Abstract
Rationale
People with cystic fibrosis (CF) experience acute worsening of respiratory symptoms and lung function known as pulmonary exacerbations. Treatment with intravenous antimicrobials is common; however, there is scant evidence to support a standard treatment duration.
Objectives
To test differing durations of intravenous antimicrobials for CF exacerbations.
Methods
STOP2 (Standardized Treatment of Pulmonary Exacerbations 2) was a multicenter, randomized, controlled clinical trial in exacerbations among adults with CF. After 7–10 days of treatment, participants exhibiting predefined lung function and symptom improvements were randomized to 10 or 14 days’ total antimicrobial duration; all others were randomized to 14 or 21 days’ duration.
Measurements and Main Results
The primary outcome was percent predicted FEV1 (ppFEV1) change from treatment initiation to 2 weeks after cessation. Among early responders, noninferiority of 10 days to 14 days was tested; superiority of 21 days compared with 14 days was compared for the others. Symptoms, weight, and adverse events were secondary. Among 982 randomized people, 277 met improvement criteria and were randomized to 10 or 14 days of treatment; the remaining 705 received 21 or 14 days of treatment. Mean ppFEV1 change was 12.8 and 13.4 for 10 and 14 days, respectively, a ‒0.65 difference (95% CI [‒3.3 to 2.0]), excluding the predefined noninferiority margin. The 21- and 14-day arms experienced 3.3 and 3.4 mean ppFEV1 changes, a difference of ‒0.10 (‒1.3 to 1.1). Secondary endpoints and sensitivity analyses were supportive.
Conclusions
Among adults with CF with early treatment improvement during exacerbation, ppFEV1 after 10 days of intravenous antimicrobials is not inferior to 14 days. For those with less improvement after one week, 21 days is not superior to 14 days.
Clinical trial registered with www.clinicaltrials.gov (NCT02781610).
Keywords: clinical trial, intravenous antibiotic therapy, respiratory infection
At a Glance
Scientific Knowledge on the Subject
The optimal duration of antibiotic treatment of a cystic fibrosis (CF) pulmonary exacerbation is unknown, and the question has been identified as an important gap in our knowledge. Current practices demonstrate considerable variance in treatment durations, offering an opportunity to define better practices.
What This Study Adds to the Field
Among adults with CF treated with intravenous antibiotics for pulmonary exacerbation, extending antibiotics beyond 14 days did not improve lung function, and for some subjects, shortening the course to 10 days resulted in similar outcomes to 14 days. This should reduce the variance in treatment practices and provide a platform for further study of the treatment of CF pulmonary exacerbations.
Cystic fibrosis (CF) is the most common life-shortening inherited disease in White individuals, affecting approximately 34,000 people in the United States (1). Advances in care for individuals with CF have resulted in dramatic survival improvements, but people with CF still have debilitating symptoms and significantly shortened life expectancies with impaired mucociliary clearance and acquisition and persistence of polymicrobial infection (2–5). Many people with CF experience episodes of clinical worsening, deemed pulmonary exacerbations (PEx), noted by increased cough and sputum production and acute loss of lung function and weight, among other signs and symptoms (6).
PEx are a major cause of morbidity linked to disease progression (7, 8) with loss of lung function, reduced quality of life (9), and diminished survival (10, 11). Approximately 40% of adults with CF were treated at least once with intravenous (i.v.) antimicrobials for PEx in a year, with a subset treated multiple times (12, 13). Complications of PEx and treatment include nephrotoxicity and ototoxicity, loss of school- or workdays, and significant healthcare costs (14–17). Recovery from these events is variable, which could be because of the etiology of the PEx, the underlying host response, or inadequate treatment (18–20). Systematic reviews of the literature revealed scant evidence upon which to base treatment recommendations for PEx and data demonstrate wide variation in treatment practice patterns, making it difficult to define best practices (13, 14, 18, 19). Although care for individuals with CF is being transformed by recent advances in therapeutics, including CF transmembrane conductance regulator (CFTR) modulators (21, 22), patients continue to receive i.v. antimicrobials for treatment of PEx. Appropriate treatment duration is one of the key unanswered questions and is fundamental to advancing our treatment and may reduce the burden for people with CF (23). PEx treatment of inadequate duration could necessitate early retreatment, yet prolonged treatment could lead to increased toxicity and complications, as well as greater cost and treatment burden and potential selection for antimicrobial resistance (15, 24–26).
The standardized treatment of pulmonary exacerbations (STOP) program was initiated to design interventional trials to identify best practices for the management of PEx in CF (27). Perspectives of patients, families, and CF clinicians about PEx were collected, and we conducted a multicenter observational pilot study of individuals with CF hospitalized for PEx (28–31). These data were used to develop the STOP2 trial, a pragmatic PEx treatment intervention trial assessing duration of i.v. antimicrobial treatment (28–31). STOP2 randomized people with CF being treated for PEx to receive differing durations of i.v. antimicrobial treatment based upon early changes in lung function and symptom scores in response to initial treatment. We hypothesized that for patients who responded robustly in the first week of treatment, a shorter course of antibiotics (10 d) would not be inferior to the most commonly used duration (14 d). In those patients that did not meet explicit criteria for a robust response, we hypothesized that 21 days of antibiotics would be superior to 14 days.
Methods
Study Design
The STOP2 trial is a divergent pragmatic clinical trial that evaluated subjects’ interim improvement in lung function, as measured by percent predicted FEV1 (ppFEV1), and symptoms, as measured by a CF-specific respiratory symptom diary, to tailor randomization to i.v. treatment duration (10 d vs. 14 d for robust responders; 14 d vs. 21 d for less robust responders). The study has been described elsewhere (31). In summary, adults (18 yr) with CF presenting with PEx and prescribed i.v. antimicrobials were eligible (complete inclusion/exclusion criteria are in the data supplement). There were three study visits. Visit 1 occurred within –3 to 1 days of i.v. antimicrobial treatment initiation. Data collected at each visit included ppFEV1 (32, 33) and respiratory symptoms via the validated chronic respiratory infection symptom score (CRISS) (34–37). Participants were randomized to an antimicrobial treatment duration at visit 2, which occurred 7–10 days after start of i.v. treatment (±1 d), when they were categorized as either early robust responders (ERR) or non-early robust responders (NERR), based upon ppFEV1 and CRISS changes from visit 1 (31). A participant with both improvement in ppFEV1 of at least 8 and a CRISS decrease of at least 11 points was allocated to ERR and randomized to receive a total of 10 (±1) or 14 (±1) days of i.v. antimicrobial treatment, while those allocated to NERR were randomized to receive a total of 14 (±1) or 21 (±3) days of i.v. antimicrobial treatment. Visit 3 occurred 2 weeks following scheduled completion of i.v. antimicrobial treatment. To reduce variation across sites, an antimicrobial selection table was provided to guide treatment based upon respiratory culture results from the preceding 2 years (see online supplement). The algorithm accounted for both methicillin-sensitive Staphylococcus aureus and methycillin-resistant S. aureus (MRSA) but assumed MRSA if susceptibility was unknown. No antimicrobial was considered “first line” but instead provided a number of choices known to have activity against the pathogen of interest. Chronic therapies were continued, and airway clearance therapies were enhanced per usual practice. Treatment could occur in the hospital or home. Although subjects with allergic bronchopulmonary aspergillosis or on chronic corticosteroids were excluded, corticosteroids for the treatment of the PEx could be used at the discretion of the treating clinician, with a request to make the decision to use steroids before randomization. Initiation or continuation of oral, i.v., or inhaled antimicrobials between scheduled end of treatment duration and visit 3 were treated as a protocol violation. The protocol was approved by the institutional review boards at participating sites (clinicaltrials.gov NCT02781610).
