Abstract
Background
Antibiotics designed to decolonize carriers of drug-resistant organisms could offer substantial population health benefits, particularly if they can help avert outbreaks by interrupting person-to-person transmission chains. However, cost effectiveness of an antibiotic is typically evaluated only according to its benefits to recipients, which can be difficult to demonstrate for carriers of an organism that may not pose an immediate health threat to the carrier.
Methods
We developed a mathematical transmission model to quantify the effects of 2 hypothetical antibiotics targeting carbapenem-resistant Enterobacteriaceae (CRE) among long-term acute care hospital inpatients: one assumed to decrease the death rate of patients with CRE bloodstream infections (BSIs) and the other assumed to decolonize CRE carriers after clinical detection. We quantified the effect of each antibiotic on the number of BSIs and deaths among patients receiving the drug (direct effect) and among all patients (direct and indirect effect) compared to usual care. We applied these results to a cost-effectiveness analysis with effectiveness outcome of life-years gained and assumed costs for antibiotic doses and for CRE BSI.
Results
The decolonizing antibiotic, once indirect effects were included, produced increased relative effectiveness and decreased relative costs compared to both usual care and the BSI treatment antibiotic. In fact, in most scenarios, the decolonizing drug was the dominant treatment strategy (ie, less costly and more effective).
Conclusions
Antibiotics that decolonize carriers of drug-resistant organisms can be highly cost-effective when considering indirect benefits within populations vulnerable to outbreaks. Public health could benefit from finding ways to incentivize development of decolonizing antibiotics in the US, where drugs with unclear direct benefits to recipients would pose difficulties in achieving FDA approval and financial benefit to the developer.
Keywords: healthcare associated infections, antimicrobial resistance, mathematical models, health economics
We used a transmission model to analyze cost-effectiveness of new antibiotics that combat drug-resistant organisms in recipients and their hospital contacts. In most scenarios, antibiotics that decolonize carriers were less costly and more effective than antibiotics that treat bloodstream infections.
Healthcare-associated infections with multi-drug resistant organisms are an urgent public health threat in the United States [1] and worldwide. The development of new antibiotics to treat life-threatening infections with bacteria resistant to existing antibiotics is of high priority [2]. Meanwhile, actions to interrupt transmission of these bacteria among healthcare patients can play a critical role in preventing large-scale outbreaks from occurring, especially if performed in settings where highly vulnerable patients are concentrated [3]. While healthcare interventions aimed at transmission mitigation are usually nonpharmaceutical, one exception could be the development and use of decolonization antibiotics [4].
Patients colonized with a drug-resistant organism (carriers) can be at increased risk of endogenously developing a dangerous infection compared to noncarriers, but also pose risk to others in their vicinity via transmission [4]. Therefore, decolonization offers both a direct benefit to the carrier receiving the antibiotic and an indirect benefit to the population of noncarriers downstream from the carrier in potential transmission chains. The direct benefits of decolonizing carriers are likely small compared to treating patients with invasive infections, as many carriers do not develop health-threatening infections from a given episode of colonization. However, when considering a decolonization regime implemented in a population vulnerable to devastating outbreaks, the overall health benefits could be comparable or perhaps greater.
The potential population benefits of decolonizing antibiotics may be misaligned with financial incentives for companies to develop them. First, an antibiotic intended for use by carriers of an organism that may not pose an immediate threat to the recipient would likely face unusually high safety standards for approval by the FDA in the U.S. Second, even if a decolonization antibiotic is approved for patient use, its spillover benefits, or “positive externalities” in economic terms, may not be incorporated into the drug’s demand. This potential failure to account economically for positive externalities would be exacerbated by individual patients or their insurers being billed for treatments that have benefits primarily at the population level. This misalignment is a potential market failure in that market forces may be resulting in the supply of decolonizing antibiotics being insufficiently provisioned relative to their potential benefits.
Our goal for this work was to quantify the potential direct and indirect benefits of the use of a decolonizing antibiotic in a high-risk setting compared to those of an antibiotic targeted for treatment of invasive infections only. Given the economic implications [5], we sought to quantify antibiotic effects not only with health outcomes (infections and deaths prevented) but also with cost-effectiveness outcomes. Cost-effectiveness analysis (CEA) measures the costs and benefits of a new treatment or prevention strategy compared to an existing approach. Examples of costs in these analyses can include treatment, progression of illness, or complications which can be paid for by an insurer or by patients out of pocket. A common effectiveness measure is the quality-adjusted life-year (QALY). Results from a CEA can be presented as incremental cost-effectiveness ratios (ICERS) which are calculated by taking the ratio of the difference in cost and the difference in effectiveness between the two strategies under consideration.
