Key Points
Immune response to the coronavirus disease 2019 vaccination is significantly reduced in patients with kidney failure compared with controls without kidney failure.
After two vaccinations, kidney transplant recipients show the lowest levels of IgGs specific to severe acute respiratory syndrome coronavirus 2, with the lowest neutralizing capacity.
These data suggest that vaccination strategies need modification in kidney transplant recipients and patients on dialysis.
Keywords: transplantation, clinical immunology, COVID-19, humoral immune response, immunity, kidney failure, kidney transplantation, renal insufficiency, SARS-CoV-2 specific antibody, vaccination, virology
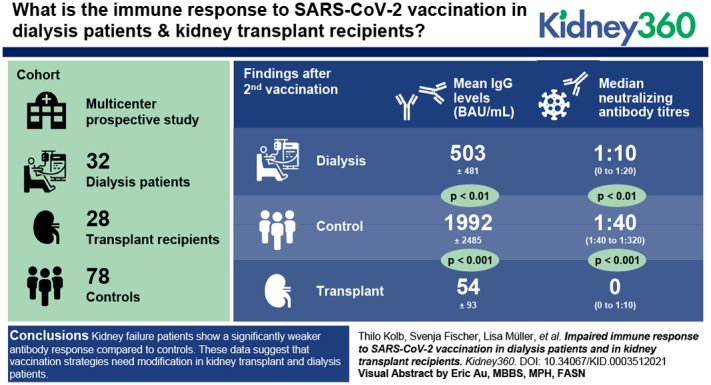
Visual Abstract
Abstract
Background
Patients with kidney failure on dialysis or after renal transplantation have a high risk for severe COVID-19 infection, and vaccination against SARS-CoV-2 is the only expedient prophylaxis. Generally, immune responses are attenuated in patients with kidney failure, however, systematic analyses of immune responses to SARS-CoV-2 vaccination in patients on dialysis and in kidney transplant recipients (KTRs) are still needed.
Methods
In this prospective, multicentric cohort study, antibody responses to COVID-19 mRNA vaccines (BNT162b2 [BioNTech/Pfizer] or mRNA-1273 [Moderna]) were measured in 32 patients on dialysis and in 28 KTRs. SARS-CoV-2–specific antibodies and neutralization capacity were evaluated and compared with controls (n=78) of a similar age range.
Results
After the first vaccination, SARS-CoV-2–specific antibodies were nearly undetectable in patients with kidney failure. After the second vaccination, 93% of the controls and 88% of patients on dialysis but only 37% of KTRs developed SARS-CoV-2–specific IgG above cutoff. Moreover, mean IgG levels were significantly lower in KTRs (54±93 BAU/ml) compared with patients on dialysis (503±481 BAU/ml; P<0.01). Both KTRs and patients on dialysis had significantly lower IgG levels compared with controls (1992±2485 BAU/ml; P<0.001 and P<0.01, respectively). Importantly, compared with controls, neutralizing antibody titers were significantly lower in KTRs and patients on dialysis. After the second vaccination, 76% of KTRs did not show any neutralization capacity against SARS-CoV-2, suggesting impaired seroprotection.
Conclusions
Patients with kidney failure show a significantly weaker antibody response compared with controls. Most strikingly, only one out of four KTRs developed neutralizing antibodies against SARS-CoV-2 after two doses of vaccine. These data suggest that vaccination strategies need modification in KTRs and patients on dialysis.
Clinical Trial registry name and registration number: Vaccination Against COVID-19 in Chronic Kidney Disease, NCT04743947
Introduction
Although great effort has been made to develop effective strategies to prevent severe courses of coronavirus disease 2019 (COVID-19), the options for treating patients are still disappointing (1). Prophylactic vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became the most effective protection against COVID-19. Patients with RRT receiving dialysis or after renal transplantation belong to the highest risk group for a severe course of COVID-19 and, consequently, are dying from COVID-19 (2,3). A general concept to protect these patients against COVID-19 is to prioritize patients on dialysis and kidney transplant recipients (KTRs) for vaccination. Until now, studies investigating immune responses to SARS-CoV-2 vaccination for patients with kidney failure are sparse (4,5). Moreover, these groups had been excluded from the main vaccine outcome studies (6). However, experience from other vaccination programs showed an impaired immune response in patients on dialysis and in KTRs taking immunosuppressive therapy (7,8). Consequently, vaccination strategies against these infectious diseases have been modified by administering higher doses or changing the dosing interval to increase the immune response (9). Therefore, it is anticipated that the immune response to SARS-CoV-2 vaccination is also less robust in patients on dialysis and in KTRs. In this prospective, multicentric observational study, we measured the humoral immune response to SARS-CoV-2 vaccination in patients on dialysis and in KTRs and compared them with a control group with no evidence of kidney failure (10). Time course and intensity of the immune response after SARS-CoV-2 vaccination needs to be known to develop future vaccination strategies for this particular patient group.
