Supplemental Digital Content is available in the text.
Keywords: dihydroxyeicosatrienoic acid, eicosanoids, epoxyeicosatrienoic acid, oxylipins, sepsis, septic shock
IMPORTANCE:
This is the largest study describing the role of P450 epoxygenase metabolites in septic shock in humans and suggests a novel therapeutic target.
Objectives:
Oxylipins are oxidative breakdown products of cell membrane fatty acids. Animal models have demonstrated that oxylipins generated by the P450 epoxygenase pathway may be implicated in septic shock pathology. However, these mediators are relatively unexplored in humans with septic shock. We aimed to determine if there were patterns of oxylipins that were associated with 28-day septic shock mortality and organ dysfunction.
Design:
Retrospective analysis of samples collected during the Vasopressin versus Norepinephrine as Initial Therapy in Septic Shock trial.
Setting:
ICUs in the United Kingdom.
PARTICIPANTS:
Adults recruited within 6 hours of onset of septic shock.
MAIN OUTCOMES AND MEASURES:
Oxylipin profiling was performed on 404 serum samples from 152 patients using liquid chromatography-mass spectrometry.
Results:
Nonsurvivors were found to have higher levels of 14,15-dihydroxyeicosatrienoic acid (DHET) at baseline than survivors (p = 0.02). Patients with 14,15-DHET levels above the lower limit of quantification of the assay were more likely to die than patients with levels below this limit (hazard ratio, 2.3; 95% CI, 1.2–4.5). Patients with measurable 14,15-DHET had higher levels of organ dysfunction and fewer renal failure-free days than those in whom it was unmeasurable. Considering samples collected over the first week of intensive care stay, measurable levels of DHET species were associated with higher daily Sequential Organ Failure Assessment scores that appeared to be accounted for predominantly by the liver component. Measurable 14,15-DHET showed positive correlation with bilirubin (rs = 0.38; p < 0.001) and lactate (rs = 0.27; p = 0.001).
CONCLUSIONS AND RELEVANCE:
The P450 epoxygenase-derived DHET species of oxylipins were associated with organ, particularly liver, dysfunction in septic shock and 14,15-DHET was associated with septic shock mortality. These results support further investigation into the role of the P450 epoxygenase-derived oxylipins in sepsis and suggest that this pathway may offer a novel therapeutic strategy in septic shock.
Septic shock remains a major worldwide health problem that is associated with significant morbidity and mortality (1). As such, improving understanding of its pathophysiology continues to be a key research interest. Oxylipins are signaling molecules formed from the enzymatic oxidation of polyunsaturated fatty acids (PUFAs) from the cell membrane (2). They are known to have a variety of paracrine and autocrine roles in health and disease, including sepsis. Eicosanoids are a well-described subgroup of oxylipins with 20 carbon atoms that are formed by cyclooxygenase and lipoxygenase enzymes and their role in sepsis has been previously investigated (3, 4). In contrast, other oxylipins, such as those generated by the cytochrome P450 superfamily, are not well studied in humans.
Endothelial cytochrome P450 epoxygenase enzymes metabolize arachidonic acid into four vasoactive regioisomers of epoxyeicosatrienoic acid (EET), which act in a paracrine manner and are released into the circulation (5, 6). These compounds are known to be vasodilatory endothelium-derived hyperpolarizing factors that activate calcium gated potassium channels (7–9). Animal models that artificially increase their expression show reduced mean arterial pressure (10). Correspondingly, blocking their synthesis increases vascular resistance in arterial vessels (11, 12). In addition, EETs are also known to be anti-inflammatory and have other organ-specific effects (6).
Recently a number of studies utilizing animal models of sepsis have shown that modulating the levels of EETs and their metabolites, the dihydroxyeicosatrienoic acids (DHETs), has an impact on survival (13–15). In particular, increasing the levels of EETs and blocking the production of DHETs, either by knockout or inhibition of soluble epoxide hydrolase (sEH), has been shown to increase survival and is associated with improved circulatory function (15) and reduced inflammatory response (14).
Previous clinical studies measuring oxylipins have been small and have found inconclusive results (16–18). In this study, we performed oxylipin profiling in a large cohort of patients with septic shock who were enrolled into the Vasopressin versus Norepinephrine as Initial Therapy in Septic Shock (VANISH) trial (19). We aimed to determine which oxylipins were associated with septic shock 28-day mortality and how these were associated with physiologic derangement and organ dysfunction.
MATERIALS AND METHODS
The VANISH Trial
The VANISH trial was a multicenter, double-blind, randomized controlled trial that compared the effect of vasopressin and norepinephrine combined with hydrocortisone or placebo on the development of renal failure in patients with septic shock (19).
Patient Cohort
Participants in the VANISH trial were recruited between February 2013 and May 2015 across 18 different sites. The cohort included patients (≥ 16 yr) who had septic shock. Patients were enrolled at admission to ICU within 6 hours of the development of shock. A full list of inclusion and exclusion criteria can be found in the Online Supplement (http://links.lww.com/CCX/A900). Patients were followed up until day 29.
