Key Points
Arteriovenous fistula (AVF) nonmaturation is a persistent problem, and there are some notable disparities in AVF maturation outcomes by sex and race.
Panel reactive antibodies (PRA) are markers of immune system reactivity that tend to be higher among female and Black patients, and are associated with greater cardiovascular mortality outside the transplant setting.
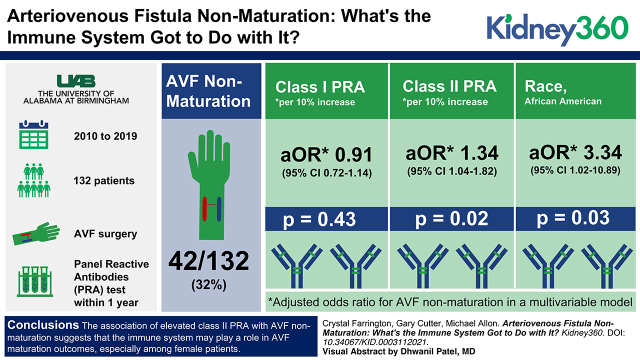
On multivariable analysis, class II PRA were independently associated with greater rates of AVF nonmaturation in this study population, suggesting a possible role for the adaptive immune system in AVF maturation outcomes.
Keywords: dialysis, arteriovenous access, arteriovenous fistula, AVF, AVF failure, dialysis access, immune system, immunology, nonmaturation, panel reactive antibodies, vascular access
Visual Abstract
Abstract
Background
Arteriovenous fistula (AVF) nonmaturation is a persistent problem, particularly among female and Black patients. Increasingly, the immune system has been recognized as an important contributor to vascular disease, but few studies have examined immune factors relative to AVF maturation outcomes. This study evaluated the association of serum panel reactive antibodies (PRA), a measure of immune system reactivity assessed in patients undergoing kidney transplant evaluation, with AVF nonmaturation.
Methods
We identified 132 patients at our institution who underwent surgical AVF placement between 2010–2019 and had PRA testing within 1 year of AVF creation. Multivariable logistic regression was used to determine the association of patient demographic and clinical factors, class I and class II PRA levels, and preoperative arterial and venous diameters with AVF maturation outcomes.
Results
AVF nonmaturation was more likely in females than males (44% versus 20%, P=0.003) and in Black than white patients (40% versus 13%, P=0.001). Class II PRA was higher in females than males (12%±23% versus 4%±13%, P=0.02). In the multivariable model, AVF nonmaturation was associated with class II PRA (adjusted odds ratio [aOR], 1.34 per 10% increase; 95% confidence interval [95% CI], 1.04 to 1.82, P=0.02) and Black race (aOR, 3.34; 95% CI, 1.02 to 10.89, P=0.03), but not with patient sex or preoperative arterial or venous diameters.
Conclusions
The association of elevated class II PRA with AVF nonmaturation suggests the immune system may play a role in AVF maturation outcomes, especially among female patients.
Introduction
Arteriovenous fistula (AVF) nonmaturation remains a significant clinical problem among patients with advanced CKD in the United States, such that 30%–60% of new AVFs are never used for dialysis (1–3). Advanced age, diabetes, and smaller preoperative vascular diameters have been associated with greater rates of AVF nonmaturation (4,5). AVF nonmaturation (including early AVF thrombosis) is more likely to occur in female and Black patients (6–10). A study using the Hemodialysis Fistula Maturation cohort reported that early AVF thrombosis was three times higher in females versus males, and twice as high in Black versus White patients, although this racial difference was not statistically significant (11). Sex and racial disparities in AVF maturation outcomes are not adequately explained by differences in clinical characteristics or preoperative blood vessel diameters, suggesting additional unidentified factors contribute to AVF nonmaturation, especially in the populations at risk for poor AVF maturation outcomes (7,12–14).
The role of the immune system in vascular diseases related to organ transplantation, such as cardiac allograft vasculopathy and transplant glomerulopathy, has been well established (15,16). HLA antibodies, clinically measured as panel reactive antibodies (PRA), may promote vascular neointimal hyperplasia (NH) in these disease processes (17). In addition, dysregulated immune activity has been linked to the pathogenesis of nontransplant-related vascular diseases, including atherosclerosis, hypertension, and systemic vasculitis (18–20). Elevated PRA levels are also independently associated with greater cardiovascular mortality, suggesting PRA may have important clinical relevance to vascular disease beyond organ transplantation (21). However, whether PRA also correlate with AVF nonmaturation has not been well studied.
The goal of this study was to evaluate elevated PRA as potential contributors to AVF nonmaturation, hypothesizing that differences in immune system reactivity (sensitization) may be a factor in greater rates of AVF nonmaturation reported among female and/or Black patients. Although determining the mechanism was outside the scope of this retrospective, epidemiologic study, we hypothesized that higher PRA levels likely contribute to the development NH in the newly created AVF, leading to blood flow–limiting stenosis and/or thrombosis and ultimately resulting in AVF nonmaturation. We retrospectively analyzed a cohort of 132 patients who underwent PRA testing within 1 year before or after AVF creation at our institution, to determine the association of PRA levels with clinical AVF maturation outcomes.
Methods
Study Setting
Approximately 550 patients with ESKD receive maintenance hemodialysis under the medical directorship of the University of Alabama at Birmingham (UAB). Four experienced surgeons created all new AVFs. Standardized preoperative vascular mapping ultrasounds were performed by trained technologists and interpreted by radiologists, and the results were provided to surgeons before the preoperative visits for AVF evaluation. Vessels were deemed suitable for an AVF in either the forearm or upper arm if the arterial diameter was ≥2.0 mm, the venous diameter ≥2.5 mm, and there was no thrombosis or stenosis of the draining veins at the proposed AVF location (22). If the AVF failed to mature within 6–8 weeks, additional interventions were performed to promote its maturation. Interventional radiologists and nephrologists performed the majority of percutaneous AVF interventions, with surgeons performing any necessary surgical interventions (e.g., revision of the anastomosis). In addition, UAB is a large transplant center, averaging >300 kidney transplants annually. PRA levels (with values ranging from 0% to 100%) are typically obtained at the time of initial transplant evaluation. Having both a substantial dialysis population and a busy transplant center at our institution provided a unique opportunity to evaluate the association between PRA levels and AVF nonmaturation. This study was granted expedited approval through the Institutional Review Board.
