Abstract
Over the past 3 decades, significant advancements in the understanding of the pathophysiology of ANCA-associated vasculitis has led to the development of a multitude of potential candidate biomarkers. Accompanied by the advent of increasingly effective therapeutic strategies, the need for a dependable biomarker to help determine the extent of disease activity and risk of relapse is ever present. Implementation of such a biomarker would enable tailored therapy, optimizing disease control while helping to mitigate unnecessary exposure to therapy and potential treatment-related damage. Although far from perfect, ANCA serology and B-cell population are the two main staple biomarker tools widely used in practice to help supplement clinical assessment. Over recent years, the application and progress of more novel biomarker tools have arisen in both organ-limited and multisystem disease, including genomics, urinary proteins, degradation products of the alternative complement system, cytokines, metabolomics, and biospectroscopy. Validation studies and clinical translation of these tools are required, with serial assessment of disease activity and determination of therapy according to biomarker status correlated with patient outcomes.
Keywords: glomerular and tubulointerstitial diseases, ANCA-associated vasculitis, biomarkers, disease activity
Introduction
Pauci-immune small-vessel vasculitis characterizes a group of relapsing diseases with potential multiorgan involvement, typically with circulating ANCA. The last decade has seen the advent of less toxic therapies and the move to a more tailored approach in the ANCA-associated vasculitides (AAV). These advances have improved patient outcomes, especially in the elderly and those with significant comorbidity, although treatment toxicity remains a significant risk (1–3). Since their first description nearly 40 years ago, ANCA have been used to aid initial diagnosis, with limited utility for disease monitoring. Given the continued therapeutic advances over the same period, it is surprising that comparatively little progress has been made in the use of laboratory biomarkers. This is particularly relevant given the role of histopathology to monitor disease activity is restricted by the risks of biopsy, and the accessibility and potential low diagnostic yield contingent on any extrarenal biopsy site (4–8). The use of imaging to monitor disease activity is equally limited. Computerized tomography is commonly used in patients with respiratory tract disease, but the radiologic features described are not specific to vasculitis (4,9,10). The time to interval change and repeated exposure to ionizing radiation further limits the use of such serial imaging for disease monitoring. More modern imaging techniques, such as positron emission tomography, are only validated for large-vessel vasculitis. These limitations are even more relevant in relapsing disease, where clinicians want to avoid both under- or over-treatment, and in distinguishing active AAV from infection. Therefore, a practical and specific biomarker that accurately correlates with systemic-disease activity remains a significant unmet need in the field. Its absence presents a significant challenge to clinicians when gauging the presence of relapsing or persistent disease. This unmet need also contrasts with the move toward less toxic and more individualized options for immunosuppressive therapy. In this primer for treating clinicians, we review currently used biomarkers in AAV and discuss their limitations. We also discuss novel biomarkers, highlight potential avenues for further research and aim to define the ideal biomarker for AAV (Figure 1). In the review that follows, PubMed and Cochrane databases were each searched using the search criteria: “ANCA” OR "anti-neutrophil cytoplasmic antibody" OR “vasculitis” OR “PR3” OR “MPO” OR “ANCA-associated” OR “renal vasculitis” AND “biomarker” OR “marker” OR “activity” OR “relapse,” with further literature searches according to the presented subsections identified. The included articles reported potential biomarker application in AAV after assessment by two authors via a consensus process. Case reports, editorials, letters to the editor, review articles, conference abstracts, and studies not published in English were excluded. Table 1 provides a summary of the current and prospective noninvasive biomarkers in AAV discussed.
Figure 1.
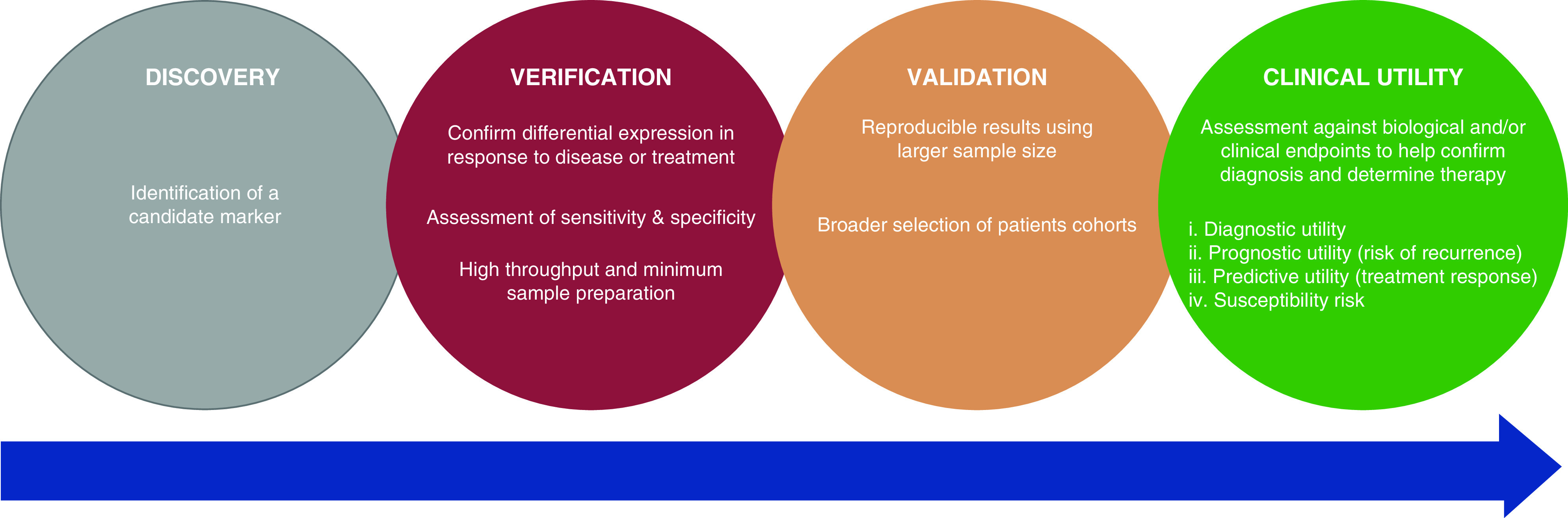
The required stages of biomarker development.
Table 1.
