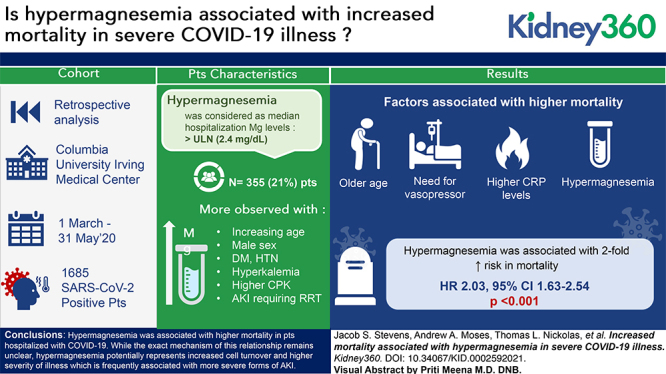
Visual Abstract
Keywords: acid/base and electrolyte disorders, coronavirus, COVID-19, hypermagnesemia, magnesium, rhabdomyolysis, SARS-CoV-2
Key Points
Hypermagnesemia is common in patients admitted with coronavirus disease 2019.
The development of hypermagnesemia in coronavirus disease 2019 is associated with renal failure and markers of high cell turnover.
In adjusted models, patients who develop hypermagnesemia have an increased risk of mortality.
Abstract
Background
Although electrolyte abnormalities are common among patients with COVID-19, very little has been reported on magnesium homeostasis in these patients. Here we report the incidence of hypermagnesemia, and its association with outcomes among patients admitted with COVID-19.
Methods
We retrospectively identified all patients with a positive test result for SARS-CoV-2 who were admitted to a large quaternary care center in New York City in spring 2020. Details of the patients’ demographics and hospital course were obtained retrospectively from medical records. Patients were defined as having hypermagnesemia if their median magnesium over the course of their hospitalization was >2.4 mg/dl.
Results
A total of 1685 patients hospitalized with COVID-19 had their magnesium levels checked during their hospitalization, and were included in the final study cohort, among whom 355 (21%) had hypermagnesemia. Patients who were hypermagnesemic had a higher incidence of shock requiring pressors (35% vs 27%, P<0.01), respiratory failure requiring mechanical ventilation (28% vs 21%, P=0.01), AKI (65% vs 50%, P<0.001), and AKI severe enough to require renal replacement therapy (18% vs 5%, P<0.001). In an adjusted multivariable model, hypermagnesemia was observed more commonly with increasing age, male sex, AKI requiring RRT, hyperkalemia, and higher CPK. Survival probability at 30 days was 34% for the patients with hypermagnesemia, compared with 65% for patients without hypermagnesemia. An adjusted multivariable time to event analysis identified an increased risk of mortality with older age, need for vasopressors, higher C-reactive protein levels, and hypermagnesemia (HR, 2.03; 95% CI, 1.63 to 2.54, P<0.001).
Conclusions
In conclusion, we identified an association between hypermagnesemia among patients hospitalized with COVID-19 and increased mortality. Although the exact mechanism of this relationship remains unclear, hypermagnesemia potentially represents increased cell turnover and higher severity of illness, which is frequently associated with more severe forms of AKI.
Introduction
Magnesium is an often-overlooked electrolyte with diverse roles in critical physiologic processes, including blood pressure regulation, muscle and nerve function, and protein synthesis. Disordered magnesium homeostasis is common among both patients who are ambulatory and hospitalized (1). Despite extracellular magnesium (<1% of total body magnesium) being an insensitive marker for magnesium depletion, many chronic conditions or medications can predispose patients to depleted total body stores over time, resulting in hypomagnesemia (2). Conversely, in the absence of renal dysfunction, hypermagnesemia is uncommon due to the high capacity for renal clearance, and is usually only seen at a GFR <20 ml/min with concurrent exogenous magnesium (3).
Electrolyte abnormalities are common among patients with coronavirus disease 2019 (COVID-19) (particularly hyperkalemia), but very little has been reported on magnesium homeostasis in these patients. Although few reports include magnesium levels among patients with COVID-19 (4,5), anecdotal reports of severe hypermagnesemia among patients who were hospitalized at our institution prompted an investigation of magnesium homeostasis among patients with COVID-19. We report the incidence of hypermagnesemia and its association with outcomes among patients admitted with COVID-19 at a large quaternary care center in New York City.
Methods
Cohort Identification
We retrospectively identified all patients with a positive test result for severe acute respiratory syndrome coronavirus 2 from either a nasopharyngeal or oropharyngeal swab PCR who were admitted to Columbia University Irving Medical Center between March 1 and May 31, 2020, who had bloodwork during their hospitalization and were followed through June 10, 2020. This study protocol was approved by the Institutional Review Board of Columbia University Irving Medical Center and the requirement for informed consent was waived.
Clinical Characteristics and Outcomes
Details of the patients’ hospital courses, demographics, clinical data, laboratory data, and clinical outcomes were obtained retrospectively from medical records. Comorbid conditions were determined by specific international classification of disease codes and are provided in Supplemental Table 1. The magnesium status was determined by the median magnesium level of each patient’s entire hospitalization, with values above the upper limit of normal at our laboratory (>2.4 mg/dl) considered hypermagnesemic, and all others were categorized as not having hypermagnesemia. Specific laboratory value cutoffs are provided in each accompanying table and figure.