Data Collection
Two dedicated dialysis access coordinators employed by the UAB Division of Nephrology maintained a prospective, computerized database of all vascular access procedures (23). A separate database of patients evaluated for kidney transplantation was maintained by the Department of Surgery. Merging these two databases by medical record number yielded 397 unique patients who underwent both kidney transplant evaluation and surgical AVF placement at UAB between January 1, 2010 and December 31, 2019. Patients were excluded from the study if (1) PRA results were not recorded in the electronic medical record (EMR) (21%); (2) PRA measurements were obtained >365 days before or after AVF placement (38%); (3) patients were younger than 18 years of age at AVF placement (<1%); (4) the AVF was placed at a non-UAB hospital (<1%); or (5) AVF outcomes were indeterminate due to early patient loss to follow-up, relocation, or death (<1%). The final cohort of 132 patients included 66 males and 66 females. The equal number of male and female patients was not predetermined (Figure 1).
Figure 1.
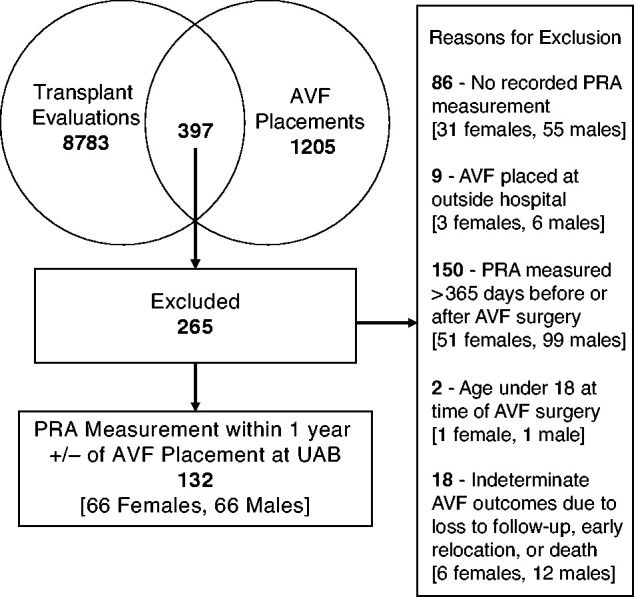
Patient inclusion and exclusion criteria for the study cohort. A total of 397 unique patients had both kidney transplant evaluation and arteriovenous fistula (AVF) placement at University of Alabama at Birmingham (UAB). Of those, 132 adult patients (66 females and 66 males) had panel reactive antibodies (PRA) measured within 1 year before or after AVF surgery.
Variables of Interest
The primary exposure of interest was PRA measured by flow cytometry or multiplex immunoassay testing within 1 year before or after AVF placement. This timeframe was prespecified in an effort to minimize bias and improve the accuracy of results by selecting the PRA level most proximal to AVF placement. The primary study outcome was clinical AVF nonmaturation, which was defined on the basis of the patient’s central venous catheter (CVC) status at the time of AVF creation. For patients with any CVC use in the study period, AVF nonmaturation was defined as nonremoval of the CVC >180 days from the date of AVF surgery. For patients who did not require any CVC use (i.e., those who were pre-ESKD, on peritoneal dialysis, or who had a working AVF or arteriovenous graft at the time of AVF creation), AVF nonmaturation was defined as the inability to initiate hemodialysis using the new AVF when the patient was clinically determined to need dialysis (Table 1). The EMRs of patients meeting study criteria were reviewed, and information regarding demographic and clinical characteristics, class I, and class II PRA percentages, and preoperative arterial and venous diameters was extracted.
Table 1.
Arteriovenous fistula nonmaturation was primarily defined on the basis of whether the patient required central venous catheter use over the study period
| Definition of AVF Nonmaturation | |
|---|---|
| 1. With any CVC use (n=91): Nonremoval of the CVC >180 days from the date of AVF creation. 2. Without any CVC use (n=41): Inability to initiate HD using the new AVF at the time HD was deemed clinically necessary, or any AVF abandonment/new access creation before HD initiation. | |
| AVF maturation outcomes | |
| Nonmaturation (n=43) • 14 patients not on dialysis at the time of AVF placement initiated HD with a CVC after AVF placement and did not have CVC removed until >180 days after the date of AVF surgery • Seven patients not on dialysis at the time of AVF placement had early AVF thrombosis and underwent AVG placement before the initiation of HD • 21 patients dialyzing with a CVC at the time of AVF placement and did not have CVC removed until >180 days after the date of AVF surgery • One patient dialyzing with an AVG at the time of AVF placement had the AVF clot 3 months after surgery, and AVF was ultimately abandoned without being used for a new AVF 4 months after the date of AVF surgery |
Successful maturation (n=99) • 30 patients not on dialysis at the time of AVF placement initiated HD with a mature AVF at a median of 124 days after AVF creation (range 49–568 days) without any CVC usage • 15 patients not on dialysis at the time of AVF placement initiated HD with a CVC and had the CVC removed ≤180 days after AVF creation • 41 patients dialyzing with a CVC at the time of AVF placement had the CVC removed ≤180 days after AVF creation • Two patients on PD initiated HD using the new AVF at 49 and 127 days after AVF creation, respectively • One patient dialyzing with an AVF at the time of AVF placement initiated HD with the new AVF 69 days after AVF creation |
Granular data regarding arteriovenous fistula maturation outcomes is listed below. AVF, arteriovenous fistula; CVC, central venous catheter; HD, hemodialysis; AVG, arteriovenous graft; PD, peritoneal dialysis.
Statistical Analysis
Baseline demographic and clinical characteristics of patients receiving an AVF were summarized and compared using a chi-squared test for categorical variables and t tests or nonparametric tests for continuous variables. P values <0.05 were considered statistically significant. Univariable analyses and multivariable logistic regression, with adjusted odds ratios (OR) and 95% confidence intervals (95% CI) for AVF nonmaturation, were performed. Receiver operator characteristic curves were generated to determine the predictive value of demographic, clinical, and ultrasound characteristics on AVF nonmaturation. Receiver operator characteristic area under the curve >0.7 was considered clinically significant.