Summary of current and prospective noninvasive biomarkers in ANCA-associated vasculitis
| Biomarker | Source | Organ System | Comment | Reference |
| ANCA | Serum | Multisystem disease | Diagnostic value in the context of clinical symptoms is well established. | |
| Persistent ANCA positivity, ANCA reappearance and the presence of anti–proteinase 3 antibodies are risk factors for relapsing disease. | 18–24 | |||
| Discordance with serology and disease activity, with seronegative disease and positivity in the absence of disease restricting its use as a reliable biomarker. | 26–33,39,41–44 | |||
| Anti-LAMP2 Ab | Serum | Multisystem disease | Initial studies suggested a potential role in pathogenesis and association with disease activity, although these findings were not corroborated in subsequent study. | 35–37 |
| Anti–tissue plasminogen Ab | Serum | Multisystem disease | Associated with ANCA seropositivity and a higher degree of acute inflammatory renal lesions. | 34 |
| Validation studies are required along with determination of its prognostic and predictive utility. | ||||
| CD19+ B-cell population | Serum | Multisystem disease | Conflicting data on the prognostic utility of B-cell reconstitution from follow-up data of several large trials. | 22,24,46,47 |
| Relapsing disease can occur despite peripheral B-cell depletion with B-cells present in tissue sites of active disease. | 48 | |||
| B-cell depletion should not provide reassurance of a reduced relapse risk and repopulation may indicate susceptibility when taken into account with other clinical parameters. | ||||
| Cytokines | Serum | Multisystem disease | CXCL-13, TIMP-1, and MMP-3 each distinguish active disease from remission with a high degree of accuracy. Further validation study is required to assess their use. | 55 |
| Conflicting data exist on the association of BAFF with disease activity. | 51–54 | |||
| Conflicting data exist on the association of Bregs, such as CD5+ B-cells, with disease activity and its prognostic utility. | 49,50 | |||
| T-cells | Serum | Multisystem disease | T-cell activity is associated with disease activity, with elevated levels of IL-2 and CD30; further validation study and assessment of its clinical utility are required. | 54 |
| ESR and acute-phase proteins | Serum | Multisystem disease | ESR and acute-phase proteins—including CRP, calprotectin, hepcidin and procalcitonin—remain nonspecific for active AAV with limited clinical use. | 55–64 |
| N/L and P/L ratio | Plasma | Multisystem disease | Both the N/L and P/L ratio are potential predictors of disease severity, but both require larger prospective study. | 57,65,66 |
| NGAL | Serum | Multisystem disease | Higher levels of NGAL are associated with relapsing disease, but this remains nonspecific and should be cautiously interpreted. | 67 |
| Endothelial cells | Plasma | Multisystem disease | Circulating necrotic endothelial cells offer a direct index of vascular damage, with a high degree of correlation in active AAV, although intensive resource requirements limit its clinical application and possibility for validation study. | 68 |
| Angiopoietin 2 | Plasma | Multisystem disease | Limited ability to distinguish active from quiescent disease or predict relapse. | 69 |
| Complement | Serum | Multisystem disease | Higher plasma concentrations of alternative complement-pathway degradation products in active disease. Prospective study is required with assessment in relapsing disease. | 71–76 |
| Urine | Renal-limited disease | Higher urinary degradation products associated with active renal vasculitis, with urinary Bb inversely correlated with the percentage of normal glomeruli. These results require validation study. | 70 | |
| mRNA | Plasma | Multisystem disease | Autoantigen gene expression is a risk factor for disease through histone depletion, hypomethylation and impaired transcriptional repression. Lower levels of DNMT1 mRNA and subsequent DNA hypomethylation is associated with active disease and a higher risk of relapse. | 88,89 |
| CD8+ T-cell transcriptional profile is predictive of relapsing disease. | 90 | |||
| Further prospective validation studies of gene-expression profiles are required. | ||||
| MCP-1 | Urine | Renal-limited disease | Prospective validation studies have demonstrated a positive association of urinary MCP-1 levels with active renal vasculitis, with a corresponding fall after remission-induction therapy. Evaluation of its clinical utility now required. | 79–82 |
| Soluble CD163 | Urine | Renal-limited disease | Higher urinary levels of soluble CD163 cleaved from macrophages and monocytes conferred a high sensitivity and specificity for active renal vasculitis compared with remission. This correlates with the degree of inflammatory lesions on histopathology in both new and relapsing ANCA-associated GN. Potential elevation can occur in infection with study of its clinical utility required. | 83,84 |
| Metabolomics | Serum | Multisystem disease | Active vasculitis is associated with a distinctive metabolomic profile of raised N-acetyl glycoproteins, LDLs/VLDLs, choline, and glycerophosphocholine; whereas glucose and amino acids were reduced compared with control groups. | 91 |
| Urine | Renal-limited disease | Raised urinary myo-inositol and hypocitraturia is present in active disease, and a ratio of the two closely associated with active renal vasculitis. Validation study and evaluation in relapsing disease required. | 92 | |
| Biospectroscopy | Urine | Renal-limited disease | Biospectroscopy offers a novel and low-cost surrogate technique of determining a sample’s metabolomic profile. One study observed the 1545 cm−1 spectral band increasing in intensity in line with glomerular inflammation and treatment response, whereas 1033 cm−1 was inversely related with the degree of fibrosis. | 101 |
Anti-LAMP2, anti–lysosomal-associated membrane antibody; Ab, antibody; CXCL-13, chemoattractant chemokine (C-X-C motif) ligand 13; TIMP-1, tissue inhibitor of metallopeptidase inhibitor 1; MMP-3, matrix metalloproteinase 3; BAFF, B-cell activating factor; Breg, regulatory B cells; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; N/L, neutrophil/lymphocyte; P/L, platelet/lymphocyte; NGAL, neutrophil gelatinase-associated lipocalin; DNMT1, DNA methyltransferase 1; MCP-1, monocyte chemoattractant protein-1.
ANCA
The diagnostic value of ANCA in the context of clinical symptoms is well established, yet its role in the prediction of relapsing disease remains debatable. Numerous studies have attempted to delineate the role of serial ANCA monitoring with varying results and a lack of consensus on reported outcomes. The subsequent discordance between ANCA serology and disease activity limits any support for its use as a reliable biomarker.
Early retrospective studies supported the relationship between ANCA and disease activity; however, their sensitivity and positive predictive value for relapse remained relatively low at 23%–28% (11–15). An initial systematic review in 2006 attempted to provide more insight, but was unable to undertake a meta-analysis and offer any meaningful conclusion due to the considerable method heterogeneity and suboptimal design of most studies for the assessment of test accuracy (16). A subsequent meta-analysis by Tomasson et al. (17) was more stringent in its study selection, identifying a modest association, at best, for persisting ANCA positivity or rising titers with the risk of relapse. Several studies with more longitudinal data, including follow-up data from large trials, have since corroborated earlier findings that persistent ANCA positivity, ANCA reappearance, and the presence of anti–proteinase 3 (anti-PR3) antibodies are risk factors for relapsing disease (18–24). Similarly, seronegativity after remission-induction therapy is associated with a longer relapse-free survival period (18,19). The positive predictive value of detectable ANCA and disease relapse increases when combined with the clinical index of suspicion for active disease (25).
Ultimately, it is the presence of seronegative disease and positivity in the absence of disease that limits the use of ANCA as a functional biomarker. Despite the compelling in vitro and limited in vivo evidence base for the pathogenicity of ANCA, the reported rate of de novo, seronegative, pauci-immune GN varies from 12% to 30%, with up to 54% of patients with limited extrarenal disease and a significant proportion of those with relapsing disease exhibiting undetectable circulating ANCA (26–33). This prompts reconsideration of the current putative pathogenesis and assays. One possibility is the presence of a novel autoantibody. One candidate is anti–tissue plasminogen autoantibodies, which are thought to be integral to the fibrinolytic system and have been observed in up to 25% of patients with anti-PR3 and anti-myeloperoxidase (anti-MPO) positivity (34). These patients tended to display more severe glomerular inflammation, microthrombi, and increased thrombotic events; however, future study is required to further evaluate its role in disease. Another potential candidate is the anti–lysosomal-associated membrane protein-2 (anti-LAMP2) autoantibody. LAMP2 is a heavily glycosylated membrane protein that plays a key role in cellular homeostasis and is coexpressed on neutrophils with MPO and PR3 as a target of ANCA. Initial studies suggested a high degree of correlation with disease activity and a potential pathogenic role in ANCA-negative disease; however, these findings were not validated on subsequent study (35–37). A second consideration is that anti-PR3 and anti-MPO antibodies may be present, but either remain below the detection limit of current enzyme immunoassays or epitope masking may confound their detection (38,39). In 2013, Roth et al. (39) undertook a study in linear ANCA epitope mapping and disease correlation. In doing so, they identified a pathogenic anti-MPO autoantibody to a new, immunodominant, sole, linear-sequence epitope in seronegative disease—the detection of which is obscured by a fragment of ceruloplasmin in serum on conventional tests (39). It is also possible that disease is mediated by IgA ANCA (40). Current mainstream ELISAs for ANCA detect IgG. Kelley et al. (40) identified the presence of IgA ANCA in a significant proportion of patients who otherwise tested negative for IgG ANCA, and demonstrated the ability of IgA ANCA to mediate disease through neutrophil stimulation.
Lastly, circulating ANCA has been detected in individuals without any known history of disease. Two case-control studies confirmed positive ANCA serology from biobank samples of asymptomatic individuals up to 19 years before disease onset (41,42). In this context, it is possible that these individuals might have been lacking the “second hit” required for disease at the time of sample collection. Similarly, anti-PR3 and anti-MPO positivity have been detected in healthy individuals and with other diagnoses, including inflammatory bowel disease, liver disease, rheumatic disease, and infection, such as tuberculosis (39,43,44). The presence of anti-MPO ANCA in healthy individuals may represent differing epitope specificity (39).
