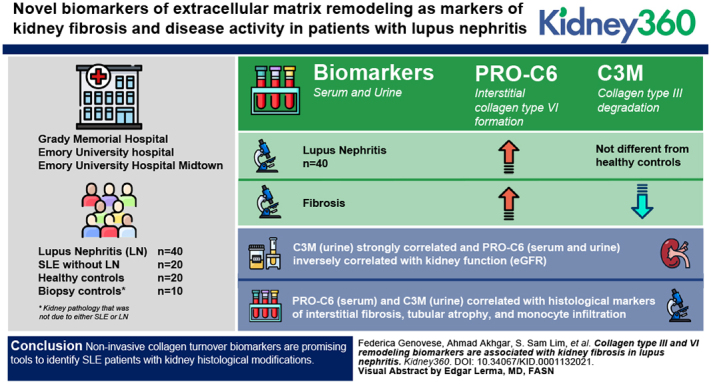
Key Points
Prognostic biomarkers that identify patients with SLE at risk of developing lupus nephritis and progressing to kidney failure are needed.
Tubulointerstitial fibrosis is an important pathologic feature of lupus nephritis and is associated with kidney disease progression.
Circulatory and urinary markers of collagen type III and type VI remodeling noninvasively reflect levels of kidney fibrosis in patients with lupus nephritis.
Keywords: chronic kidney disease, basic science, biomarkers, collagen, collagen type III, endotrophin, extracellular matrix, fibrosis, lupus nephritis
Visual Abstract
Abstract
Background
Lupus nephritis (LN) occurs in <40% of patients with SLE. Reliable biomarkers of kidney damage are needed to identify patients with SLE at risk of developing LN to improve screening, treat the disease earlier, and halt progression to kidney failure. Novel biomarkers of extracellular matrix remodeling were evaluated as markers of kidney fibrosis and disease activity in patients with LN.
Methods
Biomarkers of the interstitial collagen type III (PRO-C3) and type VI (PRO-C6) formation and of collagen type III (C3M) degradation were evaluated in the serum and urine of 40 patients with LN, 20 patients with SLE but without LN, 20 healthy controls, and ten biopsy controls (histologic kidney inflammation/damage without SLE). Their association with histologic markers of interstitial fibrosis and tubular atrophy, with inflammatory cell infiltration and with disease activity and chronicity in the patients with LN was assessed.
Results
Despite PRO-C3 (serum) and PRO-C6 (serum and urine) being significantly elevated in patients with LN compared with healthy controls, the markers did not differentiate patients with LN from those with SLE. C3M (urine) levels were not different in LN compared with the other groups. C3M (urine) strongly correlated and PRO-C6 (serum and urine) inversely correlated with kidney function (eGFR). The biomarkers of interstitial collagen turnover PRO-C6 (serum) and C3M (urine) correlated with histologic markers of interstitial fibrosis, tubular atrophy, and monocyte infiltration.
Conclusions
Noninvasive collagen turnover biomarkers are promising tools to identify patients with SLE with kidney histologic modifications.
Introduction
Lupus nephritis (LN) is a complication that can arise in <40% of patients with SLE (1). The development of kidney disease is highly predictive of increased risk of morbidity and mortality (2). Despite the availability of treatment options for patients with SLE, the 5-year cumulative incidence of ESKD was 6.4% among Black patients versus 2.5% among White patients (3). Another racial disparity has been observed on the risk of mortality after the development of LN-caused ESKD, with Black patients having a significantly increased risk of death compared to other races (4). It is therefore of paramount importance to identify the patients with SLE at the highest risk of developing LN and progressing to kidney failure, especially in certain races and ethnicities (5).
LN is the result of immune complex deposition in the glomerulus and is associated with other mechanisms that can lead to kidney injury identifiable only by histologic observation (5). The International Society of Nephrology/Renal Pathology Society have classified six LN histologic classes that help, in part, to inform the course of treatment (2,5). The presence of diffuse interstitial fibrosis, tubular atrophy, and glomerular sclerosis in the biopsy indicate a poor prognosis (6).
Kidney biopsy is the standard procedure in patients with SLE that develop significant changes in their kidney function parameters to confirm the diagnosis of LN. Some experts suggest that kidney biopsy should be considered with earlier signs of kidney involvement, because even early findings can strongly predict kidney outcome (6). Furthermore, the nature of the pathology may change in a patient, which may require a different course of treatment. In general practice, the threshold for early biopsy is not well defined, and surveillance biopsies are not common.
Despite several soluble biomarkers of kidney damage and disease activity being suggested with varied success in a number of LN cohorts (7,8), there are still no reliable markers of kidney damage and activity in clinical use that could complement or even substitute the need for baseline or repeat biopsies and be used for early identification of poor responders and for personalized treatment (9). Differentially expressed extracellular matrix (ECM) proteins have been identified in urinary proteomic studies on patients with LN (10–14); however, there is no systematic study on ECM proteins as biomarkers of kidney damage in the literature. In this study, we test for the first time in predominantly Black patients with LN noninvasive biomarkers of interstitial collagen formation and degradation that were previously associated with adverse prognosis in different CKD indications. We have selected two serological and urinary markers of collagen type III (CM3) and VI formation (PRO-C3 and PRO-C6), and a urinary marker of CM3 degradation. During fibrosis progression, the balance between collagen formation and degradation tilts toward increasing formation, deposition and crosslinking, and a lower degree of collagen degradation and scar resolution. These markers reflect this mechanism and the possibility of measuring them with a simple and fast immunoassay that allows for repeated noninvasive measurements over the course of the disease, which provides a more dynamic picture of disease progression.
Our hypotheses were: (1) serological and urinary levels of biomarkers of collagen turnover are different in patients with LN than in patients with SLE with no kidney involvement; (2) serological and urinary biomarkers of collagen turnover reflect interstitial fibrosis, and are associated with other markers of kidney disease in patients with LN; (3) serological and urinary biomarkers of collagen turnover reflect disease activity (National Institutes of Health [NIH] Activity Index and Systemic Lupus Erythematosus Disease Activity Index 2000) and histologic and disease damage (NIH Chronicity Index and Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index).
