Visual Abstract
Keywords: acute kidney injury and ICU nephrology, angiotensin-converting enzyme 2, basic science, heme oxygenase, ischemia reperfusion, lipopolysaccharide, peptidyl-dipeptidase A, urinary tract obstruction
Key Points
The healthy kidney prominently expresses angiotensin-converting enzyme 2 (ACE2) mRNA, protein, and activity, with ACE2 protein abundantly displayed in proximal tubules.
Renal ACE2 expression decreases after ischemic AKI but is induced by LPS in a heme oxygenase-1-dependent manner.
ACE2 induction/angiotensin (1–7) and angiotensin (1–7) may offer therapeutic approaches for AKI without or with coronavirus disease 2019, respectively.
Abstract
Background
The actions of angiotensin-converting enzyme 2 (ACE2) oppose those of the renin-angiotensin-aldosterone system. ACE2 may be a cytoprotectant in some tissues. This study examined ACE2 expression in models of AKI.
Methods
ACE2 mRNA and protein expression and ACE2 activity were assessed in murine ischemic AKI. Renal ACE2 mRNA expression was evaluated in LPS-induced AKI in wild-type (C57BL/6J) mice, in heme oxygenase-1+/+ and heme oxygenase-1−/− mice, and after unilateral ureteral obstruction (UUO) in wild-type mice. The effect of sex and age on renal ACE2 protein expression was also assessed.
Results
In ischemic AKI, ACE2 mRNA and protein expression and ACE2 activity were reduced as compared with such indices in the intact kidney. In ischemic AKI, ACE2, which, in health, is prominently expressed in the tubular epithelium, especially proximal tubules, is decreased in expression in these segments. Decreased ACE2 expression in AKI did not reflect reduced GFR, because ACE2 mRNA expression was unaltered after UUO. LPS induced renal ACE2 mRNA expression in wild-type mice, but this effect did not occur in heme oxygenase-1–deficient mice. In ischemic and LPS-induced AKI, renal expression of the Mas receptor was increased. In the intact kidney, renal ACE2 protein expression decreased in female mice as compared with male mice, but was unaltered with age.
Conclusion
We conclude that renal ACE2 expression is decreased in ischemic AKI, characterized by decreased GFR and abundant cell death, but is upregulated in LPS-induced AKI, an effect requiring heme oxygenase-1. Determining the significance of ACE2 expression in experimental AKI merits further study. We suggest that understanding the mechanism underlying ACE2 downregulation in AKI may offer insights relevant to COVID-19: ACE2 expression is downregulated after ACE2 mediates SARS-CoV-2 cellular entry; such downregulation is proinflammatory; and AKI commonly occurs and determines outcomes in COVID-19.
Introduction
By promoting renal reabsorption of salt and water and by its vasopressor and other effects, the renin-angiotensin-aldosterone system (RAAS) safeguards systemic and regional hemodynamics and critically contributes to homeostasis and health. However, when inordinately or inappropriately activated, components of the RAAS may promote cardiovascular, kidney, and inflammatory diseases (1). As is broadly true for virtually all biologic systems, endogenous countervailing processes concomitantly exist that mitigate the intrinsic effects of these primary systems. In the case of the RAAS, one such countervailing process is provided by angiotensin-converting enzyme 2 (ACE2): the ACE2 receptor, soluble ACE2 in the circulation, and ACE2-dependent signaling pathways exert effects—vasodilatory, antioxidant, anti-inflammatory, and antifibrotic—that offset those exerted by sustained and/or inordinate elevations in systemic or tissue levels of components of the RAAS (1–3).
Discovered in 2000, ACE2 attracted exponentially rising interest in 2020, the year the current coronavirus disease 2019 (COVID-19) pandemic began: the ACE2 surface receptor is now recognized as a critical pathway enabling intracellular invasion by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus) (2–4). The spike (S) protein of the virus engages the ACE2 receptor and undergoes priming by the serine protease TMPRSS2; the virus then enters cells and commandeers cellular machinery with attendant viral replication and cell injury. Interestingly, ACE2 expression decreases after the virus enters cells (2–4). The significance of ACE2 in COVID-19 and what influences ACE2 expression in tissues have motivated substantial investigation since the COVID-19 pandemic began.
In addition to the pulmonary epithelium, ACE2 is expressed in other cells, including cells in the kidney (2,3). Current understanding of the significance of such renal expression is limited, as is an appreciation of how such expression is altered in disease states. This study examined the expression of ACE2 in models of AKI largely for two reasons. First, this study provides the foundational steps for an investigative line that seeks to address the functional significance of ACE2 in the injured kidney. Second, insights on alterations in expression of ACE2 (and their functional significance) in the injured kidney may be relevant to COVID-19 in general, and when AKI occurs in COVID-19, in particular.
Our study examined a murine model of ischemia reperfusion injury (IRI) largely characterized by markedly reduced GFR and cell death; we also examined inflammation-induced (LPS) AKI and urinary tract obstruction. Because male sex and older age are strong risk factors for AKI, we also examined whether ACE2 expression is altered by sex differences and age. In some of these studies, we used as a comparator for changes in ACE2 expression isoforms of the heme oxygenase (HO) system, the inducible HO-1 isoform, and the constitutive HO-2 isoform (5,6).
Materials and Methods
Overview of Murine Studies
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Mayo Clinic. For all studies involving surgical procedures, buprenorphine analgesia was administered.
These studies used three well-established and commonly used murine models of AKI. In the majority of studies, male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, 10–15 weeks of age) were used. In studies examining the effect of sex, 10-week-old male and female C57BL/6J mice were used and in studies examining the effect of age, 10-week-old and 22–23-month-old male C57BL/6J mice were used. In additional studies, equal numbers of male and female age-matched HO-1+/+ and HO-1−/− mice (10–15 weeks of age) were used; HO-1+/+ and HO-1−/− mice were generated from colonies maintained as described previously (7,8).
IRI in Mice
As previously used, bilateral renal ischemia was performed in C57BL/6J mice (7–9). Briefly, the surgical procedure was performed under pentobarbital (50 mg/kg, intraperitoneally) anesthesia and on a heat-regulated surface to maintain body temperature at 37°C. Via a midline abdominal incision, gentle dissection was used to isolate each renal pedicle and ischemia (18 or 22.5 minute duration) was induced using nontraumatic clamps (straight, 10 mm, 125 g pressure micro aneurysm clip, model no. RS5426, Roboz Surgical Instruments, Rockville, MD). At 1 and 2 days after ischemia, kidneys were harvested for gene and protein assessments and plasma was collected for measurement of renal function. Sham procedures involved midline incision, but neither renal pedicle dissection nor clamping.
LPS-induced AKI in Mice
As used in our previous study, AKI was induced by the intravenous administration of LPS (10). In this study, LPS (10 mg/kg, IV, Escherichia coli, serotype 0127:B8, batch 82454, Sigma, St Louis, MO) was administered to C57BL/6J mice and at 4, 8, and 15 hours after administration, renal tissues were harvested for gene and protein assessment and plasma was collected for measurement of renal function. In studies involving HO-1+/+ and HO-1−/− mice, LPS was administered at a lower dose (2 mg/kg, intraperitoneally), and tissues and plasma were harvested at 24 hours after treatment.
