Key Points
The blood level of d-serine discriminates participants without kidney diseases, whereas the fractional excretion of d-serine is higher in diabetic nephropathy.
The combined analysis of d-serine and clinical factors correctly predicted the presence of diabetic nephropathy.
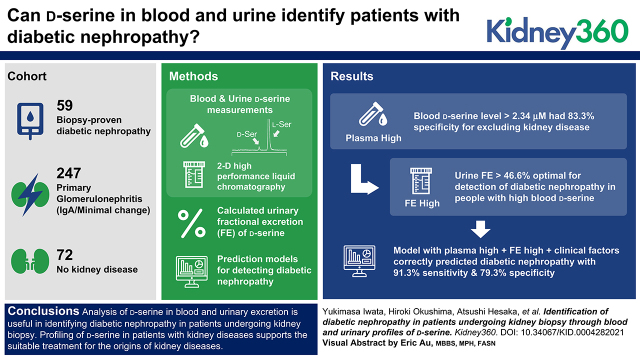
Analysis of d-serine in blood and urinary excretion is useful in identifying diabetic nephropathy in patients undergoing kidney biopsy.
Keywords: diabetes and the kidney, biomarker, chiral amino acids, d-serine, diabetes, diabetic kidney disease, diabetic nephropathy, diagnosis, glomerulonephritis, kidney biopsy
Visual Abstract
Abstract
Background
The diagnosis of diabetic nephropathy (DN), the major cause of ESKD, requires kidney biopsy. d-Serine, present only in trace amounts in humans, is a biomarker for kidney diseases and shows potential to distinguish the origin of kidney diseases, whose diagnoses usually require kidney biopsy. We extended this concept and examined the potential of d-serine in the diagnosis of DN.
Methods
We enrolled patients with biopsy sample–proven DN and primary GN (minimal change disease and IgA nephropathy) and participants without kidney disease. A total of 388 participants were included in this study, and d-serine levels in blood and urine were measured using two-dimensional high-performance liquid chromatography, and urinary fractional excretion (FE) of d-serine was calculated. Using data from 259 participants, we developed prediction models for detecting DN by logistic regression analyses, and the models were validated in 129 participants.
Results
A d-serine blood level of >2.34 μM demonstrated a high specificity of 83% (95% CI, 70% to 93%) for excluding participants without kidney diseases. In participants with a d-serine blood level >2.34 μM, the threshold of 47% in FE of d-serine provided an optimal threshold for the detection of DN (AUC, 0.85 [95% CI, 0.76 to 0.95]; sensitivity, 79% [95% CI, 61% to 91%]; specificity, 83% [95% CI, 67% to 94%]). This plasma-high and FE-high profile of d-serine in combination with clinical factors (age, sex, eGFR, and albuminuria) correctly predicted DN with a sensitivity of 91% (95% CI, 72% to 99%) and a specificity of 79% (95% CI, 63% to 80%), and outperformed the model based on clinical factors alone in the validation dataset (P<0.02).
Conclusions
Analysis of d-serine in blood and urinary excretion is useful in identifying DN in patients undergoing kidney biopsy. Profiling of d-serine in patients with kidney diseases supports the suitable treatment through the auxial diagnosis of the origins of kidney diseases.
Introduction
The diagnosis of diabetic nephropathy (DN), the major cause of ESKD, is key in clinical practice. DN is known to have a poor prognosis (1), and early diagnosis is necessary to improve the prognosis (2). Complication of GN in patients with diabetes is another matter of great concern (3,4). Chronic GN, which requires specific treatment, is highly prevalent in patients with diabetes undergoing kidney biopsy (3,4). Proteinuria is an early clinical sign of DN (5), however, it is not specific to DN and is also seen in patients who have diabetes and GN. The range of proteinuria, a known risk factor for poor prognosis, is wide in DN (6), and it is often difficult to distinguish between other diseases, such as minimal change disease (MCD), in patients with DN who have severe proteinuria (7). To determine the treatment strategy in these patients, kidney biopsy is required to differentiate DN from GN complicated by diabetes. The number of patients with diabetes is large (8), and kidney biopsy is often avoided in patients with diabetes because of their complications of cardiovascular diseases and prescriptions, such as antithrombotic or antiplatelet drugs. Therefore, GN in patients with diabetes is often overlooked. Noninvasive methods to differentiate between DN and GN is required.
d-Amino acids are emerging biomarkers of kidney diseases (9–12). d-Amino acids are mirror-imaged enantiomers (chiral bodies) of amino acids that are present only in trace amounts in nature, unlike the abundant l-amino acids (13,14). A two-dimensional high-performance liquid chromatography (2D-HPLC) system has been developed to measure the levels of d-amino acids in human samples with precision (9–11). Studies using 2D-HPLC revealed that d-serine reflects kidney function and disease activity (9,10). A previous study also revealed the levels of d-serine in blood and urine are also useful in the diagnosis of the origin of kidney diseases (15).
In this study, we investigated the potential of d-serine in detecting DN among patients with kidney diseases undergoing kidney biopsy. Because the clinical spectrum of DN is broad and secondary GN may present with a complex pathophysiology, we aimed to identify a DN-specific profile of d-serine to distinguish DN from typical primary GN. For this purpose, we profiled d-serine in three major kidney diseases for the indication of kidney biopsy: DN, IgA nephropathy (IgAN), and MCD (16).
Materials and Methods
Study Population
We prospectively enrolled consecutive patients undergoing their first kidney biopsy between 2006 and 2016 at the Department of Kidney Disease and Hypertension, Osaka General Medical Center, for diagnosis and/or treatment purposes. Biopsy specimens were routinely analyzed using light, immunofluorescence, and electron microscopy procedures. Clinical and pathologic diagnoses were established under the consensus of experienced nephrologists and pathologists. From this cohort, we extracted patients with DN or those with MCD and IgAN by referring to the Histologic Classification Scheme of Glomerular Diseases issued by the World Health Organization in 1995. We did not include patients with DN that were complicated by other forms of GN. The selection of MCD and IgAN was made on the basis of the frequency for the indication of kidney biopsy (16). The plasma and urinary levels of d-serine were measured from the samples before kidney biopsy. Separately, 60 potential living kidney transplant donors and 12 healthy volunteers were recruited at Osaka University and National Institute of Biomedical Innovation, Health and Nutrition (NIBIOHN).
