Key Points
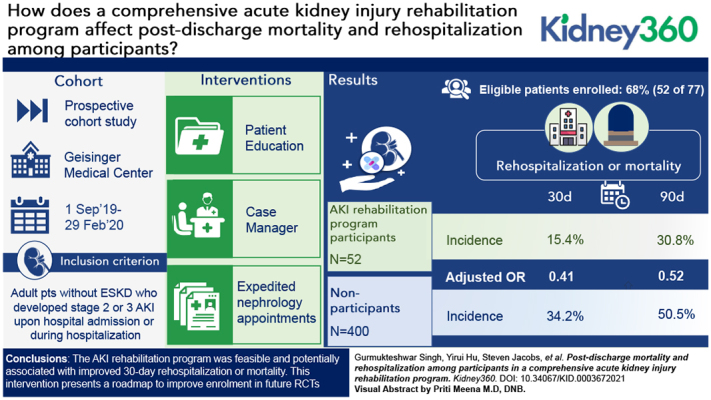
Using innovative, patient-centered interventions, 68% of eligible high-risk patients with AKI were enrolled and all came to nephrology follow-up.
Participation was associated with improvement in 30-day postdischarge rehospitalization and mortality, with similar 90-day trends.
The interventions present a roadmap for improving enrollment in AKI randomized controlled trials and should be tested further.
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, acute renal failure, aftercare, economic impact, epidemiology and outcomes, hospitalization, mortality, mortality risk, renal failure, survival
Visual Abstract
Abstract
Background
Hospitalization-associated AKI is common and is associated with markedly increased mortality and morbidity. This prospective cohort study examined the feasibility and association of an AKI rehabilitation program with postdischarge outcomes.
Methods
Adult patients hospitalized from September 1, 2019 to February 29, 2020 in a large health system in Pennsylvania with stage 2–3 AKI who were alive and not on dialysis or hospice at discharge were evaluated for enrollment. The intervention included patient education, case manager services, and expedited nephrology appointments starting within 1–3 weeks of discharge. We examined the association between AKI rehabilitation program participation and risks of rehospitalization or mortality in logistic regression analyses adjusting for comorbidities, discharge disposition, and sociodemographic and kidney parameters. Sensitivity analysis was performed using propensity score matching.
Results
Among the high-risk patients with AKI who were evaluated, 77 of 183 were suitable for inclusion. Out of these, 52 (68%) patients were enrolled and compared with 400 contemporary, nonparticipant survivors of stage 2/3 AKI. Crude postdischarge rates of rehospitalization or death were lower for participants versus nonparticipants at 30 days (15% versus 34%; P=0.01) and at 90 days (31% versus 51%; P=0.01). After multivariable adjustment, participation in the AKI rehabilitation program was associated with lower risk of rehospitalization or mortality at 30 days (OR, 0.41; 95% CI, 0.16 to 0.93), with similar findings at 90 days (OR, 0.52; 95% CI, 0.25 to 1.05). Due to small sample size, propensity-matched analyses were limited. The participants’ rehospitalization or mortality was numerically lower but not statistically significant at 30 days (18% versus 31%; P=0.22) or at 90 days (47% versus 58%; P=0.4).
Conclusions
The AKI rehabilitation program was feasible and potentially associated with improved 30-day rehospitalization or mortality. Our interventions present a roadmap to improve enrollment in future randomized trials.
Introduction
Hospitalization-associated AKI is common and associated with increased risks of mortality and morbidity, and with higher costs and length of hospital stay (1). One in five survivors of AKI are readmitted to the hospital within 30 days (2). Several gaps in the postdischarge care of these patients have been identified. Prominent among these is a lack of patient education and disease awareness. In one study, near the time of hospital discharge, 80% of survivors of AKI were unaware of their AKI diagnosis and the majority could not recognize nephrotoxic medications (3). Less than 10% of patients with AKI receive a postdischarge nephrology referral (4). Other missed opportunities include medication interventions, such as restarting renin-angiotensin-aldosterone inhibitors (5,6). Unfortunately, prospective data demonstrating the effect of interventions bridging these gaps to improve clinical outcomes in AKI remain elusive (7).
A prior retrospective study found that survivors of AKI who were seen by a nephrologist within 90 days of discharge had reduced risk of death, compared with propensity-matched survivors of AKI who did not have nephrology follow-up (8). Improved communication, patient self-management support, and guidance from a transition coach have been shown to reduce the risk of rehospitalization in patients with complex care needs (9). Protocols based on such interventions to re-engineer discharge have been applied in various healthcare settings with encouraging outcomes (10,11).
We developed an AKI rehabilitation program on the basis of these interventions, including early AKI diagnosis, intensive patient education, nurse case manager support, and expedited postdischarge nephrology follow-up. To evaluate the feasibility and preliminary effectiveness of our program, we examined outcomes of survivors of AKI who participated in the AKI rehabilitation program versus nonparticipants. We hypothesized that patients who received the AKI rehabilitation program would have decreased risk of rehospitalization or death, compared with patients who did not.
Materials and Methods
Study Setting and Study Population
The study population included adult patients without ESKD who developed stage 2 or 3 AKI upon hospital admission or during hospitalization at Geisinger Medical Center between September 1, 2019 and February 29, 2020. Geisinger Medical Center is a tertiary-care teaching hospital in central Pennsylvania. Hospitalization-associated stage 2 and 3 AKI were defined according to Kidney Disease Improving Global Outcomes (KDIGO) guidelines (12). Reference creatinine was determined using a hierarchic approach, preferably using the mean of all outpatient creatinine values 365 days before admission (Supplemental Table 1) (13).
All survivors of stage 3 AKI, regardless of the cause of AKI, were eligible for enrollment in the AKI rehabilitation program. Because the global clinical impression (“Surprise Question”) of treating clinicians has been associated with outcomes in a wide variety of patients, any survivors of stage 2 AKI who were deemed high risk by the consulting nephrologist were also potentially eligible (14). Exclusion criteria included full renal recovery before discharge, receiving dialysis at discharge, enrollment in hospice, or history of kidney transplantation.
Multilevel AKI Rehabilitation Program
The process of patient enrollment and workflow is summarized in Figure 1. Our intervention had three main components: an AKI system list for early diagnosis of hospitalization-associated AKI, a nurse case manager workflow, and expedited postdischarge nephrology follow-up.
Figure 1.