Studies of UUO in Mice
UUO was performed as described in our previous study (11). Briefly, under pentobarbital anesthesia (50 mg/kg, intraperitoneally), a midline abdominal incision was made, and the left kidney was exposed by retraction of the intestines. The left ureter was then isolated and doubly ligated using a 6–0 silk suture at the midpoint between the kidney and the bladder. Similarly, sham-operated control mice underwent the abdominal incision and retraction of the intestines without ureteral ligation.
mRNA Expression by Quantitative Real-time RT-PCR
Renal expression levels of ACE2, HO-1, and HO-2 mRNAs were measured by quantitative real-time RT-PCR, as we have previously described (8,10). Briefly, extraction of total RNA was performed with TRIzol reagent (Invitrogen, Carlsbad, CA) and further purified with the RNeasy Mini Kit (Qiagen, Valencia, CA). After reverse transcription (Transcriptor First Strand cDNA Synthesis Kit, Roche Applied Science, Indianapolis, IN) relative expression levels were measured by quantitative real-time PCR analysis using a QuantStudio 7 Real-Time PCR System (ThermoFisher Scientific, Waltham, MA). TaqMan Gene Expression Assay sets (Applied Biosystems, ThermoFisher Scientific) containing probes and primers for each target were used for the quantitation reactions; standard curves constructed for each target and housekeeping gene consisting of serial ten-fold dilutions of cDNA stocks were used. Expression of 18S rRNA was used for normalization of the expression of each target gene.
Western Blot Analysis
Western blot analysis was performed as in our previous studies (7,8). Primary antibodies against HO-1 (catalog ADI-SPA-895; Enzo Life Sciences, Farmingdale, NY), HO-2 (catalog ADI-SPA-897; Enzo Life Sciences), ACE2 (catalog NBP1–76614; Novus Biologicals, Centennial, CO), MAS1 protein (Mas receptor, catalog PA5–77282; ThermoFisher Scientific), and GAPDH (catalog 2118; Cell Signaling Technology, Danvers, MA) were used for overnight incubations at 4°C. Peroxidase-conjugated secondary antibodies were then used and an enhanced chemiluminescence method was used for band visualization.
Measurement of ACE2 Activity
ACE2 activity was measured in mouse kidney using a fluorogenic method (12,13). In this study, renal tissue was homogenized (1:10 w/v) in chilled buffer: 50 mM HEPES, pH 7.4, 150 mM NaCl, 0.5% Triton X-100, 25 µM ZnCl2, and 1mM PMSF. The homogenates were centrifuged (10,000 g, 15 minutes at 4°C) and the supernatant was assayed for protein content (BCA Protein Assay, ThermoFisher Scientific). Supernatants (2 µg protein) were assayed in 100 µl reactions containing: 50 mM 4-Morpholineethanesulfonic acid, pH 6.5, 300 mM NaCl, 10 µM ZnCl2, 20 µM captopril, 0.01% Triton X, and 30 µM substrate (Mca-APK[Dnp], Enzo Life Sciences) and an EDTA-free protease inhibitor tablet (catalog 11873580001, Roche Diagnostics). After a 5-minute preincubation at 37°C, generation of fluorescence was followed for 40 minutes using a FluoroSkan fluorescence plate reader (ThermoFisher Scientific, Waltham, MA), with an excitation wavelength of 320 nm and an emission wavelength of 415 nm. Reactions for each sample were assessed in the presence and absence of the ACE2 inhibitor MLN-4760 (250 µM, catalog 5.30616.0001, EMD Millipore, Burlington, MA) and ACE2-specific activity was determined as the fluorescence generated in the absence of the inhibitor minus the fluorescence in the presence of the inhibitor and expressed as RFU/µg protein per minute.
Immunofluorescence Staining
Immunofluorescence staining was performed on 8 μm sections cut from formalin-fixed, paraffin-embedded kidney tissues. Slides were deparaffinized and antigen retrieval with acidic citrate buffer (pH 6.0) was performed. After quenching in 100 mM NH4Cl for 20 minutes and blocking (5% normal donkey serum, 5% BSA in 0.1% Triton X-100, PBS) for 2 hours, slides were incubated with primary antibody at 4°C overnight. The next day the secondary antibody incubation was performed at room temperature for 2 hours. Washes using PBS + 0.05% Triton X were performed between the incubations. Primary antibodies against ACE2 (catalog NBPI-76614, Novus Biologicals) and NGAL (catalog AF1757, R&D Systems, Minneapolis, MN) were used. Microscope images (Zeiss Axio Observer Z1) were acquired using a 10× lens (NA 0.3) and images were prepared using Photoshop. All exposure levels were identical within the groups.
Assessment of Renal Function
Measurement of plasma levels of BUN was performed using a commercially available kit (catalog B7551–120, Pointe Scientific, Canton, MI). Plasma creatinine was measured with a Creatinine Analyzer 2 (Beckman Instruments, Fullerton, CA).
Statistics
Data are expressed as mean±SD and considered statistically significant for P<0.05. All graphs display individual data values for all measurements as dot-whisker plots. The student’s t test was used for parametric data and the Mann–Whitney U-test was used for nonparametric data.
Results
Table 1 lists values of glomerular filtration markers, serum creatinine, and BUN, at the relevant timepoints in models of AKI.
Table 1.
Renal indices in AKI models studied
| Model, n = Control, Test | Index | Control | Test | P value |
| IRI at 1 day (mouse, 22.5 min) | Plasma creatinine | 0.20±0.06 | 2.53±0.32 | 0.020 |
| n = 4, 5 | BUN | 19.3±3.1 | 141.5±12.1 | 0.016 |
| IRI at 2 days (mouse, 18 min) | Plasma creatinine | 0.18±0.04 | 2.57±0.21 | 0.003 |
| n = 6, 7 | BUN | 21.6±1.4 | 232.7±22.0 | 0.003 |
| LPS at 4 hours (mouse, 10 mg/kg) | Plasma creatinine | 0.23±0.04 | 0.27±0.05 | 0.204 |
| n = 5, 5 | BUN | 18.0±2.1 | 27.4±3.4 | 0.001 |
| LPS at 8 hours (mouse, 10 mg/kg) | Plasma creatinine | 0.23±0.04 | 0.31±0.03 | 0.027 |
| n = 5, 5 | BUN | 21.8±1.7 | 59.8±15.4 | 0.012 |
| LPS at 15 hours (mouse, 10 mg/kg) | Plasma creatinine | 0.21±0.03 | 0.39±0.05 | 0.006 |
| n = 5, 6 | BUN | 27.8±2.6 | 67.7±10.7 | 0.008 |
Data are expressed as mean±SD. IRI, ischemia reperfusion injury.
Studies in the Murine IRI Model
At day 1 after a duration of ischemia lasting 22.5 minutes, ACE2 mRNA and protein expression were reduced, whereas mRNA and protein expression of the inducible HO isoform, HO-1, was increased, and that of the constitutive HO isoform, HO-2, unchanged (Figure 1). At this time point, ACE2 activity was significantly diminished (4.30±0.09 vs 3.50±0.29 RFU/µg protein per min; P<0.02). Studies were also undertaken at a later time point after IRI, but with a shorter duration of ischemia, namely, 2 days and 18 minutes, respectively. As shown in Table 2, a similar profile was observed: ACE2 mRNA and protein expression were reduced and accompanied by decreased ACE2 activity, whereas HO-1 mRNA and protein expression was induced.
Figure 1.