The sample size calculation was performed on the basis of a previous study (15). Suppose the plasma level of d-serine was 2.8, 2.3, 2.8, and 1.5 μM for patients with IgAN, MCD, and DN and normal participants, respectively; therefore, the number needed for a one-way ANOVA F test for group effect was 11 per group. As mentioned below, we intended to divide the group of patients into prediction and validation sets; thus, we required 40 participants with each disease when the margin was set as 20%. We consecutively divided patients with each disease 2:1 into prediction and validation datasets. Clinical demographics, laboratory data, and plasma and urine samples were collected at the time of kidney biopsy. The study protocol was approved by the Ethical Committees of Osaka General Medical Center (#29-S0606), Osaka University (#16330), and NIBIOHN (#236). This study was conducted in compliance with the ethical principles of the Declaration of Helsinki, and all participants gave written informed consent. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
GFR Equations and Kidney Clearance Calculation
eGFR was calculated using the Japanese GFR equation based on serum creatinine (17). Serum and urinary creatinine were measured enzymatically. Spot urinary levels of chiral amino acids were adjusted by that of creatinine. Fractional excretion (FE; %) was calculated from clearance of substrate divided by that of creatinine. The FE is the ratio of a substrate filtered by the kidney glomerulus that is excreted in the urine. Low and high FEs indicate tubular reabsorption and excretion, respectively.
Determination of Serine Enantiomers by 2D-HPLC
Sample preparation from human plasma and urine was performed as previously described with modification (18,19). Briefly, 20-fold volumes of methanol were added to the sample, and an aliquot (10 μl of the supernatant obtained from the methanol homogenate) was placed in a brown-shaded tube. After drying the solution under reduced pressure, 20 μl of 200 mM sodium borate buffer (pH 8.0) and 5 μl of fluorescence labeling reagent (40 mM 4-fluoro-7-nitro-2,1,3-benzoxadiazole [NBD-F] in anhydrous acetonitrile [MeCN]) was added and then heated at 60°C for 2 min. An aqueous 0.1% (vol/vol) trifluoroacetic acid solution (75 μl) was added, and 2 μl of the reaction mixture was subjected to 2D-HPLC. The NBD-F derivatives of the amino acids were separated using a reverse-phase column (Singularity RP column, 1.0 mm internal diameter × 50 mm; provided by KAGAMI Inc., Osaka, Japan) with gradient elution using aqueous mobile phases containing MeCN and formic acid. To determine d- and l-serine separately, the fractions of serine were automatically collected using a multiloop valve, and transferred to an enantioselective column (Singularity CSP-001S, 1.5 mm internal diameter × 75 mm; KAGAMI Inc.). Then, d- and l-serine were separated in the second dimension by the enantioselective column. The mobile phases were a mixed solution of MeOH-MeCN–containing formic acid, and fluorescence detection of the NBD-F amino acids was carried out at 530 nm with excitation at 470 nm using two photomultiplier tubes. Target peaks were quantified by scaling the standard peak shapes (20).
Statistical Analyses
Continuous variables are presented as the median and range or interquartile range (IQR). Categoric variables are given as the ratio (%) and count. Continuous variables between multiple groups were compared using one-way ANOVA with Bonferroni post hoc test. Correlations between two variables were examined using the Pearson correlation coefficient. The association between d-serine and clinical parameters was analyzed using principal component analysis (PCA) (21). Diagnostic accuracy was examined using receiver operating characteristic curve analysis. The calculated areas under the curve (AUCs) were compared as described (22). Logistic regression analyses were performed to estimate the predictability of a binary outcome by variables by adjusting for clinical factors including age, sex, eGFR, and albuminuria. Statistical significance was defined as P<0.05. Statistical analyses were performed using STATA and R.
Results
Profiling of d-Serine in Patients with Three Kinds of Kidney Diseases Undergoing Kidney Biopsy
We profiled d-serine in patients undergoing kidney biopsy whose origin of diseases were DN, MCD, and IgAN. The median age was 59 (IQR, 47−69; n=69) in DN, 38 (IQR, 31−49; n=194) in IgAN, 61 (IQR, 40−74; n=53) in MCD, and 60 (IQR, 51−71; n=72) in participants without kidney disease (reference) (Table 1 and Supplemental Table 1). Patients with MCD and DN had worse kidney function, serum creatinine, and eGFR; higher urinary protein; and lower albumin levels, whereas those with IgAN were heterogeneous in characteristics.
Table 1.