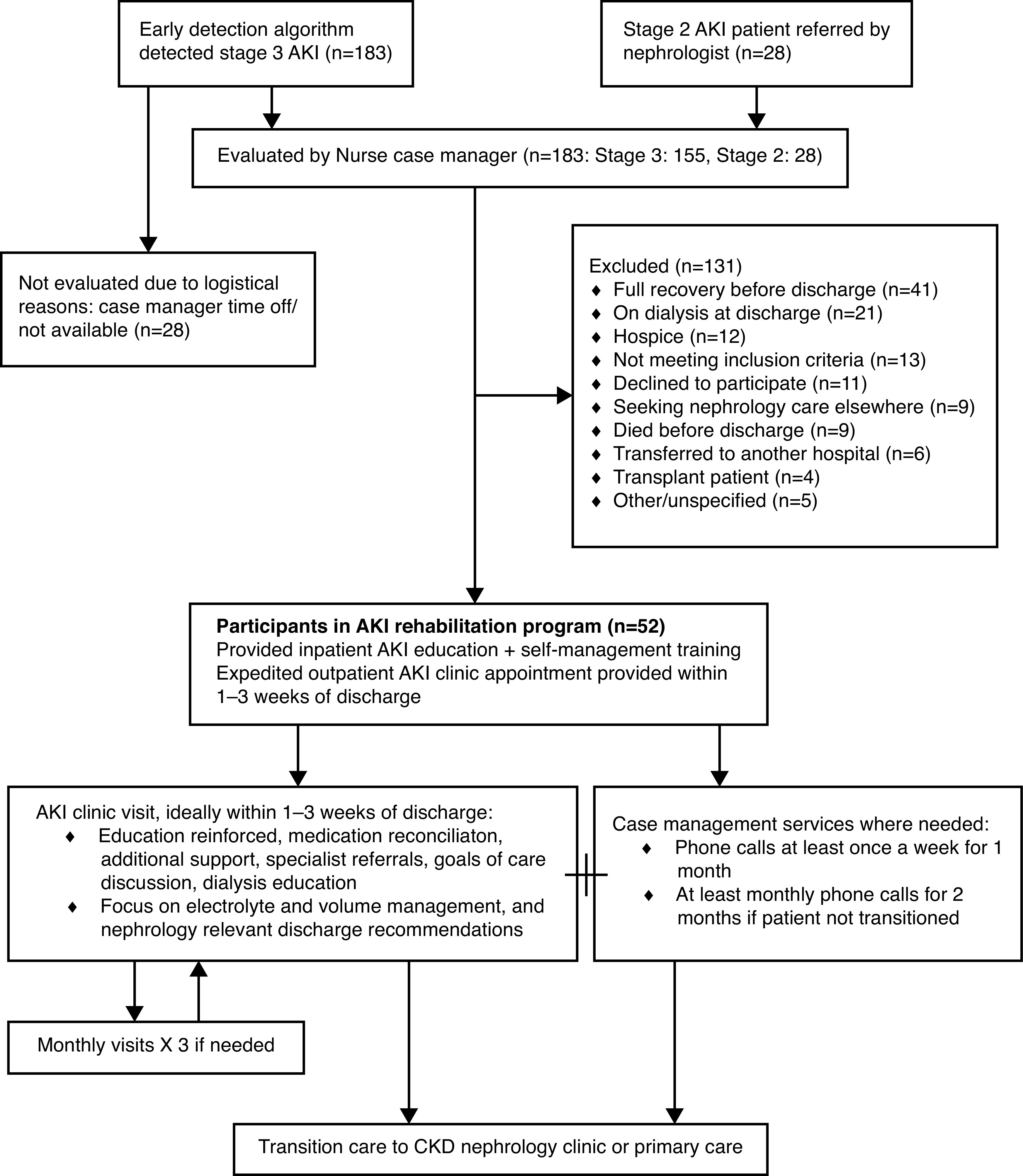
Patient enrollment and workflow for the AKI rehabilitation program.
AKI System List
A multidisciplinary team of nephrologists and informaticians developed a multipronged, systematic strategy to improve AKI diagnosis. Algorithms to determine “baseline” creatinine and classify AKI per KDIGO guidelines were built into a system list in the electronic health record (EHR; Epic Systems, Verona, WI), allowing for real-time, accurate diagnosis and staging of AKI.
Nurse Case Manager Workflow
The nurse case manager monitored the AKI system list daily to identify patients for enrollment. She also tracked high-risk patients with stage 2 AKI who were referred by the treating nephrologists. An attempt to enroll survivors of AKI was made 2–3 days before expected discharge. Treating inpatient teams were approached to ensure the patient was suitable for enrollment. After enrollment, workflow was based on prior nurse discharge advocate–based interventions shown to reduce rehospitalization (15). The nurse case manager was trained in delivering AKI education, including pathophysiology; patient-specific etiology; treatment plan; and self-management tips, including BP monitoring, volume management, nephrotoxin avoidance, medication monitoring, and sick day tips. Simulated training and teach back were performed. The principal investigator (G.S.) directly observed three patient teaching sessions. A booklet, written at an eighth grade literacy level, was provided as a guide and given to the patient after administering education. Education was also reinforced during follow-up telephone calls and appointments.
At discharge, and during AKI clinic visits, the nurse case manager monitored the patients for barriers to care, symptoms, BP/volume self-monitoring, medication discrepancies/side effects, clinical communication deficits, socioeconomic challenges, need for additional referrals, and other issues with care coordination. If issues were identified, she performed a minimum of once weekly telephone follow-up for the first 4 weeks postdischarge, followed by at least monthly phone calls for the next 2 months. Patient symptoms or care-related queries were directed to the appropriate clinician using telephone calls or electronic messaging through the EHR. Nephrologists (G.S., M.B.) were available to provide guidance in real time if needed.
As an illustration, if patients experienced conflicts with appointment schedules, the case manager would identify and try to reschedule or resolve them. Patients experiencing financial stress related to medical issues were provided community resources and appropriate referrals for medical and financial assistance. Medical transportation resources were identified in the community and made available to patients. All patients received multiple reminders of their appointments to ensure they attended them. Self-management information—such as weight monitoring, BP, blood glucose—was reviewed and any concerns or major changes were reviewed with appropriate clinical staff.
Postdischarge Nephrology Follow-Up
Follow-up appointments were scheduled in a dedicated AKI clinic within 1–3 weeks of discharge. We redesigned clinic workflows and scheduling templates to ensure that enrolled patients were routed into this dedicated AKI clinic with longer appointment slots. To ensure ongoing AKI clinic availability, we reserved at least four open slots up to 1 week before each AKI clinic. Enrolled patients were preferentially scheduled into the AKI clinic, even if they were seeing a different nephrologist before hospitalization. The AKI clinician was allotted a 1-hour slot for the first visit and 30-minute slots for subsequent visits. The nurse case manager was also available for shared visits, when needed, to address barriers to care, patient education, self-management, social determinants of health, or care coordination. A full medication reconciliation was performed, self-management education was reinforced, and appropriate medical interventions were performed. Special attention was paid to BP, electrolytes, volume management, discussions about goals of care, dialysis modality education, dialysis access planning, and multidisciplinary care coordination. To improve communication with the patient and other clinicians, an updated medication list, summary of AKI information (cause, severity, recent laboratory test results), arm vessel preservation strategy (if relevant), and other pertinent instructions were printed and given to the patient. At the discretion of the AKI nephrologist, the patient could return to the clinic for monthly visits up to two more times. Subsequently, depending on clinical needs, the patients were either discharged from nephrology care or transitioned to long-term nephrology follow-up outside the AKI clinic.