Written consent was obtained from all patients or their legal representatives. The trial was approved by the Oxford A ethics committee (reference 12/SC/0014).
Oxylipin Samples and Measurement
Serum samples were collected from patients in 11 of 18 trial sites, based on the availability of research staff, at inclusion to the trial and at up to three additional time points during the first week if they remained in ICU. Patients without samples at baseline were excluded in this analysis. Blood samples were collected from patients and serum immediately separated and frozen at –80°C prior to transfer to a central laboratory for batch oxylipin processing. Samples were analyzed at the National Phenome Centre (Imperial College London, London, United Kingdom) using ultra-high performance liquid chromatography coupled via electrospray ionization to a tandem quadrupole mass spectrometer. A validated assay for measuring lipid mediators of inflammation (20, 21) was employed, quantifying 43 oxylipins and five PUFAs. Oxylipin concentrations above or below the limits of quantification (Supplementary Table 1, http://links.lww.com/CCX/A900) were censored at the limit of quantification. Oxylipins were excluded from baseline analysis if they were unquantifiable in more than 90% of baseline samples.
Clinical Data
Demographic and clinical characteristics were recorded on inclusion to the trial. Additionally, hemodynamic and organ-specific clinical data were recorded daily for patients who remained alive and admitted to ICU, including the data required to calculate Sequential Organ Failure Assessment (SOFA) scores. A daily norepinephrine equivalent dose of vasopressors was calculated using the formula described by Goradia et al (22). Sequential oxylipin samples were matched to the corresponding daily clinical data. Outcome was recorded as status at day 29.
Statistical Analysis
Principle component analysis (PCA) and partial least squared discriminant analysis (PLS-DA) were used to explore global associations of oxylipins with 28-day survival. Oxylipin data taken at baseline were log-transformed and scaled to zero mean and unit variance prior to this multivariate analysis. PLS-DA models were cross-validated using a “leave-one-out” methodology. Oxylipins that were most strongly associated with outcome in this model were identified by inspection of the model’s loadings plot and the Variable Importance in Projection (VIP) of each oxylipin. The VIP score of a variable is calculated as a weighted sum of the squared correlation between the PLS-DA components and the original variable.
Comparisons of baseline continuous data were performed using the Mann-Whitney U test. p values from comparison of oxylipin levels between survivors and nonsurvivors were corrected for multiple comparisons using the Benjamini-Hochberg procedure for false discovery. The chi-square test was used for comparisons of categorical data with the Fisher exact test being used for cases where n value of less than 10. The association of categorical oxylipin groups, based on the threshold of quantification, and primary outcome was explored using Kaplan-Meier curves and Cox-proportional hazards models.
Classes of oxylipins found to be associated with 28-day mortality in both multivariable and univariate analysis were taken forward for further exploration. Missing daily clinical data and daily SOFA scores were imputed as described in the original VANISH trial (19). The correlation between oxylipin concentrations across all sampling time points and matched continuous clinical variables was investigated with the Spearman rank correlation coefficient. Exploration of the association of daily clinical parameters with binary oxylipin groupings was done using mixed-effects logistic models. Clinical variables were chosen to provide most clinical insight whilst minimizing colinearity (see Supplementary Table 2, http://links.lww.com/CCX/A900 for a list of included variables). The Hosmer-Lemeshow approach was used for variable selection and developing a multivariable model (23). All models were fitted using xtmelogit command in Stata 15 (StataCorp, College Station, TX). All other statistical analysis was performed in R Version 4.0.3 (R Foundation, Vienna, Austria) (24) and IBM SPSS Statistics Grad Pack Version 24 (Chicago, IL). p values of less than 0.05 were considered significant, and in all cases, two-sided tests were used.
RESULTS
Oxylipin sampling was performed in 168 of 409 patients (41%). One patient had to be excluded from further analysis as they had no associated clinical data and 15 were excluded as they did not have baseline samples collected, leaving 152 patients in the current study.
Included patients were broadly similar to patients from the trial who were not included in this analysis (Supplementary Table 3, http://links.lww.com/CCX/A900), although those patients with sampling were more likely to have chronic obstructive pulmonary disease and cirrhosis than those who were not and displayed evidence of less severe shock, as they required less fluid and lower vasopressor doses than those who did not undergo sampling. Of the included patients, the median age was 66 years (54–77 yr), 96 (63%) were male, 126 (83%) were Caucasian and the most common single source of infection was lung (n = 66 [44%]). The first blood sample (Time Point 0 [TP0]) was collected a median of 3 hours after the start of vasopressors with subsequent samples being taken at a median of 30 hours (TP1), 59 hours (TP2), and 108 hours (TP3) after the start of vasopressor support. By day 29, 39 (26%) of included patients had died.
As expected, patients who did not survive to day 29 had higher Acute Physiology and Chronic Health Evaluation II scores, a higher requirement for additional norepinephrine, lower Glasgow Coma Scores and Pao2:Fio2 ratios, and higher frequency of renal and respiratory failure than those who survived (Supplementary Table 4, http://links.lww.com/CCX/A900).
