Abstract
Enteropathogenic Escherichia coli (EPEC) was recognized as a common opportunistic pathogen of simian immunodeficiency virus-infected rhesus macaques (Macaca mulatta) with AIDS. Retrospective analysis revealed that 27 of 96 (28.1%) animals with AIDS had features of EPEC infection, and EPEC was the most frequent pathogen of the gastrointestinal tract identified morphologically. In 7.3% of animals dying with AIDS, EPEC represented the sole opportunistic agent of the gastrointestinal tract at death. In 20.8% of cases, it was seen in combination with one or more gastrointestinal pathogens, including Cryptosporidium parvum, Enterocytozoon bieneusi, Mycobacterium avium, Entamoeba histolytica, Balantidium coli, Strongyloides stercoralis, cytomegalovirus, and adenovirus. Clinically, infection was associated with persistent diarrhea and wasting and was more frequent in animals that died at under 1 year of age (P < 0.001, Fisher exact test). The organism was associated with the characteristic attaching and effacing lesion in colonic tissue sections and produced a focal adherence pattern on a HEp-2 assay but was negative for Shiga toxin production as assessed by PCR and a HeLa cell cytotoxicity assay. A 2.6-kb fragment encompassing the intimin gene was amplified and sequenced and revealed 99.2% identity to sequences obtained from human isolates (GenBank AF116899) corresponding to the epsilon intimin subtype. Further investigations with rhesus macaques may offer opportunities to study the impact of EPEC on AIDS pathogenesis and gastrointestinal dysfunction.
Worldwide, chronic diarrhea and wasting are significant causes of morbidity and mortality in patients infected with the human immunodeficiency virus (HIV) (11, 12). While a growing number of opportunistic infections have been recognized to cause these symptoms, in up to 50% of cases no etiologic agent is identified. In such patients the relative contribution of the direct effects of HIV on the gastrointestinal tract versus unrecognized pathogens has been questioned (33–35). Recently, the role of diarrheagenic bacterial infections in HIV-infected patients has been investigated with the identification of pathogenic Escherichia coli strains as potential opportunistic pathogens (17, 23, 28, 36, 37).
Six categories of diarrheagenic E. coli are defined, based on the underlying mechanism of disease pathogenesis, in vivo and in vitro growth characteristics, and the presence of specific genes encoding virulence factors (27). These include enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EaggEC), enterotoxigenic E. coli (ETEC), enterohemorrhagic E. coli (EHEC), enteroinvasive E. coli (EIEC), and diffuse adherent E. coli (DAEC). EPEC and EaggEC infections may be particularly important in children with AIDS and in individuals in underdeveloped regions where the HIV pandemic remains unchecked (11, 13, 31). Because patients with AIDS often present with multiple opportunistic pathogens and because current laboratory techniques may underestimate the true prevalence of infection, it has been difficult to ascertain the impact of pathogenic E. coli on clinical signs and disease progression. Development of an animal model to study the impact of such organisms on the host during progressive immunodeficiency might help to resolve these issues.
Rhesus macaques (Macaca mulatta) are susceptible to inoculation with the simian immunodeficiency virus (SIV) and develop an AIDS-like syndrome characterized by depletion of CD4 T lymphocytes and the occurrence of opportunistic infections (16, 21). The macaque model of AIDS has been used extensively to study aspects of disease pathogenesis and host immunity. As in humans, rhesus macaques infected with SIV develop diarrhea and wasting during AIDS. The opportunistic infections of the gastrointestinal tract seen in this setting closely approximate those seen in human patients and include Cryptosporidium parvum, Enterocytozoon bieneusi, Mycobacterium avium, Strongyloides stercoralis, Entamoeba histolytica, cytomegalovirus (CMV), and adenovirus infections (4–7, 22, 25, 26). Here we describe EPEC as a common opportunistic infection of SIV-infected rhesus macaques with AIDS. Such animals may represent a novel model with which to study the pathogenesis of EPEC infection in the normal and immunodeficient primate host.
MATERIALS AND METHODS
Index case and retrospective analysis.