Outcomes
The primary outcome was a composite outcome of 30-day rehospitalization or mortality. Other outcomes of interest included composite 90-day rehospitalization or mortality, 30-day rehospitalization, 30-day mortality, 90-day rehospitalization, and 90-day mortality. Rehospitalization was determined using EHR data. Mortality data were obtained administratively through linkage with the Social Security Death Index. Renal recovery was classified as complete if the serum creatinine improved to baseline on follow-up, and incomplete if it improved, but not to baseline.
Baseline Covariates
We defined baseline comorbidities and Charlson Comorbidity Index using International Classification of Diseases, Ninth and Tenth Revision diagnosis codes from all available outpatient and inpatient data before discharge. Additional covariates included age, sex, race/ethnicity, baseline eGFR, and discharge eGFR (calculated using the Chronic Kidney Disease Epidemiology Collaboration equation), inpatient kidney replacement therapy, length of stay, discharge disposition, intensive care unit (ICU) utilization, cardiac catheterization, surgical procedure, and RRT during admission.
Actions Taken during First AKI Clinic
To provide insights into actions taken during AKI clinic visits that may have affected outcomes, a manual chart review of the patients’ EHR was performed by a physician. Charts were examined for listed cause of AKI and interventions made, such as medication changes, care coordination, laboratory test orders, advance care planning, and imaging. The Geisinger Institutional Review Board reviewed and approved the research study.
Statistical Analyses
Patients’ demographic and clinical information were summarized between participants and nonparticipants. Mean and SD were presented for continuous variables. Frequency and corresponding percentage (%) were estimated for categoric variables. Chi-squared tests of independence were conducted to test the association of primary outcomes between groups. Multivariable logistic regression models were conducted to evaluate the association between the AKI rehabilitation program and the risk of outcomes, including 30-day rehospitalization or mortality and 90-day rehospitalization or mortality. Adjustment was performed for sociodemographic variables (age at index admission, sex, race), comorbidities (Charlson Comorbidity Index), level of care (ICU), discharge disposition (home versus nonhome), and kidney parameters (baseline eGFR, discharge eGFR, receipt of kidney replacement therapy). In sensitivity analysis, we examined the association between participation in the AKI rehabilitation program and death or rehospitalization using a propensity score–matched cohort.
Propensity score matching, using a 1:1 ratio with nearest-neighbor matching, was performed to adjust for the observed potential confounding factors including the covariates (as defined above) in the logistic regression model, including sociodemographic, comorbidity, level of care, discharge disposition, and kidney parameters. Additional variables included were length of stay for admission, hospitalizations in 1 year before index, AKI stage during index admission, and discharge diagnosis of any congestive heart failure. Covariate balance was visualized before and after propensity score matching. Statistical analyses were performed in RStudio (version 1.3.1093). P values <0.05 were considered statistically significant.
Results
From September 1, 2019 to February 29, 2020, 576 patients admitted to Geisinger Medical Center had stage 2 or 3 AKI. Out of these, 452 survived to discharge (stage 2, 279; stage 3, 173). The case manager evaluated 183 patients for enrollment: 155 patients with stage 3 AKI, and 28 patients with stage 2 AKI referred by consulting nephrologists (Figure 1). Of these, 100 patients did not meet the criteria for the AKI rehabilitation program, 11 declined to participate, nine were planning on receiving follow-up nephrology care elsewhere, six were transferred to another hospital, and five were not enrolled for other/unspecified reasons. The most common cause for not meeting the inclusion criteria was a misclassification of AKI stage in a patient with unknown baseline creatinine in our electronic medical records.
A total of 52 patients with high-risk AKI were enrolled into our AKI rehabilitation program. Patient characteristics, stratified by participation and nonparticipation in the AKI rehabilitation program, are summarized in Table 1. Patients in the AKI rehabilitation program group were more likely to have stage 3 AKI (85% versus 33%), lower eGFR at discharge (32.2±25.4 versus 54.0±34.7 ml/min per 1.73 m2), and to be discharged home (77% versus 47%), compared with nonparticipants. Participants were less likely to have spent time in the ICU during the admission (31% versus 51%). Causes of AKI for patients seen in the rehabilitation program included acute tubular necrosis (n=31), obstructive uropathy (n=6), cardiorenal syndrome (n=3), interstitial nephritis (n=2), chemotherapy/medication toxicity (n=2), rhabdomyolysis (n=2), hepatorenal syndrome (n=1), GN (n=1), atypical hemolytic uremic syndrome (n=1), renovascular disease (n=1), myeloma cast nephropathy (n=1), and preeclampsia (n=1).
Table 1.
Patient characteristics of participants versus nonparticipants in the AKI rehabilitation program
| Characteristics | Participants (n=52) | Nonparticipants (n=400) | P Value |
| AKI stage, n (%) | <0.001a | ||
| Stage 3 | 44 (85) | 131 (33) | |
| Stage 2 | 8 (15) | 269 (67) | |
| Age | 63.4 (14.4) | 63.6 (17.1) | 0.94 |
| Sex, n (%) | 0.72 | ||
| Female | 22 (42) | 184 (46) | |
| Male | 30 (58) | 216 (54) | |
| Race, n (%) | 0.29 | ||
| Non-Hispanic White | 50 (96) | 384 (96) | |
| Hispanic | 2 (4) | 16 (4) | |
| Length of stay (d) | 10.1 (7.4) | 12.9 (12.4) | 0.11 |
| Baseline eGFR (ml/min per 1.73 m2) | 64.3 (34.8) | 64.3 (31.5) | >0.99 |
| Discharge disposition, n (%) | 0.001a | ||
| Home | 40 (77) | 187 (47) | |
| Acute rehabilitation | 4 (8) | 32 (8) | |
| SNF | 8 (15) | 134 (34) | |
| LTACH/other | 0 | 47 (12) | |
| Charlson Comorbidity Index | 5.7 (4.1) | 5.9 (3.8) | 0.65 |
| Discharge eGFR (ml/min per 1.73 m2) | 32.2 (25.4) | 54.0 (34.7) | <0.001a |
| Inpatient dialysis/CRRT, n (%) | 6 (12) | 36 (9) | 0.73 |
| ICU stay during admission, n (%) | 17 (33) | 205 (51) | 0.02a |
| CHF in discharge diagnoses, n (%) | 10 (19) | 123 (31) | 0.12 |
All values indicate mean (SD) unless otherwise indicated. SNF, skilled nursing facility; LTACH, long-term acute care hospital; CRRT, continuous RRT; CHF, congestive heart failure.