AKI was defined based according to AKI Network (AKIN) definitions (6), interpreted as an absolute increase in creatinine of >0.3 mg/dl or >50% increase from baseline, and staged as follows:
AKIN stage 1: ≥0.3 mg/dl increase in creatinine within a 48-hour window or a 1.5- to 2-fold increase in creatinine compared with baseline.
AKIN stage 2: >2- to 3-fold increase in creatinine compared with baseline.
AKIN stage 3: ≥0.5 mg/dl increase in creatinine within a 48-hour window when creatinine ≥4.0 mg/dl, or >3-fold increase in creatinine compared with baseline.
Because urine output was not available for the majority of our cohort, loss of excretory function was determined solely by creatinine kinetics. Determination of baseline creatinine was made using the following hierarchy:
-
1.
median creatinine from 365 to 7 days before presentation; if none available, then:
-
2.
minimum creatinine from 7 days before presentation to the day of presentation; if none available, then:
-
3.
minimum creatinine from presentation to discharge.
-
4.
If no creatinine or only a single creatinine was measured during the index hospitalization without prior measurements, these patients were labeled as having an unknown baseline creatinine.
Statistical Analysis
Results are presented as a percent of the cohort, means and standard deviations, or median and interquartile range. Chi-squared or Fisher’s exact test were used to compare magnesium status groups on categorical variables, and t tests or Wilcoxon signed-rank test for continuous variables. Logistic regression models were used to evaluate for factors associated with the development of hypermagnesemia. Kaplan–Meier survival analysis was used to estimate and display survival stratified by hypermagnesemia status. Cox proportional hazards models were used to assess the hazard ratios for death as the outcome. Due to significant concordance between clinical illness severity as measured by the need for vasopressors, mechanical ventilation, and AKI requiring RRT (Supplemental Table 2), only AKI requiring RRT was used for the multivariable hypermagnesemia model and only vasopressor status was used in the Cox multivariable model for death. Odds ratios (OR) and hazard ratios are presented with 95% confidence intervals (95% CIs). Two sensitivity analyses were performed for both the logistic regression and Cox proportional hazard models, excluding patients who experienced any hypermagnesemia in one analysis, and excluding patients whose median magnesium level was below the lower limit of normal to evaluate for bias, by grouping patients who were normomagnesemic and hypomagnesemic together. Missing laboratory data are presented in Supplemental Table 3. No missing data were imputed. Statistical analyses were performed using Stata 16 (College Station TX).
Results
A total of 1685 patients hospitalized with COVID-19 had their magnesium levels checked during their hospital course, and were included in the final study cohort, among whom 355 (21%) had hypermagnesemia (Table 1 and Figure 1). The mean of the median magnesium levels for the group classified as not hypermagnesemic was 2.1±0.2 versus 2.8±0.5 in the hypermagnesemic group (P<0.0001; Table 2). The maximum magnesium levels were significantly higher among patients with hypermagnesemia (3.0±0.6 vs 2.3±0.5 mg/dl, P<0.001; Table 2). Time from admission to the peak serum magnesium levels was not different between the groups (4.4±5.7 days vs 4.7±5.4 days; P=0.30). Importantly, the incidence of any hypomagnesemia was low, with only 9% of patients experiencing at least one serum magnesium level below the lower limit of the normal range, and only 0.3% of patients in the hypermagnesemia group (Table 2).
Table 1.
Demographics and baseline outpatient characteristics of the cohort
| Characteristics | Total | Not Hypermagnesemic | Hypermagnesemica | |||||
| n | % | n | % | n | % | P value | ||
| N | 1685 | 100 | 1330 | 79 | 355 | 21 | ||
| Age | 65.2 | 16.30 | 64.9 | 16.42 | 66.3 | 15.79 | 0.17 | |
| Sex (female) | 679 | 40 | 577 | 43 | 102 | 29 | <0.001 | |
| Race and ethnicity | 0.14 | |||||||
| Black | 365 | 22 | 288 | 22 | 77 | 22 | ||
| White nonHispanic/Latino | 180 | 11 | 154 | 12 | 26 | 7 | ||
| White Hispanic/Latino | 210 | 12 | 167 | 13 | 43 | 12 | ||
| Other Hispanic/Latino | 557 | 33 | 438 | 33 | 119 | 34 | ||
| Other race or not reported | 373 | 22 | 283 | 21 | 90 | 25 | ||
| BMI | 0.40 | |||||||
| <20 | 108 | 6 | 88 | 6 | 20 | 6 | ||
| 20–25 | 385 | 23 | 316 | 22 | 69 | 19 | ||
| 25–30 | 515 | 31 | 515 | 36 | 120 | 34 | ||
| 30–35 | 308 | 18 | 237 | 16 | 71 | 20 | ||
| ≥35 | 242 | 14 | 193 | 13 | 49 | 14 | ||
| Missing | 127 | 8 | 101 | 7 | 26 | 7 | ||
| Medical history | ||||||||
| Hypertension | 1073 | 64 | 867 | 65 | 206 | 58 | 0.01 | |
| Diabetes mellitus | 720 | 43 | 586 | 44 | 134 | 38 | 0.03 | |
| Pulmonary disease | 299 | 18 | 244 | 18 | 55 | 15 | 0.21 | |
| CKD | 252 | 15 | 204 | 15 | 48 | 14 | 0.39 | |
| ESKD | 72 | 4 | 56 | 4 | 16 | 5 | 0.81 | |
| Liver disease | 96 | 6 | 83 | 6 | 13 | 4 | 0.06 | |
BMI, body mass index.
