Abstract
Sphingolipids are now considered not only as constitutional components of the cellular membrane but also as essential bioactive factors regulating development and physiologic functions. Ceramide is a vital intermediate of sphingolipid metabolism, synthesized by de novo and salvage pathways, producing multiple types of sphingolipids and their metabolites. Although mutations in gene-encoding enzymes regulating sphingolipid synthesis and metabolism cause distinct diseases, an abnormal sphingolipid metabolism contributes to various pathologic conditions, including kidney diseases. Excessive accumulation of glycosphingolipids and promotion of the ceramide salvage and sphingosine-1-phosphate (S1P) pathways are found in the damaged kidney. Acceleration of the sphingosine kinase/S1P/S1P receptor (SphK/S1P/S1PR) axis plays a central role in deteriorating kidney functions. The SphK/S1P/S1PR signaling impairment is also found during pregnancy complications, such as preeclampsia and intrauterine growth restriction (IUGR). This mini-review discusses the current state of knowledge regarding the role of sphingolipid metabolism on kidney diseases, and the possible involvement of preeclampsia and IUGR conditions.
Keywords: chronic kidney disease, basic science, fetal growth retardation, intrauterine growth restriction, IUGR, kidney diseases, preeclampsia, small for gestational age infant, sphingolipids
Introduction
Over several decades, studies on bioactive lipids have provided information concerning their cellular functions beyond their structural and energy-storage roles. Among a large number of bioactive lipids, sphingolipids and their metabolites have been the focus of attention (1,2). Sphingolipids are commonly distributed in the cell membrane of the eukaryotic organism and contain an 18-carbon amino alcohol, called the sphingoid base, with a fatty-acid tail or headgroup attached to the base (3,4). Sphingosine is a major sphingoid base in mammals and is often converted to a central intermediate of sphingolipid metabolism, ceramide (5). Ceramide is ester bonded with phosphatidylcholine and becomes sphingomyelin, which comprises 5%–10% of the total mammalian cell phospholipids (6). The addition of various hydrophilic headgroups to ceramide ultimately produces more complex sphingolipids. Sphingolipid variations diversify their biologic functions, and sphingolipids and their metabolites are now known to be involved in cellular signaling, regulating cell survival, growth, proliferation, differentiation, and cellular responses to inflammation by acting as cell-signaling mediators. Their mechanisms include acting as second messengers of intracellular signaling, supporting lipid-raft composition, linking transmembrane domains of the signaling protein, creating mitochondrial membrane pores, and regulating enzymatic activation as cofactors (1,7–9). Conversely, dysregulation of sphingolipid metabolism results in pathologic states, such as cancer, neurologic disease, osteoporosis, diabetes, and atherosclerosis (2,10–13).
Morbidity and mortality resulting from CKD are increasing worldwide. Abnormal lipid metabolism, including dyslipidemia and excessive accumulation of sphingolipids, has been reported to play a critical role in the pathogenesis and progression of CKD (3,14,15). Previous studies suggest a positive correlation between the onset of adult CKD and a prior history of intrauterine growth restriction (IUGR) induced by pregnancy complications (16–18). Moreover, precise sphingolipid metabolism is needed to maintain normal pregnancy. This review summarizes the pathophysiologic roles of sphingolipid metabolism in CKD and its possible role in preeclampsia and IUGR.
Sphingolipid Metabolism
De Novo Synthesis
Sphingolipid de novo synthesis occurs on the cytoplasmic side of the endoplasmic reticulum through condensation of l-serine with palmitoyl CoA (Figure 1) (19,20). The de novo sphingolipid biosynthesis is normally activated by metabolic overload of serine and/or palmitoyl CoA and by a stress stimulus, such as heat, oxidation, chemotherapeutics, cannabinoids, and TNF (21). After biosynthesis of 3-ketosphingosine and dihydrosphingosine (sphinganine), ceramide, a precursor and central molecule in sphingolipid biosynthesis, is formed by desaturation of dihydroceramide at carbon 4–carbon 5 of the sphingoid base (22).
Figure 1.
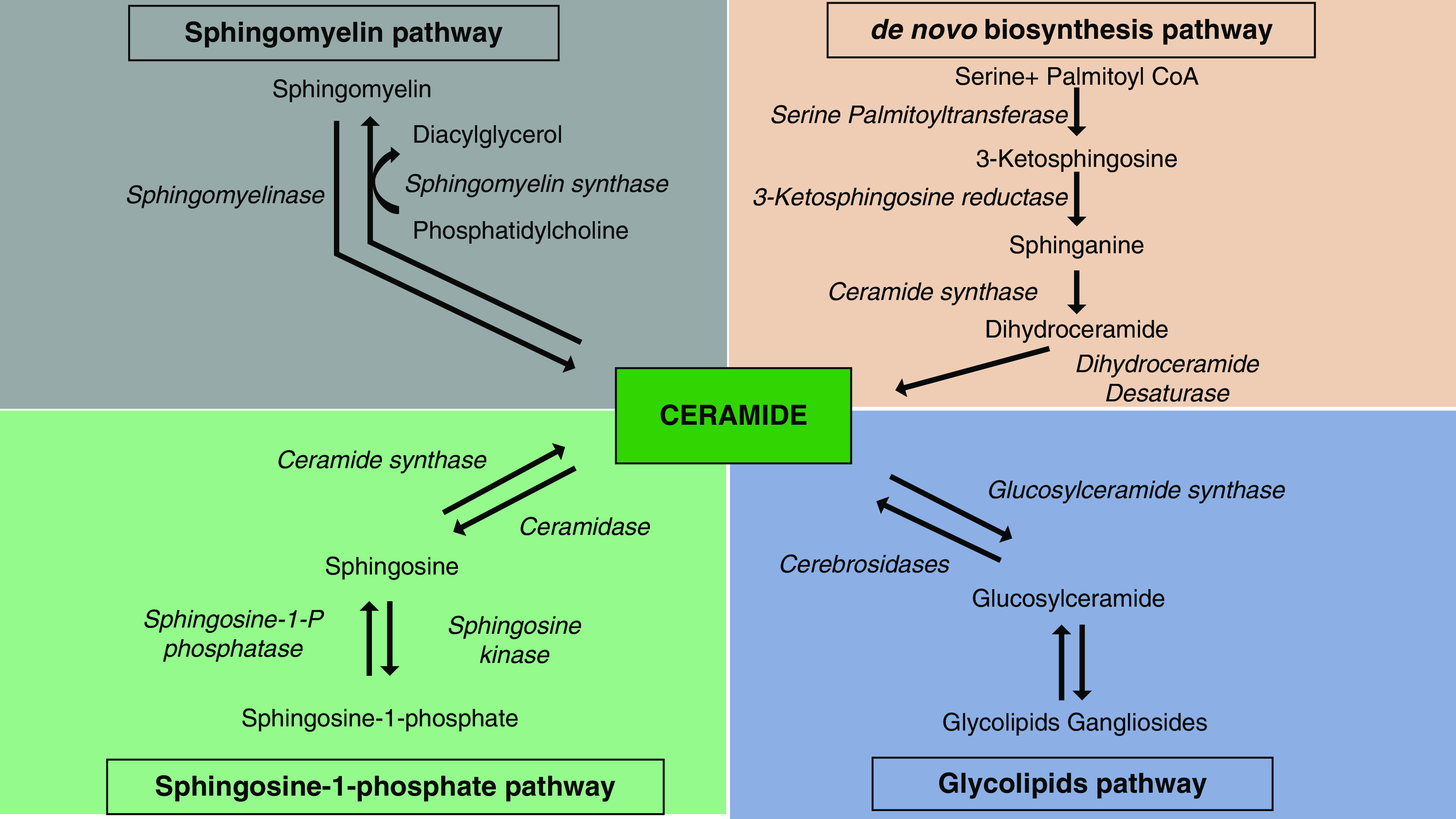
Sphingolopids are synthesized both by de novo and salvage pathways, producing various bioactive metabolites. De novo biosynthesis pathway: After condensation, serine palmitoyl CoA transferase (SPT) catalyzes the production of 3-ketodihydrosphingosine, which is a rate-limiting step in sphingolipid metabolism (114). SPT belongs to the α-axoamine synthase family and requires pyridoxal 5′ phosphate as a cofactor (115). The 3-ketodihydrosphingosine reductase converts 3-ketodihydrosphingosine to sphinganine by reducing the keto group. Sphinganine is then amino-acylated in microsomes by one of the six isoforms of dihydroceramide synthase to generate different species of dihydroceramide, depending on the length of the fatty acyl added (8,116). Sphingomyelin pathway: Sphingomyelin is formed by the addition of phosphocholine to the C1 hydroxyl group of ceramides by sphingomyelin synthase in the Golgi apparatus (117). The phosphocholine comes from phosphatidylcholine, and diacylglycerol is released from the reaction. Glycolipids pathway: Glycosphingolipids contain sugar residues attached to the C1 hydroxyl group of the sphingoid base. Another salvage pathway to produce ceramide is mediated by sphingomyelinase, which degrades sphingomyelin (sphingomyelin pathway). Sphingosine-1-phosphate pathway: Ceramide biosynthesizes sphingosine-1-phosphate by sphingosine kinase–mediated phosphorylation.
