Short abstract
Content available: Audio Recording
Listen to an audio presentation of this article.
It was only in 1998 that the first successful deceased donor liver transplant (DDLT) and a living donor LT (LDLT) were performed in India. 1 Currently, more than 1800 liver transplants (LT) are performed in India annually, in 90‐100 active LT centers. The Indian Liver Transplant Registry (ILTR, www.iltr.org) is now established and accruing prospective data from August 2019. Although there is no law or directive yet making it compulsory for all centers to contribute data in the ILTR, the Liver Transplant Society of India (LTSI) is trying its best to encourage every active center to do so. The NOTTO (National Organ and Tissue Transplant Organisation) is a regulatory body deemed to function as an apex center for all India activities of coordination and networking for procurement and distribution of Organs and Tissues in the country. Significant regional variations exist, but in general, a majority of recipients are males (80%), and most are adults (85%). Unlike in the Western world, where DDLT is predominant, LDLT is most commonly performed in India, (around 85% of cases) (personal communication, ILTR). Asia, unlike the Americas and Europe, is characterized by a dramatic diversity in social, economic, and cultural factors. In addition to religious and cultural beliefs, limited universal health‐care access, and lack of strong legislation in others, have been the stumbling blocks in the promotion of DDLT. 2 , 3 Hence, LDLT is the predominant form of liver transplantation in India.
The survival data from LTSI database will take 2‐3 years to mature. Meanwhile, we discuss published outcomes after LT from India in the current review.
Survival After LT
Survival after LT differs according to the major indications of LT: decompensated cirrhosis, hepatocellular carcinoma (HCC), acute‐on chronic liver failure (ACLF), and acute liver failure (ALF).
The main etiologies in 2137 adult LTs at our center were viral (hepatitis B or C) in 34.7%, alcoholic liver disease (ALD) in 32.7%, cryptogenic or nonalcoholic steatohepatitis related in 26.5% and autoimmune liver diseases in 5.9% of patients. The mean model for end‐stage liver disease (MELD) score before LT was 17.5 ± 6.4 (unpublished data). The overall survival among adult transplant recipients (age >18 years at the time of LT, mean age 50 ± 10 years; n = 1980 with complete follow‐up) from June 2010 to December 2019 at our center, at 1, 5 and 10 years is 84.3%, 75.5% and 72.2% respectively, as shown in Fig. 1 (unpublished data). In the pediatric population, the 1‐year and 5‐year actuarial survival at our center in the first 200 LDLTs was 95%, and 87%, respectively. 4 Same survival has been reported by other centers; however, survival is inferior in recipients older than 60 years. 5 , 6 Studies from our center showed similar survival in patients with MELD scores of <25 and ≥25 and in patients with low GRWR (with selective use of portal inflow modulation). 7 , 8
FIG 1.
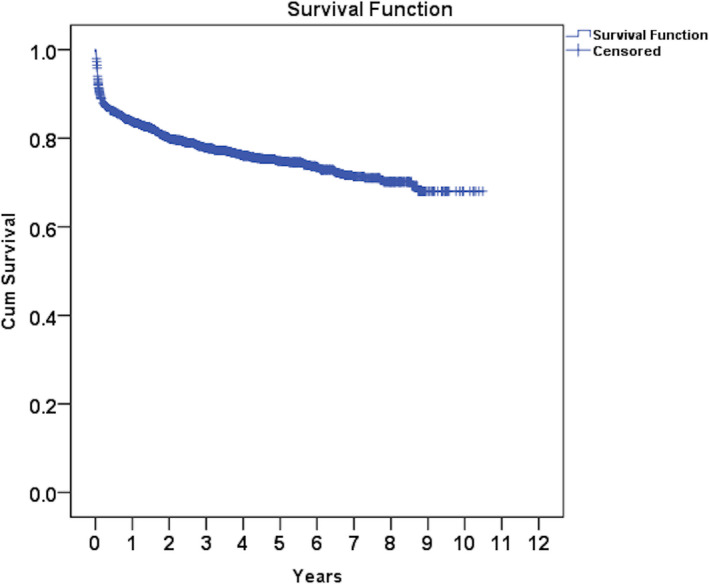
Kaplan‐Meier curve showing survival in a cohort of 1980 adult patients.