B-Cell Population and Cytokines
Data from observational studies suggest that incomplete B-cell depletion and B-cell repopulation after rituximab treatment is associated with a significantly higher relapse rate (18,45). Supporting this, follow-up data from RITUXVAS observed that B-cell repopulation accompanied all cases of relapsing disease (46). However, the trial was not powered to draw any significant conclusion from this subgroup and follow-up data from several other larger trials did not corroborate this finding. Among the RAVE cohort, B-cell population did not predict relapse with disease occuring despite undetectable CD19+ B-cells in the vast majority of relapsing cases and the presence of B-cell detectability in quiescent disease (22). Similarly, data from both the MAINRITSAN and MAINRITSAN 2 trials found that CD19+ B-cell reconstitution was not predictive of relapse (24,47). Confirmation of the tenuous association of B-cell population with disease activity comes from a case report by Ferraro et al. (48) that demonstrated relapsing disease with B-cells present in tissue sites of active disease, despite peripheral depletion. As such, B-cell depletion should not provide reassurance of a reduced relapse risk and repopulation may indicate susceptibility when taken into account with other clinical parameters.
B-cell subset populations that have drawn interest include regulatory B-cells (Bregs), such as CD5+ cells. The CD5 protein attenuates activating signals from the B-cell receptor, downregulating B-cell activity. Measurement of Bregs showed initial promise with a lower CD5+ B-cell count correlating with active disease, whereas maintaining a normal count conferred a longer relapse-free survival period (49). Data from the RAVE study observed similar findings, but subsequent analysis found that serial CD5+ B-cell count was not predictive of disease relapse, severity, or treatment failure (50).
Key cytokines may offer another predictive tool. Elevated levels of B-cell activating factor (BAFF) have been found in active disease, with a corresponding fall post-treatment (51,52). However, the few studies evaluating its predictive value found no association with disease activity and a potential inverse correlation with ANCA titer, bringing into question its role in autoantibody production (53,54). Indicators of T-cell activation have also been observed, with elevated levels of soluble IL-2 receptors and CD30 in active disease, but further study is required to elicit their potential biomarker role (54). A panel of chemokines and circulating proteins have been prospectively evaluated from patients enrolled in the RAVE trial at presentation and 6 months postremission. This identified three candidate markers: the B-lymphocyte chemoattractant chemokine (C-X-C motif) ligand 13, matrix metalloproteinase 3 (MMP-3) and tissue inhibitor of metallopeptidase inhibitor 1 (TIMP-1). These distinguished active disease from remission with an area under the curve of >0.8 and likelihood ratio of 4.3–4.6, warranting future assessment (55).
Inflammatory Markers
Traditional inflammatory markers, such as erythrocyte sedimentation rate and C-reactive protein, are nonspecific for AAV with limited clinical use (55–57). A large, cross-sectional study of the Birmingham Vasculitis Activity Score 3 (BVAS) provided confirmation of this, demonstrating a poor correlation between BVAS and C-reactive protein, with a limited role for such inflammatory markers in assessing disease activity (58). Other inflammatory markers—such as calprotectin, hepcidin, and procalcitonin—ave also been evaluated, but face the same limitation (57,59–64).
The function of activated platelets in disease propagation has drawn attention to their level as a potential gauge of disease activity. Willeke et al. (57) observed significantly higher counts in active AAV, although there was an irregularity in their findings with relatively lower levels in more severe disease. Park et al. (65) evaluated the platelet/lymphocyte ratio, identifying a value >272 as an independent predictor of severe disease; however, confounding factors could not be accounted for. Similarly, Ahn et al. (66) observed that patients exhibiting a neutrophil/lymphocyte ratio >5.9 at diagnosis tended to present with more severe disease and have a higher frequency of future relapse. Application of the neutrophil/lymphocyte ratio is needed in larger prospective studies to determine its reliability.
Neutrophil gelatinase-associated lipocalin (NGAL) provides a marker of neutrophil degranulation, with significantly higher levels found at the time of diagnosis and in relapsing AAV (67). NGAL has also been extensively investigated as an early predictor of AKI and as with the other inflammatory markers discussed, if used it should be interpreted alongside an array of other clinical parameters to help inform an assessment of disease activity.
More direct indices of vascular damage have been investigated. Analysis of circulating necrotic endothelial cells yielded promising results, with higher levels in ANCA-associated GN compared with remission and control groups, although its intensive resource requirements may have restricted clinical application (68). Investigation of angiopoietin 2 was of limited clinical utility, failing to discriminate disease activity after clinically successful treatment or to predict relapses (69).
Complement
Alternative complement-pathway activation is fundamental for the development of disease and urinary degradation products provide a potential biomarker of renal vasculitis. Gou et al. (70) found that urinary levels of Bb, C3a, C5a, and soluble C5b-9 were significantly higher in active disease, in addition to Bb—in effect—providing a surrogate marker of renal histopathology with inverse correlation with the percentage of normal glomeruli. The same group subsequently demonstrated that these degradation products were also significantly higher in plasma among patients with active multisystem disease (71). Several retrospective studies have since analyzed circulating levels of C3 and their relation to patient outcomes. A low level is present in up to 35% of patients at the time of initial diagnosis, with a higher likelihood of more severe disease and poorer renal function at presentation (72,73). Prognostically, this has been associated with a poorer renal and patient survival (74–76). Circulating markers of alternative complement-pathway activation holds promise, but studies assessing their use in relapsing disease are lacking and their role as a functional biomarker of disease activity requires further study.
In 2015, Chen et al. (77) concluded that plasma levels of complement factor H (CFH), a negative regulator of the alternative pathway, were lower in patients with active disease and were inversely correlated with renal function, renal inflammation, and BVAS. An in vitro study by the same group supported the hypothesis that higher CFH inhibited ANCA-induced neutrophil activation with reduced functional activity in patients with active disease (78). This raises the question of whether a subgroup of patients has a predisposition to disease due to an absolute or functional deficiency of CFH. Measurement of circulating CFH may help identify those patients who may be more susceptible to disease and subsequent future potential relapse.
Urinary Proteins and Chemokines
Elevated levels of urinary monocyte chemoattractant protein-1 (MCP-1) have been found among patients with active or persistent renal vasculitis, correlating with upregulated macrophage infiltration in severely inflamed glomeruli and a corresponding fall in urinary MCP-1 after successful treatment (79–82). CD163 is expressed on monocytes and macrophages, functioning as a scavenger receptor for the hemoglobin-haptoglobin complex. It also provides a surrogate marker of cell activity, with cleavage to soluble CD163 (sCD163) in a proinflammatory state. In a rodent model of disease, O’Reilly et al. (83) detected higher levels from urine in small-vessel vasculitis compared with other glomerular pathologies. Subsequent human study with an external validation cohort confirmed noticeably higher urinary levels in active disease (likelihood ratio, 20.8) (83). Evaluation of urinary sCD163 with serial renal biopsy specimen data has since demonstrated a high degree of correlation with fibrinoid necrosis and cellular crescents in those with both de novo and relapsing ANCA-associated GN compared with remission and healthy controls (84). This also lends support to the position that serial comparative analysis of non-invasive biomarkers and histopathology in renal vasculitis is potentially feasible and, arguably, needed as the ideal reference standard when determining their clinical utility in predicting outcomes and disease recurrence (85,86).