Materials and Methods
Study Population
The study was approved by the ethics committee of the Medical Faculty at the Heinrich-Heine University Düsseldorf (Düsseldorf, Germany; study numbers 2020-1237 and 2021-1287) and was carried out in line with the Declaration of Helsinki, as revised in 2013. In this multicenter, prospective, observational study, we consecutively enrolled 32 patients receiving dialysis, 28 KTRs, and 78 volunteers (controls with no evidence of kidney failure) from a nursing home who had received a vaccination appointment between December 26, 2020 and April 15, 2021, according to the effective rules of prioritization, as defined by the German government at that time (10).
Clinical data were obtained from medical records or medical questionnaires for the control group. Exclusion criteria were age <18 years, inability to give consent, and former SARS-CoV-2 infection. All participants signed a written informed consent. A total of 78 of the previously described group of 176 volunteers were selected and matched in the following manner (10). First, they should not have any evidence of kidney failure. Second, controls were selected by their date of birth. For each patient of the KTR and each patient of the dialysis group, all available controls (one to six controls) with a date of birth within 12 months of the date of birth of the patients on dialysis or KTRs were included in the analysis. Age-matched controls were not available for seven of the 28 KTRs and for one of the 32 patients on dialysis. None of the participants had a COVID-19 infection in the past, as determined by patient history and measurement of SARS-CoV-2 antibodies before vaccination.
In patients on dialysis and KTRs, we decided to perform five visits (one before vaccination, two after the first vaccination, and two after the second vaccination) to detect the vaccination-induced immune response and to monitor potential adverse events. Blood samples were taken when regarded as clinically necessary. Side effects after vaccination were scored semiquantitatively according to the sum of the following symptoms: (1) elevated temperature and fever, (2) chills, (3) pain at the injection site, (4) head/limb pain, (5) fatigue/tiredness, (6) nausea/dizziness, and (7) other complaints (unscored).
All participants were vaccinated between December 26, 2020 and April 15, 2021. For the control group, the patients on dialysis, and KTRs, SARS-CoV-2 samples for antibody levels and neutralization titers (NT) were taken as median (interquartile range [IQR]) of 19 (17–19), 20 (19–21), and 20 (19–20) days after the first vaccination and 17, 14 (13–15), and 14 (14–15) days after the second vaccination, respectively. All specimens were stored at 4°C.
Sample Processing
All samples from the participants were sequentially tested for anti–SARS-CoV-2 antibodies and for SARS-CoV-2 neutralization efficacy (NT) at the Institute of Virology, University Hospital Düsseldorf (Düsseldorf, Germany). Samples were tested for anti–SARS-CoV-2 antibodies, using the commercially available test system Anti-SARS-CoV-2 QuantiVac ELISA from Euroimmun, which measures IgG levels against the SARS-CoV-2 spike S1 subunit. According to the manufacturer’s instructions, results <25.6 binding antibody units (BAU)/ml were considered as negative, ≥25.6 BAU/ml and ≤35.2 BAU/ml as indeterminate, and >35.2 BAU/ml as positive. The upper detection limit for undiluted samples was >384 BAU/ml, the lower detection limit was <3.2 BAU/ml. For samples above the detection limit, 1:10 or 1:100 dilutions were performed in IgG sample buffer according to the manufacturer’s instruction. An immunoassay (Elecsys from Roche or Architect from Abbott) for detection of IgGs recognizing the SARS-CoV-2 nucleocapsid protein was used to detect previous SARS-CoV-2 infection.
An end point dilution neutralization test with the infectious SARS-CoV-2 isolate (EPI_ISL_425126) with 100 TCID50 units was performed in a biosafety level 3 facility, as described previously, to determine the SARS-CoV-2 neutralization capacity of the serum samples after the first and second vaccination (11). The NT was determined as the highest serum dilution without virus-induced cytopathic effect.