Macaques were housed at the New England Regional Primate Research Center in a centralized biolevel 3 containment facility in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care and Harvard Medical School's Animal Care and Use Committee.
Animals were inoculated intravenously with pathogenic strains of SIVmac, the history, preparation, and in vivo and in vitro properties of which have been described and reviewed extensively (15, 19, 20). Animals inoculated with these viruses were included in a variety of infectivity, pathogenesis, and vaccine studies and received no antiretroviral agents or antimicrobial prophylaxis. The monkeys were monitored closely and euthanatized when they were moribund or when it was deemed necessary by the veterinary staff. Complete postmortem examinations were performed on all animals, and representative samples of tissue were taken for formalin fixation, freezing, and electron microscopy. The presence of diarrhea and the degree of wasting (0, none; 1, mild; 2, moderate; and 3, severe) were recorded at the time of death.
The index case (Mm 484-97) was a 20-week-old rhesus macaque inoculated with SIVmac239 that developed profuse diarrhea and wasting. EPEC was isolated from a rectal swab obtained prior to death, and morphologic features characteristic of an attaching and effacing lesion were identified at necropsy. Following the recognition of the index case, a retrospective analysis of archived hematoxylin- and eosin-stained tissue sections and clinical records of all SIV-infected, immunodeficient macaques which died between January 1997 and December 1998 (n = 96) was conducted. A diagnosis of EPEC infection was based on characteristic morphologic features. Other opportunistic infections were confirmed through a combination of cytochemical staining, immunohistochemistry, in situ hybridization, and ultrastructural examination (10, 18, 25, 38).
Bacterial isolation and characterization.
Rectal swabs or colonic tissue obtained at necropsy (n = 18) was cultured by standard techniques. Samples were plated on MacConkey, Hektoen, and blood agar at 37°C (5% CO2) and campylobacter agar at 42°C (5% O2, 85% N, and 10% CO2). Plates were evaluated at 24, 48, and 72 h and individual bacterial colonies were identified utilizing standard biochemical techniques (API Rapid 20E; BioMerieux Vitek).
A HEp-2 adherence assay was performed as previously described (14, 39). Briefly, 2 × 106 bacteria were added to a monolayer of HEp-2 cells grown to 50 to 70% confluence. After incubation for 3 h at 37°C in cell culture medium containing 1% (wt/vol) d-mannose, the monolayer was washed with phosphate-buffered saline. Fresh tissue culture medium was then added, and the cells were incubated for an additional 3 h. The cells were washed again with phosphate-buffered saline, fixed in methanol, and stained with 20% Giemsa (Fisher Scientific, Pittsburgh, Pa.) prior to microscopic examination. Localized adherence, diffuse adherence, and aggregative adherence patterns were determined as previously described.
PCR and sequencing.
For characterization of individual bacterial clones, DNA was isolated from single colonies obtained from MacConkey-Hektoen agar and was grown overnight in broth. Amplification of DNA was performed in a 50-μl reaction volume containing 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 9.0), 0.2 mM deoxynucleoside triphosphates, 400 pmol of each primer, and 2.5 U of Amplitaq DNA Polymerase in a 9600 thermal cycler (Perkin-Elmer Cetus, Foster City, Calif.). The amplification profile consisted of 1 min at 57°C, 2 min at 72°C, and 30 s at 94°C for 35 cycles followed by a 10-min extension at 72°C. Primers directed at the intimin (eaeA), Shiga-like toxin (stx1 and stx2), and hemolysin (hlyA) genes have been described previously and were utilized to further characterize the bacterial isolates (30). For detection of bacterium-specific virulence sequences in primary fecal cultures, rectal swabs or colonic tissue was placed in Luria-Bertani broth and was grown overnight at 37°C and 5% CO2, and DNA was isolated from cell pellets (Bio-Rad). PCR was performed as described above. For further characterization of isolates, a 2.6-kb fragment encompassing the intimin gene was amplified from isolates obtained from two animals using primers SK1 and LP5 (29). The PCR products were cloned (Invitrogen, Carlsbad, Calif.) and sequenced utilizing an ABI automated sequencer (Perkin-Elmer Cetus) and forward and reverse primers.
Serotyping and Shiga toxin assay.
