Abstract
Context
Folium Ginkgo extract and tetramethylpyrazine sodium chloride injection (Xingxiong injection) is a compound preparation commonly used for treating cerebral ischaemia/reperfusion injury in ischaemic stroke in China. However, its potential mechanisms on ischaemic stroke remain unknown.
Objective
This study explores the potential mechanisms of Xingxiong injection in vivo or in vitro.
Materials and methods
Sprague-Dawley (SD) rats were randomly assigned to five groups: the sham (normal saline), the model (normal saline) and the Xingxiong injection groups (12.5, 25 or 50 mL/kg). The rats were subjected to 2 h of middle cerebral artery occlusion (MCAO) followed by reperfusion for 14 d. Xingxiong injection was administered via intraperitoneal (i.p.) injection immediately after ischaemia induction for 14 d. Afterwards, rats were sacrificed at 14 d induced by administration of Xingxiong injection.
Results
Xingxiong injection significantly reduces infarct volume (23%) and neurological deficit scores (93%) compared with the MCAO/R group. Additionally, Xingxiong injection inhibits the loss in mitochondrial membrane potential (43%) and reduces caspase-3 level (44%), decreases NOX (41%), protein carbonyl (29%), 4-HNE (40%) and 8-OhdG (41%) levels, inhibits the expression of inflammatory factors, such as TNF-α (26%), IL-1β (34%), IL-6 (39%), MCP-1 (36%), CD11a (41%) and ICAM-1 (43%). Moreover, Xingxiong injection can increase p-Akt/Akt (35%) and Nrf2 (47%) protein expression and inhibit NLRP3 (42%) protein expression.
Conclusions
Xingxiong injection prevents cerebral ischaemia/reperfusion injury via activating the Akt/Nrf2 pathway and inhibiting NLRP3 inflammasome. These findings provide experimental evidence for clinical use of drugs in the treatment of ischaemic stroke.
Keywords: Ischaemic stroke, apoptosis, inflammation, oxidative stress
Introduction
Stroke is a leading cause of mortality and serious long-term disability worldwide, which afflicted over 80 million people globally by 2016 (Zhao et al. 2018). Of all stroke events, ischaemic stroke (caused by decreased blood flow to the brain) accounts for approximately 87% in nature. The standard of care for clinical management of acute ischaemic stroke is intervention with intravenous thrombolysis via the recombinant tissue plasminogen activator (r-tPA). However, r-tPA therapy is less than ideal due to its the narrow treatment window, contraindications and complications, only 1–5% of patients currently receive tPA treatment (Aiyagari et al. 2004). Therefore, alternative therapeutic options are required.
Laboratory research has shown ischaemic stroke in response to infarct volume reduction and neurological dysfunction in a MCAO/R rat model. During cerebral ischaemia, deficiency of glucose and oxygen causes ATP depletion, which results in membrane depolarization and further induces multiple mechanisms including neuronal excitotoxicity, acidotoxicity, inflammation response, oxidant injury and mitochondrial response. Membrane depolarization also promotes intracellular calcium release, initiating the activation of apoptosis, necrosis and autophagy signalling pathways (Zhou et al. 2018). In addition, continuous calcium release accelerates reactive oxygen species (ROS) production, which triggers inflammatory responses, consequently increasing blood–brain barrier (BBB) permeability and resulting in neuronal death (Szydlowska and Tymianski 2010). Taken together, the pathophysiology of ischaemic stroke involves complicated molecular mechanisms and pathways. Drugs with only one single component generally cannot target all the above pathways; therefore, they have less efficacy and may have some adverse effects. Due to the multi-component and multi-targeted features as well as their higher safety, the experimental research and clinical application of multi-targeted medicine has exhibited great advantage in the treatment of ischaemic stroke in recent years (Zhong et al. 2005; Wang et al. 2017; Zhu et al. 2021b).
Folium Ginkgo extract and tetramethylpyrazine sodium chloride injection (Xingxiong injection) was approved in 2004 in China, where it is widely used for treating ischaemic cardiovascular or cerebrovascular diseases, peripheral circulatory disorder, hypertension, hyperlipidaemia, etc. The therapeutic effects of this Xingxiong injection were validated (Yang and Sun 2007), especially for cerebral infarction. The active ingredients in folium Ginkgo extract, flavonoids and ginkgolides, were reported to improve blood circulation and memory (Cheuvront and Carter 2003; Amieva et al. 2013). Ligustrazine phosphate was reported to exhibit antioxidant, anti-apoptotic and calcium antagonistic effects (Zhu et al. 2005; Li et al. 2010). However, the therapeutic mechanism of Xingxiong injection as a compound preparation is still not well understood due to a lack of scientific evidence indicating whether it has beneficial roles in the treatment of cerebral ischaemia/reperfusion injury.
The present study investigated the protective effects of the Xingxiong injection in a MCAO/R rat and OGD/R neuron model to clarify its potential mechanism from the aspects of anti-apoptosis, antioxidation and anti-inflammatory.
Materials and methods
Materials
Xingxiong injection was obtained from Honghe Pharmaceutical Co., Ltd. (Jilin, China). Triphenyltetrazolium chloride (TTC), poly-L-lysine, dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco’s modified Eagle’s medium/F12, foetal bovine serum (FBS), neurobasal medium and B27 were purchased from Gibco (Brooklyn, NY). 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl carbocyanine iodide (JC-1) was purchased from Beyotime Biotechnology (Shanghai, China). Caspase-3 fluorescence staining kit was purchased from BioVision (Milpitas, CA). All ELISA kits were purchased from Expandbio (Beijing, China).
Animals
Male Sprague-Dawley (SD) rats (250–280 g) were purchased from Beijing Vital River Laboratories (Beijing, China) and used in this study. All animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Chinese Academy of Medical Sciences & Peking Union Medical College (no. SYXK 2017-0020). All efforts were made to minimize the number of animals used and to ensure minimal suffering. All animals were maintained in ventilated cages at 20–25 °C and relative humidity (30–50%) with a 12 h light/dark cycle, and given free access to food and water. All animals used in this study showed clinically normal, free of apparent infection or inflammation or neurological deficits before the experimental procedures.
MCAO surgery
SD rats were anaesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) intraperitoneally (i.p.) and underwent middle cerebral artery occlusion (MCAO) procedure. Cerebral ischaemia–reperfusion (I/R) was induced in rats through middle cerebral artery occlusion/reperfusion (MCAO/R) by following a previously described method (Zhu et al. 2020, 2021c). The occlusion lasted for 2 h until reperfusion. The rats were manipulated using the same technique in the sham group, but the MCA was not occluded. The rectal temperature of rats was maintained at 37 ± 0.5 °C during the entire procedure by using a heating blanket (Sunbeam, Boca Raton, FL).