Materials and Methods
Cohort
Patients were recruited from Grady Memorial Hospital, Emory University Hospital, and Emory University Hospital Midtown in Atlanta, Georgia, and included 40 patients with LN, 20 patients with SLE but without LN, 20 healthy controls, and ten patients with kidney pathology that was not due to either SLE or LN (“biopsy controls”). All patients with SLE met the 1997 update of the 1982 American College of Rheumatology revised classification criteria for SLE, and were recruited consecutively. Those with LN had significant enough clinical suspicion by their treating physicians to warrant a kidney biopsy, which was consistent with LN (15,16). Those without LN had <500 mg proteinuria and had no clinical suspicion of LN by their treating physicians.
All patients were treated throughout the study with standard of care therapies as determined by their treating physicians. Of the ten biopsy controls, one patient was diagnosed with minimal change disease, four with secondary FSGS, two with IgA nephropathy, one with thrombotic microangiopathy, and two with HIV-associated nephropathy. Serum was available from all patients, whereas urine was available from 37 LN, 17 SLE, 19 healthy controls, and nine biopsy controls. Ethical approval was granted by the Emory University Institutional Review Board and the Grady Research Oversight Committee. All patients provided written, informed consent, and the study was conducted in accordance with the Declaration of Helsinki. Repeated serum and urine sampling were performed in the LN population at 3, 6, and 12 months (available serum from 26, 25, and 24 patients and available urine from 25, 25, and 14 patients, respectively).
Baseline measurements at the time of biopsy were used to compare the levels of biomarkers in circulation with clinical and histologic parameters on core needle biopsies. Histology was read and scored by a single study kidney pathologist (A.B.F.). The histologic parameters considered in this study were % interstitial fibrosis, % tubular atrophy, and interstitial mononuclear cell infiltration (NIH-A6) (17–19). When repeat biopsies were available, the first biopsy results were used for correlation analyses.
Biomarkers
We selected assays measuring specific fragments of ECM proteins reflecting the dynamic process of ECM turnover (Table 1). Specifically, we focused on collagen type III turnover biomarkers (PRO-C3 and C3M, reflecting collagen type III formation and degradation, respectively) previously observed to be altered and associated to adverse outcomes in CKD (20,21). Furthermore, we selected a fragment reflecting collagen type VI (PRO-C6) formation, due to a previously observed relevance of this biomarker for prognosis of CKD (22–24). C3M was measured only in urine, whereas PRO-C3 and PRO-C6 were measured in both serum and urine of the patients included in the cohort by means of ELISAs developed at Nordic Bioscience (Herlev, Denmark). The procedures for the single ELISAs have been previously detailed in the references listed in Table 1. The urinary biomarker results were normalized for levels of urinary creatinine measured by the QuantiChrom Creatinine assay kit (BioAssay Systems, Hayward, CA, USA).
Table 1.
Biomarker assays measuring specific fragments of extracellular matrix proteins
| Biomarker | Description | Serum | Urine | Reference in which Procedure Is Described |
| Formation biomarkers | ||||
| PRO-C3 | N-terminal pro-peptide of collagen type III | x | x | (36) |
| PRO-C6 | C-terminal of collagen type VI and endotrophin | x | x | (37) |
| Degradation biomarkers | ||||
| C3M | MMP-9 mediated degradation of collagen type III | x | (21,38) | |
PRO-C3, biomarker of interstitial collagen type III; PRO-C6, biomarker of interstitial collagen type VI; C3M, biomarker of collagen type III.
Statistical Analyses
The biomarkers were not normally distributed; hence, statistical differences in biomarker levels between different patient groups were calculated using the Kruskal–Wallis, test with Dunn’s t test for multiple comparison. Differences between levels of the markers at different time points were calculated using the Friedman test with Dunn’s test for multiple comparison. Statistical differences in biomarker levels between high and low NIH-Activity Index scores were calculated using the Mann–Whitney test. The correlograms of levels of the biomarkers and demographic, clinical, and histologic parameters report the rho calculated using Spearman rank correlation. Partial Spearman correlation was used to test the independent association of the levels of the biomarkers with the histologic parameters.
Results
The baseline demographic and clinical characteristics of the LN cohort can be found in Table 2. The patients with LN were predominantly young, Black women with CKD from stages I–V, but mostly mild CKD, belonging mostly to LN class III and/or IV. Most patients were treated with prednisone, hydroxychloroquine, and/or mycophenolate mofetil. The timing, method, and availability of baseline proteinuria was too variable to report.
Table 2.
Baseline demographic and clinical parameters of the examined patients
| Demographic/Clinical Characteristic | Lupus Nephritis (n=40), Mean (SD) or n (%) | SLE (n=20), Mean (SD) or n (%) | Biopsy Controls (n=12), Mean (SD) or n (%) | Healthy Controls (n=20), Mean (SD) or n (%) |
| Age, yrs | 35 (11) | 37.2 (9) | 42 (13.1) | 22.3 (7) |
| Sex, female | 33(82) | 18 (90) | 7 (58.3) | 18 (90) |
| Race | ||||
| Black | 36 (90) | 20 (100) | 6 (50) | 17 (85) |
| White | 3 (7.5) | 0 (0) | 6 (50) | 3 (15) |
| Other | 1 (2.5) | 0 (0) | 0 (0) | 0 (0) |
| Blood pressure | ||||
| Systolic | 135 (17) | 131 (12) | n/a | n/a |
| Diastolic | 80 (11) | 73 (9.1) | n/a | n/a |
| eGFR | 71 (33) | n/a | n/a | n/a |
| Platelet count | 219 (94) | 221 (73) | n/a | n/a |
| White blood cell count | 7.7 (5.3) | 4.3 (3.7) | n/a | n/a |
| Hemoglobin | 10 (1.8) | 11.2 (3.1) | n/a | n/a |
| LN class | ||||
| III or IV only | 32 (67) | n/a | n/a | n/a |
| II only | 6 (12.5) | n/a | n/a | n/a |
| V only | 6 (12.5) | n/a | n/a | n/a |
| VI only | 4 (8) | n/a | n/a | n/a |
| NIH Activity Index (range 0–24) | 7.4 (5.1) | n/a | n/a | n/a |
| NIH Chronicity Index (range 0–12) | 4.7 (2.3) | n/a | n/a | n/a |
| SLEDAI 2K (range 0–105) | 8.8 (5.6) | 3.5 (3.2) | n/a | n/a |
| SLICC/ACR damage index (range 0–47) | 0.6 (1.0) | 0.7 (1.1) | n/a | n/a |
| Concomitant treatment | ||||
| Prednisone | 27 (67.5) | 11 (55) | n/a | n/a |
| Hydroxychloroquine | 27 (67.5) | 17 (85) | n/a | n/a |
| Methotrexate | 1 (2.5) | 3 (15) | n/a | n/a |
| Cyclophosphamide | 3 (7.5) | 0 (0) | n/a | n/a |
| Mycophenolate mofetil | 19 (47.5) | 7 (35) | n/a | n/a |
| Azathioprine | 1 (2.5) | 5 (25) | n/a | n/a |
| Belimumab | 1 (2.5) | 1 (5) | n/a | n/a |
| Rituximab | 1 (2.5) | 0 (0) | n/a | n/a |
LN, lupus nephritis; NIH, National Institutes of Health; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLICC, Systemic Lupus International Collaborating Clinics; ACR, American College of Rheumatology.