In mammalian cells, ceramide is then converted into sphingomyelin or glucosylceramide, and glucosylceramide further changes into more complex glycosphingolipids. Major glycosphingolipids are glucosphingolipids and galactosphingolipids, which are attached with glucose and galactose, respectively. Glycosphingolipids are the largest subclass of sphingolipids and are often distributed in lipid rafts; they are present in the plasma membrane’s outer leaflet and, when needed, they play roles in regulating interactions between cells and protein activation (23,24). Gangliosides are a minor class of glycosphingolipids containing complex, attached sugar chains and are essential components of plasma membranes. In some instances, glycosphingolipids may contain complex sugar chains such as N-acetylgalactosamine and N-glycolylneuraminic acid (8,25). Gangliosides with one or more N-acetylneuraminic acid linkages are labeled GM1, GM2, and GM3 (one N-acetylneuraminic acid), or GD1a, GD1b, GD2, GD3, GT1b, and GQ1 (more than one N-acetylneuraminic acid) (26).
Salvage Pathway
Ceramide is also synthesized by a salvage pathway (27). In the presence of stress stimuli, sphingomyelin is broken down to ceramide and phosphocholine. This reaction is mediated by sphingomyelinase (28,29). Undesired, complex sphingolipids—such as glycosphingolipids—may be broken down in the acidic environment of lysosomes or late endosomes to ceramide (28). Complex glycosphingolipids are degraded through sequential hydrolysis of terminal hydrophilic moieties by hydrolases, where glucose or galactose is removed by β-glucosidases or galactosidase, respectively, to produce ceramide.
Ceramide is hydrolyzed by ceramidase to a sphingosine base and free fatty acid. These two products leave the lysosomes or endosomes to become recycled substrates of ceramide biosynthesis (29). Alternatively, biosynthesized sphingosine may be phosphorylated by sphingosine kinase (SphK) to form sphingosine-1-phosphate (S1P), an essential cellular signaling molecule (30). S1P can be degraded to 2-trans-hexadecenal and phosphoethanolamine by S1P lyase (31).
SphK/S1P/S1P Receptor Axis in the Kidney
Two SphK isoforms were identified: SphK1 and SphK2. These isoforms are present in the cytosol and intracellular compartments, respectively (32). SphK1 is reported to exert antiapoptotic functions in renal mesangial cells by increasing S1P levels, whereas cultured mesangial cells isolated from SphK2-knockout mice are resistant to apoptosis (33–35). In the human proximal tubular HK-2 cell line, SphK1 overexpression protects against peroxidase-induced necrosis by increasing S1P content (36).
S1P can act as an extracellular ligand for cell-membrane receptors and intracellular signaling molecules (37). Five S1P receptors (S1PRs; S1PR1–S1PR5) were identified, S1PR1–S1PR3 are detected in renal medulla and glomeruli (38,39). The protein levels of S1PR1 and S1PR2, but not S1PR3–S1PR5, are abundantly expressed in rat preglomerular microvessels (40). On the other hand, whole mouse kidneys express S1PR1–S1PR4 mRNA (but not S1PR5 mRNA) with a rank order of S1PR1>S1PR3>S1PR2>S1PR4, whereas cultured mesangial cells express all five receptors (41,42).
Sphingolipids and Kidney Diseases
Dyslipidemia, which involves high levels of LDL cholesterol and triglycerides in addition to low levels of HDL cholesterol, is a major risk factor for atherosclerotic diseases, including CKD. Alternatively, hypoalbuminemia, resulting from proteinuria and a decline in renal function, may induce the accumulation of atherogenic, triglyceride-rich lipoproteins. Recent studies have shown lipid-induced oxidation and inflammation in the kidney, so-called lipotoxicity (43).
Some genetic disorders involving disruption of sphingolipid metabolism exhibit renal damage, indicating the kidney is sensitive to sphingolipid alterations. Fabry disease is caused by α-galactosidase A mutations, which result in deficient activity of a lysosomal hydrolase and excessive accumulation of globotriaosylceramide (Gb3) in cells throughout the body, particularly cells in the kidney, heart, nervous and gastrointestinal systems, and vasculature in the skin (44). The resulting phenotype may include fatal, progressive kidney damage in hemizygous males, whereas, in some individuals, milder symptoms often appear later in life. Although the precise mechanism has not been clarified, the potential action of Gb3 has been investigated in podocytes, which accumulate more Gb3 than other renal cell types. Knocking down the α-galactosidase A gene, by RNA interference and lentiviral-transduction techniques, upregulates LC3-II and downregulates the activity of the mammalian target of rapamycin kinase in podocytes, indicating dysregulation of autophagy (45,46). The deacetylated bioactive form of Gb3 activates the NOTCH signaling pathway, leading to a proinflammatory response, dedifferentiation, and extracellular matrix accumulation via NF-κB translocation (47).