P<0.05.
Out of 77 potentially eligible patients for the program, 52 (68%) patients enrolled in the program and completed a total of 93 AKI clinic visits. The mean (SD) interval between hospital discharge and the first appointments was 14.8 (10.7) days. A single visit was required for 23 patients, two visits for 16 patients, and three visits for 13 patients. Interventions made by nephrologists during the expedited follow-up visits are summarized in Figure 2. The most common interventions included laboratory work, dietary education, and medication management (renin-angiotensin inhibitors, diuretics, antihypertensives). Although 14 patients were provided dialysis education, none of them required RRT at last follow-up in AKI clinic. At the last follow-up, 23 patients had complete renal recovery to baseline, 24 had incomplete recovery, and five showed no renal recovery.
Figure 2.
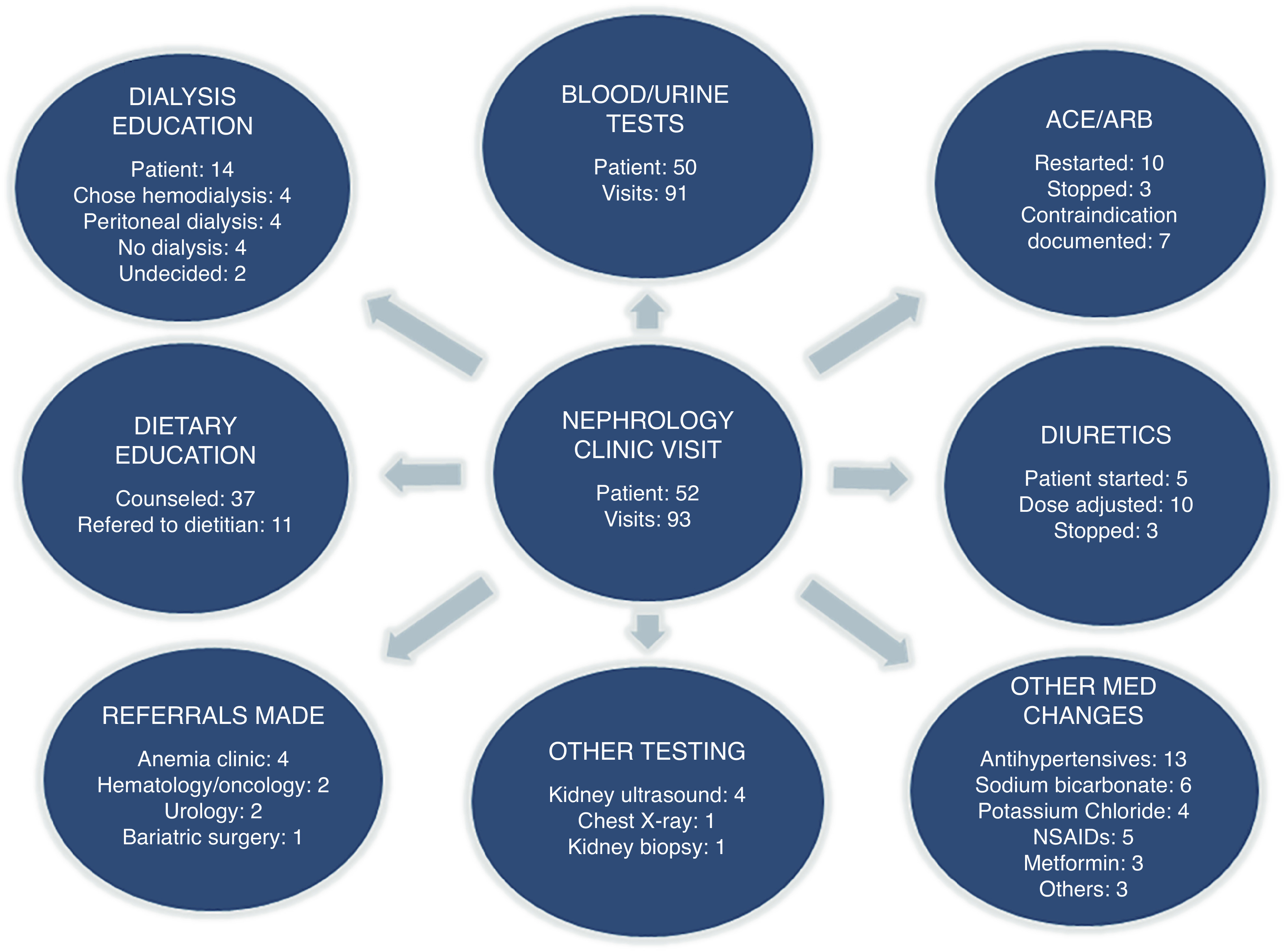
Interventions made during AKI clinic visits.
Our nurse case manager made 219 postdischarge phone contacts with enrolled patients. A total of 252 case management interventions were made to coordinate care, address patient concerns, and mitigate barriers to care (Table 2). A case management intervention was required in 46 out of 52 (88%) of patients. The most common areas of intervention included coordination of care (82 interventions), communication with clinicians about patient concerns (45 interventions), and self-management education (43 interventions). A total of 18 appointments, for ten patients, needed to be canceled or rescheduled. Our case manager used the flexible scheduling templates to arrange a suitable alternative appointment in all cases within 1–3 weeks of discharge.
Table 2.
Interventions made by nurse case manager
| Category | Intervention | Number of patients |
| Coordination of care | Laboratory workup appointment coordination | 19 |
| Appointment reminders | 18 | |
| Medication reconciliation | 17 | |
| Streamline appointment schedule | 15 | |
| Transition to CKD clinic | 7 | |
| Arrange weighing scale | 4 | |
| Obtain BP cuff | 2 | |
| Communication with clinicians | Primary care physician | 11 |
| Nephrologist | 9 | |
| Surgeon/wound care | 7 | |
| Nursing/rehabilitation facility | 6 | |
| Cardiologist | 4 | |
| Other medical specialty | 4 | |
| Urology | 2 | |
| Neurosurgery | 1 | |
| Pharmacist | 1 | |
| Self-management education | Medication adherence/side effects | 15 |
| Volume management | 12 | |
| Diet | 6 | |
| Postdischarge AKI education | 4 | |
| Hypoglycemia management | 3 | |
| Nausea/fatigue management | 3 | |
| Disease-specific plan of care | Infection | 5 |
| Depression | 4 | |
| Diabetes | 4 | |
| Dialysis | 4 | |
| Congestive heart failure | 4 | |
| Wound care | 3 | |
| Symptom detection | Depression/anxiety | 6 |
| Edema | 5 | |
| Weight gain | 4 | |
| Dysuria | 1 | |
| Chest pain | 1 | |
| Socioeconomic support | Caregiver support | 10 |
| Safety/mobility support | 9 | |
| Transportation | 5 | |
| Substance abuse | 3 | |
| Financial hardship | 2 | |
| Personal/family conflict | 2 | |
| Need-based referrals | Behavioral health | 3 |
| Home health | 2 | |
| Physical/speech therapy | 2 | |
| Cardiology | 1 | |
| Dietitian | 1 | |
| Home oxygen therapy | 1 |
Each intervention counted only once per patient, but the same patient could be included under multiple interventions.