Results
Baseline Patient Characteristics
The study cohort consisted of 132 patients with CKD who underwent AVF creation and had a PRA measured within 1 year before or after AVF creation (Figure 1). The median time (absolute value) between AVF creation and PRA measurement was 136 days (interquartile range, 69–254 days). A total of 22 patients (17%) had PRA measured before AVF placement, and 62 patients (47%) had more than one recorded PRA measurement in the EMR. In patients with >1 PRA measurement, the median number of days between subsequent PRA measurements was 864 (interquartile range, 400–1592 days). The change in class I and class II PRA over this period was ±3% or less for 75% and 87% of patients, respectively. The cohort was 50% female and 70% Black, and had a mean age of 49±13 years, with Black patients being younger than White patients (47±13 years versus 54±12 years, P=0.003). A greater number of Black females than Black males were included in the study (56% versus 44%, P=0.03). Nearly all patients (96%) had hypertension, 53% had diabetes, 14% had coronary artery disease, and 9% had peripheral vascular disease. Females were more likely than males to have an AVF placed in the upper arm (79% versus 52%, P=0.001) (Table 2).
Table 2.
Baseline demographic, clinical, and preoperative ultrasound characteristics of the study population by sex and race
| Variable | Sex | Race | All Patients (n=132) | ||||
|---|---|---|---|---|---|---|---|
| Female (n=66) |
Male (n=66) |
P Value | Black (n=93) |
White (n=39) |
P Value | ||
| Age in yr, mean±SD | 50±13 | 49±13 | 0.65 | 47±13 | 54±12 | 0.003 | 49±13 |
| Female sex, n (%) | — | 52 (79) | 14 (21) | 0.03 | 66 (50) | ||
| Black race, n (%) | 52 (79) | 41 (62) | 0.03 | — | 93 (70) | ||
| HTN, n (%) | 64 (97) | 63 (95) | 0.65 | 92 (99) | 35 (90) | 0.02 | 127 (96) |
| DM, n (%) | 36 (55) | 34 (52) | 0.73 | 49 (52) | 21 (54) | 0.90 | 70 (53) |
| CAD, n (%) | 6 (9) | 13 (20) | 0.08 | 14 (15) | 5 (13) | 0.74 | 19 (14) |
| CVD, n (%) | 7 (11) | 7 (11) | 1.0 | 11 (12) | 3 (8) | 0.47 | 14 (11) |
| PVD, n (%) | 7 (11) | 5 (8) | 0.54 | 9 (10) | 3 (8) | 0.71 | 12 (9) |
| LVEF <55%, n (%)a | 6 (9) | 14 (22) | 0.04 | 18 (20)b | 2 (2) | 0.02 | 20 (15) |
| BMI, kg/m2, mean±SD | 30.3±7.8 | 30.1±6.5 | 0.85 | 30.8±7.2 | 28.8±7.2 | 0.15 | 30.2±7.2 |
| Upper arm AVF, n (%) | 52 (79) | 34 (52) | 0.001 | 33 (35) | 13 (33) | 0.81 | 86 (65) |
| Preoperative arterial diameter in mm, mean±SD | 3.4±0.9 | 3.7±1.3 | 0.10 | 3.6±1.1 | 3.5±1.3 | 0.68 | 3.5±1.2 |
| Preoperative venous diameter in mm, mean±SD | 3.6±0.8 | 3.8±1.1 | 0.45 | 3.6±0.8 | 4.1±1.2 | 0.02 | 3.7±1.0 |
| AVF nonmaturation, n (%) | 29 (44) | 13 (20) | 0.003 | 37 (40) | 5 (13) | 0.001 | 42 (32) |
HTN, hypertension; DM, diabetes mellitus; CAD, coronary artery disease; CVD, cerebrovascular disease; PVD, peripheral vascular disease; LVEF, left ventricular ejection fraction; BMI, body mass index.
One missing value.
Three missing values.
Sex and Racial Differences in Baseline Patient Characteristics
Black patients were more likely than White patients to have hypertension (99% versus 90%, P=0.02) and left ventricular ejection fraction <55% (20% versus 5%, P=0.02). Males were more likely to have an left ventricular ejection fraction <55% compared with females (22% versus 9%, P=0.04). Otherwise, the frequency of comorbidities, including diabetes, coronary artery disease, peripheral vascular disease, and obesity, did not vary significantly by race or sex (Table 2). The mean preoperative arterial and venous diameters were comparable between male and female patients. Likewise, mean arterial diameter was similar between Black and White patients (3.6±1.1 mm versus 3.5±1.3 mm, P=0.68). Venous diameters were smaller in Black versus White patients (3.6±0.8 mm versus 4.1±1.2 mm, P=0.02), but well above the minimum threshold of 2.5 mm. (Table 2). The mean values of both class I and class II PRA were approximately three times higher in females than males (class I 17%±27% versus 6%±12%, P=0.002 and class II 12%±23% versus 4%±13%, P=0.02, respectively) and were also higher among Black versus White patients (class I 12%±22% versus 9%±20%, P=0.47 and class II 9%±20% versus 6%±16%, P=0.39, respectively), although the latter difference was not statistically significant (Table 3).
Table 3.
Association of class I and class II panel reactive antibodies with patient demographics and arteriovenous fistula maturation outcome
| Variable | Class I Panel Reactive Antibodies %, mean±SD | P Value | Class II Panel Reactive Antibodies %, mean±SDa | P Value |
|---|---|---|---|---|
| Cohort (n = 132) | 11±21 | — | 8±19 | — |
| Sex | ||||
| Female | 17±27 | 0.002 | 12±23 | 0.02 |
| Male | 6±12 | 0.002 | 4±13 | 0.02 |
| Race | ||||
| Black | 12±22 | 0.47 | 9±20 | 0.39 |
| White | 9±20 | 0.47 | 6±16 | 0.39 |
| AVF mature | ||||
| Yes | 10±20 | 0.12 | 5±14 | 0.03 |
| No | 16±24 | 0.12 | 14±26 | 0.03 |
AVF, arteriovenous fistula.