Accumulation of sphingolipids contributes to renal disorders. Although normal glomeruli express gangliosides abundantly, renal levels of glycosphingolipids (such as glucosylceramide, lactosylceramide, and ganglioside GM3) are elevated in patients with diabetic nephropathy (48–53), polycystic kidney disease, renal cell carcinoma, lupus nephritis, age-related decreased kidney function, and their experimental models (54–57). Conversely, in a model of minimal change disease induced by puromycin aminonucleoside, the amount of ganglioside GD3 and O-acetyl GD3 decreased in a time-dependent manner with the progression of proteinuria (58). Because sialoglycoproteins contribute to the glomerular filtration barrier by retaining the negative charge, decreases in gangliosides may alter glomerular permeability.
Diabetic nephropathy is characterized by albuminuria, glomerular and tubulointerstitial fibrosis, and glomerulosclerosis; this condition is a leading cause of ESKD. In patients with diabetes, high plasma levels of sphingolipids, including glycosphingolipids, ceramide, sphingosine, and sphinganine, have been observed (3,59–61). Inhibition of the formation of glucosylceramide suppresses pathologic changes in diabetic rat kidneys, suggesting a pathogenic role of glycosphingolipid (53). Sphingomyelinase phosphodiesterase acid-like 3b (SMPDL3b) in the membrane lipid raft activates the conversion of sphingomyelin to ceramide and phosphorylcholine, purportedly by modulating acid sphingomyelinase. Glomerular expression of this enzyme is enhanced in both human and mouse diabetic nephropathy. In db/db diabetic mice, ceramide levels in the renal cortex are decreased, whereas glomerular mesangial and tubular levels of sphingosine and S1P are enhanced (62–64). Increased SMPDL3b action may induce the production of other ceramide metabolites, such as glycosphingolipids and S1P, by promoting sphingomyelin conversion to ceramide. This hypothesis is also supported by AKI studies, demonstrating that sphingomyelinase activity and ceramide content increase in proportion to the extent of injury to proximal tubule cells (65–67). Moreover, a selective S1PR1 agonist, SEW2871, attenuates proteinuria in early-stage diabetic nephropathy in rats, suggesting a beneficial property of S1PR1 stimulation (68). In the pathogenesis of type 2 diabetes mellitus, ceramide is reported to participate in islet β-cell dysfunction and apoptosis (69). This report suggests that abnormal sphingolipid metabolism deteriorates diabetic nephropathy by direct effects on the kidney and glucose intolerance. Obesity plays an essential role in the onset and progression of type 2 diabetes mellitus by releasing pathogenic adipocytokines, including inflammatory cytokines. Treatment with long-chain saturated free fatty acids promotes ceramide and diacylglycerol accumulation and blocks insulin signaling in C2C12 myotubes (70). Inhibition of de novo sphingolipid synthesis suppresses inflammatory cytokine release from murine 3T3-L1 cells (71).
Unlike the diabetic kidney, renal SMPDL3b levels are low in patients with FSGS. This suggests that the accumulation of sphingomyelin may participate in FSGS pathogenesis. Fornoni et al. (72) found that serum from patients with FSGS has decreased acid-sphingomyelinase activity and SMPDL3b levels, and FSGS is associated with increases in actin cytoskeletal remodeling and apoptosis in podocytes. FSGS is the most common cause of nephrotic syndrome and results in progressive renal dysfunction (73). Soluble urokinase plasminogen activator receptor (suPAR), which is elevated in serum from patients with FSGS, activates αVβ3 integrin in podocytes, leading to a migratory phenotype. Serum suPAR levels are also elevated in patients with diabetic nephropathy. In podocytes treated with serum from patients with diabetic nephropathy, SMPDL3b interacts with suPAR to cause the podocytes to change from a migratory to an apoptotic phenotype through increasing RhoA activity (74). Taken together, regulation of sphingolipid metabolism could be a therapeutic target for glomerular diseases.
Inflammation induces the production and release of fibrogenic cytokines and growth factors, leading to fibrosis, which results in irreversible kidney dysfunction (75). S1P has been considered to play an important role in both inflammation and fibrosis. S1P, synthesized by SphK in the cytosol, is transported to the extracellular space by transporters, including ATP-binding cassette transporters. Exported S1P can bind to G protein–coupled receptors (S1PRs) on the plasma membrane, in an autocrine fashion, or to different cell types. S1P released from glomerular mesangial cells can bind to S1PR2 and S1PR3 in the fibroblasts, activating the TGF-β1/Smad pathway and triggering fibrogenic action and SphK1 production (76). Extracellular S1P can bind to S1PRs on immune cells, such as macrophages and lymphocytes, leading to inflammation. This vicious cycle is called the SphK1/S1P/S1PRs axis (15).
Furthermore, overexpression of SphK1 and S1P is found in the diabetic kidney and high glucose–treated mesangial cells (76). The activity of SphK and S1P levels are increased in isolated glomeruli of diabetic rats (77). In the pathogenesis of diabetic nephropathy, differentiation of tubular epithelial cells and fibroblasts to myofibroblasts is thought to be mediated by the SphK1/S1P/S1PRs axis, presumably via S1PR2 and subsequent Rho-kinase activation (63,76,78,79). SphK2-knockout mice exhibit less renal fibrosis than wild-type and SphK1-knockout mice 14 days after AKI induced by folic acid or unilateral ischemia reperfusion (80). Likewise, S1PR3 inhibition suppresses collagen deposition, myofibroblast differentiation, proteinuria, and leukocyte infiltration in the model of ureteral obstruction (81). FTY720, an immunosuppressive S1PR ligand that functions as an S1PR antagonist, prevents inflammatory alterations in ureteral-obstruction and angiotensin-II treatment models (82,83). However, the S1P effects are diverse, depending on receptor subtypes and pathologic conditions (84,85). For instance, the SphK1/S1P/S1PR1 axis in endothelial cells and proximal tubular cells plays important roles in protecting against renal ischemia-reperfusion injury (36,86,87). Bajwa et al. (88) demonstrated the therapeutic effects of the transfer of S1PR3-deficient, bone marrow–derived dendritic cells in renal ischemia-reperfusion injury through the expansion of splenic CD4(+)Foxp3(+) regulatory T cells.
