Gut Microbiota–Kidney Crosstalk
Distant organ crosstalk during AKI, first studied mechanistically in the lung (1), is now known to play an important role in disease outcomes. The relationship between intestinal microbiota and AKI has also been explored, resulting in exciting findings that are promising for future human therapeutics. In search of ways to reduce kidney T cells by using “cleaner,” germfree (GF) mice, it was unexpectedly found that GF mice not only retained their kidney immune cells, but also had a higher number of natural killer T (NKT) cells and reduced IL-4 levels compared with normal wild-type (WT) mice. Moreover, GF mice subjected to kidney ischemia-reperfusion (IR) injury had increased CD8 T-cell trafficking and inflammatory cytokine mediators compared with normal WT mice (2). Induction of ischemic AKI in GF mice led to a worse course of AKI, in terms of both structure and function. Conventionalizing GF mice with normal mouse stool led to normalizing T-cell and NKT populations, and relative protection from AKI, compared with GF mice (2). It was subsequently observed that early exposure to gut microbiota during development was associated with long-term changes in NKT-cell function in nonrenal tissues, and NKT cells in GF mice had a less mature phenotype with diminished activation capacity upon antigen encounter (3,4). To elucidate whether the effects were bidirectional and AKI led to changes in the gut microbiome, gut microbiota was evaluated in C57BL/6 WT mice after ischemic or nephrotoxic (cisplatin; CP) injury. DNA was isolated from the fecal pellets (n=4–5 per group) at baseline (D0) and at 3 days (D3) post-IR. The V3–V5 region of the 16S ribosomal RNA gene was amplified using the 357F/926R primer set and sequenced using the Roche/454 platform. IR and CP modified the relative abundance of specific bacterial species belonging to the phyla Actinobacteria, Bacteroidetes, Firmicutes, Tenericutes, and Verrucomicrobia (Figure 1A). Furthermore, differential abundance testing and negative binomial testing showed distinct alterations in microbial populations at the family and genus level, respectively (Figure 1B). The major bacterial families affected after CP injections included Lachnospiraceae, Lactobacillaceae, Porphyromonadaceae, and Ruminococcaceae; the families Erysipelotrichaceae, Lachnospiraceae, Porphyromonadaceae, and Ruminococcaceae were changed by IR. At the genus level, Oscillibacter, Lactobacillus, Clostridium, and Barnesiella changed after CP-induced AKI; Oscillibacter, Eisenbergiella, and Barnesiella changed after IR-induced AKI. Dimensional analysis by Brey non-metric multidimensional scaling and α-diversity analysis using the Shannon index revealed that the microbiome differentiated over time, depending on treatment (Figure 1, C and D). Further analysis showed a significant increase in the percentage of Erysipelotrichaceae incertae sedis in post-AKI (D3) samples compared with D0 samples from mice treated with IR (P=0.03) and CP (P=0.007). Conversely, the percentage of Lactobacillus decreased significantly (P=0.02) at D3 after CP treatment in comparison with D0 samples (Figure 1E).
Figure 1.
