Abstract
The T1R and T2R families of G protein-coupled receptors (GPCRs) initiate tastant perception by signaling via guanine nucleotide exchange and hydrolysis performed by associated heterotrimeric G proteins (Gαβγ). Heterotrimeric G protein signal termination is sped up by Gα-directed GTPase-accelerating proteins (GAPs) known as the Regulators of G protein Signaling (RGS proteins). Of this family, RGS21 is highly expressed in lingual epithelial cells and we have shown it acting in vitro to decrease the potency of bitterants on cultured cells. However, constitutive RGS21 loss in mice reduces organismal response to GPCR-mediated tastants—opposite to expectations arising from observed in vitro activity of RGS21 as a GAP and inhibitor of T2R signaling. Here, we show reduced quinine aversion and reduced sucrose preference by mice lacking RGS21 does not result from post-ingestive effects, as taste-salient brief-access tests confirm the reduced bitterant aversion and reduced sweetener preference seen using two-bottle choice testing. Eliminating Rgs21 expression after chemosensory system development, via tamoxifen-induced Cre recombination in eight week-old mice, led to a reduction in quinine aversive behavior that advanced over time, suggesting that RGS21 functions as a negative regulator to sustain stable bitter tastant reception. Consistent with this notion, we observed downregulation of multiple T2R proteins in the lingual tissue of Rgs21-deficient mice. Reduced tastant-mediated responses exhibited by mice lacking Rgs21 expression either since birth or in adulthood has highlighted the potential requirement for a GPCR GAP to maintain the full character of tastant signaling, likely at the level of mitigating receptor downregulation.
Keywords: bitter gustation, mouse, regulator of G protein signaling-21 (RGS21), salt gustation, sweet gustation, taste perception
Introduction
Taste perception is initiated by specialized lingual tissue cell clusters (“taste buds”) within which particular taste receptor cells express chemosensory receptors and intracellular signal transduction proteins that are collectively responsive to chemical stimuli (“tastants”). Three of the five mammalian tastes—namely, bitter, sweet, and umami—are mediated by tastant specific G protein-coupled receptors (GPCRs; reviewed in (Chandrashekar et al. 2006; Roper and Chaudhari 2017)). Sweet and umami stimuli are sensed by the T1R family of GPCR heterodimers (T1R2/T1R3 and T1R1/T1R3, respectively) (Li et al. 2002; Zhao et al. 2003); independently, bitter stimuli are sensed by the T2Rs, a much larger GPCR family (reviewed in (Bachmanov et al. 2014; Ahmad and Dalziel 2020)). Downstream signal transduction by both T1R and T2R receptors is thought to be mediated by activation of a heterotrimeric G protein complex (reviewed in (McCudden et al. 2005)), typically composed of a member of the Gα i/o subfamily (e.g. Gα gust or “gustducin-α”), Gβ 3, and Gγ 13 (reviewed in (Margolskee 2002)). This tastant-initiated signaling is transduced intracellularly by Gα nucleotide exchange, catalyzed by the activated receptor, and subsequent release of the Gβγ dimer which in turn activates phospholipase C beta2 (PLCβ2) and then TRPM5 channels (Huang et al. 1999; Zhang et al. 2003). TRPM5 channel opening leads to membrane depolarization and extracellular ATP release via CALHM1 channels (Finger et al. 2005; Taruno et al. 2013), acting in concert with CALMH3 (Ma et al. 2018), which in turn activates ionotropic purinergic receptors (P2X2, P2X3) on gustatory afferent nerve fibers (Bo et al. 1999; Vandenbeuch et al. 2015).
As in other conventional GPCR signaling cascades, tastant receptor signaling is terminated when the Gα subunit hydrolyzes its bound GTP, causing Gβγ re-association and a return to the heterotrimeric state (reviewed in (Siderovski and Willard 2005; Palmer 2007)). Therefore, downstream tastant signal transduction is critically controlled via the intrinsic rate of GTP hydrolysis by the Gα subunit, as well as any external influence on this GTPase rate. Gustducin-α is closely related to the two transducin-α subunits found within rod and cone photoreceptors (McLaughlin et al. 1992). All three of these Gα subunits have slow intrinsic GTPase activity; after discovery of the two transducin-α subunits, a large discrepancy was observed between their in vitro GTPase activity and the known rapid inactivation of phototransduction in vivo (Angleson and Wensel 1993). This timing paradox was resolved upon discovery that Regulator of G protein Signaling (RGS) proteins (reviewed in (Kimple et al. 2011)) bind Gα subunits to accelerate dramatically their rate of GTP hydrolysis (e.g. as RGS9-1 does for transducin-α subunits in vitro (He et al. 1998) and in vivo (Chen et al. 2000) in the phototransduction cascade).
Tastant signal transduction involving gustducin-α, and other Gα subunits of the Gi/o subfamily, is thought to be similarly regulated by RGS protein-mediated “GTPase-accelerating protein” (GAP) activity. For example, the gene transcript for RGS protein family member Rgs21 was originally isolated from rat foliate and fungiform papillae (von Buchholtz et al. 2004); subsequent in situ hybridization showed that virtually all cells expressing Rgs21 in rat lingual tissue also coexpress Plcβ2, suggesting that Rgs21 is expressed exclusively in Type II taste receptor cells (von Buchholtz et al. 2004). Biochemical analyses confirmed that purified RGS21 protein binds multiple different Gα subunits of the Gi/o subfamily and acts in vitro as a potent GAP for Gα i1 (Kimple et al. 2014). However, it remained unclear what role(s), if any, RGS21 plays in integrated tastant responses emanating from lingual tissue upon exposure to tastants. More recently, we described the phenotype of a mouse strain constitutively deficient in Rgs21 expression (Schroer et al. 2018), including its profound loss of avoidance of bitter compounds in two-bottle choice tests; these behavioral findings were opposite to our expectations of heightened tastant sensitivity based on the in vitro GAP activity of RGS21 being lost upon genetic ablation of Rgs21. In light of these seemingly paradoxical findings, we sought to establish whether RGS21 acts directly on gustducin-α and directly on bitterant-stimulated second messenger production. Moreover, we used conditional ablation of Rgs21 in postnatal mice to help clarify whether prior observations of blunted bitterant avoidance upon RGS21 loss resulted from either pre/perinatal developmental changes to chemosensation or, instead, from an acute loss of negative regulation of T2R signal transduction.
Materials and methods
Protein expression and coprecipitation from cultured cell lysates
Recombinant, hexahistidine (His6)-tagged RGS21 protein was purified from E. coli culture as previously described (Soundararajan et al. 2008). Monolayer cultures of the COS-7 cell line were transiently transfected with plasmid DNA encoding influenza hemagglutinin (HA)-tagged gustducin-α open-reading frame (Blake et al. 2001), and resultant whole-cell protein lysates tested for guanine nucleotide-dependent binding to immobilized His6-RGS21 protein using previously described methods of cellular lysis, nickel nitrilotriacetic acid (NTA)-agarose coprecipitation, and subsequent protein detection (Kimple et al. 2014).
cAMP accumulation assay
Denatonium benzoate-induced inhibition of forskolin-driven adenylyl cyclase activation (and resultant cAMP production) was measured in the 16HBE human bronchial airway epithelial cell line (Marano et al. 2002; Muthumalage et al. 2019). Briefly, monolayer cultures of the 16HBE cell line were transiently cotransfected using FuGENE6 (Promega) with the GloSensor cAMP-biosensor cDNA (Promega) and pcDNA3.1-based expression plasmids encoding either wild-type RGS21 open-reading frame, or a GAP-dead, loss-of-function point-mutant version (R126E a.k.a. RGS21[R>E]). Twenty-four hours post-transfection, cells were re-plated on poly-D-lysine-treated, clear-bottom, white 384-well plates at a density of 15,000 cells/well. Forty-eight hours post-transfection, culture medium was aspirated and cells were washed once before being incubated for 2 hours with 20 µl/well of equilibration medium (DMEM-based assay medium with 4% GloSensor substrate (Promega)). After 2 hours, 10 µl of 3× final concentration of denatonium benzoate (diluted in assay medium also containing 3 µM forskolin) was added to each well and allowed to incubate for 10 min before GloSensor emission was read on a MicroBeta Plate Counter (PerkinElmer). Luminescence counts were normalized to 100% maximal response in the absence of denatonium benzoate to account for any variability in GloSensor expression, transfection efficiency, and the exact number of cells per well.
