Abstract.
Significance
Whether or not capillary pericytes contribute to blood flow regulation in the brain and retina has long been debated. This was partly caused by failure of detecting the contractile protein -smooth muscle actin (-SMA) in capillary pericytes.
Aim
The aim of this review is to summarize recent developments in detecting -SMA and contractility in capillary pericytes and the relevant literature on the biology of actin filaments.
Results
Evidence suggests that for visualization of the small amounts of -SMA in downstream mid-capillary pericytes, actin depolymerization must be prevented during tissue processing. Actin filaments turnover is mainly based on de/re-polymerization rather than transcription of the monomeric form, hence, small amounts of -SMA mRNA may evade detection by transcriptomic studies. Similarly, transgenic mice expressing fluorescent reporters under the -SMA promoter may yield low fluorescence due to limited transcriptional activity in mid-capillary pericytes. Recent studies show that pericytes including mid-capillary ones express several actin isoforms and myosin heavy chain type 11, the partner of -SMA in mediating contraction. Emerging evidence also suggests that actin polymerization in pericytes may have a role in regulating the tone of downstream capillaries.
Conclusions
With guidance of actin biology, innovative labeling and imaging techniques can reveal the molecular machinery of contraction in pericytes.
Keywords: pericyte, actin, contractility, central nervous system, capillary, blood flow
1. Introduction
Since their first identification by Rouget,1 pericytes have been hypothesized to have a role in capillary blood flow regulation. This view was challenged by several counter arguments over the decades.2–5 Now, it is generally agreed that blood flow reaching to the capillary network is regulated at the precapillary sphincter and first-order capillary by contraction/relaxation of the sphincter and pericytes ensheathing the first-order capillary.6–11 Several lines of evidence have disclosed that pericytes are contractile in vivo such as the upstream vascular smooth muscle cells (vSMCs) as originally suggested.6,7,11–13 Pericytes located over the first- to fourth-order capillaries and their junctions, all of which exhibit high luminal coverage and -smooth muscle actin (-SMA) expression, have convinced most researchers that they might regulate the blood flow by contracting or relaxing.6–11,14 On the contrary, there is skepticism that the mid-capillary pericytes on downstream (≥ fourth order) capillaries, which show the morphological characteristics of mesh or thin-strand pericytes,15 could play a contractile role and contribute to blood flow regulation because their thin processes provide only limited mural coverage and express little or no -SMA.4,7,16 Some technical limitations currently preclude unambiguous clarification of these points as reviewed below.
First, capillary radius changes induced by downstream pericytes are heterogeneous. As reported by Hall et al.,8 while responders to sensory stimulation may dilate by 14% in the whisker cortex, two-thirds of the downstream capillaries either do not dilate or the diameter change induced is likely near the limit of optical resolution under in vivo imaging conditions. For a capillary (most downstream capillaries are smaller than ), a 10% change in luminal diameter results in a 500-nm difference, which can correspond to a few pixels under commonly used two-photon microscopy acquisition conditions. Motion artifacts caused by brain pulsations, respiration or movement of unanesthetized animals further complicate the specificity of measurements. Indeed, following the pioneering study by Hall et al.,8 two later studies, while replicating the rapid and robust response of the upstream capillaries to whisker stimulation, concluded that the downstream pericytes were not significantly responsive to sensory (relaxation) or optogenetic (contraction) stimulation.4,17 Nevertheless, using advanced optics with higher magnification and resolution as well as analysis tools, more recent studies succeeded to detect subtle luminal diameter changes in high-order capillaries in vivo, which typically exhibited dilation or constriction with slow onset but with prolonged duration in response to physiological (a few seconds of odor exposure)10 and optogenetic stimulation,6 respectively. Although smaller dilations (<5%) induced by physiological stimulation in the olfactory bulb or retina was difficult to assess compared to more robust changes induced by 1-min optogenetic stimulation in whisker cortex (by 20%), corresponding alterations in intra-pericytic calcium or capillary blood flow were suggestive that luminal changes measured were not artifactual.10,12 These minor diameter changes in downstream capillaries can indeed appreciably facilitate the increase in blood flow because most of the vascular bed resistance originates from small capillaries.18 The dilations observed are likely to be passive secondary to upstream hydrostatic effects but may also be eased by active, sluggish relaxation of downstream pericytes.10 Supporting the presence of a contractile capacity in downstream pericytes, a small and slowly developing luminal narrowing induced by a vasoconstrictive agent was demonstrated under more stable in vitro conditions in whole mount retina.7 Similarly, electrical stimulation of the fifth-order capillaries off the radial retinal arterioles (taken as zero order) in whole-mount retina caused a contraction by 12% along with an increase in pericyte calcium, which rapidly propagated to upstream pericytes via gap junctions.19 A propagating constriction upon stimulation of capillary pericytes including the downstream ones near the inner plexiform layer in whole-mount retina had also been reported by Peppiatt et al.20 Rapid signal transmission from downstream pericytes, which have an ideal position to monitor neuronal activity, to up and downstream endothelial and mural cells via gap junctions19 or intercellular tunnels12 can orchestrate timing of capillary diameter changes along the arteriolovenular axis to provide an optimal blood flow for the active synapses nearby downstream capillaries.10 Therefore, simultaneous temporal profiling of the luminal changes together with pericyte calcium transients could be highly informative to better understand the neurovascular coupling dynamics in the cortex in vivo as well, but currently remains technically challenging.