One missing value.
Association of Baseline Patient Characteristics with AVF Nonmaturation
AVF nonmaturation occurred in 42 of 132 patients (32%), with greater rates observed in females versus males (44% versus 20%, P=0.003), and in Black versus White patients (40% versus 13%, P=0.001) (Table 2). In total, 22 patients had a history of previous heart, liver, or kidney transplant. Of these, eight patients (36%) had nonmaturing AVFs versus 34 patients (31%) with nonmaturing AVFs in the nontransplant group (P=0.62). In total, 59 patients reported a prior blood transfusion, nine had no data regarding transfusion history, and the remaining 64 patients had no recorded history of blood transfusion. Overall, 18 patients (31%) with a history of blood transfusion had AVF nonmaturation, whereas 21 patients (33%) with no history of blood transfusion had AVF nonmaturation (P=0.31). Immunosuppressant, anticoagulant, or tobacco use were not significantly associated with AVF outcomes (Table 4). On univariable analysis, females were three times more likely to have AVF nonmaturation compared with males (OR, 3.20; 95% CI, 1.47 to 6.95, P=0.003). Additionally, women with two versus zero pregnancies were nearly five times more likely to have AVF nonmaturation (OR, 4.88; 95% CI, 1.06 to 22.38, P=0.03). Black race was associated with nearly 4.5 times greater odds of AVF nonmaturation compared with White race (OR, 4.49; 95% CI, 1.61 to 12.54, P=0.001). Preoperative venous diameters were smaller in patients with nonmaturing AVFs (3.4±0.6 mm versus 3.8±1.1 mm, P=0.01), but remained well above the minimum threshold of 2.5 mm required by our institutional protocol. Every 1 mm increase in venous diameter was associated with a 39% lower likelihood of AVF nonmaturation (OR, 0.61; 95% CI, 0.37 to 0.95, P=0.03) (Table 4). Finally, the mean preoperative arterial diameters were similar in patients whose AVFs matured versus those whose AVFs did not mature (3.6±1.2 mm versus 3.3±0.9 mm, P=0.12).
Table 4.
Unadjusted odds ratios for the likelihood of arteriovenous fistula nonmaturation on the basis of demographic, clinical, vascular, and immune factors
| Variable | Unadjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age, per 10 yr increase | 0.94 | 0.71 to 1.25 | 0.68 |
| Sex, female | 3.20 | 1.47 to 6.95 | 0.005 |
| Black race | 4.49 | 1.61 to 12.54 | 0.002 |
| HTN, yes versus no | 0.52 | 0.06 to 4.84 | 0.55 |
| DM, yes versus no | 0.59 | 0.28 to 1.24 | 0.16 |
| CAD, yes versus no | 1.36 | 0.45 to 4.07 | 0.57 |
| CVD, yes versus no | 0.59 | 0.19 to 1.81 | 0.36 |
| PVD, yes versus no | 1.44 | 0.37 to 5.64 | 0.59 |
| LVEF <55%, yes versus no | 0.95 | 0.33 to 2.67 | 0.92 |
| BMI in kg/m2, per 5 point increase, yes versus no | 0.96 | 0.74 to 1.24 | 0.78 |
| History of solid organ transplant, yes versus no | 1.39 | 0.51 to 3.63 | 0.51 |
| History of blood transfusion, yes versus no (females only) | 0.90 | 0.42 to 1.92 | |
| History of 2 versus 0 pregnancies | 4.88 | 1.06 to 22.38 | 0.03 |
| Taking immunosuppressant medications, yes versus noa | 0.80 | 0.30 to 1.93 | 0.62 |
| Current or former tobacco user, yes versus no | 0.66 | 0.30 to 1.45 | 0.30 |
| Taking anticoagulation, yes versus nob | 0.94 | 0.44 to 1.96 | 0.8 |
| Upper arm AVF location | 0.91 | 0.42 to 1.96 | 0.80 |
| Arterial diameter, per 1 mm increase | 0.78 | 0.54 to 1.09 | 0.15 |
| Venous diameter, per 1 mm increase | 0.61 | 0.37 to 0.95 | 0.03 |
| Class I PRA, per 10% increase | 1.14 | 0.96 to 1.34 | 0.12 |
| Class II PRA, per 10% increase | 1.27 | 1.06 to 1.58 | 0.01 |
HTN, hypertension; DM, diabetes mellitus; CAD, coronary artery disease; CVD, cerebrovascular disease; PVD, peripheral vascular disease; LVEF, left ventricular ejection fraction; BMI, body mass index; PRA, panel reactive antibodies.
Immunosuppressant medications included steroids, calcineurin inhibitors, and mycophenolate mofetil.
Anticoagulation included aspirin, warfarin, clopidogrel, and rivaroxaban.
Class I and class II PRA levels were higher in patients with nonmaturing versus maturing AVFs, but only the difference in class II PRA achieved statistical significance (14%±26% versus 5%±14%, P=0.03) (Table 3). In the subset of 22 patients with PRA measured before AVF placement, the mean class I PRA was 23%±22% for patients with nonmaturing AVFs versus 12%±27% for patients with maturing AVFs (P=0.03). Similarly, the mean class II PRA was 22%±33% for patients with nonmaturing AVFs versus 1%±3% for patients with maturing AVFs (P=0.02). Class I and class II PRA levels were higher in both males and females with nonmaturing AVFs compared with those whose AVFs matured, but these differences were not significant. However, PRA levels in females with nonmaturing AVFs were six times higher than in males with maturing AVFs (18%±30% versus 3%±13%, P=0.01) (Figure 2).
Figure 2.
