Abstract
AKI remains highly prevalent, yet no optimal therapy is available to prevent it or promote recovery after initial insult. Experimental studies have demonstrated that both innate and adaptive immune responses play a central role during AKI. In response to injury, myeloid cells are first recruited and activated on the basis of specific signals from the damaged microenvironment. The subsequent recruitment and activation state of the immune cells depends on the stage of injury and recovery, reflecting a dynamic and diverse spectrum of immunophenotypes. In this review, we highlight our current understanding of the mechanisms by which myeloid cells contribute to injury, repair, and fibrosis after AKI.
Keywords: acute kidney injury and ICU nephrology, AKI, basic science, CKD, dendritic cell, fibrosis, kidney repair, macrophage, myeloid cell, neutrophil
Introduction
Kidneys maintain fluid, electrolyte and acid-base balance, excrete waste products, and regulate BP with high metabolic activity, which renders them susceptible to injury from aseptic insults to asepsis. These insults cause AKI characterized by a rapid decline in renal function. AKI is common in patients who are hospitalized, especially those in the intensive care unit. Patients who survive AKI are at an 8.8-fold increased risk for developing CKD and a 3.3-fold increased risk for ESKD (1–3). However, optimal therapy has not yet been developed to prevent such injury or promote recovery after AKI. Understanding the underlying mechanism(s) of AKI will help identify novel targets and develop therapeutic strategies for AKI to mitigate the burden of this disease.
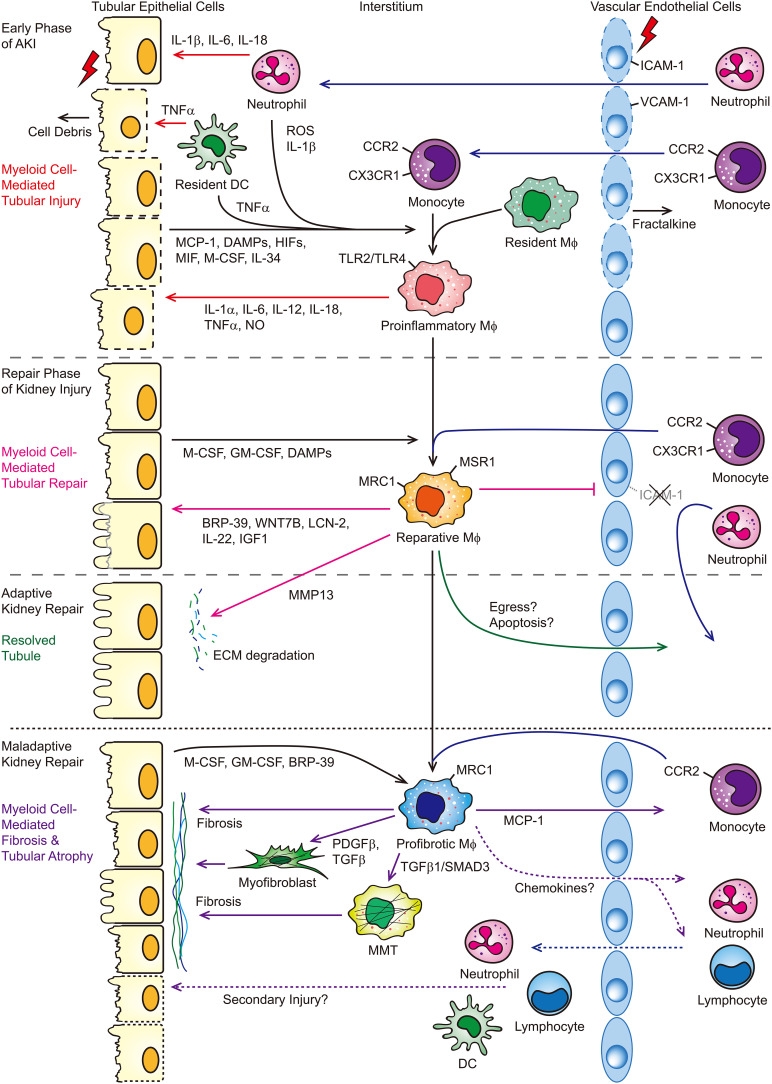
The pathophysiology of AKI ranges from diminished renal perfusion with no structural damage to intrinsic kidney disease, including vascular endothelial injury, GN, acute interstitial nephritis, acute tubular injury, and obstruction of urinary outflow with associated apoptosis of tubular cells. Recently, the advanced technology of single-cell RNA sequencing (scRNA-seq) or single-nucleus RNA-seq allows researchers to unbiasedly map the cellular complexity of the kidneys, in both human and mice, and to study the mechanism(s) of AKI at the single-cell level. For instance, unsupervised clustering of scRNA-seq data from the allograft biopsy specimen (histologically read as mixed rejection) reveals most native kidney cell types and all of the major immune cells, including monocytes, mast cells, T cells, B cells, and plasma cells (4). Consistently, the data from mouse models of AKI (i.e., ischemia-reperfusion injury [IRI], reversible unilateral ureter obstruction) show most cell types that comprise the kidney with a multitude of resident and infiltrating immune cells, including monocytes/macrophages, neutrophils, dendritic cells (DCs), T cells, B cells, and natural killer cells (5–8). The clinical and experimental evidence suggest that both myeloid and lymphoid cells are involved in immune-mediating damage to renal tubular cells and in recovery from AKI (9–12). This review will focus on the role(s) of myeloid cells in the course of AKI and kidney repair (briefly summarized in Figure 1).
Figure 1.
Myeloid cells mediate tubular injury and repair following AKI. In response to AKI, endothelial cells express fractalkine (CX3CL1) and ICAM-1 and VCAM-1 to recruit neutrophils and monocytes into the injured kidney and promote leukocyte-endothelial cell adhesion, respectively. IL-1β, IL-6, IL-18, and TNF-α released from neutrophils/dendritic cells induce apoptosis and/or necrosis of tubular epithelial cells. Proinflammatory cytokines, ROS, HIFs, and DAMPs released by degranulating PMNs and injured or dying tubular cells lead to the proinflammatory activation of macrophages. These proinflammatory macrophages then subsequently produce proinflammatory cytokines and NO, which lead to further tubular injury. The inflammatory milieu in the early phase is later reversed during the repair phase. Downregulation of ICAM-1 and VCAM-1 limits inflammatory cell infiltration. Macrophage growth and differentiation factors M-CSF and GM-CSF and DAMPs released from the tubular epithelial cells lead to the reparative activation of macrophages. BRP-39, WNT7B, LCN2, IL-22, and IGF1 secreted from reparative macrophages promote tubular cell proliferation and/or repair. In the setting of adaptive kidney repair, macrophages release MMP13 to facilitate degradation of ECM, which is initially deposited after injury to aid repair, and macrophage egress and/or apoptosis after the injury is resolved. However, in the setting of maladaptive kidney repair, macrophages persist within the kidney. Macrophage growth factors, M-CSF, GM-CSF, and BRP-39 released from sustained injured tubular epithelial cells promote further recruitment and/or retention of macrophages and polarize macrophages into a profibrotic phenotype. Profibrotic macrophages or MMTs can promote kidney fibrosis directly and indirectly by activating interstitial myofibroblasts and contribute to the secondary tubular injury. BRP-39, breast regression protein 39; DAMP, damage-associated molecular pattern; DC, dendritic cell; ECM, extracellular matrix; HIFs, hypoxia-inducible factors; ICAM-1, intracellular adhesion molecule 1; LCN-2, lipocalin-2; Mϕ, macrophage; MCP-1, monocyte chemoattractant protein-1; M-CSF, macrophage colony-stimulating factor; MIF, macrophage migration inhibitory factor; MMP13, matrix metallopeptidase 13; MMT, macrophage-to-myofibroblast transition; MRC1, mannose receptor 1; MSR1, macrophage scavenger receptor 1; NO, nitric oxide; PDGFβ, platelet-derived growth factor subunit B; ROS, reactive oxygen species; SMAD3, mothers against decapentaplegic homolog 3; TLR, Toll-like receptor; VCAM-1, vascular cell adhesion molecule-1, WNT7B, Wnt family member 7B.