The second problem is difficulty in detecting small amounts of -SMA or its mRNA in downstream pericytes. -SMA, the contractile actin isoform in vSMCs is drastically reduced in pericytes located on capillaries downstream to the first three to four branch orders both in the brain and retina when the first microvessel branching off the cortical penetrating or radial retinal arterioles is numbered as the first branch order capillary.7,16 The absence of internal elastic lamina and presence of protruding ovoid cell bodies (bump-on-a-log appearance) seems to be the best criteria to define the first capillary harboring a pericyte and may help aligning the branch order numbers in different tissues.7,15,21 As discussed above, subtle but active tonus modifications in downstream capillaries may be needed to ensure homogenization of the flow rate between neighboring branches during functional hyperemia, although they seem to be passively dilating.6,22 Moreover, restoration of the basal tonus after passive distension might also require -SMA-mediated active contraction. Supporting such possibilities, Shih’s group has recently demonstrated that high-order pericytes did contract when optogenetically stimulated (contractile capability) and that their ablation led to dilation of the underlying capillary (contribution to tonus).6 Strongly suggesting that these observations were not caused by experimental laser injury, ablation of a proximal pericyte bridging to a downstream distant pericyte led to dilation in the downstream capillary unaffected by the laser beam.6 The contractility to downstream mid-capillary pericytes can be provided by small amounts of -SMA, whose presence has been disclosed using rapid tissue fixation techniques or toxins that inhibit actin depolymerization and/or promote actin polymerization in the retina.23–25 Suggesting a physiological role for actin de/re-polymerization in downstream pericytes, intravitreal administration of vasoconstrictive agent noradrenalin in vivo induced actin polymerization in these pericytes.25,26 The actin stabilizing approaches also unveiled that 60% of NG2-DsRed-positive pericytes expressed -SMA [six times more than detected with paraformaldehyde (PFA) fixation] in the intermediate layer of the retina, whose capillaries (corresponding to the seventh to eighth orders) show the greatest dilation during light stimulation, whereas pre-venullary capillaries remained -SMA-negative.23,27 Very recently, Nelson’s group has revealed that all pericytes along the arteriolovenular axis expressed Myh11, the motor protein partner of -SMA in realizing actomyosin-mediated contraction, although they could not detect -SMA in downstream pericytes in whole-mount retinas fixed with the slowly acting fixative PFA.7 The small pool of -SMA constantly de/re-polymerizing in downstream pericytes is likely maintained by a slow actin monomer turnover, as is usually the case for actin in other cells.28 The latter view is consistent with low -SMA mRNA reported29,30 and also with low levels of fluorescent protein/reporter expression under -SMA promoter in downstream pericytes.4,7 Transgenic mouse lines expressing membrane-bound mCherry driven by the -SMA promoter have been shown to faithfully recapitulate endogenous -SMA expression rate in various embryo and adult mouse tissues.31 Indeed, not only mCherry but also GCaMP expression under the control of Acta2 promoter is reportedly low in downstream pericytes,7 supporting the idea of a weak -SMA promoter activity rather than rapid degradation of -SMA mRNA or suppression of translation by regulatory mechanisms. In other words, because the small pool of -SMA protein in downstream pericytes is mainly replenished by de/repolymerization rather than breakdown/synthesis of -SMA monomers, transcriptional and translational activity for -SMA is expectedly to be low. Regulation of -SMA expression involves a complex interaction of multiple positive and negative regulatory elements that act in a cell-type specific manner such that the precise combinatorial interactions of cis and trans acting factors determine the outcome of expression in different cells and under various physiological conditions.32–34 For example, myocardin, a potent promoter of Acta2, is expressed 134 times less in pericytes compared to arteriolar smooth muscle cells.29,30 Supporting a low mRNA content, RNA knockdown with RNA interference in the retina preferentially affected -SMA expression in pericytes downstream to the fourth-order.23 As noted above, immunohistochemistry performed after prevention of actin depolymerization shows that not all downstream pericytes are -SMA-positive in the retina,23,25 which might have diluted the average hit numbers of -SMA-mRNA from mid-capillary pericytes of the brain.35 As mentioned above, this heterogeneity in contractile capability of downstream pericytes was also noted in their response to sensory stimulation in the cortex8 and cautions against treating this population of pericytes as a homogenous group (e.g., in diameter or mRNA statistics).
In summary, before concluding that downstream pericytes do not express -SMA and are not contractile, further research seems to be warranted. While this research solves the technical challenges working with such small vessels and cells, it should also take the basic actin physiology, well characterized in cell biology, into account. Hence, we will summarize some basics of actin physiology below, which we hope may contribute to the experimental designs aiming at studying downstream pericytes.
2. Actin Isoforms in Pericytes
Actin, an evolutionarily well-conserved microfilament, is the most abundant protein (20% of the total cellular protein) in mammalian cells except for a few types.28,36,37 As a structural protein, its turnover is very slow, counted by weeks in muscle cells.28 Only 7% of the filamentous actin (F-actin) is formed from newly synthesized monomers, otherwise, the primary source is polymerization of the existing pool of monomeric globular actin (G-actin).38 G-actin has six mammalian isoforms, which are encoded by different genes but share a highly similar amino acid sequence (95% homology): the contractile -skeletal muscle actin, -cardiac muscle actin, -SMA, -enteric SMA isoforms and the cytoskeletal, -cytoplasmic actin, and -cytoplasmic actin isoforms. Most of the difference stems from the amino acid sequence at the N-terminal region, which determines the affinity to actin binding proteins that maintain the population of assembly ready actin monomers (e.g., profilin), regulate polymerization (e.g., ADF/cofilin, profilin), block the growing ends of actin filaments (e.g., gelsolin), form a nucleus for actin assembly (e.g., gelsolin, Arp2/3, and cofilin), sever actin filaments (e.g., gelsolin, ADF/cofilin), and cross-link actin filaments (e.g., Arp2/3).36,39,40
Cytoplasmic and smooth muscle isoforms of actin were detected in cultured pericytes.41 Although mid-capillary pericytes were previously reported not to express -SMA in tissue sections or in vivo,3,4 the presence of -SMA in high-order pericytes has been disclosed after prevention of F-actin depolymerization,23,25 suggesting that -SMA filaments are actively de/re-polymerized in pericytes and their rapid depolymerization during tissue processing before fixation hampers detection of thin, dispersed -SMA fibers by immunolabeling unlike the thick bundles of -SMA in upstream pericytes23,24,42,43 (Fig. 1). The latter studies23,24 also showed that short interfering RNA (-SMA-siRNA) suppressed -SMA expression preferentially in high branch order (downstream) capillary pericytes and prevented their contraction, conforming to the existence of a smaller pool of -SMA involved in contraction of downstream capillary pericytes. These findings were confirmed by recent single-cell RNA sequence of the cells of brain vasculature, which found a large amount of -SMA mRNA in vSMCs, whereas low hits were detected in pericytes.30,44
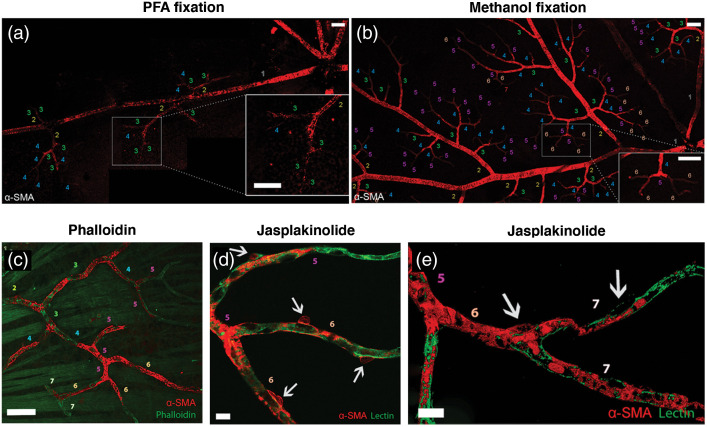
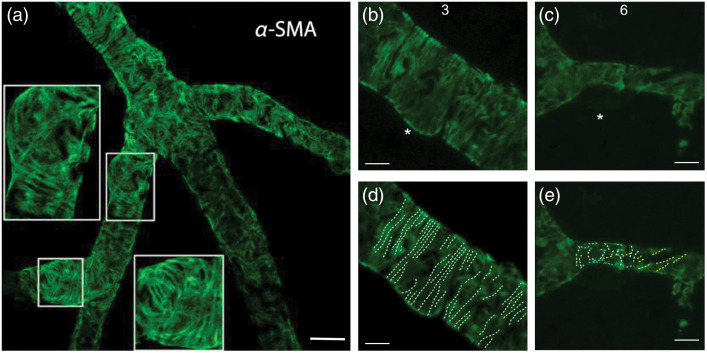
Fig. 1.