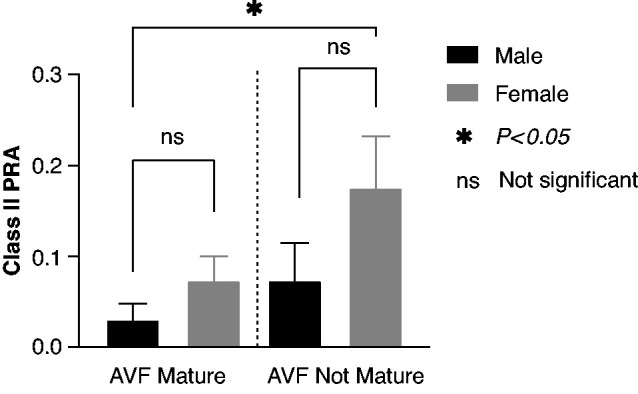
In maturing AVFs, mean class II PRA was 3%±13% in males versus 7%±16% in females (P = 0.11). In nonmaturing AVFs, mean class II PRA was 7%±15% in males versus 18%±30% in females (P=0.43). Mean class II PRA was six times higher in females with nonmaturing AVFs versus males with maturing AVFs (P=0.01). Note: in the figure, class II PRA is expressed as a decimal.
Using multivariable logistic regression to control for potential confounding factors, the odds of AVF nonmaturation were 34% greater for each absolute 10% increase in class II PRA (OR, 1.34; 95% CI, 1.04 to 1.82, P=0.02). Black race was also independently associated with over three-fold greater risk of AVF nonmaturation (OR, 3.34; 95% CI, 1.02 to 10.89, P=0.03). Females were almost twice as likely to have AVF nonmaturation compared with males, although this difference was no longer statistically significant (OR, 1.96; 95% CI, 0.80 to 4.8, P=0.14). Neither preoperative arterial nor venous diameters were significantly associated with AVF nonmaturation (Table 4 and Table 5). Seven variables (age, sex, race, preoperative arterial, and venous diameters, and class I and class II PRA) were used to construct a multivariable model. These variables were chosen because, outside of the novel introduction of class I and class II PRA, they commonly influence clinical decision making regarding a patient’s appropriateness for AVF creation. A receiver operating characteristic curve including all seven variables demonstrated an area under the curve of 0.73 (95% CI, 0.63 to 0.82, P<0.0001) (Figure 3).
Table 5.
Adjusted odds ratios for arteriovenous fistula nonmaturation in a multivariable model including demographic, vascular, and immune factors
| Variable | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age, per 10 yr increase | 1.09 | 0.76 to 1.56 | 0.64 |
| Sex, female | 1.96 | 0.80 to 4.81 | 0.14 |
| Race, Black | 3.34 | 1.02 to 10.89 | 0.03 |
| Preoperative arterial diameter, per 1 mm increase | 0.72 | 0.44 to 1.15 | 0.17 |
| Preoperative venous diameter, per 1 mm increase | 0.82 | 0.45 to 1.47 | 0.51 |
| Class I PRA, per 10% increase | 0.91 | 0.72 to 1.14 | 0.42 |
| Class II PRA, per 10% increase | 1.34 | 1.04 to 1.82 | 0.02 |
Overall P value for the model is 0.007. PRA, panel reactive antibodies.
Figure 3.
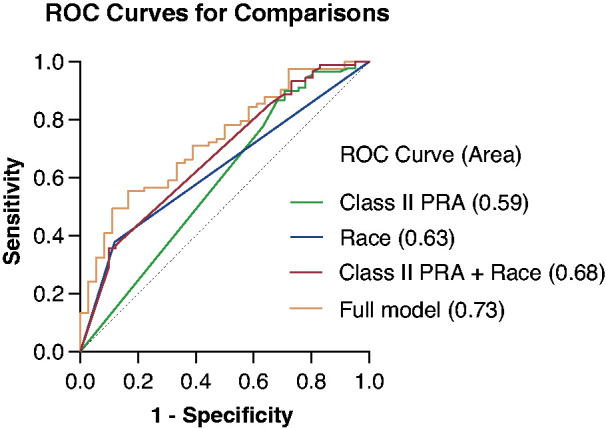
Receiver operating characteristic curves showing the predictive value of class II PRA and race alone and in combination. The full model including age, sex, race, preoperative arterial and venous diameters, and class I and class II PRA for predicting AVF nonmaturation increased the area under the curve (AUC) to 0.73 (95% confidence interval, 0.63 to 0.82, P=0.007).
Discussion
Our study identified a novel association between higher class II PRA levels and AVF nonmaturation. Female and Black patients had greater rates of AVF nonmaturation compared with male and White patients, respectively. Females also demonstrated higher class II PRA levels than males, whereas class II PRA did not vary significantly by race. It has been suggested that worse AVF maturation outcomes in females may be due to smaller baseline blood vessel diameters relative to males (9). Yet a number of subsequent studies have reported that females have inferior rates of AVF maturation than males, despite similar preoperative vascular diameters, consistent with this study’s findings (7,12–14). Of note, on multivariable analysis, female sex was no longer an independent predictor of AVF nonmaturation, whereas Black race persisted. One potential interpretation of these findings is that immune activity (as exemplified by class II PRA levels) may partially explain sex differences in AVF maturation outcomes. By contrast, PRA levels do not appear to account for racial differences in AVF maturation, which are likely due to variations in processes of care (10).
To date, PRA have had the greatest clinical relevance in solid organ transplantation, where elevated PRA have important implications for allograft function and survival (24,25). PRA develop due to previous exposure to a foreign antigen, most often from pregnancy, blood transfusion, or solid organ transplantation (26). Multiparous females (especially those with ≥3 pregnancies) are more likely to develop PRA from exposure to paternal antigens in the developing fetus (27). Higher PRA levels have been reported in Black compared with White populations, but sex imbalances between Black and White study participants may contribute to this perceived racial disparity (28,29). Elevated PRA are also associated with increased cardiovascular mortality in patients with ESKD (30). For example, a recent study observed that higher PRA was an independent predictor of cardiovascular and all-cause mortality among over 160,000 patients waitlisted for a kidney transplant (21).
