
Efficacy of rituximab is lower than suggested. For more than a decade, the efficacy of rituximab was largely supported by data derived from a large Italian cohort that included 132 patients with membranous nephropathy and nephrotic proteinuria. A detailed analysis, after a follow-up of 30.8 (interquartile range, 6.0–145.4) mon ths, showed that the nonresponder rate was 36%. Moreover, the relapse rate was approximately 30% (10). Because these were observational data, and we do not know the spontaneous remission rate, we cannot estimate the real benefit of rituximab. The Evaluate Rituximab Treatment for Idiopathic Membranous Nephropathy (GEMRITUX) study, a randomized controlled trial from France, provided better evidence (11). This study proved that rituximab was more effective than no treatment in inducing remission. At the end of follow-up (median of 23 months), the remission rate was 66% with rituximab and 45% with conservative therapy. Thus, this study confirmed the 35% failure rate. Moreover, because 45% of patients developed spontaneous remission, the attributable efficacy rate is only 38% ([66%–45%]/[100%–45%]). There has been a debate that the relatively low efficacy of rituximab might be related to the use of a low dose of rituximab (in the Italian cohort study, most patients received only one dose of 375 mg/m2 rituximab; in the GEMRITUX trial, patients received two doses of 375 mg/m2 rituximab). Indeed, a French study suggested that two doses of 1 g of rituximab were more effective (12). The recent randomized controlled Membranous Nephropathy Trial of Rituximab (MENTOR) study used a high dose of rituximab (cumulative dose, 4 g) (13). Unfortunately, the MENTOR study had a short follow-up (last observation was only 18 months after rituximab administration), and does not provide proof for efficacy on hard renal end points. Moreover, efficacy was also limited in this study: 22% of patients did not respond initially and were excluded from the study. A total of 39 of 65 patients (60%) were in partial or complete remission at the last follow-up (24 months). Stated otherwise, at 24 months, 60% of patients treated with rituximab were in partial or complete remission, as compared with 20% in the control group, which is an attributable efficacy rate of 50% ([60%– 20%]/[100%– 20%]).
-
(3)
The efficacy of rituximab in patients with kidney insufficiency is unproven. Thus, there are no data to support its use in patients with reduced eGFR. A small, retrospective study evaluated the association between baseline characteristics and proteinuria response at 3 months after rituximab treatment (14). The study included only 14 patients: six nonresponders and eight responders (defined as a proteinuria reduction of >40%). A finding of interstitial fibrosis and tubular atrophy (on review of a biopsy specimen) was a determinant of response: the tubulointerstitial score was <1.7 in responders and >1.7 in nonresponders. Of note, not unexpectedly, renal function was also different, with a serum creatinine level of 2.1 (SD, 1.0) mg/dl in nonresponders and 1.3 (SD, 0.4) mg/dl in responders. Thus, this small study suggests that rituximab is of limited benefit in patients with severe kidney dysfunction.
-
(4)
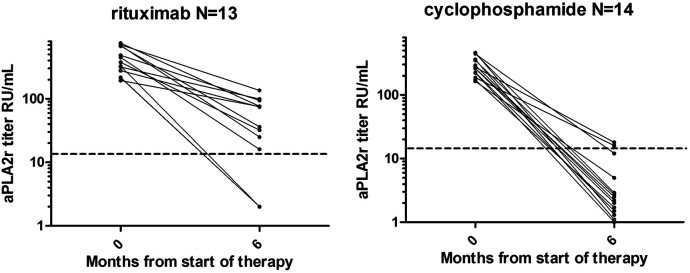
Although there are no randomized controlled trials that compare rituximab with cyclophosphamide and steroids, there are data to suggest that rituximab is inferior to cyclophosphamide and steroids. Van den Brand et al. (15) compared two European cohorts that were treated with either rituximab or cyclophosphamide and steroids. The partial remission rate was lower in the rituximab-treated cohort (adjusted hazard ratio, 0.63; 95% CI, 0.45 to 0.89). This observation was supported by the finding that rituximab was also less effective in inducing an immunologic remission than cyclophosphamide, especially in patients with high anti–phospholipase A2 receptor antibody (aPLA2R1ab) levels (16). Figure 1 illustrates the observations in patients in the highest tertile of aPLA2R1ab levels (150–776 RU/ml). aPLA2R1ab levels decreased to levels <14 RU/ml (the cutoff to define positive and negative) in 86% (12 of 14) of patients treated with cyclophosphamide, and in 23% (three of 13) of patients treated with rituximab. Interestingly, in a recent study using a cutoff of 150 RU/ml, aPLA2R1ab levels identified patients who were high risk with a specificity of 80% (17).
Figure 1.
Observations in patients in the highest tertile of aPLA2R1ab levels (150–776 RU/ml). aPLA2R1ab levels decreased to levels <14 RU/ml (the cutoff to define positive and negative) in 23% (three of 13) of patients treated with rituximab, and in 86% (12 of 14) of patients treated with cyclophosphamide. aPLA2R1ab, anti–phospholipase A2 receptor antibody.
Conclusion
There is sufficient evidence to support the notion that rituximab is not preferable to cyclophosphamide for treatment of patients with membranous nephropathy and a high risk of progressive disease. On the basis of the available data, we suggest there is a window of opportunity for rituximab in patients with membranous nephropathy, normal eGFR, nephrotic syndrome, and aPLA2R1ab levels <150 RU/ml. When discussing therapy with the individual patient, it is important to provide a balanced view, which includes a discussion of benefits and risks. The risks associated with cyclophosphamide-based therapy must include the risks associated with the concomitant use of corticosteroids. Patient characteristics such as age, obesity, glucose intolerance, and predicted rate of eGFR loss (and thus risk of ESKD) will shift the balance.