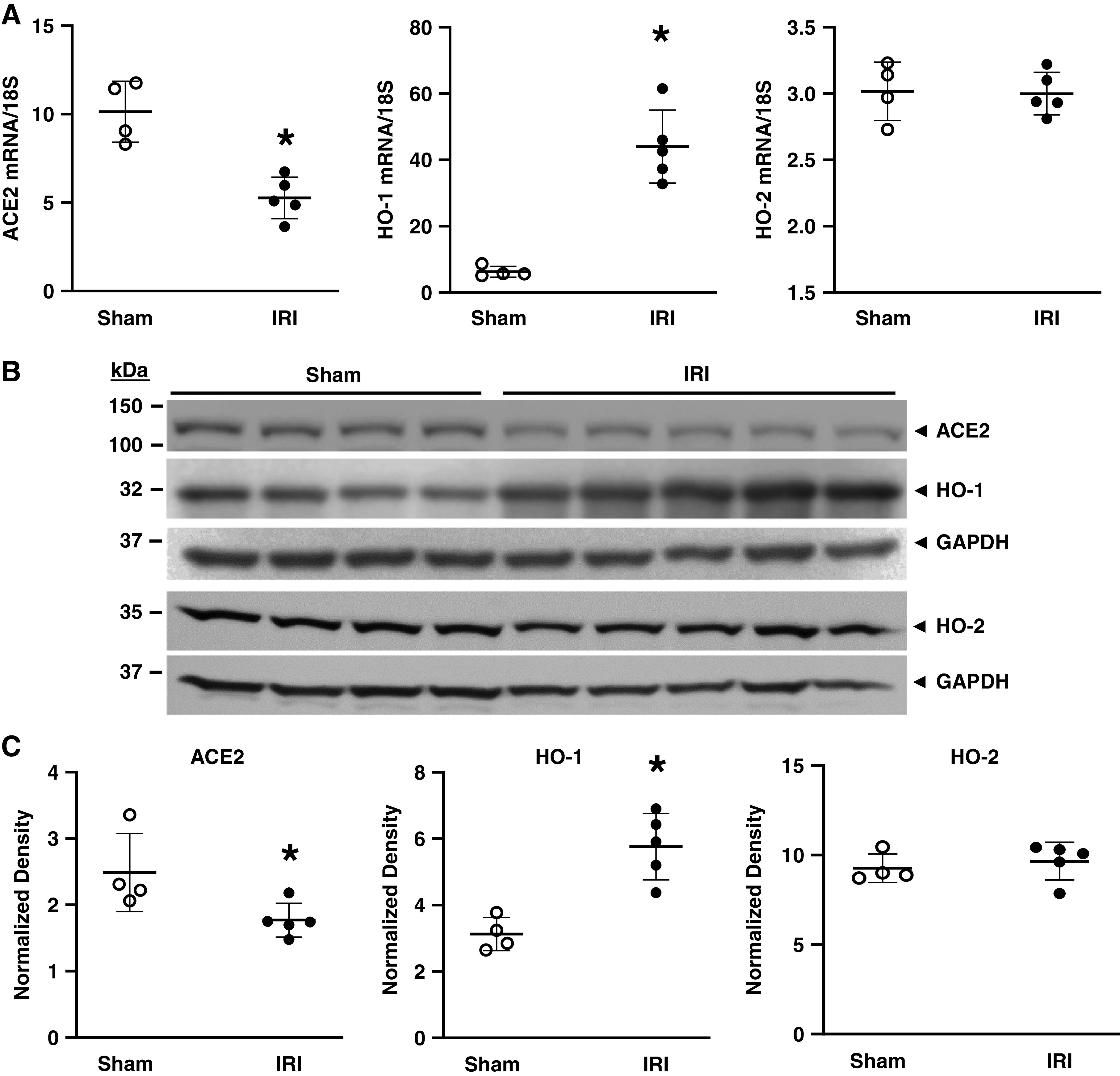
The effect of ischemia reperfusion injury (IRI) on renal angiotensin-converting enzyme 2 (ACE2), heme oxygenase-1 (HO-1), and HO-2 expression in mice 1 day after 22.5 minutes of ischemia. (A) ACE2 (left panel), HO-1 (middle panel), and HO-2 (right panel) mRNA expression in mice subjected to sham or IRI as measured by quantitative real-time RT-PCR. (B) Western blot analysis of ACE2, HO-1, and HO-2 protein expression with assessment of equal loading by immunoblotting for GAPDH. (C) Normalized densitometric analysis of Western blots of ACE2 (left panel), HO-1 (middle panel), and HO-2 (right panel) expression. n = 4 and n = 5 in sham and IRI groups, respectively; *P<0.05 versus sham group.
Table 2.
Angiotensin-converting enzyme 2 and heme oxygenase-1 expression in the murine ischemia reperfusion injury model at 2 days post ischemia
| Index, Unit | Sham | IRI (18 min) | P value |
| ACE2 mRNA/18S | 1.89±0.42 | 0.89±0.17 | 0.0001 |
| Relative expression | |||
| HO-1 mRNA/18S | 1.52±0.14 | 12.39±5.68 | 0.0034 |
| Relative expression | |||
| ACE2 protein expression | 2.82±0.28 | 1.36±0.15 | 0.0001 |
| Normalized density | |||
| HO-1 protein expression | 14.07±1.26 | 38.57±3.43 | 0.0012 |
| Normalized density | |||
| ACE2 activity | 3.05±0.29 | 2.38±0.25 | 0.0009 |
| RFU/µg protein per min |
Data are expressed as mean±SD, n = 6 and 7 for sham and IRI groups, respectively. ACE2, angiotensin-converting enzyme 2; HO-1, heme oxygenase-1.
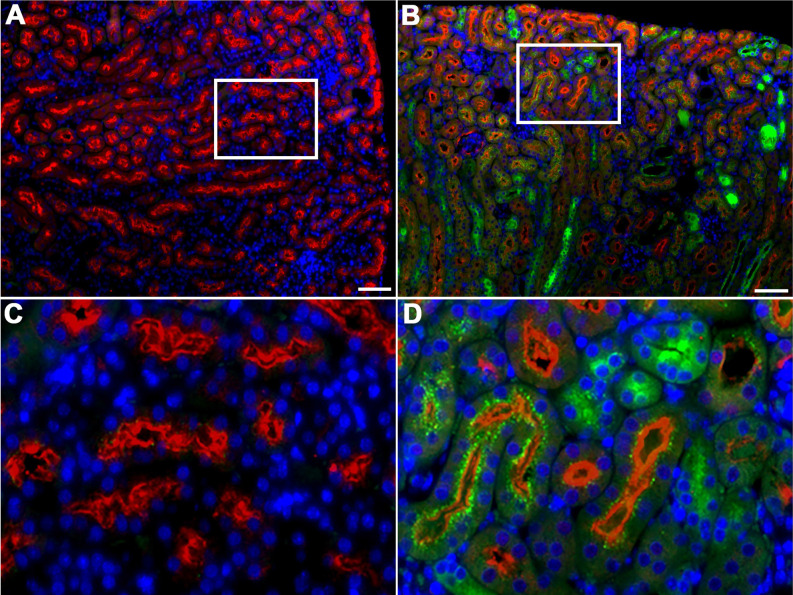
Immunofluorescence studies were undertaken at 2 days after 18 minutes of ischemia. We performed studies using NGAL as a comparator for an inducible injury-responsive gene after IRI. As shown in Figure 2, in the sham-treated (intact) kidney ACE2 was expressed in tubular epithelial cells, more prominently in the proximal tubules, with strong apical staining for ACE2. After IRI, apical ACE2 expression was markedly decreased especially in the proximal tubules; such changes after IRI were accompanied by increased tubular expression of NGAL, a marker of tubular injury.