Possible Role of Sphingolipids in Preeclampsia and IUGR
Preeclampsia is a maternal, gestational disease characterized by kidney dysfunction (involving proteinuria and hypertension after 20 weeks of gestation) and is a major cause of maternal and fetal morbidity and mortality, including IUGR. The origin of the preeclampsia pathology is believed to be in the placenta, although preeclampsia shows a high degree of heterogeneity in clinical features. Abnormal placentation (characterized by insufficient cytotrophoblast invasion of spinal arteries) and abnormal remodeling of decidual vessels limit placental perfusion, leading to release of placental factors into the maternal circulation, including soluble fms-like tyrosine kinase 1 (89–93). The soluble fms-like tyrosine kinase is believed to inhibit vasodilation and induce maternal hypertension by antagonizing the action of vascular endothelial cell growth factor to produce nitric oxide (94).
In placentas from pregnancy complicated by IUGR without preeclampsia, low ceramide and high sphingosine levels, compared with age-matched controls, are observed (95). Contrary to what is observed in IUGR pregnancy, acid ceramidase expression/activity and ceramide content are reported to increase in preeclampsia. In conjunction with the increase in de novo synthesis, ceramide overload causes excessive autophagy in cultured human trophoblast cells and in pregnant murine placentas, and necroptosis in human choriocarcinoma JEG3 cells, primary isolated cytotrophoblasts, and in human preeclamptic placentas (95–97). Progressive trophoblast cell death is a common feature of IUGR pregnancy, with or without preeclampsia. Furthermore, differences in sphingolipid metabolism may depend on trophoblast phenotypes, which are reported to be different between IUGR and preeclampsia (98). The serine palmitoyltransferase activity and the expression of sphingosine and sphingomyelin are high in chorionic arteries of the human preeclamptic placenta (99). In placental arterial endothelial cells, S1P content is reduced by heightened S1P phosphatase and lyase activity. Moreover, the equilibrium shift of S1PR expression/activity toward S1PR2 and lower S1PR1 promotes endothelial dysfunction in preeclampsia (99). The SphK/S1P/S1PR1 axis plays a crucial role in placental angiogenesis and endothelial-barrier function during pregnancy through downstream signaling of extracellular signal-regulated protein kinases 1/2 and phospholipase C (100,101). FTY720 (nonspecific agonist except for S1PR2) has been found to decrease the expression of vascular endothelial cell growth factor in human decidual natural killer (NK) cells, and to inhibit the migration and angiogenesis of decidual NK cell–mediated extravillous trophoblasts in vitro. Because S1PR5 is expressed predominantly in decidual NK cells, S1P signaling via S1PR5 may play an essential role in the angiogenic function of decidual NK cells and trophoblast migration during pregnancy (102).
The role of sphingolipids in hypertension and kidney diseases in the offspring of patients with IUGR has not been investigated, despite strong evidence that IUGR is known to increase the risk of adult cardiovascular and renal diseases. IUGR often results from placental insufficiency and is related to an increase in perinatal morbidity and mortality. IUGR is commonly defined as a fetal gap—the inability to reach growth potential which is associated with a birth weight less than the tenth percentile of the average gestational age (103,104). Offspring of patients with IUGR have been shown to have low nephron numbers (105), which causes a reduction in filtration surface area, leading to systemic hypertension and progressive renal insufficiency; sequelae becomes even more severe with confounding factors such as excess dietary sodium. Clinical and animal data regarding the maturation of renal function in offspring resulting from IUGR show birth weight is positively associated with GFR and negatively associated with BP and serum creatinine (106,107). These data suggest that offspring of those with IUGR are at risk of developing hypertension and renal failure. The long-term effects of S1P on BP may involve S1P-induced modulation of renal blood flow and renal sodium handling. S1P mediates natriuresis via the activation of S1PR1 in the renal medulla of rats (108,109). s1p1 is a candidate gene that determines the response to salt in spontaneously hypertensive, stroke-prone rats (110). Given the essential role of sphingolipids on kidney function and control of BP, which are impaired in IUGR, it is possible that the sphingolipid pathway may play a role in the pathophysiology of IUGR and requires further investigation.
Summary
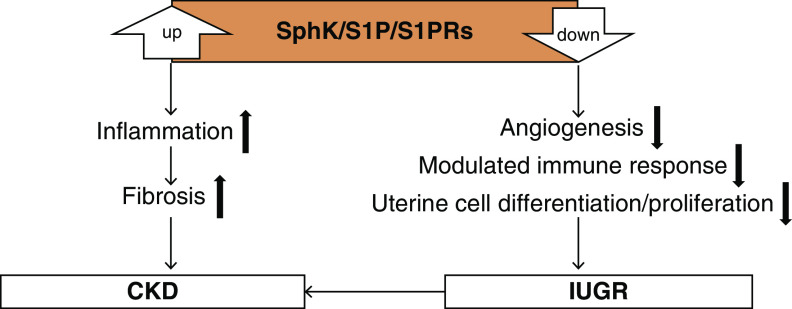
Sphingolipids are synthesized and metabolized by multiple pathways. Abnormal sphingolipid metabolism is implicated in various diseases, especially those involving the kidney. S1P is the most active sphingolipid metabolite and causes kidney inflammation and fibrosis through the SphK1/S1P/S1PRs axis. On the other hand, in fetal development, the SphK/S1P/S1PR1 axis mediates angiogenesis, suppresses the immune response to the embryo, and is involved in uterine cell differentiation and proliferation, which are essential processes required to maintain proper placenta functions. Hence, inadequate S1P action causes IUGR, increasing the risk of adult-onset diseases—such as obesity, diabetes, hypertension, and cardiovascular and kidney diseases—and resulting in a high susceptibility to kidney injury (Figure 2) (111,112). It is possible that abnormal sphingolipid metabolism may be a result of alterations caused by these diseases. However, studies using pharmacologic inhibition and gene-deletion techniques raise the notion that sphingolipid metabolism may be one of the pivotal causes of kidney diseases. Controlling sphingolipid metabolism from the fetal period into adulthood determines our lifelong fate, including that of our kidney function. The effects of S1P on the endothelial barrier and sensitivity to vasoconstrictors also depend on its concentration (113). Further studies to determine the extent and timing of SphK/S1P/S1PRs inhibition/activation and the conditions that regulate the effects of S1P will provide novel therapeutic targets against kidney disease, in general, and specifically against kidney disease induced by preeclampsia and IUGR.
Figure 2.
Roles of the SphK/S1P/S1PRs axis in CKD and IUGR. Acceleration of the SphK/S1P/S1PRs axis may induce inflammation and fibrosis, leading to CKD. On the other hand, the physiologically controlled SphK/S1P/S1PRs axis may be essential in maintaining normal pregnancy through angiogenesis, suppression of the immune response to embryos, and uterine cell differentiation and proliferation. IUGR, intrauterine growth restriction; S1P, sphingosine-1-phosphate; S1PRs, S1P receptors; SphK, sphingosine kinase.