Economic evaluations can be conducted alongside a clinical trial or using observational data. However, they are commonly performed using simulation models because no head-to-head study has been conducted of the 2 treatment strategies or because a number of considerations (such as the impact of the treatment on quality of life or the effect on healthcare costs years or decades in the future) simply cannot be measured in existing studies or databases.
While the presence of positive externalities in infectious disease treatments and prevention strategies is well-understood from a theoretical standpoint, few efforts have been made to quantify these effects for specific interventions. One reason for this is the difficulty in measuring transmission of infectious pathogens in a real-world setting. A mathematical simulation model, which can track the actions of specific hypothetical patients and healthcare providers through explicit assumptions and emergent properties, can overcome this barrier. Through this approach, we can measure indirect effects by identifying transmissions and, therefore, infections that would have been prevented through treatment or prevention efforts undertaken on other patients. These types of simulation models are called “dynamic” models because they allow interventions to affect the probability of disease both in treated and untreated individuals in a nonlinear manner over time [6]. They differ from the more common static models, which are appropriate for nontransmissible diseases. Because in these static models the probability of disease exposure is independent of intervention strategies, the probability of disease does not change over time. A recent systematic literature review showed that there are few published studies that use dynamic models for CEAs for treatment and prevention strategies of healthcare-associated infections (HAIs) [7].
For our analyses, we chose to develop a dynamic compartmental model to simulate the effect of 2 hypothetical antibiotics targeting carbapenem-resistant Enterobacteriaceae (CRE) among long-term acute care hospital (LTACH) inpatients. One antibiotic was assumed be targeted at the treatment of bloodstream infections (BSIs) and the other targeted at decolonizing clinically identified carriers. We examined these 2 drugs separately compared to no treatment, as well as head-to-head. We hypothesized that our model’s inclusion of both direct and indirect antibiotic effects would show the decolonizing antibiotic to be cost-effective and that, under certain circumstances, a decolonizing antibiotic might be more cost-effective than one used only for treatment of infections.
METHODS
Compartmental Model
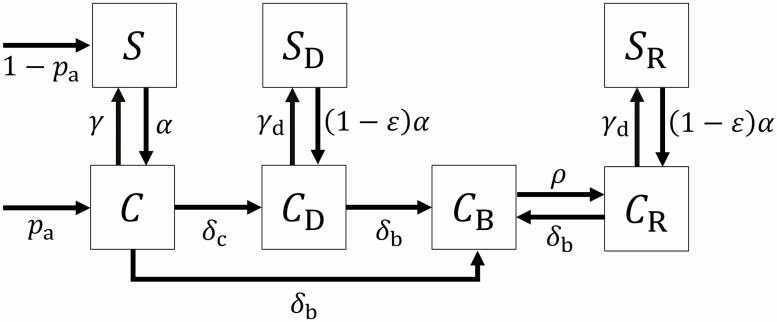
We developed a mathematical model representing the portion of LTACH inpatients in 7 different states of CRE colonization, infection, and contact precaution status (Fig. 1):
Figure 1.
Patient states and state transition rates Squares depict possible states with respect to CRE in the compartmental model. Arrows between squares depict possible state transitions during the LTACH stay and are labeled with rate parameters; arrows into squares from outside point to possible states at admission and are labeled with probabilities of each admission state. Patients in any state can be removed via death or live discharge (not depicted)—see Supplementary material for full mathematical specification.
Susceptible (): not colonized with CRE; no prior CRE detection
Colonized and undetected (): undetected CRE carrier
Colonized and clinically detected (): clinically detected CRE carrier; no prior CRE BSI
Colonized with ongoing BSI (): CRE carrier with currently unresolved BSI
Colonized with resolved BSI (): CRE carrier with prior BSI, now recovered
Susceptible and surveillance detected (): not colonized with CRE; prior CRE clinical detection; no prior CRE BSI
Colonized and surveillance detected (): not colonized with CRE; prior recovery from CRE BSI
Patients were colonized in state at admission with importation rate , with the remainder of admitted patients susceptible in state . Patients in state move to state (acquire colonization due to transmission) at the acquisition rate proportional to the prevalence of patients in one of the colonized states:
The parameter , the baseline transmission rate, defines how the acquisition rate depends on the prevalence of carriers in the LTACH. A susceptible patient acquires colonization from undetected carriers at rate , where is the prevalence of undetected carriers, and from detected carriers at rate , where , , and are the prevalence of carriers in each of the detected/infected states, is the effectiveness of contact precautions at reducing transmission compared to the baseline, and the parameters are the differential transmissibility factors associated with differential shedding due to being state .