Moran et al. (87) combined urinary MCP-1 in patients positive for urinary sCD163, with a 98% specificity and positive likelihood ratio of 19.2 for relapsing disease in the presence of new-onset proteinuria, subject to pretest probability. Both urinary proteins offer a promising non-invasive candidate biomarker that could be translated into clinical practice, although their use is limited to renal vasculitis with potential elevation in the context of infection.
mRNA
Variation in autoantigen gene expression has been confirmed as a risk factor for disease through histone depletion, hypomethylation, and impaired transcriptional repression due to reduced RUNX3 at the MPO and PRTN3 gene loci. Jones et al. (88) confirmed this link by investigating the DNA methyltransferase 1 (DNMT1) gene expression required for DNA methylation and downregulation of autoantigen expression. In doing so, they found the degree of DNMT1 mRNA positively correlated with DNA methylation and negatively correlated with PRTN3 and MPO gene expression (88). As such, a reduction in DNMT1 mRNA and DNA hypomethylation was associated with active disease and predicted a higher risk of relapse (hazard ratio, 4.55; 95% CI, 2.09 to 9.91), whereas patients exhibiting increased DNA methylation at the PRTN3 promoter in remission had a greater likelihood of a longer relapse-free survival period (88). Contrary to this, Kurz et al. (89) concluded that elevated leukocyte PR3 mRNA was not predictive of relapsing disease, although this may reflect the transrepressive effect of concurrent glucocorticoid therapy.
In 2010, McKinney et al. (90) quantified the gene-expression profiles from purified leukocytes among patients with active AAV to prospectively predict future relapse risk. This identified that transcriptional profiling of CD8+ T-cells with overexpression of mRNA encoding proteins for the IL-7 receptor pathway, T-cell receptor signaling, and expanded CD8+ T-cell memory population conferred a poorer prognosis (90). This finding has the potential for translation to clinical practice and requires validation in a prospective study with longitudinal data.
Metabolomics
Metabolomics enables the quantitative analysis of the substrates and products of metabolism to directly reflect the biochemical activity within a sample. Variation in the metabolomic profile will be reflective of changes in the underlying biochemical composition caused by physiologic processes or pathologic states. Studies applying metabolomics in AAV are limited. In 2016, Al-Ani et al. (91) analyzed the urinary metabolomic profile in a rodent model of disease using nuclear magnetic resonance spectroscopy and chemometric analysis. This identified a distinctive metabolomic profile in active disease, which resolved after successful treatment, with subsequent recurrence in relapsing disease. A large patient-cohort study by the same group yielded similar results (91). Gupta et al. (92) has since evaluated metabolomics in serum, identifying a profile that was specific to active AAV with good separation from control groups, including Takyasu arteritis and SLE. The role of metabolomics as a robust and relatively non-invasive biomarker of disease activity in AAV merits further study, but its associated costs may limit its potential application.
Biospectroscopy
Biospectroscopy provides a novel and low-cost surrogate technique of determining the metabolomic profile of a sample through one of two primary techniques: attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy and Raman spectroscopy (93,94). Irrespective of the modality used, biochemical changes caused by disease will result in a unique spectral fingerprint that is representative of the underlying pathophysiologic state. Advancements in instrumentation and standardized chemometric analysis have enabled the successful application of biospectroscopy across numerous areas of medicine, including rheumatic disease, lymphocyte subsets, cytokine monitoring, and nephrology (95–100). Its application in vasculitis is emerging, with one previous study using ATR-FTIR to identify potential urinary biomarkers in an animal model of crescentic GN and in patients with ANCA-associated GN (101). This identified the 1545 cm−1 spectral marker as a key wave-number variable, increasing in intensity in line with the degree of glomerular injury and subsiding after treatment. In parallel, the intensity of 1033 cm−1 was inversely related with the degree of fibrosis. These findings suggest that ATR-FTIR could be used as a fast, innovative method of monitoring disease progression and treatment response in renal vasculitis. The promising use of biospectroscopy to provide a robust biomarker of disease activity in AAV, which can be readily translated to clinical practice requires further study.
Conclusions
The remarkable progress in treatment strategies over the past 3 decades has been accompanied by a rising disease prevalence. Yet a reliable biomarker to detect relapsing or persistent disease is lacking, risking increasing morbidity and mortality from suboptimal disease control or unnecessary patient exposure to potentially harmful therapy. As such, there is a need for the development of a functional biomarker to enable risk stratification and individualization of treatment. Alongside the use of more innovative analytic tools, an improved understanding of the underlying immunopathogenesis and treatment targets has led to the identification of several promising novel candidate markers for renal-limited and multisystem disease. With favorable initial results, further validation studies with longitudinal data are required to elicit their potential role and translation into clinical practice.
Disclosures
F.L. Martin reports having ownership interest in Biocel UK Ltd. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
The authors would like to acknowledge the support of the Renal Department at Royal Preston Hospital Lancashire National Health Service Foundation Trust, and the team at the National Institute for Health Research Lancashire Clinical Research Facility in undertaking this work.
Author Contributions
A.P. Dhaygude, F.L. Martin, A.D. Morris, A.W. Rowbottom, and A. Woywodt reviewed and edited the manuscript; A.P. Dhaygude, F.L. Martin, and A.W. Rowbottom provided supervision; and A.D. Morris conceptualized the study, was responsible for data curation, and wrote the original draft.
References
- 1.Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, Jayne D, Harper L; European Vasculitis Study (EUVAS) Group: Early mortality in systemic vasculitis: Relative contribution of adverse events and active vasculitis. Ann Rheum Dis 69: 1036–1043, 2010. 10.1136/ard.2009.109389 [DOI] [PubMed] [Google Scholar]
- 2.Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, Jayne D, Mahr A, Westman K, Luqmani R: Damage in the ANCA-associated vasculitides: Long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis 74: 177–184, 2015. 10.1136/annrheumdis-2013-203927 [DOI] [PubMed] [Google Scholar]
- 3.Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, Jayne D, Mahr A, Westman K, Luqmani R: Glucocorticoid treatment and damage in the anti-neutrophil cytoplasm antibody-associated vasculitides: Long-term data from the European Vasculitis Study Group trials. Rheumatology (Oxford) 54: 471–481, 2015. 10.1093/rheumatology/keu366 [DOI] [PubMed] [Google Scholar]