The biomarkers were successfully measured in serum and urine with a coefficient of variation <15% between double determinations. In total, 10% of urine samples were below the lower limit of quantification (LLOQ) for C3M, 21% were below LLOQ for PRO-C3, and 7% were below LLOQ for PRO-C6. None of the serum samples were below LLOQ for PRO-C3 and PRO-C6. Some of the biomarkers had a moderate correlation with each other. Specifically, PRO-C6 in serum correlated with PRO-C6 in urine, PRO-C3 in serum, and inversely correlated with C3M in urine. Moreover, PRO-C6 in urine correlated with PRO-C3 in urine (Table 3).
Table 3.
Correlogram of baseline biomarkers.
| uPRO-C3/Crea | uPRO-C6/Crea | uC3M/Crea | PRO-C3 | PRO-C6 | |
| uPRO-C3/Crea | 0.512a | −0.024 | 0.076 | 0.207 | |
| uPRO-C6/Crea | 0.512a | −0.201 | 0.146 | 0.636a | |
| uC3M/Crea | −0.024 | −0.201 | −0.164 | −0.624a | |
| PRO-C3 | 0.076 | 0.146 | −0.164 | 0.483a | |
| PRO-C6 | 0.207 | 0.636a | −0.624a | 0.483a |
u, urine; C3M, biomarker of collagen type III; PRO-C3, biomarker of interstitial collagen type III; PRO-C6, biomarker of interstitial collagen type VI.
Moderate correlation (rho 0.4–0.7).
PRO-C3 (serum) and PRO-C6 (serum and urine) were able to discriminate patients with LN from healthy controls. However, PRO-C3 and PRO-C6 in serum and C3M in urine were not significantly different in the patients with LN compared with the patients with SLE. In contrast, PRO-C3 (urine) was decreased in patients with LN, compared with both biopsy controls and SLE. Notably, levels of PRO-C3 (serum) and PRO-C6 (serum and urine) were also elevated in the biopsy controls (Figure 1). The high levels of these markers in the biopsy controls were mostly driven by the patient with thrombotic microangiopathy and by those with HIV-associated nephropathy.
Figure 1.
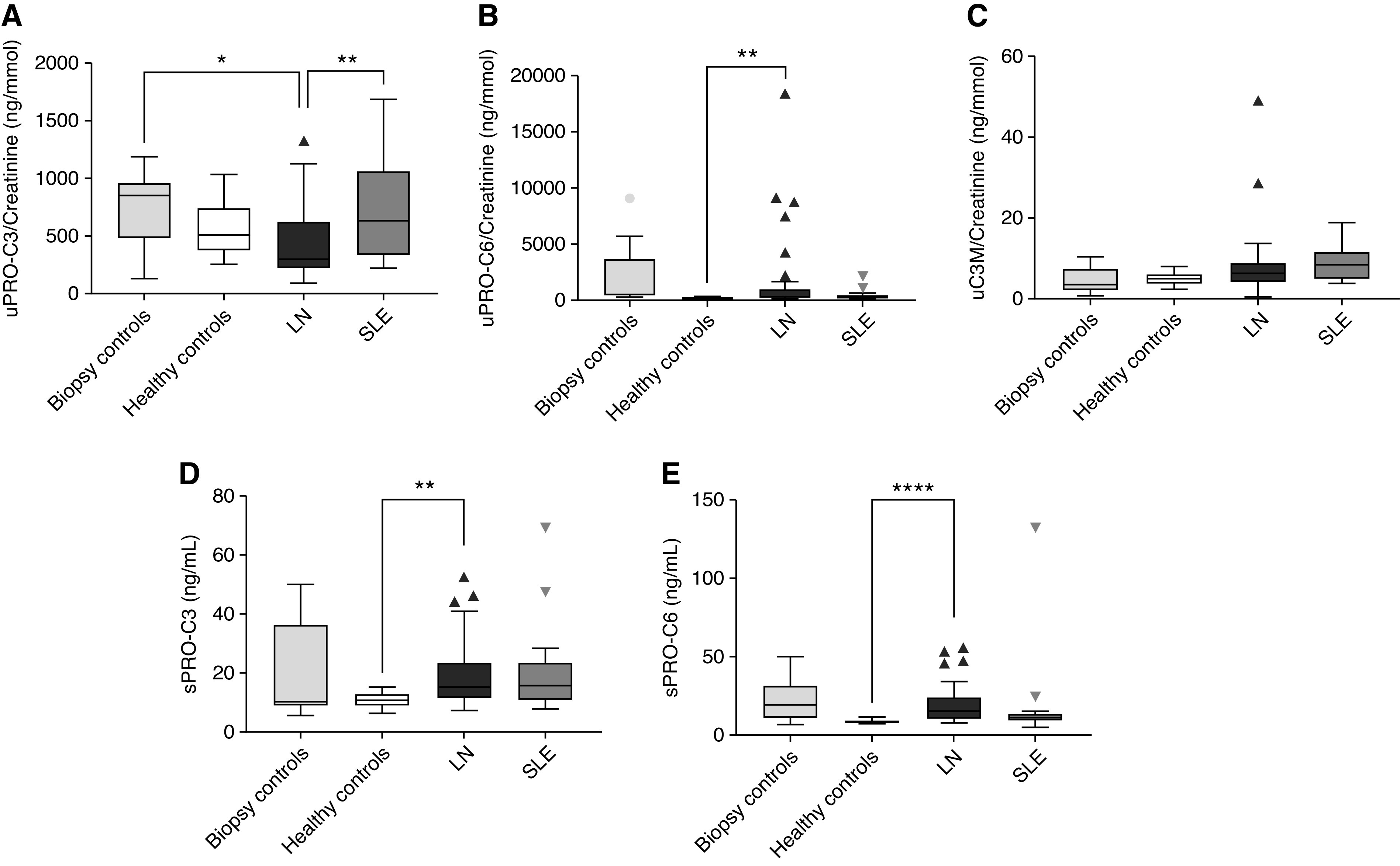
Levels of extracellular matrix biomarkers in patients with lupus nephritis (LN) compared with SLE, healthy controls, and biopsy controls. The Kruskal–Wallis with Dunn’s multiple comparison (using LN group as reference) test was used to evaluate the difference between groups. *P<0.05, **P<0.01, ****P<0.0001. (A) Urine PRO-C3, biomarker of interstitial collagen type III. (B) Urine PRO-C6, biomarker of interstitial collagen type VI. (C) C3M, biomarker of collagen type III. (D) Serum PRO-C3, biomarker of interstitial collagen type III. (E) Serum PRO-C6, biomarker of interstitial collagen type VI.