Outcomes and Sample Size
The primary endpoint for both ERR and NERR groups was absolute ppFEV1 change from visit 1 to visit 3. Secondary and safety endpoints included differences in CRISS and weight changes over the same period, FEV1 relative change, need for intravenous antimicrobial retreatment after IV completion, and adverse events (AE). Data from U.S. participants were combined with CF Foundation patient registry data to ascertain time to next PEx requiring i.v. antimicrobials and prestudy PEx clinic encounter ppFEV1 (additional details in data supplement). For ERR, we hypothesized that ppFEV1 response to 10 days of i.v. antimicrobial treatment would not be clinically inferior to that of 14 days of treatment, assuming a noninferiority margin of 3.5 ppFEV1 based on observations from the STOP pilot (31). The ERR noninferiority test was a priori designed to be conducted on the per-protocol (PP) population (38) for 93% power using STOP pilot SD = 9, two-sided α = 0.05 with 155 PP participants per arm (31). The NERR group was designed to test the hypothesis that 21 days of i.v. antimicrobial treatment would be superior to 14 days, using the intent-to-treat (ITT) population: 285 ITT participants per arm provided 91% power to detect a 2.5 ppFEV1 difference between arms (SD = 9, two-sided α = 0.05).
Statistical Analysis
ANOVA adjusted for randomization strata was used to estimate and compare change in ppFEV1 by treatment duration for each ERR and NERR study group with two-sided 95% confidence intervals (CI) and P values. Estimates were also adjusted for predefined data and safety monitoring board interim monitoring to preserve type 1 error (see online supplement for details). CRISS and weight were analyzed similarly and reported with 95% CI. Prespecified sensitivity analyses included ITT and PP analysis for ERR and NERR, respectively, in addition to last observation carried forward imputation for participants missing visit 3 spirometry. Kaplan-Meier curves, and Cox proportional hazards regression hazard ratios (HR) adjusted for randomization strata were used to compare time to next PEx requiring i.v. antimicrobials between treatment duration arms.
Results
Disposition and Treatment Adherence
The study was conducted from July 2016 through January 2020 at 57 sites in the U.S. CFF Therapeutics Development Network and 1 in Canada (see online supplement). Allocation into NERR was greater than anticipated, with 2.6 randomizing in NERR for each 1 in ERR (expected 2:1 [31]) (see Figure E1 in the supplement). After consultation with the study data and safety monitoring board, enrollment was halted to prevent gross over-enrollment in the NERR arm of the study. No additional nor unplanned analyses of ERR or NERR were conducted. In all, 1,062 PEx events were screened at visit 1, of which 982 participants were ultimately randomized at visit 2. Of 277 allocated to the ERR group, there were 140 and 137 participants randomized/assigned to each treatment group (10 d vs.14 d, respectively); of 705 allocated to the NERR study arm, 352 and 353 participants were assigned to each treatment group (21 d vs. 14 d, respectively) (Figure 1).
Figure 1.
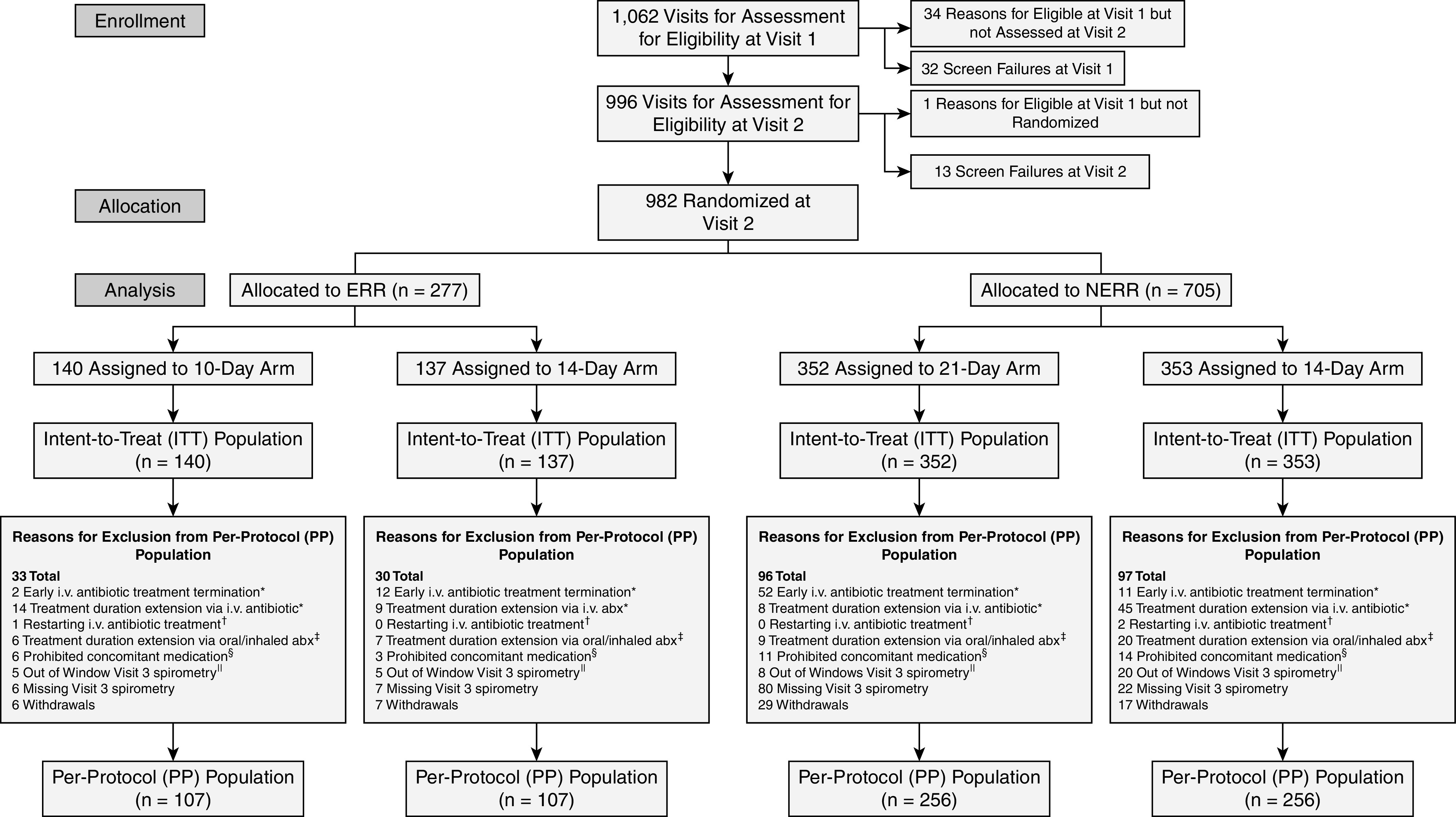
Consolidated Standards of Reporting Trials (CONSORT) diagram. Note: A participant can be excluded from the PP population for more than one reason. Exclusions due to protocol violations: *i.v. antibiotic treatment ending outside the allowed window for a deviation from assigned treatment duration: ± 1 day for 10- and 14-day assignments, ± 3 days for 21-day assignments; †restarting i.v. antibiotic treatment 1 or 2 days after the actual completion of i.v treatment; ‡any acute oral or inhaled antibiotic started before the scheduled completion of i.v treatment and continued after the scheduled completion of i.v. treatment; §any acute oral or inhaled antibiotic started after the scheduled completion of the i.v. treatment and before Visit 3, except continuation of ongoing chronic treatment of initiation of new chronic antibiotic therapy; and ∥spirometry measures done 4 or more days outside of the allowed window for Visit 3 (± 2 days). abx = antibiotics; ERR = early robust responder; NERR = non–early robust responder.