Oxylipins and 28-Day Mortality
Of the oxylipins and PUFAs measured, only 20 were quantifiable in more than 10% of baseline samples (Supplementary Table 2, http://links.lww.com/CCX/A900) and used for subsequent analysis.
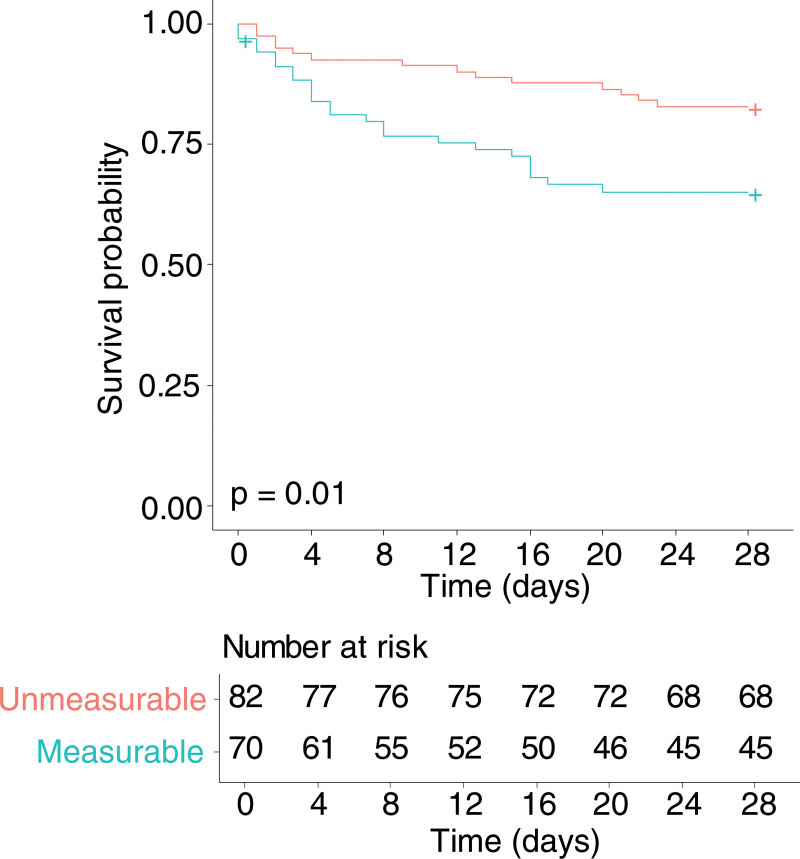
PCA of the oxylipins showed no batch effect from analysis runs or natural clustering. Although PLS-DA of baseline oxylipins showed only a limited ability to separate day 28 survivors from nonsurvivors (Supple mentary Fig. 1, http://links.lww.com/CCX/A900), within this model, 14,15-DHET (VIP 1.8) and eicosapentaenoic acid (VIP 1.7) (Supplementary Fig. 1, http://links.lww.com/CCX/A900) had the strongest association with outcome. Comparing baseline oxylipin concentrations between survivors and nonsurvivors (Table 1) found that only 14,15-DHET showed a statistically significant difference in concentration between survivors and nonsurvivors after correction for multiple comparisons. Survivors had lower baseline 14,15-DHET than nonsurvivors (250 fg/µL [250–292 fg/µL]) survivors versus 294 fg/µL ([250–474 fg/µL] nonsurvivors; p = 0.02). As 14,15-DHET was below the level of quantification in 82 patients (54%) at baseline, this could have been influencing its association with mortality. To investigate this, we divided patients into those with baseline 14,15-DHET concentrations above and below the lower level of quantification. Patients with measurable levels of 14,15-DHET had increased mortality at 28 days compared with those without measurable levels (hazard ratio, 2.3; 95% CI, 1.2–4.5; p = 0.01) (Fig. 1).
TABLE 1.