The Origins and Plasticity of Myeloid Cells in the Kidney
One of the hallmarks of most immune cells is their continuous replenishment from precursors that are ultimately derived from bone marrow–derived hematopoietic stem cells (HSCs) (13). In kidney, all myeloid cells, including granulocytes (neutrophils, eosinophils, basophils, and mast cells [in a small number]), monocytes, and DCs are derived from HSCs, except tissue-resident macrophages (Table 1). Studies also show that kidney-resident mononuclear phagocytes are derived from the yolk sac at the embryogenesis stages. The kidney macrophages are originated from HSCs and replaced continually from DC precursors (21–23).
Table 1.
The markers and origin(s) of myeloid cells in kidney
| Myeloid Cell Type | Marker(s) | Origin | Reference |
|---|---|---|---|
| Neutrophil | CD45+, CD16+, CD66b+ (homo), CD45+, Ly-6G/ Gr-1+, CD11b+ (mus) | HSCs | (14) |
| Eosinophil | CCR3+, CD125+, CD49d+, Siglec-8+ (homo), Siglec-F (mus) | HSCs | (15) |
| Basophil | FcεRIα+, CD49b+, CD203c+, Thy1+, c-Kit-, FcγR+, Siglec-2+ (homo) | HSCs | (16) |
| Mast cell | CD11b−, Lin−, c-Kithigh, MITF+, CD33+ (homo), FcεRIα+, IL-3Rα+, CD203c+ | HSCs | (17) |
| Monocyte/macrophage | CD11b+, CSF1R+, Ly-6C+, Ly-6G−, F4/80+ (mus), EMR1+ (homo), CX3CR1+, CCR2+ | HSCs | (18) |
| Resident macrophage | CD45+, CD11blow, F4/80high, Ly-6C− (yolk sac EMP-derived resident macrophage) | Yolk sac EMPs, fetal liver EMPs, and HSCs | (19) |
| Dendritic cell | CD11c+, MHC-II+, CD103+, CD11blow, CX3CR1−, F4/80−, SIRP-α− (CD103+ renal DC), CD11b+, CD103−, CX3CR1+, F4/80+, SIRP-α+ (CD11b+ DC) | HSCs | (18,20) |
homo, Homo sapiens; mus, Mus musculus; HSCs, hematopoietic stem cells; EMP, erythro-myeloid progenitor; DC, dendritic cell.
Regardless of the initial cause of injury, sepsis or kidney damage via aseptic insult, the innate immune system is first activated not only by the resident immune cells but also by a rapid influx of myeloid cells from systemic immune factors during AKI. Injury leads to oxidative stress, endoplasmic reticulum stress, and mitochondrial dysfunction in both endothelial and tubular epithelial cells, in which c-Jun N-terminal kinase, caspase cascade, and receptor-interacting protein kinase 1 pathways are activated to induce apoptosis (predominantly) and necrosis, and downstream proinflammatory cytokines are also activated to promote the inflammatory milieu (24,25). Injured or necrotic tubular cells, in turn, release damage-associated molecular patterns (DAMPs) and hypoxia-inducible factors, which are responsible for recruiting the circulating immune cells to the injured kidney. Both resident macrophages and DCs ensure inflammation by releasing proinflammatory cytokines, such as TNF-α, which is amplified by infiltrated myeloid cells through chemokines and cytokines (26,27).
Because of their heterogeneity and inherent plasticity, monocytes/macrophages may adopt different phenotypes in response to the microenvironmental stimuli, such as injury, cell debris, DAMPs, and extracellular matrix (ECM). For instance, using fate mapping, four subsets of mononuclear phagocytes (macrophages and DCs) found in the kidneys from adult mice are phenotypically, functionally, and transcriptionally distinct from each other (28). These mononuclear phagocytes exhibit unique age-dependent developmental heterogeneity. A recent scRNA-seq analysis identifies 13 subtypes of myeloid cells, and the pseudotime analysis reveals a dynamic change in monocyte and macrophage phenotypes during AKI progression after ureter obstruction and AKI regression after reversible ureter obstruction (7).
Myeloid Cell–Mediated Inflammation during Initial Kidney Injury
In the obstructive kidney or ischemic kidney after reperfusion, neutrophils are first recruited followed by expansion of macrophages and T cells, which persist beyond the reversal of obstruction or in the repair phase after IRI. In response to ischemic injury, endothelial cells increase expression of intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1, which are important determinants of leukocyte-endothelial cell adhesion (29,30). The infiltrated neutrophils release reactive oxygen species (ROS), cytokines, and proteases, which promote kidney injury (31). Genetically knocking out Icam1, using neutralizing antibody against ICAM-1, or directly depleting neutrophils, using anti–mouse neutrophil serum, protects mice against ischemic injury (32). In addition, histone secretion from ischemically injured tubular epithelial cells primes neutrophils to form neutrophil extracellular traps (NETs), which further induce tubular epithelial cell death and accelerate NETs production in fresh neutrophils (33). Pretreatment with inhibitors of NETs formation protects kidney from injury, which can be enhanced by dual inhibition of NETs formation and tubular cell necrosis (33). After cisplatin-induced AKI, neutrophil infiltration into kidneys is mediated by caspase-1–dependent proinflammatory cytokines, including IL-1β, IL-18, and IL-6. Knocking out caspase-1 protects mice against cisplatin-induced acute renal failure (34). However, individually inhibiting these cytokines or blocking neutrophil infiltration is insufficient to protect against cisplatin-induced acute renal failure (Table 2) (35), suggesting neutrophils are not essential for cisplatin-induced AKI.
Table 2.