Animal subjects and maintenance
Mice bearing a 232-kb bacterial artificial chromosome (BAC) from mouse chromosome 1 that encodes TagRFP driven by the mouse Rgs21 promoter was previously described by us (Cohen et al. 2012) and obtained from the UNC node of the Mutant Mouse Research & Research Centers (RRID: MMRRC_036805-UNC). The conditional “floxed” Rgs21 knockout mouse strain in the C57BL/6J background (containing an insertion of loxP sites on either side of Rgs21 exon 5) was maintained at West Virginia University, and its origins, maintenance, and usage in creating constitutive Rgs21-null (i.e., Rgs21Δ5/Δ5) mice have previously been described (Schroer et al. 2018). To obtain conditional Rgs21-null mice for this study, “floxed” Rgs21 mice were crossed with a ubiquitously expressed, tamoxifen (TM)-dependent Cre recombinase driver strain [CAGGCre-ER™; JAX 004682]. The CAGGCre-ER™ transgenic mouse expresses a tamoxifen-inducible Cre recombinase driven by a hybrid chicken β-actin/CMV promoter/enhancer element (Hayashi and McMahon 2002). The CAGGCre-ER™ transgene was kept in the heterozygous condition, as transgene-homozygous mice are not viable; in contrast, heterozygous CAGGCre-ER™ mice are viable, fertile, normal in size, and have no gross physical nor behavioral abnormalities (Hayashi and McMahon 2002). All procedures involving wild-type, conventional Rgs21-null, and conditional Rgs21-null mice were approved by the Institutional Animal Care and Use Committee of the West Virginia University Health Sciences Center (IACUC protocol 1608003737).
Taste-salient brief-access choice tests
Brief-access choice tests were conducted in a Davis Rig Brief Access Lickometer (16-bottle capacity legacy version; Med Associates). Mice first underwent 3 days of spout and shutter acclimation to become accustomed to drinking from the Davis rig. During spout acclimation, individual mice were placed in the Davis rig for a session lasting 15 minutes once the mouse took its first lick. On the following 2 days of training (shutter acclimation), the mouse was given access to the drinking tube with the shutter door open until 5 s after it took its first lick. The door remained closed for 7.5 s before re-opening with a different drinking tube revealed. The mouse was allowed to initiate as many trials as possible during the 15-min training session (and all subsequent test sessions). Prior to test sessions with an aversive stimuli (e.g. quinine sulfate), mice were water-deprived for 20 h to enhance drinking behavior. Following each 15-min testing session, mice were then allowed to rehydrate with ad libitum access to water for 3.75 h. Taste solutions were presented in multiple randomized blocks for the duration of each 15-min session. During each test session, the tastant/water lick ratio was calculated and averaged across the three test sessions. For sweetener testing, the ratio of concentration-specific-licks-to-total-licks was calculated to account for motivational differences between mice tested. Two-way repeated-measures ANOVA (Prism 9) was used to test for significant effects of genotype (or pre-/post-tamoxifen treatment status) on the lick ratio.
Two-bottle choice tests
Two-bottle choice tests were conducted in large Thoren mouse cages (30.80 cm × 40.60 cm × 15.88 cm). Individually housed mice were given access to two bottles containing autoclaved distilled water for 48 h prior to beginning all choice testing. Fluid was available through sipper spouts attached to 50-ml Corning conical-bottom centrifuge tubes, placed in separate bottle access slots on opposite sides of the food bin. Following the initial 48-h presentation of two bottles of water, mice were assessed over 48 h in tests with a choice between distilled water and ascending concentrations of a taste compound. The positions of the bottles were switched daily, and the fluid intakes were measured to the nearest 0.1 g by weighing the drinking bottles on an electronic balance. Preference ratio was calculated as volume of tastant solution consumed divided by volume of total solution consumed. The mice were socially housed for 7 days between each test series. At least 6 mice per genotype were assessed at each concentration of taste solution. Results were analyzed using a two-way ANOVA (genotype × concentration) with a Sidak multiple comparisons test (GraphPad Prism 9).
RNA extraction
Tongues, circumvallate (CV) papillae, and other organs and tissues were excised from mice euthanized with Fatal-Plus in accordance with guidelines from the National Institute of Health and with approval from the West Virginia University IACUC. For tongue tissue, after treatment with an enzyme cocktail consisting of Dispase (3 mg/mL; Gibco) and Elastase (2.5 mg/mL; Worthington) in Tyrode’s solution for 17 min, the epithelium was peeled from the underlying tissue. Gustatory tissue was isolated from the CV and nongustatory tissue was isolated from equivalent-sized, nontaste epithelial tissue surrounding the CV prior to being flash-frozen for subsequent RNA extraction. Tissue was homogenized in TRIzol reagent (Invitrogen) using a benchtop rotor-stator. RNA was extracted according to manufacturer’s instructions using the Direct-zol RNA MiniPrep kit from Zymo Research. Reverse transcription and genomic DNA elimination was performed using the QuantiTect Reverse Transcription Kit from Qiagen.
Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
qRT-PCR was performed to compare the expression levels of 18S rRNA and Rgs21 mRNA in conventional and conditional Rgs21-null mice and their littermate controls. Two microliters of cDNA were used in each PCR reaction using the QuantiTect SYBR Green PCR Master Mix (Qiagen). RT2 qPCR Primer Assay for Mouse 18S rRNA was purchased from Qiagen. Primers (10 µM) were designed for Rgs21 mRNA (spanning exons 4–5) sequence elements (fwd primer: 5′-TCGTAGCTGATGCACCAAAA-3′; rev primer 5′-TACAGGAAAGGCAGCCATCT-3′) and purchased from ThermoFisher Scientific. PCR was performed (initial 15 min denaturation at 95°C followed by 40 cycles of 15 s denaturation at 95°C, 30 s annealing at 60°C, and 30 s extension at 72°C) in a Qiagen Rotor Gene-Q system. We utilized the SYBR green dye qPCR technique to detect double-stranded PCR amplicons as they accumulated during PCR cycling. Melting curves were obtained after each qRT-PCR experiment to assure specificity of resultant amplicons.
Immunohistochemistry and immunoblot analyses
Tissue sections from Rgs21::TagRFP transgenic mice were obtained posteuthanasia, fixed/treated, and stained with a primary antibody against TagRFP (anti-tRFP, cat. no. FP AB233; Evrogen) using previously described methods (Cohen et al. 2012). CV papillae were isolated using published methods (Schroer et al. 2018) and then processed for immunoblot analyses using published methods (Ugawa et al. 2003; Martin et al. 2011). Commercially available antibodies against mouse Tas2r130 (C-12; Santa Cruz #sc-377364), Tas2r120 (Thermo-Fisher; #PA5-75373), Tas2r121 (D-8; #sc-514219), Tas2r104 (H-5; #sc-515693), and Tas2r137 (E-12; #sc-398489) were used at the recommended dilutions to perform immunoblot analyses, as detected with IgG isoform-appropriate, horseradish peroxidase-labeled secondary antibody (#sc-516102 or #sc-516132) and visualized using Super Signal West Pico PLUS chemiluminescent substrate (Fisher). Blots were visualized on a GE ImageQuant Blot Imager and band densitometry performed using Image J (Fiji) software. Parallel immunoblots were used to detect the housekeeping protein GAPDH for normalizing densitometric measurements to a loading control.