- 4.Gómez-Gómez A, Martínez-Martínez MU, Cuevas-Orta E, Bernal-Blanco JM, Cervantes-Ramírez D, Martínez-Martínez R, Abud-Mendoza C: Pulmonary manifestations of granulomatosis with polyangiitis. Reumatol Clin 10: 288–293, 2014. 10.1016/j.reumae.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 5.Schnabel A, Holl-Ulrich K, Dalhoff K, Reuter M, Gross WL: Efficacy of transbronchial biopsy in pulmonary vaculitides. Eur Respir J 10: 2738–2743, 1997. 10.1183/09031936.97.10122738 [DOI] [PubMed] [Google Scholar]
- 6.Diamantopoulos II, Jones NS: The investigation of nasal septal perforations and ulcers. J Laryngol Otol 115: 541–544, 2001. 10.1258/0022215011908441 [DOI] [PubMed] [Google Scholar]
- 7.Murray A, McGarry GW: The clinical value of septal perforation biopsy. Clin Otolaryngol Allied Sci 25: 107–109, 2000. 10.1046/j.1365-2273.2000.00332.x [DOI] [PubMed] [Google Scholar]
- 8.Bischof A, Jaeger VK, Hadden RDM, Luqmani RA, Pröbstel AK, Merkel PA, Suppiah R, Craven A, Collins MP, Daikeler T: Peripheral neuropathy in antineutrophil cytoplasmic antibody-associated vasculitides: Insights from the DCVAS study. Neurol Neuroimmunol Neuroinflamm 6: e615, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo JB, Im JG, Chung JW, Song JW, Goo JM, Park JH, Yeon KM: Pulmonary vasculitis: The spectrum of radiological findings. Br J Radiol 73: 1224–1231, 2000. 10.1259/bjr.73.875.11144805 [DOI] [PubMed] [Google Scholar]
- 10.D’Anza B, Langford CA, Sindwani R: Sinonasal imaging findings in granulomatosis with polyangiitis (Wegener granulomatosis): A systematic review. Am J Rhinol Allergy 31: 16–21, 2017. 10.2500/ajra.2017.31.4408 [DOI] [PubMed] [Google Scholar]
- 11.De’Oliviera J, Gaskin G, Dash A, Rees AJ, Pusey CD: Relationship between disease activity and anti-neutrophil cytoplasmic antibody concentration in long-term management of systemic vasculitis. Am J Kidney Dis 25: 380–389, 1995. 10.1016/0272-6386(95)90098-5 [DOI] [PubMed] [Google Scholar]
- 12.Davenport A, Lock RJ, Wallington T: Clinical significance of the serial measurement of autoantibodies to neutrophil cytoplasm using a standard indirect immunofluorescence test. Am J Nephrol 15: 201–207, 1995. 10.1159/000168833 [DOI] [PubMed] [Google Scholar]
- 13.Kyndt X, Reumaux D, Bridoux F, Tribout B, Bataille P, Hachulla E, Hatron PY, Duthilleul P, Vanhille P: Serial measurements of antineutrophil cytoplasmic autoantibodies in patients with systemic vasculitis. Am J Med 106: 527–533, 1999. 10.1016/S0002-9343(99)00064-9 [DOI] [PubMed] [Google Scholar]
- 14.Girard T, Mahr A, Noël LH, Cordier JF, Lesavre P, André MH, Guillevin L: Are antineutrophil cytoplasmic antibodies a marker predictive of relapse in Wegener’s granulomatosis? A prospective study. Rheumatology (Oxford) 40: 147–151, 2001. 10.1093/rheumatology/40.2.147 [DOI] [PubMed] [Google Scholar]
- 15.Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, Nachman PH: Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 143: 621–631, 2005. 10.7326/0003-4819-143-9-200511010-00005 [DOI] [PubMed] [Google Scholar]
- 16.Birck R, Schmitt WH, Kaelsch IA, van der Woude FJ: Serial ANCA determinations for monitoring disease activity in patients with ANCA-associated vasculitis: Systematic review. Am J Kidney Dis 47: 15–23, 2006. 10.1053/j.ajkd.2005.09.022 [DOI] [PubMed] [Google Scholar]
- 17.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA: Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis--a meta-analysis. Rheumatology (Oxford) 51: 100–109, 2012. 10.1093/rheumatology/ker280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dam LS, Dirikgil E, Bredewold EW, Ray A, Bakker JA, van Kooten C, Rabelink TJ, Teng YKO: Proteinase-3-anti-neutrophil cytoplasmic antibodies (PR3-ANCAs) predict relapses in ANCA-associated vasculitis patients after rituximab [published online ahead of print June 30, 2020]. Nephrol Dial Transplant 10.1093/ndt/gfaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClure ME, Wason J, Gopaluni S, Tieu J, Smith RM, Jayne DR, Jones RB: Evaluation of PR3-ANCA status after rituximab for ANCA-associated vasculitis. J Clin Rheumatol 25: 217–223, 2019. 10.1097/RHU.0000000000001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thai LH, Charles P, Resche-Rigon M, Desseaux K, Guillevin L: Are anti-proteinase-3 ANCA a useful marker of granulomatosis with polyangiitis (Wegener’s) relapses? Results of a retrospective study on 126 patients. Autoimmun Rev 13: 313–318, 2014. 10.1016/j.autrev.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Fijolek J, Wiatr E, Petroniec V, Augustynowicz-Kopec E, Bednarek M, Gawryluk D, Roszkowski-Sliz K: Antineutrophil cytoplasmic antibodies and their relationship with disease activity and presence of staphylococcal superantigens in nasal swabs in patients having granulomatosis with polyangiitis: results of a study involving 115 patients from a single center. Clin Rheumatol 38: 3297–3305, 2019. 10.1007/s10067-019-04693-0 [DOI] [PubMed] [Google Scholar]
- 22.Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Tchao NK, Viviano L, Ding L, Sejismundo LP, Mieras K, Iklé D, Jepson B, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh K, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Stone JH; Rituximab in ANCA-Associated Vasculitis-Immune Tolerance Network Research Group: Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 65: 2441–2449, 2013. 10.1002/art.38044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrier B, Pagnoux C, Perrodeau É, Karras A, Khouatra C, Aumaître O, Cohen P, Decaux O, Desmurs-Clavel H, Maurier F, Gobert P, Quémeneur T, Blanchard-Delaunay C, Bonnotte B, Carron PL, Daugas E, Ducret M, Godmer P, Hamidou M, Lidove O, Limal N, Puéchal X, Mouthon L, Ravaud P, Guillevin L; French Vasculitis Study Group: Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis 77: 1150–1156, 2018. 10.1136/annrheumdis-2017-212768 [DOI] [PubMed] [Google Scholar]
- 24.Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, Maurier F, Decaux O, Ninet J, Gobert P, Quémeneur T, Blanchard-Delaunay C, Godmer P, Puéchal X, Carron PL, Hatron PY, Limal N, Hamidou M, Ducret M, Daugas E, Papo T, Bonnotte B, Mahr A, Ravaud P, Mouthon L; French Vasculitis Study Group: Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 371: 1771–1780, 2014. 10.1056/NEJMoa1404231 [DOI] [PubMed] [Google Scholar]
- 25.Verstockt B, Bossuyt X, Vanderschueren S, Blockmans D: There is no benefit in routinely monitoring ANCA titres in patients with granulomatosis with polyangiitis. Clin Exp Rheumatol 33[Suppl 89]: S-72–S-76, 2015 [PubMed] [Google Scholar]
- 26.Hedger N, Stevens J, Drey N, Walker S, Roderick P: Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: a 10-year retrospective study. Nephrol Dial Transplant 15: 1593–1599, 2000. 10.1093/ndt/15.10.1593 [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Yu F, Wang SX, Zou WZ, Zhao MH, Wang HY: Antineutrophil cytoplasmic autoantibody-negative Pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol 18: 599–605, 2007. 10.1681/ASN.2006091021 [DOI] [PubMed] [Google Scholar]
- 28.Kim HW, Kim JW, Im CH, Shin KC, Lee EY, Lee EB, Song YW: The clinicopathologic characteristics of granulomatosis with polyangiitis (Wegener’s): A retrospective study of 45 patients in Korea. Mod Rheumatol 23: 864–871, 2013. 10.3109/s10165-012-0754-2 [DOI] [PubMed] [Google Scholar]
- 29.Lee SW, Yu MY, Baek SH, Ahn SY, Kim S, Na KY, Chae DW, Chin HJ: Long-term prognosis of anti-neutrophil cytoplasmic antibody-negative renal vasculitis: Cohort study in Korea. J Korean Med Sci 31: 542–546, 2016. 10.3346/jkms.2016.31.4.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Córdova-Sánchez BM, Mejía-Vilet JM, Morales-Buenrostro LE, Loyola-Rodríguez G, Uribe-Uribe NO, Correa-Rotter R: Clinical presentation and outcome prediction of clinical, serological, and histopathological classification schemes in ANCA-associated vasculitis with renal involvement. Clin Rheumatol 35: 1805–1816, 2016. 10.1007/s10067-016-3195-z [DOI] [PubMed] [Google Scholar]