Serotyping of bacterial isolates was conducted at the Pennsylvania State University E. coli Reference Laboratory (University Park). Shiga toxin production was assessed using the HeLa cell cytotoxicity assay as previously described (8).
Statistical analysis.
Groups were compared using a commercially available software package (Jandel Scientific, San Rafael, Calif.) by chi-square test of contingency, Fisher exact test, and the Mann-Whitney rank sum test, where appropriate.
Nucleotide sequence accession number.
The 2.6-kb fragment encompassing the intimin gene was given GenBank accession number AF301015.
RESULTS
Identification of EPEC in immunodeficient rhesus macaques.
Multiple isolates from SIV-infected macaques with diarrhea were tested for enteroadherent patterns utilizing the HEp-2 adhesion assay. A localized adherent pattern typical of EPEC was identified. DNA was isolated from these clones, and the presence of the eaeA gene was confirmed by amplification of a 384-bp product. Isolates from two animals were selected for further characterization, and a 2.6-kb fragment encompassing the intimin gene was amplified and sequenced. A BLAST similarity search revealed 99.2% identity to sequences obtained from human isolates (GenBank AF116899) corresponding to the epsilon intimin gene subtype (29). PCRs performed on isolates for stx1, stx2, and hlyA sequences were negative. Furthermore, Shiga toxin production was negative, as demonstrated through the HeLa cell toxicity test. Bacterial isolates positive for the eaeA gene and demonstrating the localized adherence pattern were serotype O156: H NM.
Morphologic features of EPEC infection in rhesus macaques.
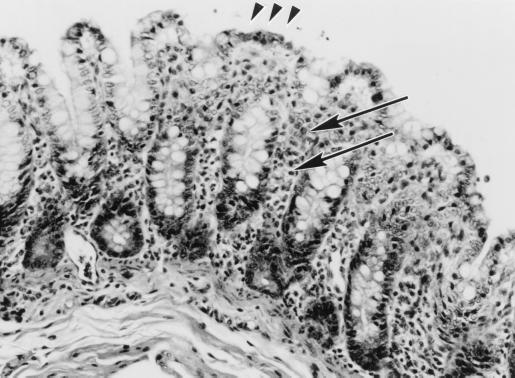
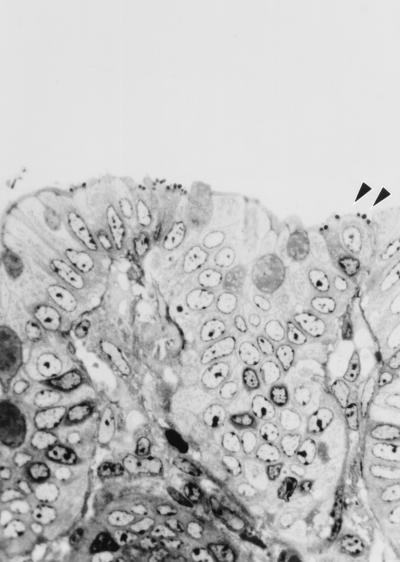
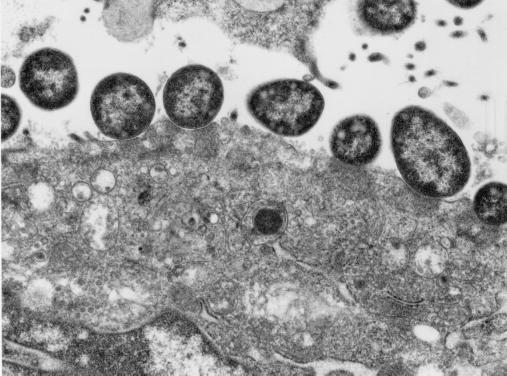
Morphologic findings were characteristic of the attaching and effacing lesion. The colonic surface epithelium appeared irregular, with bacilli intimately associated with the apical cytoplasmic membrane, and was accompanied by varying degrees of colonic crypt hyperplasia (Fig. 1). Surface epithelium variably had a cobblestone appearance, or when accompanied by necrosis, a flattened or squamous morphology. Adherent bacteria were visible by hematoxylin and eosin or toluidine blue stains (Fig. 2). There were often mild neutrophilic infiltrates and congestion of vessels within the mucosa. Individual epithelial cells with adherent bacilli often appeared rounded and vacuolated. Distribution of bacilli was limited to the colonic surface epithelium and varied from a diffuse to a locally extensive or focal pattern. Less frequently, organisms were noted in the ileum and distal jejunum. In each of these instances the colon was severely involved. In some cases multiple sections of colon needed to be evaluated to visualize the characteristic findings. Ultrastructurally there was effacement of normal microvillous architecture, and adherent bacilli were attached to the apical cytoplasmic membrane with pedestal formation and rearrangement of the underlying cytoskeleton (Fig. 3).