Results
RGS21 is highly expressed in mouse lingual epithelial cells
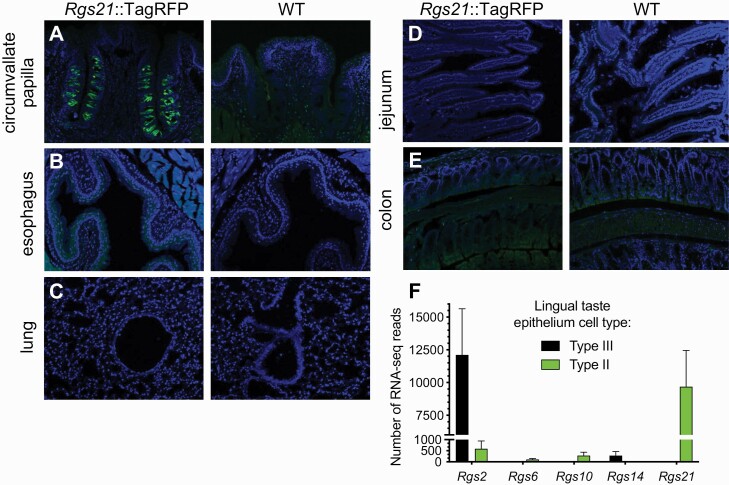
To overcome the lack of an effective anti-RGS21 antibody, we previously created a BAC transgenic mouse strain that expresses TagRFP (a monomeric red fluorescent protein variant) as driven by the wild-type Rgs21 promoter (Cohen et al. 2012). We used this transgenic strain to demonstrate coincident detection of TagRFP (as a proxy for RGS21) and gustducin-α in taste bud cells (Cohen et al. 2012). Figure 1 highlights an example of our observations of high-level expression of TagRFP from the Rgs21 promoter in taste bud cells of the circumvallate papillae of transgene-positive mice, but not wild-type mice (Fig. 1A); other types of epithelia processed and imaged under identical conditions were not seen to have such robust expression as that seen in lingual tissue (e.g. airway tissues, Fig. 1B,C; intestinal tissues, Fig. 1D,E). In a whole transcriptome profiling of distinct subpopulations of mouse taste bud cells, Sukumaran and colleagues (Sukumaran et al. 2017) reported observing Rgs21 uniquely expressed in Tas1r3+ Type II taste receptor cells; in contrast, Type III sour/high salt-receiving cells were seen to uniquely express Rgs2, a separate Rgs gene on mouse chromosome 1 which encodes a Gα q/11 attuned GAP (Kimple et al. 2009).
Fig. 1.
The Rgs21 promoter drives gene expression levels in lingual epithelium that are not equivalently observed in airway or intestinal epithelia. (A–E) Indicated tissue sections from Rgs21::TagRFP transgenic mice or wild-type (WT) mice were stained with DAPI (blue, for nuclear DNA), and anti-tRFP rabbit polyclonal primary antibody plus an Alexa Fluor-488 goat anti-rabbit secondary antibody, prior to epifluorescence imaging. (F) Rgs genes differentially expressed in Tas1r3+ Type II taste receptor cells (responsible for bitter, sweet, and umami taste modalities) versus Type III “presynaptic” taste bud cells (thought responsible for sour and high-salt taste modalities), as identified by RNA-seq transcriptomics (NCBI id SRP094673; bar-graph derived from RNA-seq count data previously published by (Sukumaran et al. 2017)).
RGS21 binds gustducin-α in vitro and reduces T2R-mediated bitterant signaling in cells
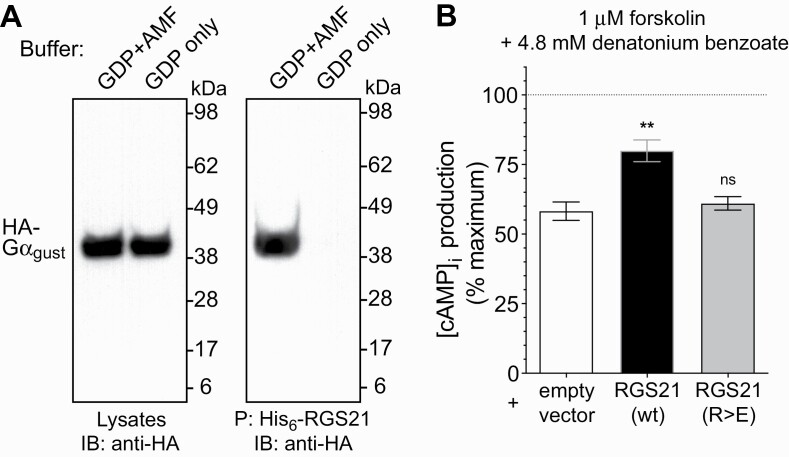
RGS21 has previously been shown (Kimple et al. 2014) to interact in vitro with Gα i2 and other Gα i/o subunits not considered to be primarily coupled to bitterant-responsive GPCRs in vivo. We therefore directly tested whether RGS21 was capable of binding to Gα gust (otherwise known as gustducin-α), which is considered the primary Gα subunit associated with T2R activation-mediated signal transduction (McLaughlin et al. 1992; Ruiz-Avila et al. 2001). We performed protein pull-down assays with recombinant His6-RGS21 protein and cellular lysates containing overexpressed, epitope-tagged Gα gust. RGS21 was observed to bind Gα gust only in its transition state mimetic form (i.e., bound with GDP and the planar anion aluminum tetrafluoride [AMF] that mimics the leaving group upon GTP hydrolysis) (Fig. 2A), consistent with the known binding affinity of RGS domains and their Gα substrates (Popov et al. 1997; Soundararajan et al. 2008). RGS21 overexpression, by transient transfection of the bitterant-responsive 16HBE cell line, was also found to reduce the inhibition of cAMP second messenger production (Fig. 2B) as caused by application of the EC50 of denatonium benzoate for this cell line (Supplementary Fig. S1). Analogous overexpression of RGS21[R>E], a GAP-dead, loss-of-function point mutant of RGS21 (Kimple et al. 2014), failed to reduce the response to denatonium benzoate (Fig. 2B).
Fig. 2.
RGS21 binds gustducin-α and reduces bitterant signaling in a bitterant-responsive cultured cell line. (A) COS7 cell monolayers were transiently transfected with plasmid DNA encoding gustducin-α tagged with an HA epitope (“HA-Gα gust”). Forty-eight hours post transfection, cell monolayers were lysed in buffer containing GDP, Mg2+ and AlF4– (“AMF”) or GDP alone, as indicated. Clarified cell lysates were incubated with 10 μg of purified His6-RGS21 protein at 4°C overnight with NTA-agarose. Samples were centrifuged, agarose beads washed four times in lysis buffer, and precipitated proteins (“P”:) eluted by boiling for 5 minutes in loading buffer prior to being resolved by SDS–PAGE electrophoresis on the basis of molecular weight (“kDa” = kiloDalton), transferred to a nitrocellulose membrane, and detected using anti-HA antibody immunoblotting (“IB”:) and chemiluminescence. (B) Overexpression of wild-type RGS21, but not a loss-of-function, point mutant RGS21, leads to inhibition of bitterant signaling-induced reduction of cAMP levels. Cultures of the 16HBE cell line were transiently transfected with GloSensor cAMP biosensor cDNA and expression plasmids containing open reading frames for wild-type (“wt”) RGS21, or Arg-126-to-Glu point mutated (“R>E”) RGS21, or empty pcDNA3.1 vector, as indicated. Inhibition of forskolin-stimulated cAMP production (“100% maximum”; dotted line) by treatment with indicated concentration of the bitterant denatonium benzoate (i.e. EC50 previously established as per Supplementary Fig. S1) was determined 24 hr post-transfection by detection of GloSensor-dependent luminescence. Asterisks denote statistically significant differences as determined by one-way ANOVA with Bonferroni’s post-test: **, P < 0.01; ns, not significant (P >> 0.05).