Patients in state could clear colonization at rate , progress to BSI at rate or other clinical detection at rate . Patients in state or . (clinically detected CRE but no ongoing BSI) could also progress to BSI onset at rate or clear colonization at rate . State patients (ongoing BSI) could recover from BSI at rate but could not fully clear colonization until after BSI recovery. Those in state or (cleared colonization after clinical detection or BSI recovery) remained under contact precautions, and we assumed that those precautions reduced their rate of CRE re-acquisition by the same factor, , that they reduced transmissibility.
We assumed patients were admitted to the LTACH at a constant rate and removed by death or live discharge at rates that could depend on the patient’s state. Death occurred at rate for BSI patients (state ) and rate for all other patients. To model live discharge, we followed results from our previous studies [3, 8], which showed that LTACH length of stay distributions are well captured by a mixture of an exponential and a gamma probability distribution. Here, we modified this framework to allow discharge dependency on disease states by superimposing a compartmental “stay-stage state” on the disease state model. A fraction of patients were admitted and remained in stay-stage state 0, where non-BSI patients followed the exponential stay distribution and were discharged at a constant rate . The remaining patients were admitted into stay-stage state 1 and followed an Erlang-distributed stay duration, which is equivalent to the gamma distribution with integer shape parameter . Patients in state 1 through 1 were not eligible for live discharge but would progress to the next sequential integer stay-stage state at constant rate , while non-BSI patients who reached stay-stage state were discharged at rate . BSI patients in disease state were not eligible for live discharge regardless of their stay-stage state.
Parameter Value Assumptions and Calibration
Values for most of our described model parameters were calibrated to published data from Chicago-area LTACHs [9], including stabilized admission and cross-sectional CRE carriage prevalence, CRE clinical detection and BSI rates, all-cause mortality rates, and length of stay statistics (Table 1). We used the pre-intervention data from the cited study to represent a LTACH without an active surveillance program to identify nonclinically detected carriers for contact isolation.
Table 1.
Data from Hayden et al [9] Used for Model Calibration
| Value (symbol) | Data |
|---|---|
| Admission CRE positivity fraction (a) |
0.206 |
| Cross-sectional facility CRE positivity fraction (f) | 0.458 |
| CRE clinical detection rate per 1000 patient days (d) | 3.7 |
| CRE BSI onset rate per 1000 patient days (b) | 0.9 |
| Inpatient mortality rate per admission (m) | 0.215 |
| Days of stay: mean; 25th, 50th, 75th percentiles | 33.8; 16, 28, 43 |
When calibrating our model to the positivity fraction data, we assumed the test sensitivity was 0.85, such that the actual CRE importation rate from admissions was and the cross-sectional prevalence of CRE carriers was .
Estimates for effectiveness of contact precautions , the clearance rate for CRE carriage , the surveillance test sensitivity , and the CRE BSI death and recovery rates and were not identifiable from these published LTACH data, so we independently obtained these values from other sources (Table 2). We assumed the effectiveness of contact precautions at reducing transmission to be 50% (ie, detected CPE carriers, assumed to be under contact precautions, transmit to other patients at a rate 50% less than undetected carriers). We used this assumption in previous publications [3, 8, 10], based on data from comparing contamination levels on healthcare provider hands after interacting with colonized patients with and without contact precautions [11]. We assumed a clearance rate of 1/387 per day [12], surveillance test sensitivity of 85% [13, 14], BSI death probability of 60.5% and mean time from BSI onset to death of 7 days [15]. We also assumed that the transmissibility parameters representing state-specific shedding effects 3. This assumption means that clinically detected CRE patients (with or without a BSI) have 3-fold higher transmissibility compared to colonized patients who have not been clinically detected. While it is plausible to assume that clinically detected patients have infections that cause them to shed more organisms, quantitative data are scarce, so we tested sensitivity to this assumption via an alternate assumption that undetected patients shed equally to clinically detected patients () (Supplemental Table 1).
Table 2.