Disclosures
All authors have nothing to disclose.
Funding
This work from S. Intapad was supported by the American Society of Nephrology Foundation for Kidney Research Norman Siegel Research Scholar grant, American Heart Association grant 16SDG27770041, National Institute of General Medical Sciences grant P20GM109036, and Tulane University start-up funding. B. Bhunu was supported by the Fulbright Association Junior Staff Development Program fellowship awarded by the United States embassy in Zimbabwe. This work is also supported by the Hoansha Foundation Grant-in-Aid for Scientific Research (to H. Toba).
Acknowledgments
The authors acknowledge Mrs. Nancy Busija for critically reading and editing the manuscript.
Author Contributions
B. Bhunu, S. Intapad, and H. Toba were responsible for funding acquisition and validation; B. Bhunu, S. Intapad, H. Toba, and R. Yokota conceptualized the study, wrote the original draft, and reviewed and edited the manuscript; and S. Intapad provided supervision.
References
- 1.Hannun YA, Obeid LM: Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150, 2008. 10.1038/nrm2329 [DOI] [PubMed] [Google Scholar]
- 2.Kroll A, Cho HE, Kang MH: Antineoplastic agents targeting sphingolipid pathways. Front Oncol 10: 833, 2020. 10.3389/fonc.2020.00833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merscher S, Fornoni A: Podocyte pathology and nephropathy - sphingolipids in glomerular diseases. Front Endocrinol (Lausanne) 5: 127, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Meer G, Voelker DR, Feigenson GW: Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol 9: 112–124, 2008. 10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrill AH Jr: De novo sphingolipid biosynthesis: A necessary, but dangerous, pathway. J Biol Chem 277: 25843–25846, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Tafesse FG, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis JC: Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem 282: 17537–17547, 2007. 10.1074/jbc.M702423200 [DOI] [PubMed] [Google Scholar]
- 7.Hakomori SI: Structure and function of glycosphingolipids and sphingolipids: Recollections and future trends. Biochim Biophys Acta 1780: 325–346, 2008. 10.1016/j.bbagen.2007.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannun YA, Obeid LM: Sphingolipids and their metabolism in physiology and disease [published correction appears in Nat Rev Mol Cell Biol 19: 673, 2018 10.1038/s41580-018-0046-6]. Nat Rev Mol Cell Biol 19: 175–191, 2018. 10.1038/nrm.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AH Jr: Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758: 1864–1884, 2006. 10.1016/j.bbamem.2006.08.009 [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S: Sphingolipids in atherosclerosis and vascular biology. Arterioscler Thromb Vasc Biol 18: 1523–1533, 1998. 10.1161/01.ATV.18.10.1523 [DOI] [PubMed] [Google Scholar]
- 11.Holland WL, Summers SA: Sphingolipids, insulin resistance, and metabolic disease: New insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29: 381–402, 2008. 10.1210/er.2007-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maceyka M, Harikumar KB, Milstien S, Spiegel S: Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22: 50–60, 2012. 10.1016/j.tcb.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pant DC, Aguilera-Albesa S, Pujol A: Ceramide signalling in inherited and multifactorial brain metabolic diseases. Neurobiol Dis 143: 105014, 2020. 10.1016/j.nbd.2020.105014 [DOI] [PubMed] [Google Scholar]
- 14.Abou Daher A, El Jalkh T, Eid AA, Fornoni A, Marples B, Zeidan YH: Translational aspects of sphingolipid metabolism in renal disorders. Int J Mol Sci 18: 2528, 2017. 10.3390/ijms18122528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Ritter JK, Li N: Sphingosine-1-phosphate pathway in renal fibrosis. Am J Physiol Renal Physiol 315: F752–F756, 2018. 10.1152/ajprenal.00596.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker DJ, Osmond C: Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1: 1077–1081, 1986. 10.1016/S0140-6736(86)91340-1 [DOI] [PubMed] [Google Scholar]
- 17.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ: Weight in infancy and death from ischaemic heart disease. Lancet 2: 577–580, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D: Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777–784, 1991 [PubMed] [Google Scholar]
- 19.Breslow DK: Sphingolipid homeostasis in the endoplasmic reticulum and beyond. Cold Spring Harb Perspect Biol 5: a013326, 2013. 10.1101/cshperspect.a013326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gault CR, Obeid LM, Hannun YA: An overview of sphingolipid metabolism: From synthesis to breakdown. Adv Exp Med Biol 688: 1–23, 2010. 10.1007/978-1-4419-6741-1_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartke N, Hannun YA: Bioactive sphingolipids: Metabolism and function. J Lipid Res 50[Suppl]: S91–S96, 2009. 10.1194/jlr.R800080-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis AC, Wallington-Beddoe CT, Powell JA, Pitson SM: Targeting sphingolipid metabolism as an approach for combination therapies in haematological malignancies [published correction appears in Cell Death Discov 5: 116, 2019 10.1038/s41420-019-0186-2]. Cell Death Discov 4: 72, 2018. 10.1038/s41420-018-0075-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall A, Róg T, Karttunen M, Vattulainen I: Role of glycolipids in lipid rafts: A view through atomistic molecular dynamics simulations with galactosylceramide. J Phys Chem B 114: 7797–7807, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Mondal S, Mukhopadhyay C: Molecular level investigation of organization in ternary lipid bilayer: A computational approach. Langmuir 24: 10298–10305, 2008. 10.1021/la8015589 [DOI] [PubMed] [Google Scholar]
- 25.D’Angelo G, Capasso S, Sticco L, Russo D: Glycosphingolipids: Synthesis and functions. FEBS J 280: 6338–6353, 2013. 10.1111/febs.12559 [DOI] [PubMed] [Google Scholar]
- 26.Hakomori S: Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem 265: 18713–18716, 1990 [PubMed] [Google Scholar]
- 27.Di Pardo A, Maglione V: Sphingolipid metabolism: A new therapeutic opportunity for brain degenerative disorders. Front Neurosci 12: 249, 2018. 10.3389/fnins.2018.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bocheńska K, Gabig-Cimińska M: Unbalanced sphingolipid metabolism and its implications for the pathogenesis of psoriasis. Molecules 25: 1130, 2020. 10.3390/molecules25051130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitatani K, Idkowiak-Baldys J, Hannun YA: The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal 20: 1010–1018, 2008. 10.1016/j.cellsig.2007.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Intapad S: Sphingosine-1-phosphate signaling in blood pressure regulation. Am J Physiol Renal Physiol 317: F638–F640, 2019. 10.1152/ajprenal.00572.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weske S, Vaidya M, Reese A, von Wnuck Lipinski K, Keul P, Bayer JK, Fischer JW, Flögel U, Nelsen J, Epple M, Scatena M, Schwedhelm E, Dörr M, Völzke H, Moritz E, Hannemann A, Rauch BH, Gräler MH, Heusch G, Levkau B: Targeting sphingosine-1-phosphate lyase as an anabolic therapy for bone loss. Nat Med 24: 667–678, 2018. 10.1038/s41591-018-0005-y [DOI] [PubMed] [Google Scholar]