When observing the effect of concomitant treatment, the 27 patients with LN treated with hydroxychloroquine had significantly lower levels of PRO-C3 and PRO-C6 (serum) than the others (data not shown). The other treatments did not have any effect on the biomarkers.
A correlogram of levels of the biomarkers and demographics and clinical parameters is presented in Table 4. Of note, two markers exhibited a strong correlation with kidney function: PRO-C6 (serum and urine) inversely correlated with eGFR and C3M (urine) correlated with eGFR. Moreover, PRO-C3 (serum) was inversely correlated with white blood cell count and hemoglobin, and PRO-C6 (serum and urine) inversely correlated with hemoglobin, which may be indicators of the severity of kidney involvement.
Table 4.
Correlogram of baseline biomarker levels and demographic and clinical parameters
| Age | Diastolic Blood Pressure | Systolic Blood Pressure | eGFR | SLICC Score | SLEDAI Score | Hemoglobin | White Blood Cell Count | Platelet count | |
| uPRO-C3/Crea | 0.577a | 0.026 | −0.260 | −0.130 | 0.031 | −0.312 | −0.118 | −0.158 | −0.054 |
| uPRO-C6/Crea | 0.351 | 0.182 | 0.268 | −0.482a | 0.114 | −0.138 | −0.493a | 0.169 | −0.140 |
| uC3M/Crea | −0.174 | −0.247 | −0.320 | 0.782b | −0.429a | 0.109 | 0.022 | −0.226 | 0.086 |
| PRO-C3 | −0.053 | −0.056 | −0.083 | −0.219 | 0.010 | −0.052 | −0.326 | −0.336 | −0.163 |
| PRO-C6 | 0.093 | 0.208 | 0.403a | −0.783b | 0.204 | 0.061 | −0.565a | 0.279 | −0.236 |
SLICC, Systemic Lupus International Collaborating Clinics; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; u, urine; PRO-C3, biomarker of interstitial collagen type III; PRO-C6, biomarker of interstitial collagen type VI; C3M, biomarker of collagen type III.
Moderate correlation (rho: 0.4–0.7).
Strong correlation (rho>0.7).
PRO-C6 (serum) was more elevated, and PRO-C3 (urine) was decreased in patients with a high NIH Activity Index (Figure 2). There was no difference in biomarker levels in patients with high versus low NIH Chronicity Index (data not shown).
Figure 2.
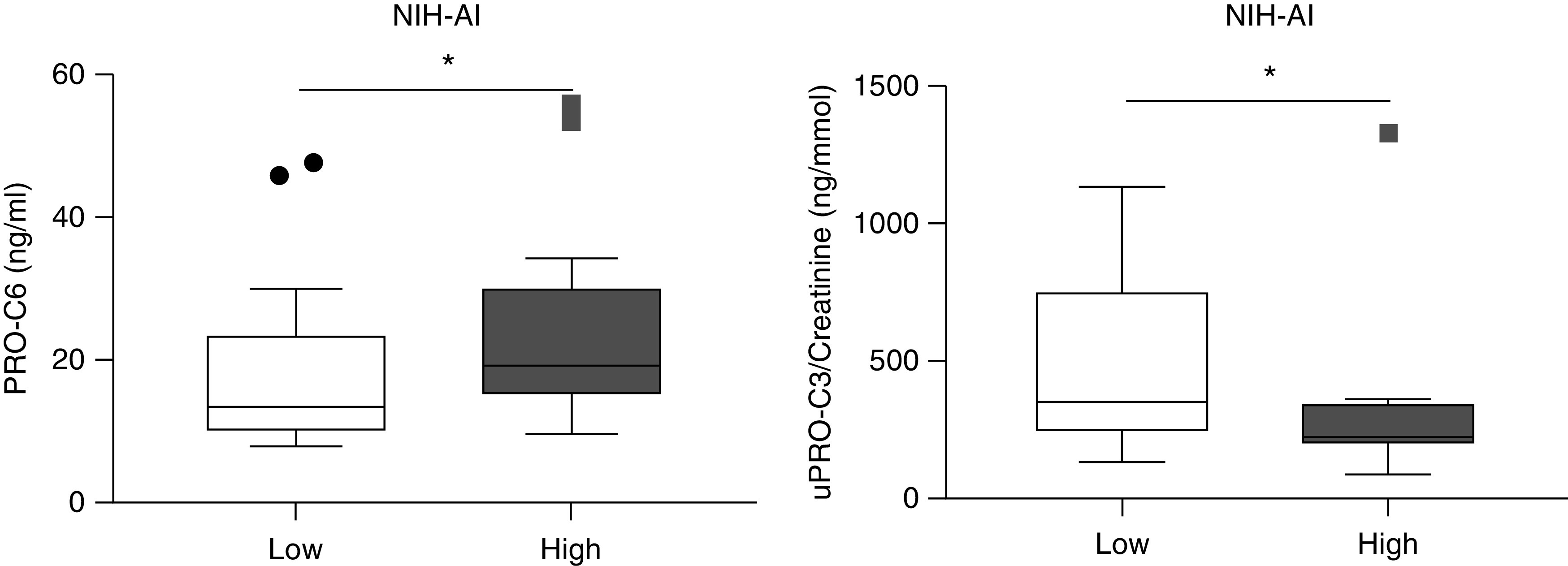
Levels of PRO-C6 (serum) and PRO-C3 (urine) in patients with high versus low National Institutes of Health Activity indices. The Mann–Whitney test was used to evaluate the difference between groups. *P<0.05. NIH-AI, National Institutes of Health activity index.