Statistical Analyses
The data were analyzed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA). The D’Agostino and Pearson omnibus normality test was performed to test normal distribution. In the case of normal distribution, t test or one-way ANOVA followed by the Holm–Sidak test for multiple comparisons was used, as appropriate. For non-normally distributed samples, data were analyzed by the nonparametric Mann–Whitney test or Kruskal–Wallis test with the post hoc Dunn test. For categoric data, the Fisher exact test and the chi-squared test were used to assess the statistical significance between groups. Correlations were tested by the Spearman rank correlation coefficient.
Results
Participants’ Characteristics
A total of 28 KTRs, 32 patients on dialysis, and 78 volunteers were enrolled in this prospective, multicenter, observational study. The median (IQR) age in KTRs was 66 (61–81) years, which was lower compared with patients on dialysis, who had a median (IQR) age of 83 (80–85) years (P<0.01), and with controls, who had a median (IQR) age of 84 (80–87) years (P<0.001). Male sex was less prevalent in controls (29%) compared with patients on dialysis (69%) and KTRs (71%) (Table 1).
Table 1.
Baseline characteristics of patients on dialysis, kidney transplant recipients, and controls without evidence of kidney failure
| Characteristics | Dialysis (n=32) | KTR (n=28) | Control (n=78) |
| General | |||
| Age (yr), median (IQR) | 83 (80–85) | 66 (61–81) | 84 (80–87) |
| Male sex, % | 69 | 71 | 29 |
| BMI (kg/m2)a | 26 | 27 | n.d. |
| Diabetes, % | 28 | 11 | n.d. |
| Vaccine | |||
| BioNTech/Pfizer, n (%) | 32 (100) | 23 (82) | 78 (100) |
| Moderna, n (%) | 0 | 5 (18) | 0 |
| Sample taken after first vaccination (d), median (IQR) | 20 (19–21) | 20 (19–20) | 19 (17–19) |
| Sample taken after second vaccination (d), median (IQR) | 14 (13–15) | 14 (14–15) | 17 |
| Dialysis | |||
| Treatment duration (yr), median (IQR) | 3 (2–6) | ||
| PD, n | 1 | ||
| HD, n | 31 | ||
| History of previous transplantation, n (%) | 2 (6) | ||
| Responder to hepatitis B vaccination, n (%) | 12 (38) | ||
| Nonresponder to hepatitis B vaccination, n (%) | 13 (40) | ||
| No hepatitis B vaccination/not determined, n (%) | 7 (22) | ||
| Immunosuppressive treatment, n (%) | 5 (16) | ||
| Transplantation | |||
| Years after transplantation (yr), median (IQR) | 10 (3–12) | ||
| History of previous transplantation, n | 2 | ||
| Baseline eGFR (ml/min per 1.73 m2), mean±SD | 46±20 | ||
| Triple immunosuppressive treatment, n (%) | 22 (79) | ||
| Dual immunosuppressive treatment, n (%) | 6 (21) | ||
| Basiliximab, n (%) | 16 (57) | ||
| Tacrolimus, n (%) | 25 (89) | ||
| Ciclosporin, n (%) | 3 (11) | ||
| Mycophenolate mofetil, n (%) | 22 (79) | ||
| Azathioprine, n (%) | 1 (4) | ||
| Prednisolone, n (%) | 27 (96) |
KTR, kidney transplant recipient; IQR, interquartile range; BMI, body mass index; n.d., not determined; PD, peritoneal dialysis; HD, hemodialysis.
Values represent mean values.
A total of 31 patients received hemodialysis and one patient received peritoneal dialysis. The median (IQR) time on dialysis was 3 (2–6) years. Two patients had a history of previous kidney transplantation, and five patients were still taking a low dose of immunosuppressive therapy.
In KTRs, the median (IQR) time after renal transplantation was 10 (3–12) years. The average eGFR at the beginning of the study was 46±20 ml/min per 1.73 m2. Of the 28 patients, 22 (79%) were treated with a triple immunosuppressive treatment (Table 1). Participants of the control group were either residents of a nursing home or their caregivers, as described previously (Table 1) (10).