Baseline characteristics of the study
| Characteristics | Diabetic Nephropathy (n=69) |
Minimal Change Disease (n=53) |
IgA Nephropathy (n=194) |
Reference (n=72) |
|---|---|---|---|---|
| Age, yr | 59 (47–69) | 61 (40–74) | 38 (31–49) | 60 (51–71) |
| Sex, M, % | 75 (52) | 53 (28) | 55 (107) | 49 (35) |
| Height, cm | 167.0 (161.0–171.5) | 161.0 (156.0–168.0) | 165.0 (157.0–171.1) | 160.2 (155.1–170.3) |
| Body weight, kg | 67.4 (58.1–74.3) | 65.0 (56.0–70.9) | 63.0 (52.6–71.4) | 60.7 (52.4–68.3) |
| Body mass index, kg/m2 | 24.2 (21.5–26.9) | 24.6 (22.3–27.5) | 22.9 (20.3–25.6) | 23.2 (20.7–26.1) |
| Systolic BP, mm Hg | 148 (127–161) | 136 (121–151) | 123 (115–134) | 123 (111–135) |
| Diastolic BP, mm Hg | 76 (66–87) | 84 (78–95) | 78 (70–86) | 76 (68–83) |
| Serum protein, g/dl | 6.2 (5.6–6.7) | 4.5 (4.1–4.9) | 7.0 (6.5–7.3) | 6.8 (6.5–7.2) |
| Serum albumin, g/dl | 3.1 (2.7–3.7) | 1.8 (1.5–2.1) | 4.1 (3.8–4.3) | 4.0 (3.9–4.3) |
| Serum creatinine, mg/dl | 1.52 (1.10–2.25) | 1.00 (0.71–1.71) | 0.89 (0.73–1.10) | 0.68 (0.61–0.79) |
| eGFR, ml/min per 1.73 m2 | 33.1 (23.4–51.7) | 58.6 (28.8–77.5) | 70.4 (53.5–82.3) | 76.6 (70.0–86.1) |
| Serum urea nitrogen, mg/dl | 25.0 (16.0–33.0) | 19.0 (11.0–41.0) | 15.0 (12.0–17.8) | 13.3 (11.3–15.9) |
| Urinary protein, g/g Cre | 2.39 (1.12–4.30) | 6.30 (4.24–12.45) | 0.31 (0.14–0.76) | 0.01 (0.00–0.03) |
| Urinary albumin, mg/g Cre | 1630.0 (829.0–2710.0) | 5220.0 (6050.0–9430.0) | 228.5 (98.9–569.0) | 10.0 (5.1–14.6) |
| Urinary NAG, IU/g Cre | 10.2 (7.4–14.4) | 24.5 (15.6–55.6) | 4.2 (2.2–8.6) | 3.2 (2.0–5.5) |
| Urinary β2-MG, µg/g Cre | 1810.3 (179.3–8528.7) | 152.4 (78.7–490.3) | 48.3 (16.4–111.2) | 154.0 (95.6–217.4) |
| Hypertension history, % | 97 (67) | 42 (22) | 35 (68) | 11 (8) |
| Diabetes history, % | 100 (69) | 11 (6) | 2 (4) | 7 (5) |
Values are presented as median (interquartile range) or percentage (n). Reference, participants without kidney disease; M, male; Cre, creatinine; NAG, N-acetyl-β-D-glucosaminidase; β2-MG, β2-microglobulin.
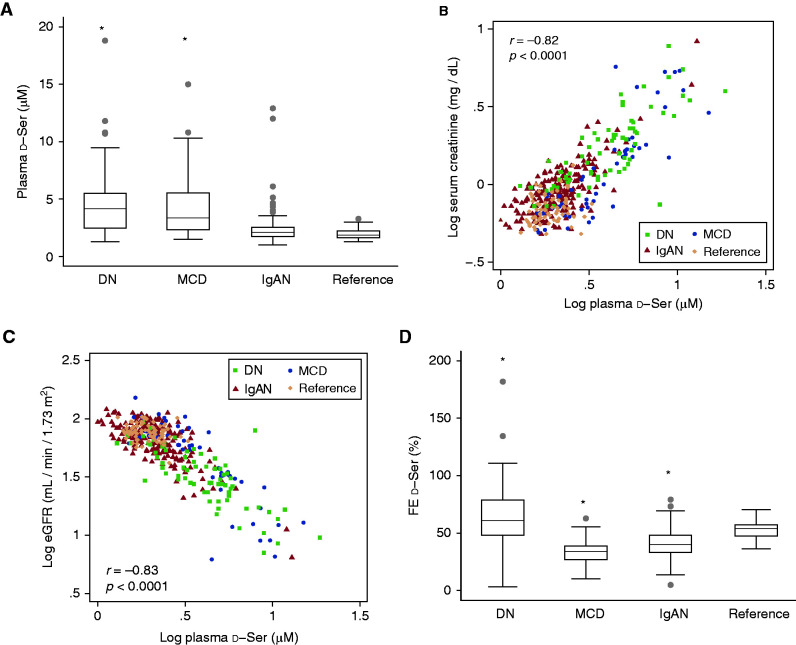
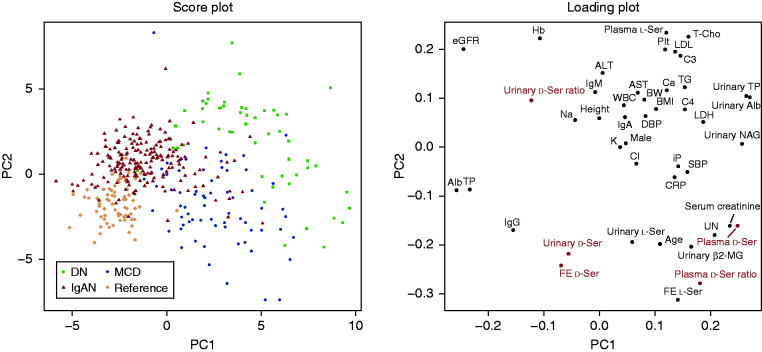
We measured blood and urinary levels of d-serine in participants. The level of d-serine in blood was significantly higher in patients with DN and MCD than in reference participants (Figure 1A), and this level correlated well with the level of serum creatinine and eGFR (Figure 1, B and C, Supplemental Figure 1, A and B). On the other hand, the urinary FE of d-serine was higher in DN and lower in MCD and IgAN, suggesting the potential of the disease-specific profile of FE (Figure 1D). PCA revealed that the plasma level of d-serine showed a relatively close profile to those of the kidney markers creatinine and cystatin C. In contrast, no biochemical parameter showed a similar profile to those of the FE of d-serine, suggesting the uniqueness of the d-serine FE as a biomarker (Figure 2).
Figure 1.
Profilings of plasma level and urinary fractional excretion (FE) of d-serine in each kidney disease. (A) Plasma level of d-serine in each kidney disease. (B and C) Scatterplot of plasma levels of d-serine with (B) serum levels of creatinine and (C) eGFR. Values are transformed to common logarithm, and original data are shown in Supplemental Figures. (D) FE of d-serine in each kidney disease. DN, diabetic nephropathy; IgAN, IgA nephropathy; MCD, minimal change disease; reference, participants without kidney disease; ser, serine. *P<0.05 versus reference, one-way ANOVA.