Figure 2.
Localization of renal ACE2 and NGAL protein expression in the kidney 2 days after IRI (18 minutes). Immunofluorescence staining for DAPI (blue), ACE2 (red), and NGAL (green) in kidneys of mice two days after sham (A) or IRI (B). There is reduced tubular ACE2 staining in kidneys of mice subjected to IRI accompanied by increased NGAL staining in tubules. High power insets of (A) and (B) are shown in (C) and (D) respectively. After IRI, apical ACE2 expression markedly decreased especially in the proximal tubules. Scale bar = 50 µm.
Studies in LPS-induced AKI
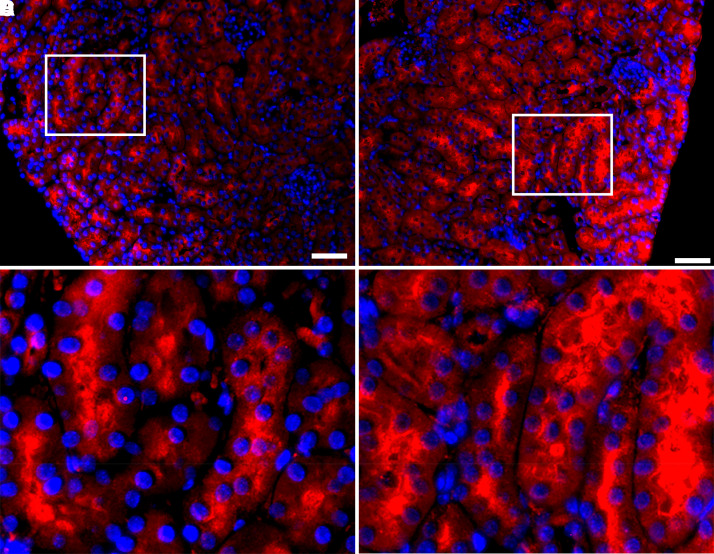
In contrast to ischemic AKI, a relatively severe model of AKI that is attended by prominent cell death and reduction in GFR, LPS-induced AKI consistently exhibited induction of ACE2 mRNA, albeit less pronounced than what is observed for HO-1 mRNA expression after the administration of LPS (Figure 3). In immunofluorescence studies, ACE2 protein expression was increased in the kidney, in particular, in proximal tubules (Figure 4).
Figure 3.

Renal HO-1 and ACE2 mRNA expression in mice after LPS administration. HO-1 and ACE2 mRNA expression was assessed in the kidneys of mice at 4, 8, and 15 hours after saline or LPS (10 mg/kg, intravenous, IV) treatment. mRNA expression was determined by quantitative real-time RT-PCR. n = 5 and n = 5–6 in saline-treated and LPS-treated groups, respectively; *P<0.05 vs saline-treated group for that timepoint.
Figure 4.
Localization of renal ACE2 protein expression in the kidney 15 hours after LPS administration. Immunofluorescence staining for DAPI (blue) and ACE2 (red) in kidneys of mice at 15 hours after saline (A) or LPS (10 mg/kg, IV) administration (B). After LPS administration, increased ACE2 expression in tubules of the murine kidney was observed. High power insets of (A) and (B) showing the localization of ACE2 expression in proximal tubules is shown in (C) and (D) respectively. Scale bar = 50 µm.
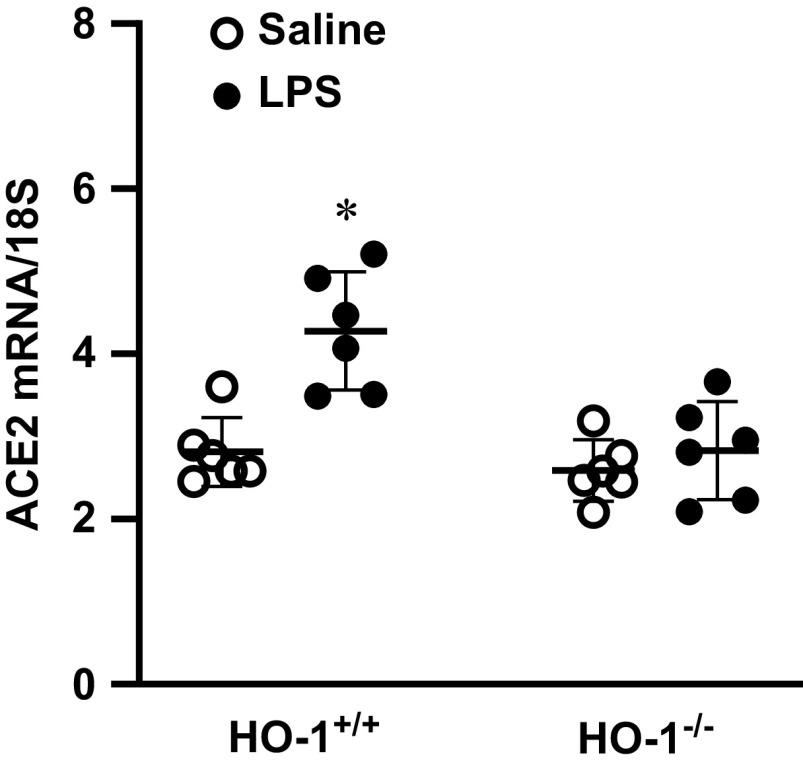
ACE2 mRNA was also examined in the kidney after the administration of LPS to HO-1+/+ and HO-1−/− mice (Figure 5). In HO-1+/+ mice, LPS induced the expression of ACE2 mRNA, whereas such induction did not occur in HO-1−/− mice.
Figure 5.
Renal ACE2 mRNA expression in HO-1+/+ and HO-1−/− mice after LPS administration. ACE2 mRNA expression was assessed in the kidneys of HO-1+/+ and HO-1−/− mice 1 day after saline or LPS (2 mg/kg, intraperitoneal, IP) treatment. mRNA expression was assessed by quantitative real-time RT-PCR. n = 6 in each group. *P<0.05 versus saline-treated HO-1+/+ group.
Expression of The Mas Receptor in the IRI Model and LPS-induced AKI
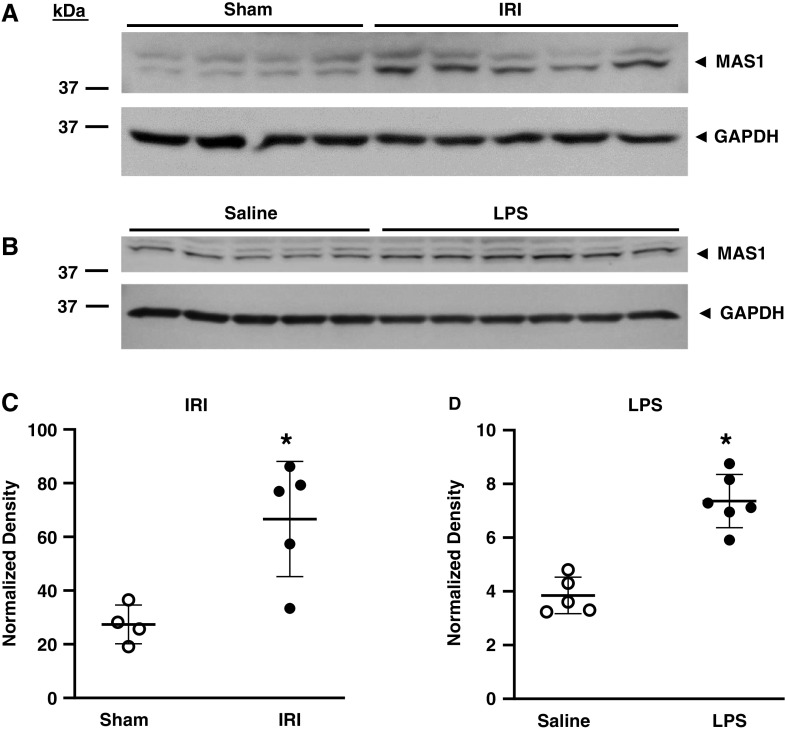
Angiotensin (1–7), the main product of ACE2 activity, acts through the Mas receptor. We thus examined protein expression of the Mas receptor in these models of AKI. As demonstrated in Figure 6, expression of Mas receptor protein was significantly increased in the kidney after IRI and LPS-induced AKI.