Defined as median admission (Mg) > upper limit of normal (2.4 mg/dl).
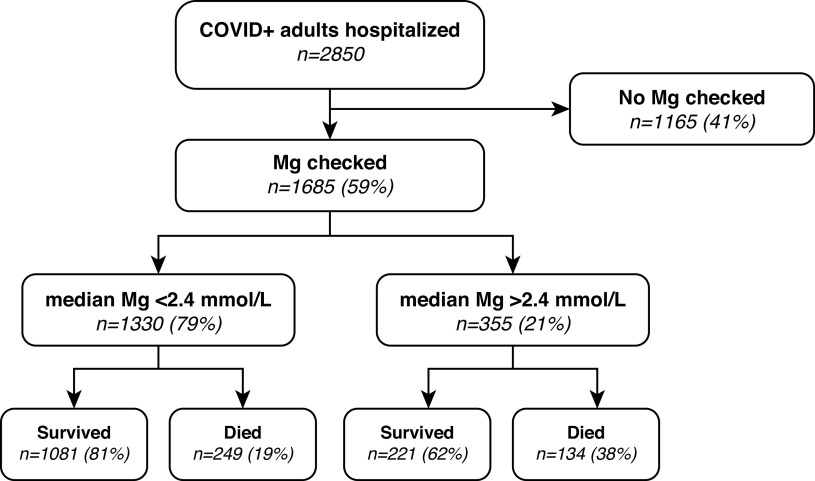
Figure 1.
Incidence and outcomes of patients who are hypermagnesemic. Of the 1685 (59%) of patients with COVID-19 admitted who had magnesium levels checked, 21% had a median magnesium level >2.4 mg/dl. In total, 38% of patients with hypermagnesemia died during their hospitalization, compared with only 19% of patients without hypermagnesemia. Mg, magnesium; COVID-19, coronavirus disease 2019.
Table 2.
Clinical outcomes, illness severity, and mortality by hypermagnesemia status: Magnesium characterization, COVID illness severity, and clinical outcomes
| Finding | Total | Not Hypermagnesemic | Hypermagnesemica | ||||
| n | % | n | % | n | % | P value | |
| N | 1685 | 100 | 1330 | 79 | 355 | 21 | |
| Characterization of magnesium | |||||||
| Mean of median magnesium levels, mg/dL | 2.2 | 0.4 | 2.1 | 0.2 | 2.8 | 0.5 | <0.001 |
| Any hypomagenesemia,%b | 159 | 9 | 154 | 9 | 5 | 0.3 | <0.001 |
| Peak magnesium, mmol/L | 2.5 | 0.6 | 2.3 | 0.5 | 3.0 | 0.6 | <0.001 |
| Time to peak magnesium (days) | 4.4 | 5.7 | 4.4 | 5.7 | 4.7 | 5.4 | 0.30 |
| Outpatient magnesium supplementation | 36 | 2 | 29 | 2 | 7 | 2 | 0.81 |
| Inpatient magnesium supplementation | 53 | 3 | 47 | 4 | 6 | 2 | 0.08 |
| Loop or Thiazide diuretic use | 48 | 3 | 41 | 3 | 7 | 2 | 0.26 |
| Additional laboratory values | |||||||
| Hypercalcemia (any),%c | 224 | 13 | 171 | 13 | 53 | 15 | 0.31 |
| Phosphate (peak), mg/dl | 4.9 | 2.3 | 4.6 | 2.0 | 6.0 | 3.0 | <0.001 |
| Potassium (peak), mmol/L | 5.4 | 1.1 | 5.3 | 1.0 | 5.7 | 1.1 | <0.001 |
| Lactate dehydrogenase (peak), U/L | 329 | 414 | 304 | 340 | 419 | 609 | <0.001 |
| Low haptoglobin,%d | 25 | 1 | 20 | 2 | 5 | 1 | 0.99 |
| Erythrocyte sedimentation rate (peak), mm/h | 89 | 34 | 87 | 35 | 96 | 30 | <0.001 |
| C-reactive protein (peak), mg/L | 183 | 105 | 175 | 106 | 213 | 97 | <0.001 |
| Alkaline phosphatase (peak), U/L | 139 | 139 | 137 | 135 | 147 | 135 | 0.25 |
| Creatine phosphokinase, % >5000 U/L | 49 | 3 | 28 | 2 | 21 | 6 | <0.001 |
| Illness severity and clinical outcomes | |||||||
| Pressors or inotropic support | 475 | 28 | 352 | 27 | 123 | 35 | <0.01 |
| Mechanical ventilation | 377 | 22 | 278 | 21 | 99 | 28 | 0.01 |
| AKIe | <0.001 | ||||||
| Unknown | 62 | 4 | 50 | 4 | 12 | 4 | |
| No AKI | 697 | 43 | 589 | 46 | 108 | 32 | |
| Any AKI | 854 | 53 | 635 | 50 | 219 | 65 | |
| Stage 1 | 322 | 20 | 248 | 19 | 74 | 22 | |
| Stage 2 | 184 | 11 | 155 | 12 | 29 | 9 | |
| Stage 3 | 348 | 22 | 232 | 18 | 116 | 34 | |
| Stage 3 + renal replacement therapy | 125 | 8 | 63 | 5 | 62 | 18 | <0.001 |
| Mortality | <0.001 | ||||||
| In-hospital death | 383 | 23 | 249 | 19 | 134 | 38 | |
| Survived to discharge or end of observation | 1302 | 77 | 1081 | 81 | 221 | 62 | |
Defined as median admission (Mg) > upper limit of normal (2.4 mg/dl).