Figure 2.
d-Serine profiles associate wtih kidney function and origins of kidney disease. Principal component (PC) analysis of d-serine profiles and clinical parameters in associations with origin of kidney diseases. The score value of each observation is plotted on the score plot to demonstrate the clusters of kidney disease origins. Profiles of d-serine are highlighted in red in the loading plot. Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BW, body weight; Cl, chloride; CRP, C-reactive protein; DBP, diastolic BP; DN, diabetic nephropathy; FE, fractional excretion; Hb, hemoglobin; IgAN, IgA nephropathy; K, potassium; LDH, lactate dehydrogenase; MCD, minimal change disease; β2-MG, β2-microglobulin; Na, sodium; NAG, N-acetyl-β-D-glucosaminidase; Plt, platelet; reference, participants without kidney disease; SBP, systolic BP; ser, serine; T-Cho, total cholesterol; TG, triglyceride; TP, total protein; UN, urea nitrogen; WBC, white blood cell.
Prediction of DN through Profiling of d-Serine
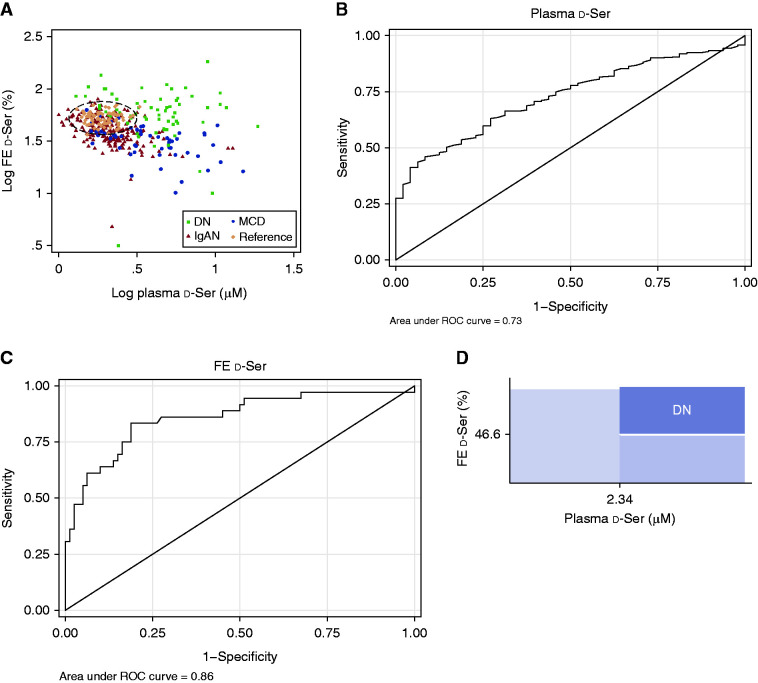
We examined whether the profile of d-serine has the capacity to distinguish the origin of kidney diseases. Patients with DN and MCD were clearly different in their profiles of d-serine; the d-serine plasma level was higher in patients with kidney disease, whereas the FE of d-serine was higher in patients with DN (Figure 3A, Supplemental Figure 2A). The profile of those with IgAN was relatively broad and there was some overlap with those of reference participants and patients with MCD in PCA. Overall, in patients with a higher plasma d-serine level, a higher FE of d-serine suggested the presence of DN.
Figure 3.
Detection of diabetic nephropathy using profiles of d-serine. (A) Scatterplot of plasma level and fractional excretion (FE) of d-serine. The dotted-ellipse represents the 95% CI of reference participants without kidney disease. Values are transformed to common logarithm, and original data are shown in Supplemental Figure 2. (B) Receiver operating characteristic (ROC) curve analysis of d-serine plasma level in eliminating the reference from kidney diseases in prediction dataset. (C) ROC curve analysis of urinary fractional excretion of d-serine in separating diabetic nephropathy (DN) from other diseases in prediction dataset with the d-serine plasma level >2.34 μM. (D) d-Serine–based thresholds for the prediction of DN. IgAN, IgA nephropathy; MCD, minimal change disease; Ser, serine.
For the analysis of this capacity of d-serine to detect DN, we consecutively divided the entire dataset into 259 participants for prediction data and 129 for validation data (in a 2:1 ratio) (Table 2 and Supplemental Table 2). We initially determined the threshold of the d-serine plasma level to exclude reference participants. Receiver operating characteristic curve analyses revealed preferable exclusion of reference participants from those with kidney diseases. The AUC for the plasma level of d-serine was 0.73 (95% CI, 0.66 to 0.79) for the exclusion of reference participants (Figure 3B). The threshold of 2.34 μM for the d-serine plasma level demonstrated a high specificity of 83% (95% CI, 70% to 93%) for excluding reference participants. The sensitivity for detecting kidney diseases is suboptimal (51%; 95% CI, 44% to 58%), because the profile of IgAN overlapped with that of the reference group. We then aimed to detect DN using FE of d-serine in participants with a plasma level of d-serine >2.34 μM. A cutoff value of 47% in FE of d-serine provided an optimal threshold for the detection of DN (AUC, 0.86 [95% CI, 0.78 to 0.94]; sensitivity, 81% [95% CI, 70% to 89%]; specificity, 83% [95% CI, 67% to 94%]; Figure 3C).
Table 2.