Figure 6.
Renal Mas receptor protein expression in mice after IRI and after LPS administration. Western blot analysis of renal Mas receptor (MAS1) protein expression was determined in mice 1 day after sham or IRI (22.5 minutes) surgery (A) and in mice 15 hours after saline or LPS (10 mg/kg, IV) treatment (B). Equal loading was assessed by immunoblotting of GAPDH, and normalized densitometric analyses for IRI studies (C) and for LPS studies (D) were performed. n = 4 and n = 5 for sham and IRI groups, respectively, in (C), and n = 5 and n = 6 for saline and LPS groups, respectively, in (D); *P<0.05.
Studies in Urinary Tract Obstruction
As GFR is variably reduced in models of AKI, with GFR more markedly reduced in ischemic AKI as compared with LPS-induced AKI, we questioned whether reduced GFR per se parallels or contributes to reduced ACE2 expression. Expression was thus examined in a model of unilateral urinary tract obstruction 7 days after obstruction is imposed. At this timepoint this model is characterized by essentially no glomerular filtration, little or no necrosis, and variable amounts of interstitial inflammation. Expression of ACE2 mRNA, like expression of HO-1 and HO-2 mRNA, was not significantly altered in the kidney in UUO (Table 3).
Table 3.
Angiotensin-converting enzyme 2, heme oxygenase-1, and heme oxygenase-2 mRNA expression in the murine unilateral ureteral obstruction model at 7 days post obstruction
| Target mRNA | Sham | Unilateral Ureteral Obstruction | P value |
| ACE2 | 3.48±0.28 | 4.14±0.89 | 0.216 |
| HO-1 | 2.26±0.65 | 3.10±1.23 | 0.152 |
| HO-2 | 2.90±0.25 | 2.76±0.31 | 0.362 |
Relative mRNA expression is standardized for 18S rRNA expression. Data are expressed as mean±SD, n = 6 and 9 for sham and unilateral ureteral obstruction groups, respectively. ACE2, angiotensin-converting enzyme 2; HO-1, heme oxygenase-1; HO-2, heme oxygenase-2.
Effect of Sex and Age on ACE2 Expression
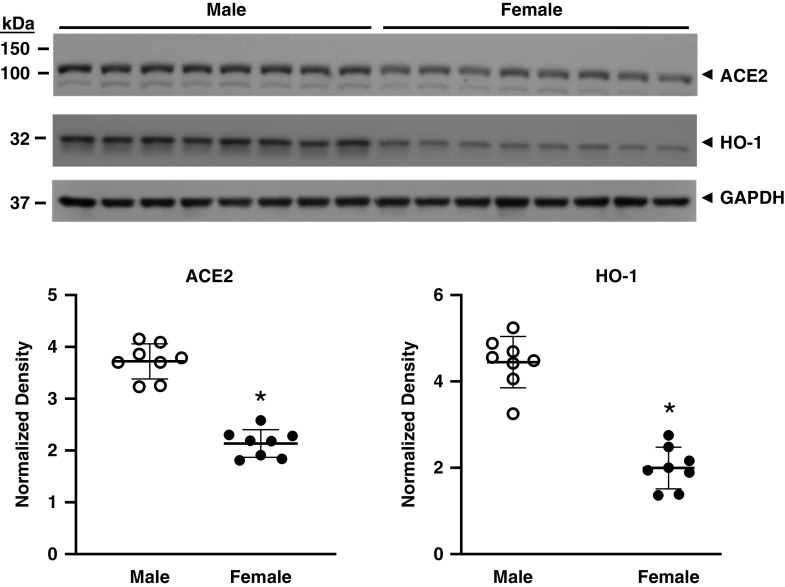
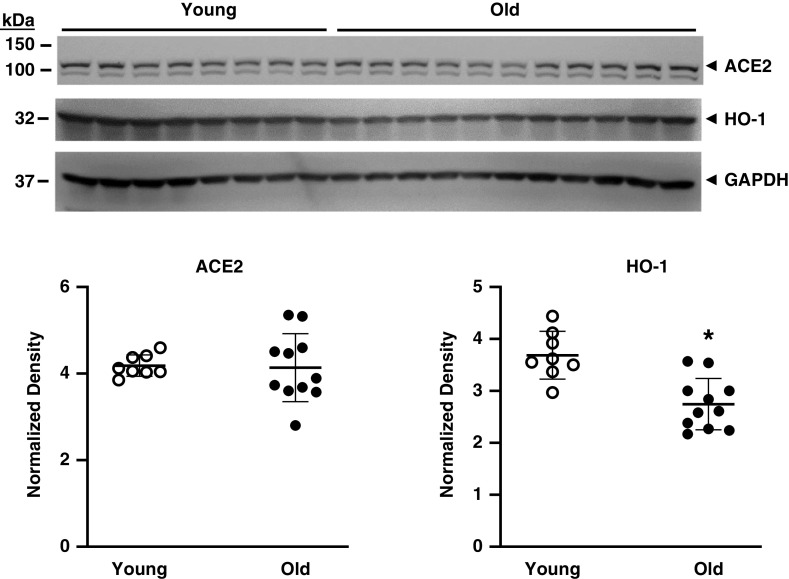
Sex and age are major determinants of sensitivity to AKI, with male sex and older age significantly predisposing to AKI (14–17). We thus studied their effects on expression of ACE2 protein in the kidney. Female mice as compared with similarly aged male mice exhibited a significantly lower expression of ACE2 protein and HO-1 protein in the kidney (Figure 7). ACE2 protein expression was unaltered in old male mice as compared with young male mice, whereas there was a significant reduction in HO-1 protein expression with aging (Figure 8).
Figure 7.
The effect of sex on renal ACE2 and HO-1 protein expression in mice. Western blot analysis of renal ACE2 and HO-1 protein expression was determined in male and female mice with assessment of equal loading by immunoblotting for GAPDH. Normalized densitometric analysis for ACE2 (left panel) and HO-1 (right panel) is displayed below the Western blots. n = 8 in each group; *P<0.05 versus male group.
Figure 8.
The effect of age on renal ACE2 and HO-1 protein expression in mice. Western blot analysis of renal ACE2 and HO-1 protein expression was determined in young (10 weeks old) and old (22–23 months old) male mice with assessment of equal loading by immunoblotting for GAPDH. Normalized densitometric analysis for ACE2 (left panel) and HO-1 (right panel) is displayed below the Western blots. n = 8 and n = 11 in young and old groups, respectively; *P<0.05.