Defined as median admission (Mg) < upper limit of normal (1.7 mg/dl).
Defined as any (calcium) > upper limit of normal (>10.3 mg/dl).
Defined as any (haptoglobin) < 20 U/L.
n=1613, excluding ESKD n=72.
Patients with hypermagnesemia had similar age, race, and body mass index compared with those without hypermagnesemia. However, they were less likely to be female (29% vs 43%, P<0.001), or have comorbid hypertension (58% vs 65%, P=0.01) or diabetes (38% vs 44%, P=0.03) (Table 1). Additionally, patients who experienced hypermagnesemia were noted to have higher peak phosphorus concentrations, higher serum potassium levels, and more commonly had creatine phosphokinase >5000 U/L, and no differences were observed in peak alkaline phosphatase levels or incidence of hypercalcemia (Table 2). Several markers of inflammation that were being measured routinely on patients with COVID-19 early in the pandemic were higher among patients with hypermagnesemia, including lactate dehydrogenase, erythrocyte sedimentation rate, and C-reactive protein (CRP) (Table 2).
During their COVID-19 hospitalization, patients with hypermagnesemia also had a higher incidence of shock requiring pressors (35% vs 27%, P<0.01), respiratory failure requiring mechanical ventilation (28% vs 21%, P=0.01), AKI (65% vs 50%, P<0.001), and AKI severe enough to require renal replacement therapy (18% vs 5%; Table 2). There were no differences in the use of either outpatient (before hospitalization) or inpatient magnesium supplementation between the two groups.
Univariate logistic regression identified factors associated with hypermagnesemia (n=1371; Table 3). Hypermagnesemia was more common among men (OR, 1.90; 95% CI, 1.47 to 2.45, P<0.001) in addition to being associated with more severe infections as evidenced by the need for vasopressors (OR, 1.47; 95% CI, 1.15 to 1.89, P<0.01), mechanical ventilation (OR, 1.46; 95% CI, 1.12 to 1.91, P=0.01) and the development of developing AKI requiring RRT (OR, 4.26; 95% CI, 2.93 to 6.18, P<0.001). Several markers of cell turnover and illness severity (including hyperkalemia, creatine phosphokinase (CPK), lactate dehydrogenase [LDH], and CRP) were also associated with hypermagnesemia. In a multivariable model, hypermagnesemia was observed more commonly with increasing age, male sex, AKI requiring RRT, hyperkalemia, and higher CPK, whereas LDH and CRP were no longer significantly associated with hypermagnesemia. Interestingly, patients who were hypermagnesemic had lower odds of having either hypertension or diabetes, both in univariate and multivariate models (Table 3). Sensitivity analysis excluding patients with any hypomagnesemia showed small differences in race, history of diabetes, and CPK; however, subsequent analysis only excluding patients with a median magnesium <1.7 mg/dl provided results more similar to the primary multivariable model (Supplemental Table 4).
Table 3.