Comparison of -SMA immunolabeling in retinal capillary pericytes under (a) PFA and (b) methanol fixation. Each capillary segment is numbered corresponding to its branching order. Faster fixation of retinas with methanol at shows clear -SMA immunoreactivity in up to sixth order microvessels. Prevention of -SMA depolymerization in vivo with phalloidin or jasplakinolide revealed further -SMA immunolabeling in high order retinal capillaries. (c) Phalloidin, intravitreally injected for preventing F-actin depolymerization in vivo, was fluorescent-tagged (green) and revealed -SMA immunolabeling (red) in sixth and seventh order retinal capillaries on whole-mount retinas ex vivo. (d) and (e) F-actin stabilization in vivo with Jasplakinolide further increased the number of -SMA-positive pericytes and an enhanced -SMA immunolabeling intensity by promoting polymerization as well as inhibiting depolymerization (red) in sixth and seventh order retinal capillaries, which were visualized with lectin (green). It is noteworthy that the branch ordering in this study took the central retinal artery but not the radial retinal arterioles as the zero-order (also in other retina figures below), hence, the branch numbers are one order higher compared to the studies on cerebral vasculature, which should be considered when comparing results. After jasplakinolide, not all downstream pericytes were -SMA-positive, but the ratio of -SMA-positivity within NG2-DsRed-positive pericytes increased to 78.7%, 60.4%, and 55.0% in the superficial, intermediate and deeper retinal vascular layers, respectively. Arrows point to pericyte somas and numbers indicate the branch order. Image in (e) is surface rendered. Scale bars: in (a)–(c), in (d) and (e). Adapted from Alarcon-Martinez et al.23 with permission.
3. Visualizing Actin in Fixed and Live Tissues
Actin is found in polymerized [polymeric/filamentous(F)] or depolymerized [monomeric/globular(G)] forms. Phalloidin, obtained from the mushroom Amanita phalloides, binds to monomers in actin filaments by interacting with two neighboring monomers.45,46 Therefore, fluorescently conjugated form of phalloidin makes F-actin visible but not G-actin [Figs. 1(c), 2(a), 2(b), and 4(a)]. Affinity of phalloidin is unaffected by the length of actin filaments or the interaction with tropomyosin and myosin because of its small molecular size (0.6 nm),45–47 ensuring reproducible labeling independent of the physiological state. However, it should be noted that phalloidin cannot differentiate between actin isoforms; for the latter purpose, specific immunofluorescent staining is currently the only solution. SiR-actin is a recently introduced cell-permeable alternative48 that can help live tissue imaging, but it has F-actin stabilization effect, hence, can interfere with actin dynamics in live cells. Whereas phalloidin binds to filamentous actin, deoxyribonuclease I (DNase I) enzyme has a natural affinity for the monomeric form.49 It binds to G-actin with 1:1 ratio and its enzymatic activity stops upon binding. Its fluorescently conjugated forms are used in combination with phalloidin to assess monomeric and polymeric states of actin.
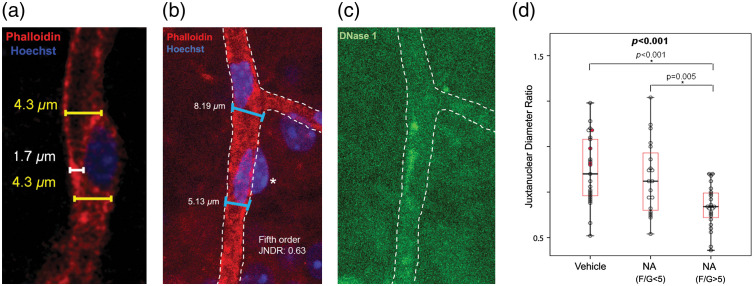
Fig. 2.
Noradrenalin-induced actin polymerization is associated with capillary diameter decrease. Filamentous actin (F-actin) was labeled with phalloidin and globular actin (G-actin) with DNase I. (a) Image illustrates how juxtanuclear capillary diameter was measured. Measurements under the soma (white) were not preferred because they give misleading values in some instances where the pericyte nucleus is superimposed on vessel lumen in maximum projection images. Juxtanuclear values were then divided by the initial diameter of each capillary segment at the proximal branching point to yield the juxtanuclear diameter ratio. (b) and (c) The ratio of the diameter near pericyte soma to the diameter at the branch origin is defined as the juxtanuclear diameter ratio. Image illustrates how these diameters are measured in a fifth-order pericyte with F/G-actin signal ratio above 5. Asterisk in (b) shows the index pericyte’s soma. JNDR: juxtanuclear diameter ratio. (d) In vascular orders of 5 and 6, which had significantly increased F/G-actin signal ratio values in response to noradrenaline administered into the vitreous, a cutoff F/G value of 5 was able to differentiate noradrenaline and vehicle treated groups. See Kureli et al.25 for details. Figure and part of its legend were reproduced from Kureli et al.25 with permission.
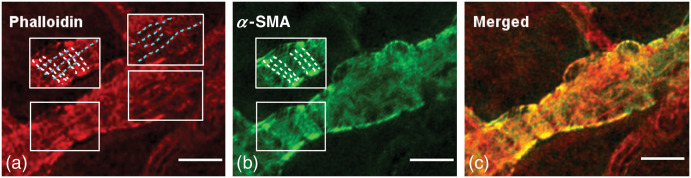
Fig. 4.
(a)–(c) -SMA in the processes of a pericyte on the third-order retinal vessel runs around the vascular axis (white-dashed lines), whereas non--SMA isoforms of actin can be traced along the longitudinal axis of the vessel (cyan-dashed lines). It is noteworthy that the absence of -SMA immunostaining of the phalloidin-positive longitudinal filaments in contrast to circular ones in inset. Scale bar: . Adapted from Kureli et al.,25 with permission.
Immunofluorescent staining with antibodies against actin is the conventional method of labeling actin in fixed tissues.50 Although nonspecific binding and background staining could sometimes be problematic, this is the only method for differentiating actin isoforms, such as -SMA and -actin. Background labeling in the cytoplasm partly originates from antibodies binding to freely dispersed G-actin in addition to the filamentous structures. The major challenge for immunofluorescent labeling of -SMA filaments is their tendency to rapidly depolymerize before tissue fixation when using slow fixatives such as PFA as mentioned above. Low amounts of loosely organized -SMA filaments in mid-capillary pericytes appear to be particularly vulnerable compared to the -SMA filaments in upstream pericytes and vSMCs.23 Whether or not this vulnerability has a biological significance is currently unknown. However, these pericytes show substantial F-actin polymerization25 and slowly contract when stimulated compared to upstream pericytes,6 suggesting that F-actin de/re-polymerization may have a particular role in mid-capillary pericytes. The labeling performance of antibodies is dependent on the fixation method;51 for instance, methanol fixation is superior for labeling -SMA in retinal tissues23 and a combination of formaldehyde, glutaraldehyde and saponin is superior for staining -actin in rat adenocarcinoma cells.52 It should be noted that methanol fixation might not be applicable for the brain tissue due to its high lipid content. The comparison of fixatives for imaging pericyte -SMA in different tissues, including the brain, requires further research.
A different set of tools is required for actin imaging in live cells and tissues. GFP tagging of monomeric actin was developed but GFP-actin was not efficiently incorporated into the polymerized form.53 Therefore, genetically encoded actin-binding peptides derived from organisms such as yeasts or humans were fused to fluorescent proteins. LifeAct, a 17-aminoacid peptide from yeast fused with GFP, is such a tool that labels actin in eukaryotic cells.54 LifeAct can be expressed in cultured cells and a transgenic mouse line that expresses LifeAct fused to eGFP is also available.55 LifeAct has been successful to label F-actin in axon terminals and growth cones,56 in endothelial cells of retina and also in vSMCs and pericytes of skin and skeletal muscle.57 Recently, LifeAct has been reported to interfere with actin dynamics in vivo, impacting stress fiber functioning and cytoskeletal architecture.58,59 Some actin binding proteins, like cofilin, have also been shown to interfere with binding of LifeAct to F-actin.60 Because LifeAct, binds to both F-actin and G-actin,51,54 unlike phalloidin, background fluorescence may be a problem.