Accumulating evidence suggests the immune system plays a critical part in tissue maintenance and repair beyond its traditional role of distinguishing self from non-self and eradicating pathogens (31). In recent years, the immune system has been recognized as an important contributor to a number of cardiovascular diseases, including hypertension, atherosclerosis, and the vasculitides (15,16,18–20,32). Although the intact vascular endothelium is not immunologically reactive, if the blood vessels become damaged, further vascular injury may occur through a variety of innate and adaptive immune mechanisms (33,34). We postulate that vascular injury from surgery itself may trigger immune activity in the vessels used to create the AVF, and thereby promote stenosis or thrombosis in the developing AVF, resulting in its nonmaturation.
AVF nonmaturation is often attributed to aggressive venous NH (35). In NH, vascular endothelial injury induces the recruitment of inflammatory cytokines and prothrombotic circulating factors, resulting in the proliferation of vascular smooth muscle cells that can lead to blood flow–limiting stenosis (36,37). Nevertheless, the mechanisms leading to NH in nonmaturing AVFs are not fully understood (38). Interestingly, the hallmark lesion of cardiac transplant vasculopathy is arterial NH. In this case, class II HLA antibodies (measured clinically as PRA) are crucial to developing NH by inducing proinflammatory cytokines and activating cellular signaling pathways that lead to dedifferentiation and proliferation of vascular smooth muscle cells and occlusion of the vessel (15). Kidney transplant glomerulopathy is likewise characterized by vascular NH, and promoted by class II HLA antibodies (39). Furthermore, class II HLA are expressed in atherosclerotic plaques, whose precursor lesion is NH (40,41). These associations raise the question whether similar immune mechanisms may contribute to NH in AVF nonmaturation.
Currently, limited evidence suggests a role for the immune system in AVF nonmaturation. Elevated C-reactive protein, a marker of inflammation linked with innate immune activity, has been linked with venous NH and endothelial dysfunction in the context of AVF failure (42–44). However, C-reactive protein is nonspecific, is chronically elevated (<10–50 mg/L) in patients with ESKD, and may be acutely elevated for other reasons, such as infection (45). Few prior studies have evaluated the role of the adaptive immune system in AVF nonmaturation. Although PRA might be directly involved in the pathogenesis of NH leading to AVF nonmaturation, an AVF is created with native rather than foreign vessels, so it seems most likely PRA would rather play an indirect role in AVF nonmaturation through proinflammatory effects.
The strengths of our study include its innovative conceptual approach to AVF nonmaturation as a partially immune-mediated phenomenon. At present, there are no serological biomarkers widely used in clinical practice to predict AVF maturation outcomes. If our findings are confirmed by additional prospective studies, class II PRA may serve as a novel immune biomarker to assist clinicians in determining the risk of AVF nonmaturation. This study also offers a unique perspective on sex disparities in AVF maturation by considering a potentially modifiable biologic mechanism that may explain why females have poor AVF maturation outcomes relative to males.
We present our findings as an intriguing new avenue for research into the biologic mechanism of AVF nonmaturation, but recognize their limitations. First, the study cohort was small, only 132 patients, making definitive conclusions premature regarding the use of PRA as a biomarker for AVF nonmaturation on the basis of these results. Second, PRA was measured at variable time periods within 1 year before or after AVF creation and the optimal timing of PRA measurement around AVF creation remains unclear. Third, as a single-center, retrospective study, our results may have limited external validity. Fourth, the patient population was restricted to those who had been referred for kidney transplant evaluation and had PRA levels measured. This cohort was somewhat younger, and likely healthier, than the general hemodialysis population, which may further limit generalizability. Finally, the results in our predominantly Black study cohort may not apply to patients of other races and ethnicities. However, because Black patients have worse AVF outcomes, they represent an important population of interest, so we view the greater proportion of Black patients in our study as a strength as well.
Elevated PRA are associated with vascular pathology in both transplant-related and nontransplant-related vascular diseases, but their role in AVF nonmaturation has yet to be fully explored. Class II PRA are higher in females than males. Black race and higher levels of class II PRA are independently associated with greater rates of AVF nonmaturation. Our study suggests that adaptive immune system activity may contribute to the greater rates of AVF nonmaturation among female patients on hemodialysis. Further study is needed to evaluate the relationship between PRA and/or other immune factors to AVF nonmaturation, and examine how sex differences in immune system activity may contribute to disparities in AVF maturation outcomes.
Disclosures
G. Cutter reports serving on Data and Safety Monitoring Boards for AstraZeneca, Avexis Pharmaceuticals, Biolinerx, Brainstorm Cell Therapeutics, Bristol Meyers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Mapi Pharmaceuticals, Merck, Merck/Pfizer, Mitsubishi Tanabe Pharma Holdings, Neurim, Novartis, Opko Biologics, Ophazyme, Sanofi-Aventis, Reata Pharmaceuticals, Teva Pharmaceuticals, VielaBio Inc., National Heart, Lung, and Blood Institute (Protocol Review Committee), National Institute of Child Health and Human Development (Obstetrical-Fetal Pharmacology Research Unit oversight committee); reports serving on Consulting or Advisory Boards for Alexion, Antisense Therapeutics, Biodelivery Sciences International, Biogen, Genzyme, Genentech, GW Pharmaceuticals, Immunic, Klein-Buendel Incorporated, Medimmune/Viela Bio, Medday, Merck/Serono, Neurogenesis, Novartis, Osmotica Pharmaceuticals, Perception Neurosciences, Recursion/Cerexis Pharmaceuticals, Regeneron, Reckover Pharmaceuticals, Roche, SAB Biotherapeutics, and TG Therapeutics; and reports being employed by the University of Alabama at Birmingham, and is President of Pythagoras, Inc., a private consulting company located in Birmingham, Alabama. M. Allon is a consultant for CorMedix and reports being supported by grant R01013818 from National Institute on Minority Health and Health Disparities. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
We thank Dr. Vineeta Kumar (University of Alabama at Birmingham) for providing expertise in the interpretation of PRA results.
Author Contributions
M. Allon and C. Farrington conceptualized the study; C. Farrington was responsible for data curation; G. Cutter and C. Farrington were responsible for the formal analysis; C. Farrington was responsible for investigation; M. Allon and C. Farrington were responsible for the methodology; G. Cutter was responsible for the resources; M. Allon, G. Cutter provided supervision; M. Allon, G. Cutter, and C. Farrington were responsible for the validation; C. Farrington wrote the original draft; and M. Allon, G. Cutter, and C. Farrington reviewed and edited the manuscript.