Logistic regression analysis of characteristics and outcomes associated with hypermagnesemia (n=1371)
| Variable | Univariate | Multivariable | ||||
| Odds Ratio | 95% Confidence Interval | P value | Odds Ratio | 95% Confidence Interval | P value | |
| Demographics | ||||||
| Age (per 10 years) | 1.05 | 0.98 to 1.13 | 0.17 | 1.17 | 1.06 to 1.29 | <0.01 |
| Sex (reference: female) | 1.90 | 1.47 to 2.45 | <0.001 | 2.11 | 1.56 to 2.87 | <0.001 |
| Race (reference: White)a | 1.32 | 0.99 to 1.76 | 0.06 | 1.32 | 0.95 to 1.85 | 0.10 |
| PMHx | ||||||
| HTN | 0.74 | 0.58 to 0.94 | 0.01 | 0.71 | 0.51 to 0.98 | <0.05 |
| DM | 0.77 | 0.61 to 0.98 | 0.03 | 0.73 | 0.53 to 0.99 | <0.05 |
| ESKD | 1.07 | 0.61 to 1.90 | 0.81 | 1.64 | 0.87 to 3.10 | 0.13 |
| Illness severity | ||||||
| Pressors | 1.47 | 1.15 to 1.89 | <0.01 | |||
| Mechanical ventilation | 1.46 | 1.12 to 1.91 | 0.01 | |||
| AKI requiring RRT | 4.26 | 2.93 to 6.18 | <0.001 | 3.08 | 1.97 to 4.81 | <0.001 |
| Labs | ||||||
| Any hypercalcemia, mg/dlb | 1.19 | 0.85 to 1.66 | 0.31 | 0.88 | 0.59 to 1.31 | 0.52 |
| Any hyperkalemia, mmol/Lc | 2.19 | 1.72 to 2.80 | <0.001 | 1.59 | 1.17 to 2.16 | <0.05 |
| Creatine phosphokinase (per 1000 U/L) | 1.05 | 1.02 to 1.08 | <0.01 | 1.03 | 1.00 to 1.06 | <0.05 |
| Lactate dehydrogenase (per 100 U/L) | 1.06 | 1.03 to 1.08 | <0.001 | 1.03 | 1.00 to 1.06 | 0.08 |
| Alkaline phosphatase (per 10 U/L) | 1.00 | 1.00 to 1.01 | 0.25 | 1.00 | 0.99 to 1.01 | 0.43 |
| C-reactive protein (per 10 mg/L) | 1.03 | 1.02 to 1.05 | <0.001 | 1.01 | 0.99 to 1.02 | 0.39 |
PMHx, Past medical history; HTN, hypertension; DM, diabetes.
Reference group White non-Hispanic/Latino and White Hispanic/Latino.
Hypercalcemia defined as corrected calcium >ULN (10.3 mg/dl).
Hyperkalemia defined as potassium >ULN (5.1 mmol/L).
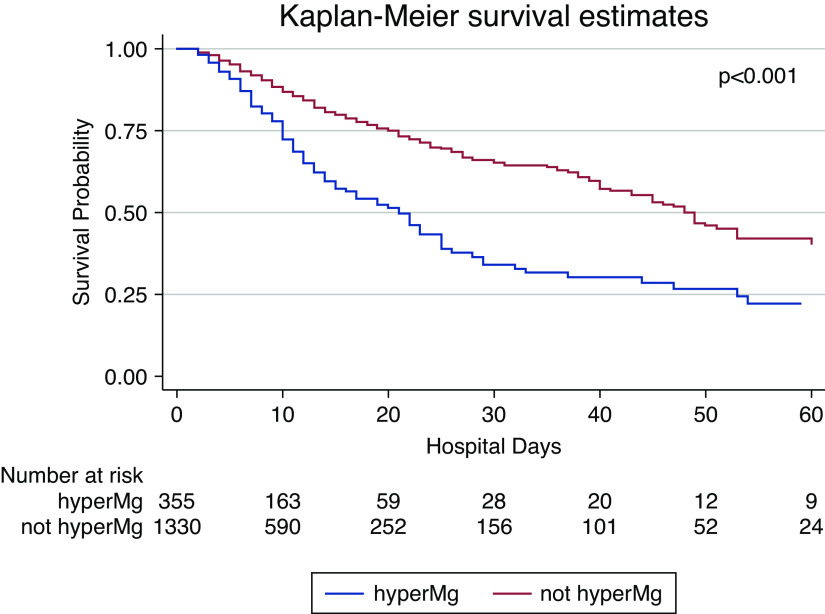
Survival probability at 30 days was 34% for the patients with hypermagnesemia, compared with 65% for patients without hypermagnesemia. By the end of follow-up, a greater proportion of patients with hypermagnesemia had died (38% vs 19%, P<0.001; Table 2). Time to event analyses identified an increased risk of mortality with older age, hypertension, diabetes, need for vasopressors, higher CRP levels, and hypermagnesemia. Hypermagnesemia was associated with a two-fold increase in mortality (Figure 2), an effect size that was similar even after adjusting for age, sex, race, comorbidities, and illness severity (n=1594; hazard ratio, 2.03; 95% CI, 1.63 to 2.54, P<0.001; Table 4). Additional time-to-event analyses were performed grouping patients by tertiles of their median magnesium levels during their hospitalization, which revealed significant lower survival probability for patients across tertiles (P<0.001; Supplemental Figure 1). Finally, sensitivity analysis excluding patients with any hypomagnesemia showed only slight differences and subsequent analysis only excluding patients with a median magnesium <1.7 mg/dl did not show any significant differences from the primary multivariable model (Supplemental Table 5).
Figure 2.
Patient survival curves by hypermagnesemia status. Kaplan–Meier curve for patient survival over time from admission for all patients stratified by those who had a median magnesium less than the upper limit of normal (red) and those who had a median magnesium greater than the upper limit of normal (>2.4 mg/dl; blue), P<0.001. HyperMg, hypermagnesemia.
Table 4.