Characteristics of prediction and validation data
| Characteristics | Prediction Data (n=259) |
Validation Data (n=129) |
P Value |
|---|---|---|---|
| Disease | >0.99 | ||
| Diabetic nephropathy | 18 (46) | 18 (23) | |
| Minimal change disease | 14 (36) | 13 (17) | |
| IgA nephropathy | 50 (129) | 50 (65) | |
| Reference | 19 (48) | 19 (24) | |
| Age, yr | 47 (35–66) | 47 (37–64) | 0.51 |
| Sex, M, % | 59 (153) | 54 (69) | 0.33 |
| Height, cm | 165.0 (158.0–171.0) | 162.0 (156.0–170.8) | 0.12 |
| Body weight, kg | 64.4 (55.0–72.6) | 63.0 (52.8–71.8) | 0.28 |
| Body mass index, kg/m2 | 23.5 (20.8–26.0) | 23.6 (20.5–26.6) | 0.83 |
| Systolic BP, mm Hg | 128 (118–146) | 123 (114–141) | 0.10 |
| Diastolic BP, mm Hg | 79 (71–88) | 78 (68–84) | 0.04 |
| Serum protein, g/dl | 6.8 (6.0–7.2) | 6.8 (5.9–7.1) | 0.47 |
| Serum albumin, g/dl | 3.9 (3.2–4.2) | 3.9 (3.1–4.2) | 0.64 |
| Serum creatinine, mg/dl | 0.92 (0.72–1.24) | 0.87 (0.69–1.17) | 0.88 |
| eGFR, ml/min per 1.73 m2 | 65.8 (44.9–79.9) | 67.9 (46.2–80.1) | 0.78 |
| Serum urea nitrogen, mg/dl | 15.7 (12.0–20.0) | 14.6 (12.0–19.1) | 0.80 |
| Urinary protein, g/g Cre | 0.41 (0.07–2.06) | 0.49 (0.11–2.31) | 0.54 |
| Urinary albumin, mg/g Cre | 306.0 (49.6–1450.0) | 371.0 (63.8–1620.0) | 0.72 |
| Urinary NAG, IU/g Cre | 5.6 (2.7–11.7) | 6.2 (2.8–12.2) | 0.99 |
| Urinary β2-MG, µg/g Cre | 101.3 (32.0–254.8) | 111.2 (36.1–314.0) | 0.13 |
| Hypertension history, % | 43 (126) | 41 (39) | 0.59 |
| Diabetes history, % | 29 (84) | 20 (19) | >0.99 |
Values are presented as median (interquartile range) or percentage (n). Reference, participants without kidney disease; M, male; Cre, creatinine; NAG, N-acetyl-β-D-glucosaminidase; β2-MG, β2-microglobulin.
Because the profile of IgAN overlapped with that of the reference group, we performed a sensitivity analysis without IgAN. Analysis excluding IgAN revealed that the same thresholds for d-serine were optimal. The threshold of 2.34 μM in the plasma level of d-serine is persistently preferable for the exclusion of the reference group (specificity, 83%; 95% CI, 70% to 93%) with improved detection of kidney diseases (sensitivity, 74% [95% CI, 64% to 83%]; AUC, 0.87 [95% CI, 0.81 to 0.93]; Supplemental Figure 3A). The threshold of 47% in FE of d-serine also provided an optimal threshold for the detection of DN (AUC, 0.85 [95% CI, 0.76 to 0.95]; sensitivity, 79% [95% CI, 61% to 91%]; specificity, 83% [95% CI, 67% to 94%]; Supplemental Figure 3B). Overall, these two threshold values provided the opportunity to detect DN (Figure 3D). The plasma-high and FE-high profile of d-serine was likely to represent DN, and we call this profile the DN-d-Ser profile.
Utility of the d-Serine Profile in Detecting DN
We examined the utility of the DN-d-Ser profile for detecting DN. We developed a logistic regression model by adjusting for clinical factors, including age, sex, eGFR, and albuminuria. Logistic regression analyses revealed that the DN-d-Ser profile had a high predictability for DN (adjusted odds ratio, 14.7; 95% CI, 6.0 to 36.1; Supplemental Table 3).
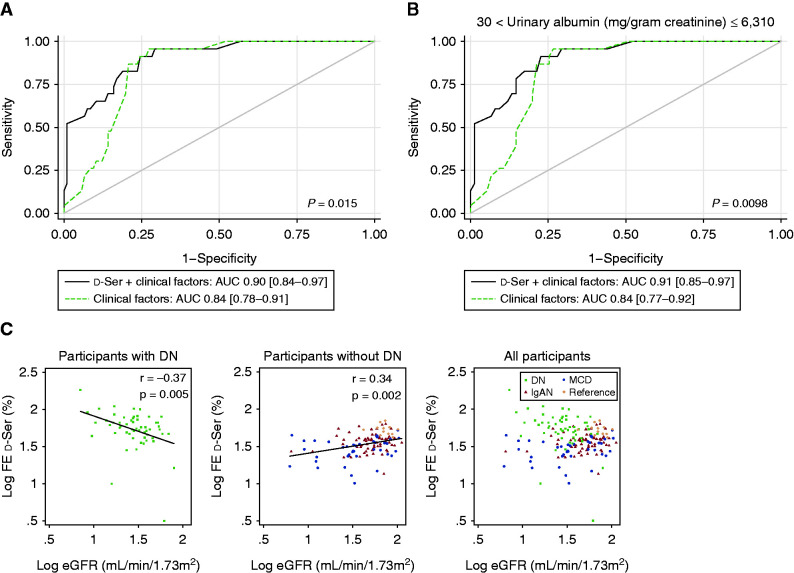
We validated the utility of the DN-d-Ser profile in the detection of DN using the validation data. The logistic regression model, developed by adjusting for the clinical factors in the prediction dataset, performed well in the validation dataset, with a sensitivity of 91% (95% CI, 72% to 99%), a specificity of 79% (95% CI, 63% to 80%), and an AUC of 0.90 (95% CI, 0.84 to 0.97) (Figure 4A). This model outperformed the prediction model developed only for the clinical factors (AUC, 0.84; 95% CI, 0.78 to 0.91; P<0.02; Figure 4A).
Figure 4.