Specific depletion of myeloid cells and its outcomes in the rodent models of AKI and kidney repair
| Rodent Model(s) | Phase of Injury | Targeting Cell(s) | Depletion Method(s) | Major Finding(s) | Reference |
|---|---|---|---|---|---|
| Cisplatin-induced AKI | Early phase | Neutrophil | Injection (i.p.) of neutralizing Ab (RB6-8C5) against Ly-6G on days −1, 0, +1, and +2 relative to the day of cisplatin injection | Neutrophil depletion insufficiently protects against cisplatin-induced ARF. | (35) |
| Early phase | Macrophage | Injection (i.v.) of liposomal-encapsulated clodronate 2 days before and 1 day after cisplatin injection | Depletion of CD11b+ macrophages insufficiently protects against cisplatin-induced ARF. | (36) | |
| Early phase | Macrophage | Knocking out Cx3cr1 or administration of neutralizing Ab against CX3CR1 either 1 hour or 1 day after cisplatin injection | Blockage of CX3CR1 is insufficient to prevent cisplatin-induced ARF. | (36) | |
| IRI | Early phase | Myeloid phagocytes (circulating monocyte, tissue macrophage, CD11c+ dendritic cell, and neutrophil) | Injection (i.p.) of liposomal-encapsulated clodronate on two successive days before IRI | Depletion of myeloid phagocytes before IRI protects tubular epithelial cells from injury and ameliorates the loss of renal function induced by IRI. | (37,38, 39,40) |
| Early phase | Macrophage | Knocking out Ccr2, Ccl2, or Cx3cr1 | Deletion of Ccr2 and Cx3cr1, but not Ccl2, suppresses macrophage infiltration and protects kidneys from IRI. | (41,42) | |
| Early phase | Macrophage | Injection (i.p.) of neutralizing Ab against CX3CR1 1 hour before IRI | Neutralizing Ab against CX3CR1 partially suppresses macrophage infiltration and protects kidneys from IRI. | (43) | |
| Early phase | Macrophage | Administration (orally) of CCR2 antagonist (RS-504393) every 12 hours from the day of IRI or propagermanium, which targets glycosylphosphatidylinositol-anchored proteins that are closely associated with CCR2, from 8 days before IRI | Both RS-504393 and propagermanium suppress macrophage infiltration and protects against tubular necrosis after IRI. | (42) | |
| Early phase | Macrophage | Knocking out Mif | Deletion of Mif reduces kidney macrophage accumulation in the area of damaged tubules and associated inflammation and protects kidneys from IRI. | (44) | |
| Early phase | Leukocyte | Injection (s.c.) of CXCR4 antagonists, plerixafor (AMD3100) or its monocyclam analogue (AMD3465) 3.5 hours after IRI every 12 hours for 2 days | CXCR4 antagonists suppress CD11b+ (neutrophils and monocytes) and CD4+ lymphocytes 24 hours after IRI, diminish epithelial and endothelial injury and interstitial inflammation, and ameliorate the loss of renal function induced by IRI. | (45) | |
| Adaptive repair phase | Myeloid phagocytes (circulating monocyte, tissue macrophage, CD11c+ dendritic cell, and neutrophil) | Injection (i.p.) of liposomal-encapsulated clodronate on two successive days 2 days after IRI | Depletion of myeloid phagocytes on day 3 after IRI diminishes tubular recovery from IRI, as shown in persistent luminal casts and decreased regenerating tubules. | (37) | |
| Adaptive repair phase | Macrophage | Injection (i.v.) diphtheria toxin to CD11b-DTR allele mice on day 3 and day 5 after IRI | Depletion of macrophages on day 3 and day 5 leads to a striking failure of normal regeneration of kidney tubule epithelium and normal functional recovery of the kidneys. | (46) | |
| Adaptive repair phase | Macrophage and dendritic cell | Injection (i.v.) diphtheria toxin to γ-GT Cre:CSF-1f/f:DTR mice | Proximal tubule–specific depletion of Csf1 decreases reparative macrophage polarization, delays functional and structural recovery, and increases interstitial fibrosis. | (47) | |
| Adaptive repair phase | F4/80+, CD11c+ dendritic cell | Injection (i.v.) of liposomal-encapsulated clodronate on day 1 and day 3 | Depletion of dendritic cells is associated with persistent kidney injury, apoptosis, inflammation, and impaired tubular cell proliferation. | (48) | |
| Maladaptive repair phase | Macrophage | Injection (i.v.) of liposomal-encapsulated clodronate on day 3 every 5 days thereafter until 8 weeks or every 7 days thereafter until 4 weeks after IRI | Depletion of macrophages attenuates interstitial fibrosis, inflammation, and the renal function impairment in the long-term (8 weeks) follow-up after IRI. | (49,50) | |
| Maladaptive repair phase | Macrophage | Knocking out Ccr2 or injection (i.p.) of CCR2 antagonist (RS102895) on day 7 after IRI and every 12 hours for 7 days | Blockage of MCP-1/CCR2 signaling markedly decreases macrophage infiltration and attenuates interstitial fibrosis and sustained inflammation during AKI-to-CKD transition. | (51) | |
| Maladaptive repair phase | Macrophage | Knocking out Il34 | Deletion of Il34 markedly suppresses macrophage proliferation and protects kidneys from AKI and subsequent CKD. | (52) | |
| Proximal tubule injury–mediated by diphtheria toxin in the Ggt1 DTR transgenic mice | Adaptive repair phase | Macrophage and dendritic cell | Injection (i.p.) of liposomal-encapsulated clodronate after DT injection every 3 days or injection of DT in the Ggt1/CD11c DTR double transgenic mice | Depletion of macrophages/dendritic cells induces a striking increase of tubular injury and kidney dysfunction and delays recovery after injury. | (53) |
| Adaptive repair phase | Macrophage and dendritic cell | Knocking out Csf1 or administration of CSF-1R inhibitor (GW2580, an inhibitor of c-fms, via gastric gavage) twice per day | Blocking CSF-1/CSF-1R signaling decreases macrophage/dendritic cell proliferation, markedly inhibits macrophage phenotype transition (from proinflammatory to reparative), and delays recovery from proximal tubule injury. | (53) | |
| Adaptive repair phase | Macrophage and dendritic cell | Injection (i.v.) diphtheria toxin to γ-GT Cre:CSF-1f/f:DTR mice | Proximal tubule–specific depletion of Csf1 decreases reparative macrophage polarization, delays functional and structural recovery, and increases interstitial fibrosis. | (47) | |
| Rhabdomyolysis (intramuscular injection of glycerol)-induced AKI | Early phase and maladaptive repair phase | Macrophage | Injection (i.p.) of liposomal-encapsulated clodronate 1 day before or after the glycerol injection | Depletion of macrophage before rhabdomyolysis improves animal survival and the eGFR at day 2 and subsequently attenuates interstitial fibrosis and inflammation 7 months after rhabdomyolysis. | (54) |
i.p., intraperitoneal; Ab, antibody; ARF, acute renal failure; i.v., intravenous; IRI, ischemic-reperfusion injury; Mif, macrophage migration inhibitory factor; s.c., subcutaneous; DTR, diphtheria toxin receptor; MCP-1, monocyte chemoattractant protein-1; DT, diphtheria toxin.
One of the major cell types that accumulate around injured tubules in the kidney after AKI is the mononuclear phagocyte or macrophage (37). After neutrophils, the early recruitment of monocytes to the kidney after ischemic or obstructive injury is primarily mediated by CCR2 (the chemokine receptor for CCL2) and CX3CR1 (the chemokine receptor for CX3CL1) (41). At the single-cell level, both Ly6c2+ proinflammatory macrophages and reparative arginase-1+ (Arg1+) macrophages, which predominantly accumulate in the kidney 2 days after obstructive injury, express Cx3cr1 and Ccr2, suggesting these macrophages are derived from recruited monocytes (7). Consistently, in the kidney 2 days after ischemic injury, proximal tubular epithelial cells of failed repair are projected to signal monocytes via a variety of chemokines and proinflammatory cytokines, including Ccl2, Ccl5, Ccl7, Ccl8, Cxcl10, Csf1, and Tnf (6). The immunostaining of kidney sections 1 day after ischemic injury confirms that monocyte chemoattractant protein-1 (also known as Ccl2) is predominantly expressed in the tubular epithelial cells (42), whereas CX3CL1 (also known as fractalkine) is highly expressed in the injured endothelial cells (43). In response to proinflammatory cytokines, tubular epithelial cells upregulate macrophage colony-stimulating factor 1, IL-34, and macrophage migration inhibitory factor (Mif), which signal macrophage proliferation and survival through CSF-1 receptor and CD74, respectively (44,52,53,55,56). Knocking out Ccr2 or Mif or depleting macrophages protects mice against ischemic injury in the early phase (Table 2) (37,38,41–45), suggesting macrophages are detrimental in the early phase of AKI. Kidney-resident macrophages (KRMs) that are undifferentiated from the infiltrating macrophages present as a distinct cellular subpopulation after AKI (57). However, injury can result in KRMs reprogramming transcriptomes more closely to those found in kidney development (postnatal day 7), i.e., enriched Wingless-type MMTV integration site family (Wnt) signaling, indicating that mechanisms involved in kidney development may be functioning after injury in KRMs (57).