Survival analysis showing hazard ratios for death (n=1594)
| Variable | Univariate | Multivariable | ||||
| Hazard Ratio | 95% Confidence Interval | P value | Hazard Ratio | 95% Confidence Interval | P value | |
| Demographics | ||||||
| Age (per 10 years) | 1.67 | 1.53 to 1.81 | <0.001 | 1.86 | 1.68 to 2.06 | <0.001 |
| Sex (reference: female) | 1.17 | 0.94 to 1.44 | 0.16 | 1.24 | 0.99 to 1.55 | 0.06 |
| Race (reference: White) | 0.89 | 0.71 to 1.12 | 0.33 | 1.01 | 0.80 to 1.28 | 0.92 |
| PMHx | ||||||
| HTN | 1.69 | 1.35 to 2.11 | <0.001 | 1.07 | 0.83 to 1.38 | 0.60 |
| DM | 1.45 | 1.18 to 1.77 | <0.001 | 1.22 | 0.97 to 1.52 | 0.08 |
| ESRD | 0.97 | 0.55 to 1.68 | 0.90 | 1.36 | 0.78 to 2.40 | 0.28 |
| Illness severity | ||||||
| Pressors | 1.81 | 1.44 to 2.26 | <0.001 | 1.98 | 1.53 to 2.55 | <0.001 |
| Mechanical Ventilation | 1.24 | 0.99 to 1.55 | 0.06 | |||
| AKI requiring RRT | 1.27 | 0.98 to 1.64 | 0.07 | |||
| Labs | ||||||
| C-reactive protein (per 10 mg/L) | 1.03 | 1.02 to 1.04 | <0.001 | 1.01 | 1.00 to 1.03 | 0.03 |
| Hypermagnesemiaa | 2.14 | 1.73 to 2.64 | <0.001 | 2.03 | 1.63 to 2.54 | <0.001 |
PMHx, Past medical history; HTN, hypertension; DM, xx.
Defined as median (Mg) > upper limit of normal (2.4 mg/dl).
Discussion
Our observational study demonstrates that hypermagnesemia in patients hospitalized with COVID-19 is common, with nearly one in five patients having an elevated median serum magnesium level. The presence of hypermagnesemia was associated with greater illness severity, and patients who develop hypermagnesemia experience twice the mortality rate seen in patients with normal magnesium concentrations, suggesting hypermagnesemia may serve as a useful prognostic marker in patients hospitalized with COVID-19.
Given magnesium’s role as a primarily intracellular ion, hypermagnesemia may either reflect elevated total body stores (resulting from exogenous administration, decreased clearance, or both), or a shift in magnesium between compartments. In our cohort, markers of inflammation and high cell turnover (such as potassium, phosphate, LDH, erythrocyte sedimentation rate, CRP, CPK) were all higher in the hypermagnesemia group, suggesting the elevated magnesium level may simply reflect increased cell turnover, rather than a true total body excess. The high rate of lymphocyte death and lymphocyte cell turnover combined with the underrecognized complication of rhabdomyolysis may explain the high serum magnesium observed, particularly in individuals with severe COVID-19. Although bone serves as a large magnesium reservoir, markers of elevated bone turnover, such as alkaline phosphate, were not associated with the development of hypermagnesemia, making bone an unlikely source of the elevated serum magnesium in our cohort. We did not observe any differences in either the outpatient magnesium medications that patients were receiving before hospitalization or inpatient magnesium supplementation between the two groups, making excess administration an unlikely cause. Another consideration is that these patients developed hypermagnesemia secondary to decreased renal clearance (i.e., AKI). Although this certainly remains a possibility, it is worth noting the hypermagnesemia group did have higher incidences of AKI, but it was disproportionately skewed toward higher severity AKI and, accordingly, this group also had higher rates of renal replacement therapy. Therefore, although high rates of AKI leading to decreased native renal magnesium clearance might have been an explanation for the development of hypermagnesemia in this group, the higher rates of extracorporeal magnesium clearance must also be considered. Further, the high incidence of hypermagnesemia appears to be much higher than would be traditionally encountered among patients hospitalized for other reasons who develop severe AKI. As a result, the high incidence of hypermagnesemia among patients hospitalized with COVID-19 appears to be secondary to a combination of increased cell turnover and decreased renal clearance. Other smaller cohorts have also reported a higher incidence of both rhabdomyolysis and hypermagnesemia in COVID-19 nonsurvivors compared with survivors (4). Another smaller study of 20 patients reported a 25% incidence of hypermagnesemia in their cohort, but also noted that 50% of their patients developed hypomagnesemia (5).