Comparison of Baseline Oxylipin Concentration Between 28-Day Septic Shock Survivors and Nonsurvivors
| Oxylipin | 28-Day Survivors (Median [IQR], fg/µL) | 28-Day Nonsurvivors (Median [IQR], fg/µL) | p | Adjusted p |
|---|---|---|---|---|
| C20:3 (dihomo-γ-linolenic acid) | 2.0 × 105 (1.5 × 105–2.8 × 105) | 1.6 × 105 (1.2 × 105–2.4 × 105) | 0.07 | 0.18 |
| C20:5 (eicosapentaenoic acid) | 3.3 × 105 (2.2 × 105–5.8 × 105) | 2.7 × 105 (1.7 × 105–4.0 × 105) | 0.01 | 0.08 |
| 9(S)-HODE | 5,000 (5,000–5,000) | 5,000 (5,000–5,000) | 0.70 | 0.74 |
| 13(S)-HODE | 10,126 (7,101–16,699) | 11,749 (7,740–22,720) | 0.29 | 0.36 |
| 12(S)-hydroxyeicosapentaenoic acid | 703 (500–3,491) | 2,243 (500–8,163) | 0.10 | 0.18 |
| 5,6-EET | 250 (250–250) | 250 (250–250) | 0.53 | 0.62 |
| 11,12-EET | 250 (250–269) | 250 (250–250) | 0.24 | 0.32 |
| 5(S)-HETE | 1,269 (963–1,908) | 1,454 (888–1,852) | 0.81 | 0.81 |
| 8(S)-HETE | 292 (250–582) | 424 (250–976) | 0.11 | 0.18 |
| 11(R)-HETE | 376 (253–662) | 578 (269–1,094) | 0.10 | 0.18 |
| 12(R)-HETE | 4,028 (1,172–16,254) | 7,741 (2,664–30,969) | 0.07 | 0.18 |
| 15(S)-HETE | 1,183 (817–1,904) | 1,745 (1,070–3,485) | 0.02 | 0.14 |
| 8,9-DHET | 250 (250–250) | 250 (250–267) | 0.24 | 0.32 |
| 11,12-DHET | 250 (250–250) | 250 (250–317) | 0.04 | 0.16 |
| 14,15-DHET | 250 (250–292) | 294 (250–474) | 0.001 | 0.02 |
| 14-HDoHE | 2,636 (1,128–7,856) | 4,700 (1,750–21,351) | 0.04 | 0.16 |
| 17(S)-HDoHE | 500 (500–599) | 500 (500–841) | 0.10 | 0.18 |
| LTB4 | 250 (250–250) | 250 (250–250) | 0.15 | 0.23 |
| 12-oxo-LTB4 | 500 (500–500) | 500 (500–500) | 0.10 | 0.18 |
| Thromboxane B2 | 513 (250–3,074) | 512 (250–5,019) | 0.58 | 0.64 |
DHET = dihydroxyeicosatrienoic acid, EET = epoxyeicosatrienoic acid, HDoHE = hydroxydocosahexaenoic acid, HETE = hydroxyeicosatetraenoic acid, HODE = hydroxyoctadecadienoic acid, IQR = interquartile range, LTB4 = leukotriene B4.
Data given as median and IQR. Raw and adjusted p values are given, adjustment was performed using the Benjamini-Hochberg procedure. Adjusted p values in bold are those less than 0.05.
Figure 1.
Kaplan-Meier survival curve for patients with concentrations of 14,15-dihydroxyeicosatrienoic acid (DHET) above (blue line, n = 70) versus below (red line, n = 82) the lower limit of quantification at inclusion to the study. Data were censored at day 28. Patients with quantifiable levels of 14,15-DHET had higher mortality (hazard ratio, 2.3; 95% CI, 1.2–4.5; p = 0.01). Crosses indicated censored data.
Consistent with the mortality outcome, those patients with measurable, compared with unmeasurable, levels of 14,15-DHET had higher mean total SOFA scores (6 [4–9] vs 4 [3–6]; p < 0.001), fewer renal failure-free days in those who developed renal failure or died (6 [0–24] vs 18 [5–26]; p = 0.02), but shorter ICU (6 d [3–10 d] vs 9 d [3–18 d]; p = 0.02) and hospital (16 d [7–34 d] vs 24 d [12–47 d]; p = 0.007) stays. No statistically significant differences were observed for the duration of shock (49 hr [23–92 hr] vs 37 hr [20–84 hr]; p = 0.23), duration of renal failure (4 d [1–8 d] vs 4 d [2–11 d]; p = 0.78), or mechanical ventilation (5 d [2–9 d] vs 7 d [2–16 d]; p = 0.14), the occurrence of renal failure (48% vs 40%; p = 0.30), requirement for inotropes (13% vs 12%; p = 0.90), or likelihood of being weaned off vasopressors (90% vs 95%; p = 0.22).
Association With Clinical Characteristics
In an attempt to understand the pathologic involvement in septic shock of the DHET family of oxylipins (5,6-DHET, 8,9-DHET, 11,12-DHET, 14,15-DHET) and their measured precursors (5,6-EET and 11,12-EET), their association with physiologic parameters across sequential samples was explored. A total of 404 sequential samples were collected from the 152 patients, including 152 on inclusion (TP0), 115 at TP1, 78 at TP2, and 59 at TP3.
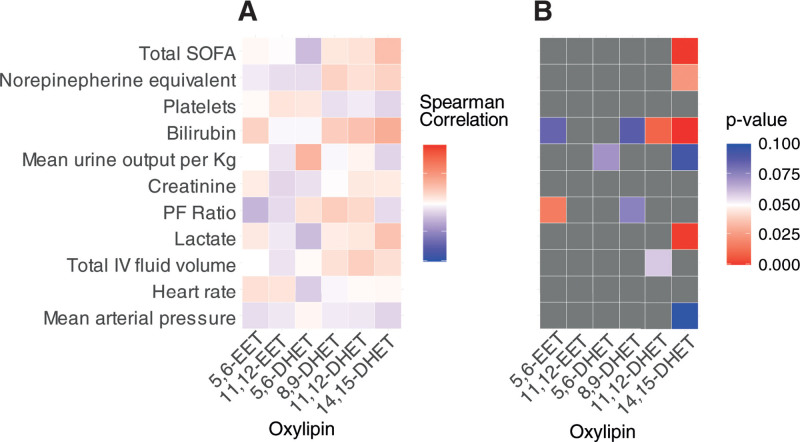
When analyzing only those samples where oxylipin concentrations could be quantified, both 11,12-DHET and 14,15-DHET showed significant but weak positive correlation with bilirubin (rs = 0.29; p = 0.01 and rs = 0.38; p < 0.001, respectively) across all available samples. Positive correlations were also seen between 14,15-DHET and lactate (rs = 0.27; p = 0.001), vasopressor requirement (rs = 0.19; p = 0.02), and total SOFA score (rs = 0.29; p < 0.001) (Fig. 2). Patterns of correlation were consistent for 14,15-DHET when samples were considered by each individual day of sampling post baseline (Supplementary Fig. 2, http://links.lww.com/CCX/A900).