- 31.Holle JU, Gross WL, Holl-Ulrich K, Ambrosch P, Noelle B, Both M, Csernok E, Moosig F, Schinke S, Reinhold-Keller E: Prospective long-term follow-up of patients with localised Wegener’s granulomatosis: Does it occur as persistent disease stage? Ann Rheum Dis 69: 1934–1939, 2010. 10.1136/ard.2010.130203 [DOI] [PubMed] [Google Scholar]
- 32.Morgan MD, Szeto M, Walsh M, Jayne D, Westman K, Rasmussen N, Hiemstra TF, Flossmann O, Berden A, Höglund P, Harper L; European Vasculitis Society: Negative anti-neutrophil cytoplasm antibody at switch to maintenance therapy is associated with a reduced risk of relapse. Arthritis Res Ther 19: 129, 2017. 10.1186/s13075-017-1321-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith RM, Jones RB, Guerry MJ, Laurino S, Catapano F, Chaudhry A, Smith KG, Jayne DR: Rituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 64: 3760–3769, 2012. 10.1002/art.34583 [DOI] [PubMed] [Google Scholar]
- 34.Berden AE, Nolan SL, Morris HL, Bertina RM, Erasmus DD, Hagen EC, Hayes DP, van Tilburg NH, Bruijn JA, Savage CO, Bajema IM, Hewins P: Anti-plasminogen antibodies compromise fibrinolysis and associate with renal histology in ANCA-associated vasculitis. J Am Soc Nephrol 21: 2169–2179, 2010. 10.1681/ASN.2010030274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kain R, Tadema H, McKinney EF, Benharkou A, Brandes R, Peschel A, Hubert V, Feenstra T, Sengölge G, Stegeman C, Heeringa P, Lyons PA, Smith KG, Kallenberg C, Rees AJ: High prevalence of autoantibodies to hLAMP-2 in anti-neutrophil cytoplasmic antibody-associated vasculitis. J Am Soc Nephrol 23: 556–566, 2012. 10.1681/ASN.2011090920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peschel A, Basu N, Benharkou A, Brandes R, Brown M, Rees AJ, Kain R: Autoantibodies to hLAMP-2 in ANCA-negative pauci-immune focal necrotizing GN. J Am Soc Nephrol 25: 455–463, 2014. 10.1681/ASN.2013030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth AJ, Brown MC, Smith RN, Badhwar AK, Parente O, Chung H, Bunch DO, McGregor JG, Hogan SL, Hu Y, Yang JJ, Berg EA, Niles J, Jennette JC, Preston GA, Falk RJ: Anti-LAMP-2 antibodies are not prevalent in patients with antineutrophil cytoplasmic autoantibody glomerulonephritis. J Am Soc Nephrol 23: 545–555, 2012. 10.1681/ASN.2011030273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tateyama K, Kodama S, Kishibe K, Harabuchi Y, Suzuki M: A novel strategy with combined assays for detection of anti-neutrophil cytoplasmic antibody (ANCA) in clinically ANCA-negative granulomatosis with polyangiitis patients. Auris Nasus Larynx 44: 735–741, 2017. 10.1016/j.anl.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 39.Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, McGregor J, Burkart M, Hogan SL, Hu Y, Winnik W, Nachman PH, Stegeman CA, Niles J, Heeringa P, Kitching AR, Holdsworth S, Jennette JC, Preston GA, Falk RJ: Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 123: 1773–1783, 2013. 10.1172/JCI65292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelley JM, Monach PA, Ji C, Zhou Y, Wu J, Tanaka S, Mahr AD, Johnson S, McAlear C, Cuthbertson D, Carette S, Davis JC Jr, Dellaripa PF, Hoffman GS, Khalidi N, Langford CA, Seo P, St Clair EW, Specks U, Stone JH, Spiera RF, Ytterberg SR, Merkel PA, Edberg JC, Kimberly RP: IgA and IgG antineutrophil cytoplasmic antibody engagement of Fc receptor genetic variants influences granulomatosis with polyangiitis. Proc Natl Acad Sci U S A 108: 20736–20741, 2011. 10.1073/pnas.1109227109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson SW, Arbogast CB, Baker TP, Owshalimpur D, Oliver DK, Abbott KC, Yuan CM: Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J Am Soc Nephrol 22: 1946–1952, 2011. 10.1681/ASN.2010090928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson SW, Owshalimpur D, Yuan CM, Arbogast C, Baker TP, Oliver D, Abbott KC: Relation between asymptomatic proteinase 3 antibodies and future granulomatosis with polyangiitis. Clin J Am Soc Nephrol 8: 1312–1318, 2013. 10.2215/CJN.10411012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houben E, Bax WA, van Dam B, Slieker WAT, Verhave G, Frerichs FCP, van Eijk IC, Boersma WG, de Kuyper GTM, Penne EL: Diagnosing ANCA-associated vasculitis in ANCA positive patients: A retrospective analysis on the role of clinical symptoms and the ANCA titre. Medicine (Baltimore) 95: e5096, 2016. 10.1097/MD.0000000000005096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flores-Suárez LF, Cabiedes J, Villa AR, van der Woude FJ, Alcocer-Varela J: Prevalence of antineutrophil cytoplasmic autoantibodies in patients with tuberculosis. Rheumatology (Oxford) 42: 223–229, 2003. 10.1093/rheumatology/keg066 [DOI] [PubMed] [Google Scholar]
- 45.Cartin-Ceba R, Golbin JM, Keogh KA, Peikert T, Sánchez-Menéndez M, Ytterberg SR, Fervenza FC, Specks U: Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): Ten-year experience at a single center. Arthritis Rheum 64: 3770–3778, 2012. 10.1002/art.34584 [DOI] [PubMed] [Google Scholar]
- 46.Jones RB, Furuta S, Tervaert JWC, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, Walsh M, Westman K, Jayne DR; European Vasculitis Society (EUVAS): Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis 74: 1178–1182, 2015. 10.1136/annrheumdis-2014-206404 [DOI] [PubMed] [Google Scholar]
- 47.Charles P, Terrier B, Perrodeau É, Cohen P, Faguer S, Huart A, Hamidou M, Agard C, Bonnotte B, Samson M, Karras A, Jourde-Chiche N, Lifermann F, Gobert P, Hanrotel-Saliou C, Godmer P, Martin-Silva N, Pugnet G, Matignon M, Aumaitre O, Viallard JF, Maurier F, Meaux-Ruault N, Rivière S, Sibilia J, Puéchal X, Ravaud P, Mouthon L, Guillevin L; French Vasculitis Study Group: Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: Results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2) [published correction appears in Ann Rheum Dis 78: e101, 2019 10.1136/annrheumdis-2017-212878corr1]. Ann Rheum Dis 77: 1143–1149, 2018. 10.1136/annrheumdis-2017-212878 [DOI] [PubMed] [Google Scholar]
- 48.Ferraro AJ, Smith SW, Neil D, Savage COS: Relapsed Wegener’s granulomatosis after rituximab therapy--B cells are present in new pathological lesions despite persistent ‘depletion’ of peripheral blood. Nephrol Dial Transplant 23: 3030–3032, 2008. 10.1093/ndt/gfn318 [DOI] [PubMed] [Google Scholar]
- 49.Bunch DOD, McGregor JG, Khandoobhai NB, Aybar LT, Burkart ME, Hu Y, Hogan SL, Poulton CJ, Berg EA, Falk RJ, Nachman PH: Decreased CD5+ B cells in active ANCA vasculitis and relapse after rituximab. Clin J Am Soc Nephrol 8: 382–391, 2013. 10.2215/CJN.03950412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unizony S, Lim N, Phippard DJ, Carey VJ, Eli M, Tchao NK, Iklé D, Asare AL, Merkel PA, Monach PA, Seo P, St Clair EW, Langford CA, Spiera R, Hoffman GS, Kallenberg CGM, Specks U, Stone JH: Peripheral CD5+ B cells in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 67: 535–544, 2015. 10.1002/art.38916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krumbholz M, Specks U, Wick M, Kalled SL, Jenne D, Meinl E: BAFF is elevated in serum of patients with Wegener’s granulomatosis. J Autoimmun 25: 298–302, 2005. 10.1016/j.jaut.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 52.Nagai M, Hirayama K, Ebihara I, Shimohata H, Kobayashi M, Koyama A: Serum levels of BAFF and APRIL in myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis: association with disease activity. Nephron Clin Pract 118: c339–c345, 2011. 10.1159/000323393 [DOI] [PubMed] [Google Scholar]