Drug treatment
The rats were randomly assigned to five experimental groups: (1) a sham group; (2) an MCAO/R group injected with an isovolumetric solvent (saline) for 14 days; groups injected with (3) 12.5, (4) 25 and (5) 50 mL/kg/day Xingxiong injection (Honghe Pharmaceutical Co., Ltd., Jilin, China) for 14 days after MCAO. For drug administration, Xingxiong injection or vehicle (0.9% normal saline) was given immediately after MCAO surgery. At 14 d post-injection, some brain tissues and serum were collected in −80 °C for subsequent experiments.
TTC staining
Triphenyltetrazolium chloride staining was conducted 7 d post-stroke based on the methods described previously (Zhu et al. 2020, 2021a). Cerebral infarct size was measured using Image-Pro Plus version 5.0 analysis software. Infarct areas of all sections were added to derive the total infarct area, which was multiplied by the thickness of the brain sections to obtain the infarct volume. The corrected infarct volume was calculated as before to compensate for the effect of brain oedema. Repeat three times for each group.
Histopathology staining
Histopathological staining (14 d postreperfusion) was conducted based on previously described methods (Wang et al. 2016). The brain samples were embedded in paraffin and coronally dissected into 5 μm thick sections. Then, paraffin sections were stained with H&E and Nissl staining to reveal the histopathological lesions.
TUNEL staining
Neuronal apoptosis was detected by TUNEL staining with the neural marker NeuN (Abcam, Cambridge, UK). The brain slices (n = 3 for each group) were incubated with proteinase K (20 μg/mL) for 15 min and then stained using a TUNEL kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s instructions. The nucleus was separately stained using DAPI (0.1 μg/mL). Images of the TUNEL-positive neuronal cells were captured using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Enzyme-linked immunosorbent assay
Right cortex tissues, serum and cell lysis solution were collected from each sample, and the total protein was extracted using a protein extraction reagent supplemented with protease and phosphatase inhibitor cocktails (ComWin Biotech, Beijing, China). The total protein concentration of tissue and cell samples was determined by a BCA kit (ComWin Biotech, Beijing, China). The expression level of TNF-α, IL-1β, IL-6, MCP-1, CD11a, ICAM-1, caspase-1, NOX, PC, 4-HNE and 8-OHdG was assessed using enzyme-linked immunosorbent assay (ELISA) kits according to the previous method (Xie et al. 2019). All ELISA kits were purchased from Expandbio (Beijing, China).
Western blot analysis
Western blot analysis was conducted as previously described (Xie et al. 2019; Zhu et al. 2021d). Right cortex tissues were collected from each rat (n = 3), and the total protein was extracted using a protein extraction reagent supplemented with protease and phosphatase inhibitor cocktails (ComWin Biotech, Beijing, China). The total protein concentration of each sample was determined by a BCA kit (ComWin Biotech, Beijing, China). Equal amounts of protein were separated using SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked before being incubated overnight at 4 °C with the appropriate primary antibodies against the following proteins: NLRP3 (A5652, 1:1000), IL-1β (Ab16288, 1:1000), Nrf2 (A0674, 1:1000), HO-1 (A1346, 1:1000), Akt (CST4685, 1:1000), p-Akt (CST4060T, 1:1000) and β-actin (EXP0036 F, 1:2000). Then, the membranes were washed three times and incubated with the appropriate secondary antibodies. The blots were visualized using enhanced chemiluminescence western blot detection kits (ComWin Biotech, Beijing, China). The blot densities were measured by Image J software (Bethesda, MD).
Cell culture
Primary cortical neurons were separated from cerebral cortices of rat foetuses (embryonic day 18) and were cultured on poly-l-lysine-coated plates referencing to a previously method with some modifications (Meng et al. 2014a). Dissociated cortical neurons grown in neurobasal medium supplemented with 2% B27 and 2 mM l-glutamine and changed every other day by replacing half of the medium. Cortical neurons were seeded with a density of 1 × 105 cells/cm2 for neurite outgrowth and differentiation. The neuron purity identification was assessed by immunofluorescence using the antibody against microtubule-associated protein-2 (MAP2), a marker of terminal neuronal differentiation. Dulbecco’s modified Eagle’s medium/F12, FBS, neurobasal medium and B27 were purchased from Gibco (Brooklyn, NY).
Oxygen-glucose deprivation/re-oxygenation
OGD/R was conducted with primary cortical neurons grown to mimic cerebral I/R injury in vitro. This technique was conducted according to previously described method with minor modification (Lu et al. 2011; Meng et al. 2014b). Briefly, primary cortical neurons cultured in glucose-free Locke’s medium under hypoxia for 2 h and then removed from the anaerobic chamber (TYPE c; Coy Laboratory Products, Inc., Grass Lake, MI) to a normoxic environment with the medium replaced by normal medium and were allowed to re-oxygenate for 24 h.
Detection of caspase-3 activity
The activity of caspase-3 was detected according to the previous method (Lu et al. 2019).
Mitochondrial membrane potential determination
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl carbocyanine iodide (JC-1) staining was used to determine the changes in mitochondrial membrane potential by Multiscan Spectrum analysis. Cells were pre-treated with concentrations of Xingxiong injection (1, 2 and 4 μL/mL). The cells were challenged with OGD/R after rinsing twice with PBS. After above processes, the cells were loaded 2 mM JC-1 (Beyotime Biotechnology, Shanghai, China) and incubated for 30 min at 37 °C in the dark. Fluorescence intensity was detected at 514/585 nm excitation wave length and 529/590 nm emission wavelength for monomer/J-aggregates (green light/red light) using Multiscan Spectrum (Tecan, Männedorf, Switzerland) (Meng et al. 2014b).
Statistical analysis
Data were obtained from at least three independent experiments and expressed as means ± standard deviation (SD). Data were analysed using one-way ANOVA, followed by Tukey’s test with GraphPad Prism 6.0 (SPAA, Inc., Chicago, IL), and the graphical presentation of data was also undertaken with GraphPad Prism (SPAA, Inc., Chicago, IL). p < 0.05 was considered statistically significant.