Table 5 shows the correlogram of levels of the biomarkers and the histologic parameters examined in this study. C3M in urine inversely correlated with interstitial fibrosis and tubular atrophy, whereas PRO-C6 in serum correlated with interstitial fibrosis and interstitial mononuclear cell infiltration. When eGFR was added as a confounder to the partial correlation analyses, the correlations of C3M and PRO-C6 with the histologic features of fibrosis and inflammation lost their significance (data not shown).
Table 5.
Correlogram of baseline biomarker levels and histologic measurements in patients with lupus nephritis
| % Interstitial Fibrosis | % Tubular Atrophy | Interstitial Mononuclear Cell Infiltration | |
| uPRO-C3/Crea | 0.192 | 0.189 | 0.059 |
| uPRO-C6/Crea | 0.384 | 0.363 | 0.356 |
| uC3M/Crea | −0.430a | −0.503a | −0.257 |
| PRO-C3 | 0.372 | 0.280 | 0.302 |
| PRO-C6 | 0.412a | 0.377 | 0.477a |
u, urine; PRO-C3, biomarker of interstitial collagen type III; PRO-C6, biomarker of interstitial collagen type VI; C3M, biomarker of collagen type III.
Moderate correlation (rho: 0.4–0.7).
The longitudinal analysis of the biomarkers showed consistent levels over time. PRO-C6 (serum) appeared higher at 12 months; however, the significance was driven by an outlier (Figure 3).
Figure 3.
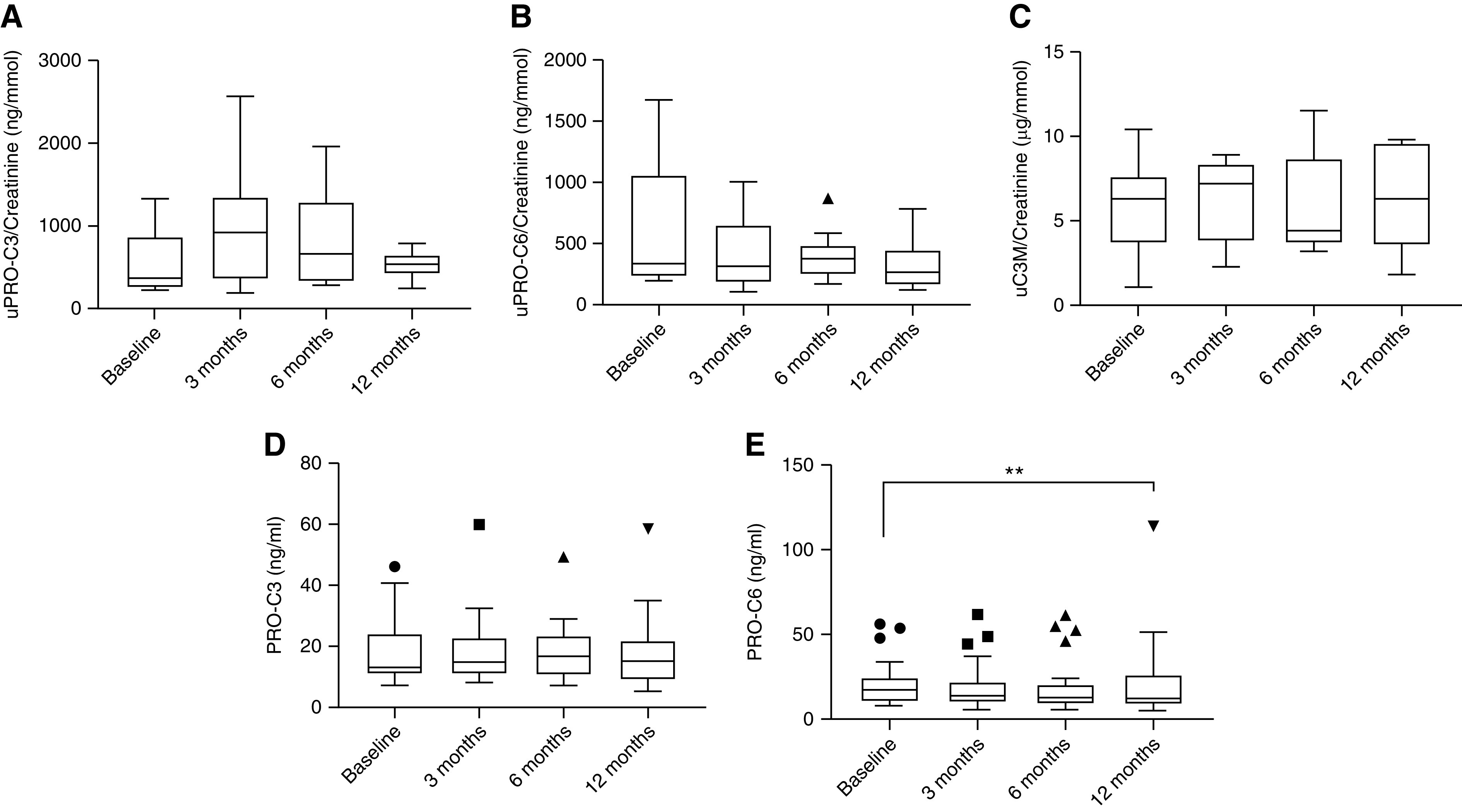
Levels of extracellular matrix biomarkers in patients with LN at multiple time points. The Friedman with Dunn’s multiple comparison (using baseline as reference) test was used to evaluate the difference between groups. **P<0.01. (A) Urine PRO-C3, biomarker of interstitial collagen type III. (B) Urine PRO-C6, biomarker of interstitial collagen type VI. (C) Urine C3M, biomarker of collagen type III. (D) Serum PRO-C3, biomarker of interstitial collagen type III. (E) Serum PRO-C6, biomarker of interstitial collagen type VI.