Discussion
To the best of our knowledge, this study provides the first comparative analysis of ACE2 expression in ischemic AKI and less severe models of AKI. We demonstrate that in ischemic AKI, a model attended by markedly reduced GFR and by abundant cell death, ACE2 mRNA and protein expression and ACE2 activity were all significantly reduced at days 1 and 2 after IRI. A prior study in rats showed that ACE2 mRNA expression was decreased at 4 hours after IRI with ACE2 activity unaltered at this timepoint (18). Although not extending beyond this early 4 hour timepoint after IRI, these studies demonstrated that intrarenal angiotensin (1–7) levels were decreased at this time point after IRI (18). The decreased ACE2 expression we observed did not simply reflect generalized compromise in gene transcription and translation in a severely damaged kidney as other genes such as HO-1 were concomitantly induced and translated into protein. Decreased expression of ACE2 was neither consistently paralleled by, nor could it be ascribed to, decreased GFR per se because expression of ACE2 mRNA was unaltered in urinary tract obstruction in which glomerular filtration ceases. The basis for this downregulation after renal IRI is unknown. In other tissues, angiotensin II (19), endothelin-1 (19,20), ERK1/ERK2 (19), HIF1α (21), and FGF23 (22) may all decrease ACE2 mRNA and/or ACE2 activity. We speculate that such molecular species, which are elevated in plasma and/or kidney in AKI, may underlie the suppression of ACE2 that occurs in AKI.
LPS induced ACE2 mRNA at the early timepoints in our study. As is generally accepted, LPS causes renal vasoconstriction, decreased GFR, and a pronounced upregulation of inflammatory and vasoconstrictive genes; however, unlike ischemic AKI, little in the way of cell death occurs in this model. We suggest that cells progressing toward or evincing death pathways (for example, necrosis, apoptosis, necroptosis, ferroptosis, among others), as occurs in IRI, relinquish processes essential in maintaining the endogenous expression of ACE2 mRNA and protein, or activate processes that suppress ACE2 expression.
This inductive effect of LPS was also observed in studies undertaken in HO-1+/+ and HO-1−/− mice, in that HO-1+/+ mice evinced induction of ACE2 mRNA in response to LPS, although the dose of LPS previously used was considerably less (2 mg/kg versus 10 mg/kg) (10). Interestingly, HO-1−/− mice failed to demonstrate induction of ACE2 mRNA as observed in HO-1+/+ mice. This raises at least two possibilities. First, the kidney in HO-1−/− mice may be more sensitive to LPS (10), and the failure of induction of ACE2 mRNA may simply reflect greater histologic damage. However, in our prior studies, there was little histologic injury or cell death in the kidney in either HO-1+/+ or HO-1−/− mice subjected to this dose of LPS and at that time point (10). Second, it is possible that HO-1 gene expression may be involved in some as yet unresolved way in maintaining ACE2 mRNA expression. In this regard, our prior studies demonstrate that HO-1 and HO-2 exert counter-regulatory effects to those exerted by angiotensin II (23,24). It is possible that such counter-regulatory effects of HO systems involve the support of ACE2 expression, a consideration that will be examined in future studies. HO-1 exerts its cellular effects via such mechanisms as the generation of bile pigments, the production of carbon monoxide, the fostering of cellular iron homeostasis, and the reduction of oxidant stress (5,6). We speculate that the involvement of HO-1 in enabling the induction of ACE2 by LPS may reflect such effects of HO-1.
ACE2, a homolog of ACE, generates angiotensin (1–7) either directly from angiotensin II or, indirectly, as ACE2 converts angiotensin I to angiotensin (1–9), the latter then converted to angiotensin (1–7) by ACE (1–3). Angiotensin (1–7) largely acts through the Mas receptor to exert effects that are vasodilatory, anti-inflammatory, and antifibrotic; similar effects may be exerted by angiotensin- (1–9) acting through the AT2R receptor. ACE2 thus generates products with actions that oppose those exerted by the RAAS, in particular, angiotensin II. These counter-regulatory effects of ACE2 are relevant to COVID-19 because they oppose the effects of ambient levels of angiotensin II that exist in tissues and plasma in COVID-19. Although earlier studies demonstrated increased levels of angiotensin II in COVID-19 (25), more recent studies indicate that levels of renin, angiotensin II, and aldosterone are not significantly altered in COVID-19 (26,27). Interestingly, those patients with COVID-19 who develop AKI exhibit more than a two-fold increase in plasma renin and aldosterone as compared with those patients who do not develop AKI (28). In COVID-19, expression of ACE2 decreases after cellular invasion by SARS-CoV-2, and, indeed, plasma levels of angiotensin (1–7) are decreased in COVID-19 (29). Such ACE2 downregulation reduces both the anti-inflammatory effects of ACE2 and ACE2-mediated catabolism of angiotensin II. In this way, decreased ACE2 activity may promote inflammation and the cytokine storm that occur in COVID-19.
Our findings regarding Mas receptor expression merit comment. Notably, in experimental AKI whether attended by decreased ACE2 expression (IRI) or increased ACE2 expression (LPS), Mas receptor expression is increased. The functional significance of such augmented expression remains to be resolved. We speculate that irrespective of what is occurring upstream as regards ACE2 expression, increased Mas receptor expression may be a default response to renal injury, and one that enables the beneficial effects of angiotensin (1–7) whatever the ambient levels of angiotensin-(1–7).
Salutary effects of ACE2 have been shown in models of tissue injury. In models of lung injury, ACE2−/− mice as compared with ACE2+/+ mice exhibit heightened injury, whereas the administration of recombinant ACE2 protein or mesenchymal stem cells overexpressing ACE2 reduces lung injury (30–32). Genetic deficiency of ACE2 exaggerates liver injury (33) and muscle weakness (34) in relevant disease models. Beneficial effects of ACE2 have been described in models of systemic hypertension, diabetic kidney injury, and atherosclerosis (reviewed in 1–3). Relevant to the kidney are studies in ACE2−/− mice, which demonstrate a heightened sensitivity to AKI induced by IRI (35). These latter studies, undertaken before the COVID-19 pandemic, speak to the functional significance of the findings observed in AKI in this study and the need to comprehensively examine the precise role of ACE2 in the evolution of AKI in representative AKI models, in the transition of AKI to CKD, and in models of CKD including chronic urinary tract obstruction.
Male sex and older age are strong risk factors for AKI (14–17) and COVID-19 (36,37). The examination of such risk factors in this study thus provides a bridge that connects (1) the intact healthy kidney to (2) the intact kidney with risk factors such as age and male sex to (3) the kidney with “mild” AKI (LPS-induced) and, finally, to (4) the kidney with “severe” AKI (IRI). We observed that ACE2 protein expression was reduced in female mice as compared with male mice of similar age. This finding, in conjunction with the recent findings regarding renal ACE2 activity in female mice (38), may reflect lower activity of the RAAS that generally occurs in females as compared with males, and, accordingly, less of a need for a counter-regulatory system such as ACE2. In our studies, renal expression of HO-1 was also decreased in female mice as compared with male mice. We observed no effect of age on renal expression of ACE2 protein, whereas renal HO-1 protein expression decreased with age. As best as we can tell, these observations on sex-related and age-related changes in HO-1 protein expression are new to the literature. We offer the following speculation. Angiotensin II induces HO-1 (23), and it is possible the decreased HO-1 expression with age reflects the fact that the basal activity of RAAS (including angiotensin II) generally decreases with age (39). RAAS activity is generally higher in male as compared with female sex (40), and we speculate that this may underlie the lower HO-1 expression observed in female mice as compared with male mice.