Diabetic nephropathy-specific profile of d-serine improves the detection of diabetic nephropathy (DN). (A and B) Receiver operating characteristic curve analysis of plasma-high and fractional excretion (FE)–high profile of d-serine with clinical factors (age, sex, eGFR, and albuminuria) for the detection of diabetic nephropathy (DN) in validation dataset of (A) whole range of albuminuria and (B) albuminuria between 30 and 6310 mg/g creatinine. (C) Relation of FE of d-serine with eGFR in patients with plasma level of d-serine >2.34 μM. Scatterplots are shown in participants with or without DN, and in all participants. Values are transformed to common logarithm. AUC, area under curve; IgAN, IgA nephropathy; MCD, minimal change disease; reference, participants without kidney disease; Ser, serine.
The range of proteinuria is broad in patients with DN, and patients often present with moderate or severe proteinuria (albuminuria of 30 − 300 or >300 mg/g creatinine, respectively) (21). The maximum level of albuminuria was 6310 mg/g creatinine in patients with DN in this study. The level of proteinuria is wide in patients with DN, and it is important to detect DN in patients with higher level of proteinuria. To examine this, we performed relevant sensitivity analyses. In the analysis of several ranges of albuminuria, the DN-d-Ser profile consistently outperformed the prediction model developed only for clinical factors (Figure 4B and Supplemental Figure 4, A–C). Overall, the DN-d-Ser profile provides additional information on classic clinical factors in the detection of DN in patients undergoing kidney biopsy.
Characteristic FE of d-Serine in Patients with DN
The FE of d-serine clearly distinguished DN from MCD and IgAN in patients with d-serine plasma levels >2.34 μM. We further investigated what the FE of d-serine reflects at this range of plasma d-serine. We noticed that the FE of d-serine and eGFR correlated inversely in patients with DN; i.e., the FE of d-serine increased in patients with worse eGFR (Figure 4C). In contrast, the FE of d-serine correlated positively in the remaining participants. Opposite urinary excretion dynamics of d-serine was noted between DN and other kidney diseases in patients with decreased GFR.
Because the FE of d-serine is a measure for tubular function of reabsorption, we examined the associations with tubular injury markers in these patients. The FE of d-serine positively correlated with the urinary level of β2-microglobulin in patients with DN, whereas it correlated inversely with the urinary level of N-acetyl-β-D-glucosaminidase in the remaining participants. The FE of d-serine decreased in participants with higher urinary level of N-acetyl-β-D-glucosaminidase (Supplemental Figure 5, A and B). Overall, the FE of d-serine differentially associated with kidney function and tubular injury markers depending on the origin of kidney diseases and, thus, this may facilitate the detection of DN.
Discussion
In this study, we demonstrated the utility of the d-serine profile in the diagnosis of DN. The blood and urinary profile of d-serine was characteristic in patients with DN, which may assist in diagnosis without kidney biopsy. This study extended the concept that the profile of d-serine varies depending on the origin of kidney disease, and may assist in the diagnosis of DN. Profiling d-serine in patients with kidney diseases will allow us to distinguish DN from other kinds of kidney diseases and to determine the most suitable treatment for the origin of kidney disease.
Presence of proteinuria due to diabetes is a risk for mortality and ESKD, and patients with diabetes and proteinuria are clinically diagnosed as having diabetic kidney disease (DKD) (22). DKD usually includes CKD caused by diabetes or DN, but not by GN. Profiling of d-serine provides a powerful tool for differentiating the disease spectrums of DKD and GN with diabetes. For patients with DKD who may need to avoid having a kidney biopsy, profiling of d-serine may help differentiating DN and, thus, may improve their prognosis. Previously, the d-serine blood level was reported to correlate well with GFR, because the kidney tightly regulates the dynamics of d-serine (10,12,23). Indeed, blood levels of creatinine and d-serine correlated well in this study, suggesting that the d-serine blood level is precisely regulated, even in the presence of kidney diseases. Because the profile of d-serine also reflects key information, such as kidney function and the prognosis of CKD (12), monitoring d-serine improves comprehensive management of DN.
The profile of d-serine varies by the type of kidney disease. The intrabody dynamics of d-serine are balanced between synthesis through a racemic reaction in the brain (24); food intake, which is potentially produced by microbiota (25); and urinary excretion (10,12,23). The proximal tubules of the kidney respond to serine with chiral selectivity, and the efficacy of reabsorption of d-serine is lower than that of l-serine; the FE is 60% for d-serine versus 1% for l-serine in those without kidney disease (12). d-Serine that is taken up by proximal tubules, in turn, is required to maintain kidney function (26). In the presence of DN, urinary excretion of d-serine increases, and detection of these changes emerged as a useful biomarker for the diagnosis of DN. Even in participants with lower ranges of plasma d-serine (<2.34 μM), the FE of d-serine tended to be higher and still had the potential to distinguish DN (Figure 3A).
Increased FE of d-serine in the presence of reduced eGFR is unique in DN, because FE of d-serine is not usually correlated with eGFR (10,12,23). This phenomenon clearly suggests a functional change of tubules in DN. Tubulointestinal lesions, which are often seen in patients with DN (27,28), may worsen reabsorption of proximal tubules and, thus, promote excretion of d-serine. This is in line with the weak but positive correlation between FE of d-serine and urinary β2-microglobulin, a marker for reduced reabsorption. Increased FE of d-serine was not seen in patients with IgAN and MCD who had reduced eGFR, probably because the pathologic lesions are relatively constricted to glomeruli and the reabsorption of d-serine in the remnant nephrons may be preserved. Each type of kidney disease affects kidney tubular reabsorption differently, which is reflected by the opposite effect of d-serine on FE.
There were some limitations in this study. The DN-d-Ser profile was characterized in typical cases of kidney biopsy, and including several kidney diseases in the analysis will strengthen the results obtained by this study. The primary GN studied in this study was exclusively IgAN and MCD, and it may be desirable to examine patients undergoing kidney biopsies with other GNs, such as membranous nephropathy, or secondary GN. Additionally, the analysis of patients with DN complicated by chronic GN may establish a specific d-serine profile to distinguish pure DN from complicated cases, aiding in the decision for kidney biopsy and selection of treatment. Although kidney biopsy is often avoided in patients with diabetes, inclusion of more patients with diabetes may strengthen the results obtained in this study. Whereas kidney biopsy is usually performed only once, monitoring of d-serine can be performed repeatedly. Once the efficacy of the repeat monitoring of d-serine is demonstrated, longitudinal assessment of d-serine profiles may be useful for the assessment of treatment efficacy in DN. These limitations form key concepts that need to be investigated in future studies.