Results
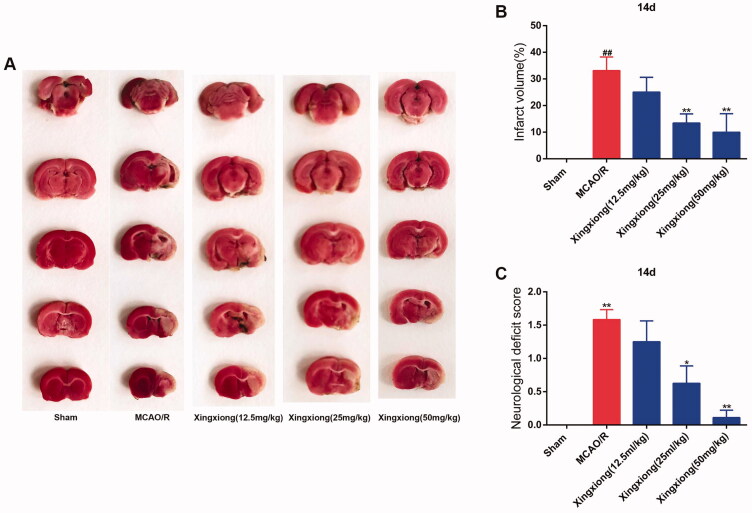
Reduction of cerebral infarction volumes and neurologic deficits by Xingxiong injection in rats
The cerebral infarction was examined 14 d after MCAO surgery performed on the right side in rats through TTC staining. Figure 1(A,B) shows typical photographs and quantitative data of TTC staining of the sham group, MCAO/R group, Xingxiong injection (12.5, 25 and 50 mL/kg). Administration of Xingxiong injection produced reductions in the infarct volumes compared to MCAO/R group. After treatment of Xingxiong injection at the dosages of 25 and 50 mL/kg for 14 d, infarction volumes significantly decrease than those in the MCAO/R group. In addition, Figure 1(C) shows a visible improvement in neurologic deficit in Xingxiong group.
Figure 1.
Xingxiong injection reduces the infarction volume and neurologic deficits in rats subjected to MCAO. (A) Effects of Xingxiong injection on infarction volumes (n = 3). (B) Quantitative analysis of cerebral infarct volumes. (C) Neurological score in rats with MCAO/R at 14 d after reperfusion (n = 3). Data are expressed as the mean ± SD and were analysed by ANOVA. ##p < 0.01 vs. sham group; *p < 0.05 and **p < 0.01 vs. MCAO/R group.
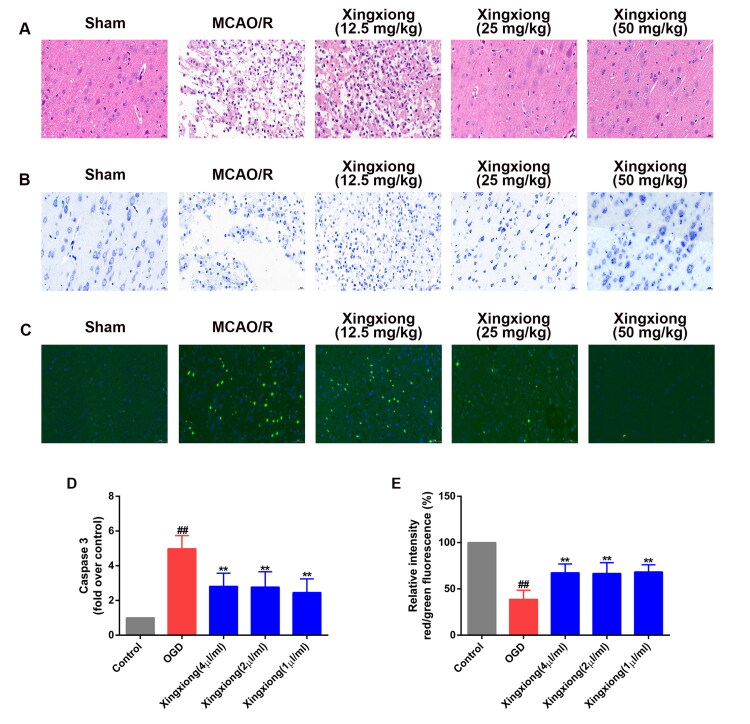
Suppression of ischaemia-induced neuronal injury by Xingxiong injection in rats
H&E-stained slides of rat brain sections were detected under a light microscope (Figure 2(A)). Rats in sham group have many neurons present in the pyknotic nuclei, whereas MCAO/R rats have pale nuclei in the cortex regions. After treatment of Xingxiong injection at the dosages of 25 and 50 mL/kg for 14 d, pyknotic nuclei significantly reduce than those in the MCAO/R group. In addition, Nissl staining shows that most neurons in the cortex region in the MCAO/R group had a shrunken phenotype, weak staining and were scattered irregularly. Similarly, Xingxiong injection at the dosages of 25 and 50 mL/kg for 14 d results in strong staining and regularly arranged neurons in the cortex.
Figure 2.
Xingxiong injection reduces neuronal injury and apoptosis of rats with MCAO/R and primary cortical neurons with OGD/R. (A) H&E staining in the cortex region in different groups. Scale bar: 200 μm, n = 3; (B) Nissl staining in the cortex region in different groups. Scale bar: 200 μm, n = 3; (C) immunofluorescence for TUNEL-positive neurons in the cortex, n = 3. Scale bar: 50 μm. (D) Caspase-3 activity of primary cortical neurons with OGD/R was measured using a fluorometric assay. (F) Primary cortical neurons were dyed with JC-1 and then detected using a fluorescence microscope. Data are expressed as the mean ± SD and were analysed by ANOVA. ##p < 0.01 vs. control group; **p < 0.01 vs. OGD/R group.
Suppression of ischaemia/hypoxia-induced apoptosis by Xingxiong injection in vivo and in vitro
To further explore the neuropathological alterations in ischaemic brains from all groups, neuronal apoptosis in the ischaemic brain tissues was examined after 14 d reperfusion following 2 h of MCAO. The immunofluorescent results showed that the MCAO/R-induced rats exhibited more TUNEL-positive neurons in the ischaemic cortex regions than the sham group (Figure 2(C)). However, Xingxiong injection at the dosages of 25 and 50 mL/kg treatment decreased the number of TUNEL-positive neurons in the ischaemic brain.
The expression of active caspase-3 of the primary cortical neurons separated from E18 SD was detected. The result showed that the expression of active caspase-3 was up-regulated in the OGD-treated group compared with the control group. However, the expression levels of caspase-3 were significantly suppressed by the treatment of Xingxiong injection (Figure 2(D)). In addition, mitochondrial membrane potential of the primary cortical neurons, detected with the ratio of red and green, was suppressed by the treatment of OGD while the effects were dramatically reversed by treatment of Xingxiong injection (Figure 2(E)).