AKI is relatively common and a determinant of outcomes in COVID-19. Such AKI reflects, in part, hemodynamic alterations and inflammation (36,37). Another mechanism involves infection of the kidney by SARS-CoV-2 as shown in two recent studies (41,42), with abundant renal expression of ACE2 serving as the receptor for cellular entry of SARS-CoV-2. Moreover, the SARS-CoV-2 virus in the kidney is infectious and capable of viral replication (41). SARS-CoV-2 often localizes to the renal proximal tubule, a major site of injury in COVID-19 and AKI in general. This is also the site of highest ACE2 expression. On the basis of our findings of decreased ACE2 expression in AKI, we speculate that such decrement in expression in AKI, when it occurs in COVID-19, may amplify SARS-CoV-2–induced inflammation and injury.
In summary, this study assessed the expression of ACE2 in the acutely injured kidney. The functional significance of these findings, of downregulation of ACE2 in models of necrotic AKI (IRI) and upregulation in non-necrotic AKI (LPS), merit further examination by relevant genetic and nongenetic approaches. Whatever the outcome—ACE2 as a protectant against or a perpetrator of AKI—would be of therapeutic interest because there is a sizable pharmaceutical initiative in developing inducers and inhibitors of ACE2. In AKI occurring in settings other than COVID-19, induction of ACE2 and administration of angiotensin (1–7) may offer a protective strategy. However, in COVID-19, because ACE2 is the receptor for internalizing SARS-CoV-2, such induction may prove deleterious; this risk may be obviated by administering angiotensin (1–7), the potentially cytoprotective product of ACE2. We also speculate that understanding the basis for suppressed expression of ACE2 in AKI may offer insights germane to AKI in COVID-19.
Disclosures
A. Agarwal reports having consultancy agreements with Akebia Therapeutics, Dynamed, and Reata Pharmaceuticals; reports having an ownership interest in Goldilocks Therapeutics, Inc.; reports receiving funding Genzyme/Sanofi Fabry Fellowship Award; reports receiving honoraria from Akebia Therapeutics, Emory, the University of Southern California, and Vanderbilt; reports being a scientific advisor or member of the Editorial Board of American Journal of Physiology-Renal Physiology, Kidney International, and Lab Investigation; reports serving on the Advisory Board of and Alpha Young, LLC, Angion, Goldilocks Therapeutics, a New York–based company investigating delivery of drugs in the kidney using nanotechnology for acute and chronic kidney disease, and on the External evaluation panel for the Kidney Precision Medicine Program; and reports other interests/relationships as his wife, Lisa Curtis, was President-elect for Women in Nephrology (2018–2019). J. Grande reports being a member of the editorial board of the Editorial Board for Biochemistry and Molecular Biology Education Journal. K. Nath reports being a member of the editorial board of JASN and editor-in-chief of Mayo Clinic Proceedings. All remaining authors have nothing to disclose.
Funding
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK11916 (to K. Nath), and R01 DK059600 and P30 DK079337 (to A. Agarwal), National Institute of Allergy and Infectious Diseases grant R01 AI100911 (to J.P. Grande), and National Heart, Lung, and Blood Institute grant R01 HL136348 (to V.D. Garovic).
Footnotes
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/K360/2021_08_09_KID0001562021.mp3
Author Contributions
A. Agarwal, A. Croatt, M. Barry, K. Nath, and R. Singh conceptualized the study; A. Ackerman, A. Croatt, J. Grande, K. Nath, and R. Singh were responsible for data curation; A. Ackerman, A. Agarwal, A. Croatt, J. Grande, K. Nath, and R. Singh were responsible for formal analysis; A. Agarwal, V. Garovic, J. Grande, and K. Nath were responsible for funding acquisition; A. Croatt and K. Nath were responsible for investigation; A. Ackerman, A. Croatt, J. Grande, K. Nath, and R. Singh were responsible for methodology; A. Croatt and K. Nath were responsible for project administration; K. Nath was responsible for resources; A. Ackerman, A. Croatt, and R. Singh were responsible for the software; A. Croatt and K. Nath provided supervision; A. Ackerman, A. Croatt, K. Nath, and R. Singh were responsible for validation; K. Nath were responsible for visualization; A. Croatt and K. Nath wrote the original draft; and all authors reviewed and edited the manuscript.
References
- 1.Paz Ocaranza M, Riquelme JA, García L, Jalil JE, Chiong M, Santos RAS, Lavandero S: Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol 17: 116–129, 2020. 10.1038/s41569-019-0244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY: Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ Res 126: 1456–1474, 2020. 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparks MA, South AM, Badley AD, Baker-Smith CM, Batlle D, Bozkurt B, Cattaneo R, Crowley SD, Dell’Italia LJ, Ford AL, Griendling K, Gurley SB, Kasner SE, Murray JA, Nath KA, Pfeffer MA, Rangaswami J, Taylor WR, Garovic VD: Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: Pressing needs and best research practices. Hypertension 76: 1350–1367, 2020. 10.1161/HYPERTENSIONAHA.120.15948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groß S, Jahn C, Cushman S, Bär C, Thum T: SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J Mol Cell Cardiol 144: 47–53, 2020. 10.1016/j.yjmcc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agarwal A, Bolisetty S: Adaptive responses to tissue injury: Role of heme oxygenase-1. Trans Am Clin Climatol Assoc 124: 111–122, 2013 [PMC free article] [PubMed] [Google Scholar]
- 6.Nath KA: Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens 23: 17–24, 2014. 10.1097/01.mnh.0000437613.88158.d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA: MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int 68: 611–622, 2005. 10.1111/j.1523-1755.2005.00439.x [DOI] [PubMed] [Google Scholar]
- 8.Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA: Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 72: 1073–1080, 2007. 10.1038/sj.ki.5002471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nath KA, Grande JP, Croatt AJ, Frank E, Caplice NM, Hebbel RP, Katusic ZS: Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am J Pathol 166: 963–972, 2005. 10.1016/S0002-9440(10)62318-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA: Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1-/- mice. Am J Pathol 170: 1820–1830, 2007. 10.2353/ajpath.2007.061093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarró E, Durán M, Rico A, Bou-Teen D, Fernández-Majada V, Croatt AJ, Nath KA, Salcedo MT, Gundelach JH, Batlle D, Bram RJ, Meseguer A: Cyclophilins A and B oppositely regulate renal tubular epithelial cell phenotype. J Mol Cell Biol 12: 499–514, 2020. 10.1093/jmcb/mjaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosa RM, Colucci JA, Yokota R, Moreira RP, Aragão DS, Ribeiro AA, Arita DY, Watanabe IK, Palomino Z, Cunha TS, Casarini DE: Alternative pathways for angiotensin II production as an important determinant of kidney damage in endotoxemia. Am J Physiol Renal Physiol 311: F496–F504, 2016. 10.1152/ajprenal.00121.2014 [DOI] [PubMed] [Google Scholar]
- 13.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D: ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006. 10.2337/db06-0033 [DOI] [PubMed] [Google Scholar]