Discussion
Noninvasive biomarkers reflecting the burden and activity of kidney fibrosis in patients with SLE would be an important tool to identify patients with LN or at risk of developing LN, to monitor disease progression and to inform the right course of treatment. Although ECM proteins have been identified in the urinary proteome of patients with LN in several studies (10–14), this is the first time a systematic approach to evaluate biomarkers of ECM remodeling in LN has been undertaken, particularly in a high-risk population. We identified two promising biomarkers of kidney fibrosis: the noninvasive biomarkers of collagen type VI formation and collagen type III degradation were significantly associated with either histologic extent of interstitial fibrosis, tubular atrophy, and/or interstitial mononuclear cell infiltration in LN.
The examined cohort included LN patients enrolled in an observational study in Atlanta. The prevalence of women in the cohort reflects the prevalence of LN in the American population, where the female-to-male ratio ranges between 8:1 and 15:1; the frequency of LN is highest in the Black population (5,25).
Levels of the biomarker of collagen formation PRO-C6 in serum were more elevated in patients exhibiting greater interstitial fibrosis. On the contrary, lower levels of the collagen degradation marker C3M in urine indicated advanced histologic fibrosis and tubular atrophy.
The levels of these markers were not significantly different in patients with SLE compared with patients with LN. The 20 patients with SLE included as a control group did not have clinically significant evidence of kidney disease to warrant a kidney biopsy. However, we cannot determine if there was some degree of renal fibrosis, particularly in those with high biomarker levels.
These observations, and the fact that PRO-C6 (serum and urine) and C3M (urine) were strongly associated with kidney function in opposite directions, with higher PRO-C6 and lower C3M found in patients with more advanced kidney impairment, confirm previous findings in nonlupus CKD cohorts (20–24,26–28). Further confirmation of our findings comes from urinary proteomic studies where collagen type VI was one of the few ECM proteins found to be upregulated (13), and collagen type III, specifically the fragment corresponding to the epitope recognized by C3M, was downregulated (14).
The strong collinearity of serum PRO-C6 and urine C3M with eGFR is likely to be the cause of the lack of correlation of the fibrosis biomarkers when eGFR is included in the partial correlation model. However, eGFR and the markers of fibrosis reflect different biologic aspects of kidney disease progression. Kidney fibrosis is an integral part of the kidney tissue remodeling that causes loss of kidney function, and noninvasive markers of kidney fibrosis have an intrinsic value in patients in which remodeling of the kidney tissue have not yet started affecting the less sensitive markers of kidney function (29).
We also observed that PRO-C6 in serum was elevated in patients with higher disease activity (NIH Activity Index >9). This suggests PRO-C6 is not only a marker of increased burden of fibrosis in the kidneys, but also reflects an active disease, which is associated with a worse prognosis and faster progression. PRO-C6 detects the C-terminus of the propeptide of collagen type VI α3 chain. Part of this propeptide is further processed to give rise to a bioactive molecule, endotrophin, that has been implicated in several detrimental processes in cancer progression, insulin resistance, fibrosis, and inflammation (30–35). Because PRO-C6 can detect both formation of collagen type VI and endotrophin, the high levels of this biomarker may reflect not only a process of fibrogenesis, but also the release of this multifunctional matrikine, which may have a role in triggering or advancing the disease.
In LN patients treated with hydroxychloroquine, levels of PRO-C3 and PRO-C6 were lowered in serum, but not in urine, suggesting this treatment may be associated with systemic changes in ECM remodeling and worth further exploration.
A limitation of the use of these collagen biomarkers is the lack of specificity for LN, because the elevation of PRO-C3 and PRO-C6 (serum) was observed in patients with LN, SLE, or the biopsy control group as compared with healthy controls. However, the markers reflect a higher rate of tissue remodeling in patients with a profibrotic profile, rather than of a mechanism specific to the LN pathogenesis. Surprisingly, levels of PRO-C3 (urine) were significantly lower in patients with LN compared with the other groups. Combined with the finding that this biomarker was also decreased in patients with high activity index, the data suggest the presence of lower levels of the collagen type III formation biomarker in urine might be indicative of a phenotype of LN patients with advanced disease. However, given the variety of histologic classes represented in the LN population, the sample size is too small to be conclusive.
This study presented several limitations: this cohort was not collected with the scope of evaluating biomarkers, hence the sample size is small, and a systematic collection of clinical data is missing on some parameters (for instance, albuminuria), which hinders the control for confounders. Moreover, there were no follow-up clinical data, hence we were not able to evaluate the prognostic potential of the investigated biomarkers that showed promising results in other kidney indications in LN. This study represented a first approach to evaluate markers of ECM remodeling in LN. The data obtained in this study will need to be confirmed and further validated in larger LN studies meant for biomarker discovery and validation.
In conclusion, we have identified a biomarker of interstitial collagen formation, PRO-C6, and a biomarker of interstitial collagen degradation, C3M, as promising biomarkers reflecting histologic alterations, and possibly degree of disease activity in LN patients. These biomarkers describe the dynamic processes of tissue turnover in real time; patients presenting with altered levels of these biomarkers in serum or urine are at higher risk of having high disease activity, and, therefore, faster disease progression. These biomarkers could potentially be used for patient stratification, to identify the phenotypes of patients with LN at higher risk of disease progression, and to help elaborate the best therapeutic plan, contributing to the application of personalized medicine in this highly heterogeneous disease.