FIG. 1.
Microscopic appearance of EPEC infection in the colon of a rhesus macaque, using hematoxylin and eosin stain, characterized by irregular mucosal surface (arrowheads) and inflammatory cell infiltrates (arrows) within the lamina propria. Magnification, ×94.
FIG. 2.
Attaching and effacing lesion in colonic tissue of a rhesus macaque with adherent bacteria (arrowheads), using toluidine blue stain. Magnification, ×282.
FIG. 3.
Ultrastructural appearance of attaching and effacing lesion in colonic tissue of a rhesus macaque. Magnification, ×15,000.
Retrospective analysis of animals with attaching and effacing lesions.
To investigate the epizoology of EPEC infection, a retrospective analysis of animals dying with AIDS (n = 96) over a 2-year period was conducted. Age at death, days of survival, and the occurrence of other opportunistic infections of the gastrointestinal tract in animals with and without the attaching and effacing lesions are summarized in Table 1. As previously described, wasting and diarrhea were common clinical findings for rhesus macaques with AIDS. A variety of opportunistic infections of the gastrointestinal tract were identified at death, including C. parvum, M. avium, E. bieneusi, S. stercoralis, Balantidium coli, CMV, adenovirus, and E. histolytica. Diagnosis of opportunistic infections was based on morphologic features in combination with cytologic stains, ultrastructural examination, immunohistochemistry, and in situ hybridization, when indicated. A total of 65 of 72 (90.2%) animals with diarrhea had morphologic evidence of gastrointestinal opportunistic infections compared to 9 of 24 (37.5%) animals without diarrhea.
TABLE 1.
Retrospective analysis of attaching and effacing lesions in immunodeficient rhesus macaquesa
| Parameter | No. of animals (%)
|
||
|---|---|---|---|
| AE lesion present | AE lesion absent | Total | |
| Diarrheab | 27 (100.0) | 45 (65.2) | 72 (75.0) |
| Wasting | 21 (77.8) | 51 (73.9) | 72 (75.0) |
| C. parvum | 6 (22.2) | 19 (27.5) | 25 (26.0) |
| M. avium | 4 (17.8) | 17 (24.6) | 21 (21.9) |
| E. bieneusi | 4 (17.8) | 10 (14.5) | 14 (14.6) |
| Cytomegalovirus | 4 (17.8) | 7 (10.1) | 11 (11.5) |
| Adenovirusc | 10 (37.0) | 10 (14.5) | 20 (20.8) |
| E. histolytica | 1 (3.7) | 7 (10.1) | 8 (8.3) |
Mean age (in days) at time of inoculation of animals with attaching and effacing lesions (n = 27) was 985, and that of animals without lesions (n = 69) was 1,390. The mean age of all animals (n = 96) was 1,268. The mean number of days of survival for animals with attaching and effacing lesions was 436, and that for animals without lesions was 478. The mean number of days of survival for all animals was 466. AE lesion, attaching and effacing lesion.
P < 0.001 (chi-square test).
P < 0.003 (chi-square test).