Rgs21 allele ablation requires homozygosity to engender blunted aversion to bitterant and blunted appetitive response to low-dose NaCl
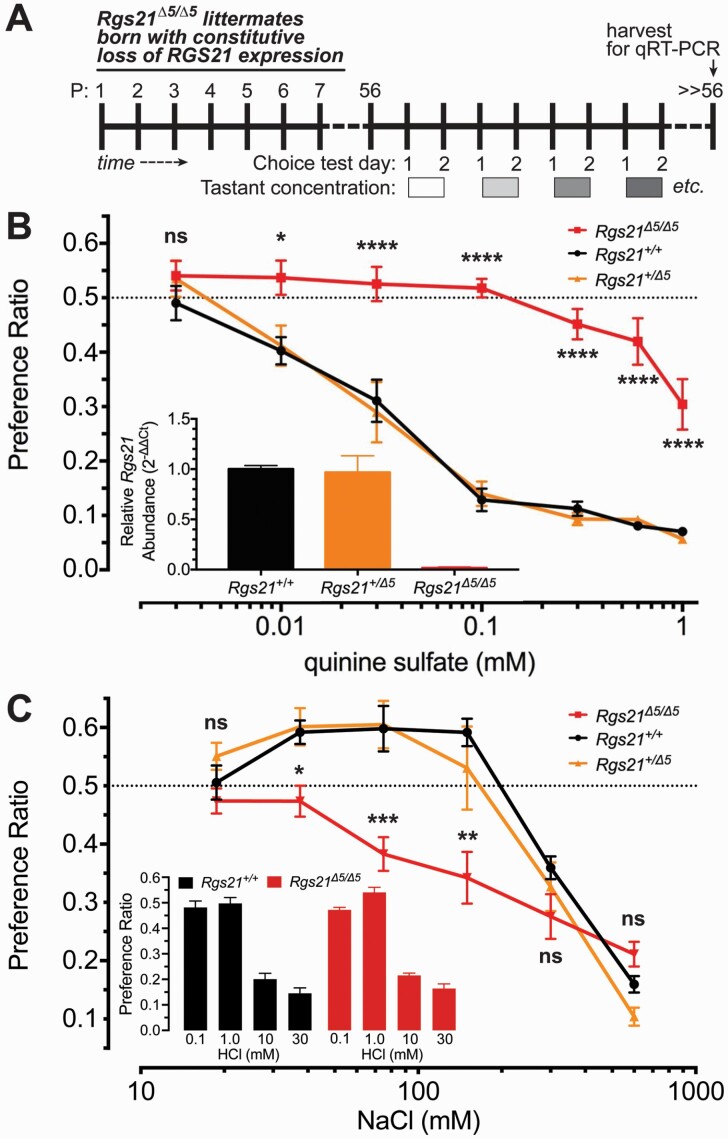
We previously demonstrated that complete Rgs21 ablation and resultant RGS21 expression loss in mice leads to a dramatic blunting of their bitterant sensitivity, as assessed by two-bottle choice testing with both quinine- and denatonium-containing drinking water (Schroer et al. 2018); this effect of complete RGS21 loss is not shared with all bitterant-responsive tissues (e.g. mouse airway smooth muscle, as evaluated using methods described in Devallance et al. 2018; see Supplementary Fig. S2). In an initial attempt to establish whether reducing (but not eliminating) Rgs21 gene expression would also engender demonstrable changes to mouse bitterant responsiveness at the level of peripheral lingual chemosensation, we bred the conventional Rgs21 knockout allele (i.e., ubiquitous, CMV-driven, Cre-mediated excision of Rgs21 exon 5; (Schroer et al. 2018)) using heterozygous crosses (Rgs21+/Δ5 females x Rgs21+/Δ5 males). In challenging resultant, 8-week-old heterozygous Rgs21+/Δ5 F1 progeny alongside Rgs21+/+ and Rgs21Δ5/Δ5 littermates (Fig. 3A), the heterozygous Rgs21+/Δ5 mice were found to have normal aversion to quinine in two-bottle choice tests (Fig. 3B), equivalent to that previously shown by Rgs21+/+ mice (Schroer et al. 2018). Heterozygous Rgs21+/Δ5 mice and wild-type Rgs21+/+ mice also shared a biphasic, concentration-dependent preference/aversion response to the salt NaCl in two-bottle choice tests, whereas Rgs21Δ5/Δ5 littermates were found to lack the appetitive behavioral response to lower NaCl concentrations (Fig. 3C), as previously reported (Schroer et al. 2018). The latter blunting of appetitive behavior towards low-dose NaCl upon RGS21 loss does not appear to reflect any overall organismal pathology in salt handling (e.g. renal salt clearance as measured by urine specific gravity; Supplementary Fig. S3) or any change in salt detection threshold (e.g. use of prior LiCl exposure to sensitize avoidance of low-dose NaCl in two-bottle choice testing; Supplementary Fig. S4). Blunted appetitive behavior towards low NaCl also does not appear to reflect a global effect of RGS21 loss on all tastant-driven chemosensation, as aversion to the sour tastant hydrochloric acid (specifically, [H+]) was seen to be unchanged upon RGS21 loss (Fig. 3C inset).
Fig. 3.
Only bi-allelic ablation of Rgs21 expression blunts aversion to the bitterant quinine and blunts preference for low concentrations of NaCl salt. (A) Schematic representation of timing of two-bottle choice tests performed with 8-week-old (postnatal [P] day 56) constitutive Rgs21 knockout mice (Rgs21Δ5/Δ5) and littermate controls (wild-type Rgs21+/+ and heterozygous Rgs21+/Δ5 F1 progeny). (B, C) Tests of two-bottle choice for an ascending concentration series of the bitterant quinine sulfate (B) or the salt NaCl (C) versus unadulterated drinking water were performed with heterozygous Rgs21+/Δ5 F1 progeny (n = 6) alongside Rgs21+/+ (n = 11) and Rgs21Δ5/Δ5 (n = 10) littermates. A preference ratio of 0.5 (dashed line) indicates indifference towards the tastant solution relative to water. Each test was assessed with a two-way ANOVA and differences between the groups in preference or aversion to specific concentrations of tastant solution were determined using Sidak posthoc test to correct for multiple comparisons (*, P < 0.05; **, P < 0.01, ***, P < 0.001, ****, P < 0.0001; ns, not significant [P >> 0.05]). Wild-type and Rgs21+/Δ5 mice appeared to have no difference in preference at any concentration tested. Inset bar graph of panel B, Data from qRT-PCR (SYBR Green detection) of the Rgs21 mRNA transcript, which is seen to be completely absent in Rgs21Δ5/Δ5 mice, but equally detected in tongue tissue from both Rgs21+/+ and Rgs21+/Δ5 mice. Inset bar graph of panel C, Data from two-bottle choice testing for an ascending concentration series of the sour tastant hydrochloric acid (HCl), indicating no significant difference in aversion at the 10 mM or 30 mM concentrations between the two indicated genotypes (n = 7 for both groups).