Mechanistically, in response to the local microenvironment with ROS and DAMPs released from neutrophils and injured/necrotic tubular cells, respectively, the recruited monocytes/macrophages and resident mononuclear phagocytes have been shown to differentiate into a proinflammatory state, partially mediated through Toll-like receptors (TLR2 and TLR4), and release proinflammatory cytokines, including IL-6, IL-12, and TNF-α and nitric oxide (NO) (11,24,37). NO can interact with ROS to generate cytotoxic peroxynitrites that leads to oxidative stress and apoptosis in tubular epithelial cells (11). However, similar to neutrophils, depleting macrophages or blocking macrophage infiltration is insufficient to protect mice against cisplatin-induced AKI (Table 2) (36), suggesting the role of myeloid cells in the pathogenicity of cisplatin-induced nephrotoxicity may differ from that of ischemic and obstructive injuries. Together, the current results suggest that macrophages are classically activated (M1) to promote kidney injury in the early phase of acute ischemic and obstructive injury.
In addition to macrophages, prolonged exposure of DCs to proinflammatory cytokines and/or DAMPs triggers DC maturation to activate T cells (58) and renal DCs to produce IFN-α, TNF-α, and IL-6 (59,60), suggesting DCs have a proinflammatory role in renal ischemic injury; however, some studies show that kidney DCs prevent ischemic tissue damage (61–63). For instance, blocking TNF-α via neutralizing binding protein or a pegylated form of soluble TNF receptor type 1 protects mice against renal ischemic and obstructive injury (64,65). Knocking out IL-6, IFN-γ/IL-12, or inducible NO synthase (iNOS; an enzyme to catalyze the production of NO) protects mice against renal ischemic injury (66–69). The data generated from the bulk RNA-seq analyses indicate that these oxidative stress- and hypoxia-initiated cascades of stress and immediate transcriptional responses in the early phase of AKI are largely conserved between mice and humans (70).
Myeloid Cell–Mediated Adaptive Kidney Repair
The inflammatory milieu is later reversed by the infiltrated/infiltrating cells that promote a reparative microenvironment by secreting anti-inflammatory cytokines. These cells include proreparative (also known as alternatively activated M2) macrophages that predominate between days 3 and 7 after ischemic injury (37). At the single-cell level, mannose receptor 1+ (Mrc1+) macrophages and Ccr2+ macrophages are predominantly found in the kidney 7 days after obstructive injury (7). Those Mrc1+ macrophages express multiple scavenger receptors, including Mrc1, Fcrls, Stab1, and Igf1, but downregulate MHC-II, suggesting the monocytes transition to a reparative state that can play a role in scavenging debris/excess ECM and kidney repair. Consistently, evidence shows that IFN-γ–primed proinflammatory macrophages switch to an anti-inflammatory (reparative) phenotype in the kidney 7 days after ischemic injury by upregulating Arg1, Mrc1, macrophage scavenger receptor 1, and Igf1 via STAT6 activation but downregulating iNos expression (37,71). Depleting macrophages during the repair phase diminishes kidney repair after ischemic injury (Table 2) (37,46,72), which can be recovered with reinjection of macrophages after depletion (73).
Mechanistically, tubular epithelial cells are involved in the activation of reparative macrophages through the production of macrophage growth factors, including macrophage colony-stimulating factor 1 and GM-CSF. Reparative macrophages are activated to express factors, including WNT7b, BRP-39 (also known as YKL-40, the orthologous human protein), and IL-22, that directly promote tubular repair after ischemic injury (46,74,75). For instance, depleting GM-CSF using neutralizing antibody during the repair phase attenuates the reparative macrophage activation and suppresses tubular proliferation after ischemic injury (71). DAMP released from injured and necrotic tubular epithelial cells induces proinflammatory macrophages via TLR4 to express IL-22, which promotes tubular epithelial cell proliferation and kidney repair (75). Of note, the downstream signaling of TLR4 is time dependent: early blockade of TLR4/IL-22 signaling prevents tubular necrosis, and late blockade of TLR4/IL-22 impairs tubular regeneration (75). In addition, macrophages can promote tubular repair indirectly. For instance, vascular-resident CD169+ macrophages prevent excessive inflammation in the kidney after ischemic injury by downregulating ICAM-1 expression on vascular endothelial cells (76). Depleting CD169+ cells enhances endothelial ICAM-1 expression, resulting in irreversible renal damage associated with infiltration of a large number of neutrophils (76).
Similarly to macrophages, DCs may contribute to the recovery process by a dynamic phenotype change from a proinflammatory to anti-inflammatory (expressing high levels of IL-10) state with modulation of immune response (48). After ischemic injury, oxidative stress can also activate DCs to express IFN regulatory factor 4, which attenuates secretion of proinflammatory cytokines from resident antigen-presenting cells and prevents excess ischemic damage (77). Depleting DCs during the repair phase leads to persistence of the inflammatory milieu, tubular injury, and apoptosis in both ischemic and nephrotoxic nephritis models (Table 2) (48,78).
Myeloid-derived suppressor cells are a heterogeneous population of cells, generally composed of progenitors and precursors of DCs, macrophages, and granulocytes at various stages of differentiation. Myeloid-derived suppressor cells can also be recruited into the injured kidneys after the interaction of CXCL1 and CXCL2 with their receptor CXCR2 (79,80) to protect the kidney from ischemic injury by suppressing effector T-cell activation and subsequently downregulating production of proinflammatory cytokines (IFN-γ, IL-1β, and IL-6) (80).
Myeloid Cell–Mediated Maladaptive Kidney Repair
In the event of optimal repair, deactivation or egress of macrophages is required for resolution of inflammation after ischemic injury. However, persistent parenchymal inflammation or more severe injury leads to macrophage persistence, which is strongly associated with renal fibrosis, tubular atrophy, and progressive CKD (81,82). For instance, knocking out IRAK-M, a cell-intrinsic pathway that suppresses TLR/IL-1R signaling, leads to persistence of proinflammatory macrophages and late tubular atrophy (83). Sustained tubular injury after ischemic injury leads to tubular expression of GM-CSF and BRP-39, which, in turn, activate interstitial macrophages to express monocyte chemoattractant protein-1 to recruit monocytes/macrophages and transition to a profibrotic phenotype, respectively (51,84). These profibrotic macrophages, a MRC1+ (CD206+) subset of reparative macrophages, persist in the kidney interstitium adjacent to nonrepaired tubules and promote kidney fibrosis directly or indirectly through myofibroblast activation in both human and mouse models (50,51,84–88). Long-term depletion of macrophages or blockade of certain macrophage homing signaling pathways attenuates late renal fibrosis after ischemic injury (Table 2) (49–51), which is also shown in the obstructive injury model (unilateral ureter obstruction) (89–91).
Macrophages isolated from kidney at the late stage of ischemic injury reveal high-level expression of Lgals3 (also known as galectin-3), Pdgfb, Tgfb1, Tgfb2, and Egf (profibrotic growth factors), but low-level expression of Nos2 and Arg1 (proinflammatory and reparative phenotype markers) (84). Myeloid cell–specific knockout of Lgals3 reduces late fibrosis severity but does not alter macrophage recruitment in the kidney after obstructive injury, suggesting galectin-3 promotes interstitial fibrosis through fibroblast/myofibroblast activation. Macrophages are also a major source for matrix metalloproteinases (MMPs) (6,7), which play a complex role in the development of kidney fibrosis by promoting fibrosis and degrading ECM. For instance, whole-body knockout of Mmp2 and Mmp9, but not Mmp12, promotes macrophage accumulation and kidney fibrosis after obstructive injury (40,92,93), suggesting MMP2 and MMP9, but not MMP12, are required for macrophage migration within the tubulointerstitium. Macrophage-specific knockout of twist-related protein 1, a transcription factor that regulates the expression of several MMPs, such as MMP13, to facilitate ECM degradation, promotes kidney fibrosis after obstructive injury (94,95). In addition, macrophages may potentially promote renal fibrosis directly via macrophage-myofibroblast transition (MMT), which is driven by TGF-β1/SMAD3 signaling via an Src-centric regulatory network (96). These MMT cells are recognized by coexpressing macrophage marker CD68 and myofibroblast marker α-smooth muscle actin in the diseased kidneys in both mouse obstructive model and human biopsy specimen (96–98). The MMTs may serve as a key checkpoint in the progression of chronic inflammation to renal fibrosis (99). In contrast, evidence shows that DCs do not directly promote kidney fibrosis after obstructive injury (100), suggesting fibrosis is mainly driven by profibrotic macrophages. However, DCs adopt a proinflammatory phenotype and become more effective antigen-presenting cells to activate T cells after ischemic and obstructive injury (62,100,101).