Overall, 13.4% of ERR and 16.5% of NERR participants received antimicrobial durations outside those allowed by the protocol (Figure 1). In the ERR group, antimicrobial treatment duration protocol deviations were more likely to be of excessive durations in the 10-day treatment group (n = 14, 10%), whereas duration deviations in the 14-day treatment group were of both shorter (n = 12, 8.8%) and longer (n = 9, 6.5%) treatment durations than allowed (Figure E2). In the NERR study arm, 15.9% and 17% of participants had treatment duration protocol violations in the 14-day and 21-day treatment groups, respectively. Among NERR participants randomized to receive 14 days of treatment, 11 (3.3%) had treatments stopped prematurely and 45 (12.7%) received treatments exceeding protocol specifications. In contrast, 52 participants in the 21-day treatment group (14.8%) had antimicrobial treatment stopped prematurely and 8 (2.3%) were treated for durations beyond protocol specifications. Additional information regarding treatment duration compliance and visit timing relative to antimicrobial treatment is provided in Figure E2 and Table E1. For the PP analysis, there were 107 participants in each assigned duration for ERR and 256 in each assigned duration for NERR participants. No differences in subject demographics or treatment parameters were observed between treatment groups in either the ITT or PP populations (Table 1 and Table E4). Baseline respiratory microbiology, antimicrobials used, and airway clearance methods were similar across arms (Tables E2 and E3). Declines in ppFEV1 from pre-PEx 6-month average (derived from the CFFPR) to study enrollment were larger for ERR (mean = −9.2) than NERR (mean = −2.5) but indistinguishable by randomization arm (Table 1).
Table 1.
Baseline Characteristics and Treatment Parameters
| Early Robust Responder per Protocol Population | Non–early Robust Responder Intent to Treat Population | |||||
|---|---|---|---|---|---|---|
| Duration assignment | 10 d | 14 d | Total | 14 d | 21 d | Total |
| N | 107 | 107 | 214 | 353 | 352 | 705 |
| Sex, male (%) | 61 (57) | 54 (50.5) | 115 (53.7) | 161 (45.6) | 181 (51.4) | 342 (48.5) |
| Mean age, yr (SD) | 26.6 (6.3) | 27.1 (8.9) | 26.9 (7.7) | 31.8 (10.5) | 31.5 (9.6) | 31.7 (10.1) |
| Genotype | ||||||
| F508del homozygous, n (%) | 46 (43.0) | 55 (51.4) | 101 (47.2) | 171 (48.4) | 174 (49.4) | 345 (48.9) |
| F508del heterozygous, n (%) | 45 (42.1) | 40 (37.4) | 85 (39.7) | 127 (36.0) | 135 (38.4) | 262 (37.2) |
| Other/UK, n (%) | 16 (14.9) | 12 (11.2) | 28 (13.1) | 55 (15.6) | 43 (12.2) | 98 (13.9) |
| Body mass index, kg/m2, mean (SD) | 21.7 (4.4) | 21.6 (4.0) | 21.6 (4.2) | 22.3 (4.1) | 22.4 (3.9) | 22.3 (4.0) |
| ppFEV1 | ||||||
| Visit 1, mean (SD) | 49.5 (18.6) | 49.8 (19.6) | 49.7 (19.1) | 49.5 (21.2) | 50.0 (20.3) | 49.7 (20.7) |
| Visit 1 < 50%, n (%) | 60 (56.1) | 61 (57.0) | 121 (56.5) | 192 (54.4) | 194 (55.1) | 386 (54.8) |
| Average 6 mo prior in CFFPR | ||||||
| n | 98 | 91 | 189 | 334 | 328 | 662 |
| Mean (SD) | 60.2 (17.9) | 60.3 (19.3) | 60.3 (18.6) | 51.8 (20.0) | 53.2 (20.1) | 52.5 (20.0) |
| Change from average 6 mo prior to visit 1, mean (SD) | −9.3 (9.3) | −9.2(8.1) | −9.2 (8.7) | −2.2 (10.3) | −2.9 (6.4) | −2.5 (8.6) |
| History of PEx in last year | ||||||
| 0–1, n (%) | 43 (40.2) | 45 (42.1) | 88 (41.1) | 145 (41.1) | 144 (40.9) | 289 (41.0) |
| ⩾2, n (%) | 64 (59.8) | 62 (57.9) | 126 (58.9) | 208 (58.9) | 208 (59.1) | 416 (59.0) |
| Chronic oral antibiotics (%) | 59 (55.1) | 55 (51.4) | 114 (53.3) | 197 (55.8) | 206 (58.5) | 403 (57.2) |
| CFTR modulator* | ||||||
| None, n (%) | 73 (68.2) | 72 (67.3) | 145 (67.8) | 214 (60.6) | 206 (58.5) | 420 (59.6) |
| Highly effective, n (%) | 7 (6.5) | 3 (2.8) | 10 (4.7) | 25 (7.1) | 28 (8.0) | 53 (7.5) |
| CF-related diabetes | ||||||
| Insulin dependent, n (%) | 26 (24.3) | 24 (22.4) | 50 (23.4) | 105 (29.7) | 116 (33.0) | 221 (31.3) |
| Non-insulin dependent, n (%) | 11 (10.3) | 12 (11.2) | 23 (10.7) | 28 (7.9) | 34 (9.7) | 62 (8.8) |
| Allergic bronchopulmonary aspergillosis, n (%) | 16 (15.0) | 9 (8.4) | 25 (11.7) | 21 (5.9) | 36 (10.2) | 57 (8.1) |
| NTM treated in last 2 yr, n (%) | 6 (5.6) | 7 (6.5) | 13 (6.1) | 21 (5.9) | 15 (4.3) | 36 (5.1) |
| Pneumothorax in last 2 yr, n (%) | 3 (2.8) | 2 (1.9) | 5 (2.3) | 4 (1.1) | 8 (2.3) | 12 (1.7) |
| Treatment location prior to randomization | ||||||
| Any hospital, n (%) | 92 (86) | 90 (84.1) | 182 (85) | 265 (75.1) | 266 (75.6) | 531 (75.3) |
| All at home, n (%) | 15 (14) | 17 (15.9) | 32 (15) | 88 (24.9) | 86 (24.4) | 174 (24.7) |
| Antibiotics initiated† | ||||||
| Intravenous, n (%) | 107 (100) | 107 (100) | 214 (100) | 353 (100) | 352 (100) | 705 (100) |
| Oral, n (%) | 29 (27.1) | 24 (22.4) | 53 (24.8) | 99 (28.0) | 82 (23.3) | 181 (25.7) |
| Inhaled, n (%) | 2 (1.9) | 5 (4.7) | 7 (3.3) | 9 (2.5) | 11 (3.1) | 20 (2.8) |
| Steroids initiated† | 13 (12.1) | 10 (9.3) | 23 (10.7) | 33 (9.3) | 41 (11.6) | 74 (10.5) |
Definition of abbreviations: CF = cystic fibrosis; CFFPR = Cystic Fibrosis Foundation patient registry; CFTR = cystic fibrosis transmembrane conductance regulator; NTM = nontuberculosis Mycobacterium; PEx = pulmonary exacerbations; ppFEV1 = percent of predicted FEV1.
Highly effective include ivacaftor, elexacaftor/tezacaftor/ivacaftor; other CFTR modulators include tezacaftor/ivacaftor and lumacaftor/ivacaftor.
Initiated on or after day of intravenous antibiotics start and before randomization.