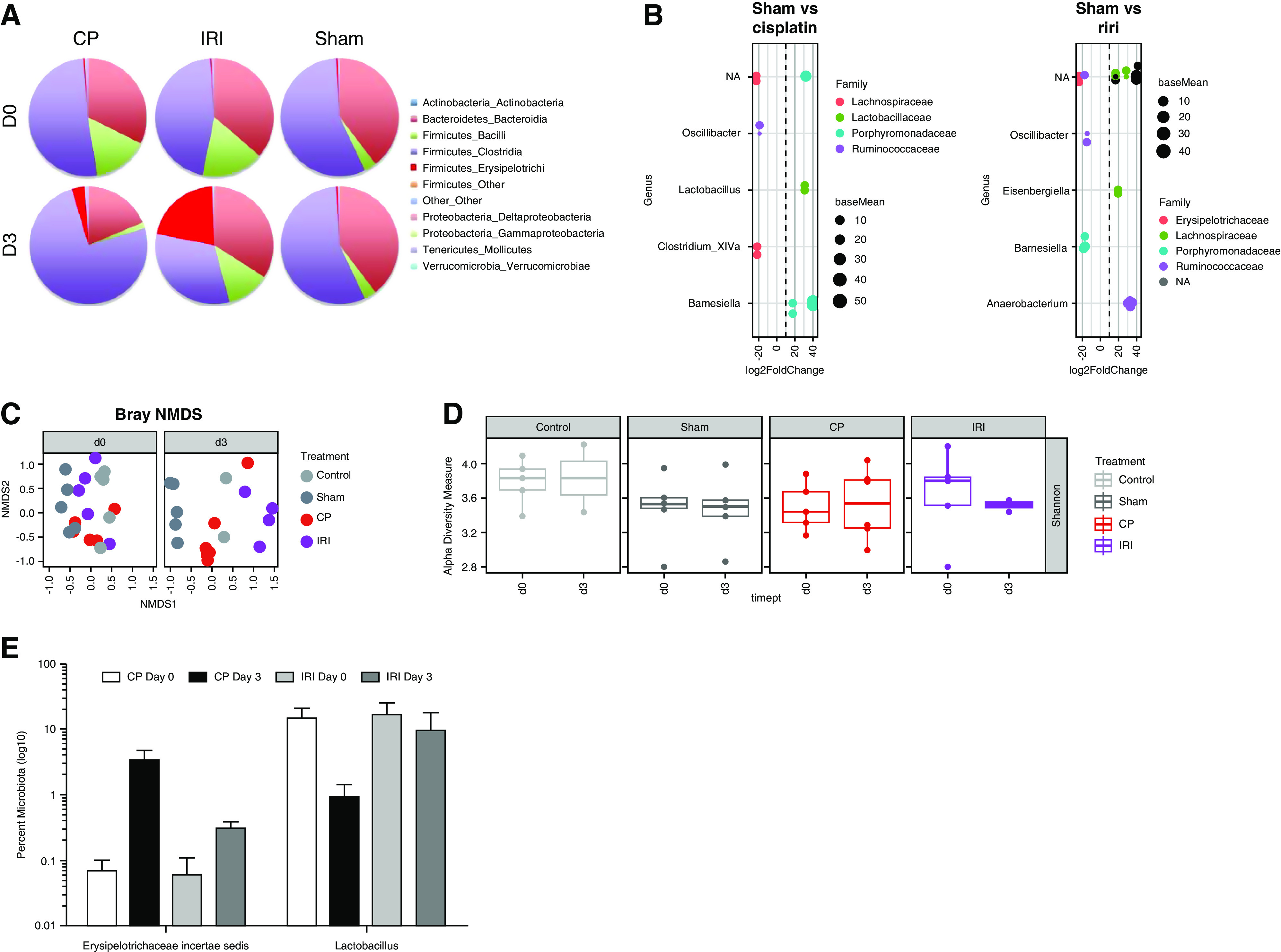
Renal ischemia-reperfusion injury (IRI) and cisplatin treatment change gut microbial populations. Under an approved animal protocol, AKI was induced in male, 8- to 10-week-old, C57BL/6 mice by 30 minutes of bilateral IRI or 30 mg/kg cisplatin (CP) injection. The gut microbiota was then studied at baseline (D0) and 72 hours (D3) post-AKI using 16S sequencing. (A) IRI and CP affected relative abundance of bacterial species belonging to the phyla Actinobacteria, Bacteroidetes, Firmicutes, Tenericutes, and Verrucomicrobia. (B) The vertical dot plots represent differential abundance testing between sham and CP mice or sham and IRI mice (x axis, fold change; size, base mean), showing distinct alterations in microbial populations at the family level. The genera identified on the y axis are those that were affected by AKI, using negative binomial testing. There were no significant operational taxonomic units with species-level information. (C and D) Dimensional analysis by Brey non-metric multidimensional scaling (NMDS) and α-diversity analysis using the Shannon index suggests the microbiome differentiates itself over time, depending on treatment. (E) Percentage change in Erysipelotrichaceae incertae sedis and Lactobacillus populations after IRI and CP-induced AKI. Timept, time point. NA, not available.