Model Parameter Values
| Parameter | Value | Source |
|---|---|---|
| CRE BSI onset rate of CRE carriers | 0.0017 / d | calibration [9] |
| CRE non-BSI clinical detection rate of CRE carriers | 0.0066 / d | calibration [9] |
| Non-BSI death rate | 0.0058 / d | calibration [9] |
| Exponential length of stay probability | 0.44 | calibration [9] |
| Exponential non-BSI discharge rate | 0.020 / d | calibration [9] |
| Erlang stage transition rate / non-BSI discharge rate | 0.15 / d | calibration [9] |
| Erlang shape parameter | 5 | calibration [9] |
| Baseline CRE transmission rate | 0.044 / d | calibration [9] |
| Contact precaution effectiveness | 0.5 | literature [11] |
| Clinical state CRE transmissibility factors | 3 | assumption |
| BSI death rate... | ||
| ...without BSI treatment antibiotic | 0.086 / d | literature [15] |
| ...with BSI treatment antibiotic | 0.043 / d | assumption [15] |
| BSI recovery rate... | ||
| ...without BSI treatment antibiotic | 0.056 / d | literature [15] |
| ...with BSI treatment antibiotic | 0.100 / d | assumption [15] |
| CRE clearance rate... | ||
| ...without decolonization antibiotic | 1 / (387 d) | literature [12] |
| ...with decolonization antibiotic | 1 / (3 d) | assumption |
| Equilibrium CRE acquisition rate, without new antibiotics (α)... | ||
| ...with [9] |
0.0259 / d | calibration [9] |
| ...with | 0.0190 / d | model result |
| ...with | 0.0115 / d | model result |
| ...with | 0.0102 / d | model result |
| Equilibrium CRE acquisition rate, with BSI treatment antibiotic ... | ||
| ...with | 0.0195 / d | model result |
| ...with | 0.0122 / d | model result |
| ...with | 0.0110 / d | model result |
| Equilibrium CRE acquisition rate, with decolonization antibiotic ... | ||
| ...with | 0.0120 / d | model result |
| ...with | 0.0040 / d | model result |
| ...with | 0.0015 / d | model result |
With those values fixed, we simultaneously calibrated the CRE importation rate , acquisition rate , BSI progression rate , progression rate to non-BSI clinical detection , non-BSI death rate , and live discharge parameters to match the equilibrium cross-sectional carriage prevalence (scaled by the assumed test sensitivity), clinical detection incidence, BSI onset incidence, and overall death and length of stay statistics reported in the published data [9] (Table 1). Then we solved for the baseline transmission rate that produced the calibrated acquisition rate (Table 2 and Supplemental Methods).
New Antibiotic Effects Model
For the BSI treatment antibiotic, we assumed the new antibiotic would be given to all patients with CRE BSI at detection. The effect of the drug was to decrease the BSI death rate and to change the BSI recovery rate such that the probability of death from BSI would match an estimate of BSI death probability from carbapenem-susceptible Enterobacteriaceae (29.8%) [15] and the average time to death would remain 7 days.
For the decolonizing antibiotic, we assumed the new antibiotic would be given to all patients with clinical detection of CRE at detection. The effect of the drug was to increase the clearance rate of CRE carriage such that the average time to clearance was 3 days. Patients with active BSI were still assumed to be ineligible for full clearance until after the BSI was resolved.
For each antibiotic, we quantified its effect by comparing the equilibrium reached by the calibrated model without new antibiotics to the equilibrium reached by the model with parameter values altered by the antibiotic, for a given constant CRE importation rate. We tested 3 different importation probabilities (, , and ) to represent different scenarios of endemicity of CRE in the healthcare region of the LTACH. For a given value, we first calculated the equilibrium acquisition rate consistent with the calibrated transmission rate and other parameters. Then we calculated the rates of death and CRE BSI per admission among all patients and among recipients of the antibiotic (Supplementary Material). The antibiotic effects were quantified as the reduction in the number of BSIs and deaths among antibiotic recipients (direct effect) and among all patients (total effect, ie, direct plus indirect effect) compared to usual care.
For each antibiotic type, we also generated results assuming that, in addition to the primary modeled drug effects described above, the antibiotics decreased transmissibility from patients receiving them while still carrying CRE. Specifically, for the BSI treatment antibiotic we reduced and by 90%, and for the decolonizing antibiotic we reduced , , and by 90% (Supplemental Table 2).