Disclosures
J. Wetzels reports receiving research funding from Alexion and Chemocentryx; serving as a member of the editorial board of Kidney International; serving on a speakers bureau for Novartis; having consultancy agreements with Novartis and Travere; receiving honoraria from Travere and UpToDate; and having other interests in/relationships with a patient advisory organization in The Netherlands. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
A.-E. van de Logt wrote the original draft and was responsible for data curation; and J. Wetzels conceptualized the study and reviewed and edited the manuscript.
Footnotes
References
- 1.van de Logt AE, Hofstra JM, Wetzels JF: Pharmacological treatment of primary membranous nephropathy in 2016. Expert Rev Clin Pharmacol 9: 1463–1478, 2016. 10.1080/17512433.2016.1225497 [DOI] [PubMed] [Google Scholar]
- 2.Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, Sasdelli M, Redaelli B, Grassi C, Pozzi C, Bizzarri D, Banfi G: A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604, 1995. 10.1038/ki.1995.453 [DOI] [PubMed] [Google Scholar]
- 3.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007. 10.1681/ASN.2007020166 [DOI] [PubMed] [Google Scholar]
- 4.van den Brand JA, van Dijk PR, Hofstra JM, Wetzels JF: Long-term outcomes in idiopathic membranous nephropathy using a restrictive treatment strategy. J Am Soc Nephrol 25: 150–158, 2014. 10.1681/ASN.2013020185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease Improving Global Outcomes (KDIGO) : KDIGO clinical practice guideline for glomerulonephritis. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-English.pdf. Accessed January 2, 2020 [Google Scholar]
- 6.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL; North America Nephrotic Syndrome Study Group : Cyclosporine in patients with steroid-resistant membranous nephropathy: A randomized trial. Kidney Int 59: 1484–1490, 2001. 10.1046/j.1523-1755.2001.0590041484.x [DOI] [PubMed] [Google Scholar]
- 7.Goumenos DS, Kalliakmani P, Tsakas S, Sotsiou F, Vlachojannis JG: The remission of nephrotic syndrome with cyclosporin treatment does not attenuate the progression of idiopathic membranous nephropathy. Clin Nephrol 61: 17–24, 2004. 10.5414/CNP61017 [DOI] [PubMed] [Google Scholar]
- 8.Praga M, Barrio V, Juárez GF, Luño J; Grupo Español de Estudio de la Nefropatía Membranosa: Tacrolimus monotherapy in membranous nephropathy: A randomized controlled trial. Kidney Int 71: 924–930, 2007. 10.1038/sj.ki.5002215 [DOI] [PubMed] [Google Scholar]
- 9.Kalliakmani P, Koutroulia E, Sotsiou F, Vlachojannis JG, Goumenos DS: Benefit and cost from the long-term use of cyclosporine-A in idiopathic membranous nephropathy. Nephrology (Carlton) 15: 762–767, 2010. 10.1111/j.1440-1797.2010.01301.x [DOI] [PubMed] [Google Scholar]
- 10.Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pellé T, Gaspari F, Suardi F, Gagliardini E, Orisio S, Benigni A, Ronco P, Remuzzi G: Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 26: 2545–2558, 2015. 10.1681/ASN.2014070640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, Michel PA, Mihout F, Dussol B, Matignon M, Mousson C, Simon T, Ronco P; GEMRITUX Study Group: Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J Am Soc Nephrol 28: 348–358, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seitz-Polski B, Dahan K, Debiec H, Rousseau A, Andreani M, Zaghrini C, Ticchioni M, Rosenthal A, Benzaken S, Bernard G, Lambeau G, Ronco P, Esnault VLM: High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol 14: 1173–1182, 2019. 10.2215/CJN.11791018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Jüni P, Cattran DC; MENTOR Investigators : Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 381: 36–46, 2019. 10.1056/NEJMoa1814427 [DOI] [PubMed] [Google Scholar]
- 14.Ruggenenti P, Chiurchiu C, Abbate M, Perna A, Cravedi P, Bontempelli M, Remuzzi G: Rituximab for idiopathic membranous nephropathy: Who can benefit? Clin J Am Soc Nephrol 1: 738–748, 2006. 10.2215/CJN.01080905 [DOI] [PubMed] [Google Scholar]
- 15.van den Brand JAJG, Ruggenenti P, Chianca A, Hofstra JM, Perna A, Ruggiero B, Wetzels JFM, Remuzzi G: Safety of rituximab compared with steroids and cyclophosphamide for idiopathic membranous nephropathy. J Am Soc Nephrol 28: 2729–2737, 2017. 10.1681/ASN.2016091022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Logt AE, Dahan K, Rousseau A, van der Molen R, Debiec H, Ronco P, Wetzels J: Immunological remission in PLA2R-antibody-associated membranous nephropathy: Cyclophosphamide versus rituximab. Kidney Int 93: 1016–1017, 2018. 10.1016/j.kint.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 17.van de Logt AE, Justino J, Vink C, van den Brand J, Debiec H, Lambeau G, Wetzels JF,: Anti-PLA2R1 antibodies as prognostic biomarker in membranous nephropathy [published online ahead of print April 21, 2021]. Kidney Int Rep 10.1016/j.ekir.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]