Disclosures
A. Akhgar reports having an ownership interest in and receiving research funding from AstraZeneca. A. Farris reports receiving research funding from MedImmune Inc. and the NIH. D. Sinibaldi reports having an ownership interest in AstraZeneca. F. Genovese and M. Karsdal repost being full-time employees at Nordic Bioscience and holding stocks in Nordic Bioscience; Nordic Bioscience is a privately owned, small to medium-sized enterprise partly focused on the development of biomarkers. The patents for the assays described in the paper are owned by Nordic Bioscience. J. Cobb reports having consultancy agreements with the American College of Rheumatology and NephCure; reports receiving research funding from Akebia and Vertex; reports receiving honoraria from Aurinia. S. Lim reports receiving research funding from Bristol Myers Squibb, GlaxoSmithKline, MedImmune, and UCB; reports being a scientific advisor or member of Accordant, GlaxoSmithKline, and Pfizer; reports other interests/relationships with American College of Rheumatology, Lupus Foundation of America, Systemic Lupus International Collaborating Clinics. W. White reports having an ownership interest in and receiving research funding from AstraZeneca. None of the authors received fees, bonuses, or other benefits for the work described in the manuscript. All remaining authors have nothing to disclose.
Funding
This work was supported by AstraZeneca.
Footnotes
See related editorial, “Collagen Remodeling Biomarkers in Lupus Nephritis,” on pages 1395–1398.
Author Contributions
A. Akhgar, F. Genovese, S. Lim, and W. White conceptualized the study; A. Akhgar, A. Farris, and F. Genovese were responsible for data curation; A. Farris, F. Genovese, and D. Sinibaldi were responsible for formal analysis; M. Battle, J. Cobb, A. Farris, F. Genovese, and S. Lim were responsible for investigation; F. Genovese was responsible for methodology and wrote the original draft; A. Akhgar, M. Battle, J. Cobb, A. Farris, M. Karsdal, S. Lim, D. Sinibaldi, and W. White reviewed and edited the manuscript; M. Battle, M. Karsdal, and S. Lim were responsible for resources; S. Lim, M. Karsdal, and W. White provided supervision; M. Karsdal, D. Sinibaldi, and W. White were responsible for funding acquisition; W. White was responsible for project administration; and all authors approved the final version of the manuscript.
References
- 1.Hanly JG, O’Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, Bae SC, Bernatsky S, Clarke AE, Wallace DJ, Merrill JT, Isenberg DA, Rahman A, Ginzler EM, Fortin P, Gladman DD, Sanchez-Guerrero J, Petri M, Bruce IN, Dooley MA, Ramsey-Goldman R, Aranow C, Alarcón GS, Fessler BJ, Steinsson K, Nived O, Sturfelt GK, Manzi S, Khamashta MA, Van Vollenhoven RF, Zoma AA, Ramos-Casals M, Ruiz-Irastorza G, Sam Lim S, Stoll T, Inanc M, Kalunian KC, Kamen DL, Maddison P, Peschken CA, Jacobsen S, Askanase A, Theriault C, Thompson K, Farewell V: The frequency and outcome of lupus nephritis: Results from an international inception cohort study. Rheumatol 55: 252–262, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu F, Haas M, Glassock R, Zhao M-H: Redefining lupus nephritis: Clinical implications of pathophysiologic subtypes. Nat Rev Nephrol 13: 483–495, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Plantinga L, Lim SS, Patzer R, McClellan W, Kramer M, Klein M, Pastan S, Gordon C, Helmick C, Drenkard C: Incidence of end-stage renal disease among newly diagnosed systemic lupus erythematosus patients: The Georgia lupus registry. Arthritis Care Res 68: 357–365, 2016. 10.1002/acr.22685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nee R, Martinez-Osorio J, Yuan CM, Little DJ, Watson MA, Agodoa L, Abbott KC: Survival disparity of African American versus non-African American patients with ESRD due to SLE. Am J Kidney Dis 66: 630–637, 2015. 10.1053/j.ajkd.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Almaani S, Meara A, Rovin BH: Update on lupus nephritis. Clin J Am Soc Nephrol 12: 825–835, 2017. 10.2215/CJN.05780616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moroni G, Depetri F, Ponticelli C: Lupus nephritis: When and how often to biopsy and what does it mean? J Autoimmun 74: 27–40, 2016. 10.1016/j.jaut.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 7.Aragón CC, Tafúr RA, Suárez-Avellaneda A, Martínez MT, Salas AL, Tobón GJ: Urinary biomarkers in lupus nephritis. J Transl Autoimmun 3: 100042, 2020. 10.1016/j.jtauto.2020.100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caster DJ, Powell DW: Utilization of biomarkers in lupus nephritis.Adv Chronic Kidney Dis 26: 351–359, 2019. 10.1053/j.ackd.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayoub I, Cassol C, Almaani S, Rovin B, Parikh SV: The kidney biopsy in systemic lupus erythematosus: A view of the past and a vision of the future. Adv Chronic Kidney Dis 26: 360–368, 2019. 10.1053/j.ackd.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 10.Nicolaou O, Kousios A, Hadjisavvas A, Lauwerys B, Sokratous K, Kyriacou K: Biomarkers of systemic lupus erythematosus identified using mass spectrometry-based proteomics: A systematic review. J Cell Mol Med 21: 993–1012, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Chen YD, Gu W: Urinary proteomics as a novel tool for biomarker discovery in kidney diseases. J Zhejiang Univ Sci B 11: 227–237, 2010. 10.1631/jzus.B0900327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley S, Vanarsa K, Soliman S, Habazi D, Pedroza C, Gidley G, Zhang T, Mohan S, Der E, Suryawanshi H, Tuschl T, Buyon J, Putterman C, Mok CC, Petri M, Saxena R, Mohan C: Comprehensive aptamer-based screening identifies a spectrum of urinary biomarkers of lupus nephritis across ethnicities. Nat Commun 11: 2197, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sui W, Tang D, Zou G, Chen J, Ou M, Zhang Y, Dai Y: Differential proteomic analysis of renal tissue in lupus nephritis using iTRAQ reagent technology. Rheumatol Int 32: 3537–3543, 2012. 10.1007/s00296-011-2207-1 [DOI] [PubMed] [Google Scholar]