- 53.Bader L, Koldingsnes W, Nossent J: B-lymphocyte activating factor levels are increased in patients with Wegener’s granulomatosis and inversely correlated with ANCA titer. Clin Rheumatol 29: 1031–1035, 2010. 10.1007/s10067-010-1526-z [DOI] [PubMed] [Google Scholar]
- 54.Sanders JSF, Huitma MG, Kallenberg CGM, Stegeman CA: Plasma levels of soluble interleukin 2 receptor, soluble CD30, interleukin 10 and B cell activator of the tumour necrosis factor family during follow-up in vasculitis associated with proteinase 3-antineutrophil cytoplasmic antibodies: Associations with disease activity and relapse. Ann Rheum Dis 65: 1484–1489, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monach PA, Warner RL, Tomasson G, Specks U, Stone JH, Ding L, Fervenza FC, Fessler BJ, Hoffman GS, Iklé D, Kallenberg CG, Krischer J, Langford CA, Mueller M, Seo P, St Clair EW, Spiera R, Tchao N, Ytterberg SR, Johnson KJ, Merkel PA: Serum proteins reflecting inflammation, injury and repair as biomarkers of disease activity in ANCA-associated vasculitis. Ann Rheum Dis 72: 1342–1350, 2013. 10.1136/annrheumdis-2012-201981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang H, Xin M, Zhao L, Wang L, Sun M, Wang J: Serum creatinine level and ESR values associated to clinical pathology types and prognosis of patients with renal injury caused by ANCA-associated vasculitis. Exp Ther Med 14: 6059–6063, 2017. 10.3892/etm.2017.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willeke P, Kümpers P, Schlüter B, Limani A, Becker H, Schotte H: Platelet counts as a biomarker in ANCA-associated vasculitis. Scand J Rheumatol 44: 302–308, 2015. 10.3109/03009742.2015.1006247 [DOI] [PubMed] [Google Scholar]
- 58.Suppiah R, Mukhtyar C, Flossmann O, Alberici F, Baslund B, Batra R, Brown D, Holle J, Hruskova Z, Jayne DR, Judge A, Little MA, Palmisano A, Stegeman C, Tesar V, Vaglio A, Westman K, Luqmani R: A cross-sectional study of the Birmingham Vasculitis Activity Score version 3 in systemic vasculitis. Rheumatology (Oxford) 50: 899–905, 2011. 10.1093/rheumatology/keq400 [DOI] [PubMed] [Google Scholar]
- 59.Přikryl P, Hrušková Z, Konopásek P, Hladinová Z, Tesař V, Vokurka M: Serum hepcidin is increased in ANCA-associated vasculitis and correlates with activity markers. Physiol Res 67: 945–954, 2018. 10.33549/physiolres.933765 [DOI] [PubMed] [Google Scholar]
- 60.Martinez Valenzuela L, Draibe J, Quero Ramos M, Fulladosa Oliveras X, Melilli E, Cruzado Garrit JM, Torras Ambrós J: Calprotectin as a smoldering activity detection tool and renal prognosis biomarker in ANCA associated vasculitis. PLoS One 13: e0205982, 2018. 10.1371/journal.pone.0205982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zycinska K, Wardyn KA, Zielonka TM, Tyszko P, Straburzynski M: Procalcitonin as an indicator of systemic response to infection in active pulmonary Wegener’s granulomacytosis. J Physiol Pharmacol 59[Suppl 6]: 839–844, 2008 [PubMed] [Google Scholar]
- 62.Brunkhorst R, Eberhardt OK, Haubitz M, Brunkhorst FM: Procalcitonin for discrimination between activity of systemic autoimmune disease and systemic bacterial infection. Intensive Care Med 26[Suppl 2]: S199–S201, 2000. 10.1007/s001340051144 [DOI] [PubMed] [Google Scholar]
- 63.Herrmann K, Schinke S, Csernok E, Moosig F, Holle JU: Diagnostic value of procalcitonin in ANCA-Associated Vasculitis (AAV) to differentiate between disease activity, infection and drug hypersensitivity. Open Rheumatol J 9: 71–76, 2015. 10.2174/1874312901409010071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eberhard OK, Haubitz M, Brunkhorst FM, Kliem V, Koch KM, Brunkhorst R: Usefulness of procalcitonin for differentiation between activity of systemic autoimmune disease (systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody-associated vasculitis) and invasive bacterial infection. Arthritis Rheum 40: 1250–1256, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Park HJ, Jung SM, Song JJ, Park YB, Lee SW: Platelet to lymphocyte ratio is associated with the current activity of ANCA-associated vasculitis at diagnosis: A retrospective monocentric study. Rheumatol Int 38: 1865–1871, 2018. 10.1007/s00296-018-4125-y [DOI] [PubMed] [Google Scholar]
- 66.Ahn SS, Jung SM, Song JJ, Park YB, Lee SW: Neutrophil to lymphocyte ratio at diagnosis can estimate vasculitis activity and poor prognosis in patients with ANCA-associated vasculitis: A retrospective study. BMC Nephrol 19: 187, 2018. 10.1186/s12882-018-0992-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen M, Wang F, Zhao MH: Circulating neutrophil gelatinase-associated lipocalin: a useful biomarker for assessing disease activity of ANCA-associated vasculitis. Rheumatology (Oxford) 48: 355–358, 2009. 10.1093/rheumatology/ken500 [DOI] [PubMed] [Google Scholar]
- 68.Woywodt A, Streiber F, de Groot K, Regelsberger H, Haller H, Haubitz M: Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet 361: 206–210, 2003. 10.1016/S0140-6736(03)12269-6 [DOI] [PubMed] [Google Scholar]
- 69.Monach PA, Kümpers P, Lukasz A, Tomasson G, Specks U, Stone JH, Cuthbertson D, Krischer J, Carette S, Ding L, Hoffman GS, Iklé D, Kallenberg CG, Khalidi NA, Langford CA, Seo P, St Clair EW, Spiera R, Tchao N, Ytterberg SR, Haubitz M, Merkel PA: Circulating angiopoietin-2 as a biomarker in ANCA-associated vasculitis. PLoS One 7: e30197, 2012. 10.1371/journal.pone.0030197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gou SJ, Yuan J, Wang C, Zhao MH, Chen M: Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol 8: 1884–1891, 2013. 10.2215/CJN.02790313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gou SJ, Yuan J, Chen M, Yu F, Zhao MH: Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 83: 129–137, 2013. 10.1038/ki.2012.313 [DOI] [PubMed] [Google Scholar]
- 72.Choi H, Kim Y, Jung SM, Song JJ, Park YB, Lee SW: Low serum complement 3 level is associated with severe ANCA-associated vasculitis at diagnosis. Clin Exp Nephrol 23: 223–230, 2019. 10.1007/s10157-018-1634-7 [DOI] [PubMed] [Google Scholar]
- 73.Manenti L, Vaglio A, Gnappi E, Maggiore U, Allegri L, Allinovi M, Urban ML, Delsante M, Galetti M, Nicastro M, Pilato FP, Buzio C: Association of serum C3 concentration and histologic signs of thrombotic microangiopathy with outcomes among patients with ANCA-associated renal vasculitis. Clin J Am Soc Nephrol 10: 2143–2151, 2015. 10.2215/CJN.00120115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Augusto JF, Langs V, Demiselle J, Lavigne C, Brilland B, Duveau A, Poli C, Chevailler A, Croue A, Tollis F, Sayegh J, Subra JF: Low serum complement C3 levels at diagnosis of renal ANCA-associated vasculitis is associated with poor prognosis. PLoS One 11: e0158871, 2016. 10.1371/journal.pone.0158871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villacorta J, Diaz-Crespo F, Acevedo M, Cavero T, Guerrero C, Praga M, Fernandez-Juarez G: Circulating C3 levels predict renal and global outcome in patients with renal vasculitis. Clin Rheumatol 35: 2733–2740, 2016. 10.1007/s10067-016-3384-9 [DOI] [PubMed] [Google Scholar]
- 76.Crnogorac M, Horvatic I, Kacinari P, Ljubanovic DG, Galesic K: Serum C3 complement levels in ANCA associated vasculitis at diagnosis is a predictor of patient and renal outcome. J Nephrol 31: 257–262, 2018. 10.1007/s40620-017-0445-3 [DOI] [PubMed] [Google Scholar]