Primary Outcome
By design, mean ppFEV1 increased substantially by visit 2 among ERR participants (criteria for ERR was ⩾8 ppFEV1 and ⩾11 CRISS points’ improvement from visit 1) and declined slightly by visit 3 (Figure 2A), as had been observed in a previous study (29, 31). Mean ppFEV1 changes from baseline (visit 1) to visit 3, adjusted for randomization strata, were 12.8 and 13.4 for 10 and 14 days, respectively. Ten days was not clinically inferior to 14 days of treatment with respect to change in ppFEV1 from visit 1 to visit 3 for the primary PP analysis (difference ‒0.7, 95% CI [‒3.3 to 2.0], P = 0.0164, rejecting the null hypothesis of 10 d −3.5 ppFEV1 inferior to 14 days in favor of the alternative hypothesis: 10 d is not inferior to 14 d). All sensitivity analyses, including using the ITT population, supported the alternative hypothesis of noninferiority (Figure 3A).
Figure 2.
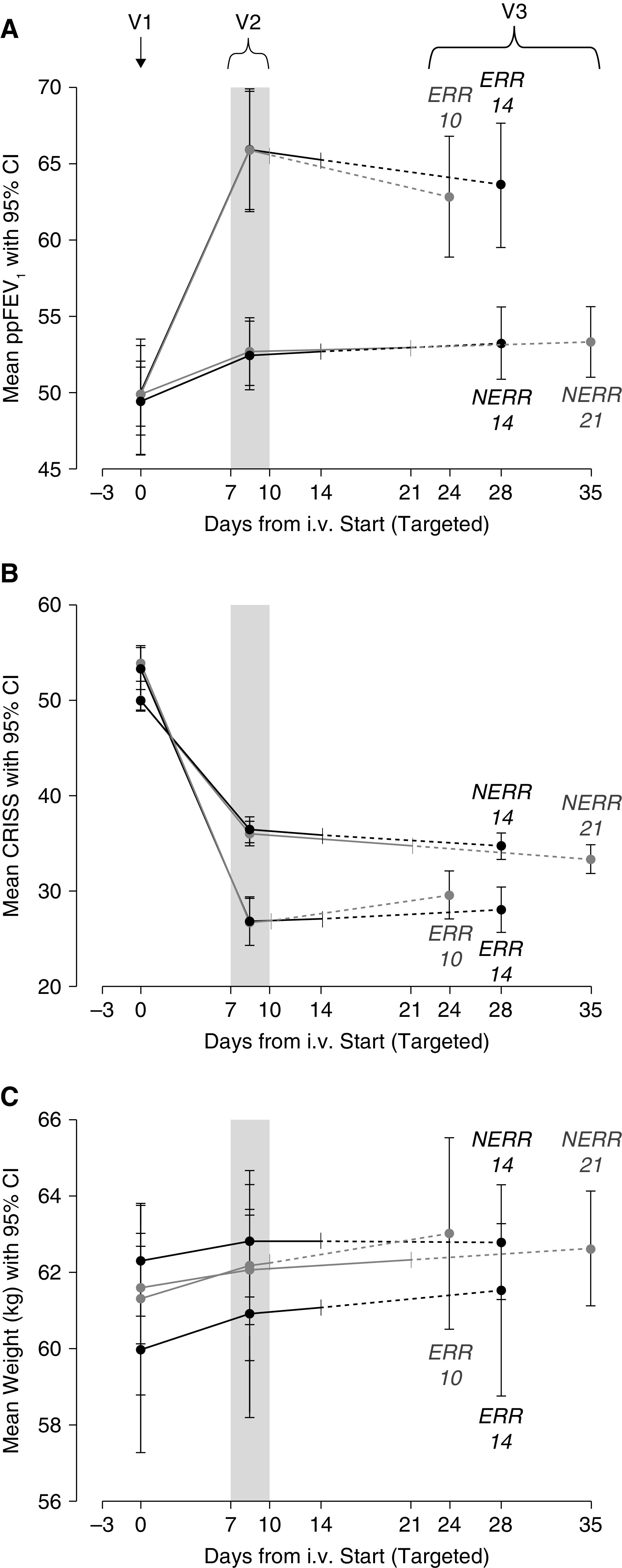
Outcomes by visit, allocation, and antimicrobial treatment duration: (A) Primary outcome ppFEV1. (B) Chronic respiratory infection symptom score (CRISS). (C) Weight. V1 = visit 1 at start of intravenous antimicrobials; V2 = visit 2 when randomization occurs; and V3 = visit 3 was targeted for 14 days after scheduled end of intravenous antimicrobial treatment. Table E1 in the online supplement provides realized timing of each visit by allocation and duration assignment. CI = confidence interval; ERR = early robust responder; NERR = non–early robust responder; ppFEV1 = percent of predicted FEV1.
Figure 3.
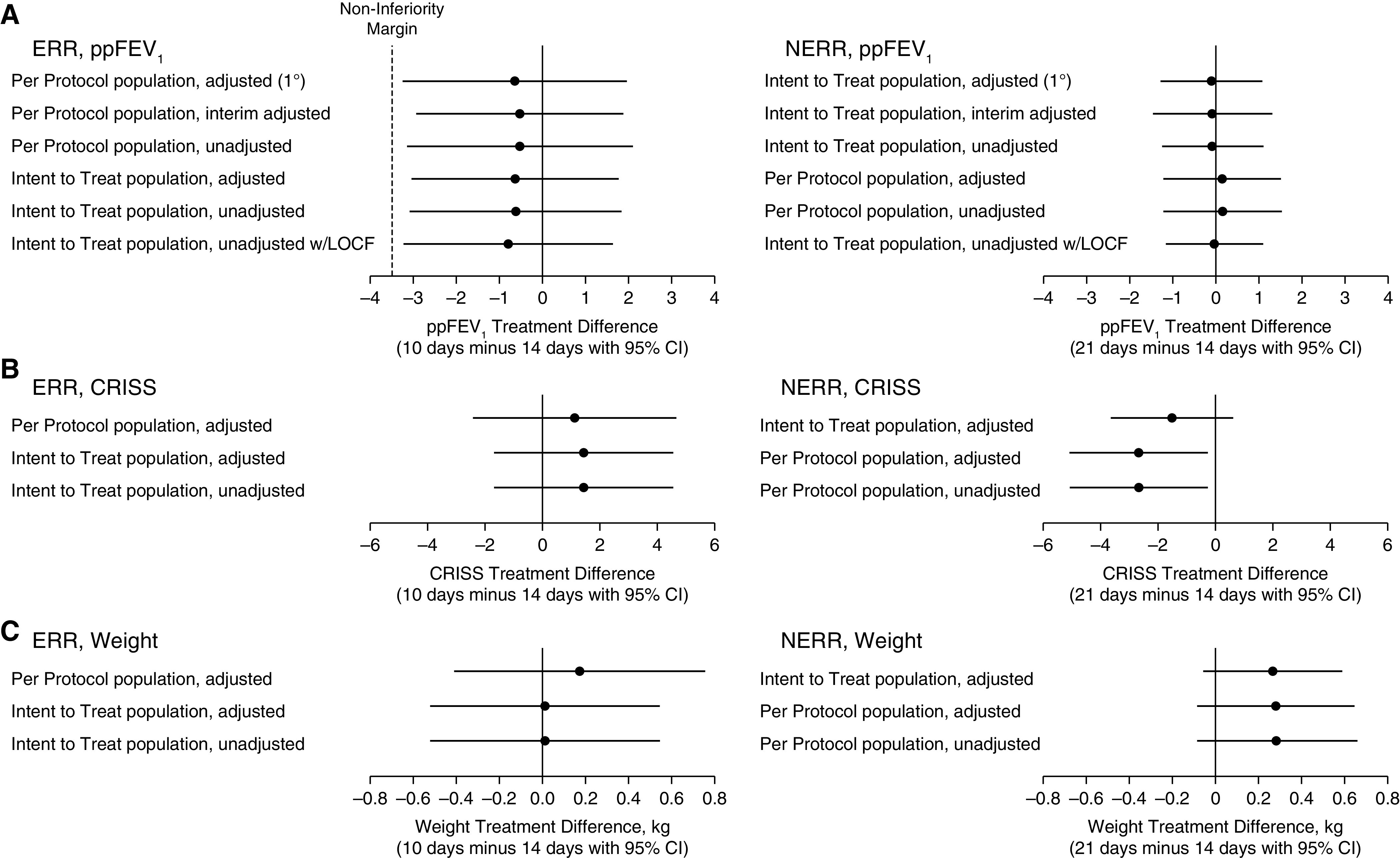
Point estimates and 95% confidence intervals for treatment differences in sensitivity analysis populations: (A) Primary endpoint ppFEV1 change from visit 1 to visit 3; (B) CRISS change from visit 1 to visit 3; (C) weight change from visit 1 to visit 3. Adjusted = ANOVA adjusted for randomization strata: ppFEV1 < 50%, location of intravenous treatment, corticosteroid use, and history of PEx in the year before. Interim adjusted = bias adjustment for interim analyses (according to prespecified boundary parameters). CI = confidence interval; CRISS = Chronic Respiratory Infection Symptom Score; ERR = early robust responder; LOCF = last observation carried forward; NERR = non–early robust responder; PEx = pulmonary exacerbations; ppFEV1 = percent of predicted FEV1.