Gut Microbiota, Amino Acids, Antibiotics, and AKI
Further studies have elegantly examined AKI and microbiome interactions. Worse outcome of AKI in GF mice was confirmed in a study that associated AKI-induced gut dysbiosis with reduced D-amino acid oxidase activity and altered d-serine metabolism (5). Oral administration of d-serine reduced F4/80+ cells in the kidneys and protected from tubular injury after IR. However, antibiotic pretreatment of WT mice, performed to deplete gut microbiota, protected from IR-induced kidney injury (6). Antibiotic-treated mice also had reduced F4/80 macrophage populations and chemokine receptors CX3C chemokine receptor 1 and C-C chemokine receptor 2 in the F4/80+ renal-resident macrophages. Additionally, mRNA levels of TNF-α, IL-6, monocyte chemoattractant protein-1, and macrophage inflammatory protein-2α were significantly reduced in antibiotic-treated mice (6). The reason for the discrepancy between worsened AKI outcomes in GF mice and improved AKI outcomes in antibiotic-treated mice is unclear. It is possible that antibiotics selectively depleted deleterious gut microbiota, with subsequent enrichment of protective gut microbiota, whereas there was altered maturation of kidney immune cells and function in GF mice.
Role of Gut Microbiota in Immune-System Development and Function
Gut microbiota has also been found to be instrumental in the development, induction, and function of T cells, with dysbiosis leading to imbalances in T-cell subpopulations. These dysregulations in gut microbiota–immune system interactions result in the development of complex immune–mediated diseases, including inflammatory bowel disease, rheumatoid arthritis, type 1 and 2 diabetes mellitus, asthma, and cancer. In addition to established immune-mediated diseases, dysregulated gut microbiota–immune cell interactions influence the outcome of acute tissue injury in nonrenal organs, such as during models of traumatic and ischemic brain injury, myocardial infarction, and acute liver injury. One study demonstrated that colonization of GF mice reduced stroke volume and poststroke neuroinflammation by priming immune responses (7). They found that lymphocyte priming, particularly of T cells by gut microbiota, was the key mechanism that affected stroke outcome. They also observed an increase in CD4, regulatory T, and T helper 17 cells in the poststroke intestines, and an overall increased T-cell population in ischemic brain of colonized mice. In an experimental cardiac study, depletion of gut microbiota, using antibiotics before injury, resulted in significant mortality in mice after inducing myocardial infarction (8). This study found a significant reduction in myeloid cells and neutrophils in the hearts of antibiotic-treated mice before and after myocardial infarction, which was restored after microbiota reconstitution. Additionally, CD4+Foxp3− T cells, regulatory T cells, and B cells were absent from the hearts of these mice after antibiotic treatment. In a study on experimental acute liver injury, there was enrichment of Lactobacillus that activated IL-22 production by intestinal innate lymphoid cells, which subsequently promoted IL-10 and TGFβ production by regulatory dendritic cells (9).