- 14.Grams ME, Sang Y, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP, Naimark D, Oien C, Smith DH, Coresh J, Sarnak MJ, Stengel B, Tonelli M; CKD Prognosis Consortium: A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis 66: 591–601, 2015. 10.1053/j.ajkd.2015.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson S, Eldadah B, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kimmel PL, Molitoris BA, Murthy M, O’Hare AM, Schmader KE, High KP: Acute kidney injury in older adults. J Am Soc Nephrol 22: 28–38, 2011. 10.1681/ASN.2010090934 [DOI] [PubMed] [Google Scholar]
- 16.Neugarten J, Golestaneh L, Kolhe NV: Sex differences in acute kidney injury requiring dialysis. BMC Nephrol 19: 131, 2018. 10.1186/s12882-018-0937-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neugarten J, Sandilya S, Singh B, Golestaneh L: Sex and the risk of AKI following cardio-thoracic surgery: A meta-analysis. Clin J Am Soc Nephrol 11: 2113–2122, 2016. 10.2215/CJN.03340316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, de Sousa LP, Teixeira MM, Santos RA, Simões e Silva AC, Ribeiro Vieira MA: ACE2-angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond) 119: 385–394, 2010. 10.1042/CS20090554 [DOI] [PubMed] [Google Scholar]
- 19.Gallagher PE, Ferrario CM, Tallant EA: Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 295: H2373–H2379, 2008. 10.1152/ajpheart.00426.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Li Y, Zeng Y, Wu R, Ou J: Endothelin-1 downregulates angiotensin-converting enzyme-2 expression in human bronchial epithelial cells. Pharmacology 91: 297–304, 2013. 10.1159/000350395 [DOI] [PubMed] [Google Scholar]
- 21.Zhang R, Wu Y, Zhao M, Liu C, Zhou L, Shen S, Liao S, Yang K, Li Q, Wan H: Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 297: L631–L640, 2009. 10.1152/ajplung.90415.2008 [DOI] [PubMed] [Google Scholar]
- 22.Quarles LD: Fibroblast growth factor 23 and α-Klotho co-dependent and independent functions. Curr Opin Nephrol Hypertens 28: 16–25, 2019. 10.1097/MNH.0000000000000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haugen EN, Croatt AJ, Nath KA: Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int 58: 144–152, 2000. 10.1046/j.1523-1755.2000.00150.x [DOI] [PubMed] [Google Scholar]
- 24.Nath KA, Hernandez MC, Croatt AJ, Katusic ZS, Juncos LA: Heme oxygenase activity as a determinant of the renal hemodynamic response to low-dose ANG II. Am J Physiol Regul Integr Comp Physiol 299: R1183–R1191, 2010. 10.1152/ajpregu.00212.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L: Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63: 364–374, 2020. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry BM, Benoit S, Lippi G, Benoit J: Letter to the Editor - Circulating plasma levels of angiotensin II and aldosterone in patients with coronavirus disease 2019 (COVID-19): A preliminary report. Prog Cardiovasc Dis 63: 702–703, 2020. 10.1016/j.pcad.2020.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rieder M, Wirth L, Pollmeier L, Jeserich M, Goller I, Baldus N, Schmid B, Busch HJ, Hofmann M, Kern W, Bode C, Duerschmied D, Lother A: Serum ACE2, angiotensin II, and aldosterone levels are unchanged in patients with COVID-19. Am J Hypertens 34: 278–281, 2021. 10.1093/ajh/hpaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudoignon E, Moreno N, Deniau B, Coutrot M, Longer R, Amiot Q, Mebazaa A, Pirracchio R, Depret F, Legrand M: Activation of the renin-angiotensin-aldosterone system is associated with acute kidney injury in COVID-19. Anaesth Crit Care Pain Med 39: 453–455, 2020. 10.1016/j.accpm.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry BM, Benoit JL, Berger BA, Pulvino C, Lavie CJ, Lippi G, Benoit SW: Coronavirus disease 2019 is associated with low circulating plasma levels of angiotensin 1 and angiotensin 1,7. J Med Virol 93: 678–680, 2021. 10.1002/jmv.26479 [DOI] [PubMed] [Google Scholar]
- 30.He H, Liu L, Chen Q, Liu A, Cai S, Yang Y, Lu X, Qiu H: Mesenchymal stem cells overexpressing angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced lung injury. Cell Transplant 24: 1699–1715, 2015. 10.3727/096368914X685087 [DOI] [PubMed] [Google Scholar]
- 31.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui C-C, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM: Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rey-Parra GJ, Vadivel A, Coltan L, Hall A, Eaton F, Schuster M, Loibner H, Penninger JM, Kassiri Z, Oudit GY, Thébaud B: Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J Mol Med (Berl) 90: 637–647, 2012. 10.1007/s00109-012-0859-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osterreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM, Brenner DA: Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology 50: 929–938, 2009. 10.1002/hep.23104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nozato S, Yamamoto K, Takeshita H, Nozato Y, Imaizumi Y, Fujimoto T, Yokoyama S, Nagasawa M, Takeda M, Hongyo K, Akasaka H, Takami Y, Takeya Y, Sugimoto K, Mogi M, Horiuchi M, Rakugi H: Angiotensin 1-7 alleviates aging-associated muscle weakness and bone loss, but is not associated with accelerated aging in ACE2-knockout mice. Clin Sci (Lond) 133: 2005–2018, 2019. 10.1042/CS20190573 [DOI] [PubMed] [Google Scholar]
- 35.Fang F, Liu GC, Zhou X, Yang S, Reich HN, Williams V, Hu A, Pan J, Konvalinka A, Oudit GY, Scholey JW, John R: Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PLoS One 8: e71433, 2013. 10.1371/journal.pone.0071433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW: Extrapulmonary manifestations of COVID-19. Nat Med 26: 1017–1032, 2020. 10.1038/s41591-020-0968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, Rimmelé T, Zarbock A, Bell S, Bihorac A, Cantaluppi V, Hoste E, Husain-Syed F, Germain MJ, Goldstein SL, Gupta S, Joannidis M, Kashani K, Koyner JL, Legrand M, Lumlertgul N, Mohan S, Pannu N, Peng Z, Perez-Fernandez XL, Pickkers P, Prowle J, Reis T, Srisawat N, Tolwani A, Vijayan A, Villa G, Yang L, Ronco C, Kellum JA: COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. [Published correction appears in Nat Rev Nephrol, 2020] Nat Rev Nephrol 16: 747–764, 2020. 10.1038/s41581-020-00356-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji H, de Souza AMA, Bajaj B, Zheng W, Wu X, Speth RC, Sandberg K: Sex-specific modulation of blood pressure and the renin-angiotensin system by ACE (angiotensin-converting enzyme) 2. Hypertension 76: 478–487, 2020. 10.1161/HYPERTENSIONAHA.120.15276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsunoda K, Abe K, Goto T, Yasujima M, Sato M, Omata K, Seino M, Yoshinaga K: Effect of age on the renin-angiotensin-aldosterone system in normal subjects: Simultaneous measurement of active and inactive renin, renin substrate, and aldosterone in plasma. J Clin Endocrinol Metab 62: 384–389, 1986. 10.1210/jcem-62-2-384 [DOI] [PubMed] [Google Scholar]
- 40.Radin MJ, Holycross BJ, Sharkey LC, Shiry L, McCune SA: Gender modulates activation of renin-angiotensin and endothelin systems in hypertension and heart failure. J Appl Physiol 92: 935–940, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, Nörz D, Heinrich F, Meißner K, Wichmann D, Kluge S, Gross O, Pueschel K, Schröder AS, Edler C, Aepfelbacher M, Puelles VG, Huber TB: SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396: 597–598, 2020. 10.1016/S0140-6736(20)31759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB: Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020. 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]