Discussion and Outlook
To date, the roles of myeloid cells in AKI and kidney repair remain heavily studied in rodent models of kidney injury using ischemia/reperfusion, ureteral obstruction, or nephrotoxin administration. By manipulating models and controlling time, researchers can reveal the biologic responses that lead to inflammation, cell death/proliferation, kidney repair, and long-term fibrosis after the injury. Among the myeloid cells (Table 1), the roles of eosinophils and basophils in AKI have been reported in a small number of case reports and observational studies (102,103). In contrast, mast cells have been shown to not necessarily be involved in the development of kidney pathology, but could have a beneficial role in restoration of normal kidney homeostasis (104). Neutrophils, macrophages, and DCs become the major players in response to AKI and kidney repair (Table 2). Consistently, an increase in the number of neutrophils and macrophages has also been observed in the biopsy specimens from patients with sepsis-induced AKI, acute tubular necrosis and acute tubular injury, and DCs in the biopsy specimens from patients with GN (105–108). However, the substantial increase of understanding rodent AKI has not led to effective therapies to treat patients with AKI. The disconnection between rodent models and human AKI may be due to many causes, including, but not limited to, distinct pathogenic pathways that are activated by different initial stressors and distinct biologic and immune responses that are not identical between rodent models and human diseases. For instance, in the rodent models of AKI, myeloid cells, such as macrophages and DCs, generally promote kidney injury in the early phase, kidney repair afterwards, and kidney fibrosis in the maladaptive kidney repair after IRI; however, depletion of macrophages or DCs does not protect kidneys from cisplatin-induced AKI, suggesting the involvement of myeloid cells is likely different not only depending on the time but also the types of injury.
Recently, kidney tissues obtained from patients with diabetes undergoing partial or radical nephrectomy, kidney transplant biopsy, normal kidney tissues obtained at least 2 cm away from tumor tissue, and fetal kidneys from gestational weeks 7–25 have been subjected to the scRNA-seq or single-nucleus RNA-seq to understand the pathogenesis of diabetic nephropathy, chronic transplant rejection, and kidney development in humans (4,109–113). The application of single-cell technologies on human AKI biopsy specimens holds the potential to fill the gap for understanding the pathogenesis of human AKI, especially the roles of myeloid cells in AKI, and how it may differ from those in the rodent models of AKI. Such technology, however, relies on tissue dissociation and does not offer spatial context, e.g., what specific type(s) of myeloid cells are adjacent to the tubular epithelial/endothelial cells at the point of injury, apoptosis, necrosis, repair, proliferation, or fibrosis (to the interstitial fibroblast/myofibroblasts). Recent advanced mass cytometry technology, i.e., imaging mass cytometry supports simultaneous detection of >40 protein markers on a single section of formalin-fixed, paraffin-embedded kidney biopsy specimen, termed Kidney-MAPPS (multiplexed antibody-based profiling with preservation of spatial context) (114). In addition to single-cell technologies, the application of Kidney-MAPPS holds the potential to greatly expand our understanding of the role of myeloid cells in human AKI.
Disclosures
The author has nothing to disclose.
Funding
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant K01 DK120783.
Author Contributions
L. Xu conceptualized the study, wrote the original draft, and reviewed and edited the manuscript.
References
- 1.Chawla LS, Kimmel PL: Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int 82: 516–524, 2012. 10.1038/ki.2012.208 [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012. 10.1038/ki.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikizler TA, Parikh CR, Himmelfarb J, Chinchilli VM, Liu KD, Coca SG, Garg AX, Hsu CY, Siew ED, Wurfel MM, Ware LB, Faulkner GB, Tan TC, Kaufman JS, Kimmel PL, Go AS: A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int 99: 456–465, 2021. 10.1016/j.kint.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H, Malone AF, Donnelly EL, Kirita Y, Uchimura K, Ramakrishnan SM, Gaut JP, Humphreys BD: Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol 29: 2069–2080, 2018. 10.1681/ASN.2018020125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H, Kirita Y, Donnelly EL, Humphreys BD: Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: Rare cell types and novel cell states revealed in fibrosis. J Am Soc Nephrol 30: 23–32, 2019. 10.1681/ASN.2018090912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD: Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A 117: 15874–15883, 2020. 10.1073/pnas.2005477117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway BR, O’Sullivan ED, Cairns C, O’Sullivan J, Simpson DJ, Salzano A, Connor K, Ding P, Humphries D, Stewart K, Teenan O, Pius R, Henderson NC, Bénézech C, Ramachandran P, Ferenbach D, Hughes J, Chandra T, Denby L: Kidney single-cell atlas reveals myeloid heterogeneity in progression and regression of kidney disease. J Am Soc Nephrol 31: 2833–2854, 2020. 10.1681/ASN.2020060806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudman-Melnick V, Adam M, Potter A, Chokshi SM, Ma Q, Drake KA, Schuh MP, Kofron JM, Devarajan P, Potter SS: Single-cell profiling of AKI in a murine model reveals novel transcriptional signatures, profibrotic phenotype, and epithelial-to-stromal crosstalk. J Am Soc Nephrol 31: 2793–2814, 2020. 10.1681/ASN.2020010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonavia A, Singbartl K: A review of the role of immune cells in acute kidney injury. Pediatr Nephrol 33: 1629–1639, 2018. 10.1007/s00467-017-3774-5 [DOI] [PubMed] [Google Scholar]
- 10.Gharaie Fathabad S, Kurzhagen JT, Sadasivam M, Noel S, Bush E, Hamad ARA, Rabb H: T Lymphocytes in Acute Kidney Injury and Repair. Semin Nephrol 40: 114–125, 2020. 10.1016/j.semnephrol.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Huen SC, Cantley LG: Macrophages in renal injury and repair. Annu Rev Physiol 79: 449–469, 2017. 10.1146/annurev-physiol-022516-034219 [DOI] [PubMed] [Google Scholar]
- 12.Huen SC, Cantley LG: Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol 30: 199–209, 2015. 10.1007/s00467-013-2726-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultze JL, Mass E, Schlitzer A: Emerging principles in myelopoiesis at homeostasis and during infection and inflammation. Immunity 50: 288–301, 2019. 10.1016/j.immuni.2019.01.019 [DOI] [PubMed] [Google Scholar]
- 14.Micanovic R, Chitteti BR, Dagher PC, Srour EF, Khan S, Hato T, Lyle A, Tong Y, Wu X-R, El-Achkar TM: Tamm-Horsfall protein regulates granulopoiesis and systemic neutrophil homeostasis. J Am Soc Nephrol 26: 2172–2182, 2015. 10.1681/ASN.2014070664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulkerson PC, Rothenberg ME: Origin, regulation and physiological function of intestinal oeosinophils. Best Pract Res Clin Gastroenterol 22: 411–423, 2008. 10.1016/j.bpg.2007.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min B: Basophils: What they ‘can do’ versus what they ‘actually do’. Nat Immunol 9: 1333–1339, 2008. 10.1038/ni.f.217 [DOI] [PubMed] [Google Scholar]
- 17.Kitamura Y, Shimada M, Hatanaka K, Miyano Y: Development of mast cells from grafted bone marrow cells in irradiated mice. Nature 268: 442–443, 1977. 10.1038/268442a0 [DOI] [PubMed] [Google Scholar]