Retrospective analysis revealed that 27 of 96 (28.1%) animals had attaching and effacing lesions consistent with EPEC infection, and EPEC was the most frequent pathogen of the gastrointestinal tract identified morphologically. There was no significant difference in survival or degree of wasting between animals with and without EPEC lesions; however, animals with EPEC had significantly higher rates of diarrhea at death (P < 0.001, chi-square test). Clinically, EPEC infection was characterized by persistent nonhemorrhagic diarrhea accompanied by tenesmus and significant weight loss. Animals with EPEC were younger and had a higher incidence of intestinal adenovirus infection than those without EPEC lesions. There was no significant difference in the occurrence of other opportunistic infections, including C. parvum, M. avium, E. bieneusi, CMV, and E. histolytica. While coinfections of the gastrointestinal tract were frequent, in 7 of 96 (7.3%) cases, EPEC was the only opportunistic infection recognized morphologically. In five of these animals, infection was associated with diarrhea and wasting, suggesting that EPEC infection in and of itself may be associated with significant clinical signs.
EPEC was recognized with greater frequency in animals that died at <1 year of age (P < 0.001, Fisher exact test). A comparison of clinical features and opportunistic infections of animals dying with AIDS at >1 and <1 year of age is presented in Table 2. Animals dying at <1 year of age had a shorter mean survival time (124 days versus 520 days) but no significant difference in the incidence of diarrhea or wasting. There was a marked difference in the incidence of the various opportunistic infections of the gastrointestinal tract. M. avium, E. bieneusi, CMV, and E. histolytica were absent in animals dying at under 1 year of age. In contrast, the incidence of EPEC and adenovirus infection of the gastrointestinal tract was significantly greater in animals that died at less than 1 year of age.
TABLE 2.
Comparison of opportunistic infections in animals dying of AIDS at <1 and >1 year of agea
| Parameter | No. of animals (%)
|
|
|---|---|---|
| <1 year | >1 year | |
| EPEC presentb | 9 (69.2) | 18 (21.7) |
| Diarrhea | 11 (92.3) | 61 (73.5) |
| Wasting | 7 (53.8) | 65 (78.3) |
| C. parvum | 2 (15.4) | 23 (27.7) |
| M. avium | 0 (0) | 21 (25.3) |
| E. bieneusi | 0 (0) | 14 (16.8) |
| Cytomegalovirus | 0 (0) | 11 (13.3) |
| Adenovirus | 7 (53.8) | 13 (15.7) |
| E. histolytica | 0 (0) | 8 (9.6) |
Mean age (in days) at time of inoculation of animals that died at an age of <1 year (n = 13) was 84, and that of animals that died at an age of >1 year (n = 83) was 1,558. Mean number of days of survival for animals that died at an age of <1 year was 124, and that for animals that died at an age of >1 year was 520.
P < 0.001 (Fisher exact test).
DISCUSSION
Diarrhea during AIDS is a frequent clinical sign in both HIV-infected humans and SIV-infected rhesus macaques. While a number of etiologic agents may be responsible, for as many as 50% of human patients the cause remains unknown. The relative contribution of direct HIV- or SIV-induced gastrointestinal pathology versus unrecognized opportunistic pathogens remains unresolved. Recently, DAEC has been implicated as one such potential pathogen in humans (23, 28, 32).
Here we describe EPEC as a common enteric infection of SIV-infected infant and adult rhesus macaques with AIDS. In 7.3% of animals dying with AIDS it represented the sole opportunistic agent of the gastrointestinal tract at death. In 20.8% of cases it was seen in combination with one or more gastrointestinal pathogens, including C. parvum, E. bieneusi, M. avium, E. histolytica, B. coli, S. stercoralis, CMV, and adenovirus. Morphologic alterations, including crypt hyperplasia, epithelial defects, and inflammatory cell infiltrates, suggest that EPEC played a significant role in producing illness and clinical signs in these animals.
A consensus definition of EPEC organisms has been reached and includes the histologic presence of the attaching and effacing lesion and demonstrated absence of Shiga toxin production (27). The organism identified in these rhesus macaques produced the characteristic attaching and effacing lesion in colonic sections and a focal adherence pattern on HEp-2 assay. Furthermore, cell toxicity assay and PCR could not demonstrate Shiga toxin production.