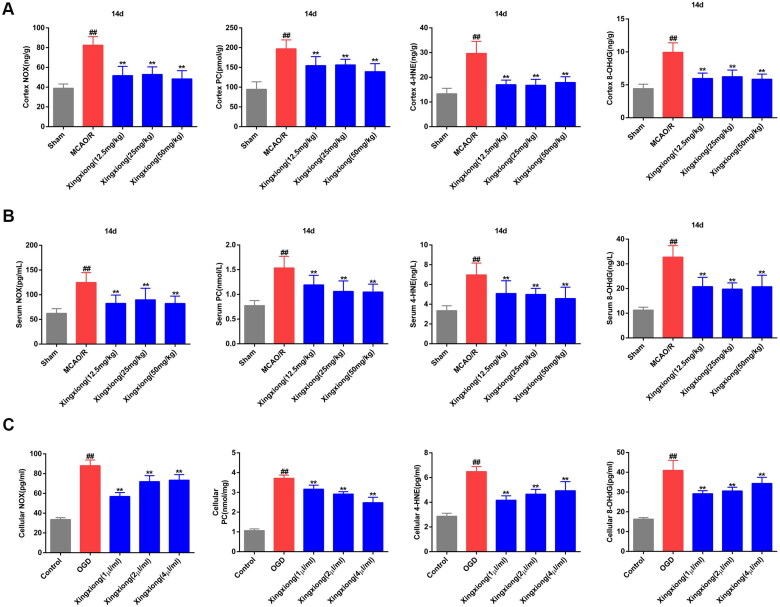
Suppression of ischaemia/hypoxia-induced oxidative stress by Xingxiong injection in vivo and in vitro
Results of ELISA of ischaemia/hypoxia-induced oxidative stress in vivo and in vitro are shown in Figure 3. Compared with the sham group, MCAO/R treatment resulted in a significantly increase in the level of NOX, PC, 4-HNE and 8-OHdG in rat cortex (Figure 3(A)) and serum (Figure 3(B)) after MCAO surgery for 14 d. Furthermore, these factors also displayed a notable increase in OGD-treated primary cortical neurons (Figure 3(C)). However, Xingxiong injection substantially downregulated the levels of NOX, PC, 4-HNE and 8-OHdG in the serum and cortex of MCAO-treated rats and in OGD-treated primary cortical neurons.
Figure 3.
Xingxiong injection inhibits oxidative stress in rat cortex and serum with MCAO/R and in primary cortical neurons with OGD/R. Effect of Xingxiong injection treatment on NOX, PC, 4-HNE and 8-OHdG in rat cortex (A) and serum (B) with MCAO/R (n = 8) and in primary cortical neurons (C) with OGD/R (n = 3). Data are expressed as the mean ± SD and were analysed by ANOVA. ##p < 0.01 vs. sham or control group; **p < 0.01 vs. MCAO/R and OGD/R group.
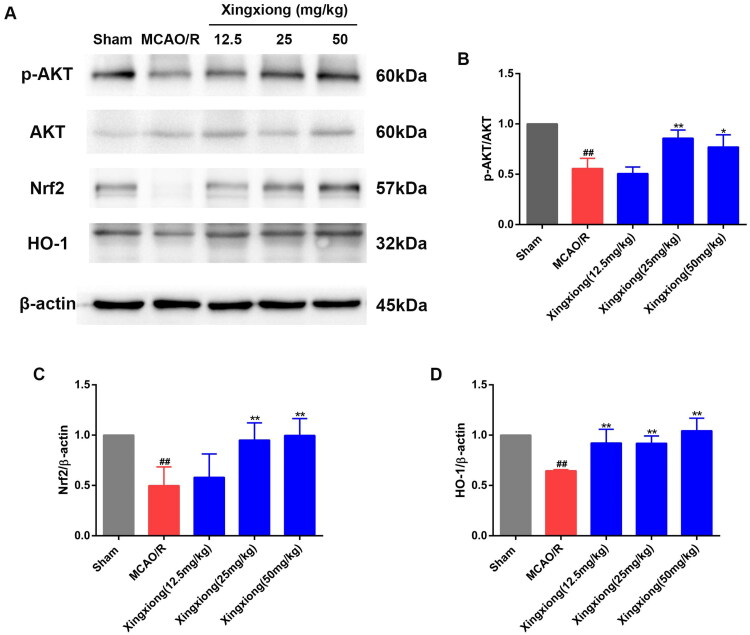
Xingxiong injection reduces oxidative stress by activating the Akt/Nrf2/HO-1 pathway
In view of the fact that HO-1 is an important antioxidant enzyme, we examined cortex HO-1 protein expression. Figure 4 shows that MCAO/R rats had lower HO-1 expression level than rats in the sham group. However, Xingxiong injection treatment for 14 d significantly increased HO-1 expression level. Nrf2 directly regulates the activity of HO-1 promoter. Western blot results reveal that Nrf2 expression level was higher in rats treated with Xingxiong injection than rats in the MCAO/R group. Moreover, activation of the PI3K/Akt signal transduction pathway could promote Nrf2 nuclear translocation (Zhai et al. 2018). We aimed to assess if PI3K/Akt signal transduction pathway is involved in the mechanisms underlying the Xingxiong injection-mediated reduction of oxidative stress. Western blot results also reveal that the expression level of phospho-Akt (p-Akt) were higher in rats pre-treatment with Xingxiong injection than rats in the MCAO/R group, indicating that Xingxiong injection could promote Akt phosphorylation in the cortex region.
Figure 4.
Xingxiong injection reduces oxidative stress by activating the Akt/Nrf2/HO-1 pathway. Representative images of immunoblotting (A) and quantification of the relative protein levels of p-AKT/AKT (B), Nrf2 (C) and HO-1 (D) in the infarct cortex region of the different groups on day 14 after MCAO surgery. n = 3 in each group. Data are expressed as the mean ± SD and were analysed by ANOVA. ##p < 0.01 vs. sham group; *p < 0.05, **p < 0.01 vs. MCAO/R group.