In conclusion, d-serine harbored key information for the diagnosis of DN. Profiling d-serine improves both the diagnosis of DN without kidney biopsy and the estimation of kidney function. By providing vital information for clinicians, monitoring d-serine may improve the clinical outcomes of those with DN.
Disclosures
A. Hesaka reports having ownership interest in KAGAMI Inc. Y. Isaka reports serving on a speakers bureau for Astellas Pharma Inc., AstraZeneca, Kissei Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., Mitsubishi Tanabe Pharma, and Otsuka Pharmaceutical Co. Ltd.; and receiving research funding from Chugai Pharma Co. Ltd., Kyowa Kirin Co. Ltd., Mitsubishi Tanabe Pharma, and Otsuka Pharmaceutical Co. Ltd. T. Ikeda, M. Mita, and M. Nakane report being cofounders of KAGAMI Inc., a startup company working on chiral amino acids analysis and research for medical application. T. Kimura reports having ownership interest in KAGAMI Inc., and receiving research funding from Kyowa Hakko Kirin Co. Ltd., Shiseido Co. Ltd, and KAGAMI Inc. All remaining authors have nothing to disclose.
Funding
This work was supported by Japan Society for the Promotion of Science grants 17H04188 and 21H02935, Japan Agency of Medical Research and Development grant JP20gm5010001, Osaka Kidney Foundation grant OKF19-0010, and KAGAMI Inc.
Acknowledgments
We thank Mr. Hiroshi Imoto, Mr. Eiichi Negishi, Mr. Shoto Ishigo and Ms. Yukie Terashi for techincal support.
Author Contributions
A. Hesaka, T. Hayashi, M. Horio, T. Ikeda, R. Imamura, Y. Isaka, Y. Iwata, M. Kawamura, T. Kimura, M. Mita, M. Nakane, H. Okushima, and S. Takahara were responsible for investigation; T. Hayashi, Y. Isaka and T. Kimura provided supervision; Y. Iwata, T. Kimura, and H. Okushima wrote the original draft and were responsible for formal analysis; T. Kimura conceptualized the study and was responsible for funding acquisition, project administration, and validation; and T. Kimura and Y. Tanaka were responsible for visualization.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0004282021/-/DCSupplemental.
Plasma level of d-serine and kidney function. Download Supplemental Figure 1, PDF file, 904 KB (903.7KB, pdf)
Scatter plots of plasma level and fractional excretion (FE) of d-serine in patients with all participants. Download Supplemental Figure 2, PDF file, 904 KB (903.7KB, pdf)
Detection of diabetic nephropathy (DN) using profiles of d-serine in participants without IgA nephropathy. Download Supplemental Figure 3, PDF file, 904 KB (903.7KB, pdf)
Combination of d-serine and clinical factors for the detection of diabetic nephropathy. Download Supplemental Figure 4, PDF file, 904 KB (903.7KB, pdf)
Relation of fractional excretion (FE) of d-serine and clinical parameters in patients with plasma level of d-serine above 2.34 µM. Download Supplemental Figure 5, PDF file, 904 KB (903.7KB, pdf)
Baseline characteristics of the study. Download Supplemental Table 1, PDF file, 904 KB (903.7KB, pdf)
Characteristics of prediction and validation data. Download Supplemental Table 2, PDF file, 904 KB (903.7KB, pdf)
Logistic regression analysis of d-serine profile in the detection of diabetic nephropathy (DN). Download Supplemental Table 3, PDF file, 904 KB (903.7KB, pdf)
References
- 1.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R; RENAAL Study Investigators : The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL study. Kidney Int 63: 1499–1507, 2003. 10.1046/j.1523-1755.2003.00885.x [DOI] [PubMed] [Google Scholar]
- 2.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004. 10.1111/j.1523-1755.2004.00653.x [DOI] [PubMed] [Google Scholar]
- 3.Fiorentino M, Bolignano D, Tesar V, Pisano A, Biesen WV, Tripepi G, D’Arrigo G, Gesualdo L; ERA-EDTA Immunonephrology Working Group : Renal biopsy in patients with diabetes: A pooled meta-analysis of 48 studies. Nephrol Dial Transplant 32: 97–110, 2017. 10.1093/ndt/gfw070 [DOI] [PubMed] [Google Scholar]
- 4.Bermejo S, Garcia-Carro C, Soler MJ: Diabetes and renal disease-should we biopsy? Nephrol Dial Transplant 36: 1384–1386, 2021. 10.1093/ndt/gfz248 [DOI] [PubMed] [Google Scholar]
- 5.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP : Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003. 10.1046/j.1523-1755.2003.00712.x [DOI] [PubMed] [Google Scholar]
- 6.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J; ADVANCE Collaborative Group : Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009. 10.1681/ASN.2008121270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John GT, Date A, Korula A, Jeyaseelan L, Shastry JC, Jacob CK: Nondiabetic renal disease in noninsulin-dependent diabetics in a south Indian Hospital. Nephron 67: 441–443, 1994. 10.1159/000188019 [DOI] [PubMed] [Google Scholar]
- 8.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH: Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 316: 602–610, 2016. 10.1001/jama.2016.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura T, Hamase K, Miyoshi Y, Yamamoto R, Yasuda K, Mita M, Rakugi H, Hayashi T, Isaka Y: Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci Rep 6: 26137, 2016. 10.1038/srep26137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hesaka A, Sakai S, Hamase K, Ikeda T, Matsui R, Mita M, Horio M, Isaka Y, Kimura T: D-Serine reflects kidney function and diseases. Sci Rep 9: 5104, 2019. 10.1038/s41598-019-41608-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesaka A, Yasuda K, Sakai S, Yonishi H, Namba-Hamano T, Takahashi A, Mizui M, Hamase K, Matsui R, Mita M, Horio M, Isaka Y, Kimura T: Dynamics of D-serine reflected the recovery course of a patient with rapidly progressive glomerulonephritis. CEN Case Rep 8: 297–300, 2019. 10.1007/s13730-019-00411-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura T, Hesaka A, Isaka Y: D-Amino acids and kidney diseases. Clin Exp Nephrol 24: 404–410, 2020. 10.1007/s10157-020-01862-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs HA: Metabolism of amino-acids: Deamination of amino-acids. Biochem J 29: 1620–1644, 1935. 10.1042/bj0291620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takahashi K: The presence of free D-serine in rat brain. FEBS Lett 296: 33–36, 1992. 10.1016/0014-5793(92)80397-Y [DOI] [PubMed] [Google Scholar]