Table 3 shows the frequency and proportions experiencing the rehospitalization and mortality outcomes in participants in the AKI rehabilitation program and nonparticipants. Participants in the AKI rehabilitation program had significantly lower crude rates of both composite outcomes and mortality outcomes than nonparticipants: for 30-day rehospitalization or death, 15% versus 34% (P=0.01); for 90-day rehospitalization or death, 31% versus 51% (P=0.01); for 30-day mortality, 2% versus 12% (P=0.03); for 90-day mortality, 6% versus 21% (P=0.01) (Table 3). In multivariable logistic regression analyses, participation in the AKI rehabilitation program was associated with lower risk of 30-day rehospitalization or mortality (odds ratio, 0.41; 95% CI, 0.16 to 0.93; P=0.04), with similar findings for 90-day rehospitalization or mortality (odds ratio, 0.52; 95% CI, 0.25 to 1.05; P=0.07), although the 95% CI crossed one for the latter outcome (Table 4).
Table 3.
Rehospitalization and mortality outcomes of participants of the AKI rehabilitation program and nonparticipants
| Outcome | n (%) | P Valuea | |
| Participants (N=52) | Nonparticipants (N=400) | ||
| 30-Day rehospitalization or mortality | 8 (15) | 137 (34) | 0.01 |
| 30-Day rehospitalization | 7 (14) | 111 (28) | 0.04 |
| 30-Day mortality | 1 (2) | 48 (12) | 0.03 |
| 90-Day rehospitalization or mortality | 16 (31) | 202 (51) | 0.01 |
| 90-Day rehospitalization | 15 (29) | 167 (42) | 0.10 |
| 90-Day mortality | 3 (6) | 82 (21) | 0.01 |
P values were determined on the basis of chi-squared tests, except for values less than five, where Fisher exact tests were used.
Table 4.
Factors associated with 30-day and 90-day rehospitalization or mortality on multivariable logistic regression
| Variables | 30-Day Rehospitalization or Mortality, OR (95% CI) | P Value | 90-Day Rehospitalization or Mortality, OR (95% CI) | P Value |
| Participation in AKI rehabilitation clinic | 0.41 (0.16 to 0.93) | 0.04 | 0.52 (0.25 to 1.05) | 0.07 |
| Age (years) | 0.98 (0.97 to 1.0) | 0.05 | 0.98 (0.96 to 0.99) | 0.006 |
| Male versus female | 1.14 (0.69 to 1.64) | 0.78 | 1.18 (0.79 to 1.77) | 0.41 |
| Non-Hispanic White versus another race | 1.65 (0.51 to 6.83) | 0.43 | 0.86 (0.28 to 2.63) | 0.78 |
| Stage 3 versus stage 2 AKI | 0.96 (0.56 to 1.56) | 0.82 | 0.85 (0.53 to 1.37) | 0.51 |
| Charlson Comorbidity Index | 1.14 (1.06 to 1.22) | 0.0004 | 1.16 (1.08 to 1.24) | 0.0001 |
| Length of stay (d) | 0.99 (0.97 to 1.01) | 0.44 | 0.997 (0.98 to 1.02) | 0.72 |
| Dialysis received during admission | 1.02 (0.34 to 2.15) | 0.76 | 0.53 (0.22 to 1.27) | 0.16 |
| Discharge eGFR (ml/min per 1.73 m2) | 0.999 (0.99 to 1.01) | 0.55 | 0.996 (0.99 to 1.00) | 0.33 |
| ICU admission | 1.07 (0.68 to 1.80) | 0.68 | 0.90 (0.57 to 1.41) | 0.63 |
| CHF in discharge diagnoses | 0.74 (0.45 to 1.20) | 0.20 | 0.81 (0.5 to 1.30) | 0.39 |
| Admissions within 1-year before index | 0.42 (0.06 to 2.26) | 0.29 | 0.12 (0.01 to 0.88) | 0.08 |
| Discharge disposition | ||||
| Rehabilitation versus home | 0.76 (0.28 to 1.89) | 0.58 | 1.16 (0.51 to 2.55) | 0.72 |
| SNF versus home | 1.89 (1.12 to 3.21) | 0.02 | 1.82 (1.10 to 3.02) | 0.02 |
| LTACH versus home | 3.4 (1.45 to 8.04) | 0.005 | 4.1 (1.75 to 10.19) | 0.64 |
OR, odds ratio; ICU, intensive care unit; CHF, congestive heart failure; SNF, skilled nursing facility; LTACH, long-term acute care hospital.
In sensitivity analyses using 1:1 propensity score matching, we were able to match 45 of 52 (87%) participants in the AKI rehabilitation program. Among the 45 matched pairs, the covariates were well balanced on the basis of the covariate balance plot, with most standardized mean differences <0.15 (Supplemental Table 2). Results in propensity score–matched analyses were similar in direction, although not statistically significant for 30-day rehospitalization or mortality (18% versus 31%; P=0.22) and 90-day rehospitalization or mortality (47% versus 58%; P=0.4) (Supplemental Table 3).
Discussion
In this pilot study, enrollment in a comprehensive AKI rehabilitation program was associated with a lower risk of rehospitalization or mortality at 30 days after discharge, as compared with usual care. These findings could have important implications given the very high morbidity and mortality experienced by survivors of AKI. In terms of implications for healthcare systems and payers, costs of rehospitalization are significant, often exceeding the initial hospitalization (16). If our findings can be confirmed in a randomized controlled trial, this intervention could translate into significant improvement in patient outcomes and cost savings.