- 18.Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J: Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat Rev Nephrol 10: 625–643, 2014. 10.1038/nrneph.2014.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen Y, Yan H-R, Wang B, Liu B-C: Macrophage heterogeneity in kidney injury and fibrosis. Front Immunol 12: 681748, 2021. 10.3389/fimmu.2021.681748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teteris SA, Engel DR, Kurts C: Homeostatic and pathogenic role of renal dendritic cells. Kidney Int 80: 139–145, 2011. 10.1038/ki.2011.129 [DOI] [PubMed] [Google Scholar]
- 21.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F: A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311: 83–87, 2006. 10.1126/science.1117729 [DOI] [PubMed] [Google Scholar]
- 22.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M: In vivo analysis of dendritic cell development and homeostasis. Science 324: 392–397, 2009. 10.1126/science.1170540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F: A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012. 10.1126/science.1219179 [DOI] [PubMed] [Google Scholar]
- 24.Zuk A, Bonventre JV: Acute kidney injury. Annu Rev Med 67: 293–307, 2016. 10.1146/annurev-med-050214-013407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S: Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012. 10.1038/ki.2011.450 [DOI] [PubMed] [Google Scholar]
- 26.Jang HR, Rabb H: Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015. 10.1038/nrneph.2014.180 [DOI] [PubMed] [Google Scholar]
- 27.Kinsey GR, Li L, Okusa MD: Inflammation in acute kidney injury. Nephron, Exp Nephrol 109: e102–e107, 2008. 10.1159/000142934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salei N, Rambichler S, Salvermoser J, Papaioannou NE, Schuchert R, Pakalniškytė D, Li N, Marschner JA, Lichtnekert J, Stremmel C, Cernilogar FM, Salvermoser M, Walzog B, Straub T, Schotta G, Anders HJ, Schulz C, Schraml BU: The kidney contains ontogenetically distinct dendritic cell and macrophage subtypes throughout development that differ in their inflammatory properties. J Am Soc Nephrol 31: 257–278, 2020. 10.1681/ASN.2019040419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, Yao B, Zhang MZ, Harris RC, Duffy KJ, Erickson-Miller CL, Sutton TA, Haase VH: Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest 124: 2396–2409, 2014. 10.1172/JCI69073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida T, Yamashita M, Iwai M, Hayashi M: Endothelial Krüppel-like factor 4 mediates the protective effect of statins against ischemic AKI. J Am Soc Nephrol 27: 1379–1388, 2016. 10.1681/ASN.2015040460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolisetty S, Agarwal A: Neutrophils in acute kidney injury: Not neutral any more. Kidney Int 75: 674–676, 2009. 10.1038/ki.2008.689 [DOI] [PubMed] [Google Scholar]
- 32.Kelly KJ, Williams WW Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV: Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996. 10.1172/JCI118498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakazawa D, Kumar SV, Marschner J, Desai J, Holderied A, Rath L, Kraft F, Lei Y, Fukasawa Y, Moeckel GW, Angelotti ML, Liapis H, Anders HJ: Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol 28: 1753–1768, 2017. 10.1681/ASN.2016080925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faubel S, Ljubanovic D, Reznikov L, Somerset H, Dinarello CA, Edelstein CL: Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int 66: 2202–2213, 2004. 10.1111/j.1523-1755.2004.66010.x [DOI] [PubMed] [Google Scholar]
- 35.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL: Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322: 8–15, 2007. 10.1124/jpet.107.119792 [DOI] [PubMed] [Google Scholar]
- 36.Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL: Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther 324: 111–117, 2008. 10.1124/jpet.107.130161 [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. 10.1681/ASN.2009060615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK: Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006. 10.1093/ndt/gfk047 [DOI] [PubMed] [Google Scholar]
- 39.Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J: Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int 82: 928–933, 2012. 10.1038/ki.2012.207 [DOI] [PubMed] [Google Scholar]
- 40.Abraham AP, Ma FY, Mulley WR, Ozols E, Nikolic-Paterson DJ: Macrophage infiltration and renal damage are independent of matrix metalloproteinase 12 in the obstructed kidney. Nephrology (Carlton) 17: 322–329, 2012. 10.1111/j.1440-1797.2012.01567.x [DOI] [PubMed] [Google Scholar]
- 41.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE Jr, Lobo PI, Okusa MD: The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008. 10.1038/ki.2008.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H: CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol 14: 2503–2515, 2003. 10.1097/01.ASN.0000089563.63641.A8 [DOI] [PubMed] [Google Scholar]
- 43.Oh DJ, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, Faubel S, Edelstein CL: Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol 294: F264–F271, 2008. 10.1152/ajprenal.00204.2007 [DOI] [PubMed] [Google Scholar]
- 44.Li JH, Tang Y, Lv J, Wang XH, Yang H, Tang PMK, Huang XR, He ZJ, Zhou ZJ, Huang QY, Klug J, Meinhardt A, Fingerle-Rowson G, Xu AP, Zheng ZH, Lan HY: Macrophage migration inhibitory factor promotes renal injury induced by ischemic reperfusion. J Cell Mol Med 23: 3867–3877, 2019. 10.1111/jcmm.14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuk A, Gershenovich M, Ivanova Y, MacFarland RT, Fricker SP, Ledbetter S: CXCR4 antagonism as a therapeutic approach to prevent acute kidney injury. Am J Physiol Renal Physiol 307: F783–F797, 2014. 10.1152/ajprenal.00685.2013 [DOI] [PubMed] [Google Scholar]
- 46.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010. 10.1073/pnas.0912228107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Chang J, Yao B, Niu A, Kelly E, Breeggemann MC, Abboud Werner SL, Harris RC, Zhang MZ: Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int 88: 1274–1282, 2015. 10.1038/ki.2015.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MG, Boo CS, Ko YS, Lee HY, Cho WY, Kim HK, Jo SK: Depletion of kidney CD11c+ F4/80+ cells impairs the recovery process in ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 25: 2908–2921, 2010. 10.1093/ndt/gfq183 [DOI] [PubMed] [Google Scholar]
- 49.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK: Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 23: 842–852, 2008. 10.1093/ndt/gfm694 [DOI] [PubMed] [Google Scholar]
- 50.Kim MG, Kim SC, Ko YS, Lee HY, Jo SK, Cho W: The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One 10: e0143961, 2015. 10.1371/journal.pone.0143961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu L, Sharkey D, Cantley LG: Tubular GM-CSF promotes late MCP-1/CCR2-mediated fibrosis and inflammation after ischemia/reperfusion injury. J Am Soc Nephrol 30: 1825–1840, 2019. 10.1681/ASN.2019010068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baek JH, Zeng R, Weinmann-Menke J, Valerius MT, Wada Y, Ajay AK, Colonna M, Kelley VR: IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest 125: 3198–3214, 2015. 10.1172/JCI81166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC: CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. 10.1172/JCI60363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belliere J, Casemayou A, Ducasse L, Zakaroff-Girard A, Martins F, Iacovoni JS, Guilbeau-Frugier C, Buffin-Meyer B, Pipy B, Chauveau D, Schanstra JP, Bascands JL: Specific macrophage subtypes influence the progression of rhabdomyolysis-induced kidney injury. J Am Soc Nephrol 26: 1363–1377, 2015. 10.1681/ASN.2014040320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, Wada T, Schwarting A, Stanley ER, Kelley VR: CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest 119: 2330–2342, 2009. 10.1172/JCI39087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isbel NM, Hill PA, Foti R, Mu W, Hurst LA, Stambe C, Lan HY, Atkins RC, Nikolic-Paterson DJ: Tubules are the major site of M-CSF production in experimental kidney disease: Correlation with local macrophage proliferation. Kidney Int 60: 614–625, 2001. 10.1046/j.1523-1755.2001.060002614.x [DOI] [PubMed] [Google Scholar]