Alternative probes are also available. Utrophin (UtrCH), an actin binding protein from humans, can bind to actin globules, hence; serve as a genetically encoded label for F-actin.61 It has not yet been tested in nervous system, but in Drosophila, a concentration-dependent disintegration of actin cytoskeleton has been reported.62 Experience with another probe, F-tractin from the rat,63 is relatively limited compared to the others. But its size is considerably larger than LifeAct and UtrCH, hence, can interfere with other F-actin binding proteins and alter actin cytoskeleton structure.51,64 It should be noted that not all strategies label the same actin subsets in live cells. For instance, LifeAct cannot be incorporated into filopodia-like structures, whereas GFP-actin can be. On the other hand, LifeAct can better demonstrate stress fibers than GFP-actin.
4. Actin Polymerization and Depolymerization
There is a highly dynamic balance between two actin forms, F-actin and G-actin in live cells, which rapidly shifts toward the polymerized form to promote contractility upon mechanical or chemical stimulation.65,66 Steady-state polymerization-depolymerization activity, that is, adding new monomers to the barbed end while removing monomers from the pointed end of filaments, is called “treadmilling” and the growth rate is generally slower than 1 subunit per second.28,67 This mechanism provides motility to cells by way of formation of lamellipodia. The durability of filaments may range from seconds to days depending on the activity; for example, having a faster turnover in lamellipodia and more stable state in myofibrils. Polymerization rate is also affected by local monomer concentrations. The concentrations are heterogenous in polarized cells, and local translation events contribute to this heterogeneity.68
F-actin polymerization is one of the universal cellular mechanisms providing contractility, motility, and tonus; therefore, it likely plays a role in pericyte contraction as well, as does it in vSMCs25,69 (Fig. 2). Unlike upstream pericytes, rapid depolymerization of -SMA suggests that polymerization may regulate the length of contractile -SMA filaments in downstream pericytes.25 Polymerization of cytoskeletal actins can also function as an independent but parallel mechanism to actomyosin-mediated contraction by providing cellular stiffness against mechanical deformation of vSMCs during contraction and a lattice between contracting stress fibers and extracellular matrix to transform the tension developed by actomyosin cross bridge cycling to vessel constriction.65,70–72 It likely contributes to vSMC tonus because blockage of actin polymerization induces relaxation in vessels without affecting actomyosin-mediated contraction.73 Rho kinase, in addition to the actomyosin-mediated contraction, also regulates actin polymerization via activation of LIM kinase, which phosphorylates cofilin, preventing depolymerization.71,74 This pathway has recently been shown to be involved in optogenetically-induced slow contraction of downstream pericytes and suggested to play a role in pericyte tone as well.6 Supporting this view, another activator of the Rho kinase pathway, vasoconstrictor noradrenalin has also been shown to induce actin polymerization in downstream pericytes.25 The slow and sustained contraction and relaxation characteristics of downstream pericytes favor a mechanism involving actin de/re-polymerization especially in the prolonged phase as well as in pericyte tonus. However, further research is needed to elucidate the relative contribution and timing of actomyosin- and polymerization-mediated contraction/relaxation mechanisms in pericytes.
Differential labeling of F-actin and G-actin in the same cell (see below) allows calculation of F/G actin ratio from fluorescence intensities and this ratio is significantly higher in capillaries with smaller juxtanuclear diameters relative to the segment origin25 (Fig. 2).
5. Organization of Actin in vSMCs and Pericytes
We can take vSMCs as the proxy cells to gain insight into the contractile machinery and actin cytoskeleton in pericytes. In vSMCs, actin filaments are attached to focal adhesion points (dense plaques) on the plasma membrane to transmit the intracellular forces to the extracellular matrix69,75 (Fig. 3). The connection to extracellular matrix generates the constrictive force necessary to narrow the vessel lumen.72,76 Phosphorylation of focal adhesion associated proteins (e.g., vinculin, paxillin, and talin) upon mechanical stimulation starts a reaction that results in actin polymerization.69,77 The intracellular anchoring points of F-actin bundles are called dense bodies, which also contain -actinin for cross-linking the filaments.
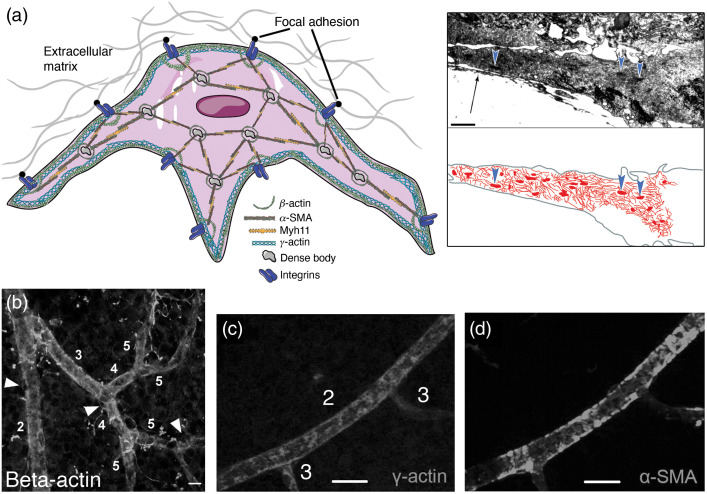
Fig. 3.
Putative organization of actin filaments in pericytes. The diagram is based on the available pericyte data and is inspired by extensive data from SMCs. - and -cytoplasmic actins are concentrated in cortical/submembranous regions, whereas -cytoplasmic actin is also located around dense bodies. -SMA, along with myosin filaments, stretches between focal adhesion points and dense bodies, forming the contractile stress fibers. This figure is produced using Servier medical art (http://www.servier.com). Inset shows a transmission electron micrograph of a cultured retinal pericyte process, harboring actin filaments and dense bodies in cytoplasm (arrowheads). Arrow indicates the extracellular matrix elements. Microfilaments and dense bodies are hand-outlined in red to depict their organization inside the pericyte process. Inset adapted with modifications from Schor et al.78 (b) Immunolabeling for cytoskeletal -actin, which runs along the cortex of the vessel in addition to diffuse labeling. Scale bar: . Adapted from Kureli et al.25 (c) and (d) - cytoplasmic actin and -SMA distribution in vessels after intravitreally injected phalloidin. Despite phalloidin stabilization of F-actin filaments, gamma-actin remained detectable only on ≤fourth-order branches unlike α-SMA. Scale bar: . (c) and (d) Adapted from Alarcon-Martinez et al.,23 with permission.
In vSMCs, contractile and cytoskeletal actin filaments are differentially organized.69 -SMA together with myosin II forms the contractile structures called stress fibers.72 Stress fibers in vSMCs are less orderly compared to sarcomeres of skeletal muscles such that stress fibers spreading out in different directions are anchored to cell membrane via focal adhesion points as well as to dense bodies in the cytoplasm69,72,79 (Fig. 3). Stress fibers formed by -SMA are also reported in pericytes; however, their organization is not as extensively studied as in vSMCs80 (Figs. 4 and 5). Pericytes do not express the contractile -SMA isoform mRNA (Actg2), which is the predominant form of the stress fibers in enteric and urethral smooth muscle cells.44 Of the cytoskeletal isoforms, -actin stretches along the cortex under the plasma membrane and is concentrated around dense bodies in the cytoplasm, whereas -cytoplasmic actin is mainly localized to the cortical (submembranous) region of the cell36,69,72 (Fig. 3). and cytoplasmic actins constitute more dynamic actin populations compared to -SMA.36 Cytoskeletal actin isoforms contribute to contractility by reorganizing the cytoskeleton for better force transmission, as shown in isolated large cerebral arteries.37,72,81,82 A parallel organization of cytoskeletal actin isoforms to the vSMCs has been detected in cultured bovine retinal microvascular pericytes in vitro41,80,83 and in whole mount retinas ex vivo23,25 (Figs. 3 and 4). Transmission electron microscopy of rat and human brain pericytes also disclosed the presence of actin filament network organized around focal adhesion points and dense bodies in the plasma membrane and cytoplasm, respectively.84,85
Fig. 5.