- 32.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH Jr, Milstien S, Spiegel S: SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem 280: 37118–37129, 2005. 10.1074/jbc.M502207200 [DOI] [PubMed] [Google Scholar]
- 33.Hofmann LP, Ren S, Schwalm S, Pfeilschifter J, Huwiler A: Sphingosine kinase 1 and 2 regulate the capacity of mesangial cells to resist apoptotic stimuli in an opposing manner. Biol Chem 389: 1399–1407, 2008. 10.1515/BC.2008.160 [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S: Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem 278: 40330–40336, 2003. 10.1074/jbc.M304455200 [DOI] [PubMed] [Google Scholar]
- 35.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S: Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem 278: 46832–46839, 2003. 10.1074/jbc.M306577200 [DOI] [PubMed] [Google Scholar]
- 36.Park SW, Kim M, Kim M, D’Agati VD, Lee HT: Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int 80: 1315–1327, 2011. 10.1038/ki.2011.281 [DOI] [PubMed] [Google Scholar]
- 37.Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk M: Sphingosine-1-phosphate: Extracellular mediator or intracellular second messenger? Biochem Pharmacol 58: 201–207, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Imasawa T, Kitamura H, Ohkawa R, Satoh Y, Miyashita A, Yatomi Y: Unbalanced expression of sphingosine 1-phosphate receptors in diabetic nephropathy. Exp Toxicol Pathol 62: 53–60, 2010. 10.1016/j.etp.2009.02.068 [DOI] [PubMed] [Google Scholar]
- 39.Zhu Q, Xia M, Wang Z, Li PL, Li N: A novel lipid natriuretic factor in the renal medulla: Sphingosine-1-phosphate. Am J Physiol Renal Physiol 301: F35–F41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan Z, Singletary ST, Cook AK, Hobbs JL, Pollock JS, Inscho EW: Sphingosine-1-phosphate evokes unique segment-specific vasoconstriction of the renal microvasculature. J Am Soc Nephrol 25: 1774–1785, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awad AS, Ye H, Huang L, Li L, Foss FW Jr, Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Gennero I, Fauvel J, Nieto M, Cariven C, Gaits F, Briand-Mésange F, Chap H, Salles JP: Apoptotic effect of sphingosine 1-phosphate and increased sphingosine 1-phosphate hydrolysis on mesangial cells cultured at low cell density. J Biol Chem 277: 12724–12734, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Rutledge JC, Ng KF, Aung HH, Wilson DW: Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat Rev Nephrol 6: 361–370, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Dunn TM, Tifft CJ, Proia RL: A perilous path: The inborn errors of sphingolipid metabolism. J Lipid Res 60: 475–483, 2019. 10.1194/jlr.S091827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liebau MC, Braun F, Höpker K, Weitbrecht C, Bartels V, Müller RU, Brodesser S, Saleem MA, Benzing T, Schermer B, Cybulla M, Kurschat CE: Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS One 8: e63506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Niño MD, Carpio D, Sanz AB, Ruiz-Ortega M, Mezzano S, Ortiz A: Lyso-Gb3 activates Notch1 in human podocytes. Hum Mol Genet 24: 5720–5732, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A: NF-kappaB in renal inflammation. J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Hoon DS, Okun E, Neuwirth H, Morton DL, Irie RF: Aberrant expression of gangliosides in human renal cell carcinomas. J Urol 150: 2013–2018, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Iwamori M, Shimomura J, Tsuyuhara S, Nagai Y: Gangliosides of various rat tissues: Distribution of ganglio-N-tetraose-containing gangliosides and tissue-characteristic composition of gangliosides. J Biochem 95: 761–770, 1984. 10.1093/oxfordjournals.jbchem.a134667 [DOI] [PubMed] [Google Scholar]
- 50.Reivinen J, Holthöfer H, Miettinen A: A cell-type specific ganglioside of glomerular podocytes in rat kidney: An O-acetylated GD3. Kidney Int 42: 624–631, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Saito M, Sugiyama K: Gangliosides in rat kidney: Composition, distribution, and developmental changes. Arch Biochem Biophys 386: 11–16, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Shayman JA, Radin NS: Structure and function of renal glycosphingolipids. Am J Physiol 260: F291–F302, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA: A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest 91: 797–803, 1993. 10.1172/JCI116299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatterjee S, Alsaeedi N, Hou J, Bandaru VV, Wu L, Halushka MK, Pili R, Ndikuyeze G, Haughey NJ: Use of a glycolipid inhibitor to ameliorate renal cancer in a mouse model. PLoS One 8: e63726, 2013. 10.1371/journal.pone.0063726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deshmukh GD, Radin NS, Gattone VH 2nd, Shayman JA: Abnormalities of glycosphingolipid, sulfatide, and ceramide in the polycystic (cpk/cpk) mouse. J Lipid Res 35: 1611–1618, 1994. 10.1016/S0022-2275(20)41159-9 [DOI] [PubMed] [Google Scholar]
- 56.Nowling TK, Mather AR, Thiyagarajan T, Hernández-Corbacho MJ, Powers TW, Jones EE, Snider AJ, Oates JC, Drake RR, Siskind LJ: Renal glycosphingolipid metabolism is dysfunctional in lupus nephritis. J Am Soc Nephrol 26: 1402–1413, 2015. 10.1681/ASN.2014050508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hernández-Corbacho MJ, Jenkins RW, Clarke CJ, Hannun YA, Obeid LM, Snider AJ, Siskind LJ: Accumulation of long-chain glycosphingolipids during aging is prevented by caloric restriction. PLoS One 6: e20411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holthöfer H, Reivinen J, Solin ML, Haltia A, Miettinen A: Decrease of glomerular disialogangliosides in puromycin nephrosis of the rat. Am J Pathol 149: 1009–1015, 1996 [PMC free article] [PubMed] [Google Scholar]
- 59.Górska M, Dobrzyń A, Baranowski M: Concentrations of sphingosine and sphinganine in plasma of patients with type 2 diabetes. Med Sci Monit 11: CR35–CR38, 2005 [PubMed] [Google Scholar]
- 60.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP: Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58: 337–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kremer GJ, Atzpodien W, Schnellbacher E: Plasma glycosphingolipids in diabetics and normals. Klin Wochenschr 53: 637–638, 1975 [DOI] [PubMed] [Google Scholar]