Suppression of ischaemia/hypoxia-induced inflammation by Xingxiong injection in vivo and in vitro
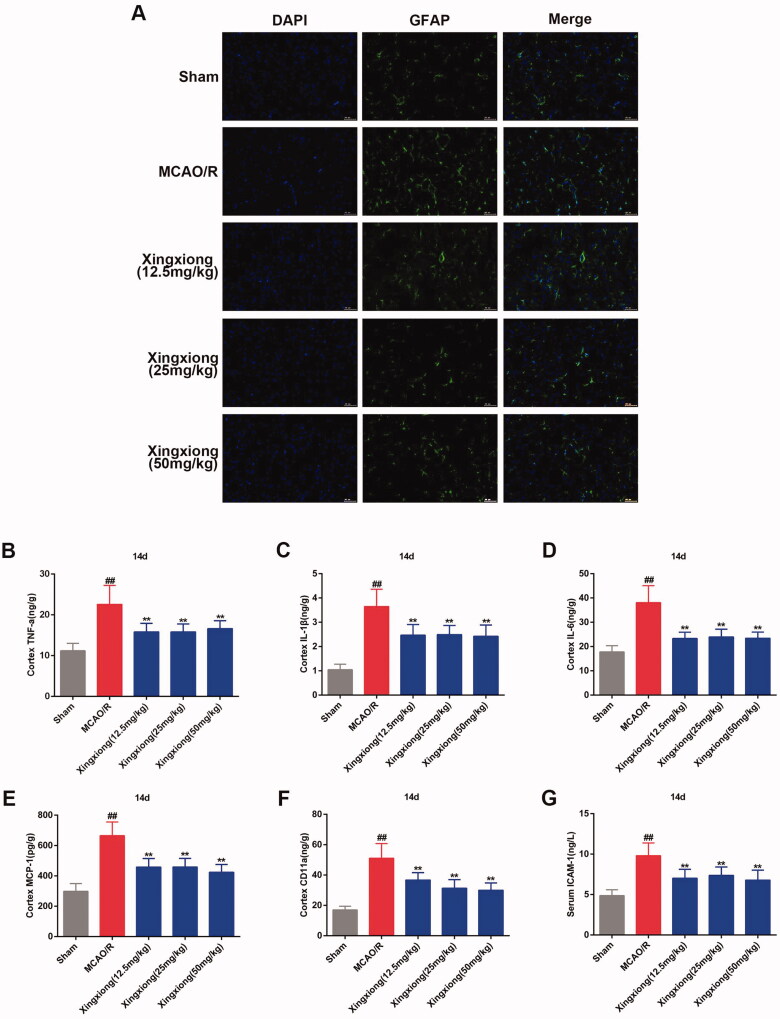
Cerebral ischaemia promotes the activation and proliferation of astrocytes, and the reactive astrocytes produce and release inflammatory mediators, such as IL-6, TNF-α and IL-1β (Liu and Chopp 2016; Pekny et al. 2016). In the present study, the increased reactive astrocytes that were labelled with GFAP was observed in the MCAO/R-induced cortex region were attenuated by Xingxiong injection (Figure 5(A,B)).
Figure 5.
Xingxiong injection inhibits inflammatory response in rat cortex with MCAO/R. (A) Immunofluorescence for GFAP (14 days postreperfusion) in the cortex. Scale bar: 50 μm. Effect of Xingxiong injection treatment on cortex TNF-α (B), IL-1β (C), IL-6 (D), MCP-1 (E), CD11a (F) and ICAM-1 (G) in rats with MCAO/R. Data are expressed as the mean ± SD and were analysed by ANOVA. ##p < 0.01 vs. sham group; **p < 0.01 vs. MCAO/R group.
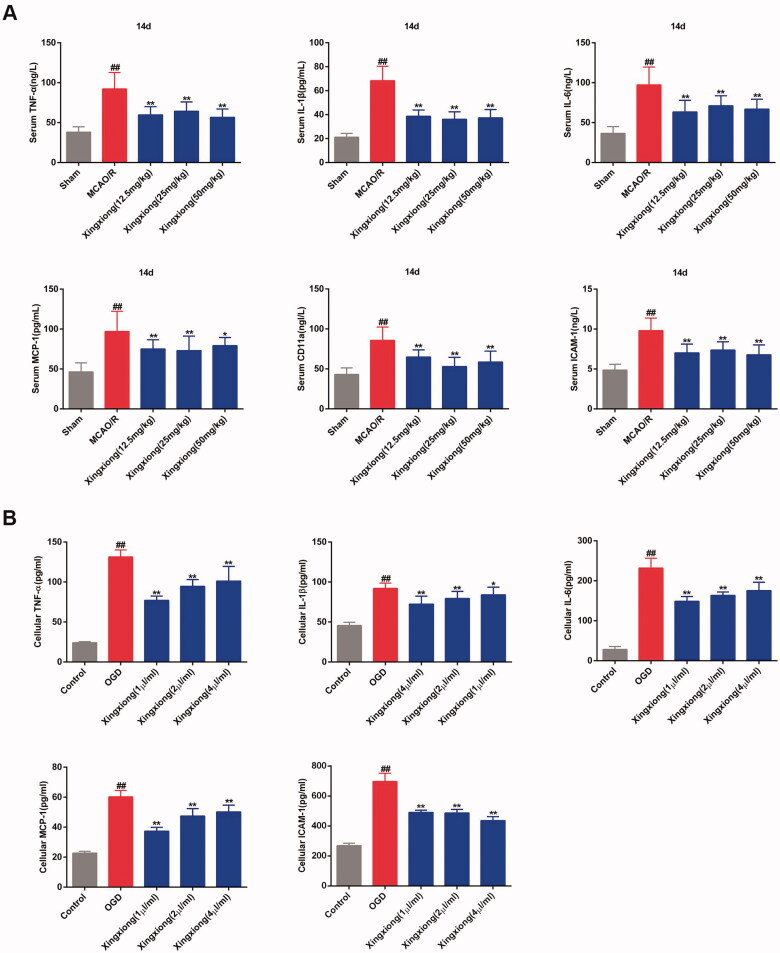
Results of ELISA of ischaemia/hypoxia-induced inflammation in vivo and in vitro are shown in Figures 5(C) and 6. The expression of excessive inflammatory factors, such as TNF-a, IL-1β, IL-6, MCP-1, CD11a and ICAM-1 was induced by ischaemia in rat cortex (Figure 5(B)) and serum (Figure 6(A)) after MCAO surgery for 14 d. There excessive inflammatory factors with the exception of CD11a were up-regulated expressed in the OGD-treated primary cortical neurons in vitro (Figure 6(B)). However, the expression levels of these inflammatory cytokines in the cortex and serum of MCAO-treated rats and in OGD-treated primary cortical neurons were inhibited by Xingxiong injection.
Figure 6.
Xingxiong injection inhibits the expression of inflammatory factors in rat serum with MCAO/R and in primary cortical neurons with OGD/R. Effect of Xingxiong injection treatment on TNF-α, IL-1β, IL-6, MCP-1, CD11a and ICAM-1 in rat serum (A) with MCAO/R (n = 8) and in primary cortical neurons (C) with OGD/R (n = 3). Data are expressed as the mean ± SD and were analysed by ANOVA. ##p < 0.01 vs. sham or control group; *p < 0.05, **p < 0.01 vs. MCAO/R and OGD/R group.