Blunted tastant sensitivity upon Rgs21 ablation is proximal to post-ingestive chemosensation
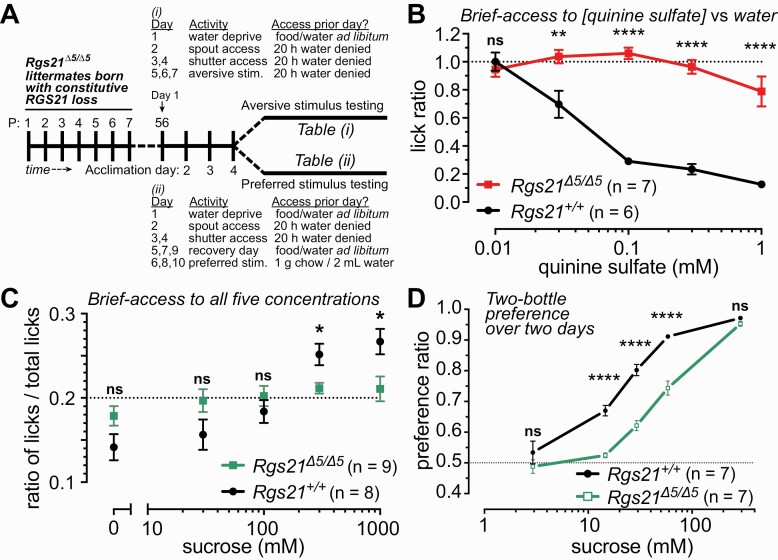
As choices for or against particular tastants can be heavily influenced by stimulatory or aversive post-oral signals and effects (e.g. (Zukerman et al. 2011)), we also performed brief-access taste tests (schematized in Fig. 4A) to minimize the influence of post-oral factors in assessing behavioral responses of the individual Rgs21-deficient mouse strains. Consistent with the results from two-bottle choice testing (Fig. 3), bi-allelic Rgs21 deficiency led to a nearly complete loss of aversion to quinine in 30-minute exposures to quinine-containing water versus unadulterated water (Fig. 4B). Bi-allelic Rgs21 deficiency was also seen in brief-access testing to eliminate preference for higher concentrations of the caloric/carbohydrate sweetener sucrose (Fig. 4C), consistent with blunted preference to sucrose and to the noncaloric sweetener SC45647 observed in two-bottle choice testing (Fig. 4D and (Schroer et al. 2018)).
Fig. 4.
Taste-salient brief-access tests of quinine and sucrose responsiveness of mice bearing long-term constitutive loss of RGS21 expression. (A) Schematic representation of timing of brief-access tests for quinine (“aversive stimulus”; Table (i)) and for sucrose (“preferred stimulus”; Table (ii)) performed with 8-week-old (postnatal [P] day 56) constitutive Rgs21 knockout mice (Rgs21Δ5/Δ5) and wild-type Rgs21+/+ littermate controls. (B) Lick ratios (between indicated quinine sulfate concentration vs unadulterated water) were analyzed by two-way ANOVA with Sidak posthoc testing for differences by genotype (n = 6 for Rgs21+/+ and n = 7 for Rgs21Δ5/Δ5; ns, not significant [P >> 0.05]; **, P < 0.01; ****, P < 0.0001). (C) Data from brief-access to sucrose are reported as the ratio of concentration-specific-licks-to-total-licks to account for motivational differences between mice tested (i.e., the horizontal dashed line represents the expected value of the ratio [0.2] from completely random licking of all 5 concentrations). (D) Two-bottle choice preferences of Rgs21Δ5/Δ5 (n = 7) and wild-type mice (n = 7) for ascending concentration series of sucrose, as previously conducted for quinine sulfate and NaCl (Fig. 3; (Schroer et al. 2018)). Each test was assessed with a two-way ANOVA. Differences between the groups at specific concentrations of taste solution were determined using Sidak posthoc test to correct for multiple comparisons (* P < 0.05; ** P < 0.01; **** P < 0.0001).
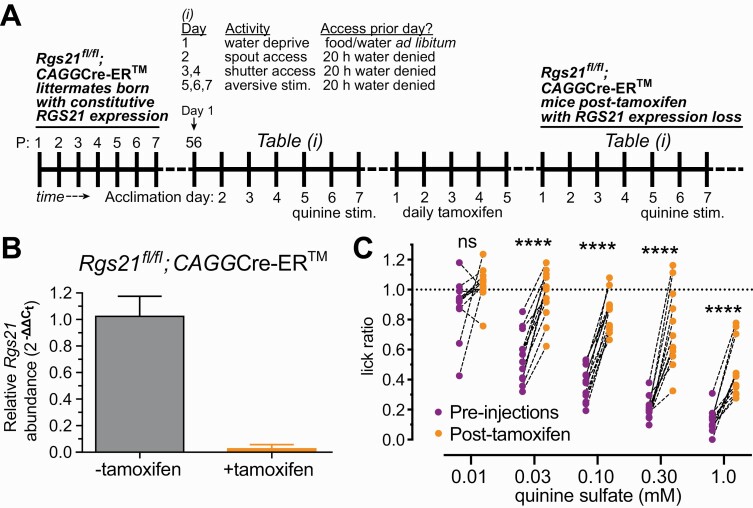
In an independent means of eliminating RGS21 expression, the original “floxed” Rgs21 knockout mouse strain was bred to an ubiquitously expressed, but tamoxifen (TM)-dependent Cre recombinase driver strain (CAGGCre-ERTM; (Hayashi and McMahon 2002)) (Fig. 5A). Treatment of 8-week old Rgs21fl/fl; CAGGCre-ER™ mice with daily intraperitoneal injections of tamoxifen over five days led to complete loss of Rgs21 expression relative to sham-treated control mice (Fig. 5B). Similar to the results using mice with constitutive Rgs21 deficiency (Rgs21Δ5/Δ5 mice, Fig. 4B), conditional loss of Rgs21 led to significant intra-animal reductions in quinine avoidance at all but the lowest quinine sulfate concentration examined in brief-access testing (0.03 to 1.0 mM quinine sulfate; Fig. 5C).
Fig. 5.
Taste-salient brief-access tests of quinine responsiveness of mice bearing short-term conditional loss of RGS21 expression. (A) Schematic representation of timing of pre- and post-tamoxifen brief-access tests for quinine (“aversive stimulus”; Table (i)) performed with 8-week-old (postnatal [P] day 56) conditional Rgs21 knockout mice (Rgs21fl/fl;CAGGCre-ER). (B) To demonstrate the degree of Rgs21 expression loss achieved by tamoxifen treatment, Rgs21fl/fl;CAGGCre-ER mice were IP injected once a day for five consecutive days, either with 75 mg/kg of tamoxifen or corn oil (vehicle only). Seven days after the final injection, tongues were excised and RNA was isolated for qRT-PCR analysis of Rgs21 mRNA expression (normalized to 18S rRNA levels) in CV papillae (panel B; n = 4 per treatment group; unpaired Student t test, P = 0.007). (C) Twelve individual mice, homozygous for the loxP site-flanked exon 5 allele of Rgs21 (Rgs21fl/fl) and heterozygous for the ubiquitiously expressed, tamoxifen-inducible Cre recombinase transgene (CAGGCre-ER), were profiled for quinine responsiveness in brief-access tests performed before (“pre-injections”) and 1 week after five daily intraperitoneal injections of 75 mg/kg tamoxifen to induce Rgs21 exon 5 excision. Repeated-measures ANOVA with Sidak posthoc testing was performed to establish significance of intra-animal changes pre- vs post-tamoxifen in aversion to indicated quinine sulfate concentration (n = 12; ns, not significant [P >> 0.05]; ****, P < 0.0001).