Whether the hypermagnesemia is merely evidence of severe illness or is in the contributory pathway for the poor prognosis cannot be determined from our analysis. Although there are many papers touting the protective effect magnesium supplementation may have in outcomes of patients with COVID-19 (putting forth magnesium’s anti-inflammatory, antioxidative, antispasmodic, vasodilatory, and neuroprotective effects), these are purely speculative (7–11). Few studies have attempted to investigate whether magnesium plays a role in outcomes in COVID-19, but all have methodological limitations (e.g., small sample size, not randomized, selection bias, and confounding) (12,13). There are also conflicting reports of the role magnesium homeostasis plays in the expression of IL-6 and other cytokines in non–COVID-19 critical illness, with some studies reporting the proinflammatory effect of hypomagnesemia leading to increased production of IL-1β, IL-6, and TNF-α and PAI-1 (11,14,15), whereas one study reported patients treated with magnesium had lower levels of TNF-α, but higher levels of IL-6, IL-4, and IL-10 (16). Unfortunately, there were too few patients in our cohort with available cytokine levels (in particular IL-6) to make any meaningful conclusions about the association with magnesium. One alternative explanation for the high incidence and poor outcomes associated with hypermagnesemia is from transcellular shift of magnesium from the intracellular to extracellular space in response to stress hormones (11), which is plausible given the association between vasopressor use and the development of hypermagnesemia in our cohort. Finally, one compelling physiologic explanation for the worse outcomes seen in hypermagnesemia is magnesium’s known vasoplegic effect on vascular smooth muscle (17). Magnesium has important antagonistic effects to calcium and functions as a physiologic calcium channel blocker, leading to a reduction of blood pressure (e.g., the standard of care in the treatment of pre-eclampsia). As a result, in the absence of additional data, our findings would argue against the use of magnesium supplementation in patients with COVID-19 as some have proposed.
Although this is the largest and most granular description of magnesium homeostasis in patients with COVID-19, it is not without limitations. First, the observational nature of the study introduces the possibility of residual confounders that were not identified. Second, because the categorization of groups in our analysis was defined by their median magnesium level, there is a possibility for temporal bias because the sequence of the event of hypermagnesemia with other clinical outcomes was not necessarily uniform. Finally, our study design precludes any determination of whether treating hypermagnesemia confers any clinical benefit by altering the clinical trajectory of these patients. Despite these limitations, the novelty of the question, large sample size, detailed description, and extensive laboratory data are strengths of this study.
In conclusion, we identified an association between hypermagnesemia among patients hospitalized with COVID-19 and increased mortality. Although the exact mechanism of this relationship remains unclear, this finding potentially represents increased cell turnover and severity of illness, which is frequently associated with more severe forms of AKI. Additional prospective studies are needed to understand: (1) the mechanisms that underlie this association, (2) the potential role of magnesium concentrations as a prognostic marker, and (3) whether correction of hypermagnesemia alters the prognosis of patients with serious severe acute respiratory syndrome coronavirus 2 infections.
Disclosures
S. Mohan reports having consultancy agreements with Angion Biomedica; reports receiving research funding from Angion Biomedica; reports being a scientific advisor or having membership as Deputy Editor, Kidney International Reports (International Society of Nephrology); Vice Chair, United Network for Organ Sharing, Data Advisory committee; member of the Scientific Registry of Transplant Recipients Visiting Committee, ASN Quality committee, and the Angion Pharma scientific advisory board. T. L. Nickolas reports having consultancy agreements with Pharmacosmos; reports receiving research funding from Amgen; reports having patents and inventions from Columbia University, which has licensed patents on neutrophil gelatinase-associated lipocalin to Abbott Diagnostics and Alere; reports being a scientific advisor or member of Amgen, and Pharmacosmos. All remaining authors have nothing to disclose.
Funding
This work is supported by is supported by the National Center for Advancing Translational Sciences grant KL2 TR001874 to S. Husain and National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK114893, R01-MD014161, and U01-DK116066 to S. Mohan.
Author Contributions
S. Mohan, A. Moses, T. Nickolas, and J. Stevens conceptualized the study and reviewed and edited the manuscript; A. Moses and J. Stevens were responsible for data curation; S. Husain, S. Mohan, and J. Stevens were responsible for formal analysis; S. Mohan, A. Moses, and J. Stevens were responsible for the investigation; S. Mohan, A. Moses, S. Husain, and J. Stevens were responsible for the methodology; S. Husain and S. Mohan provided supervision; S. Husain and J. Stevens were responsible for validation; S. Husain, S. Mohan, and J. Stevens were responsible for visualization; S. Husain, S. Mohan, A. Moses, and J. Stevens wrote the original draft.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0002592021/-/DCSupplemental
International classification of disease codes used for determining medical history. Download Supplemental Table 1, PDF file, 256 KB (255KB, pdf)