References
- 1.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI; Dialysis Access Consortium Study Group : Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008. 10.1001/jama.299.18.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon BS: Why don’t fistulas mature? Kidney Int 70: 1413–1422, 2006. 10.1038/sj.ki.5001747 [DOI] [PubMed] [Google Scholar]
- 3.Woodside KJ, Bell S, Mukhopadhyay P, Repeck KJ, Robinson IT, Eckard AR, Dasmunshi S, Plattner BW, Pearson J, Schaubel DE, Pisoni RL, Saran R: Arteriovenous fistula maturation in prevalent hemodialysis patients in the United States: A national study. [Published correction appears in Am J Kidney Dis, 72: 314, 2018] Am J Kidney Dis 71: 793–801, 2018. 10.1053/j.ajkd.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui MA, Ashraff S, Carline T: Maturation of arteriovenous fistula: Analysis of key factors. Kidney Res Clin Pract 36: 318–328, 2017. 10.23876/j.krcp.2017.36.4.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrington CA, Robbin ML, Lee T, Barker-Finkel J, Allon M: Early predictors of arteriovenous fistula maturation: A novel perspective on an enduring problem. J Am Soc Nephrol 31: 1617–1627, 2020. 10.1681/ASN.2019080848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashar K, Zafar A, Elsheikh S, Healy DA, Clarke-Moloney M, Casserly L, Burke PE, Kavanagh EG, Walsh SR: Predictive parameters of arteriovenous fistula functional maturation in a population of patients with end-stage renal disease. PLoS One 10: e0119958, 2015. 10.1371/journal.pone.0119958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson WJ, Barker J, Allon M: Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol 3: 437–441, 2008. 10.2215/CJN.03480807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui MA, Ashraff S, Santos D, Rush R, Carline T, Raza Z: Predictive parameters of arteriovenous fistula maturation in patients with end-stage renal disease. Kidney Res Clin Pract 37: 277–286, 2018. 10.23876/j.krcp.2018.37.3.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003. 10.1046/j.1523-1755.2003.00740.x [DOI] [PubMed] [Google Scholar]
- 10.Qian J, Lee T, Thamer M, Zhang Y, Crews DC, Allon M: Racial disparities in the arteriovenous fistula care continuum in hemodialysis patients. Clin J Am Soc Nephrol 15: 1796–1803, 2020. 10.2215/CJN.03600320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farber A, Imrey PB, Huber TS, Kaufman JM, Kraiss LW, Larive B, Li L, Feldman HI; HFM Study Group : Multiple preoperative and intraoperative factors predict early fistula thrombosis in the Hemodialysis Fistula Maturation Study. J Vasc Surg 63: 163–70.e6, 2016. 10.1016/j.jversus.2015.07.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A: Arteriovenous fistulae versus. arteriovenous grafts: A retrospective review of 1,700 consecutive vascular access cases. J Vasc Access 9: 231–235, 2008. 10.1177/112972980800900402 [DOI] [PubMed] [Google Scholar]
- 13.Wen M, Li Z, Li J, Zhou W, Liu Y, Liu H, Chen G: Risk factors for primary arteriovenous fistula dysfunction in hemodialysis patients: A retrospective survival analysis in multiple medical centers. Blood Purif 48: 276–282, 2019. 10.1159/000500045 [DOI] [PubMed] [Google Scholar]
- 14.Praehauser C, Breidthardt T, Moser-Bucher CN, Wolff T, Baechler K, Eugster T, Dickenmann M, Gurke L, Mayr M: The outcome of the primary vascular access and its translation into prevalent access use rates in chronic haemodialysis patients. Clin Kidney J 5: 339–346, 2012. 10.1093/ckj/sfs055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvani S, Trayssac M, Augé N, Thiers JC, Calise D, Krell HW, Sallusto F, Kamar N, Rostaing L, Thomsen M, Nègre-Salvayre A, Salvayre R: A key role for matrix metalloproteinases and neutral sphingomyelinase-2 in transplant vasculopathy triggered by anti-HLA antibody. Circulation 124: 2725–2734, 2011. 10.1161/CIRCULATIONAHA.111.021790 [DOI] [PubMed] [Google Scholar]
- 16.Filippone EJ, McCue PA, Farber JL: Transplant glomerulopathy. Mod Pathol 31: 235–252, 2018. 10.1038/modpathol.2017.123 [DOI] [PubMed] [Google Scholar]
- 17.Valenzuela NM, Reed EF: The link between major histocompatibility complex antibodies and cell proliferation. Transplant Rev (Orlando) 25: 154–166, 2011. 10.1016/j.trre.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bomfim GF, Cau SBA, Bruno AS, Fedoce AG, Carneiro FS: Hypertension: A new treatment for an old disease? Targeting the immune system. Br J Pharmacol 176: 2028–2048, 2019. 10.1111/bph.14436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gisterå A, Hansson GK: The immunology of atherosclerosis. Nat Rev Nephrol 13: 368–380, 2017. 10.1038/nrneph.2017.51 [DOI] [PubMed] [Google Scholar]
- 20.Chimenti MS, Ballanti E, Triggianese P, Perricone R: Vasculitides and the complement system: A comprehensive review. Clin Rev Allergy Immunol 49: 333–346, 2015. 10.1007/s12016-014-8453-8 [DOI] [PubMed] [Google Scholar]
- 21.Sapir-Pichhadze R, Tinckam KJ, Laupacis A, Logan AG, Beyene J, Kim SJ: Immune sensitization and mortality in wait-listed kidney transplant candidates. J Am Soc Nephrol 27: 570–578, 2016. 10.1681/ASN.2014090894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001. 10.1046/j.1523-1755.2001.00031.x [DOI] [PubMed] [Google Scholar]