Mean ppFEV1 continued to increase from visit 2 to visit 3 among NERR participants, but overall ppFEV1 improvement was less than that seen in the ERR population (Figure 2A). Those receiving 21 days of antimicrobial treatment experienced a mean ppFEV1 change of 3.3 compared with 3.4 in those treated for 14 days (adjusted for randomization strata). There was no evidence of a difference in lung function improvement between 21 and 14 days for the primary ITT analysis (mean difference –0.1, 95% CI [–1.3 to 1.1], P = 0.568) or any of the sensitivity analyses (Figure 3A), including the PP population, despite unintended over-enrollment into this NERR group.
Secondary and Safety Outcomes
Respiratory symptoms declined as measured by the CRISS by the minimal clinically important difference of 11 points or more with treatment in all treatment groups, with no significant differences between assigned durations in ERR or NERR (Figures 2B and 3B) (34–37). Weight increased in all groups with no significant difference between the groups after completion of treatment (Figures 2C and 3C). There was no difference in the proportion of subjects who were retreated for a PEx with i.v. antimicrobials by the end of the study (<2% in all groups) or within 30 days of i.v. treatment cessation (⩽5% in all groups) (Table E5). Relative change in FEV1 (L) was, in magnitude, larger than absolute change in ppFEV1, but no differences arose between groups; and change in ppFEV1 from pre-PEx 6-month average to end of study did not differ by treatment duration (Table E5).
There was no difference in the number of AE between ERR duration groups; 21 AE among 18 participants (12.9%) were observed in the 10-day group, and 16 AE among 12 participants (8.8%) were observed in the 14-day group (Table 2). There were few serious AE (SAE) overall: 4 participants in the ERR 10-day group and 1 subject in the 14-day group had SAE, all thought to be unrelated to the treatment. There was an increase in the number of AE in the NERR 21-day group (73 among 47 participants) compared with the 14-day group (40 among 33 participants) with 25 of 33 excess AEs in the 21-day arm being mild or moderate severity. 24 of 26 SAEs in the 21-day arm were deemed unrelated, compared with 15 of 21 SAEs in the 14-day arm (Table 2), gastrointestinal disorders, fever, and elevated liver function tests being the most common (for full AE description, see Tables E6 and E7).
Table 2.
Adverse Events
| Early Robust Responder Intent to Treat Population | Non–early Robust Responder Intent to Treat Population | |||||
|---|---|---|---|---|---|---|
| Randomized i.v. duration | 10 d | 14 d | Total | 14 d | 21 d | Total |
| N | 140 | 137 | 277 | 353 | 352 | 705 |
| Total number AEs | 21 | 16 | 37 | 40 | 73 | 113 |
| Maximum severity of AE | ||||||
| Mild | 4 | 4 | 8 | 6 | 19 | 25 |
| Moderate | 14 | 10 | 24 | 15 | 27 | 42 |
| Severe | 3 | 2 | 5 | 16 | 24 | 40 |
| Life-threatening | 0 | 0 | 0 | 3 | 3 | 6 |
| Participants with any AE, n (%) | 18 (12.9) | 12 (8.8) | 30 (10.8) | 33 (9.3) | 47 (13.4) | 80 (11.3) |
| Person-weeks follow-up | 347.71 | 427.71 | 775.42 | 1105.71 | 1412.00 | 2517.71 |
| AE rate (AEs/person-week) | 0.060 | 0.037 | 0.039 | 0.036 | 0.052 | 0.045 |
| Participants with any SAE, n (%) | 4 (2.9) | 1 (0.7) | 5 (1.8) | 19 (5.4) | 19 (5.4) | 38 (5.4) |
| Total number of SAEs | 4 | 1 | 5 | 21 | 26 | 47 |
| Relatedness of SAE | ||||||
| Unrelated | 4 | 1 | 5 | 15 | 24 | 39 |
| Possibly | 0 | 0 | 0 | 4 | 1 | 5 |
| Probably | 0 | 0 | 0 | 2 | 0 | 2 |
| Definitely | 0 | 0 | 0 | 0 | 1 | 1 |
| AE occurring in 5% of participants | ||||||
| Infective pulmonary exacerbation, n (%) | 9 (6.4) | 5 (3.6) | 14 (5.1) | 13 (3.7) | 13 (3.7) | 26 (3.7) |
Definition of abbreviations: AE = adverse event; SAE = serious adverse event.
Time to next PEx requiring i.v. antimicrobials was ascertained for 961 of 982 randomized in STOP2 (9 were at Canadian sites or did not link to the U.S. CFF patient registry; an additional 12 had study PEx ending after 12/31/2019, the date of the last available registry data). A total of 86% had an event or at least one year of follow-up. In the ERR PP population, 153 of 209 (73.2%) had a subsequent PEx, and in the NERR ITT population, 512 of 689 (74.3%) had a PEx in follow-up. The Kaplan-Meier estimated median time to next PEx was 204 days for ERR overall, 179 days for ERR-10 (n = 104, 95% CI [140–255]), and 227 days for ERR-14 (n = 105, 95% CI [183–301] with no increased hazard in 10 days compared with 14 (HR = 1.14, 95% CI [0.83–1.58]). NERR median time to next PEx was 210 days: 202 days for NERR-14 (n = 346, 95% CI [172–228]) and 232 days for NERR-21 (n = 343, 95% CI [202–274]) with no significant difference in 21 days compared with 14 (HR = 0.90, 95% CI [0.75–1.07]) (Figure 4).
Figure 4.