Mechanism of Gut Microbiota–Immune System Interactions
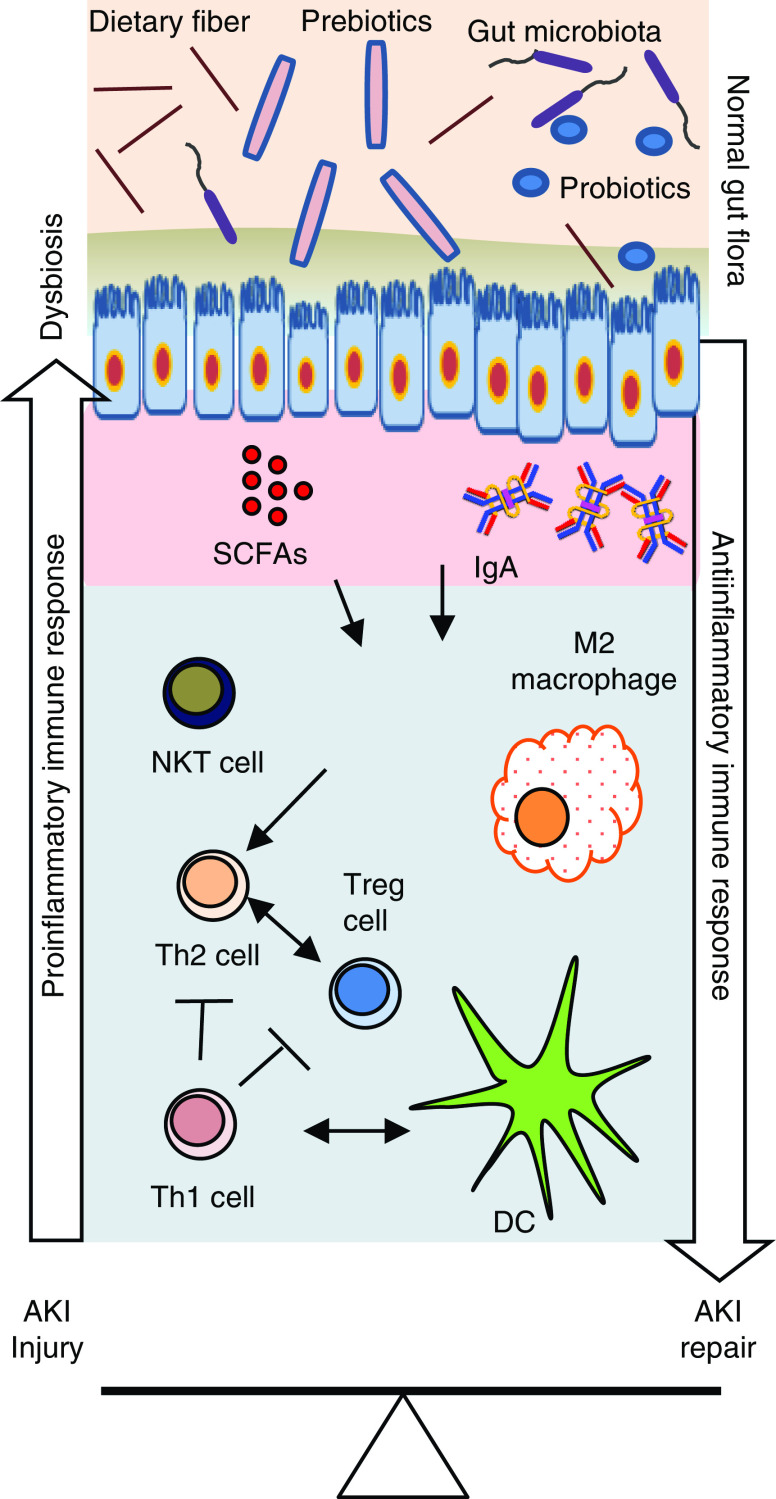
The mechanism by which gut microbiota interacts with kidney immune cells is likely complex and unclear, but short-chain fatty acids (SCFAs) appear to play important roles (Figure 2) (10). Gut microbiota produce SCFAs—such as acetate, propionate, and butyrate—as the fermentation byproducts of dietary fibers. SCFA ligation and activation of various G protein–coupled receptors (such as GPR109a, free fatty acid receptor 2, free fatty acid receptor 3, and olfactory receptor-78) are important mechanisms by which they modulate immune-cell function. SCFAs can further modulate the activity of histone acetyltransferase and deacetylase and the hypoxia-inducible factor. Exogenous administration of SCFAs was found to improve kidney function during experimental AKI and contrast-induced nephropathy (10). It is likely that dysregulation in gut microbiota and microbiome-associated metabolites affect T-cell functions and antibody-mediated humoral immunity. Acetate treatment ameliorated sepsis-induced AKI by inhibiting NADPH-oxidase signaling in T cells, suggesting that SCFAs act through immune-cell regulation (11). Furthermore, in vitro SCFA treatment was found to modulate the inflammatory process by decreasing dendritic-cell maturation and inhibiting CD4 and CD8 T-cell proliferation, and could be a potential mechanism through which gut microbiota interact and modulate T-cell functions in vivo (10). Recent data demonstrated that diet can induce post-translational modifications to the microbial proteome that, in turn, affect microbial metabolite production and, ultimately, kidney function (12). The lack of microbes in GF mice has also been shown to affect differentiation of regulatory T cells due to the absence of SCFA production (13).