In a large cohort of 405 patients with cirrhosis and HCC (accepted for LDLT using our expanded selection criteria), the 5‐year recurrence‐free survival was 70%. Following variables predicted recurrence: pre‐LT AFP ≥100 ng/mL, tumor burden beyond the UCSF criteria, and tumor 18FDG‐PET avidity. 9
ACLF is associated with high short‐term mortality without LT. We published our experience with LDLT in 218 ACLF patients. A total of 80% of the ACLF‐1 and 72.7% of ACLF‐2 group underwent LDLT, whereas only 35% of the ACLF‐3 group could have LDLT. The MELD scores were 24.1 ± 4.3, 28.5 ± 4.5 and 34.4 ± 8.1 for ACLF‐1, 2 and 3, respectively. While there was a trend toward worse 1‐year post transplant survival with increasing number of organ failures, the difference was not statistically significant: 92.9%, 85.4%, and 75.6%, respectively in ACLF‐1, 2, and 3 (p=0.15). Furthermore, among patients in the ACLF‐3 group, survival at 90 days was only 5.9% in those who could not undergo LDLT. 10
Various Indian studies have shown 64%‐88% survival after LT in patients with ALF. 11 , 12 , 13 , 14 , 15 , 16
Immunological Outcomes
In a series of 1372 adult LDLTs from our center, the incidence of acute cellular rejection was 13.8% in genetically related, and 17.5% in genetically unrelated LDLTs (p=0.072). Chronic rejection developed in 37 (2.6%) recipients at 25 (11‐45) months after LT. Multivariate analysis revealed that genetically unrelated donors (odds ratio: 3.88, 95% confidence interval: 1.80–8.34, p = 0.001) and history of acute cellular rejection (odds ratio: 3.39, 95% CI: 1.68–6.81, p = 0.001) were predictors of chronic rejection. 17 Expense of immunosuppression is indeed a key factor in the post operative period, which may lead to discontinuation or non adherence in some recipients. Individual centers can make an impact by modifying protocols and using branded generic immunosuppressants (which have regularly been found to be efficacious) to reduce expenses. It has been observed that Indian patients have a lower requirement of immunosuppression compared to their Western counterparts. Also, with the high overall prevalence of infections, it is good strategy to keep the immunosuppression to a minimum. 18
Complications and Disease Recurrence
Reported overall incidence of biliary complications in recipients post LDLT from high volume centers in India ranges between 11% and 14%. 19 , 20 The incidence is higher in recipients with multiple bile duct anastomoses. It has been indicated, however, that with adequate use of endoscopic and percutaneous interventional radiology techniques, complications may be successfully dealt with, without impacting overall graft or patient survival. 21 As regards donor biliary complications, reported incidence in one series was 2.5%, with grade II complications in 2%, and grade III in 0.6%. 22 Furthermore, no definite association between biliary complications and type of graft, or portal or biliary anatomy was found. 23 The incidence of vascular complications (hepatic artery thrombosis and portal vein thrombosis) in the recipients post LDLT range between 1% and 3%, with a slightly higher incidence in the pediatric population. 4 , 24 , 25
Prevention or treatment of Hepatitis B or C recurrence has ceased to be a significant problem given the availability of potent antiviral medications. The hepatitis B recurrence was 1.1% [with high genetic barrier antivirals and short‐term hepatitis B immunoglobulin (HBIG)] and 8% with antivirals alone in recipients with selective HBIG strategy. 26 , 27 A recent study showed only 3.3% histological recurrence (diagnosed on on‐demand biopsy) of hepatitis C in direct acting antiviral era. 28
ALD is one of the major indications for LT in India. In a study of 408 liver transplants (26% of total LTs) for ALD, the overall rate of relapse was 9.5% at a median of 34.7 (15‐57.6) months; 23% of those who relapsed were heavy drinkers. 29
There is no published data on recurrence of autoimmune diseases after LT from India. In the only published experience of LT for CLD secondary to primary sclerosing cholangitis, PSC recurrence was seen in 20% at a median follow‐up of 59 (29‐101) months after LDLT. 30
Non‐Liver Related Morbidity
In a series from our center, out of 2100 adult LDLTs, 21 (1%) patients developed de novo malignancy (DNM) at a median follow‐up of 42 (32‐73) months. The most common sites of DNM were oropharyngeal (n = 7) and lung (n = 4). 31 This is in contrast to the Western world where skin malignancies are the most common de novo malignancies after LT, and is in accordance with the non‐transplant data from the East where aerodigestive malignancies are the most common de novo neoplasms after LT.
Post transplant metabolic syndrome (PTMS) is common due to weight gain and the effect of immunosuppression. In a study from our center, among 82 patients, PTMS was present in 52%, and sarcopenic obesity was present in 88% at a follow‐up 24 months. 32 Another study found new‐onset diabetes after LT in 38.5% and PTMS in 29%. 33 To the best of our knowledge, no study from India has looked at chronic kidney disease and cardiovascular outcomes after LT in long‐term.