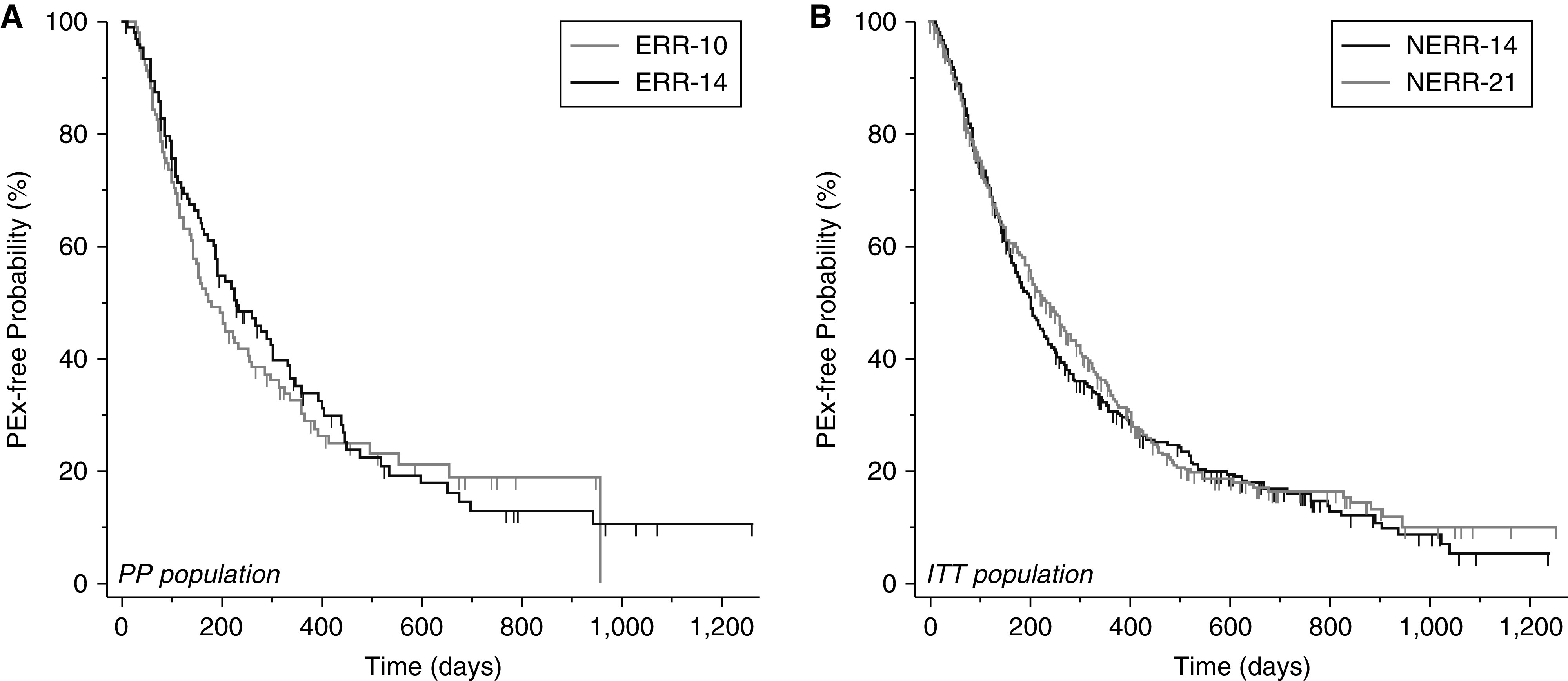
Kaplan-Meier survival curves of time to next PEx requiring intravenous antimicrobials ascertained from the U.S. Cystic Fibrosis Foundation patient registry: (A) Early robust responders per protocol (10 d, n = 104; 14 d, n = 105). (B) Non–early robust responders, intent to treat (14 d, n = 346; 21 d, n = 343). ERR = early robust responder; ITT = intent to treat; PEx = pulmonary exacerbations; PP = per protocol; NERR = non–early robust responder.
Discussion
In this study comparing treatment durations of i.v. antimicrobial treatment for a PEx in adults with CF based on initial response to treatment, there was no evidence of benefit from longer treatment durations in either study group in improvement in lung function, respiratory symptoms, or weight. Although there was a nominal increase in adverse events in the longest treatment duration, there was a low overall rate of adverse events and no significant differences between treatment arms. These results are similar in many ways to those of other high-profile antibiotic trials in conditions other than CF that demonstrated extended treatment durations may not be warranted (39–42).
This is the first large-scale randomized, controlled study of the treatment of CF PEx. In designing this study, we addressed the perceptions of clinicians and patients/families that shorter treatment durations could result in inadequate recovery or early relapse, while longer treatment durations might be associated with diminishing improvement in clinical outcomes and unnecessary cost and toxicity. The timing of randomization (i.e., after evaluating initial response to therapy) prevented rapidly improving patients from extended treatment durations, as well as more slowly improving patients from shortened treatment durations. Our primary endpoint was measured two weeks after completion of i.v. antibiotics to ensure that a decline in FEV1 that might occur with inadequate treatment was not missed. We observed no difference in acute retreatment with antibiotics after initial i.v. antibiotic cessation. Also of note, all patients had improvements in key secondary endpoints like respiratory symptoms and weight, with no clear differences seen between treatment arms. A common measure of PEx treatment success is the time to next pulmonary exacerbation, and no differences were observed across the antimicrobial durations in this trial. Not unlike what many observational studies and trials of exacerbation treatments have shown, the median time to next PEx in STOP2 was over six months after the end of i.v. treatment, and therefore it is unlikely that the subsequent event is the result of any success or failure of the preceding PEx treatment (12, 19, 43). For the CF community, establishing a standard treatment duration will allow for systematic evaluations of other gaps in PEx treatment knowledge without having to account for variable antibiotic durations (6). Furthermore, a widely accepted antibiotic treatment length has the potential to entice new investigational therapies in this area; the lack of standardized treatment protocols in CF PEx has discouraged drug development specifically targeted toward these acute respiratory events.
Our study has several limitations. Given that treatment duration could not have been blinded without extreme cost, 6.5–16.4% of subjects had protocol deviations related to treatment duration. The “open-label” intervention also had the potential to impact adjunctive therapies after randomization approximately one week into treatment and may have influenced outcomes, particularly subjective ones such as symptoms. In the ERR study arm, the primary analysis evaluated only those subjects who were treated per protocol, as missing data and protocol violations in the ITT population would have the potential to bias results toward noninferiority of the 10-day treatment arm. There are varying and conflicting opinions on which primary analytic population to use for a noninferiority trial (44–46). We opted to prioritize conservatism, choosing the most stringent test to reject the inferiority hypothesis, at the risk of making inference about “real world” implementation of i.v. antimicrobial duration. Predefined sensitivity analyses that include the ITT population and examination of outcomes by duration adherence and withdrawal indicate that in this study, conclusions are consistent regardless of analysis population. Our noninferiority margin of 3.5 ppFEV1 was prespecified and chosen to maintain over 70% of a previously observed treatment effect (31). The anticipated large response in the ERR group justifies the relatively larger margin than that used in the NERR superiority hypothesis because those individuals were only expected to improve 3–7 ppFEV1 over the course of treatment (31). For the NERR study arm, all subjects in the ITT populations were included in the analysis, as PP analyses could have biased the results toward superiority of the 21-day treatment duration if, as was the case, there were earlier dropouts from the extended treatment arm. Furthermore, our conclusions are supported by multiple sensitivity analyses, including analyses using 6-month pre-PEx lung function as “baseline,” suggesting the robustness of our results. PEx diagnosis is often accompanied by a “drop” in FEV1, which may confound with, or account for, an individual’s lung function response to i.v. treatment; however, STOP pilot data showed that many individuals diagnosed with PEx have apparently experienced no FEV1 reduction relative to previous values, or previous measures were missing entirely (29). Those data also indicated that most tended to “recover” FEV1 proportional to the amount that they had lost, which was confirmed here in STOP2. The ERR participants lost, on average, 9.2 ppFEV1 from 6 months prior, and on average, their response was 13 ppFEV1, whereas NERR participants averaged a 2.5 ppFEV1 drop from 6 months prior to PEx start and then gained 3.5 ppFEV1 with i.v. treatment. Randomization was effective, and within ERR and NERR grouping, the drop in lung function from 6 months prior did not differ by randomized duration.