Reduced SARS-CoV-2 Spike–Specific IgG Levels in Patients on Dialysis and KTRs after the First and Second Vaccination
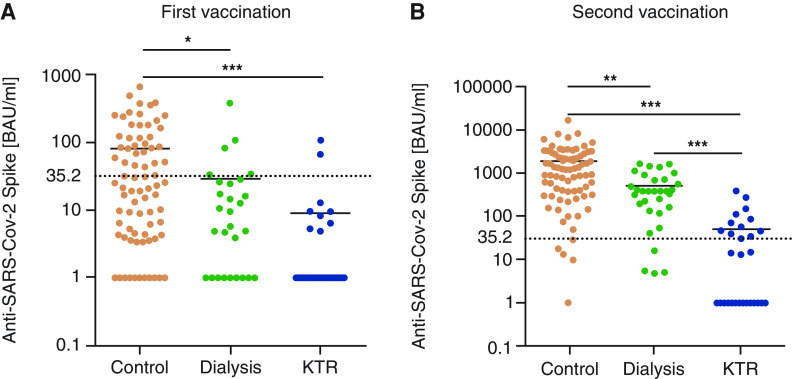
After the first vaccination, mean vaccination-induced SARS-CoV-2 spike S1–specific IgG levels were significantly lower in patients on dialysis (30±72 BAU/ml; P<0.05) and in KTRs (10±24 BAU/ml; P<0.001) compared with controls (81±125 BAU/ml), but levels did not differ between patients on dialysis and KTRs (Figure 1A). IgG levels above the cutoff (>35.2 BAU/ml) were only detected in two out of 28 KTRs (7%) and in three out of 32 patients on dialysis (11%), but were detected in 33 out of 78 controls (42%) after the first vaccination.
Figure 1.
Anti SARS-CoV-2 spike protein-specific antibody titers after first and second vaccination. Antibody titers >35.2 BAU/ml were considered a positive immune response to vaccination. Antibody titers below the detection limit were set to 1.0. Antibody titers after the (A) first and (B) second vaccination were significantly higher in controls compared with patients on dialysis and kidney transplant recipients (KTRs). Moreover, mean antibody titers after the second vaccination were significantly lower in KTRs compared with the dialysis group. For comparison of three groups, data were analyzed by the nonparametric Kruskal–Wallis Test with post hoc Dunn test. *P<0.05, **P<0.01, ***P<0.001. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
After the second vaccination, IgG levels increased significantly in all groups. Nevertheless, differences in the vaccination-induced immune response, as determined by SARS-CoV-2 spike S1–specific IgG, became even more evident between the three groups. IgG levels were still significantly lower in KTRs (54±93 BAU/ml) compared with the patients on dialysis (503±481 BAU/ml; P<0.001) and the controls (1922±2485 BAU/ml; P<0.001) (Figure 1B). Moreover, IgG levels were significantly lower in patients on dialysis compared with controls (P<0.01). IgG levels were found to be positive (>35.2 BAU/ml) in 73 out of 78 controls (94%) and in 28 out of 32 patients on dialysis (88%), but only in ten out of 28 KTRs (36%).
To define cofactors influencing the immune response after SARS-CoV-2 vaccination, differences between KTRs with positive SARS-CoV-2 spike S1–specific IgGs and KTRs with lower SARS-CoV-2 levels were analyzed. As shown in Table 2, KTRs with positive IgG levels had been transplanted for a longer median time and were more often treated with a dual immunosuppressive therapy, compared with KTRs with IgG levels <35.2 BAU/ml. Furthermore, to test whether age affects the IgG response in patients with kidney failure, a correlation analysis was performed. We did not find any correlation between IgG levels and age in patients on dialysis (r=−0.06; P=0.74) or in KTRs (r=0.07; P=0.73). eGFR did not correlate with the IgG response in KTRs (r=−0.10; P=0.63). Five out of 28 KTRs did not receive antimetabolite immunosuppression therapy (Table 1). Four of these five patients (80%) showed a positive immune response, whereas only six out of 23 (26%) KTRs treated with mycophenolate or azathioprine showed a positive immune response (P<0.05).
Table 2.