Despite widespread infection in the colony, the agent has gone largely unrecognized as a cause of diarrhea in immunodeficient rhesus macaques. The reasons for this are likely multifactorial. The true prevalence of EPEC infection in both humans and animals is probably underestimated, as most clinical laboratories do not attempt to isolate and identify lactose-fermenting organisms from fecal specimens. A definitive diagnosis of EPEC requires a systematic approach including the use of adhesion assays, biopsies, and/or molecular identification of virulence genes. Furthermore, the diagnosis in immunodeficient humans and animals may be obscured by the presence of multiple agents, and EPEC is likely to be missed unless a specific effort is made to identify it.
EPEC infection was more frequent in neonatal and infant rhesus macaques. In humans a striking age susceptibility is recognized, with clinical disease seen primarily in infants under 2 years of age (24). Furthermore, case control studies have shown a strong correlation between isolation of EPEC from human infants and diarrhea. There was a marked difference in the incidence of various opportunistic agents between animals greater than and less than 1 year of age at death. Such differences have previously been noted in SIV-infected neonates, and the propensity to develop recurrent or persistent bacterial infections is a characteristic feature of AIDS in both human and macaque infants (9). The reasons neonates and infants are more susceptible may relate to a lack of preexisting immunity as well as detrimental effects of SIV on mucosal immunity.
EPEC isolates have recently been subdivided into several genotypes based on sequence similarities in the intimin gene (1–3). Sequence variability in the carboxy terminus or polypeptide binding domain may be responsible for differences in host range and tissue distribution. Sequencing of a 2.6-kb fragment encompassing the intimin gene of rhesus macaque isolates revealed 99.2% identity to sequences from an existing human isolate of the epsilon subtype. Isolates of the epsilon subtype have previously been identified in humans and cattle and have been associated with EHEC and the hemolytic-uremic syndrome (29). Rhesus macaque isolates were negative for stx1 and stx2 sequences by PCR and failed to demonstrate Shiga toxin production through HeLa cell cytotoxicity assay.
Recognition of EPEC infection in rhesus macaques is important for several reasons. The agent may adversely affect primate colony health, confound experimental results, and represent a potential zoonosis. Furthermore, investigations in rhesus macaques may offer opportunities to study the impact of EPEC on AIDS pathogenesis and provide a novel animal model with which to study the effect of EPEC on gastrointestinal dysfunction.
ACKNOWLEDGMENTS
Financial support was provided by Public Health Service grants RR00168, RR0700, and RO1AI41889. A. Lackner is the recipient of an Elizabeth Glaser Scientist award.
REFERENCES
- 1.Adu-Bobie J, Frankel G, Bain C, Goncalves A G, Trabulsi L R, Douce G, Knutton S, Dougan G. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J Clin Microbiol. 1998;36:662–668. doi: 10.1128/jcm.36.3.662-668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adu-Bobie J, Trabulsi L R, Carneiro-Sampaio M M, Dougan G, Frankel G. Identification of immunodominant regions within the C-terminal cell binding domain of intimin α and intimin β from enteropathogenic Escherichia coli. Infect Immun. 1998;66:5643–5649. doi: 10.1128/iai.66.12.5643-5649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agin T S, Wolf M K. Identification of a family of intimins common to Escherichia coli causing attaching-effacing lesions in rabbits, humans, and swine. Infect Immun. 1997;65:320–326. doi: 10.1128/iai.65.1.320-326.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskin G B. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am J Pathol. 1987;129:345–352. [PMC free article] [PubMed] [Google Scholar]