This study was designed pragmatically with limited exclusion criteria to increase the generalizability of the results. However, the results of this study may not be applicable to pediatric patients, patients with more severe PEx (e.g., requiring care in the intensive care unit), or patients with frequent i.v.-treated PEx, all of whom were excluded from the study. Differing treatment practices and outcomes at nonstudy sites or outside the United States may also limit generalizability to those groups. The study protocol attempted to control for some of the many treatment factors that could impact the response to treatment, especially considering the nonblinded nature of the intervention. For example, antibiotic selection differs greatly among people with CF (19), likely because of sputum culture results, antibiotic tolerances/allergies, and previous patient experience with PEx. We provided an antimicrobial selection guideline in an attempt to reduce this variability and show that antimicrobial selection was similar across groups. Additional PEx interventions (e.g., inhaled antibiotics) that were initiated after treatment arm assignment were treated as a protocol violation. Clinicians had informed us of their usual practices with PEx, so we encouraged continuation of chronic therapies and maximizing airway clearance therapies in line with these practices (30). In addition, there are challenges to the definition of an exacerbation and the criterion of decision to use IV antibiotics leading to practice variation. However, the size of our study (number of subjects), and the allocation schema likely buffer the impact of that heterogeneity. Finally, we did not study even shorter durations. Observational studies have noted poorer outcomes if treatment is too short. For example, in the United States, treatment with i.v. antibiotics for less than 9 days and treatment entirely as an outpatient have both been associated with an increased risk of retreatment with i.v. antibiotics within 30 days of PEx treatment completion, despite similar patient characteristics at i.v. antibiotic initiation (12, 47). Here we demonstrate that shortening the course of therapy for selected patients (early responders) may be appropriate, although we did not study treatments of <9 days, based on clinician and patient survey data (31). Implementing a shorter course would remain at the discretion of the treating physician and person with CF as CRISS or spirometry lab- acquired FEV1 may not be formally or practically assessed one week into PEx treatment.
In conclusion, this pragmatic trial of IV antimicrobial treatment durations for PEx in adults with CF demonstrated that, for patients with an early clinical response, 10 days is not inferior to 14 days for change in lung function, and there is no evidence of clinical inferiority for symptom or weight changes or time to next PEx. For patients with an attenuated early response, there is no evidence 21 days is superior to 14 days in lung function, symptom recovery weight gain, or prevention of subsequent PEx. This does not mean that 10 days would also be equivalent to 21 days of therapy, as we tested durations in two different populations based on their initial response to treatment. Adverse events were relatively rare and did not differ among treatment durations. Furthermore, this is the first such study to address treatment duration in a chronic infection, in which the goal of treatment is not eradication of the causal bacteria. Future studies of treatment of PEx can use a fixed duration of i.v. antimicrobials to limit confounding by treatment duration and ensure proper interpretation of the results.
Acknowledgments
Acknowledgment
The authors thank Jean Kirihara, Valeria Beckett, and Renee Russell for their contributions to the execution of STOP2. They also thank the participants, families, care providers, and clinic and research coordinators at participating STOP2 sites and throughout the CFF Therapeutic Development Network for their contributions to the study.
Footnotes
A complete list of the STOP2 trial investigators is provided in the Appendix in the online supplement.
Supported by grants from the Cystic Fibrosis Foundation: FLUME15A0, FLUME17AB0, HELTSH15A0, GOSS15A0, SANDER14A0, SANDER17AB0, WEST15A0, WEST17AB0, and WEST175Y (the STOP2 study); National Center for Advancing Translational Sciences of the National Institutes of Health: UL1 TR001450 (P.A.F.); and the National Institutes of Health: P30 DK089507 (C.H.G. and S.L.H.).
Author Contributions: C.H.G., S.L.H., N.E.W., D.B.S., D.R.V., and P.A.F. conceived and conducted the trial, interpreted the data, and drafted the manuscript. M.S., R.J., T.L.B., B.F., and B.C.M. played a role in data acquisition and interpretation of work. M.S., D.R.V., and S.L.H. conducted analyses. All authors reviewed, revised, and approved the final version submitted for publication and agree to be accountable for the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202102-0461OC on September 1, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
on behalf of the STOP2 Investigators:
Gregory Omlor, Brenda Bourne, Dion Roberts, Vicki Roberts, Samya Nasr, Dawn Kruse, Rachel Linnemann, Tsion Hailemichael, Caralee Forseen, Heidi Stapp, Natalie West, S. Patel, A. Claudio, Jerimiah Lysinger, Amy Harmala, George Solomon, Latona Kersh, Karen Miller, Dixie Durham, Ahmet Uluer, Robert Fowler, Carla Frederick, Nadine Caci, Charlotte Teneback, Julie Sweet, Michael Parkins, Clare Smith, Jennifer Goralski, Kelsey Haywood, Patrick Flume, Caroline Brailsford, Dana Albon, Christie Aderholt, Kimberly McBennett, Cindy Schaefer, Alpa Patel, April Hunt, Raksha Jain, Lauren Schumacher, Hari Polenakovik, Linda Clark, Jerry Nick, Katie Poch, Dana Kissner, James Cahill, Jorge Lascano, Erin Silverman, John McArdle, Alison Champagne, Robert Vender, Lisa Allwein, Lance Cohen, Norma (Jean) Barton, Tara Barto, Ami Patel, Cynthia Brown, Nia Vorhees, Michael Crosser, Lawrence Scott, Alix Ashare, Barbara Rodgers, Robert Zanni, Lisa Koval, Andrew Braun, Sophia Chiron Stevens, Maria Tupayachi Ortiz, Patricia Graham, Julie Biller, Erin Hubertz, Kathryn Moffett, Tammy Clark, Rebecca Griffith, Nancy Martinez, Sabiha Hussain, Fei Chen, Marie Egan, Catalina Guzman, Janice Wang, Aileen Espinal, Patricia Walker, Anne Kukral, Emily DiMango, Sarah Fracasso Francis, Carlos Milla, Colleen Dunn, Subramanyam Chittivelu, Ashley Scott, Daniel Dorgan, Sharon Ng, Joseph Pilewski, Rose Lanzo, Nauman Chaudary, Ryan Hayden, Steven Scofield, Barb Johnson, Brian Morrissey, Brandt Robinson, Douglas Conrad, Jenna Mielke, Moira Aitken, Chami Sanlors, Ravi Nayak, Freda Branch, Daniel Rosenbluth, Molly Siegel, Anil Ghimire, Mary Forell, Cori Daines, Monica Varela, Leslie Couch, Rebekah Hibbard, Allen Dozor, Armando Ramirez, Victor Ortega, Kathryn Kennedy, David Fish, and Karen Longtine
References
- 1. Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation patient registry: design and methods of a national observational disease registry. Ann Am Thorac Soc . 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 2. Jain M, Goss CH. Update in cystic fibrosis 2013. Am J Respir Crit Care Med . 2014;189:1181–1186. doi: 10.1164/rccm.201402-0203UP. [DOI] [PubMed] [Google Scholar]
- 3. MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, et al. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med . 2014;161:233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers . 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elborn JS. Cystic fibrosis. Lancet . 2016;388:2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 6. Goss CH. Acute pulmonary exacerbations in cystic fibrosis. Semin Respir Crit Care Med . 2019;40:792–803. doi: 10.1055/s-0039-1697975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr . 2007;151:134–139.e1. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 8. Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J . 2012;40:61–66. doi: 10.1183/09031936.00159111. [DOI] [PubMed] [Google Scholar]
- 9. Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest . 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 10. de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax . 2011;66:680–685. doi: 10.1136/thx.2011.161117. [DOI] [PubMed] [Google Scholar]