Differences in baseline characteristics between KTRs with SARS-CoV-2 spike S1–specific IgG >35.2 BAU/ml versus those with <35.2 BAU/ml
| Characteristics | KTR SARS-CoV-2 Spike S1–Specific IgG Level | |
| >35.2 BAU/ml (n=10) | <35.2 BAU/ml (n=18) | |
| General | ||
| Age (yr), median (IQR) | 74 (64–81) | 65 (60–76) |
| Male sex, % | 70 | 72 |
| BMI (kg/m2)b | 25 | 26 |
| Diabetes, % | 10 | 11 |
| Malignancy, % | 50 | 39 |
| Transplantation | ||
| Time after transplantation (yr), median (IQR) | 12 (12–19) | 6 (3–11)a |
| History of previous transplantation | 1 | 1 |
| Baseline eGFR (ml/min per 1.73 m2), mean±SD | 44±23 | 47±18 |
| History of rejection within a year before vaccination, n (%) | 0 (0) | 0 (0) |
| Primary renal disease, n (%) | ||
| Glomerular | 6 (60) | 6 (33) |
| Vascular | 0 (0) | 2 (11) |
| Interstitial | 2 (20) | 1 (6) |
| Polycystic kidney disease | 2 (20) | 4 (22) |
| Diabetes | 0 (0) | 2 (11) |
| Other | 0 (0) | 3 (17) |
| Immunosuppression, n (%) | ||
| Triple therapy | 5 (50) | 17 (94) |
| Dual therapy | 5 (50) | 1 (6)a |
| Basiliximab | 5 (20) | 11 (44) |
| Tacrolimus | 8 (80) | 17 (94) |
| Ciclosporin | 2 (20) | 1 (6) |
| Mycophenolate mofetil | 6 (60) | 16 (89) |
| Azathioprine | 0 (0) | 1 (6) |
| Prednisolone | 9 (90) | 18 (100) |
KTR, kidney transplant recipient; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IQR, interquartile range; BMI, body mass index.
Significant difference between groups (P<0.05) using Mann–Whitney test.
Values represent mean values.
Reduced SARS-CoV-2 Neutralizing Capacity in Patients with Kidney Failure Compared with Controls
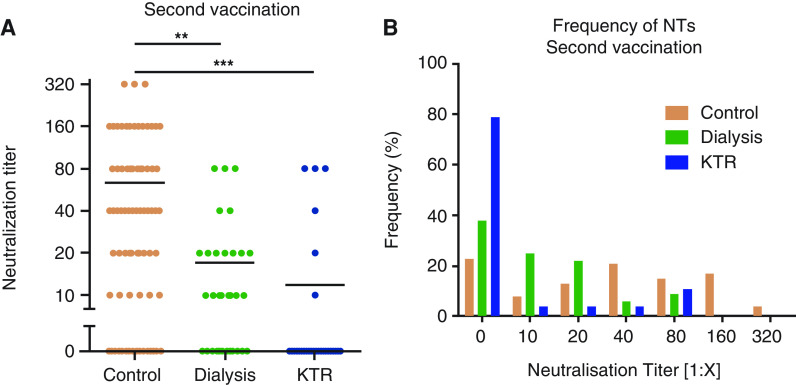
To further characterize the specific humoral immune response after COVID-19 vaccination in patients with kidney failure, neutralization capacity was determined in patients on dialysis and in KTRs and compared with controls. After the first dose of vaccination, neutralizing antibodies were not detectable in patients on dialysis or KTRs, and in only four out of 78 controls (4%). After the second vaccination, the frequency of detectable neutralizing capacity increased in all three groups. Median (IQR) NTs were significantly lower in patients on dialysis (1:10 [0 to 1:20]; P<0.01) and KTRs (0 [0 to 1:10]; P<0.001) compared with controls (1:40 [1:10 to 1:320]) (Figure 2A). Importantly, neutralizing responses were detected in 60 out of 78 controls (77%) and in 20 out of 32 patients on dialysis (63%), but only in six out of 28 KTRs (21%), suggesting an impaired seroprotection in KTRs (P<0.001 versus control; P<0.01 versus dialysis) (Figure 2B). Because of the low numbers of KTRs with neutralizing antibodies, a clear correlation between any cofactors and the occurrence of neutralizing antibodies could not be determined.
Figure 2.
Median titer and frequency of neutralizing antibodies after second vaccination. (A) The mean titers of neutralizing antibodies were significantly lower in patients on dialysis and KTRs, compared with controls, after the second vaccination. (B) Frequencies of neutralizing antibodies in controls, patients on dialysis, and KTRs after the second vaccination. For comparison of three groups, data were analyzed by the nonparametric Kruskal–Wallis test with post hoc Dunn test. **P<0.01, ***P<0.001. NT, neutralization titer.