Figure 2.
An overview of gut microbiota–immune cell interactions in the kidney affecting injury and repair processes after AKI. Gut microbiota produce short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, that interact with multiple G protein–coupled receptors on kidney epithelial cells. Similar interactions are likely involved in immune cells that modulate their number, immune function, and metabolism in the kidney. Normal gut microbiota promote the anti-inflammatory milieu by increasing T helper 2 (Th2) cell, regulatory T (Treg) cell, and M2 macrophage populations that protect kidneys from AKI. However, AKI-induced dysbiosis and translocation of bacterial products across the leaky intestine promote a proinflammatory immune environment, such as increased Th1 cells, M1 macrophages, and activated dendritic cells (DCs). Activated DCs secrete proinflammatory cytokines, such as IL-12 and IL-6, and skew the differentiation of naive CD4 T cells and maturation of B cells. Th17-inducing bacteria may promote Th17 immunity via IL-17A/IL-17F induction, which may involve signaling mediated by the Toll-like receptor ligands. Additionally, IgA produced by plasma cells residing in the gut epithelium modulates response to colonization by specific commensal bacteria. SCFAs also regulate cytokine expression in T cells and generation of Treg cells through histone deacetylase inhibition. Therapeutic and supplemental use of pre-, pro-, and postbiotics could be helpful in normalizing bacterial composition in the gut and in protecting the kidneys from AKI. NKT, natural killer T cell.
Conclusions
The gut microbiota is an exciting frontier in medicine. The metagenome of the microbiota can be changed through dietary modifications and administration of pre-, pro-, and postbiotics, providing unique opportunities to develop novel therapeutics for AKI treatment. Furthermore, potential therapeutic effects of AKI-specific microbiota on systemic immune dysfunction or distant organ damage in AKI remain to be explored. Although promising, diet- and SCFA-based AKI therapy should be investigated more carefully because of the evidence for potential deleterious effects, such as immune-cell activation, T cell–mediated ureteritis, and hydronephrosis (14). Diet-based, post-translational modifications of the gut microbiota proteome and metabolites should be further explored to understand effects on AKI pathophysiology (12). Because reduced diversity of gut microbiota occurs in recipients of kidney transplants, elucidating the effect of gut microbiota on AKI could also have significant implications for allografts (15).
Disclosures
H. Rabb reports being a nephrology associate editor for the Journal of Clinical Investigation. J. White reports being employed by and having ownership interest in Resphera Biosciences. All remaining authors have nothing to disclose.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK123342 (to H. Rabb).
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
S. Gharaie, K. Lee, S. Noel, and H. Rabb wrote the original draft; F. Mohammad, S. Noel, and J. White were responsible for formal analysis; S. Noel was responsible for methodology; H. Rabb conceptualized the study, provided supervision, and was responsible for funding acquisition and project administration; and all authors reviewed and edited the manuscript.