Figure 2.
Correlation between measurable dihydroxyeicosatrienoic acids (DHETs) or their precursor epoxyeicosatrienoic acids (EETs) and daily clinical variables. A, Correlation matrix showing the Spearman rho correlation coefficients for oxylipin concentrations of the DHETs and their precursor EETs where quantities were above the lower limit of quantification. Data combines all sampled time points over the first week of ICU stay and their matched continuous clinical variables. B, Corresponding p value matrix for the correlation coefficients shown in (A), colored boxes represent p < 0.1 and gray boxes are all those greater than 0.1. Significant p values are considered less than 0.05 (red). PF = Pao2:Fio2 ratio, SOFA = Sequential Organ Failure Assessment.
Considering the EETs and DHETs as measurable or unmeasurable across all time points of sampling, measurable 5,6-EET and 11,12-EET at any time were both associated with lower creatinine concentrations at enrollment into the study (odds ratios [ORs], 0.67; 0.47–0.95; p = 0.03 and 0.72; 0.55–0.94; p = 0.02, respectively). Measurable 8,9-DHET and 11,12-DHET were both associated with higher bilirubin at enrollment (OR, 1.55; 1.22–1.98; p < 0.001 and 1.50; 1.21–1.86; p < 0.001) and higher daily lactate (OR, 1.28; 1.04–1.50; p = 0.02 and OR, 1.46; 1.15–1.85; p = 0.002). Ability to measure 14,15-DHET had positive association with total daily SOFA score (OR, 1.27; 1.12–1.45; p < 0.001), daily bilirubin (OR, 1.14; 1.02–1.29; p = 0.03), a past medical history of chronic obstructive pulmonary disease (OR, 6.23; 1.26–30.64; p = 0.02), and a source of infection other than lung, abdominal, or soft tissue (OR, 3.56; 1.56–8.12; p = 0.003). All associations remained when the models were corrected for age, sex, and body mass index. Due to the low rates of responses for 5,6-DHET across sequential time points, associations were not explored for this mediator.
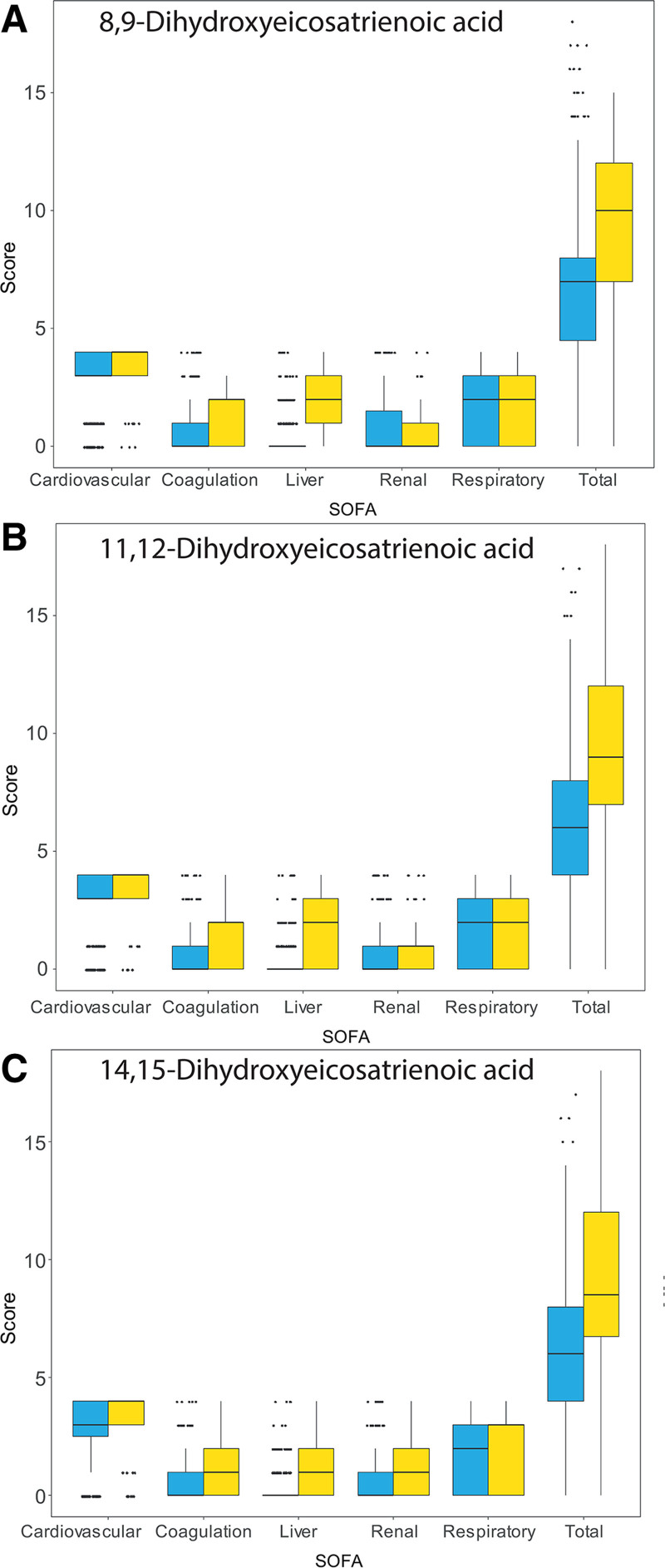
As suggested by the models above, the higher daily total SOFA scores associated with measurable 14,15-DHET were predominantly made up of a higher liver component. Although not significant in the models above, similar trends were also seen with 8,9-DHET and 11,12-DHET (Fig. 3). These associations remained when individual days of sampling postbaseline sample were considered (Supplementary Fig. 3, http://links.lww.com/CCX/A900).