- 62.Brunskill EW, Potter SS: Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. BMC Nephrol 13: 70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishizawa S, Takahashi-Fujigasaki J, Kanazawa Y, Matoba K, Kawanami D, Yokota T, Iwamoto T, Tajima N, Manome Y, Utsunomiya K: Sphingosine-1-phosphate induces differentiation of cultured renal tubular epithelial cells under Rho kinase activation via the S1P2 receptor. Clin Exp Nephrol 18: 844–852, 2014 [DOI] [PubMed] [Google Scholar]
- 64.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J: Altered adipose and plasma sphingolipid metabolism in obesity: A potential mechanism for cardiovascular and metabolic risk. Diabetes 55: 2579–2587, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Zager RA, Burkhart KM, Johnson A: Sphingomyelinase and membrane sphingomyelin content: Determinants of proximal tubule cell susceptibility to injury. J Am Soc Nephrol 11: 894–902, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Zager RA, Conrad DS, Burkhart K: Ceramide accumulation during oxidant renal tubular injury: Mechanisms and potential consequences. J Am Soc Nephrol 9: 1670–1680, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Zager RA, Conrad S, Lochhead K, Sweeney EA, Igarashi Y, Burkhart KM: Altered sphingomyelinase and ceramide expression in the setting of ischemic and nephrotoxic acute renal failure. Kidney Int 53: 573–582, 1998. 10.1046/j.1523-1755.1998.00772.x [DOI] [PubMed] [Google Scholar]
- 68.Awad AS, Rouse MD, Khutsishvili K, Huang L, Bolton WK, Lynch KR, Okusa MD: Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int 79: 1090–1098, 2011. 10.1038/ki.2010.544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lang F, Ullrich S, Gulbins E: Ceramide formation as a target in beta-cell survival and function. Expert Opin Ther Targets 15: 1061–1071, 2011. 10.1517/14728222.2011.588209 [DOI] [PubMed] [Google Scholar]
- 70.Chavez JA, Summers SA: Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 419: 101–109, 2003. 10.1016/j.abb.2003.08.020 [DOI] [PubMed] [Google Scholar]
- 71.Hamada Y, Nagasaki H, Fujiya A, Seino Y, Shang QL, Suzuki T, Hashimoto H, Oiso Y: Involvement of de novo ceramide synthesis in pro-inflammatory adipokine secretion and adipocyte-macrophage interaction. J Nutr Biochem 25: 1309–1316, 2014. 10.1016/j.jnutbio.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 72.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW 3rd: Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3: 85ra46, 2011. 10.1126/scitranslmed.3002231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitiyakara C, Eggers P, Kopp JB: Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44: 815–825, 2004. 10.1016/S0272-6386(04)01081-9 [DOI] [PubMed] [Google Scholar]
- 74.Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, Mitrofanova A, Leclercq F, Faul C, Li J, Kretzler M, Nelson RG, Lehto M, Forsblom C, Groop PH, Reiser J, Burke GW, Fornoni A, Merscher S: Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol 26: 133–147, 2015. 10.1681/ASN.2013111213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toba H, Lindsey ML: Extracellular matrix roles in cardiorenal fibrosis: Potential therapeutic targets for CVD and CKD in the elderly. Pharmacol Ther 193: 99–120, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lan T, Liu W, Xie X, Xu S, Huang K, Peng J, Shen X, Liu P, Wang L, Xia P, Huang H: Sphingosine kinase-1 pathway mediates high glucose-induced fibronectin expression in glomerular mesangial cells. Mol Endocrinol 25: 2094–2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geoffroy K, Troncy L, Wiernsperger N, Lagarde M, El Bawab S: Glomerular proliferation during early stages of diabetic nephropathy is associated with local increase of sphingosine-1-phosphate levels. FEBS Lett 579: 1249–1254, 2005. 10.1016/j.febslet.2004.12.094 [DOI] [PubMed] [Google Scholar]
- 78.Lan T, Shen X, Liu P, Liu W, Xu S, Xie X, Jiang Q, Li W, Huang H: Berberine ameliorates renal injury in diabetic C57BL/6 mice: Involvement of suppression of SphK-S1P signaling pathway. Arch Biochem Biophys 502: 112–120, 2010 [DOI] [PubMed] [Google Scholar]
- 79.Shiohira S, Yoshida T, Sugiura H, Nishida M, Nitta K, Tsuchiya K: Sphingosine-1-phosphate acts as a key molecule in the direct mediation of renal fibrosis. Physiol Rep 1: e00172, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bajwa A, Huang L, Kurmaeva E, Ye H, Dondeti KR, Chroscicki P, Foley LS, Balogun ZA, Alexander KJ, Park H, Lynch KR, Rosin DL, Okusa MD: Sphingosine kinase 2 deficiency attenuates kidney fibrosis via IFN-γ. J Am Soc Nephrol 28: 1145–1161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S: Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol 22: 1064–1075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su K, Zeng P, Liang W, Luo Z, Wang Y, Lv X, Han Q, Yan M, Chen C: FTY720 attenuates angiotensin II-induced podocyte damage via inhibiting inflammatory cytokines. Mediators Inflamm 2017: 3701385, 2017. 10.1155/2017/3701385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thangada S, Shapiro LH, Silva C, Yamase H, Hla T, Ferrer FA: Treatment with the immunomodulator FTY720 (fingolimod) significantly reduces renal inflammation in murine unilateral ureteral obstruction. J Urol 191[Suppl]: 1508–1516, 2014. 10.1016/j.juro.2013.10.072 [DOI] [PubMed] [Google Scholar]
- 84.Dupre TV, Siskind LJ: The role of sphingolipids in acute kidney injury. Adv Biol Regul 70: 31–39, 2018. 10.1016/j.jbior.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 85.Kurano M, Tsuneyama K, Morimoto Y, Nishikawa M, Yatomi Y: Apolipoprotein M suppresses the phenotypes of IgA nephropathy in hyper-IgA mice. FASEB J 33: 5181–5195, 2019 [DOI] [PubMed] [Google Scholar]
- 86.Park SW, Kim M, Kim JY, Brown KM, Haase VH, D’Agati VD, Lee HT: Proximal tubule sphingosine kinase-1 has a critical role in A1 adenosine receptor-mediated renal protection from ischemia. Kidney Int 82: 878–891, 2012. 10.1038/ki.2012.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perry HM, Huang L, Ye H, Liu C, Sung SJ, Lynch KR, Rosin DL, Bajwa A, Okusa MD: Endothelial sphingosine 1-phosphate receptor-1 mediates protection and recovery from acute kidney injury. J Am Soc Nephrol 27: 3383–3393, 2016. 10.1681/ASN.2015080922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bajwa A, Huang L, Kurmaeva E, Gigliotti JC, Ye H, Miller J, Rosin DL, Lobo PI, Okusa MD: Sphingosine 1-phosphate receptor 3-deficient dendritic cells modulate splenic responses to ischemia-reperfusion injury. J Am Soc Nephrol 27: 1076–1090, 2016. 10.1681/ASN.2015010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brosens IA, Robertson WB, Dixon HG: The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1: 177–191, 1972 [PubMed] [Google Scholar]