Prior population-based studies have reported 30-day rehospitalization rates of 20%–25% and 30- day mortality rates of 6%–18% among survivors of AKI (2,17–19). High 90-day mortality rates between 20% and 30% have also been described (20,21). We observed similarly high rates of rehospitalization and mortality among nonparticipants in the AKI rehabilitation clinic. Despite having a higher proportion of stage 3 AKI and lower mean discharge eGFR, our participant group had a lower incidence of rehospitalization and mortality in crude and multivariable-adjusted analyses. Findings were similar, although not significant, in a propensity score–matched analysis. However, findings for 30- and 90-day risk of rehospitalization or mortality were similar in direction to the multivariable logistic regression.
There is widespread interest in testing interventions to improve AKI outcomes in randomized controlled trials. The Caring for Outpatients after Acute Kidney Injury consortium is one such attempt to test interventions to reduce morbidity in patients who survive hospitalization for stage 2 and 3 AKI (22). However, an attempt at a similar randomized controlled trial across four hospitals in Toronto, Canada encountered several challenges (23). Only 71 out of 269 (26%) eligible patients could be randomized. The primary reason for declining participation were difficulties with coordination of care and care fatigue. On the other hand, by starting education and case management services during admission, and providing outpatient comprehensive case management, we were able to enroll 52 out of 77 (68%) eligible patients. In the randomized trial, even among the 34 patients randomized to AKI follow-up clinic, only 24 (71%) came to the visit. On the other hand, all 52 of our enrolled patients attended clinic visits. Although ten of our patients had to cancel and reschedule 18 appointments, our case manager was able to coordinate alternative appointments. By using flexible scheduling templates, we were able to ensure nephrologist availability. Other interventions that might have improved patient engagement and mitigated care fatigue included timely, symptom-based interventions, interdisciplinary communication, regular check-ins, expedited care requests, and attention to socioeconomic barriers to care. Hence, our study creates a roadmap to overcome barriers to enrollment in future studies.
A previous, pragmatic, cluster randomized trial, including an AKI electronic detection and alerting system, an inpatient care bundle, and healthcare worker education, failed to show an improvement in 30-day mortality (24). However, our intervention was distinct in that we focused on post-AKI care with significant patient education, self-management training, and nurse case manager support beginning in the hospital, along with expedited 1- to 3-week nephrology follow-up. Our findings are consistent with a retrospective study showing an association between early outpatient nephrology follow-up and lower postdischarge mortality in survivors of AKI (8).
Whereas an electronic survey found that Canadian nephrologists would frequently recommend postdischarge follow-up, only 24% of survivors of AKI were seen by a nephrologist within 1 year (25). An analysis of US veterans similarly found that only 9% of survivors of AKI receive a postdischarge nephrology referral (4). Likewise, in our cohort, only 38 patients (10%) in the comparison group were seen by a nephrologist in 3 months postdischarge. Prior studies have also identified gaps in postdischarge communication in patients with AKI (26). Our intervention was successful in addressing these gaps by improving care coordination with nurse case manager telephone calls and early nephrology follow-up.
A possible mechanism for improving postdischarge hospitalization-associated AKI outcomes might be preventing recurrent AKI. Up to 25% of survivors of AKI are rehospitalized with recurrent AKI within 12 months. Recurrent AKI is also associated with other poor outcomes, such as accelerated kidney function decline (27). Using multiple preventive interventions as a bundle has been shown to reduce the incidence and severity of AKI in inpatients (28). Our outpatient intervention included many of the AKI preventive measures included in the KDIGO clinical practice guideline for AKI (29). Unfortunately, we were unable to examine recurrent AKI as an outcome because follow-up creatinine values were not systematically collected among nonparticipants. Regardless, it is easily conceivable that application of these measures as an outpatient bundled intervention could lead to similar results.
Use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACE/ARB) after AKI has been associated with lower mortality, although it remains uncertain whether restarting ACE/ARB affects risk of recurrent AKI (30,31). Restarting ACE/ARB and ongoing monitoring was a common intervention performed in our clinic. Other common actions that may have prevented adverse outcomes included medication reconciliation, symptom assessment, improved communication, and assistance with navigating socioeconomic barriers. These issues are particularly important for survivors of AKI, who are at high risk for polypharmacy and serious postdischarge medication side effects, such as hypoglycemia (32). In patients with impaired kidney function, medication discrepancy and risk for potential harm is significant, which could be prevented by outpatient medication reconciliation in the nephrology clinic (33,34).
We modeled our case manager intervention on prior work done in postdischarge trials not specific to AKI. Prior randomized trials have shown that comprehensive discharge planning and home follow-up after discharge can reduce rehospitalization and decrease the cost of care in other settings where patients are at high risk of rehospitalization (35). Our case manager acted as a “transition coach,” who starts educating the patient about AKI self-management in the hospital and works to improve postdischarge communication and follow-up care coordination (9). We adopted some components of the re-engineered hospital discharge program as applicable to AKI care in a nephrology-centric setting (15). However, implementing all components of this complex intervention requires multilevel, organizational commitment and can be challenging (36). Given the increasing penetration of value-based care, and increasing financial penalties for rehospitalization, we are hopeful there will be more support from healthcare systems to allow multicenter implementation and rigorous evaluation (37). Interventions, such as the one in our study, could lead to significant improvements in patients’ self-perceived knowledge about AKI, and potentially patient outcomes (38).
Our single-center, nonrandomized pilot study needs further testing in a larger, more heterogenous population, and preferably as a randomized controlled trial, to verify that this approach improves outcomes. Due to the nonrandomized nature of the study, it is likely that the patients enrolled in our AKI rehabilitation program were inherently different from our comparison patients, leading to confounding bias, and these differences may not have been fully accounted for despite our statistical adjustment. Due to the small sample size, a propensity score–matched analysis was possible for only 45 out of the 52 participants in the program. We could have missed rehospitalizations if the patient was admitted to a different healthcare system. However, we believe this effect is negligible because almost all hospitals in our patient catchment area are owned by Geisinger Health or we have access to hospitalization data through the EHR interface. We only included patients with stage 2 AKI who were deemed high risk by the consulting nephrologist. In the absence of standardized, externally validated tools to predict AKI readmissions, we chose the global clinical impression (Surprise Question) of treating clinicians (14). Although this approach is not standardized, it seemed to truly select for high-risk patients with stage 2 AKI, because their rehospitalization and mortality rates were comparable with, or higher than, participants with stage 3 AKI (Supplemental Table 4).
In conclusion, our intervention—which included real-time AKI diagnosis, in-hospital education, case management, and expedited nephrology follow-up—was feasible and was associated with improvement in outcomes in survivors of high-risk AKI. Our interventions were clearly defined and should be reproducible in future studies. Our enrollment rates and patient retention rates were much more robust when compared with other studies. Given the paucity of interventional studies in this patient population, we believe these encouraging findings deserve further study as part of a randomized controlled trial.