(a) -SMA fiber bundles form circular string-like structures reminiscent of contractile stress fiber organization. Insets: 2× magnified. (b)–(e) Bundles are differentially organized in pericytes from third and sixth retinal vascular orders. The organization of the -SMA labeling within the cytoplasm of pericyte processes suggest a vectoral structure, functioning in capillary diameter changes. It is noteworthy that in the (b) and (d) third order, bundles regularly run rather circumferential (white), while in the (c) and (e) sixth-order bundle orientation becomes irregular with some bundles running oblique (yellow) or parallel (cyan) to the longitudinal capillary axis. Scale bar: in (a), in (b-e).
6. Pharmacological Tools for Modulating Actin Dynamics
A number of pharmacological agents, mostly natural toxins, are commonly used to investigate actin polymerization-depolymerization dynamics (Table 1). Besides their role in modulating actin-related cellular mechanisms, these can also serve as tools to improve their microscopic imaging. Phalloidin, which binds to filamentous actin as noted above, stabilizes its structure by inhibiting its depolymerization.45,46 Pretreatment with fluorescent-tagged phalloidin before perfusing the animal improves F-actin labeling in pericytes by preventing depolymerization and help detect F-actin without immunostaining.23 Sea sponge-derived jasplakinolide, which stabilizes F-actin and additionally induces polymerization can also further improve F-actin detection in mid-capillary pericytes expressing small/undetectable amounts of -SMA23,86 (Fig. 1).
Table 1.
Pharmacological agents for manipulation of actin polymerization.
| Natural toxin | Action | Net effect |
|---|---|---|
| Latrunculin | Binds G-actin monomers to prevent polymerization | Depolymerization |
| Cytochalasin | Binds to barbed (+) end of F-actin to prevent polymerization | Depolymerization |
| Phalloidin | Stabilizes F-actin by inhibiting depolymerization | Stabilization/polymerization |
| Jasplakinolide | Stabilizes F-actin and induces polymerization | Polymerization |
Other pharmacological tools with different mechanisms are also available to modulate actin machinery. Latrunculins are derived from sea sponge.87 They bind to G-actin monomers at a 1:1 ratio and prevent their polymerization.88 Cytochalasins, which are derived from fungi, preclude polymerization by binding to the barbed (+) end of actin filaments and preventing addition of new subunits.89,90 The aforementioned drugs have been employed in several studies to induce or suppress actin polymerization.23,36
7. Myosin II and Actomyosin Cross-Bridge Cycling
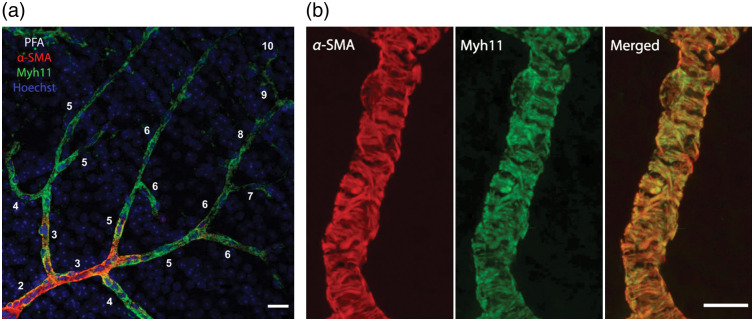
Myosin is the principal cellular motor protein. Among 35 subclasses,91 myosin II functions in cellular contraction and motility.92,93 Head region of its heavy chain, which is highly conserved across species,94 harbors the actin-binding site and ATP catalytic activity required for actomyosin-mediated contraction.92 Among the myosin heavy chain isoforms, type 11 is considered to be smooth muscle specific. Pericytes also express myosin II,95 and recent studies indicate that the predominant isoform is Myh117,30,44,96 (Fig. 6). Since -SMA and Myh11 are functionally coupled and tightly co-localized, detecting Myh11 may be particularly useful to study stress fibers in pericytes because myosin does not have the above-mentioned labeling issues for -SMA and is a stable protein that does not have rapid polymerization-depolymerization dynamics.
Fig. 6.
(a) Myh11 labeling is unaffected by fixation method. In this PFA-fixed retina tissue, despite gradual tapering in -SMA signal toward downstream branches, Myh11 labeling continued all along the whole vascular tree scale bar: . (b) The tight overlap between -SMA and Myh11 proteins is illustrated on a third branch order retinal vessel display the organization of fibrillary bundles in pericyte processes suggestive of tension generating stress fibers. Scale bar: . Z-stack maximum projection images of thick vascular sections. Use of nanosecondaries for immunolabeling -SMA and Myh11 provided better resolution and delineation of the fibers by standard confocal microscopy.
Actin-myosin cross-bridge cycling is accepted as the main contractile mechanism for muscle cells. Upon actin-myosin interaction, power stroke moves myosin along the actin filament.97 ATP binding to myosin head is essential for detachment of myosin head from actin filament. When bound ATP is hydrolyzed, the cycle restarts and myosin head can bind actin once again. Phosphorylation of regulatory light chain (RLC) of myosin II by myosin light chain kinase, which is activated by cytosolic calcium elevation, is the major regulatory mechanism of contraction in vSMCs.92,98 RLC phosphorylation is also enhanced by inhibition of myosin light chain phosphatase, which is activated by small G protein RhoA/Rho kinase pathway.99 Rho kinase also promotes actin polymerization via activation of LIM kinase.74 Rho activation induces pericyte contractility in cultures.71 Suppression of pericyte contractility with blebbistatin,96 a myosin II inhibitor or by fasudil,6 a Rho kinase inhibitor suggests that pathways mediating contraction in pericytes are similar to vSMCs.
8. Conclusion
Presence of contractile proteins like -SMA and Myh11, suggests that pericytes on capillaries including the mid-capillary ones may play differentiated roles in blood flow regulation and neurovascular coupling. Whereas the upstream ensheathing type pericytes contribute to regulation of the blood flow to match the tissue demand by contracting and relaxing, the downstream pericytes may facilitate homogenization of capillary flow to optimize the oxygen extraction by subtly changing capillary resistance. Increasing evidence indicates that molecular machinery of the contractile apparatus in pericytes share striking similarities with vSMCs. Application of cutting-edge imaging and labeling techniques together with the well-established knowledge about actin biology in vSMCs, can advance our understanding of neurovascular coupling in microcirculation.
Acknowledgments
We thank our colleagues who contributed to the work from which figures are re-used, and Jens Peter Gabriel his expert help in the acquisition of Fig. 5(a). Turgay Dalkara’s research is supported by the Turkish Academy of Sciences and the Scientific and Technological Research Council of Turkey (TÜBİTAK) (Grant No. 120S425).