- 15.Okushima H, Iwata Y, Hesaka A, Sugimori E, Ikeda T, Nakane M, Mita M, Hayashi T, Isaka Y, Kimura T: Intra-body dynamics of D-serine reflects the origin of kidney diseases. Clin Exp Nephrol 25: 893–901, 2021. 10.1007/s10157-021-02052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, Tsuruya K, Kiyomoto H, Iida H, Sasaki T, Higuchi M, Hattori M, Oka K, Kagami S, Kawamura T, Takeda T, Hataya H, Fukasawa Y, Fukatsu A, Morozumi K, Yoshikawa N, Shimizu A, Kitamura H, Yuzawa Y, Matsuo S, Kiyohara Y, Joh K, Nagata M, Taguchi T, Makino H; Committee for Standardization of Renal Pathological Diagnosis; Committee for Kidney Disease Registry; Japanese Society of Nephrology : Japan Renal Biopsy Registry and Japan Kidney Disease Registry: Committee Report for 2009 and 2010. Clin Exp Nephrol 17: 155–173, 2013. 10.1007/s10157-012-0746-8 [DOI] [PubMed] [Google Scholar]
- 17.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators Developing the Japanese Equation for Estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009. 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi Y, Hamase K, Tojo Y, Mita M, Konno R, Zaitsu K: Determination of D-serine and D-alanine in the tissues and physiological fluids of mice with various D-amino-acid oxidase activities using two-dimensional high-performance liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2506–2512, 2009. 10.1016/j.jchromb.2009.06.028 [DOI] [PubMed] [Google Scholar]
- 19.Hamase K, Miyoshi Y, Ueno K, Han H, Hirano J, Morikawa A, Mita M, Kaneko T, Lindner W, Zaitsu K: Simultaneous determination of hydrophilic amino acid enantiomers in mammalian tissues and physiological fluids applying a fully automated micro-two-dimensional high-performance liquid chromatographic concept. J Chromatogr A 1217: 1056–1062, 2010. 10.1016/j.chroma.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 20.Hamase K, Ikeda T, Ishii C, Ishigo S, Masuyama K, Akita T, Furusho A, Takahashi M, Ide T, Mita M: Determination of trace amounts of chiral amino acids in complicated biological samples using two-dimensional high-performance liquid chromatography with an innovative “shape-fitting” peak identification/quantification method. Chromatography (Basel) 39: 147–152, 2018. 10.15583/jpchrom.2018.019 [DOI] [Google Scholar]
- 21.Levin A, Stevens PE: Summary of KDIGO 2012 CKD guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85: 49–61, 2014. 10.1038/ki.2013.444 [DOI] [PubMed] [Google Scholar]
- 22.Persson F, Rossing P: Diagnosis of diabetic kidney disease: State of the art and future perspective. Kidney Int Suppl (2011) 8: 2–7, 2018. 10.1016/j.kisu.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T, Hesaka A, Isaka Y: Utility of d-serine monitoring in kidney disease. Biochim Biophys Acta Proteins Proteomics 1868: 140449, 2020. 10.1016/j.bbapap.2020.140449 [DOI] [PubMed] [Google Scholar]
- 24.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H: Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol 510: 641–654, 2008. 10.1002/cne.21822 [DOI] [PubMed] [Google Scholar]
- 25.Sasabe J, Miyoshi Y, Rakoff-Nahoum S, Zhang T, Mita M, Davis BM, Hamase K, Waldor MK: Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat Microbiol 1: 16125, 2016. 10.1038/nmicrobiol.2016.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesaka A, Tsukamoto Y, Nada S, Kawamura M, Ichimaru N, Nakane M, Mita M, Okuzaki D, Okada M, Isaka Y, Kimura T: D-Serine mediates cellular proliferation for kidney remodeling [published online ahead of print August 16, 2021]. Kidney360 10.34067/KID.0000832021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017. 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J: Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl 68: S82–S86, 2005. 10.1111/j.1523-1755.2005.09915.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma level of d-serine and kidney function. Download Supplemental Figure 1, PDF file, 904 KB (903.7KB, pdf)
Scatter plots of plasma level and fractional excretion (FE) of d-serine in patients with all participants. Download Supplemental Figure 2, PDF file, 904 KB (903.7KB, pdf)
Detection of diabetic nephropathy (DN) using profiles of d-serine in participants without IgA nephropathy. Download Supplemental Figure 3, PDF file, 904 KB (903.7KB, pdf)
Combination of d-serine and clinical factors for the detection of diabetic nephropathy. Download Supplemental Figure 4, PDF file, 904 KB (903.7KB, pdf)
Relation of fractional excretion (FE) of d-serine and clinical parameters in patients with plasma level of d-serine above 2.34 µM. Download Supplemental Figure 5, PDF file, 904 KB (903.7KB, pdf)
Baseline characteristics of the study. Download Supplemental Table 1, PDF file, 904 KB (903.7KB, pdf)
Characteristics of prediction and validation data. Download Supplemental Table 2, PDF file, 904 KB (903.7KB, pdf)
Logistic regression analysis of d-serine profile in the detection of diabetic nephropathy (DN). Download Supplemental Table 3, PDF file, 904 KB (903.7KB, pdf)