Disclosures
A.R. Chang reports receiving grant support from the National Kidney Foundation (NKF) via the NKF Patient Network; having consultancy agreements with Novartis (as consultant); receiving research funding from Novo Nordisk (investigator-sponsored study); receiving honoraria from Reata; and serving as a scientific advisor for, or member of, Reata and Relypsa. K. Ho reports having other interests in/relationships with the American Society of Nephrology and NKF, and having patents and inventions with Partners Healthcare. H.L. Kirchner reports having consultancy agreements: with Baylor College of Medicine and Guthrie Research Foundation. All remaining authors have nothing to disclose.
Funding
This work was supported by Geisinger Health Plan/Geisinger Clinic Quality Pilot Fund grant 62303702 (to G. Singh, Y. Hu, J. Brown, M. Bermudez, K. Ho, and J.A. Green).
Acknowledgments
The authors acknowledge Ms. Christina Yule (research project manager), Ms. Lisa Jones (nurse case manager), and Dr. Evan Norfolk and Ms. Karri Bickert for departmental, scheduling, and administrative support.
Author Contributions
M. Bermudez, A.R. Chang, J.A. Green, K. Ho, Y. Hu, H.L. Kirchner, and G. Singh conceptualized the study; M. Bermudez, J. George, J.A. Green, S. Jacobs, and G. Singh were responsible for investigation; M. Bermudez, J.A. Green, K. Ho, and G. Singh were responsible for funding acquisition; J. Brown, A.R. Chang, Y. Hu, and G. Singh reviewed and edited the manuscript; J. Brown and S. Jacobs were responsible for data curation; A.R. Chang, J.A. Green, Y. Hu, H.L. Kirchner, and G. Singh were responsible for methodology; A.R. Chang and Y. Hu were responsible for formal analysis; A.R. Chang, Y. Hu, and G. Singh wrote the original draft; A.R. Chang, H.L. Kirchner, and G. Singh provided supervision; A.R. Chang and S. Jacobs were responsible for validation; J. George was responsible for project administration; H.L. Kirchner and G. Singh were responsible for visualization.
Supplemental Material
This article contains Supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003672021/-/DCSupplemental.
Hierarchy for determining baseline reference creatinine. Download Supplemental Table 1, PDF file, 24 KB (23KB, pdf)
Comparison of groups before and after propensity score matching. Download Supplemental Table 2, PDF file, 24 KB (23KB, pdf)
Rehospitalization and mortality outcomes of propensity score-matched AKI rehabilitation program participants and non-participants. Download Supplemental Table 3, PDF file, 24 KB (23KB, pdf)
Characteristics of stage 2 and stage 3 AKI patients in the AKI rehabilitation program. Download Supplemental Table 4, PDF file, 24 KB (23KB, pdf)
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005. 10.1681/ASN.2004090740 [DOI] [PubMed] [Google Scholar]
- 2.Silver SA, Harel Z, McArthur E, Nash DM, Acedillo R, Kitchlu A, Garg AX, Chertow GM, Bell CM, Wald R: 30-day readmissions after an acute kidney injury hospitalization. Am J Med 130: 163–172.e4, 2017. 10.1016/j.amjmed.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 3.Siew ED, Parr SK, Wild MG, Levea SL, Mehta KG, Umeukeje EM, Silver SA, Ikizler TA, Cavanaugh KL: Kidney disease awareness and knowledge among survivors ofacute kidney injury. Am J Nephrol 49: 449–459, 2019. 10.1159/000499862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siew ED, Peterson JF, Eden SK, Hung AM, Speroff T, Ikizler TA, Matheny ME: Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol 23: 305–312, 2012. 10.1681/ASN.2011030315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hines A, Li X, Ortiz-Soriano V, Saleh S, Litteral J, Ruiz-Conejo M, Wald R, Silver SA, Neyra JA: Use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and acute kidney disease after an episode of AKI: A multicenter prospective cohort study. Am J Nephrol 51: 266–275, 2020. 10.1159/000505893 [DOI] [PubMed] [Google Scholar]
- 6.Qiao Y, Shin JI, Sang Y, Inker LA, Secora A, Luo S, Coresh J, Alexander GC, Jackson JW, Chang AR, Grams ME: Discontinuation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Mayo Clin Proc 94: 2220–2229, 2019. 10.1016/j.mayocp.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siew ED, Liu KD, Bonn J, Chinchilli V, Dember LM, Girard TD, Greene T, Hernandez AF, Ikizler TA, James MT, Kampschroer K, Kopp JB, Levy M, Palevsky PM, Pannu N, Parikh CR, Rocco MV, Silver SA, Thiessen-Philbrook H, Wald R, Xie Y, Kimmel PL, Star RA: Improving care for patients after hospitalization with AKI. J Am Soc Nephrol 31: 2237–2241, 2020. 10.1681/ASN.2020040397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, Ray JG, Luo J, Li P, Quinn RR, Forster A, Perl J, Bell CM: Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013. 10.1038/ki.2012.451 [DOI] [PubMed] [Google Scholar]
- 9.Coleman EA, Parry C, Chalmers S, Min SJ: The care transitions intervention: Results of a randomized controlled trial. Arch Intern Med 166: 1822–1828, 2006. 10.1001/archinte.166.17.1822 [DOI] [PubMed] [Google Scholar]
- 10.Clancy CM: Reengineering hospital discharge: A protocol to improve patient safety, reduce costs, and boost patient satisfaction. Am J Med Qual 24: 344–346, 2009. 10.1177/1062860609338131 [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz RE, Fang Z, Helfand BKI, Jones RN, Schreiber R, Paasche-Orlow MK: Project ReEngineered Discharge (RED) lowers hospital readmissions of patients discharged from a skilled nursing facility. J Am Med Dir Assoc 14: 736–740, 2013. 10.1016/j.jamda.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 12.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group: Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 17: 204, 2013. 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siew ED, Matheny ME: Choice of reference serum creatinine in defining acute kidney injury. Nephron 131: 107–112, 2015. 10.1159/000439144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings KS, Marks S, Lum HD: The surprise question as a prognostic tool #360. J Palliat Med 21: 1529–1530, 2018. 10.1089/jpm.2018.0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, Forsythe SR, O’Donnell JK, Paasche-Orlow MK, Manasseh C, Martin S, Culpepper L: A reengineered hospital discharge program to decrease rehospitalization: A randomized trial. Ann Intern Med 150: 178–187, 2009. 10.7326/0003-4819-150-3-200902030-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey MK, Weiss AJ, Barrett ML, Jiang HJ: Characteristics of 30-Day All-Cause Hospital Readmissions, 2010–2016: Statistical Brief #248. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, Rockville, MD, Agency for Healthcare Research and Quality (US), 2006 [Google Scholar]