Blunted sensitivity to bitterant upon Rgs21 ablation increases over time post-excision
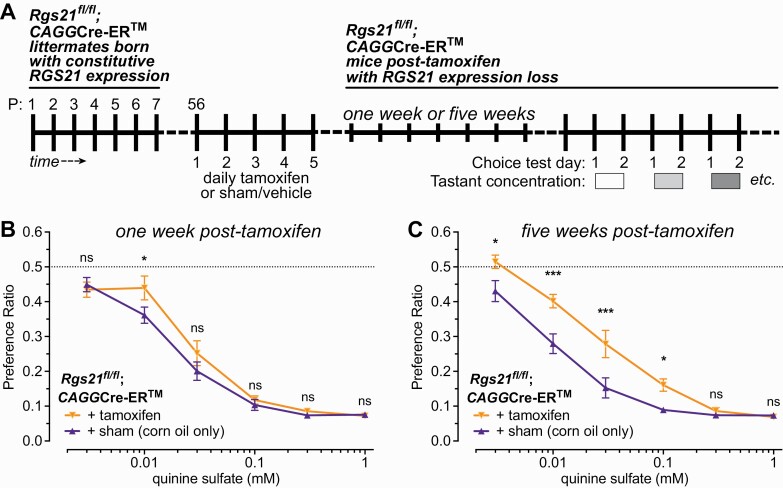
As a five-day tamoxifen regimen applied to Rgs21fl/fl;CAGGCre-ER mice led to demonstrable reductions in both CV papillae Rgs21 expression and brief-access quinine avoidance (Fig. 5), we then measured quinine aversion in two-bottle choice testing after one or five weeks post-tamoxifen treatment (Fig. 6A). A statistically significant reduction in avoidance of 0.01 mM quinine sulfate in drinking water was observed after one week of tamoxifen treatment (Fig. 6B), and this reduction was enhanced after five weeks: both at the 0.01 mM quinine sulfate concentration, and at lower (0.03 mM) and higher (0.03 mM, 0.1 mM) concentrations (Fig. 6C).
Fig. 6.
As measured in two-bottle choice testing (schematized in panel A), acute, tamoxifen-induced Rgs21 excision leads to a detectable deficit in quinine aversion after one week (B), and an increased deficit after five weeks (C). Eight-week old mice were either sham injected (corn oil only) (n = 10 mice) or injected (n = 11 mice) with 75 mg/kg of tamoxifen dissolved in corn oil once a day for 5 consecutive days (*, P < 0.05; ***, P < 0.001). A preference ratio of 0.5 (dashed line) indicates indifference towards the tastant solution relative to plain water.
Rgs21 loss leads to lower levels of T2R bitterant receptor expression
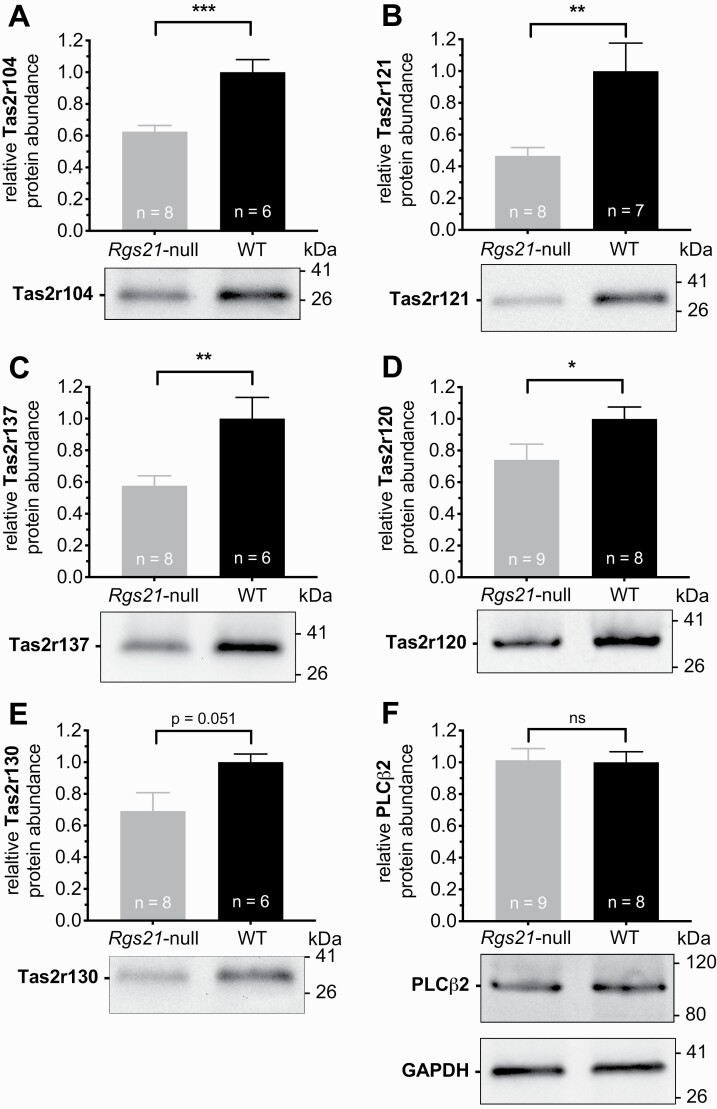
Prolonged GPCR activation normally leads to long-term downregulation that generally involves receptor internalization, destruction, and a resultant decrease in cellular steady-state receptor levels (Luttrell and Lefkowitz 2002; Rajagopal and Shenoy 2018). In this light, a potential cause of reduced bitterant responsiveness observed in mice upon loss of Rgs21 expression (e.g. Figs. 3–5; (Schroer et al. 2018)) is that an initial increase in T2R signaling (due to loss of negative regulation; e.g. Fig. 2B) leads over time to T2R downregulation and a resultant decrease in bitterant sensitivity. We, therefore, examined the levels of five different Tas2r proteins (using validated commercial antibodies; see Supplementary Fig. S5) within CV papillae isolates from wild-type and Rgs21-deficient mice. As normalized to GAPDH protein levels, we observed that four of the five (Tas2r120, Tas2r121, Tas2r137, Tas2r104) were significantly reduced in expression relative to their levels seen in wild-type littermate controls (Fig. 7A–D); the fifth tested T2R (Tas2r130) trended towards being lower as well in Rgs21-deficient mice, but this observation did not reach statistical significance (Fig. 7E; P = 0.0511 on Student t test). In contrast to the reduction in T2R levels observed, the levels of PLCβ2 were seen to be unaltered between wild-type and Rgs21-deficient mice (Fig. 7F).
Fig. 7.
Steady-state levels of multiple mouse Tas2r bitterant receptor family members are lower upon constitutive RGS21 loss relative to wild-type C57Bl/6J mice. Representative immunoblots of CV papillae tissue from multiple Rgs21-null mice and wild-type (WT) littermate controls are presented underneath bar-graphs summarizing accumulated quantitative densitometry data from multiple immunoblotting experiments, normalized to GAPDH level detection (numbers of animals sampled [“n”] indicated within each bar). Relative mobility (kDa values on right side of immunoblot examples) was identified by comigrating protein standards on SDS–PAGE. Data analyzed by unpaired Student t tests (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant [P >> 0.05]).