Biographies
Şefik E. Erdener is a medical doctor of neurology; he received his PhD in neuroscience at Hacettepe University, with his work on the interactions between cortical spreading depression and migraine headache. He did his postdoctoral fellowship at Massachusetts General Hospital and Boston University from 2016 to 2018. He is currently an associate professor at Hacettepe University. He is interested in application of optical imaging techniques to evaluate microcirculatory dysfunction and neuroinflammatory signaling in migraine, ischemia, and neurodegenerative disorders.
Gülce Küreli received her MD–PhD at Hacettepe University in 2020. Her PhD study focused on the contractile mechanism of retinal pericytes. She is now a postdoctoral fellow at Boston University Neurophotonics Center, working on long-term follow-up and management of microcirculatory disturbance in stroke.
Turgay Dalkara is a professor of neurology and director of neuroscience PhD program at the Institute of Neurological Sciences and Psychiatry, Hacettepe University, Ankara, Turkey. He received his MD degree in neurology and PhD (pharmacology) trainings at Hacettepe University, and did his postdoctoral fellowship at the Montreal Neurological Institute, McGill University. He joined the Massachusetts General Hospital, Harvard Medical School, in 1992 and held a joint appointment until 2020. He is a member of the European, the World (TWAS), and the Turkish Academy of Sciences.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Contributor Information
Şefik E. Erdener, Email: evrenerdener@gmail.com.
Gülce Küreli, Email: gulcekureli@gmail.com.
Turgay Dalkara, Email: tdalkara@hacettepe.edu.tr.
References
- 1.Rouget C., “Memoire sur le developpement la structure et les proprietes physiologiques des capillaires sanguins et lymphatiques,” Arch. Physiol. Norm. Path. 5, 603–663 (1873). [Google Scholar]
- 2.Sims D. E., “The pericyte--a review,” Tissue Cell 18(2), 153–174 (1986). 10.1016/0040-8166(86)90026-1 [DOI] [PubMed] [Google Scholar]
- 3.Nehls V., Drenckhahn D., “Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin,” J. Cell Biol. 113(1), 147–154 (1991). 10.1083/jcb.113.1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill R. A., et al. , “Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes,” Neuron 87(1), 95–110 (2015). 10.1016/j.neuron.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grutzendler J., Nedergaard M., “Cellular control of brain capillary blood flow: in vivo imaging veritas,” Trends Neurosci. 42(8), 528–536 (2019). 10.1016/j.tins.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann D. A., et al. , “Brain capillary pericytes exert a substantial but slow influence on blood flow,” Nat. Neurosci. 24(5), 633–645 (2021). 10.1038/s41593-020-00793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzales A. L., et al. , “Contractile pericytes determine the direction of blood flow at capillary junctions,” Proc. Natl. Acad. Sci. U. S. A. 117(43), 27022–27033 (2020). 10.1073/pnas.1922755117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall C. N., et al. , “Capillary pericytes regulate cerebral blood flow in health and disease,” Nature 508(7494), 55–60 (2014). 10.1038/nature13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biesecker K. R., et al. , “Glial cell calcium signaling mediates capillary regulation of blood flow in the retina,” J. Neurosci. 36(36), 9435–9445 (2016). 10.1523/JNEUROSCI.1782-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rungta R. L., et al. , “Vascular compartmentalization of functional hyperemia from the synapse to the pia,” Neuron 99(2), 362–375.e4 (2018). 10.1016/j.neuron.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grubb S., Lauritzen M., Aalkjaer C., “Brain capillary pericytes and neurovascular coupling,” Comp. Biochem. Physiol. A Mol. Integr. Physiol. 254, 110893 (2021). 10.1016/j.cbpa.2020.110893 [DOI] [PubMed] [Google Scholar]
- 12.Alarcon-Martinez L., et al. , “Interpericyte tunnelling nanotubes regulate neurovascular coupling,” Nature 585(7823), 91–95 (2020). 10.1038/s41586-020-2589-x [DOI] [PubMed] [Google Scholar]
- 13.Alarcon-Martinez L., Yemisci M., Dalkara T., “Pericyte morphology and function,” Histol. Histopathol. 36(6), 633–643 (2021). 10.14670/HH-18-314 [DOI] [PubMed] [Google Scholar]
- 14.Yemisci M., et al. , “Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery,” Nat. Med. 15(9), 1031–1037 (2009). 10.1038/nm.2022 [DOI] [PubMed] [Google Scholar]
- 15.Grant R. I., et al. , “Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex,” J. Cereb. Blood Flow Metab. 39(3), 411–425 (2019). 10.1177/0271678X17732229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann D. A., et al. , “Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice,” Neurophotonics 2(4), 041402 (2015). 10.1117/1.NPh.2.4.041402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai C., et al. , “Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses,” Proc. Natl. Acad. Sci. U. S. A. 115(25), E5796–E5804 (2018). 10.1073/pnas.1707702115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blinder P., et al. , “The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow,” Nat. Neurosci. 16(7), 889–897 (2013). 10.1038/nn.3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs-Oller T., et al. , “The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes,” Cell Discov. 6, 39 (2020). 10.1038/s41421-020-0180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peppiatt C. M., et al. , “Bidirectional control of CNS capillary diameter by pericytes,” Nature 443(7112), 700–704 (2006). 10.1038/nature05193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attwell D., et al. , “What is a pericyte?” J. Cereb. Blood Flow Metab. 36(2), 451–455 (2016). 10.1177/0271678X15610340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B., et al. , “More homogeneous capillary flow and oxygenation in deeper cortical layers correlate with increased oxygen extraction,” Elife 8, e42299 (2019). 10.7554/eLife.42299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alarcon-Martinez L., et al. , “Capillary pericytes express alpha-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection,” Elife 7, e34861 (2018). 10.7554/eLife.34861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alarcon-Martinez L., et al. , “Retinal ischemia induces alpha-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion,” Acta Neuropathol. Commun. 7(1), 134 (2019). 10.1186/s40478-019-0761-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kureli G., et al. , “F-actin polymerization contributes to pericyte contractility in retinal capillaries,” Exp. Neurol. 332, 113392 (2020). 10.1016/j.expneurol.2020.113392 [DOI] [PubMed] [Google Scholar]
- 26.Hirata H., Tatsumi H., Sokabe M., “Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner,” J. Cell Sci. 121(17), 2795–2804 (2008). 10.1242/jcs.030320 [DOI] [PubMed] [Google Scholar]