- 17.Horkan CM, Purtle SW, Mendu ML, Moromizato T, Gibbons FK, Christopher KB: The association of acute kidney injury in the critically ill and postdischarge outcomes: A cohort study*. Crit Care Med 43: 354–364, 2015. 10.1097/CCM.0000000000000706 [DOI] [PubMed] [Google Scholar]
- 18.Thakar CV, Parikh PJ, Liu Y: Acute kidney injury (AKI) and risk of readmissions in patients with heart failure. Am J Cardiol 109: 1482–1486, 2012. 10.1016/j.amjcard.2012.01.362 [DOI] [PubMed] [Google Scholar]
- 19.Iwagami M, Moriya H, Doi K, Yasunaga H, Isshiki R, Sato I, Mochida Y, Ishioka K, Ohtake T, Hidaka S, Noiri E, Kobayashi S: Seasonality of acute kidney injury incidence and mortality among hospitalized patients. Nephrol Dial Transplant 33: 1354–1362, 2018. 10.1093/ndt/gfy011 [DOI] [PubMed] [Google Scholar]
- 20.Wiersema R, Eck RJ, Haapio M, Koeze J, Poukkanen M, Keus F, van der Horst ICC, Pettilä V, Vaara ST: Burden of acute kidney injury and 90-day mortality in critically ill patients. BMC Nephrol 21: 1, 2019. 10.1186/s12882-019-1645-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006. 10.1681/ASN.2005060668 [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services : Caring for outpatients after acute kidney injury (COPE-AKI). Grants & Funding. https://grants.nih.gov/grants/guide/rfa-files/rfa-dk-20-012.html. Accessed December, 2020
- 23.Silver S, Adhikari N, Bell C, Chan C, Harel Z, Kitchlu A, Meraz Muñoz A, Norman P, Perez A, Zahirieh A, Wald R: Nephrologist follow-up versus usual care after an acute kidney injury hospitalization (FUSION) [published online ahead of print May 21, 2021]. Clin J Am Soc Nephrol 10.2215/CJN.17331120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selby NM, Casula A, Lamming L, Stoves J, Samarasinghe Y, Lewington AJ, Roberts R, Shah N, Johnson M, Jackson N, Jones C, Lenguerrand E, McDonach E, Fluck RJ, Mohammed MA, Caskey FJ: An organizational-level program of intervention for AKI: A pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol 30: 505–515, 2019. 10.1681/ASN.2018090886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsanji DJ, Pannu N, Manns BJ, Hemmelgarn BR, Tan Z, Jindal K, Scott-Douglas N, James MT: Disparity between nephrologists’ opinions and contemporary practices for community follow-up after AKI hospitalization. Clin J Am Soc Nephrol 12: 1753–1761, 2017. 10.2215/CJN.01450217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE: Hospital discharge communications during care transitions for patients with acute kidney injury: A cross-sectional study. BMC Health Serv Res 16: 449, 2016. 10.1186/s12913-016-1697-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, Hung AM, Fly J, Speroff T, Ikizler TA, Matheny ME: Predictors of recurrent AKI. J Am Soc Nephrol 27: 1190–1200, 2016. 10.1681/ASN.2014121218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A: Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial [published correction appears in Intensive Care Med 43: 1949, 2017]. Intensive Care Med 43: 1551–1561, 2017. 10.1007/s00134-016-4670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61: 649–672, 2013. 10.1053/j.ajkd.2013.02.349 [DOI] [PubMed] [Google Scholar]
- 30.Brar S, Ye F, James MT, Hemmelgarn B, Klarenbach S, Pannu N; Interdisciplinary Chronic Disease Collaboration: Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med 178: 1681–1690, 2018. 10.1001/jamainternmed.2018.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu CY, Liu KD, Yang J, Glidden DV, Tan TC, Pravoverov L, Zheng S, Go AS: Renin-angiotensin system blockade after acute kidney injury (AKI) and risk of recurrent AKI. Clin J Am Soc Nephrol 15: 26–34, 2020. 10.2215/CJN.05800519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung AM, Siew ED, Wilson OD, Perkins AM, Greevy RA Jr, Horner J, Abdel-Kader K, Parr SK, Roumie CL, Griffin MR, Ikizler TA, Speroff T, Matheny ME: Risk of hypoglycemia following hospital discharge in patients with diabetes and acute kidney injury. Diabetes Care 41: 503–512, 2018. 10.2337/dc17-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim J, Hazzan AD, Mathew AT, Sakhiya V, Zhang M, Halinski C, Fishbane S: Medication discrepancies in late-stage chronic kidney disease. Clin Kidney J 11: 507–512, 2018. 10.1093/ckj/sfx135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips M, Wilson JA, Aly A, Wood M, Poyah P, Drost S, Hiltz A, Carver H: An evaluation of medication reconciliation in an outpatient nephrology clinic. CANNT J 26: 29–33, 2017 [PubMed] [Google Scholar]
- 35.Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, Schwartz JS: Comprehensive discharge planning and home follow-up of hospitalized elders: A randomized clinical trial. JAMA 281: 613–620, 1999. 10.1001/jama.281.7.613 [DOI] [PubMed] [Google Scholar]
- 36.Sullivan JL, Shin MH, Engle RL, Yaksic E, VanDeusen Lukas C, Paasche-Orlow MK, Starr LM, Restuccia JD, Holmes SK, Rosen AK: Evaluating the implementation of project re-engineered discharge (RED) in five veterans health administration (VHA) hospitals. Jt Comm J Qual Patient Saf 44: 663–673, 2018. 10.1016/j.jcjq.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 37.Desai NR, Ross JS, Kwon JY, Herrin J, Dharmarajan K, Bernheim SM, Krumholz HM, Horwitz LI: Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA 316: 2647–2656, 2016. 10.1001/jama.2016.18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortiz-Soriano V, Alcorn JL 3rd, Li X, Elias M, Ayach T, Sawaya BP, Malluche HH, Wald R, Silver SA, Neyra JA: A survey study of self-rated patients’ knowledge about AKI in a post-discharge AKI clinic. Can J Kidney Health Dis 6: 2054358119830700, 2019. 10.1177/2054358119830700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hierarchy for determining baseline reference creatinine. Download Supplemental Table 1, PDF file, 24 KB (23KB, pdf)
Comparison of groups before and after propensity score matching. Download Supplemental Table 2, PDF file, 24 KB (23KB, pdf)
Rehospitalization and mortality outcomes of propensity score-matched AKI rehabilitation program participants and non-participants. Download Supplemental Table 3, PDF file, 24 KB (23KB, pdf)
Characteristics of stage 2 and stage 3 AKI patients in the AKI rehabilitation program. Download Supplemental Table 4, PDF file, 24 KB (23KB, pdf)