Cost-Effectiveness Analyses
We used our compartmental model to conduct CEAs of 3 comparisons using a lifetime time horizon: (1) the treatment antibiotic vs. usual care, (2) the decolonizing antibiotic vs. usual care, and (3) the decolonizing antibiotic vs. the treatment antibiotic. Our effectiveness measure was life-years (LYs) gained. Because our model considered patients in an LTACH, we assumed that patients entering our model were age 68. According to actuarial life tables from the US Social Security Administration, the life expectancy for a 68-year-old individual is 15.77 for males and 18.07 for females. Because the individuals in our model have been admitted to an LTACH and therefore have had higher mortality rates than the general population, we lowered the value of our LY input to 12 years, which amounted to 10.25 years after applying a 3% annual discount rate.
We included costs from the VA perspective which included the cost of the antibiotic and the cost of a healthcare-associated CRE BSI. The assumed cost of the new drug was $4000 and the cost of a CRE BSI was $54 614. For the decolonization scenario, a $25 surveillance test was performed on all patients in order to detect CRE carriage.
RESULTS
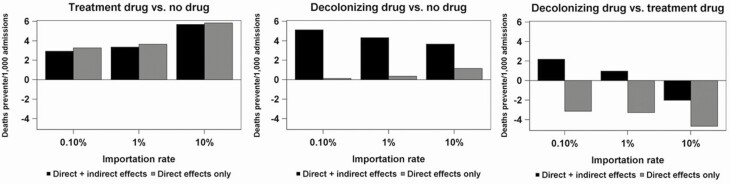
Figure 2 shows the results for deaths prevented across a range of importation levels of CRE (0.10%, 1%, and 10%) for the 3 comparisons. Deaths prevented by the BSI treatment antibiotic are mostly captured by its direct effect on recipients. Including indirect effects slightly reduces the total death-reduction benefit compared to considering only direct effects. This was because we assumed that the BSI treatment antibiotic has no effect on transmissibility and BSI-recovered patients remain colonized in the facility, thus the higher recovery rate produced slightly higher facility transmission rates. For the decolonization antibiotic, on the other hand, including indirect effects led to 2- to 40-fold increases in deaths prevented compared to only including its direct effects. When comparing the 2 antibiotics, with importation rates of 0.10% and 1%, the inclusion of indirect effects resulted in the decolonizing antibiotic preventing more deaths than the BSI treatment antibiotic.
Figure 2.
Model results—deaths prevented
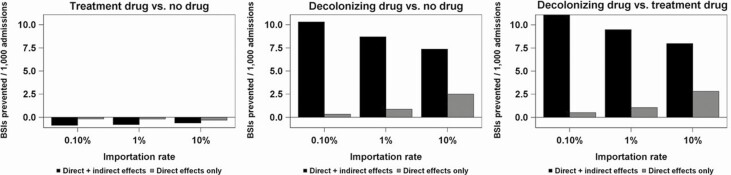
The BSIs prevented from each analysis are presented in Fig. 3. The direct effect for this outcome was slightly negative for the BSI treatment antibiotic because it cannot prevent the recipient’s first BSI (it is administered after onset), and recovered recipients who otherwise might have died have a small risk of contracting a second BSI. The indirect effect for the BSI treatment antibiotic on BSIs prevented was also slightly negative due to transmission from successfully treated but still colonized patients. For the decolonization antibiotic, inclusion of the indirect effect yields BSI prevented results that are 4- to 30-fold larger as when only considering direct effects. In the head-to-head comparison, under all importation rate assumptions and whether indirect effects were included or not, the decolonizing drug led to fewer BSIs (ie, more BSIs prevented) than the treatment drug.
Figure 3.
Model results—bloodstream infections prevented
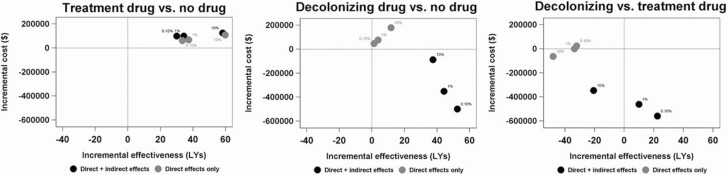
In the CEAs for the BSI treatment antibiotic, relative to usual care, the addition of indirect effects led to slightly higher incremental costs and slightly lower incremental effectiveness (Fig. 4). However, in all scenarios, the incremental cost-effectiveness ratios were quite small (ranging from $1796/LY to $1821/LY when only direct effects were considered and from $2134/LY to $3287/LY when indirect effects were included), indicating that this treatment antibiotic would be cost-effective. In the case of the decolonizing antibiotic, compared to usual care, the incremental effectiveness was positive regardless of whether indirect effects were included. But the inclusion of indirect effects caused the incremental costs to become negative. In other words, when both direct and indirect effects were included, the decolonizing antibiotic was dominant compared to usual care. In the head-to-head comparison of the decolonizing and BSI treatment antibiotics, when only direct effects were considered in the analysis, the costs were relatively equal but the effectiveness clearly favored the BSI treatment antibiotic. However, with indirect effects included, the decolonizing antibiotic was substantially less costly than the BSI treatment, and, with importation rates of 0.10% and 1%, the decolonizing antibiotic dominated (ie, was both less costly and more effective than) the BSI treatment antibiotic.