- 27.Kornfield T. E., Newman E. A., “Regulation of blood flow in the retinal trilaminar vascular network,” J. Neurosci. 34(34), 11504–11513 (2014). 10.1523/JNEUROSCI.1971-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollard T. D., “Actin and actin-binding proteins,” Cold Spring Harb. Perspect. Biol. 8(8) (2016). 10.1101/cshperspect.a018226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He L., et al. , “Analysis of the brain mural cell transcriptome,” Sci. Rep. 6, 35108 (2016). 10.1038/srep35108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanlandewijck M., et al. , “A molecular atlas of cell types and zonation in the brain vasculature,” Nature 554(7693), 475–480 (2018). 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- 31.Armstrong J. J., et al. , “Characterization of bacterial artificial chromosome transgenic mice expressing mCherry fluorescent protein substituted for the murine smooth muscle alpha-actin gene,” Genesis 48(7), 457–463 (2010). 10.1002/dvg.20638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack C. P., Owens G. K., “Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5’ and first intron promoter regions,” Circ. Res. 84(7), 852–861 (1999). 10.1161/01.RES.84.7.852 [DOI] [PubMed] [Google Scholar]
- 33.Gan Q., et al. , “Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha-actin expression,” Circ. Res. 101(9), 883–892 (2007). 10.1161/CIRCRESAHA.107.154831 [DOI] [PubMed] [Google Scholar]
- 34.Gomez D., Swiatlowska P., Owens G. K., “Epigenetic control of smooth muscle cell identity and lineage memory,” Arterioscler. Thromb. Vasc. Biol. 35(12), 2508–2516 (2015). 10.1161/ATVBAHA.115.305044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chasseigneaux S., et al. , “Isolation and differential transcriptome of vascular smooth muscle cells and mid-capillary pericytes from the rat brain,” Sci. Rep. 8(1), 12272 (2018). 10.1038/s41598-018-30739-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H. R., et al. , “Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent,” Am. J. Physiol. Cell Physiol. 295(3), C768–C778 (2008). 10.1152/ajpcell.00174.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S. H., Dominguez R., “Regulation of actin cytoskeleton dynamics in cells,” Mol. Cells 29(4), 311–325 (2010). 10.1007/s10059-010-0053-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Condeelis J., Singer R. H., “How and why does beta-actin mRNA target?” Biol. Cell 97(1), 97–110 (2005). 10.1042/BC20040063 [DOI] [PubMed] [Google Scholar]
- 39.Dugina V. B., Shagieva G. S., Kopnin P. B., “Biological role of actin isoforms in mammalian cells,” Biochemistry (Mosc) 84(6), 583–592 (2019). 10.1134/S0006297919060014 [DOI] [PubMed] [Google Scholar]
- 40.dos Remedios C. G., et al. , “Actin binding proteins: regulation of cytoskeletal microfilaments,” Physiol. Rev. 83(2), 433–473 (2003). 10.1152/physrev.00026.2002 [DOI] [PubMed] [Google Scholar]
- 41.Hoock T. C., Newcomb P. M., Herman I. M., “ actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury,” J. Cell Biol. 112(4), 653–664 (1991). 10.1083/jcb.112.4.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skalli O., et al. , “Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes,” J. Histochem. Cytochem. 37(3), 315–321 (1989). 10.1177/37.3.2918221 [DOI] [PubMed] [Google Scholar]
- 43.Bandopadhyay R., et al. , “Contractile proteins in pericytes at the blood-brain and blood-retinal barriers,” J. Neurocytol. 30(1), 35–44 (2001). 10.1023/A:1011965307612 [DOI] [PubMed] [Google Scholar]
- 44.Zeisel A., et al. , “Molecular architecture of the mouse nervous system,” Cell 174(4), 999–1014 e1022 (2018). 10.1016/j.cell.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dancker P., et al. , “Interaction of actin with phalloidin: polymerization and stabilization of F-actin,” Biochim. Biophys. Acta 400(2), 407–414 (1975). 10.1016/0005-2795(75)90196-8 [DOI] [PubMed] [Google Scholar]
- 46.Oda T., Namba K., Maeda Y., “Position and orientation of phalloidin in F-actin determined by X-ray fiber diffraction analysis,” Biophys. J. 88(4), 2727–2736 (2005). 10.1529/biophysj.104.047753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De La Cruz E. M., Pollard T. D., “Transient kinetic analysis of rhodamine phalloidin binding to actin filaments,” Biochemistry 33(48), 14387–14392 (1994). 10.1021/bi00252a003 [DOI] [PubMed] [Google Scholar]
- 48.D’Este E., et al. , “STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons,” Cell Rep. 10(8), 1246–1251 (2015). 10.1016/j.celrep.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 49.Hitchcock S. E., “Actin deoxyroboncuclease I interaction. Depolymerization and nucleotide exchange,” J. Biol. Chem. 255(12), 5668–5673 (1980). 10.1016/S0021-9258(19)70681-4 [DOI] [PubMed] [Google Scholar]
- 50.Lazarides E., Weber K., “Actin antibody: the specific visualization of actin filaments in non-muscle cells,” Proc. Natl. Acad. Sci. U. S. A. 71(6), 2268–2272 (1974). 10.1073/pnas.71.6.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melak M., Plessner M., Grosse R., “Actin visualization at a glance,” J. Cell Sci. 130(3), 525–530 (2017). 10.1242/jcs.189068 [DOI] [PubMed] [Google Scholar]
- 52.DesMarais V., et al. , “Optimizing leading edge F-actin labeling using multiple actin probes, fixation methods and imaging modalities,” Biotechniques 66(3), 113–119 (2019). 10.2144/btn-2018-0112 [DOI] [PubMed] [Google Scholar]
- 53.Doyle T., Botstein D., “Movement of yeast cortical actin cytoskeleton visualized in vivo,” Proc. Natl. Acad. Sci. U. S. A. 93(9), 3886–3891 (1996). 10.1073/pnas.93.9.3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riedl J., et al. , “Lifeact: a versatile marker to visualize F-actin,” Nat. Methods 5(7), 605–607 (2008). 10.1038/nmeth.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riedl J., et al. , “Lifeact mice for studying F-actin dynamics,” Nat. Methods 7(3), 168–169 (2010). 10.1038/nmeth0310-168 [DOI] [PubMed] [Google Scholar]
- 56.Ladt K., Ganguly A., Roy S., “Axonal actin in action: imaging actin dynamics in neurons,” Methods Cell Biol. 131, 91–106 (2016). 10.1016/bs.mcb.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fraccaroli A., et al. , “Visualization of endothelial actin cytoskeleton in the mouse retina,” PLoS One 7(10), e47488 (2012). 10.1371/journal.pone.0047488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flores L. R., et al. , “Lifeact-GFP alters F-actin organization, cellular morphology and biophysical behavior,” Sci. Rep. 9(1), 3241 (2019). 10.1038/s41598-019-40092-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belyy A., et al. , “Structure of the Lifeact-F-actin complex,” PLoS Biol. 18(11), e3000925 (2020). 10.1371/journal.pbio.3000925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munsie L. N., et al. , “Lifeact cannot visualize some forms of stress-induced twisted F-actin,” Nat. Methods 6(5), 317 (2009). 10.1038/nmeth0509-317 [DOI] [PubMed] [Google Scholar]
- 61.Burkel B. M., von Dassow G., Bement W. M., “Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin,” Cell Motil. Cytoskeleton 64(11), 822–832 (2007). 10.1002/cm.20226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spracklen A. J., et al. , “The pros and cons of common actin labeling tools for visualizing actin dynamics during Drosophila oogenesis,” Dev. Biol. 393(2), 209–226 (2014). 10.1016/j.ydbio.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schell M. J., Erneux C., Irvine R. F., “Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus,” J. Biol. Chem. 276(40), 37537–37546 (2001). 10.1074/jbc.M104101200 [DOI] [PubMed] [Google Scholar]