- 77.Chen SF, Wang FM, Li ZY, Yu F, Zhao MH, Chen M: Plasma complement factor H is associated with disease activity of patients with ANCA-associated vasculitis [published correction appears in Arthritis Res Ther 19: 82, 2017 10.1186/s13075-017-1298-9]. Arthritis Res Ther 17: 129, 2015. 10.1186/s13075-015-0656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen SF, Wang FM, Li ZY, Yu F, Chen M, Zhao MH: Complement factor H inhibits anti-neutrophil cytoplasmic autoantibody-induced neutrophil activation by interacting with neutrophils. Front Immunol 9: 559, 2018. 10.3389/fimmu.2018.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tam FWK, Sanders JS, George A, Hammad T, Miller C, Dougan T, Cook HT, Kallenberg CG, Gaskin G, Levy JB, Pusey CD: Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant 19: 2761–2768, 2004. 10.1093/ndt/gfh487 [DOI] [PubMed] [Google Scholar]
- 80.Ohlsson S, Bakoush O, Tencer J, Torffvit O, Segelmark M: Monocyte chemoattractant protein 1 is a prognostic marker in ANCA-associated small vessel vasculitis. Mediators Inflamm 2009: 584916, 2009. 10.1155/2009/584916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lieberthal JG, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, Koening CL, Langford CA, Maksimowicz-McKinnon K, Seo P, Specks U, Ytterberg SR, Merkel PA, Monach PA; Vasculitis Clinical Research Consortium: Urinary biomarkers in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol 40: 674–683, 2013. 10.3899/jrheum.120879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jönsson N, Erlandsson E, Gunnarsson L, Pettersson Å, Ohlsson S: Monocyte chemoattractant protein-1 in antineutrophil cytoplasmic autoantibody-associated vasculitis: Biomarker potential and association with polymorphisms in the MCP-1 and the CC chemokine receptor-2 gene. Mediators Inflamm 2018: 6861257, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Reilly VP, Wong L, Kennedy C, Elliot LA, O’Meachair S, Coughlan AM, O’Brien EC, Ryan MM, Sandoval D, Connolly E, Dekkema GJ, Lau J, Abdulahad WH, Sanders JS, Heeringa P, Buckley C, O’Brien C, Finn S, Cohen CD, Lindemeyer MT, Hickey FB, O’Hara PV, Feighery C, Moran SM, Mellotte G, Clarkson MR, Dorman AJ, Murray PT, Little MA: Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 27: 2906–2916, 2016. 10.1681/ASN.2015050511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aendekerk JP, Timmermans SAMEG, Busch MH, Potjewijd J, Heeringa P, Damoiseaux JGMC, Reutelingsperger CP, van Paassen P; Limburg Renal Registry: Urinary soluble CD163 and disease activity in biopsy-proven ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol 15: 1740–1748, 2020. 10.2215/CJN.07210520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hruskova Z, Honsova E, Berden AE, Rychlik I, Lanska V, Zabka J, Bajema IM, Tesar V: Repeat protocol renal biopsy in ANCA-associated renal vasculitis. Nephrol Dial Transplant 29: 1728–1732, 2014. 10.1093/ndt/gfu042 [DOI] [PubMed] [Google Scholar]
- 86.Hauer HA, Bajema IM, Hagen EC, Noël LH, Ferrario F, Waldherr R, van Houwelingen HC, Lesavre P, Sinico RA, van der Woude F, Gaskin G, Verburgh CA, de Heer E, Bruijn JA: Long-term renal injury in ANCA-associated vasculitis: An analysis of 31 patients with follow-up biopsies. Nephrol Dial Transplant 17: 587–596, 2002. 10.1093/ndt/17.4.587 [DOI] [PubMed] [Google Scholar]
- 87.Moran SM, Monach PA, Zgaga L, Cuthbertson D, Carette S, Khalidi NA, Koening CL, Langford CA, McAlear CA, Moreland L, Pagnoux C, Seo P, Specks U, Sreih A, Wyse J, Ytterberg SR, Merkel PA, Little MA; Vasculitis Clinical Research Consortium: Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 35: 283–291, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones BE, Yang J, Muthigi A, Hogan SL, Hu Y, Starmer J, Henderson CD, Poulton CJ, Brant EJ, Pendergraft WF 3rd, Jennette JC, Falk RJ, Ciavatta DJ: Gene-specific DNA methylation changes predict remission in patients with ANCA-associated vasculitis. J Am Soc Nephrol 28: 1175–1187, 2017. 10.1681/ASN.2016050548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurz T, Weiner M, Skoglund C, Basnet S, Eriksson P, Segelmark M: A myelopoiesis gene signature during remission in anti-neutrophil cytoplasm antibody-associated vasculitis does not predict relapses but seems to reflect ongoing prednisolone therapy. Clin Exp Immunol 175: 215–226, 2014. 10.1111/cei.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McKinney EF, Lyons PA, Carr EJ, Hollis JL, Jayne DRW, Willcocks LC, Koukoulaki M, Brazma A, Jovanovic V, Kemeny DM, Pollard AJ, Macary PA, Chaudhry AN, Smith KG: A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med 16: 586–591, 1p following 591, 2010. 10.1038/nm.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Ani B, Fitzpatrick M, Al-Nuaimi H, Coughlan AM, Hickey FB, Pusey CD, Savage C, Benton CM, O’Brien EC, O’Toole D, Mok KH, Young SP, Little MA: Changes in urinary metabolomic profile during relapsing renal vasculitis. Sci Rep 6: 38074, 2016. 10.1038/srep38074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gupta L, Jain A, Ekbote GG, Mishra R, Kumar D, Gulerla A, Raj R, Kumar U: NMR based serum metabolomics revealed distinctive metabolic patterns of ANCA- associated vasculitis. Int J Rheum Dis 22[S3]: 40–226, 2019 [Google Scholar]
- 93.Trevisan J, Angelov PP, Carmichael PL, Scott AD, Martin FL: Extracting biological information with computational analysis of Fourier-transform infrared (FTIR) biospectroscopy datasets: Current practices to future perspectives. Analyst (Lond) 137: 3202–3215, 2012. 10.1039/c2an16300d [DOI] [PubMed] [Google Scholar]
- 94.Morais CLM, Lima KMG, Singh M, Martin FL: Tutorial: Multivariate classification for vibrational spectroscopy in biological samples. Nat Protoc 15: 2143–2162, 2020. 10.1038/s41596-020-0322-8 [DOI] [PubMed] [Google Scholar]
- 95.Shaw RA, Kotowich S, Eysel HH, Jackson M, Thomson GTD, Mantsch HH: Arthritis diagnosis based upon the near-infrared spectrum of synovial fluid. Rheumatol Int 15: 159–165, 1995. 10.1007/BF00301774 [DOI] [PubMed] [Google Scholar]
- 96.Chen M, McReynolds N, Campbell EC, Mazilu M, Barbosa J, Dholakia K, Powis SJ: The use of wavelength modulated Raman spectroscopy in label-free identification of T lymphocyte subsets, natural killer cells and dendritic cells. PLoS One 10: e0125158, 2015. 10.1371/journal.pone.0125158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamińska A, Winkler K, Kowalska A, Witkowska E, Szymborski T, Janeczek A, Waluk J: SERS-based immunoassay in a microfluidic system for the multiplexed recognition of interleukins from blood plasma: towards picogram detection. Sci Rep 7: 10656, 2017. 10.1038/s41598-017-11152-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carvalho CS, Martin AA, Santo AME, Andrade LEC, Pinheiro MM, Cardoso MAG, et al. : A rheumatoid arthritis study using Raman spectroscopy. Theor Chem Acc 130: 1211–1220, 2011. 10.1007/s00214-011-0905-0 [DOI] [Google Scholar]
- 99.Prentice BM, Caprioli RM, Vuiblet V: Label-free molecular imaging of the kidney. Kidney Int 92: 580–598, 2017. 10.1016/j.kint.2017.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varma VK, Kajdacsy-Balla A, Akkina SK, Setty S, Walsh MJ: A label-free approach by infrared spectroscopic imaging for interrogating the biochemistry of diabetic nephropathy progression. Kidney Int 89: 1153–1159, 2016. 10.1016/j.kint.2015.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu MC, Rich P, Foreman L, Smith J, Yu MS, Tanna A, Dibbur V, Unwin R, Tam FWK: Label free detection of sensitive mid-infrared biomarkers of glomerulonephritis in urine using Fourier transform infrared spectroscopy. Sci Rep 7: 4601, 2017. 10.1038/s41598-017-04774-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