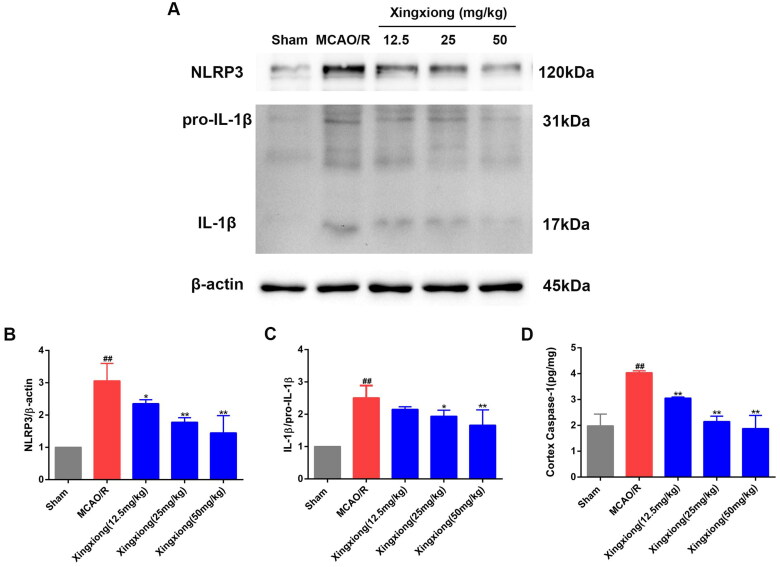
Xingxiong injection inhibits NLRP3 inflammasome activation
As shown in Figure 7(A–C), the active IL-1β protein expression level was observably upregulated in the cortex of MCAO/R rats, which suggested activation of NLRP3 inflammasome in the cortex. In addition, result of ELISA of ischaemia-induced caspase-1 expression level in cortex tissue is shown in Figure 7(D). The result shows that the caspase-1 expression level in cortex was significantly decreased in the Xingxiong injection-treated rats compared with the MCAO/R-treated rats. Therefore, we detected the protein expression level of NLRP3 in the cortex. As expected, NLRP3 expression level in the cortex was significantly increased in MCAO/R-treated rats compared with the rats in the sham group. However, rats pre-treated with Xingxiong injection exhibit a significant decrease in NLRP3 expression level in the cortex compared with the MCAO/R group.
Figure 7.
Xingxiong injection reduces inflammatory response by inhibiting NLRP3 inflammasome. Representative images of immunoblotting (A) and quantification of the relative protein levels of NLRP3 (B) and IL-1β (C) in the infarct cortex region of the different groups on day 14 after MCAO surgery, n = 3 in each group. Effect of Xingxiong injection treatment on caspase-1 in cortex region (D) with MCAO/R by ELISA (n = 3). Data are expressed as the mean ± SD and were analysed by ANOVA. ##p < 0.01 vs. sham group; *p < 0.05, **p < 0.01 vs. MCAO/R group.
Discussion
Ischaemic stroke was mainly caused by cerebral ischaemia and reperfusion injury (Jamison et al. 2008; Xie et al. 2018). After ischaemic stroke, the pathophysiological responses of the brain were predominated by BBB perturbations, free radical production, brain oedema and excitatory amino acid toxicity in the early acute phase of ischaemic injury, followed by inflammation and neuronal apoptosis in the middle stage, and then angiogenesis and neurogenesis, which provide the critical neurovascular substrates for neuronal remodelling, in the delayed phase (Arai et al. 2009). In the present study, MCAO/R rat model was used to explore the effects and mechanisms of Xingxiong injection following acute ischaemic stroke. Results show that infarct volume, neurological deficit, neuronal injury, neuronal apoptosis and glial activation were improved in the MCAO/R-treated rats by Xingxiong injection treatment. Moreover, the Xingxiong injection-treated rats exhibited decreased NOX, protein carbonyl, 4-HNE, 8-OHdG and various inflammatory factors compared with the MCAO/R group. In addition, we also found that Nrf2 pathway and NLRP3 inflammasome were involved in the neuroprotective effects of Xingxiong injection against ischaemic stroke in vivo. These results indicated that activation of Nrf2 pathway and inhibition of NLRP3 inflammasome may be the molecular mechanisms against oxidative stress and inflammation after ischaemic stroke challenge.
Mitochondria, one of the most important organs in brain neurons, played an important role in regulating Ca2+ homeostasis in cytoplasm, maintaining intracellular pH and generating ATP through the tricarboxylic acid cycle and electron transport chains. Disorders of ROS and Ca2+ overload can cause the integrity of the mitochondrial inner membrane to be damaged, the mitochondrial membrane potential (Δψm) depolarized and decreased, and the proteinic pores (mitochondrial permeability transition pore, MPTP) in the membrane to open. Subsequently, numerous small molecules (relative molecular mass <1.5 kDa) pass through the membrane to increase the osmotic pressure of mitochondrial matrix proteins, resulting in mitochondrial outer membrane damage and mitochondrial membrane potential depolarization. These changes further induce mitochondrial apoptosis and activate caspase cascade (Jonas et al. 2015; Mnatsakanyan et al. 2017). The activation of caspase-3 pathway caused by ischaemia would induce neurons apoptosis, thereby leading to the neurological dysfunction. The present study demonstrated that Xingxiong injection treatment reduced TUNEL positive cells, increased mitochondrial membrane potential and lowered the activity of caspase-3. Therefore, inhibiting apoptosis might be an important mechanism of Xingxiong injection treatment for playing a role of neuroprotective effect.
Cerebral I/R injury induced the expression of NOX, PC, 4-HNE and 8-OHdG, which might represent the response of oxidative stress suffering from ischaemic insult. Oxidative stress refers to a condition in which the imbalance favouring ROS/RNS over antioxidative ability causes cellular injury and pathologic change (Zhou et al. 2018). ROS/RNS are mainly generated in the ischaemic penumbra, particularly after reperfusion and are continuously removed through powerful free radical scavenging systems (e.g., SOD, GSH, CAT, GSH-Px and NADP/NADPH) to maintain a dynamic balance (Zhou et al. 2019). Under the condition of ischaemia/reperfusion, increased generation of active free radicals can induce lipid peroxidation, protein denaturation, intracellular Ca2+ overload, enzyme inactivation and DNA damage and mitochondrial damage, which promotes cytochrome-C (Cyt-C) and apoptosis inducing factor (AIF) in mitochondria release, cellular signalling pathway disorder and downstream apoptotic cascade reaction (Sherif and Al-Shaalan 2018). In addition, oxidative stress exacerbates the inflammatory response (Bahar et al. 2017) and triggers the activation of matrix metalloproteinases (Ito et al. 2017), thereby disrupting the integrity of BBB and accelerating neuronal apoptosis and white matter damage. The present study has shown that Xingxiong injection treatment significantly inhibits the increased levels of oxidative stress markers (such as NOX, protein carbonyl, 4-HNE and 8-OHdG) induced by MCAO/R in rats in vivo and OGD/R in primary cortical neurons in vitro. These results indicated that the neuroprotective effects of Xingxiong injection were partly caused by oxidative stress inhibition.