- 11. Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol . 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. VanDevanter DR, Morris NJ, Konstan MW. IV-treated pulmonary exacerbations in the prior year: an important independent risk factor for future pulmonary exacerbation in cystic fibrosis. J Cyst Fibros . 2016;15:372–379. doi: 10.1016/j.jcf.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry. 2020. https://www.cff.org/Research/researcher-resources/patient-Registry/
- 14. Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med . 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 15. Jackson AD, Jackson AL, Fletcher G, Doyle G, Harrington M, Zhou S, et al. Estimating direct cost of cystic fibrosis care using Irish registry healthcare resource utilisation data, 2008-2012. Pharmacoeconomics . 2017;35:1087–1101. doi: 10.1007/s40273-017-0530-4. [DOI] [PubMed] [Google Scholar]
- 16. Quon BS, Mayer-Hamblett N, Aitken ML, Smyth AR, Goss CH. Risk factors for chronic kidney disease in adults with cystic fibrosis. Am J Respir Crit Care Med . 2011;184:1147–1152. doi: 10.1164/rccm.201105-0932OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gleser MA, Zettner EM. Negative hearing effects of a single course of IV aminoglycoside therapy in cystic fibrosis patients. Int J Audiol . 2018;57:917–924. doi: 10.1080/14992027.2018.1514537. [DOI] [PubMed] [Google Scholar]
- 18. Flume PA, Wainwright CE, Elizabeth Tullis D, Rodriguez S, Niknian M, Higgins M, et al. Recovery of lung function following a pulmonary exacerbation in patients with cystic fibrosis and the G551D-CFTR mutation treated with ivacaftor. J Cyst Fibros . 2018;17:83–88. doi: 10.1016/j.jcf.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 19. Heltshe SL, Goss CH, Thompson V, Sagel SD, Sanders DB, Marshall BC, et al. Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. Thorax . 2016;71:223–229. doi: 10.1136/thoraxjnl-2014-206750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med . 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med . 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet . 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rowbotham NJ, Smith S, Leighton PA, Rayner OC, Gathercole K, Elliott ZC, et al. The top 10 research priorities in cystic fibrosis developed by a partnership between people with CF and healthcare providers. Thorax . 2018;73:388–390. doi: 10.1136/thoraxjnl-2017-210473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harruff EE, Kil J, Ortiz MGT, Dorgan D, Jain R, Poth EA, et al. Ototoxicity in cystic fibrosis patients receiving intravenous tobramycin for acute pulmonary exacerbation: ototoxicity following tobramycin treatment. J Cyst Fibros . 2021;20:288–294. doi: 10.1016/j.jcf.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 25. Suthoff E, Mainz JG, Cox DW, Thorat T, Grossoehme DH, Fridman M, et al. Caregiver burden due to pulmonary exacerbations in patients with cystic fibrosis. J Pediatr . 2019;215:164–171.e2. doi: 10.1016/j.jpeds.2019.08.038. [DOI] [PubMed] [Google Scholar]
- 26. Franz N, Rapp H, Hansen RN, Gold LS, Goss CH, Lechtzin N, et al. Health care costs related to home spirometry in the eICE randomized trial. J Cyst Fibros . 2021 doi: 10.1016/j.jcf.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burgel PR, Reid DW, Aaron SD. A first step to STOP cystic fibrosis exacerbations. J Cyst Fibros . 2017;16:529–531. doi: 10.1016/j.jcf.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 28. Sanders DB, Solomon GM, Beckett VV, West NE, Daines CL, Heltshe SL, et al. STOP Study Group. Standardized treatment of pulmonary exacerbations (STOP) study: observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros . 2017;16:592–599. doi: 10.1016/j.jcf.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. VanDevanter DR, Heltshe SL, Spahr J, Beckett VV, Daines CL, Dasenbrook EC, et al. STOP Study Group. Rationalizing endpoints for prospective studies of pulmonary exacerbation treatment response in cystic fibrosis. J Cyst Fibros . 2017;16:607–615. doi: 10.1016/j.jcf.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. West NE, Beckett VV, Jain R, Sanders DB, Nick JA, Heltshe SL, et al. STOP investigators. Standardized treatment of pulmonary exacerbations (STOP) study: physician treatment practices and outcomes for individuals with cystic fibrosis with pulmonary exacerbations. J Cyst Fibros . 2017;16:600–606. doi: 10.1016/j.jcf.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heltshe SL, West NE, VanDevanter DR, Sanders DB, Beckett VV, Flume PA, et al. STOP Study Group. Study design considerations for the standardized treatment of pulmonary exacerbations 2 (STOP2): a trial to compare intravenous antibiotic treatment durations in CF. Contemp Clin Trials . 2018;64:35–40. doi: 10.1016/j.cct.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J . 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 34. Goss CH, Caldwell E, Gries KS, Leidy NK, Edwards T, Flume PA, et al. Validation of a novel patient-reported respiratory symptoms instrument in cystic fibrosis CFRSD-CRISS Pediatr Pulmonol 2013. 48 295 296 22553136 [Google Scholar]
- 35. Gold LS, Patrick DL, Hansen RN, Beckett V, Goss CH, Kessler L. Correspondence between symptoms and preference-based health status measures in the STOP study. J Cyst Fibros . 2019;18:251–264. doi: 10.1016/j.jcf.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bennett AV, Patrick DL, Lymp JF, Edwards TC, Goss CH. Comparison of 7-day and repeated 24-hour recall of symptoms of cystic fibrosis. J Cyst Fibros . 2010;9:419–424. doi: 10.1016/j.jcf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lechtzin N, Mayer-Hamblett N, West NE, Allgood S, Wilhelm E, Khan U, et al. eICE Study Team. Home monitoring of patients with cystic fibrosis to identify and treat acute pulmonary exacerbations: eICE study results. Am J Respir Crit Care Med . 2017;196:1144–1151. doi: 10.1164/rccm.201610-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mauri L, D’Agostino RB., Sr Challenges in the design and interpretation of noninferiority trials. N Engl J Med . 2017;377:1357–1367. doi: 10.1056/NEJMra1510063. [DOI] [PubMed] [Google Scholar]
- 39. Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, et al. PneumA Trial Group. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA . 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 40. Uranga A, España PP, Bilbao A, Quintana JM, Arriaga I, Intxausti M, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med . 2016;176:1257–1265. doi: 10.1001/jamainternmed.2016.3633. [DOI] [PubMed] [Google Scholar]
- 41. Oosterheert JJ, Bonten MJ, Schneider MM, Buskens E, Lammers JW, Hustinx WM, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ . 2006;333:1193. doi: 10.1136/bmj.38993.560984.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Capellier G, Mockly H, Charpentier C, Annane D, Blasco G, Desmettre T, et al. Early-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatment. PLoS One . 2012;7:e41290. doi: 10.1371/journal.pone.0041290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tangpricha V, Lukemire J, Chen Y, Binongo JNG, Judd SE, Michalski ES, et al. Vitamin D for the immune system in cystic fibrosis (DISC): a double-blind, multicenter, randomized, placebo-controlled clinical trial. Am J Clin Nutr . 2019;109:544–553. doi: 10.1093/ajcn/nqy291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matilde Sanchez M, Chen X. Choosing the analysis population in non-inferiority studies: per protocol or intent-to-treat. Stat Med . 2006;25:1169–1181. doi: 10.1002/sim.2244. [DOI] [PubMed] [Google Scholar]
- 45. Wiens BL, Zhao W. The role of intention to treat in analysis of noninferiority studies. Clin Trials . 2007;4:286–291. doi: 10.1177/1740774507079443. [DOI] [PubMed] [Google Scholar]
- 46. Matsuyama Y. A comparison of the results of intent-to-treat, per-protocol, and g-estimation in the presence of non-random treatment changes in a time-to-event non-inferiority trial. Stat Med . 2010;29:2107–2116. doi: 10.1002/sim.3987. [DOI] [PubMed] [Google Scholar]
- 47. VanDevanter DR, Flume PA, Morris N, Konstan MW. Probability of IV antibiotic retreatment within thirty days is associated with duration and location of IV antibiotic treatment for pulmonary exacerbation in cystic fibrosis. J Cyst Fibros . 2016;15:783–790. doi: 10.1016/j.jcf.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