- 57.Lever JM, Hull TD, Boddu R, Pepin ME, Black LM, Adedoyin OO, Yang Z, Traylor AM, Jiang Y, Li Z, Peabody JE, Eckenrode HE, Crossman DK, Crowley MR, Bolisetty S, Zimmerman KA, Wende AR, Mrug M, Yoder BK, Agarwal A, George JF: Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight 4: e125503, 2019. 10.1172/jci.insight.125503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurts C, Ginhoux F, Panzer U: Kidney dendritic cells: Fundamental biology and functional roles in health and disease. Nat Rev Nephrol 16: 391–407, 2020. 10.1038/s41581-020-0272-y [DOI] [PubMed] [Google Scholar]
- 59.Deng B, Lin Y, Chen Y, Ma S, Cai Q, Wang W, Li B, Liu T, Zhou P, He R, Ding F: Plasmacytoid dendritic cells promote acute kidney injury by producing interferon-α. Cell Mol Immunol 18: 219–229, 2021. 10.1038/s41423-019-0343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71: 619–628, 2007. 10.1038/sj.ki.5002132 [DOI] [PubMed] [Google Scholar]
- 61.Cho WY, Choi HM, Lee SY, Kim MG, Kim HK, Jo SK: The role of Tregs and CD11c(+) macrophages/dendritic cells in ischemic preconditioning of the kidney. Kidney Int 78: 981–992, 2010. 10.1038/ki.2010.266 [DOI] [PubMed] [Google Scholar]
- 62.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int 68: 1096–1108, 2005. 10.1111/j.1523-1755.2005.00502.x [DOI] [PubMed] [Google Scholar]
- 63.Lech M, Avila-Ferrufino A, Allam R, Segerer S, Khandoga A, Krombach F, Garlanda C, Mantovani A, Anders HJ: Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J Immunol 183: 4109–4118, 2009. 10.4049/jimmunol.0900118 [DOI] [PubMed] [Google Scholar]
- 64.Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR: Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol 277: R922–R929, 1999. 10.1152/ajpregu.1999.277.3.R922 [DOI] [PubMed] [Google Scholar]
- 65.Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, Meldrum KK: TNF-alpha mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol 288: F406–F411, 2005. 10.1152/ajprenal.00099.2004 [DOI] [PubMed] [Google Scholar]
- 66.Ling H, Edelstein C, Gengaro P, Meng X, Lucia S, Knotek M, Wangsiripaisan A, Shi Y, Schrier R: Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am J Physiol 277: F383–F390, 1999. 10.1152/ajprenal.1999.277.3.F383 [DOI] [PubMed] [Google Scholar]
- 67.de Paiva VN, Monteiro RM, Marques VP, Cenedeze MA, Teixeira VP, dos Reis MA, Pacheco-Silva A, Câmara NO: Critical involvement of Th1-related cytokines in renal injuries induced by ischemia and reperfusion. Int Immunopharmacol 9: 668–672, 2008. 10.1016/j.intimp.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 68.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY: Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005. 10.1681/ASN.2003090757 [DOI] [PubMed] [Google Scholar]
- 69.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH: IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19: 1106–1115, 2008. 10.1681/ASN.2007070744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, Chen Y, Li M, Dessing MC, Parvez RK, Cippà PE, Krautzberger AM, Saribekyan G, Smith AD, McMahon AP: Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2: e94716, 2017. 10.1172/jci.insight.94716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huen SC, Huynh L, Marlier A, Lee Y, Moeckel GW, Cantley LG: GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol 26: 1334–1345, 2015. 10.1681/ASN.2014060612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu L, Faubel S, He Z, Andres Hernando A, Jani A, Kedl R, Edelstein CL: Depletion of macrophages and dendritic cells in ischemic acute kidney injury. Am J Nephrol 35: 181–190, 2012. 10.1159/000335582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vinuesa E, Hotter G, Jung M, Herrero-Fresneda I, Torras J, Sola A: Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J Pathol 214: 104–113, 2008. 10.1002/path.2259 [DOI] [PubMed] [Google Scholar]
- 74.Schmidt IM, Hall IE, Kale S, Lee S, He CH, Lee Y, Chupp GL, Moeckel GW, Lee CG, Elias JA, Parikh CR, Cantley LG: Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol 24: 309–319, 2013. 10.1681/ASN.2012060579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kulkarni OP, Hartter I, Mulay SR, Hagemann J, Darisipudi MN, Kumar Vr S, Romoli S, Thomasova D, Ryu M, Kobold S, Anders HJ: Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J Am Soc Nephrol 25: 978–989, 2014. 10.1681/ASN.2013050528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karasawa K, Asano K, Moriyama S, Ushiki M, Monya M, Iida M, Kuboki E, Yagita H, Uchida K, Nitta K, Tanaka M: Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J Am Soc Nephrol 26: 896–906, 2015. 10.1681/ASN.2014020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lassen S, Lech M, Römmele C, Mittruecker HW, Mak TW, Anders HJ: Ischemia reperfusion induces IFN regulatory factor 4 in renal dendritic cells, which suppresses postischemic inflammation and prevents acute renal failure. J Immunol 185: 1976–1983, 2010. 10.4049/jimmunol.0904207 [DOI] [PubMed] [Google Scholar]
- 78.Scholz J, Lukacs-Kornek V, Engel DR, Specht S, Kiss E, Eitner F, Floege J, Groene HJ, Kurts C: Renal dendritic cells stimulate IL-10 production and attenuate nephrotoxic nephritis. J Am Soc Nephrol 19: 527–537, 2008. 10.1681/ASN.2007060684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xing YF, Cai RM, Lin Q, Ye QJ, Ren JH, Yin LH, Li X: Expansion of polymorphonuclear myeloid-derived suppressor cells in patients with end-stage renal disease may lead to infectious complications. Kidney Int 91: 1236–1242, 2017. 10.1016/j.kint.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 80.Zhang C, Wang S, Li J, Zhang W, Zheng L, Yang C, Zhu T, Rong R: The mTOR signal regulates myeloid-derived suppressor cells differentiation and immunosuppressive function in acute kidney injury. Cell Death Dis 8: e2695, 2017. 10.1038/cddis.2017.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, Howie AJ, Adu D, Cockwell P: The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int 69: 1189–1197, 2006. 10.1038/sj.ki.5000212 [DOI] [PubMed] [Google Scholar]
- 82.Puthumana J, Thiessen-Philbrook H, Xu L, Coca SG, Garg AX, Himmelfarb J, Bhatraju PK, Ikizler TA, Siew E, Ware LB, Liu KD, Go AS, Kaufman JS, Kimmel PL, Chinchilli VM, Cantley L, Parikh CR: Biomarkers of inflammation and repair in kidney disease progression. J Clin Invest 131: e139927, 2021. 10.1172/JCI139927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lech M, Gröbmayr R, Ryu M, Lorenz G, Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA, Anders HJ: Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy. J Am Soc Nephrol 25: 292–304, 2014. 10.1681/ASN.2013020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montgomery TA, Xu L, Mason S, Chinnadurai A, Lee CG, Elias JA, Cantley LG: Breast regression protein-39/chitinase 3-like 1 promotes renal fibrosis after kidney injury via activation of myofibroblasts. J Am Soc Nephrol 28: 3218–3226, 2017. 10.1681/ASN.2017010110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin SL, Castaño AP, Nowlin BT, Lupher ML Jr, Duffield JS: Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009. 10.4049/jimmunol.0901473 [DOI] [PubMed] [Google Scholar]