Figure 4.
Cost-effectiveness analysis results Note: The assumed CRE importation level for each particular model run is indicated next to each dot
Our results were somewhat sensitive to reducing the assumed relative transmissibility of clinically detected/infected CRE patients compared to undetected carriers. Under these alternate assumptions, the indirect effects of the decolonizing antibiotic were diminished, such that it prevented fewer deaths than the BSI treatment antibiotic under each example importation rate (Supplemental Fig. 1). The BSI prevention results were similar (Supplemental Fig. 2), and the decolonizing antibiotic was no longer dominant compared to the BSI treatment drug at low importation rates (Supplemental Fig. 3). When adding the additional assumption that each antibiotic reduces transmission from recipients by 90% while still colonized, the outcome comparisons were similar to our main analysis in deaths prevented (Supplemental Fig. 4) and BSIs prevented (Supplemental Fig. 5), and the decolonizing antibiotic was dominant compared to the BSI treatment antibiotic at low importation rates (Supplemental Fig. 6).
DISCUSSION
We constructed a dynamic simulation model and used this model to conduct a CEA for the use of a decolonization antibiotic for clinically detected CRE carriers in a LTACH. When comparing the new antibiotic to no drug or to a drug for treatment of CRE BSI, we found lower incremental cost and higher incremental effectiveness, largely attributable to the inclusion of indirect effects via within-facility transmission reduction. In fact, in many scenarios, the size of the indirect effect was larger than that of the direct effect.
This finding demonstrates the importance of considering the nonlinear effects of transmission reduction when conducting economic evaluations of infection treatment and prevention strategies. Our results show that if these indirect effects were neglected, the new antibiotic would appear to lead to an increase in cost and an improvement in effectiveness. However, when quantifying the full population effect we found a number of plausible situations in which these new drugs became the dominant strategy by yielding cost savings as well. This can have important ramifications in this era of rising healthcare costs. While many new technologies are shown to be cost-effective (ie, yielding QALYs or LYs at an incremental cost below $100 000), it is rare that an intervention can achieve true cost savings. Demonstrating the magnitude of this cost savings can provide government decision makers with estimates of the amount of investment needed to overcome the market failure caused by financial incentives being misaligned, due to the presence of significant positive externalities.
An important insight from our analysis is that the decolonizing agent led to fewer BSIs than the treatment drug regardless of whether these calculations included indirect effects. Also, due to the effect of transmission reduction on reducing large-scale outbreak risk, the decolonizing agent led to fewer infection-related deaths than the treatment agent in low importation scenarios, which are most commonly present in acute care hospitals. In economic analyses, the inclusion of these indirect effects led the decolonizing drug to be less costly than the treatment drug under all importation rate assumptions and even dominant when importation rates were assumed to be low.
There is little incentive for companies to pursue development of antibiotics targeted for decolonization, likely because of the low or unclear immediate direct benefit to most potential recipients. This lack of well-documented direct benefit would create difficulties for FDA approval in the U.S., especially if there are potentially harmful side effects to the recipient. The safety profile required for approval of a decolonizing antibiotic might be held to high standards, similar to those for a vaccine to be administered to healthy subjects. The lower direct benefit also poses economic difficulties when the drug cost is borne by the recipient or the recipient’s insurer, but the benefits manifest largely among nonrecipients. Finding systemic ways to alleviate these barriers to development could prove highly beneficial for public health.
If developed and available for wide use in healthcare facilities, the market for such decolonizing drugs could be substantially larger than for infection treatment agents, especially for endemic organisms for which carriage is more common than infection. Identification of carriage for some high-priority organisms like CRE remains scarce in many regions, where use of a decolonizing antibiotic would be infrequent. However, our findings suggest that decolonizing those infrequently identified carriers could contribute substantially to preventing explosive outbreaks in high-risk facilities.