- 90.Robertson WB, Brosens I, Dixon HG: The pathological response of the vessels of the placental bed to hypertensive pregnancy. J Pathol Bacteriol 93: 581–592, 1967. 10.1002/path.1700930219 [DOI] [PubMed] [Google Scholar]
- 91.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A: Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 98: 648–655, 1991. 10.1111/j.1471-0528.1991.tb13450.x [DOI] [PubMed] [Google Scholar]
- 92.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ: Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 91: 950–960, 1993. 10.1172/JCI116316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hecht JL, Zsengeller ZK, Spiel M, Karumanchi SA, Rosen S: Revisiting decidual vasculopathy. Placenta 42: 37–43, 2016. 10.1016/j.placenta.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 94.Tomimatsu T, Mimura K, Matsuzaki S, Endo M, Kumasawa K, Kimura T: Preeclampsia: Maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci 20: 4246, 2019. 10.3390/ijms20174246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chauvin S, Yinon Y, Xu J, Ermini L, Sallais J, Tagliaferro A, Todros T, Post M, Caniggia I: Aberrant TGFβ signalling contributes to dysregulation of sphingolipid metabolism in intrauterine growth restriction. J Clin Endocrinol Metab 100: E986–E996, 2015. 10.1210/jc.2015-1288 [DOI] [PubMed] [Google Scholar]
- 96.Melland-Smith M, Ermini L, Chauvin S, Craig-Barnes H, Tagliaferro A, Todros T, Post M, Caniggia I: Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 11: 653–669, 2015. 10.1080/15548627.2015.1034414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bailey LJ, Alahari S, Tagliaferro A, Post M, Caniggia I: Augmented trophoblast cell death in preeclampsia can proceed via ceramide-mediated necroptosis. Cell Death Dis 8: e2590, 2017. 10.1038/cddis.2016.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Newhouse SM, Davidge ST, Winkler-Lowen B, Demianczuk N, Guilbert LJ: In vitro differentiation of villous trophoblasts from pregnancies complicated by intrauterine growth restriction with and without pre-eclampsia. Placenta 28: 999–1003, 2007. 10.1016/j.placenta.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 99.Del Gaudio I, Sasset L, Lorenzo AD, Wadsack C: Sphingolipid signature of human feto-placental vasculature in preeclampsia. Int J Mol Sci 21: 1019, 2020. 10.3390/ijms21031019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skaznik-Wikiel ME, Kaneko-Tarui T, Kashiwagi A, Pru JK: Sphingosine-1-phosphate receptor expression and signaling correlate with uterine prostaglandin-endoperoxide synthase 2 expression and angiogenesis during early pregnancy. Biol Reprod 74: 569–576, 2006. 10.1095/biolreprod.105.046714 [DOI] [PubMed] [Google Scholar]
- 101.Del Gaudio I, Sreckovic I, Zardoya-Laguardia P, Bernhart E, Christoffersen C, Frank S, Marsche G, Illanes SE, Wadsack C: Circulating cord blood HDL-S1P complex preserves the integrity of the feto-placental vasculature. Biochim Biophys Acta Mol Cell Biol Lipids 1865: 158632, 2020 [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, Dunk CE, Lye SJ: Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum Reprod 28: 3026–3037, 2013. 10.1093/humrep/det339 [DOI] [PubMed] [Google Scholar]
- 103.The American College of Obstetricians and Gynecologists : ACOG practice bulletin no. 134: Fetal growth restriction. Obstet Gynecol 121: 1122–1133, 2013. Available at: 10.1097/01.AOG.0000429658.85846.f9 [DOI] [PubMed] [Google Scholar]
- 104.Sharma D, Shastri S, Sharma P: Intrauterine growth restriction: Antenatal and postnatal aspects. Clin Med Insights Pediatr 10: 67–83, 2016. 10.4137/CMPed.S40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int 63: 2113–2122, 2003. 10.1046/j.1523-1755.2003.00018.x [DOI] [PubMed] [Google Scholar]
- 106.Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frölich M, van der Heijden BJ; Dutch POPS-19 Collaborative Study Group: Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 16: 2762–2768, 2005. 10.1681/ASN.2004090783 [DOI] [PubMed] [Google Scholar]
- 107.Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM: Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int 74: 187–195, 2008. 10.1038/ki.2008.153 [DOI] [PubMed] [Google Scholar]
- 108.Bischoff A, Meyer Zu Heringdorf D, Jakobs KH, Michel MC: Lysosphingolipid receptor-mediated diuresis and natriuresis in anaesthetized rats. Br J Pharmacol 132: 1925–1933, 2001. 10.1038/sj.bjp.0703969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Czyborra C, Bischoff A, Michel MC: Indomethacin differentiates the renal effects of sphingosine-1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedebergs Arch Pharmacol 373: 37–44, 2006. 10.1007/s00210-006-0037-6 [DOI] [PubMed] [Google Scholar]
- 110.Graham D, McBride MW, Gaasenbeek M, Gilday K, Beattie E, Miller WH, McClure JD, Polke JM, Montezano A, Touyz RM, Dominiczak AF: Candidate genes that determine response to salt in the stroke-prone spontaneously hypertensive rat: Congenic analysis. Hypertension 50: 1134–1141, 2007. 10.1161/HYPERTENSIONAHA.107.095349 [DOI] [PubMed] [Google Scholar]
- 111.Barker DJ: The origins of the developmental origins theory. J Intern Med 261: 412–417, 2007. 10.1111/j.1365-2796.2007.01809.x [DOI] [PubMed] [Google Scholar]
- 112.Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST: Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res 90: 285–294, 2011. 10.1093/cvr/cvq363 [DOI] [PubMed] [Google Scholar]
- 113.Kerage D, Brindley DN, Hemmings DG: Review: Novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta 35[Suppl]: S86–S92, 2014. 10.1016/j.placenta.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 114.Breslow DK, Weissman JS: Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol Cell 40: 267–279, 2010. 10.1016/j.molcel.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mashima R, Okuyama T, Ohira M: Biosynthesis of long chain base in sphingolipids in animals, plants and fungi. Future Sci OA 6: FSO434, 2019. 10.2144/fsoa-2019-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mullen TD, Hannun YA, Obeid LM: Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem J 441: 789–802, 2012. 10.1042/BJ20111626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cowart LA: A novel role for sphingolipid metabolism in oxidant-mediated skeletal muscle fatigue. Focus on “Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue”. Am J Physiol Cell Physiol 299: C549–C551, 2010. 10.1152/ajpcell.00236.2010 [DOI] [PubMed] [Google Scholar]