Figure 3.
Box plots (median [bold line], interquartile range [IQR] [box], ±1.5 × IQR [whiskers] and outliers [points]) showing the total Sequential Organ Failure Assessment (SOFA) score and its component parts combining all sampled time points. The neurologic component was not recorded due to difficulties in its measurement in sedated patients. The highest total SOFA score possible was therefore 20. Data are shown for all samples taken over approximately the first week of ICU stay matched to daily clinical information. Blue boxes combine all samples where the oxylipin concentration was below the level of quantification and yellow where they were above the level of quantification. A, 8,9-dihydroxyeicosatrienoic acid (DHET). B, 11,12-DHET. C, 14,15-DHET. Mixed-effects logistic models showed higher daily total SOFA scores were associated with detectable 14,15-DHET (odds ratio [OR], 1.27; 1.12–1.45; p < 0.001).
DISCUSSION
We have reported the largest study of oxylipins in septic shock to date. Although the EETs were mostly undetectable, their DHET metabolites were found to be discriminant in separating nonsurvivors from survivors. Patients with measurable 14,15-DHET had twice the risk of death of those in whom it was too low to measure. Additionally, our data suggest that whilst having measurable 5,6-EET or 11,12-EET is associated with a lower baseline creatinine, having measurable DHETs is associated with a higher lactate, higher bilirubin concentration, and liver dysfunction, hinting that these two species of oxylipins may have opposite associations with organ dysfunction.
Comparable human data in the literature is limited, with studies either analyzing a very small number of patients (17) or focusing on proresolving mediators without analyzing P450 epoxygenase-generated oxylipins (16). As such, the majority of relevant data come from preclinical studies.
EETs are derived from arachidonic acid via cytochrome P450 enzymes. They act in a paracrine manner after release into the circulation (5, 6) and are known to be vasodilatory (7–9). Animal models have shown that EETs reduce mean arterial pressure (10) and that blocking their synthesis increases vascular resistance (11, 12). EETs are highly labile and are rapidly metabolized in vivo to their DHET counterparts by sEH (6). These were initially thought to be inactive (6) but some studies have shown them to be vasoactive, especially in the coronary circulation (25, 26). Although our findings support a role for the EET-DHET pathways in the pathogenesis of sepsis in humans, we found little evidence to support their involvement in pathologic vasodilation or severity of shock, with 14,15-DHET only having a week correlation with vasopressor requirement.
Instead, our data suggest that DHETs may be related to other sepsis-induced organ failure, especially liver dysfunction. Although this association has not been demonstrated before in humans, 14,15-DHET has been found to correlate with alpha-fetoprotein levels in chronic hepatitis B infection (27). Hepatic cytochrome p450 enzymes are known to be downregulated in sepsis, whereas sEH activity may instead be upregulated (28–30). The combination of these changes to enzymic activity in severe sepsis could lead to a reduction in EET levels due to a combination of reduced production and increased metabolism.
In addition to their vasodilatory actions, EETs are known to have a number of immunomodulatory and organ-specific effects, which may be protective in sepsis (6). Indeed, animal models of sepsis where EETs are stabilized, either by pharmacological inhibition or genetic knockout of sEH, show improved survival, associated with either measurable increases in serum EET levels or decreases in their DHET counterparts (14, 15, 31). However, some of these studies (15, 31) used a lipopolysaccharide challenge model of sepsis, which may be of limited comparability to human sepsis. Nonetheless, sEH inhibition has also been shown to reduce sepsis-induced transaminitis (14). Correspondingly, inhibition of sEH may be beneficial in attenuating several other, nonsepsis-related, inflammatory liver diseases (32).