Conclusions
The survival and outcomes after LT in India are similar to those from leading centers elsewhere. It is hoped that over the next 2 years, inclusive survival data will emerge from the Indian Liver Transplant Registry.
References
- 1. Sibal A, Rajasekar MR, Soin AS. Liver transplantation in the developing world. Indian J Pediatr 1999;66(1 Suppl):S120‐S123. [PubMed] [Google Scholar]
- 2. Hibi T, Wei Chieh AK, Chi‐Yan Chan A, Bhangui P. Current status of liver transplantation in Asia. Int J Surg 2020. Oct;82S:4‐8. 10.1016/j.ijsu.2020.05.071. Epub 2020 Jun 11 PMID: 32535264. [DOI] [PubMed] [Google Scholar]
- 3. Rela M, Reddy MS. Living donor liver transplant (LDLT) is the way forward in Asia. Hepatol Int 2017;11:148‐151. 10.1007/s12072-016-9780-z. [DOI] [PubMed] [Google Scholar]
- 4. Mohan N, Karkra S, Rastogi A, et al. Outcome of 200 pediatric living donor liver transplantations in India. Indian Pediatr 2017;54:913‐918. [DOI] [PubMed] [Google Scholar]
- 5. Pamecha V, Sasturkar SV, Sinha PK, Mohapatra N, Patil N. Biliary reconstruction in adult living donor liver transplantation: the all‐knots‐outside technique. Liver Transpl 2020. 10.1002/lt.25862. [DOI] [PubMed] [Google Scholar]
- 6. Nidoni R, Kandagaddala R, Agarwal S, Dey R, Chikkala BR, Gupta S. Living donor liver transplant in patients aged 60 years or older: experience from a large volume centre in India. J Clin Exp Hepatol 2021;11:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soin AS, Yadav SK, Saha SK, et al. Is Portal inflow modulation always necessary for successful utilization of small volume living donor liver grafts? Liver Transpl 2019;25:1811‐1821. [DOI] [PubMed] [Google Scholar]
- 8. Yadav SK, Saraf N, Saigal S, et al. High MELD score does not adversely affect outcome of living donor liver transplantation: experience in 1000 recipients. Clin Transplant 2017;31(8):e13006. 10.1111/ctr.13006. [DOI] [PubMed] [Google Scholar]
- 9. Bhangui P, Saigal S, Gautam D, et al. Incorporating tumor biology to predict hepatocellular carcinoma recurrence in patients undergoing living donor liver transplantation using expanded selection criteria. Liver Transpl 2021;27:209‐221. [DOI] [PubMed] [Google Scholar]
- 10. Yadav SK, Saraf N, Chodhary NS, et al. Living donor liver transplantation for acute‐on‐chronic liver failure. Liver Transpl 2019;25:459‐468. [DOI] [PubMed] [Google Scholar]
- 11. Mallick S, Nair K, Thillai M, et al. Liver transplant in acute liver failure ‐ looking back over 10 years. J Clin Exp Hepatol 2020;10:322‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pamecha V, Vagadiya A, Sinha PK, et al. Living donor liver transplantation for acute liver failure: donor safety and recipient outcome. Liver Transpl 2019;25:1408‐1421. [DOI] [PubMed] [Google Scholar]
- 13. Mehrotra S, Mehta N, Rao PS, Lalwani S, Mangla V, Nundy S. Live donor liver transplantation for acute liver failure: a single center experience. Indian J Gastroenterol 2018;37:25‐30. [DOI] [PubMed] [Google Scholar]
- 14. Choudhary NS, Saigal S, Saraf N, et al. Good outcome of living donor liver transplantation in drug‐induced acute liver failure: A single‐center experience. Clin Transplant 2017;31:e12907. 10.1111/ctr.12907. [DOI] [PubMed] [Google Scholar]
- 15. Saraf V, Pande S, Gopalakrishnan U, et al. Acute liver failure due to zinc phosphide containing rodenticide poisoning: Clinical features and prognostic indicators of need for liver transplantation. Indian J Gastroenterol 2015;34:325‐329. [DOI] [PubMed] [Google Scholar]
- 16. Bavikatte AP, Sudhindran S, Dhar P, et al. Live donor liver transplantation for antitubercular drug‐induced acute liver failure. Indian J Gastroenterol 2017;36:56‐61. [DOI] [PubMed] [Google Scholar]