Discussion
Our initial report of the phenotype of Rgs21-deficient mice (Schroer et al. 2018) included evidence of normal lingual tissue and taste bud development, yet profound behavioral insensitivity to the bitter compounds quinine and denatonium. These altered behaviors were in direct opposition to expectations of observing heightened bitterant sensitivity based on earlier biochemical and in vitro evidence of RGS21 as a negative regulator of GPCR signal transduction. Here, we confirmed gustducin-α as a target of RGS21 action, as well as that action being able to reduce intracellular effector function downstream of bitterant-stimulated T2R activation (specifically, the inhibition of cAMP production in the bitterant-responsive cultured cell line 16HBE). We also confirmed the loss of bitterant avoidance and sweetener preference, first observed in multiple-day two-bottle choice testing (Schroer et al. 2018), is also apparent in taste-salient brief-access (30 min) testing of Rgs21-deficient mice, thereby eliminating a loss of long-term post-ingestive effects of tastant consumption as a possible explanation for the paradoxical behavior of bitterant insensitivity shown by Rgs21-deficient mice in light of RGS21 being an established negative regulator of T2R signaling.
When first faced with this paradox (Schroer et al. 2018), we speculated that normal exposure to tastants at the lingual epithelium in the absence of RGS21’s negative regulation may lead to compensatory desensitization and/or downregulation of element(s) of the tastant response machinery (e.g. the tastant receptor(s) themselves and/or the purinergic receptors responsive to heightened ATP release). Here we have demonstrated that several Tas2r proteins are expressed at lower levels in the lingual epithelium of Rgs21-deficient mice relative to wild-type mice. In parallel, acute reduction of RGS21 expression via tamoxifen-induced Cre recombination resulted in the same paradoxical behavioral phenotype of bitterant insensitivity, and this insensitivity advanced over time (i.e., seen more profoundly blunted at five weeks vs one week), suggestive of a time-dependent change to extant tastant response machinery upon RGS21 loss. These new observations strengthen the argument that loss of a negative regulator of tastant receptor signaling leads, over time, to a downregulation of those tastant receptors (and/or other downstream component(s); e.g. as modeled in Supplementary Fig. S6). The timing of this receptor downregulation would necessarily have to be rapid, owing to the rapid cycle time of replenishment of mature taste receptor cells by lingual tissue-resident stem cells (e.g. the life span of the average rat taste bud cell is reported to be ~10 days (Beidler and Smallman 1965), leading to the suggestion that roughly 10% of cells are new to each taste bud each day in adulthood (Miura and Barlow 2010; Barlow 2015)).
Our original speculation was advanced by observations within Rgs21-deficient mice of accelerated signal decay (emblematic of short-term receptor desensitization) across the first thirty seconds of tastant-elicited neuronal output (Schroer et al. 2018): Rgs21Δ5/Δ5 mice with constitutive RGS21 loss exhibited a reduced ratio of the last 10 s to the first 10 s of recorded chorda tympani nerve response amplitude upon exposure to bitter (and sweet and umami) taste stimuli, suggesting that these mice have a less-prolonged signal output after initial tastant/T2R (or tastant/T1R) engagement. Desensitization of activated GPCR signaling to G protein-mediated output commonly occurs via the sequential actions of G protein receptor kinases (i.e. activated receptor phosphorylation) and β-arrestin proteins (i.e. activated receptor binding) that preclude return of the G protein heterotrimer and ultimately lead to receptor internalization and degradation (Luttrell and Lefkowitz 2002; Rajagopal and Shenoy 2018). Recent evidence suggests that T2R activation by structurally distinct agonists can differentially elicit long-term downregulation via β-arrestin recruitment (e.g. (Woo et al. 2019) for TAS2R14 in human airway smooth muscle). Furthermore, both of these aforementioned “agents” of GPCR desensitization and downregulation (GRKs and β-arrestins) are expressed in mouse taste bud cells (Supplementary Fig. S7; Shandilya 2016), and so their actions could be enhanced in the absence of RGS21, similar to the shifted balance in Rgs12-deficient mice between G protein- vs GRK/β-arrestin-mediated signals emanating from the activated kappa opioid receptor (Gross et al. 2019; Kaski et al. 2021). RGS21 may, therefore, play a similar role in dampening tastant-initiated G protein signaling so that the mammalian gustatory system is not overwhelmed when tastants are too abundant on the lingual epithelium—a state of being overwhelmed that leads ultimately to chronic downregulation of tastant receptor and/or downstream components. In the absence of RGS21 protein GAP activity opposing G protein signaling downstream of activated tastant receptors, normal environmental exposure to receptor-activating tastants may lead to a heightened degree of receptor desensitization and downregulation.
Further work is clearly needed to establish the direct mechanisms causing the decreases in Tas2r protein levels we have reported here as being observed in the lingual tissue of RGS21-null mice, as well as to extend these findings to T1R signaling (given the blunting of sweetener preference) and to salt signaling via potentially shared, G protein-related signaling components (given observed loss of low-dose NaCl appetitive response, but no loss of sour aversion, upon Rgs21 ablation). The latter observation remains the most puzzling in the context of RGS21 being a “regulator of G protein signaling,” as low salt detection is considered to be performed in a G protein-independent fashion by the amiloride-sensitive epithelial sodium channel (ENaC) (Roper 2015; Shekdar et al. 2021), whereas it is only the aversive response to high salt concentration that is thought to evoke T2R-expressing Type II and/or amiloride-insensitive Type III taste cells (Chandrashekar et al. 2010; Lewandowski et al. 2016).
Supplementary Material
Acknowledgments
We thank Kim Wix (WVU) for provision of animal care assistance throughout the conduct of this investigation, Brian Buckley (UNC) for assistance with anti-TagRFP IHC studies, and Dr. Harish Radhakrishna (Georgia Tech; Chromocell) for continual enthusiasm and guidance provided throughout these on-going studies of the Rgs21-null mouse phenotype.
Contributor Information
Adam B Schroer, Department of Neuroscience, West Virginia University School of Medicine, 64 Medical Center Drive, Morgantown, WV 26506, USA.
Kayla W Branyan, Division of Exercise Physiology, West Virginia University School of Medicine, 64 Medical Center Drive, Morgantown, WV 26506, USA.
Joshua D Gross, Department of Cell Biology, Duke University Medical Center, 307 Research Drive, Durham, NC 27710, USA.
Paul D Chantler, Division of Exercise Physiology, West Virginia University School of Medicine, 64 Medical Center Drive, Morgantown, WV 26506, USA.
Adam J Kimple, Department of Otolaryngology and Marsico Lung Institute, UNC School of Medicine , 170 Manning Drive, Chapel Hill, NC 27599-7070, USA.
Aurelie Vandenbeuch, Department of Otolaryngology, University of Colorado–Denver, Anschutz Medical Campus, 12700 E. 19th Avenue, Aurora, CO 80045, USA.
David P Siderovski, Department of Pharmacology & Neuroscience, Graduate School of Biomedical Sciences, University of North Texas Health Science Center, 3500 Camp Bowie Blvd, Fort Worth, TX 76107, USA.
Conflict of interest
None declared.
Funding
This work was supported, in part, by National Institutes of Health (NIH) grant R01 DA048153 [to D.P.S.], and by E.J. Van Liere and William W. Fleming Pharmacology Trust endowments [to D.P.S. and A.B.S., respectively].