Although an initial pro-inflammatory response is vital to combat microbial pathogens in sepsis, it has long been considered that an exaggerated or dysregulated response may result in excessive tissue damage (33). In a murine cecal ligation and puncture model, boosting EET levels by inhibiting sEH, significantly reduced the systemic inflammatory response via inactivation of mitogen-activated protein kinase signaling pathways, resulting in reduced levels of the pro-inflammatory cytokines interleukin-1β and tumor necrosis factor-α (14). Not only was this associated with improved survival, subsequent in vitro work showed this response to be dependent on 14,15-EET, as application of an EET receptor blocker abolished the immunomodulatory effects. Likewise, others have shown that stabilizing EET levels can decrease pro-inflammatory signaling by reducing the expression of triggering receptors expressed on myeloid cells-1 (34). Finally, EETs are known to inhibit M1 macrophage polarization and preserve M2 polarization, which is associated with reduced pro-inflammatory cytokine expression and improved phagocytosis and pathogen clearance (14, 35). Taken together, these data support the possible beneficial immunomodulatory effects of EETs in sepsis and, in particular, on sepsis-induced liver dysfunction. Our results demonstrate an association of the DHETs with mortality and liver dysfunction, which may be explained by a more rapid breakdown of the protective EETs.
Although this was the largest study of oxylipins in sepsis to date, the VANISH trial was not designed or powered for the purpose of oxylipin analysis. As only a fraction of the trial participants underwent blood sampling, it is possible that the included participants are not fully representative of a generalized septic shock population. This is supported by the higher rates of cirrhosis and chronic obstructive pulmonary disease and lower severity of shock in included patients compared with the rest of the trial population, potentially limiting the generalizability of our findings. Similarly, it is possible that we under-detected oxylipin associations with mortality as the study was not powered for this analysis. Also, several oxylipins were below the quantification limit, which meant they were not able to be accurately analyzed. The fact that such small numbers of patients had detectable EETs complicates the analysis and understanding of the pathologic relationship between EETs and DHETS in sepsis, for example 8,9-EET and 14,15-EET were unquantifiable in all samples and 5,6-EET and 11,12-EET were quantifiable in a minority. Future work should endeavor to explore these oxylipins using more sensitive assays. Finally, this work only addresses concentrations of oxylipins in the circulation. It is possible that oxylipin relationships, activity and associations are different in different tissue beds such as the liver, where many of these are produced.
CONCLUSIONS
This exploratory analysis has demonstrated a possible association between the cytochrome P450 epoxygenase metabolites with septic shock mortality and organ, especially liver, dysfunction in humans. This association warrants further exploration and raises the possibility that inhibition of the sEH enzyme may represent a possible future therapeutic strategy in septic shock.
ACKNOWLEDGMENTS
We would like to thank the National Phenome Centre at Imperial College London for assistance performing the oxylipin measurements.
Supplementary Material
Footnotes
Current address for Drs. Gordon and Antcliffe: Division of Anaesthetics, Pain Medicine and Intensive Care, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, London, United Kingdom.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the Medical Research Council and National Institute for Health Research (NIHR) (grant number MC_PC_12025), the NIHR Imperial Biomedical Research Centre, and also by the U.K. Intensive Care Foundation.
Prof. Gordon reports that he has consulted for GlaxoSmithKline and 30 Respiratory, with funds paid to his institution. This article presents independent research funded by the U.K. National Institute for Health Research (NIHR), an NIHR Clinician Scientist Award (NIHR/CS/009/007), and an NIHR Research Professor award (RP-2015-06-018) held by Dr. Gordon. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
REFERENCES
- 1.Angus DC, van der Poll T: Severe sepsis and septic shock. N Engl J Med. 2013; 369:840–851 [DOI] [PubMed] [Google Scholar]
- 2.Mosblech A, Feussner I, Heilmann I: Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem. 2009; 47:511–517 [DOI] [PubMed] [Google Scholar]
- 3.Aronoff DM: Cyclooxygenase inhibition in sepsis: Is there life after death? Mediators Inflamm. 2012; 2012:696897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard GR, Wheeler AP, Russell JA, et al. : The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med. 1997; 336:912–918 [DOI] [PubMed] [Google Scholar]
- 5.Capdevila JH, Falck JR, Harris RC: Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000; 41:163–181 [PubMed] [Google Scholar]
- 6.Bellien J, Joannides R: Epoxyeicosatrienoic acid pathway in human health and diseases. J Cardiovasc Pharmacol. 2013; 61:188–196 [DOI] [PubMed] [Google Scholar]
- 7.Archer SL, Gragasin FS, Wu X, et al. : Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003; 107:769–776 [DOI] [PubMed] [Google Scholar]
- 8.Fleming I, Rueben A, Popp R, et al. : Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007; 27:2612–2618 [DOI] [PubMed] [Google Scholar]