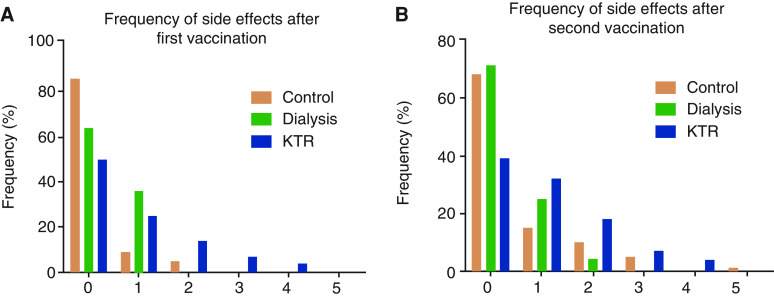
Postvaccination Reactions in Patients on Dialysis and KTRs
To assess postvaccination reactions in kidney failure and to detect a potential effect of the COVID-19 vaccination on renal function in KTRs, patients were seen 2–4 days after each vaccination. Reactions to vaccination after the first and second dose did not differ substantially (Figure 3, A and B). In 19 KTRs, eGFR values were measured before vaccination (43±16 ml/min per 1.73 m2) and after the first (42±16 ml/min per 1.73 m2) and second (40±16 ml/min per 1.73 m2) vaccination. eGFR did not differ significantly between the three time points.
Figure 3.
Frequencies of the side effects after the first and second vaccination.
Discussion
In this prospective, multicenter study, we showed an impaired immune response to the mRNA COVID-19 vaccines BNT162b2 (BioNTech/Pfizer) and mRNA-1273 (Moderna) in patients receiving RRT compared with controls with a similar age range. To our knowledge, this is the first prospective study that analyses the humoral immune response to COVID-19 vaccination by measuring IgG levels and their neutralizing capacity in patients on dialysis and KTRs, in comparison with participants without kidney failure. It is well known that patients on dialysis or after kidney transplantation show a reduced immune response to different vaccines, such as hepatitis B or influenza A virus subtype H1N1 (7,8). The impaired humoral and cellular immunity may be caused by accumulation of uremic toxins in kidney failure and by the chronic intake of immunosuppressive drugs (12–15). Here, we showed clearly that the humoral immune response, measured by SARS-CoV-2 spike–specific IgG levels and the neutralizing capacity of the antibodies, was almost undetectable in patients with kidney failure 2–3 weeks after the first vaccination, and this was significantly reduced compared with controls. Although some studies showed protection against COVID-19 infection after the first injection, our results indicate that patients receiving RRT by dialysis or renal transplantation are much less protected after the first vaccination (16–19).
Two weeks after the second vaccination, a time point when full protection can be assumed (20,21), mean SARS-CoV-2 spike–specific IgG levels were significantly lower in patients on dialysis and even lower in KTRs compared with controls. This is highlighted by our finding that only 37% of KTRs had developed IgG levels above the cutoff 2 weeks after the second vaccination. In addition to IgG levels as a classic parameter for assessing humoral immune response, we measured the neutralizing capacities of these antibodies. Recent studies have shown that neutralizing antibodies are crucial for defense against viruses like SARS-CoV-2 (A. S. Iyer et al., unpublished observations; https://doi.org/10.1101/2020.07.18.20155374) (22). High neutralizing antibody levels seem to be relevant for protection against novel circulating SARS-CoV-2 escape variants (S. Jangra et al., unpublished observations; https://doi.org/10.1101/2021.01.26.21250543) (23,24). Whereas 77% of controls and 63% of patients on dialysis developed neutralizing antibodies, only 24% of the KTRs showed some neutralization capacity after the second vaccination. Although the percentage of patients on dialysis who attained neutralizing capacity was not statistically different to that of the controls, the median neutralizing antibody titers were significantly lower, suggesting less seroprotection against SARS-CoV-2.
The highly significant lower levels of SARS-CoV-2 spike–specific IgGs and neutralizing antibodies in KTRs suggest specific underlying mechanisms. Previous studies have addressed the role of immunosuppressive drugs, renal function, and age. Two observational studies in transplant patients showed a correlation between a reduced immune response to SARS-CoV-2 vaccination with the use of antimetabolite immunosuppression, such as mycophenolate and azathioprine, and with impaired renal function (25,26). In this study, we also found a positive signal for a reduced immune response and use of antimetabolite immunosuppression. There was no correlation between IgG levels and kidney function or age in KTRs.
Postvaccination reactions were assessed in all three groups. Both vaccinations were well tolerated, and kidney function was not affected in KTRs. Therefore, vaccination against SARS-CoV-2 by an mRNA vaccine appears to be safe in patients with kidney failure.