HO-1 is a powerful antioxidant that catalyses the degradation of biliverdin, iron and carbon monoxide. Inducing the expression of HO-1 can enhance the antioxidant stress ability of neurons (Meng et al. 2014b). Previous studies have shown that transcription factor Nrf2 is a key factor in regulating the expression of HO-1, which is regulated by PI3K/Akt (Martin et al. 2004).
In our study, higher expression levels of p-Akt/Akt, Nrf2 and HO-1 were observed in rats treated with Xingxiong injection than in the MCAO/R group, indicating that Xingxiong injection treatment activates the Akt/Nrf2 pathway and promotes HO-1 expression.
Inflammatory cascades, triggered by energy depletion and necrotic neuron death play an important role in ischaemia/reperfusion process. Inflammatory response is activated by blood-borne leukocytes that penetrate the brain during the ischaemic process (Kamel and Iadecola 2012). Ischaemia/reperfusion injury induces ROS production, glutamate toxicity, Ca2+ overload, platelets and endothelial cell activation, and releases inflammatory cytokines and pro-inflammatory mediators including TNF-a, IL-1β, IL-6, ICAM-1, CD11a, MCP-1 and anti-inflammatory mediators including IL-4, IL-10 in ischaemic brain (Boehncke 2018). TNF-α is a potent stimulator of microglial activation closely related to inflammatory response. TNF-α aggravates the inflammatory response after cerebral ischaemia, promotes vascular endothelial cells damage and thrombosis formation. TNF-α is also an important promoter of inflammatory factors, which can upregulate the expression of other inflammatory factors (such as IL-1β, IL-6, IL-8, etc.) and intercellular adhesion factor-1 (ICAM-1), promoting the transfer of inflammatory cells to nervous tissue and aggravating neurological dysfunction (Gong et al. 2019). In our research, MCAO/R injury significantly deteriorated neurological function. These phenomena were accompanied by the enhanced expression of a series of pro-inflammatory cytokines such as TNF-a, IL-1β, IL-6, ICAM-1, CD11a and MCP-1. In contrast, Xingxiong injection inhibits all above-mentioned activities induced by MCAO/R challenge both in vivo and in vitro study. The increased protein level of ICAM-1 observed from the serum and cortex following MCAO/R challenge is consistent with previous research manifesting that high ICAM-1 level was more likely to have previous stroke and increased haemorrhagic transformation risk (Wu et al. 2018). This increase was triggered by MCAO/R challenge and inflammatory cytokine overproduction, which was dramatically limited by Xingxiong injection treatments, proved that Xingxiong injection reduced cerebral ischaemia/reperfusion injury by inhibiting inflammatory response.
Inflammasomes play an important role in the inflammatory response after ischaemic stroke. Among them, NLRP3 is the most representative inflammasomes, which has become a research topic of great interest. After ischaemic stroke, DAMPs released by necrotic cells in the ischaemic core and changes in intracellular microenvironment can trigger the formation of inflammasomes complexes, activating caspase-1 and mediating the secretion of IL-1β and IL-18, and finally cause cell death through inflammation and/or apoptosis. Western blot analysis confirmed that Xingxiong injection downregulates the protein expression of NLRP3 and IL-1β/pro-IL-1β. ELISA results also show that Xingxiong injection significantly downregulates the caspase-1 expression levels in cortex and serum. The data suggest that Xingxiong injection exerts anti-inflammatory effects after ischaemic stroke via the inhibition of NLRP3 activation.
Conclusions
The current study showed that treatment with Xingxiong injection inhibits apoptosis, oxidative stress and inflammation after ischaemic stroke. The mechanisms by which Xingxiong injection exert antioxidant and anti-inflammatory effects involved the upregulation of Akt/Nrf2 pathway and inhibition of NLRP3 inflammasome. These findings clarified the mechanism underlying the function of Xingxiong injection for the clinical treatment of ischaemic stroke.
Funding Statement
This research was supported by the National Key R&D Plan (No. 2018YFC1707408), Central Public-Interest Scientific Institution Basal Research Fund (No. 2018PT35030) and Major Projects of the National Natural Science Foundation of China (No. 81891012).
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Aiyagari V, Gujjar A, Zazulia AR, Diringer MN.. 2004. Hourly blood pressure monitoring after intravenous tissue plasminogen activator for ischemic stroke: does everyone need it? Stroke. 35(10):2326–2330. [DOI] [PubMed] [Google Scholar]
- Amieva H, Meillon C, Helmer C, Barberger-Gateau P, Dartigues JF.. 2013. Ginkgo biloba extract and long-term cognitive decline: a 20-year follow-up population-based study. PLOS One. 8(1):e52755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Jin G, Navaratna D, Lo EH.. 2009. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 276(17):4644–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar E, Kim JY, Yoon H.. 2017. Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-κB and HO-1/Nrf2 pathways. Int J Mol Sci. 18(9):1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehncke WH. 2018. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. 9:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuvront SN, Carter R 3rd.. 2003. Ginkgo and memory. JAMA. 289(5):547. [DOI] [PubMed] [Google Scholar]
- Gong P, Zhang Z, Zou Y, Tian Q, Han S, Xu Z, Liao J, Gao L, Chen Q, Li M.. 2019. Tetramethylpyrazine attenuates blood–brain barrier disruption in ischemia/reperfusion injury through the JAK/STAT signaling pathway. Eur J Pharmacol. 854:289–297. [DOI] [PubMed] [Google Scholar]
- Ito F, Yamada Y, Shigemitsu A, Akinishi M, Kaniwa H, Miyake R, Yamanaka S, Kobayashi H.. 2017. Role of oxidative stress in epigenetic modification in endometriosis. Reprod Sci. 24(11):1493–1502. [DOI] [PubMed] [Google Scholar]