- 5.Baskin G B. Cryptosporidiosis of the conjunctiva in SIV-infected rhesus monkeys. J Parasitol. 1996;82:630–632. [PubMed] [Google Scholar]
- 6.Baskin G B, Murphey-Corb M, Watson E A, Martin L N. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet Pathol. 1988;25:456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- 7.Baskin G B, Soike K. Adenovirus infection in SIV-infected macaques. J Infect Dis. 1989;160:905–907. doi: 10.1093/infdis/160.5.905. [DOI] [PubMed] [Google Scholar]
- 8.Beutin L, Zimmermann S, Gleier K. Rapid detection and isolation of Shiga-like toxin (verocytotoxin)-producing Escherichia coli by direct testing of individual enterohemolytic colonies from washed sheep blood agar plates in the VTEC-RPLA assay. J Clin Microbiol. 1996;34:2812–2814. doi: 10.1128/jcm.34.11.2812-2814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohm R P, Jr, Martin L N, Davison-Fairburn B, Baskin G B, Murphey-Corb M. Neonatal disease induced by SIV infection of the rhesus monkey (Macaca mulatta) AIDS Res Hum Retrovir. 1993;9:1131–1137. doi: 10.1089/aid.1993.9.1131. [DOI] [PubMed] [Google Scholar]
- 10.Carville A, Mansfield K, Widmer G, Lackner A, Kotler D, Weiss P, Gumbo T, Sarbah S, Tzipori S. Development and application of genetic probes for detection of Enterocytozoon bieneusi in formalin-fixed stools and in intestinal biopsy specimens from infected patients. Clin Diagn Lab Immunol. 1997;4:405–408. doi: 10.1128/cdli.4.4.405-408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chintu C, DuPont H L, Kaile T, Mahmoud M, Marani S, Baboo K S, Mwansa W, Sakala-Kazembe F, Sunkutu R, Zumla A. Human immunodeficiency virus-associated diarrhea and wasting in Zambia: selected risk factors and clinical associations. Am J Trop Med Hyg. 1998;59:38–41. doi: 10.4269/ajtmh.1998.59.38. [DOI] [PubMed] [Google Scholar]
- 12.Clerinx J, Bogaerts J, Taelman H, Habyarimana J B, Nyirabareja A, Ngendahayo P, Van de Perre P P. Chronic diarrhea among adults in Kigali, Rwanda: association with bacterial enteropathogens, rectocolonic inflammation, and human immunodeficiency virus infection. Clin Infect Dis. 1995;21:1282–1284. doi: 10.1093/clinids/21.5.1282. [DOI] [PubMed] [Google Scholar]
- 13.Colebunders R, Francis H, Mann J M, Bila K M, Izaley L, Kimputu L, Behets F, van der Groen G G, Quinn T C, Curran J W. Persistent diarrhea, strongly associated with HIV infection in Kinshasa, Zaire. Am J Gastroenterol. 1987;82:859–864. [PubMed] [Google Scholar]
- 14.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of E. coli HEp-2 adherence patterns with type and duration of diarrhea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 15.Daniel M D, Letvin N L, King N W, Kannagi M, Sehgal P K, Hunt R D. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- 16.Desrosiers R C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 17.Hii J H, Guccion J G, Gilbert C L. Enteroadherent eaeA-positive Escherichia coli associated with chronic AIDS-related diarrhea. Ann Intern Med. 1996;125:523. doi: 10.7326/0003-4819-125-6-199609150-00040. [DOI] [PubMed] [Google Scholar]
- 18.Ilyinskii P O, Daniel M D, Simon M A, Lackner A A, Desrosiers R C. The role of upstream U3 sequences in the pathogenesis of simian immunodeficiency virus-induced AIDS. J Virol. 1994;68:5933–5944. doi: 10.1128/jvi.68.9.5933-5944.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R C. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 20.Kestler H W, Li Y, Naidu Y M, Butler C V, Ochs M F, Jaenel G, King N W, Daniel M D, Desrosiers R C. Comparison of simian immunodeficiency isolates. Nature. 1988;331:619–621. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- 21.King N W, Chalifoux L V, Ringler D J, Wyand M S, Sehgal P K, Daniel M D, Letvin N L, Desrosiers R C, Blake B J, Hunt R D. Comparative biology of natural and experimental SIVmac infection in macaque monkeys: a review. J Med Primatol. 1990;19:109–118. [PubMed] [Google Scholar]
- 22.King N W, Hunt R D, Letvin N L. Histopathologic changes in macaques with an acquired immunodeficiency syndrome (AIDS) Am J Pathol. 1983;113:382–388. [PMC free article] [PubMed] [Google Scholar]