References
- Ahmad R, Dalziel JE. 2020. G protein-coupled receptors in taste physiology and pharmacology. Front Pharmacol. 11:587664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angleson JK, Wensel TG. 1993. A GTPase-accelerating factor for transducin, distinct from its effector cGMP phosphodiesterase, in rod outer segment membranes. Neuron. 11(5):939–949. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Bosak NP, Lin C, Matsumoto I, Ohmoto M, Reed DR, Nelson TM. 2014. Genetics of taste receptors. Curr Pharm Des. 20(16):2669–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow LA. 2015. Progress and renewal in gustation: new insights into taste bud development. Development. 142(21):3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler LM, Smallman RL. 1965. Renewal of cells within taste buds. J Cell Biol. 27(2):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake BL, Wing MR, Zhou JY, Lei Q, Hillmann JR, Behe CI, Morris RA, Harden TK, Bayliss DA, Miller RJ, et al. 2001. G beta association and effector interaction selectivities of the divergent G gamma subunit G gamma(13). J Biol Chem. 276(52):49267–49274. [DOI] [PubMed] [Google Scholar]
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G. 1999. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 10(5):1107–1111. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. 2006. The receptors and cells for mammalian taste. Nature. 444(7117):288–294. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. 2010. The cells and peripheral representation of sodium taste in mice. Nature. 464(7286):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Burns ME, He W, Wensel TG, Baylor DA, Simon MI. 2000. Slowed recovery of rod photoresponse in mice lacking the GTPase accelerating protein RGS9-1. Nature. 403(6769):557–560. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Buckley BK, Kosloff M, Garland AL, Bosch DE, Cheng G Jr, Radhakrishna H, Brown MD, Willard FS, Arshavsky VY, et al. 2012. Regulator of G-protein signaling-21 (RGS21) is an inhibitor of bitter gustatory signaling found in lingual and airway epithelia. J Biol Chem. 287(50):41706–41719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVallance E, Branyan KW, Lemaster K, Olfert IM, Smith DM, Pistilli EE, Frisbee JC, Chantler PD. 2018. Aortic dysfunction in metabolic syndrome mediated by perivascular adipose tissue TNFα- and NOX2-dependent pathway. Exp Physiol. 103(4):590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. 2005. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 310(5753):1495–1499. [DOI] [PubMed] [Google Scholar]
- Gross JD, Kaski SW, Schmidt KT, Cogan ES, Boyt KM, Wix K, Schroer AB, McElligott ZA, Siderovski DP, Setola V. 2019. Role of RGS12 in the differential regulation of kappa opioid receptor-dependent signaling and behavior. Neuropsychopharmacology. 44(10):1728–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. 2002. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 244(2):305–318. [DOI] [PubMed] [Google Scholar]
- He W, Cowan CW, Wensel TG. 1998. RGS9, a GTPase accelerator for phototransduction. Neuron. 20(1):95–102. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. 1999. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 2(12):1055–1062. [DOI] [PubMed] [Google Scholar]
- Kaski SW, White AN, Gross JD, Siderovski DP. 2021. Potential for kappa-opioid receptor agonists to engineer nonaddictive analgesics: a narrative review. Anesth Analg. 132(2):406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimple AJ, Bosch DE, Giguère PM, Siderovski DP. 2011. Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets. Pharmacol Rev. 63(3):728–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimple AJ, Garland AL, Cohen SP, Setola V, Willard FS, Zielinski T, Lowery RG, Tarran R, Siderovski DP. 2014. RGS21, a regulator of taste and mucociliary clearance? Laryngoscope. 124(3):E56–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimple AJ, Soundararajan M, Hutsell SQ, Roos AK, Urban DJ, Setola V, Temple BR, Roth BL, Knapp S, Willard FS, et al. 2009. Structural determinants of G-protein alpha subunit selectivity by regulator of G-protein signaling 2 (RGS2). J Biol Chem. 284(29):19402–19411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski BC, Sukumaran SK, Margolskee RF, Bachmanov AA. 2016. Amiloride-insensitive salt taste is mediated by two populations of type III taste cells with distinct transduction mechanisms. J Neurosci. 36(6):1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. 2002. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 99(7): 4692–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. 2002. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 115(Pt 3):455–465. [DOI] [PubMed] [Google Scholar]
- Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, Niisato N, Marunaka Y, Lee RJ, Hoff H, et al. 2018. CALHM3 Is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron. 98(3):547–561.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano F, Boland S, Bonvallot V, Baulig A, Baeza-Squiban A. 2002. Human airway epithelial cells in culture for studying the molecular mechanisms of the inflammatory response triggered by diesel exhaust particles. Cell Biol Toxicol. 18(5):315–320. [DOI] [PubMed] [Google Scholar]
- Margolskee RF. 2002. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 277(1):1–4. [DOI] [PubMed] [Google Scholar]
- Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. 2011. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One. 6(8):e24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. 2005. G-protein signaling: back to the future. Cell Mol Life Sci. 62(5):551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SK, McKinnon PJ, Margolskee RF. 1992. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 357(6379):563–569. [DOI] [PubMed] [Google Scholar]
- Miura H, Barlow LA. 2010. Taste bud regeneration and the search for taste progenitor cells. Arch Ital Biol. 148(2):107–118. [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T, Lamb T, Friedman MR, Rahman I. 2019. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci Rep. 9(1):19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RK. 2007. The pharmacology and signaling of bitter, sweet, and umami taste sensing. Mol Interv. 7(2):87–98. [DOI] [PubMed] [Google Scholar]
- Popov S, Yu K, Kozasa T, Wilkie TM. 1997. The regulators of G protein signaling (RGS) domains of RGS4, RGS10, and GAIP retain GTPase activating protein activity in vitro. Proc Natl Acad Sci U S A. 94(14):7216–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Shenoy SK. 2018. GPCR desensitization: acute and prolonged phases. Cell Signal. 41:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD. 2015. The taste of table salt. Pflugers Arch. 467(3):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD, Chaudhari N. 2017. Taste buds: cells, signals and synapses. Nat Rev Neurosci. 18(8):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Avila L, Wong GT, Damak S, Margolskee RF. 2001. Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in alpha-gustducin. Proc Natl Acad Sci U S A. 98(15):8868–8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer AB, Gross JD, Kaski SW, Wix K, Siderovski DP, Vandenbeuch A, Setola V. 2018. Development of full sweet, umami, and bitter taste responsiveness requires regulator of G protein signaling-21 (RGS21). Chem Senses. 43(5):367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandilya J, Gao Y, Nayak TK, Roberts SG, Medler KF. 2016. AP1 transcription factors are required to maintain the peripheral taste system. Cell Death Dis. 7(10):e2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekdar K, Langer J, Venkatachalan S, Schmid L, Anobile J, Shah P, Lancaster A, Babich O, Dedova O, Sawchuk D. 2021. Cell engineering method using fluorogenic oligonucleotide signaling probes and flow cytometry. Biotechnol Lett. 43(5):949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS. 2005. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 1(2):51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundararajan M, Willard FS, Kimple AJ, Turnbull AP, Ball LJ, Schoch GA, Gileadi C, Fedorov OY, Dowler EF, Higman VA, et al. 2008. Structural diversity in the RGS domain and its interaction with heterotrimeric G protein alpha-subunits. Proc Natl Acad Sci U S A. 105(17):6457–6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran SK, Lewandowski BC, Qin Y, Kotha R, Bachmanov AA, Margolskee RF. 2017. Whole transcriptome profiling of taste bud cells. Sci Rep. 7(1):7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, et al. 2013. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 495(7440):223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S. 2003. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J Neurosci. 23(9):3616–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE, Kinnamon SC. 2015. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol. 593(5):1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Buchholtz L, Elischer A, Tareilus E, Gouka R, Kaiser C, Breer H, Conzelmann S. 2004. RGS21 is a novel regulator of G protein signalling selectively expressed in subpopulations of taste bud cells. Eur J Neurosci. 19(6):1535–1544. [DOI] [PubMed] [Google Scholar]
- Woo JA, Castaño M, Goss A, Kim D, Lewandowski EM, Chen Y, Liggett SB. 2019. Differential long-term regulation of TAS2R14 by structurally distinct agonists. FASEB J. 33(11):12213–12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. 2003. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 112(3):293–301. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. 2003. The receptors for mammalian sweet and umami taste. Cell. 115(3):255–266. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, Sclafani A. 2011. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol. 301(6):R1635–R1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.