- 23.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, Rhynes VK, Robbin ML, Saddekni S, Zeigler ST: A multidisciplinary approach to hemodialysis access: Prospective evaluation. Kidney Int 53: 473–479, 1998. 10.1046/j.1523-1755.1998.00761.x [DOI] [PubMed] [Google Scholar]
- 24.Mishra MN, Baliga KV: Significance of panel reactive antibodies in patients requiring kidney transplantation. Saudi J Kidney Dis Transpl 24: 495–499, 2013. 10.4103/1319-2442.111019 [DOI] [PubMed] [Google Scholar]
- 25.Gosset C, Viglietti D, Rabant M, Vérine J, Aubert O, Glotz D, Legendre C, Taupin JL, Duong Van-Huyen JP, Loupy A, Lefaucheur C: Circulating donor-specific anti-HLA antibodies are a major factor in premature and accelerated allograft fibrosis. Kidney Int 92: 729–742, 2017. 10.1016/j.kint.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 26.Iniotaki-Theodoraki A: The role of HLA class I and class II antibodies in renal transplantation. Nephrol Dial Transplant 16(Suppl 6): 150–152, 2001. 10.1093/ndt/16.suppl_6.150 [DOI] [PubMed] [Google Scholar]
- 27.Densmore TL, Goodnough LT, Ali S, Dynis M, Chaplin H: Prevalence of HLA sensitization in female apheresis donors. Transfusion 39: 103–106, 1999. 10.1046/j.1537-2995.1999.39199116901.x [DOI] [PubMed] [Google Scholar]
- 28.Morris AA, Cole RT, Veledar E, Bellam N, Laskar SR, Smith AL, Gebel HM, Bray RA, Butler J: Influence of race/ethnic differences in pre-transplantation panel reactive antibody on outcomes in heart transplant recipients. J Am Coll Cardiol 62: 2308–2315, 2013. 10.1016/j.jacc.2013.06.054 [DOI] [PubMed] [Google Scholar]
- 29.Cooper TY, Jordan CL, Willimon CM, Land GA: Comparison of panel-reactive antibody levels in Caucasian and African American renal transplant candidates. Transplantation 60: 327–330, 1995. 10.1097/00007890-199508270-00004 [DOI] [PubMed] [Google Scholar]
- 30.Magnussen C, Ruebsamen N, Ojeda FM, Rybczynski M, Kobashigawa J, Reichenspurner H, Bernhardt AM, Schnabel RB: Sex differences in preformed panel-reactive antibody levels and outcomes in patients undergoing heart transplantation. Clin Transplant 33: e13572, 2019. 10.1111/ctr.13572 [DOI] [PubMed] [Google Scholar]
- 31.Sattler S: The role of the immune system beyond the fight against infection. Adv Exp Med Biol 1003: 3–14, 2017. 10.1007/978-3-319-57613-8_1 [DOI] [PubMed] [Google Scholar]
- 32.Baganha F, de Jong A, Jukema JW, Quax PHA, de Vries MR: The role of immunomodulation in vein graft remodeling and failure. J Cardiovasc Transl Res 14: 100–109, 2020. 10.1007/s12265-020-10001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan CC, Gao PJ: Role of complement-related inflammation and vascular dysfunction in hypertension. Hypertension 73: 965–971, 2019. 10.1161/HYPERTENSIONAHA.118.11210 [DOI] [PubMed] [Google Scholar]
- 34.Al-Soudi A, Kaaij MH, Tas SW: Endothelial cells: From innocent bystanders to active participants in immune responses. Autoimmun Rev 16: 951–962, 2017. 10.1016/j.autrev.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 35.Roy-Chaudhury P, Lee TC: Vascular stenosis: Biology and interventions. Curr Opin Nephrol Hypertens 16: 516–522, 2007. 10.1097/MNH.0b013e3282efa57f [DOI] [PubMed] [Google Scholar]
- 36.Lee T: Novel paradigms for dialysis vascular access: Downstream vascular biology--is there a final common pathway? Clin J Am Soc Nephrol 8: 2194–2201, 2013. 10.2215/CJN.03490413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, Samaha A, Munda R: Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis 50: 782–790, 2007. 10.1053/j.ajkd.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 38.Lee T, Roy-Chaudhury P: Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis 16: 329–338, 2009. 10.1053/j.ackd.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Husain S, Sis B: Advances in the understanding of transplant glomerulopathy. Am J Kidney Dis 62: 352–363, 2013. 10.1053/j.ajkd.2012.10.026 [DOI] [PubMed] [Google Scholar]
- 40.Söderlund J, Forsblom C, Ilonen J, Thorn LM, Wadén J, Parkkonen M, Groop PH; FinnDiane Study Group : HLA class II is a factor in cardiovascular morbidity and mortality rates in patients with type 1 diabetes. Diabetologia 55: 2963–2969, 2012. 10.1007/s00125-012-2670-6 [DOI] [PubMed] [Google Scholar]
- 41.Haudenschild C: Morphology of intimal hyperplasia. J Vasc Surg 10: 591–592, 1989. 10.1016/0741-5214(89)90157-2 [DOI] [Google Scholar]
- 42.Khavanin Zadeh M, Mohammadipour S, Omrani Z: Correlation between CRP and early failure of arteriovenous fistula (AVF). Med J Islam Repub Iran 29: 219, 2015 [PMC free article] [PubMed] [Google Scholar]
- 43.Stirbu O, Gadalean F, Pitea IV, Ciobanu G, Schiller A, Grosu I, Nes A, Bratescu R, Olariu N, Timar B, Tandrau MC: C-reactive protein as a prognostic risk factor for loss of arteriovenous fistula patency in hemodialyzed patients. J Vasc Surg 70: 208–215, 2019. 10.1016/j.jversus.2018.10.100 [DOI] [PubMed] [Google Scholar]
- 44.Black S, Kushner I, Samols D: C-reactive Protein. J Biol Chem 279: 48487–48490, 2004. 10.1074/jbc.R400025200 [DOI] [PubMed] [Google Scholar]
- 45.Kalantar-Zadeh K: Inflammatory marker mania in chronic kidney disease: Pentraxins at the crossroad of universal soldiers of inflammation. Clin J Am Soc Nephrol 2: 872–875, 2007. 10.2215/CJN.02750707 [DOI] [PubMed] [Google Scholar]