- 64.Belin B. J., Goins L. M., Mullins R. D., “Comparative analysis of tools for live cell imaging of actin network architecture,” Bioarchitecture 4(6), 189–202 (2014). 10.1080/19490992.2014.1047714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunst S. J., Zhang W., “Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction,” Am. J. Physiol. Cell Physiol. 295(3), C576–C587 (2008). 10.1152/ajpcell.00253.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehta D., Gunst S. J., “Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle,” J. Physiol. 519(3), 829–840 (1999). 10.1111/j.1469-7793.1999.0829n.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wegner A., “Head to tail polymerization of actin,” J. Mol. Biol. 108(1), 139–150 (1976). 10.1016/S0022-2836(76)80100-3 [DOI] [PubMed] [Google Scholar]
- 68.Skruber K., Read T. A., Vitriol E. A., “Reconsidering an active role for G-actin in cytoskeletal regulation,” J. Cell Sci. 131(1), 203760 (2018). 10.1242/jcs.203760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohanian J., Pieri M., Ohanian V., “Non-receptor tyrosine kinases and the actin cytoskeleton in contractile vascular smooth muscle,” J. Physiol. 593(17), 3807–3814 (2015). 10.1113/jphysiol.2014.284174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang D. D., “Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling,” Respir. Res. 16, 134 (2015). 10.1186/s12931-015-0296-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kutcher M. E., Herman I. M., “The pericyte: cellular regulator of microvascular blood flow,” Microvasc. Res. 77(3), 235–246 (2009). 10.1016/j.mvr.2009.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamin R., Morgan K. G., “Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell,” J. Physiol. 590(17), 4145–4154 (2012). 10.1113/jphysiol.2012.232306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boels P. J., Pfitzer G., “Relaxant effect of phalloidin on Triton-skinned microvascular and other smooth muscle preparations,” J. Muscle Res. Cell Motil. 13(1), 71–80 (1992). 10.1007/BF01738430 [DOI] [PubMed] [Google Scholar]
- 74.Maekawa M., et al. , “Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase,” Science 285(5429), 895–898 (1999). 10.1126/science.285.5429.895 [DOI] [PubMed] [Google Scholar]
- 75.North A. J., et al. , “Complementary distributions of vinculin and dystrophin define two distinct sarcolemma domains in smooth muscle,” J. Cell Biol. 120(5), 1159–1167 (1993). 10.1083/jcb.120.5.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schor A. M., et al. , “Pericytes derived from the retinal microvasculature undergo calcification in vitro,” J. Cell Sci. 97(3), 449–461 (1990). 10.1242/jcs.97.3.449 [DOI] [PubMed] [Google Scholar]
- 77.Opazo Saez A., et al. , “Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane,” Am. J. Physiol. Cell Physiol. 286(2), C433–C447 (2004). 10.1152/ajpcell.00030.2003 [DOI] [PubMed] [Google Scholar]
- 78.Pavalko F. M., et al. , “Phosphorylation of dense-plaque proteins talin and paxillin during tracheal smooth muscle contraction,” Am. J. Physiol. 268(3 Pt 1), C563–C571 (1995). 10.1152/ajpcell.1995.268.3.C563 [DOI] [PubMed] [Google Scholar]
- 79.Kassianidou E., Kumar S., “A biomechanical perspective on stress fiber structure and function,” Biochim. Biophys. Acta 1853(11 Pt B), 3065–3074 (2015). 10.1016/j.bbamcr.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herman I. M., D’Amore P. A., “Microvascular pericytes contain muscle and nonmuscle actins,” J. Cell Biol. 101(1), 43–52 (1985). 10.1083/jcb.101.1.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cipolla M. J., Gokina N. I., Osol G., “Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior,” FASEB J. 16(1), 72–76 (2002). 10.1096/cj.01-0104hyp [DOI] [PubMed] [Google Scholar]
- 82.Moreno-Dominguez A., et al. , “Cytoskeletal reorganization evoked by Rho-associated kinase- and protein kinase C-catalyzed phosphorylation of cofilin and heat shock protein 27, respectively, contributes to myogenic constriction of rat cerebral arteries,” J. Biol. Chem. 289(30), 20939–20952 (2014). 10.1074/jbc.M114.553743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeNofrio D., Hoock T. C., Herman I. M., “Functional sorting of actin isoforms in microvascular pericytes,” J. Cell Biol. 109(1), 191–202 (1989). 10.1083/jcb.109.1.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le Beux Y. J., Willemot J., “Actin- and myosin-like filaments in rat brain pericytes,” Anat. Rec. 190(4), 811–826 (1978). 10.1002/ar.1091900404 [DOI] [PubMed] [Google Scholar]
- 85.Ho K. L., “Ultrastructure of cerebellar capillary hemangioblastoma. IV. Pericytes and their relationship to endothelial cells,” Acta Neuropathol. 67(3-4), 254–264 (1985). 10.1007/BF00687810 [DOI] [PubMed] [Google Scholar]
- 86.Sawitzky H., et al. , “The anti-proliferative agent jasplakinolide rearranges the actin cytoskeleton of plant cells,” Eur. J. Cell Biol. 78(6), 424–433 (1999). 10.1016/S0171-9335(99)80085-5 [DOI] [PubMed] [Google Scholar]
- 87.Holzinger A., Blaas K., “Actin-dynamics in plant cells: the function of actin-perturbing substances: jasplakinolide, chondramides, phalloidin, cytochalasins, and latrunculins,” Methods Mol. Biol. 1365, 243–261 (2016). 10.1007/978-1-4939-3124-8_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coue M., et al. , “Inhibition of actin polymerization by latrunculin A,” FEBS Lett. 213(2), 316–318 (1987). 10.1016/0014-5793(87)81513-2 [DOI] [PubMed] [Google Scholar]
- 89.Brown S. S., Spudich J. A., “Mechanism of action of cytochalasin: evidence that it binds to actin filament ends,” J. Cell Biol. 88(3), 487–491 (1981). 10.1083/jcb.88.3.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flanagan M. D., Lin S., “Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin,” J. Biol. Chem. 255(3), 835–838 (1980). 10.1016/S0021-9258(19)86105-7 [DOI] [PubMed] [Google Scholar]
- 91.Odronitz F., Kollmar M., “Drawing the tree of eukaryotic life based on the analysis of 2,269 manually annotated myosins from 328 species,” Genome Biol. 8(9), R196 (2007). 10.1186/gb-2007-8-9-r196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heissler S. M., Sellers J. R., “Various themes of myosin regulation,” J. Mol. Biol. 428(9 Pt B), 1927–1946 (2016). 10.1016/j.jmb.2016.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sellers J., “Myosins: a diverse superfamily,” Biochim. Biophys. Acta – Mol. Cell Res. 1496(1), 3–22 (2000). 10.1016/S0167-4889(00)00005-7 [DOI] [PubMed] [Google Scholar]
- 94.Lee K. H., et al. , “Interacting-heads motif has been conserved as a mechanism of myosin II inhibition since before the origin of animals,” Proc. Natl. Acad. Sci. U. S. A. 115(9), E1991–E2000 (2018). 10.1073/pnas.1715247115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sweeney M. D., Ayyadurai S., Zlokovic B. V., “Pericytes of the neurovascular unit: key functions and signaling pathways,” Nat. Neurosci. 19(6), 771–783 (2016). 10.1038/nn.4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kureli G., Erdener S. E., Dalkara T., “Organization of contractile proteins in pericytes suggest different flow regulatory functions,” in SfN Global Connectome; 11–13 Jan 2021; a Virtual Event (2021). [Google Scholar]
- 97.Rauscher A. A., et al. , “Targeting myosin by blebbistatin derivatives: optimization and pharmacological potential,” Trends Biochem. Sci. 43(9), 700–713 (2018). 10.1016/j.tibs.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 98.Somlyo A. P., Somlyo A. V., “Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II,” J. Physiol. 522(2), 177–185 (2000). 10.1111/j.1469-7793.2000.t01-2-00177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kimura K., et al. , “Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase),” Science 273(5272), 245–248 (1996). 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]