- Jamison JT, Kayali F, Rudolph J, Marshall M, Kimball SR, DeGracia DJ.. 2008. Persistent redistribution of poly-adenylated mRNAs correlates with translation arrest and cell death following global brain ischemia and reperfusion. Neuroscience. 154(2):504–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA, Porter GA Jr, Beutner G, Mnatsakanyan N, Alavian KN.. 2015. Cell death disguised: the mitochondrial permeability transition pore as the c-subunit of the F(1)F(O) ATP synthase. Pharmacol Res. 99:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel H, Iadecola C.. 2012. Brain–immune interactions and ischemic stroke: clinical implications. Arch Neurol. 69(5):576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WM, Liu HT, Li XY, Wu JY, Xu G, Teng YZ, Ding ST, Yu C.. 2010. The effect of tetramethylpyrazine on hydrogen peroxide-induced oxidative damage in human umbilical vein endothelial cells. Basic Clin Pharmacol Toxicol. 106:45–52. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chopp M.. 2016. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol. 144:103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Luo Y, Zhou P, Yang K, Sun G, Sun X.. 2019. Ginsenoside compound K protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury via inhibition of nuclear factor-κB, p38, and JNK MAPK pathways. J Ginseng Res. 43(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Jiang Y, Zhou Z, Yue X, Wei N, Chen Z, Ma M, Xu G, Liu X.. 2011. Intranasal ginsenoside Rb1 targets the brain and ameliorates cerebral ischemia/reperfusion injury in rats. Biol Pharm Bull. 34(8):1319–1324. [DOI] [PubMed] [Google Scholar]
- Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A.. 2004. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 279(10):8919–8929. [DOI] [PubMed] [Google Scholar]
- Meng X, Sun G, Ye J, Xu H, Wang H, Sun X.. 2014a. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: a novel mechanism of Nrf2/ARE signaling activation. Free Radic Res. 48(4):445–460. [DOI] [PubMed] [Google Scholar]
- Meng X, Wang M, Wang X, Sun G, Ye J, Xu H, Sun X.. 2014b. Suppression of NADPH oxidase- and mitochondrion-derived superoxide by notoginsenoside R1 protects against cerebral ischemia–reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways. Free Radic Res. 48(7):823–838. [DOI] [PubMed] [Google Scholar]
- Mnatsakanyan N, Beutner G, Porter GA, Alavian KN, Jonas EA.. 2017. Physiological roles of the mitochondrial permeability transition pore. J Bioenerg Biomembr. 49(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A.. 2016. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 131(3):323–345. [DOI] [PubMed] [Google Scholar]
- Sherif IO, Al-Shaalan NH.. 2018. Vildagliptin attenuates hepatic ischemia/reperfusion injury via the TLR4/NF-κB signaling pathway. Oxid Med Cell Longev. 2018:3509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M.. 2010. Calcium, ischemia and excitotoxicity. Cell Calcium. 47(2):122–129. [DOI] [PubMed] [Google Scholar]
- Wang M, Sun GB, Du YY, Tian Y, Liao P, Liu XS, Ye JX, Sun XB.. 2017. Myricitrin protects cardiomyocytes from hypoxia/reoxygenation injury: involvement of heat shock protein 90. Front Pharmacol. 8:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Huang Y, Cheng Y, Tan Y, Wu F, Wu J, Shi H, Zhang H, Yu X, Gao H, et al. 2016. Endoplasmic reticulum stress-induced neuronal inflammatory response and apoptosis likely plays a key role in the development of diabetic encephalopathy. Oncotarget. 7(48):78455–78472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BN, Wu J, Hao DL, Mao LL, Zhang J, Huang TT.. 2018. High serum sICAM-1 is correlated with cerebral microbleeds and hemorrhagic transformation in ischemic stroke patients. Br J Neurosurg. 32(6):631–636. [DOI] [PubMed] [Google Scholar]
- Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G, Sun X.. 2018. Protective effects and target network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: a comprehensive overview of experimental studies. Cells. 7(12):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Zhu T, Dong X, Nan F, Meng X, Zhou P, Sun G, Sun X.. 2019. HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-κB signaling pathways. Biomolecules. 9:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WB, Sun YQ.. 2007. Clinical observation on effect of Floium Ginkgo extract and tertram ethypyrazine sodium chloride injection in treating acute cerebral infarction: a report of 32 cases. Lishizhen Med and Materia Med Res. 2007:2063–2064. [Google Scholar]
- Zhai Y, Meng X, Luo Y, Wu Y, Ye T, Zhou P, Ding S, Wang M, Lu S, Zhu L, et al. 2018. Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Oncotarget. 9(10):9344–9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZY, Jing-Ping MA, Neurology DO.. 2018. The autophagy in rats on cerebral ischemia reperfusion injury and the influence of it on dl-3n-butyphthalide through LKB-1/AMPK pathway. World Latest Med Inform. 18:30–32. [Google Scholar]
- Zhong SQ, Sun LJ, Yan YZ, Sun YQ, Zhong YY.. 2005. Effect of Xuesaitong soft capsule on hemorrheology and in auxiliarily treating patients with acute cerebral infarction. Chin J Integr Med. 11:128–131. [DOI] [PubMed] [Google Scholar]
- Zhou P, Xie W, Sun Y, Dai Z, Li G, Sun G, Sun X.. 2019. Ginsenoside Rb1 and mitochondria: a short review of the literature. Mol Cell Probes. 43:1–5. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Lu J, Liu WW, Manaenko A, Hou X, Mei Q, Huang JL, Tang J, Zhang JH, Yao H, et al. 2018. Advances in stroke pharmacology. Pharmacol Ther. 191:23–42. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Zhang GH, Yang N, Rowlands DK, Wong HY, Tsang LL, Chung YW, Chan HC.. 2005. Activation of apical CFTR and basolateral Ca+2-activated K + channels by tetramethylpyrazine in Caco-2 cell line. Eur J Pharmacol. 510(3):187–195. [DOI] [PubMed] [Google Scholar]
- Zhu T, Meng XB, Dong DX, Zhao LY, Qu MW, Sun GB, Sun XB.. 2021a. Xuesaitong injection (lyophilized) combined with aspirin and clopidogrel protect against focal cerebral ischemic/reperfusion injury in rats by suppressing oxidative stress and inflammation and regulating the NOX2/IL-6/STAT3 pathway. Ann Palliat Med. 10(2):1650–1667. [DOI] [PubMed] [Google Scholar]
- Zhu T, Wang L, Feng Y, Sun G, Sun X. 2021b. Classical active ingredients and extracts of Chinese herbal medicines: pharmacokinetics, pharmacodynamics, and molecular mechanisms for ischemic stroke. Oxid Med Cell Longev. 7:8868941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Wang L, Tian F, Zhao X, Pu XP, Sun GB, Sun XB.. 2020. Anti-ischemia/reperfusion injury effects of notoginsenoside R1 on small molecule metabolism in rat brain after ischemic stroke as visualized by MALDI-MS imaging. Biomed Pharmacother. 129:110470. [DOI] [PubMed] [Google Scholar]
- Zhu T, Wang L, Xie W, Meng X, Feng Y, Sun G, Sun X.. 2021c. Notoginsenoside R1 improves cerebral ischemia/reperfusion injury by promoting neurogenesis via the BDNF/Akt/CREB pathway. Front Pharmacol. 12:615998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Xie WJ, Wang L, Jin XB, Meng XB, Sun GB, Sun XB.. 2021d. Notoginsenoside R1 activates the NAMPT-NAD+-SIRT1 cascade to promote postischemic angiogenesis by modulating Notch signaling. Biomed Pharmacother. 140:111693. [DOI] [PubMed] [Google Scholar]