- 9.Huang A, Sun D, Jacobson A, et al. : Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005; 96:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CR, Imig JD, Edin ML, et al. : Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010; 24:3770–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halcox JP, Narayanan S, Cramer-Joyce L, et al. : Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. Am J Physiol Heart Circ Physiol. 2001; 280:H2470–H2477 [DOI] [PubMed] [Google Scholar]
- 12.Bellien J, Joannides R, Iacob M, et al. : Evidence for a basal release of a cytochrome-related endothelium-derived hyperpolarizing factor in the radial artery in humans. Am J Physiol Heart Circ Physiol. 2006; 290:H1347–H1352 [DOI] [PubMed] [Google Scholar]
- 13.Samokhvalov V, Jamieson KL, Vriend J, et al. : CYP-epoxygenase metabolites of docosahexaenoic acid protect HL-1 cardiac cells against LPS-induced cytotoxicity through SIRT1. Cell Death Discov. 2015; 1:15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Tang Y, Yu J, et al. : sEH Inhibitor TPPU ameliorates cecal ligation and puncture-induced sepsis by regulating macrophage functions. Shock. 2020; 53:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong R, Hu D, Yang Y, et al. : EETs reduces LPS-induced hyperpermeability by targeting GRP78 mediated Src activation and subsequent Rho/ROCK signaling pathway. Oncotarget. 2017; 8:50958–50971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalli J, Colas RA, Quintana C, et al. : Human sepsis eicosanoid and proresolving lipid mediator temporal profiles: Correlations with survival and clinical outcomes. Crit Care Med. 2017; 45:58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamaguchi M, Wu HN, Tanaka M, et al. : A case series of the dynamics of lipid mediators in patients with sepsis. Acute Med Surg. 2019; 6:413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antcliffe DB, Wolfer AM, O’Dea KP, et al. : Profiling inflammatory markers in patients with pneumonia on intensive care. Sci Rep. 2018; 8:14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon AC, Mason AJ, Thirunavukkarasu N, et al. ; VANISH Investigators. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: The VANISH Randomized Clinical Trial. JAMA. 2016; 316:509–518 [DOI] [PubMed] [Google Scholar]
- 20.Wolfer AM, Gaudin M, Taylor-Robinson SD, et al. : Development and validation of a high-throughput ultrahigh-performance liquid chromatography-mass spectrometry approach for screening of oxylipins and their precursors. Anal Chem. 2015; 87:11721–11731 [DOI] [PubMed] [Google Scholar]
- 21.Wolfer AM, Scott AJ, Rueb C, et al. : Longitudinal analysis of serum oxylipin profile as a novel descriptor of the inflammatory response to surgery. J Transl Med. 2017; 15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goradia S, Sardaneh AA, Narayan SW, et al. : Vasopressor dose equivalence: A scoping review and suggested formula. J Crit Care. 2021; 61:233–240 [DOI] [PubMed] [Google Scholar]
- 23.Jewell NP: Statistics for Epidemiology. New York, Chapman and Hall/CRC, 2003 [Google Scholar]
- 24.R Core Team. R: A Language and Environment for Statistical Computing. Available at: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing. Accessed June 10, 2021
- 25.Oltman CL, Weintraub NL, VanRollins M, et al. : Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998; 83:932–939 [DOI] [PubMed] [Google Scholar]
- 26.Lu T, Katakam PV, VanRollins M, et al. : Dihydroxyeicosatrienoic acids are potent activators of Ca(2+)-activated K(+) channels in isolated rat coronary arterial myocytes. J Physiol. 2001; 534:651–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y, Fang J, Zou L, et al. : Omega-6-derived oxylipin changes in serum of patients with hepatitis B virus-related liver diseases. Metabolomics. 2018; 14:26. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Wang W, Hu Y, et al. : Prolonged soluble epoxide hydrolase reactivity in brain endothelial cells is associated with long cognitive deficits in sepsis. Mol Neurobiol. 2020; 57:2846–2855 [DOI] [PubMed] [Google Scholar]
- 29.Harris TR, Hammock BD: Soluble epoxide hydrolase: Gene structure, expression and deletion. Gene. 2013; 526:61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacob A, Zhou M, Wu R, et al. : The role of hepatic cytochrome P-450 in sepsis. Int J Clin Exp Med. 2009; 2:203–211 [PMC free article] [PubMed] [Google Scholar]
- 31.Samokhvalov V, Jamieson KL, Darwesh AM, et al. : Deficiency of soluble epoxide hydrolase protects cardiac function impaired by LPS-induced acute inflammation. Front Pharmacol. 2018; 9:1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warner J, Hardesty J, Zirnheld K, et al. : Soluble epoxide hydrolase inhibition in liver diseases: A review of current research and knowledge gaps. Biology (Basel). 2020; 9:E124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry H, Zhou J, Zhong Y, et al. : Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013; 27:669–684 [PMC free article] [PubMed] [Google Scholar]
- 34.Dong L, Zhou Y, Zhu ZQ, et al. : Soluble epoxide hydrolase inhibitor suppresses the expression of triggering receptor expressed on myeloid cells-1 by inhibiting NF-kB activation in murine macrophage. Inflammation. 2017; 40:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai M, Wu L, He Z, et al. : Epoxyeicosatrienoic acids regulate macrophage polarization and prevent LPS-induced cardiac dysfunction. J Cell Physiol. 2015; 230:2108–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.