- 17. Choudhary NS, Saha SK, Saigal S, et al. Do recipients of genetically related donors have better outcomes after living donor liver transplantation? J Clin Exp Hepatol 2020;10:334‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sudhindran S, Aboobacker S, Menon RN, Unnikrishnan G, Sudheer OV, Dhar P. Cost and efficacy of immunosuppression using generic products following living donor liver transplantation in India. Indian J Gastroenterol 2012;31:20‐23. [DOI] [PubMed] [Google Scholar]
- 19. Vij V, Makki K, Chorasiya VK, Sood G, Singhal A, Dargan P. Targeting the Achilles' heel of adult living donor liver transplant: Corner‐sparing sutures with mucosal eversion technique of biliary anastomosis. Liver Transpl 2016;22:14‐23. [DOI] [PubMed] [Google Scholar]
- 20. Rammohan A, Govil S, Vargese J, Kota V, Reddy MS, Rela M. Changing pattern of biliary complications in an evolving liver transplant unit. Liver Transpl 2017;23:478‐486. [DOI] [PubMed] [Google Scholar]
- 21. Bhangui P, Saha S. The high‐end range of biliary reconstruction in living donor liver transplant. Curr Opin Organ Transplant 2019;24:623‐630. [DOI] [PubMed] [Google Scholar]
- 22. Pamecha V, Bharathy KG, Kumar S, Sasturkar SV, Sinha PK. Biliary complications after living donor hepatectomy: A first report from India. Liver Transpl 2016;22:607‐614. [DOI] [PubMed] [Google Scholar]
- 23. Shaji Mathew J, Manikandan K, Santosh Kumar KY, et al. Biliary complications among live donors following live donor liver transplantation. Surgeon 2018;16:214‐219. [DOI] [PubMed] [Google Scholar]
- 24. Soin AS. Smoothing the path: reducing biliary complications, addressing small‐for‐size syndrome, and making other adaptations to decrease the risk for living donor liver transplant recipients. Liver Transpl 2012. Nov;18(Suppl 2):S20‐S24. 10.1002/lt.23541. [DOI] [PubMed] [Google Scholar]
- 25. Mehta NN, Mangla V, Varma V, et al. Minimizing hepatic artery thrombosis and establishing safety of grafts with dual arteries in living donor liver transplantation. Transplant Proc 2018;50: 1378‐1385. [DOI] [PubMed] [Google Scholar]
- 26. Choudhary NS, Saraf N, Saigal S, et al. Low‐dose short‐term hepatitis B immunoglobulin with high genetic barrier antivirals: the ideal post‐transplant hepatitis B virus prophylaxis? Transpl Infect Dis 2015;17:329‐333. [DOI] [PubMed] [Google Scholar]
- 27. Wadhawan M, Gupta S, Goyal N, Taneja S, Kumar A. Living related liver transplantation for hepatitis B‐related liver disease without hepatitis B immune globulin prophylaxis. Liver Transpl 2013;19:1030‐1035. [DOI] [PubMed] [Google Scholar]
- 28. Choudhary NS, Saraf N, Saigal S, et al. Outcome of hepatitis C‐related liver transplantation in direct‐acting antiviral era. Indian J Gastroenterol 2020;39:539‐543. [DOI] [PubMed] [Google Scholar]
- 29. Saigal S, Choudhary NS, Yadav SK, et al. Lower relapse rates with good post‐transplant outcome in alcoholic liver disease: experience from a living donor liver transplant center. Indian J Gastroenterol 2016;35:123‐128. [DOI] [PubMed] [Google Scholar]
- 30. Choudhary NS, Saigal S, Thummala S, et al. Good long‐term outcomes in patients with primary sclerosing cholangitis undergoing living donor liver transplantation. J Clin Exp Hepatol 2020;10:442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tiwari A, Saigal S, Choudhary NS, et al. De novo malignancy after living donor liver transplantation: a large volume experience. J Clin Exp Hepatol 2020;10:448‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choudhary NS, Saigal S, Saraf N, et al. Sarcopenic obesity with metabolic syndrome: a newly recognized entity following living donor liver transplantation. Clin Transplant 2015;29:211‐215. [DOI] [PubMed] [Google Scholar]
- 33. Oommen T, Arun CS, Kumar H, et al. Incidence of new‐onset diabetes and posttransplant metabolic syndrome after liver transplantation ‐ a prospective study from South India. Indian J Endocrinol Metab 2020;24:165‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]