Illness severity concordance. Download Supplemental Table 2, PDF file, 256 KB (255KB, pdf)
Missing data. Download Supplemental Table 3, PDF file, 256 KB (255KB, pdf)
Sensitivity analysis of the logistic regression analysis of characteristics and outcomes associated with developing hypermagnesemia, excluding patients with hypomagnesemia. Download Supplemental Table 4, PDF file, 256 KB (255KB, pdf)
Sensitivity analysis of the survival analysis showing hazard ratios for death excluding patients with hypomagnesemia. Download Supplemental Table 5, PDF file, 256 KB (255KB, pdf)
Patient survival curves by median magnesium tertiles. Kaplan–Meier curve for patient survival over time from admission for all patients stratified by tertiles of median magnesium (lowest values = tertile 1 (blue) whereas highest values = tertile 3 (green); P<0.001). Download Supplemental Figure 1, PDF file, 256 KB (255KB, pdf)
References
- 1.Whang R, Hampton EM, Whang DD: Magnesium homeostasis and clinical disorders of magnesium deficiency. Ann Pharmacother 28: 220–226, 1994. 10.1177/106002809402800213 [DOI] [PubMed] [Google Scholar]
- 2.Arnaud MJ: Update on the assessment of magnesium status. Br J Nutr 99[Suppl 3]: S24–S36, 2008. 10.1017/S000711450800682X [DOI] [PubMed] [Google Scholar]
- 3.Coburn JW, Popovtzer MM, Massry SG, Kleeman CR: The physicochemical state and renal handling of divalent ions in chronic renal failure. Arch Intern Med 124: 302–311, 1969. 10.1001/archinte.1969.00300190042007 [DOI] [PubMed] [Google Scholar]
- 4.Ouyang SM, Zhu HQ, Xie YN, Zou ZS, Zuo HM, Rao YW, Liu XY, Zhong B, Chen X: Temporal changes in laboratory markers of survivors and non-survivors of adult inpatients with COVID-19. BMC Infect Dis 20: 952, 2020. 10.1186/s12879-020-05678-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarvazad H, Cahngaripour SH, Eskandari Roozbahani N, Izadi B: Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. New Microbes New Infect 38: 100807, 2020. 10.1016/j.nmni.2020.100807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007. 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang CF, Ding H, Jiao RQ, Wu XX, Kong LD: Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol 886: 173546, 2020. 10.1016/j.ejphar.2020.173546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kempen TATG, Deixler E: SARS-CoV-2: Influence of phosphate and magnesium, moderated by vitamin D, on energy (ATP) metabolism and on severity of COVID-19. Am J Physiol Endocrinol Metab 320: E2–E6, 2021. 10.1152/ajpendo.00474.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace TC: Combating COVID-19 and building immune resilience: A potential role for magnesium nutrition? J Am Coll Nutr 39: 685–693, 2020. 10.1080/07315724.2020.1785971 [DOI] [PubMed] [Google Scholar]
- 10.Younes B, Alshawabkeh AD, Jadallah ARR, Awwad EF, Tarabsheh TMI: Magnesium sulfate extended infusion as an adjunctive treatment for complicated COVID-19 infected critically ill patients. EAS J Anesthesiol Crit Care 2: 97–101, 2020 [Google Scholar]
- 11.Iotti S, Wolf F, Mazur A, Maier JA: The COVID-19 pandemic: Is there a role for magnesium? Hypotheses and perspectives. Magnes Res 33: 21–27, 2020. 10.1684/mrh.2020.0465 [DOI] [PubMed] [Google Scholar]
- 12.Procter BC, Ross C, Pickard V, Smith E, Hanson C, McCullough PA: Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med 21: 611–614, 2020. 10.31083/j.rcm.2020.04.260 [DOI] [PubMed] [Google Scholar]
- 13.Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, Wong HM, Tern PJW, Chandran M, Chay JWM, Nagarajan C, Sultana R, Low JGH, Ng HJ: Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 79–80: 111017, 2020. 10.1016/j.nut.2020.111017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper ID, Crofts CAP, DiNicolantonio JJ, Malhotra A, Elliott B, Kyriakidou Y, Brookler KH: Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: Rationale for clinical management. Open Heart 7: e001356, 2020. 10.1136/openhrt-2020-001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maier JA, Castiglioni S, Locatelli L, Zocchi M, Mazur A: Magnesium and inflammation: Advances and perspectives [published online ahead of print November 18, 2020]. Semin Cell Dev Biol 2020 [DOI] [PubMed] [Google Scholar]
- 16.Chung HS, Park CS, Hong SH, Lee S, Cho ML, Her YM, Sa GJ, Lee J, Choi JH: Effects of magnesium pretreatment on the levels of T helper cytokines and on the severity of reperfusion syndrome in patients undergoing living donor liver transplantation. Magnes Res 26: 46–55, 2013. 10.1684/mrh.2013.0338 [DOI] [PubMed] [Google Scholar]
- 17.Altura BM, Altura BT, Carella A, Gebrewold A, Murakawa T, Nishio A: Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol 65: 729–745, 1987. 10.1139/y87-120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
International classification of disease codes used for determining medical history. Download Supplemental Table 1, PDF file, 256 KB (255KB, pdf)
Illness severity concordance. Download Supplemental Table 2, PDF file, 256 KB (255KB, pdf)
Missing data. Download Supplemental Table 3, PDF file, 256 KB (255KB, pdf)
Sensitivity analysis of the logistic regression analysis of characteristics and outcomes associated with developing hypermagnesemia, excluding patients with hypomagnesemia. Download Supplemental Table 4, PDF file, 256 KB (255KB, pdf)
Sensitivity analysis of the survival analysis showing hazard ratios for death excluding patients with hypomagnesemia. Download Supplemental Table 5, PDF file, 256 KB (255KB, pdf)
Patient survival curves by median magnesium tertiles. Kaplan–Meier curve for patient survival over time from admission for all patients stratified by tertiles of median magnesium (lowest values = tertile 1 (blue) whereas highest values = tertile 3 (green); P<0.001). Download Supplemental Figure 1, PDF file, 256 KB (255KB, pdf)