There are several strengths in this study. First, samples from all patients were analyzed in the same laboratory using an identical protocol for quantification of IgG levels and neutralizing capacity. Second, all three groups were of a similar age range, with the lowest median age in the KTRs. Because KTRs had also the lowest immune response to vaccination, an age-dependent bias toward better immunity in this group can be excluded (10). Third, the study was performed in a controlled, prospective manner with a similar protocol including time points of sampling and analysis in patients on dialysis and KTRs.
The limitations of this study are the relatively low number of KTRs and the lack of exploration into the cellular immune response in the cohorts.
However, this study offers enough evidence that the humoral immune response to SARS-CoV-2 vaccination is significantly impaired in patients on dialysis and in KTRs, compared with controls. This suggests the need for modification of vaccination strategies for patients with kidney failure. In line with this suggestion, a recent observational study reported an additional effect of a third vaccination in KTRs with markedly attenuated antibody response after the second vaccination (27). In that study, six of 30 KTRs (20%) had low detectable SARS-CoV-2 antibodies levels, whereas the others did not show any response. This immune response pattern is similar to that found in our case series of 28 KTRs (five of 28 KTRs with low detectable SARS-CoV-2 antibodies levels <35.2 BAU/ml). Interestingly, those KTRs with detectable SARS-CoV-2 antibodies levels after the second vaccination showed an effective immune response after the third vaccination (27). In contrast, the majority of patients with no detectable antibodies after the second vaccination did not benefit from the third vaccination. Thus, it would be plausible to recommend a third vaccination in KTRs with detectable SARS-CoV-2 antibody levels after the second vaccination. Furthermore, it will be essential to identify modifiable factors to enhance the chance of a sufficient immune response to vaccination in KTRs. Our findings underpin the recommendation to vaccinate all patients against SARS-CoV-2 on the transplant waiting list.
Disclosures
L.C. Rump reports having consultancy agreements with, and receiving honoraria from, Bayer, Boehringer, Medtronic, and ReCor. M. Schmitz reports receiving honoraria from Daiichi-Sankyo. J. Stegbauer reports having other interests in/relationships with American Heart Association High Blood Pressure, German Society of Nephrology, and German Society of Hypertension; receiving honoraria from AstraZeneca, Bayer Life Science, and Boehringer; serving on the editorial board of Experimental and Clinical Endocrinology & Diabetes and Kidney360; and receiving research funding from German Research Foundation. All remaining authors have nothing to disclose.
Funding
This work was supported by the Medizinische Fakultät, Heinrich-Heine-Universität Düsseldorf Forschungskommission of the Medical Faculty, the Ministerium für Kultur und Wissenschaft des Landes Nordrhein-Westfalen (Ministry of Culture and Science of North Rhine-Westphalia; VIRus Alliance NRW), and Bundesministerium für Bildung und Frauen grant COVIM 01KX2021.
Acknowledgments
The authors thank Mrs. Yvonne Dickschen for technical assistance, and Mrs. Liliane Janssen and Mrs. Natascha Rapp for their support for organizing the visits.
Footnotes
See related editorial, “SARS-CoV-2 Vaccination: The Time Is Now,” on pages 1402–1404.
Author Contributions
O. Adams, M. Andree, N. Lübke, L. Müller, and H. Schaal were responsible for methodology; O. Adams, N. Lübke, L. Müller, and J. Stegbauer were responsible for data curation; M. Andree, S. Fischer, J. Hillebrandt, N. Lübke, L. Müller, J. Stegbauer, and J. Timm were responsible for formal analysis; K. W. Dreyling, S. Fischer, G. Hetzel, J. Hillebrandt, K. Ivens, N. Lübke, L. Müller, L. C. Rump, C. Schmidt, M. Schmitz, J. Stegbauer, J. Timm, and L. Weiland reviewed and edited the manuscript; K. W. Dreyling, S. Fischer, J. Hillebrandt, K. Ivens, M. Kittel, T. Kolb, L. Koster, S. Kücükköylü, C. Schmidt, M. Schmitz, and L. Weiland were responsible for investigation; G. Hetzel was responsible for resources; K. Ivens, L. C. Rump, H. Schaal, J. Stegbauer, and J. Timm conceptualized the study; T. Kolb, L. C. Rump, J. Stegbauer, and J. Timm wrote the original draft; and J. Timm was responsible for funding acquisition.