A region or facility with currently low CRE rates might be tempted to forego use of decolonizing antibiotics until detections rise above some threshold. However, our prior modeling study showed that even short delays in mitigation response to emerging CRE outbreaks can result in the intervention’s benefits materializing slowly, with many more cumulative transmissions occurring compared to immediate or preemptive intervention [3].
While this is the first study to provide evidence that externalities are an important aspect of economic evaluations of new treatment or prevention strategies for MDRO bacteria, several studies have explored this phenomenon within the context of influenza. For example, Lugner et al. (2010) compared dynamic and static models of anti-viral therapy during an influenza pandemic [6]. They showed that, in dynamic models, ICERs can vary by the size of the pandemic, the proportion of cases that are treated, and age-specific clinical attack rates. Pradas-Velasco et al. (2008) also compared static and dynamic CEA models [16]. They modeled an influenza epidemic and found that the indirect effect of vaccination can be greater than the direct effect, indicating the importance of taking positive externalities into account in such models.
Our study had limitations. As with any modeling study, projected results depend on assumptions that may not be accurate. While we matched our assumptions to published data wherever possible, some key parameter value assumption did not have strong data-based justification. In particular, the relative transmissibility of clinically detected or invasively infected CRE patients compared to undetected carriers in hospitals is unknown, as the transmitter to an acquisition is rarely identified definitively during CRE outbreaks. This assumption is important for quantifying the indirect, transmission-reduction effect of antibiotic administration when the antibiotic is directed toward clinical cases only. We showed that when our default assumptions for post-infection transmissibility were changed, the indirect antibiotic effects were altered more strongly for the decolonizing antibiotic (Supplemental Figures), suggesting that our results for that drug are less robust to that key source of uncertainty. In addition, given that the medications explored in our model are hypothetical, the values of the inputs used in our calculations of cost-effectiveness (ie, LYs gained, cost of treatments, and cost of CRE BSIs) are our best guesses of the actual values of these parameters. In sensitivity analyses, we explored ranges for each which yielded similar results to those reported here.
Another limitation is that our results may not extrapolate to settings other than the highly vulnerable LTACHs on which our model was based, where high transmissibility and long length of stay likely amplify the projected indirect effects of the decolonization drug. However, given the high importance attached to LTACHs for their role in potentially driving regional outbreaks of CRE and other high-urgency, drug-resistant threats [17], it may be of equally high importance to consider the potential benefits of new antibiotics and other interventions introduced in those settings [3].
Our model does not represent potentially important nuances and difficulties of the administration of new antibiotics in real-world settings. For example, imperfect compliance with or refusal of our assumed antibiotic administration plans could alter resulting impacts. We also quantified the new antibiotic impacts using the long-term equilibrium reached after the antibiotic has been regularly administered for some time, which ignores the transitional period after the antibiotic is first introduced during which the benefits might not yet be realized.
We assumed that the treatment and decolonizing antibiotic would be targeted to patients with BSI and to CRE carriers identified by a positive clinical test, respectively. These were chosen as illustrative test cases that could be matched to incidence data. In a real-world setting, the target recipients would vary; for example, it would also be possible to identify candidates for decolonization via active surveillance. As regular surveillance for CRE is not normally performed outside of special interventions [9] a CEA for this scenario would have to include the costs of performing surveillance, in addition to the costs of antibiotic administration to those who test positive, and likewise would need to separate the benefits due to decolonization vs. the other benefits of detecting carriers, such as implementing contact precautions [8] to reduce transmission before decolonization is achieved.
In conclusion, we found that use of a decolonization antibiotic could be highly cost-effective when accounting for its effect on reducing transmission in high risk settings. In some instances, administering a decolonization antibiotic could be a dominant, cost-saving strategy compared to a new antibiotic for treating invasive infections. The fact that the benefits of decolonization manifested largely at the population level rather than among antibiotic recipients could explain the lack of incentive for decolonization drug development. Finding ways to alleviate this potential market inefficiency could be highly beneficial for public health.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Glossary
Nonstandard Abbreviations:
- BSI
bloodstream infection
- CEA
cost-effectiveness analysis
- CRE
carbapenem-resistant Enterobacteriaceae
- HAI
healthcare-associated infections
- ICER
incremental cost-effectiveness ratio
- LTACH
long-term acute care hospital
- LY
life years
- QALY
quality-adjusted life-year
Notes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Acknowledgments. The authors thank John A. Jernigan and Rachel B. Slayton for inspiring our pursuit of the topic of this work and for helpful input to the manuscript. This material is the result of work supported with resources and the use of facilities at the George E. Wahlen Department of Veterans Affairs Medical Center, Salt Lake City, Utah.