- 23.Kotler D P, Giang T T, Thiim M, Nataro J P, Sordillo E M, Orenstein J M. Chronic bacterial enteropathy in patients with AIDS. J Infect Dis. 1995;171:552–558. doi: 10.1093/infdis/171.3.552. [DOI] [PubMed] [Google Scholar]
- 24.Levine M M, Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- 25.Mansfield K G, Carville A, Shvetz D, Mackey J, Tzipori S, Lackner A A. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian immunodeficiency virus inoculated macaques with hepatobiliary disease. Am J Pathol. 1997;150:1395–1405. [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield K G, Pauley D, Young H L, Lackner A A. Mycobacterium avium complex in macaques with AIDS is associated with a specific strain of simian immunodeficiency virus and prolonged survival after primary infection. J Infect Dis. 1995;172:1149–1152. doi: 10.1093/infdis/172.4.1149. [DOI] [PubMed] [Google Scholar]
- 27.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orenstein J M, Kotler D P. Diarrheogenic bacterial enteritis in acquired immune deficiency syndrome: a light and electron microscopy study of 52 cases. Hum Pathol. 1995;26:481–492. doi: 10.1016/0046-8177(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 29.Oswald E, Schmidt H, Morabito S, Karch H, Marches O, Caprioli A. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect Immun. 2000;68:64–71. doi: 10.1128/iai.68.1.64-71.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton A W, Paton J C. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 1998;36:598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavia A T, Long E G, Ryder R W, Nsa W, Puhr N D, Wells J G, Martin P, Tauxe R V, Griffin P M. Diarrhea among African children born to human immunodeficiency virus 1-infected mothers: clinical, microbiologic and epidemiologic features. Pediatr Infect Dis J. 1992;11:996–1003. doi: 10.1097/00006454-199211120-00002. [DOI] [PubMed] [Google Scholar]
- 32.Polotsky Y, Nataro J P, Kotler D P, Orenstein J M. Adherence patterns of bacterial diarrheal agents in AIDS, Adv. Exp Med Biol. 1997;412:367–371. doi: 10.1007/978-1-4899-1828-4_59. [DOI] [PubMed] [Google Scholar]
- 33.Riecken E O, Zeitz M, Ullrich R. Non-opportunistic causes of diarrhoea in HIV infection. Bailliere's Clin Gastroenterol. 1990;4:385–403. doi: 10.1016/0950-3528(90)90008-5. [DOI] [PubMed] [Google Scholar]
- 34.Ullrich R, Riecken E O, Zeitz M. Human immunodeficiency virus-induced enteropathy. Immunol Res. 1991;10:456–464. doi: 10.1007/BF02919742. [DOI] [PubMed] [Google Scholar]
- 35.Ullrich R, Zeitz M, Heise W, L'age M, Hoffken G, Riecken E O. Small intestinal structure and function in patients infected with human immunodeficiency virus (HIV): evidence for HIV-induced enteropathy. Ann Intern Med. 1989;111:15–21. doi: 10.7326/0003-4819-111-1-15. [DOI] [PubMed] [Google Scholar]
- 36.Wanke C A, Cohan D, Thummakul T, Jongwuitiwes S, Grayson M L, Hammer S M, Hanvanich M. Diarrheal disease in patients infected with human immunodeficiency virus in Bangkok, Thailand. Am J Trop Med Hyg. 1999;60:871–874. doi: 10.4269/ajtmh.1999.60.871. [DOI] [PubMed] [Google Scholar]
- 37.Wanke C A, Mayer H, Weber R, Zbinden R, Watson D A, Acheson D. Enteroaggregative Escherichia coli as a potential cause of diarrheal disease in adults infected with human immunodeficiency virus. J Infect Dis. 1998;178:185–190. doi: 10.1086/515595. [DOI] [PubMed] [Google Scholar]
- 38.Wyand M S, Ringler D J, Naidu Y M, Mattmuller M, Chalifoux L V, Sehgal P K, Daniel M D, Desrosiers R C, King N W. Cellular localization of simian immunodeficiency virus in lymphoid tissues: II. In situ hybridization. Am J Pathol. 1989;134:385–393. [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto T, Endo S, Yokota T, Echeverria P. Characteristics of adherence of enteroaggregative Escherichia coli to human and animal mucosa. Infect Immun. 1991;59:3722–3739. doi: 10.1128/iai.59.10.3722-3739.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]