References
- 1.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H: Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367, 1999. 10.1046/j.1523-1755.1999.00460.x [DOI] [PubMed] [Google Scholar]
- 2.Jang HR, Gandolfo MT, Ko GJ, Satpute S, Racusen L, Rabb H: Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. Am J Physiol Renal Physiol 297: F1457–F1465, 2009. 10.1152/ajprenal.90769.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS: Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336: 489–493, 2012. 10.1126/science.1219328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingender G, Hiss M, Engel I, Peukert K, Ley K, Haller H, Kronenberg M, von Vietinghoff S: Neutrophilic granulocytes modulate invariant NKT cell function in mice and humans. J Immunol 188: 3000–3008, 2012. 10.4049/jimmunol.1101273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakade Y, Iwata Y, Furuichi K, Mita M, Hamase K, Konno R, Miyake T, Sakai N, Kitajima S, Toyama T, Shinozaki Y, Sagara A, Miyagawa T, Hara A, Shimizu M, Kamikawa Y, Sato K, Oshima M, Yoneda-Nakagawa S, Yamamura Y, Kaneko S, Miyamoto T, Katane M, Homma H, Morita H, Suda W, Hattori M, Wada T: Gut microbiota-derived D-serine protects against acute kidney injury. JCI Insight 3: e97957, 2018. 10.1172/jci.insight.97957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emal D, Rampanelli E, Stroo I, Butter LM, Teske GJ, Claessen N, Stokman G, Florquin S, Leemans JC, Dessing MC: Depletion of gut microbiota protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 28: 1450–1461, 2017. 10.1681/ASN.2016030255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh V, Sadler R, Heindl S, Llovera G, Roth S, Benakis C, Liesz A: The gut microbiome primes a cerebroprotective immune response after stroke. J Cereb Blood Flow Metab 38: 1293–1298, 2018. 10.1177/0271678X18780130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang TWH, Chen HC, Chen CY, Yen CYT, Lin CJ, Prajnamitra RP, Chen LL, Ruan SC, Lin JH, Lin PJ, Lu HH, Kuo CW, Chang CM, Hall AD, Vivas EI, Shui JW, Chen P, Hacker TA, Rey FE, Kamp TJ, Hsieh PCH: Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 139: 647–659, 2019. 10.1161/CIRCULATIONAHA.118.035235 [DOI] [PubMed] [Google Scholar]
- 9.Nakamoto N, Amiya T, Aoki R, Taniki N, Koda Y, Miyamoto K, Teratani T, Suzuki T, Chiba S, Chu PS, Hayashi A, Yamaguchi A, Shiba S, Miyake R, Katayama T, Suda W, Mikami Y, Kamada N, Ebinuma H, Saito H, Hattori M, Kanai T: Commensal Lactobacillus controls immune tolerance during acute liver injury in mice. Cell Rep 21: 1215–1226, 2017. 10.1016/j.celrep.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 10.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, Bassi EJ, Moraes-Vieira PM, Hiyane MI, Rodas AC, Peron JP, Aguiar CF, Reis MA, Ribeiro WR, Valduga CJ, Curi R, Vinolo MA, Ferreira CM, Câmara NO: Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 26: 1877–1888, 2015. 10.1681/ASN.2014030288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Harbi NO, Nadeem A, Ahmad SF, Alotaibi MR, AlAsmari AF, Alanazi WA, Al-Harbi MM, El-Sherbeeny AM, Ibrahim KE: Short chain fatty acid, acetate ameliorates sepsis-induced acute kidney injury by inhibition of NADPH oxidase signaling in T cells. Int Immunopharmacol 58: 24–31, 2018. 10.1016/j.intimp.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 12.Lobel L, Cao YG, Fenn K, Glickman JN, Garrett WS: Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 369: 1518–1524, 2020. 10.1126/science.abb3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY: Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455, 2013. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Goergen CJ, HogenEsch H, Kim CH: Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J Immunol 196: 2388–2400, 2016. 10.4049/jimmunol.1502046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swarte JC, Douwes RM, Hu S, Vich Vila A, Eisenga MF, van Londen M, Gomes-Neto AW, Weersma RK, Harmsen HJM, Bakker SJL: Characteristics and dysbiosis of the gut microbiome in renal transplant recipients. J Clin Med 9: 386, 2020. 10.3390/jcm9020386 [DOI] [PMC free article] [PubMed] [Google Scholar]