- 14.Wei R, Gao B, Shih F, Ranger A, Dearth A, Mischak H, Siwy J, Wisniacki N, Petri M, Burkly LC: Alterations in urinary collagen peptides in lupus nephritis subjects correlate with renal dysfunction and renal histopathology. Nephrol Dial Transplant 32: 1468–1477, 2017. 10.1093/ndt/gfw446 [DOI] [PubMed] [Google Scholar]
- 15.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25: 1271–1277, 1982. 10.1002/art.1780251101 [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 17.Austin HA 3rd, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, Decker JL, Balow JE: Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med 75: 382–391, 1983. 10.1016/0002-9343(83)90338-8 [DOI] [PubMed] [Google Scholar]
- 18.Austin HA 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE: Diffuse proliferative lupus nephritis: Identification of specific pathologic features affecting renal outcome. Kidney Int 25: 689–695, 1984. 10.1038/ki.1984.75 [DOI] [PubMed] [Google Scholar]
- 19.Zappitelli M, Duffy CM, Bernard C, Gupta IR: Evaluation of activity, chronicity and tubulointerstitial indices for childhood lupus nephritis. Pediatr Nephrol 23: 83–91, 2008. 10.1007/s00467-007-0619-7 [DOI] [PubMed] [Google Scholar]
- 20.Genovese F, Rasmussen DGK, Karsdal MA, Jesky M, Ferro C, Fenton A, Cockwell P: Imbalanced turnover of collagen type III is associated with disease progression and mortality in high-risk chronic kidney disease patients. Clin Kidney J 14: 593–604, 2020. 10.1093/ckj/sfz174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genovese F, Boor P, Papasotiriou M, Leeming DJ, Karsdal MA, Floege J: Turnover of type III collagen reflects disease severity and is associated with progression and microinflammation in patients with IgA nephropathy. Nephrol Dial Transplant 31: 472–479, 2016. 10.1093/ndt/gfv301 [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen DGK, Fenton A, Jesky M, Ferro C, Boor P, Tepel M, Karsdal MA, Genovese F, Cockwell P: Urinary endotrophin predicts disease progression in patients with chronic kidney disease. Sci Rep 7: 17328, 2017. 10.1038/s41598-017-17470-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen DGK, Hansen TW, von Scholten BJ, Nielsen SH, Reinhard H, Parving H-H, Tepel M, Karsdal MA, Jacobsen PK, Genovese F, Rossing P: Higher collagen VI formation is associated with all-cause mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care 41: 1493–1500, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Fenton A, Jesky MD, Ferro CJ, Sørensen J, Karsdal MA, Cockwell P, Genovese F: Serum endotrophin, a type VI collagen cleavage product, is associated with increased mortality in chronic kidney disease. PLoS One 12: e0175200, 2017. 10.1371/journal.pone.0175200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C: The incidence and prevalence of systemic lupus erythematosus, 2002-2004: The Georgia Lupus Registry. Arthritis Rheumatol 66: 357–368, 2014. 10.1002/art.38239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilemann-Lyberg S, Rasmussen DGK, Hansen TW, Tofte N, Winther SA, Holm Nielsen S, Theilade S, Karsdal MA, Genovese F, Rossing P: Markers of collagen formation and degradation reflect renal function and predict adverse outcomes in patients with type 1 diabetes. Diabetes Care 42: 1760–1768, 2019. 10.2337/dc18-2599 [DOI] [PubMed] [Google Scholar]
- 27.Stribos EGD, Nielsen SH, Brix S, Karsdal MA, Seelen MA, van Goor H, Bakker SJL, Olinga P, Mutsaers HAM, Genovese F: Non-invasive quantification of collagen turnover in renal transplant recipients. PLoS One 12: e0175898, 2017. 10.1371/journal.pone.0175898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen DGK, Boesby L, Nielsen SH, Tepel M, Birot S, Karsdal MA, Kamper AL, Genovese F: Collagen turnover profiles in chronic kidney disease. Sci Rep 9: 16062, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P: The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 7: 4, 2014. 10.1186/1755-1536-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, Goncalves Marangoni R, Nicoloro SM, Czech MP, Varga J, Ploug T, An Z, Scherer PE: Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun 5: 3485, 2014. 10.1038/ncomms4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun K, Park J, Kim M, Scherer PE: Endotrophin, a multifaceted player in metabolic dysregulation and cancer progression, is a predictive biomarker for the response to PPARγ agonist treatment. Diabetologia 60: 24–29, 2017. 10.1007/s00125-016-4130-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bu D, Crewe C, Kusminski CM, Gordillo R, Ghaben AL, Kim M, Park J, Deng H, Xiong W, Liu X-Z, Lønning PE, Halberg N, Rios A, Chang Y, Gonzalez A, Zhang N, An Z, Scherer PE: Human endotrophin as a driver of malignant tumor growth. JCI Insight 4: e125094 2019. 10.1172/jci.insight.125094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park J, Morley TS, Scherer PE: Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol Med 5: 935–948, 2013. 10.1002/emmm.201202006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J, Scherer PE: Endotrophin: A novel factor linking obesity with aggressive tumor growth. Oncotarget 3: 1487–1488, 2012. 10.18632/oncotarget.796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C, Kim M, Lee JH, Oh J, Shin H, Lee SM, Scherer PE, Kwon HM, Choi JH, Park J: COL6A3-derived endotrophin links reciprocal interactions among hepatic cells in the pathology of chronic liver disease. J Pathol 247: 99–109, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Nielsen MJ, Nedergaard AF, Sun S, Veidal SS, Larsen L, Zheng Q, Suetta C, Henriksen K, Christiansen C, Karsdal MA, Leeming DJ: The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res 5: 303–315, 2013 [PMC free article] [PubMed] [Google Scholar]
- 37.Sun S, Henriksen K, Karsdal MA, Byrjalsen I, Rittweger J, Armbrecht G, Belavy DL, Felsenberg D, Nedergaard AF: Collagen type III and VI turnover in response to long-term immobilization. PLoS One 10: e0144525, 2015. 10.1371/journal.pone.0144525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barascuk N, Veidal SS, Larsen L, Larsen DV, Larsen MR, Wang J, Zheng Q, Xing R, Cao Y, Rasmussen LM, Karsdal MA: A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin Biochem 43: 899–904, 2010. 10.1016/j.clinbiochem.2010.03.012 [DOI] [PubMed] [Google Scholar]