References
- 1.Thoguluva Chandrasekar V, Venkatesalu B, Patel HK, Spadaccini M, Manteuffel J, Ramesh M: Systematic review and meta-analysis of effectiveness of treatment options against SARS-CoV-2 infection. J Med Virol 93: 775–785, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, Sanderson C, McKee M, Troeger C, Ong KL, Checchi F, Perel P, Joseph S, Gibbs HP, Banerjee A, Eggo RM; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group: Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob Health 8: e1003–e1017, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B: Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: 430–436, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikizler TA, Coates PT, Rovin BH, Ronco P: Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int 99: 1275–1279, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozen-Zvi B, Yahav D, Agur T, Zingerman B, Ben-Zvi H, Atamna A, Tau N, Mashraki T, Nesher E, Rahamimov R: Antibody response to mRNA SARS-CoV-2 vaccine among kidney transplant recipients - Prospective cohort study [published online ahead of print May 3, 2021]. Clin Microbiol Infect [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glenn DA, Hegde A, Kotzen E, Walter EB, Kshirsagar AV, Falk R, Mottl A: Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. Kidney Int Rep 6: 1407–1410, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, Stefanidis I: Factors affecting effectiveness of vaccination against hepatitis B virus in hemodialysis patients. World J Gastroenterol 20: 12018–12025, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen A, Grund S, Hetzel G, Ivens K, Sümmchen HA, Zgoura P, Hengel H, Adams O, Rump LC: Noncontrolled trial of monovalent AS03A-adjuvanted vaccine for 2009 pandemic influenza A(H1N1) in long-term dialysis patients and transplant recipients. Am J Kidney Dis 59: 471–473, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Kausz A, Pahari D: The value of vaccination in chronic kidney disease. Semin Dial 17: 9–11, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, Ostermann PN, Robitzsch R, Hauka S, Walker A, Menne C, Grutza R, Timm J, Adams O, Schaal H: Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination [published online ahead of print April 27, 2021]. Clin Infect Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller L, Ostermann PN, Walker A, Wienemann T, Mertens A, Adams O, Andree M, Hauka S, Lübke N, Keitel V, Drexler I, Di Cristanziano V, Hermsen DF, Kaiser R, Boege F, Klein F, Schaal H, Timm J, Senff T: Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting. Eur J Clin Microbiol Infect Dis 40: 1063–1071, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espi M, Koppe L, Fouque D, Thaunat O: Chronic kidney disease-associated immune dysfunctions: Impact of protein-bound uremic retention solutes on immune cells. Toxins (Basel) 12: 300, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krueger KM, Ison MG, Ghossein C: Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am J Kidney Dis 75: 417–425, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Kunisaki KM, Janoff EN: Influenza in immunosuppressed populations: A review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis 9: 493–504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurts C, Panzer U, Anders HJ, Rees AJ: The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 13: 738–753, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD: BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384: 1412–1423, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, de Lusignan S, Docherty AB, Ford D, Hobbs FR, Joy M, Katikireddi SV, Marple J, McCowan C, McGagh D, McMenamin J, Moore E, Murray JL, Pan J, Ritchie L, Shah SA, Stock S, Torabi F, Tsang RS, Wood R, Woolhouse M, Robertson C, Sheikh A: Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 397: 1646–1657, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, Martzloff J, Perrin P, Moulin B, Fafi-Kremer S, Caillard S: Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int 99: 1487–1489, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM: Immunogenicity of a single dose of SARS-CoV-2 messenger rna vaccine in solid organ transplant recipients. JAMA 325: 1784–1786, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S: Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 397: 1819–1829, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group: Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383: 2603–2615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotkin SA: Correlates of protection induced by vaccination. Clin Vaccine Immunol 17: 1055–1065, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, Feldman J, Pavlovic MN, Gregory DJ, Poznansky MC, Sigal A, Schmidt AG, Iafrate AJ, Naranbhai V, Balazs AB: Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 184: 2372–2383.e9, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzmina A, Khalaila Y, Voloshin O, Keren-Naus A, Boehm-Cohen L, Raviv Y, Shemer-Avni Y, Rosenberg E, Taube R: SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host Microbe 29: 522–528.e2, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM: Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 325: 2204–2206, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, Katchman E, Levi S, Houri I, Lubezky N, Shibolet O, Katchman H: Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol 75: x435–438, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, Segev DL: Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: A case series [published online ahead of print June 15, 2021]. Ann Intern Med [DOI] [PMC free article] [PubMed] [Google Scholar]