- 86.Duffield JS: Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014. 10.1172/JCI72267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bellón T, Martínez V, Lucendo B, del Peso G, Castro MJ, Aroeira LS, Rodríguez-Sanz A, Ossorio M, Sánchez-Villanueva R, Selgas R, Bajo MA: Alternative activation of macrophages in human peritoneum: Implications for peritoneal fibrosis. Nephrol Dial Transplant 26: 2995–3005, 2011. 10.1093/ndt/gfq771 [DOI] [PubMed] [Google Scholar]
- 88.Toki D, Zhang W, Hor KL, Liuwantara D, Alexander SI, Yi Z, Sharma R, Chapman JR, Nankivell BJ, Murphy B, O’Connell PJ: The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation. Am J Transplant 14: 2126–2136, 2014. 10.1111/ajt.12803 [DOI] [PubMed] [Google Scholar]
- 89.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H: Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol 165: 237–246, 2004. 10.1016/S0002-9440(10)63292-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T: Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 172: 288–298, 2008. 10.2353/ajpath.2008.070726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia Y, Entman ML, Wang Y: CCR2 regulates the uptake of bone marrow-derived fibroblasts in renal fibrosis. PLoS One 8: e77493, 2013. 10.1371/journal.pone.0077493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan TK, Zheng G, Hsu TT, Lee SR, Zhang J, Zhao Y, Tian X, Wang Y, Wang YM, Cao Q, Wang Y, Lee VW, Wang C, Zheng D, Alexander SI, Thompson E, Harris DC: Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest 93: 434–449, 2013. 10.1038/labinvest.2013.3 [DOI] [PubMed] [Google Scholar]
- 93.Nishida M, Okumura Y, Ozawa S, Shiraishi I, Itoi T, Hamaoka K: MMP-2 inhibition reduces renal macrophage infiltration with increased fibrosis in UUO. Biochem Biophys Res Commun 354: 133–139, 2007. 10.1016/j.bbrc.2006.12.165 [DOI] [PubMed] [Google Scholar]
- 94.Ren J, Zhang J, Rudemiller NP, Griffiths R, Wen Y, Lu X, Privratsky JR, Gunn MD, Crowley SD: Twist1 in infiltrating macrophages attenuates kidney fibrosis via matrix metallopeptidase 13-mediated matrix degradation. J Am Soc Nephrol 30: 1674–1685, 2019. 10.1681/ASN.2018121253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ren J, Crowley SD: Twist1: A double-edged sword in kidney diseases. Kidney Dis 6: 247–257, 2020. 10.1159/000505188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang S, Meng XM, Ng YY, Ma FY, Zhou S, Zhang Y, Yang C, Huang XR, Xiao J, Wang YY, Ka SM, Tang YJ, Chung AC, To KF, Nikolic-Paterson DJ, Lan HY: TGF-β/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget 7: 8809–8822, 2016. 10.18632/oncotarget.6604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meng XM, Wang S, Huang XR, Yang C, Xiao J, Zhang Y, To KF, Nikolic-Paterson DJ, Lan HY: Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis 7: e2495, 2016. 10.1038/cddis.2016.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang PM, Zhou S, Li CJ, Liao J, Xiao J, Wang QM, Lian GY, Li J, Huang XR, To KF, Ng CF, Chong CC, Ma RC, Lee TL, Lan HY: The proto-oncogene tyrosine protein kinase Src is essential for macrophage-myofibroblast transition during renal scarring. Kidney Int 93: 173–187, 2018. 10.1016/j.kint.2017.07.026 [DOI] [PubMed] [Google Scholar]
- 99.Tang PM, Nikolic-Paterson DJ, Lan HY: Macrophages: Versatile players in renal inflammation and fibrosis. Nat Rev Nephrol 15: 144–158, 2019. 10.1038/s41581-019-0110-2 [DOI] [PubMed] [Google Scholar]
- 100.Snelgrove SL, Kausman JY, Lo C, Lo C, Ooi JD, Coates PT, Hickey MJ, Holdsworth SR, Kurts C, Engel DR, Kitching AR: Renal dendritic cells adopt a pro-inflammatory phenotype in obstructive uropathy to activate T cells but do not directly contribute to fibrosis. Am J Pathol 180: 91–103, 2012. 10.1016/j.ajpath.2011.09.039 [DOI] [PubMed] [Google Scholar]
- 101.Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H: Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol 285: F87–F94, 2003. 10.1152/ajprenal.00026.2003 [DOI] [PubMed] [Google Scholar]
- 102.Tariq A, Okamato K, Tariq A, Rosenberg AZ, Soliman KM, Ploth DW, Atta MG, McMahon BA: Eosinophilia and risk of incident end stage kidney disease. BMC Nephrol 21: 14, 2020. 10.1186/s12882-020-1685-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mack M, Rosenkranz AR: Basophils and mast cells in renal injury. Kidney Int 76: 1142–1147, 2009. 10.1038/ki.2009.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blank U, Essig M, Scandiuzzi L, Benhamou M, Kanamaru Y: Mast cells and inflammatory kidney disease. Immunol Rev 217: 79–95, 2007. 10.1111/j.1600-065X.2007.00503.x [DOI] [PubMed] [Google Scholar]
- 105.Aslan A, van den Heuvel MC, Stegeman CA, Popa ER, Leliveld AM, Molema G, Zijlstra JG, Moser J, van Meurs M: Kidney histopathology in lethal human sepsis. Crit Care 22: 359, 2018. 10.1186/s13054-018-2287-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim M-G, Lim K, Lee YJ, Yang J, Oh SW, Cho WY, Jo S-K: M2 macrophages predict worse long-term outcomes in human acute tubular necrosis. Sci Rep 10: 2122, 2020. 10.1038/s41598-020-58725-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palmer MB, Vichot AA, Cantley LG, Moeckel GW: Quantification and localization of M2 macrophages in human kidneys with acute tubular injury. Int J Nephrol Renovasc Dis 7: 415–419, 2014. 10.2147/IJNRD.S66936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Segerer S, Heller F, Lindenmeyer MT, Schmid H, Cohen CD, Draganovici D, Mandelbaum J, Nelson PJ, Gröne HJ, Gröne EF, Figel AM, Nössner E, Schlöndorff D: Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney Int 74: 37–46, 2008. 10.1038/ki.2008.99 [DOI] [PubMed] [Google Scholar]
- 109.Hochane M, van den Berg PR, Fan X, Bérenger-Currias N, Adegeest E, Bialecka M, Nieveen M, Menschaart M, Chuva de Sousa Lopes SM, Semrau S: Single-cell transcriptomics reveals gene expression dynamics of human fetal kidney development. PLoS Biol 17: e3000152, 2019. 10.1371/journal.pbio.3000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liao J, Yu Z, Chen Y, Bao M, Zou C, Zhang H, Liu D, Li T, Zhang Q, Li J, Cheng J, Mo Z: Single-cell RNA sequencing of human kidney. Sci Data 7: 4, 2020. 10.1038/s41597-019-0351-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Hu J, Liu D, Zhou S, Liao J, Liao G, Yang S, Guo Z, Li Y, Li S, Chen H, Guo Y, Li M, Fan L, Li L, Lin A, Zhao M: Single-cell analysis reveals immune landscape in kidneys of patients with chronic transplant rejection. Theranostics 10: 8851–8862, 2020. 10.7150/thno.48201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang P, Chen Y, Yong J, Cui Y, Wang R, Wen L, Qiao J, Tang F: Dissecting the global dynamic molecular profiles of human fetal kidney development by single-cell RNA sequencing. Cell Rep 24: 3554–3567.e3, 2018. 10.1016/j.celrep.2018.08.056 [DOI] [PubMed] [Google Scholar]
- 113.Wilson PC, Wu H, Kirita Y, Uchimura K, Ledru N, Rennke HG, Welling PA, Waikar SS, Humphreys BD: The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci U S A 116: 19619–19625, 2019. 10.1073/pnas.1908706116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh N, Avigan ZM, Kliegel JA, Shuch BM, Montgomery RR, Moeckel GW, Cantley LG: Development of a 2-dimensional atlas of the human kidney with imaging mass cytometry. JCI Insight 4: e129477, 2019. 10.1172/jci.insight.129477 [DOI] [PMC free article] [PubMed] [Google Scholar]