Financial support. This work was supported by the Centers for Disease Control and Prevention [MIND-Healthcare Program award number U01CK000538] and the Department of Veterans Affairs Health Services Research and Development Service [Center of Innovation—Informatics, Decision-Enhancement and Analytic Sciences (IDEAS) 2.0 Center award number I50HX001240].
Supplement sponsorship. This supplement is sponsored by the Informatics, Decision-Enhancement and Analytic Sciences (IDEAS) Center.
Potential conflicts of interest. D. J. A. Toth and M. H. Samore report grants from Pfizer, Inc., outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Damon J A Toth, Department of Veterans Affairs Salt Lake City Health Care System, Salt Lake City, UT, USA; Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA; Department of Mathematics, University of Utah, Salt Lake City, UT, USA.
Matthew H Samore, Department of Veterans Affairs Salt Lake City Health Care System, Salt Lake City, UT, USA; Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA.
Richard E Nelson, Department of Veterans Affairs Salt Lake City Health Care System, Salt Lake City, UT, USA; Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, UT, USA.
References
- 1. CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC, 2019. [Google Scholar]
- 2. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics, Geneva: World Health Organization, 2017. [Google Scholar]
- 3. Toth DJA, Khader K, Slayton RB, et al. The potential for interventions in a long-term acute care hospital to reduce transmission of carbapenem-resistant enterobacteriaceae in affiliated healthcare facilities. Clin Infect Dis 2017; 65:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Septimus EJ, Schweizer ML. Decolonization in prevention of health care-associated infections. Clin Microbiol Rev 2016; 29:201–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roope LSJ, Smith RD, Pouwels KB, et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019; 364:eaau4679. [DOI] [PubMed] [Google Scholar]
- 6. Lugnér AK, Mylius SD, Wallinga J. Dynamic versus static models in cost-effectiveness analyses of anti-viral drug therapy to mitigate an influenza pandemic. Health Econ 2010; 19:518–31. [DOI] [PubMed] [Google Scholar]
- 7. Nelson RE, Deka R, Khader K, Stevens VW, Schweizer ML, Rubin MA. Dynamic transmission models for economic analysis applied to health care-associated infections: A review of the literature. Am J Infect Control 2017; 45:1382–7. [DOI] [PubMed] [Google Scholar]
- 8. Toth DJA, Khader K, Beams A, Samore MH. Model-based assessment of the effect of contact precautions applied to surveillance-detected carriers of carbapenemase-producing enterobacteriaceae in long-term acute care hospitals. Clin Infect Dis 2019; 69:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayden MK, Lin MY, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenters Program . Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2015; 60:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slayton RB, Toth D, Lee BY, et al. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities–United States. MMWR Morb Mortal Wkly Rep 2015; 64:826–31. [PMC free article] [PubMed] [Google Scholar]
- 11. DalBen MF, Teixeira Mendes E, Moura ML, et al. A model-based strategy to control the spread of carbapenem-resistant enterobacteriaceae: simulate and implement. Infect Control Hosp Epidemiol 2016; 37:1315–22. [DOI] [PubMed] [Google Scholar]
- 12. Zimmerman FS, Assous MV, Bdolah-Abram T, Lachish T, Yinnon AM, Wiener-Well Y. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am J Infect Control 2013; 41:190–4. [DOI] [PubMed] [Google Scholar]
- 13. Adler A, Navon-Venezia S, Moran-Gilad J, Marcos E, Schwartz D, Carmeli Y. Laboratory and clinical evaluation of screening agar plates for detection of carbapenem-resistant Enterobacteriaceae from surveillance rectal swabs. J Clin Microbiol 2011; 49:2239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathers AJ, Poulter M, Dirks D, Carroll J, Sifri CD, Hazen KC. Clinical microbiology costs for methods of active surveillance for Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Infect Control Hosp Epidemiol 2014; 35:350–5. [DOI] [PubMed] [Google Scholar]
- 15. Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 2014; 20:1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pradas-Velasco R, Antoñanzas-Villar F, Martínez-Zárate MP. Dynamic modelling of infectious diseases: an application to the economic evaluation of influenza vaccination. Pharmacoeconomics 2008; 26:45–56. [DOI] [PubMed] [Google Scholar]
- 17. Lin MY, Lyles-Banks RD, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenters Program . The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2013; 57:1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.