Abstract
Background:
Childhood psychotic-like experiences (PLEs) often precede the development of later severe psychopathology. This study examined whether childhood PLEs are associated with several psychopathology-related polygenic scores (PGS), and additionally examined possible neural and behavioral mechanisms.
Methods:
Adolescent Brain Cognitive DevelopmentSM Study baseline data from children with European ancestry (n=4,650; ages 9-10; 46.8% female) were used to estimate associations between PLEs (i.e., both total and presence of significantly distressing) and PGS for psychopathology (i.e., schizophrenia, psychiatric cross-disorder risk, PLEs) and related phenotypes (i.e., educational attainment [EDU], birth-weight, inflammation). We also assessed whether variability in brain structure indices (i.e., volume, cortical thickness, surface area), as well as behaviors proximal to PGS (e.g., cognition for EDU), indirectly linked PGS to PLEs using mediational models.
Results:
Total and significantly distressing PLEs were associated with EDU and cross-disorder PGS (all %ΔR2s=0.202-0.660%; pFDRs<0.006). Significantly distressing PLEs were also associated with higher schizophrenia and PLEs PGS (both %ΔR2=0.120-0.216%; pFDRs<0.03). There was evidence global brain volume metrics and cognitive performance indirectly linked EDU PGS to PLEs (estimated proportion mediated= 3.33-32.22%).
Conclusions:
Total and significantly distressing PLEs were associated with genomic risk indices of broad-spectrum psychopathology risk (i.e., EDU and cross-disorder PGS). Significantly distressing PLEs were also associated with genomic risk for psychosis (i.e., schizophrenia, PLEs). Global brain volume metrics and PGS-proximal behaviors represent promising putative intermediary phenotypes that may indirectly link genomic risk to psychopathology. Broadly, polygenic scores derived from genome-wide association studies of adult samples generalize to indices of psychopathology risk among children.
Keywords: psychotic-like experiences, polygenic, schizophrenia, educational attainment, psychopathology, MRI
Introduction
Psychotic-like experiences (PLEs) are nonclinical schizophrenia-spectrum symptoms that include perceptual abnormalities and mild delusional thoughts. They commonly occur in children (~10% of children and adolescents) and are considered a dimensional, transdiagnostic marker of significant psychopathology risk (e.g., odds ratio ~3), including conversion to adult psychotic disorders in some children (1,2). Indeed, supporting the potential validity of PLEs as markers of psychopathology and psychosis-specific risk, PLEs are associated with a range of risk factors (e.g., family history of psychotic disorders, developmental milestone delays, cognition, and neural correlates) within the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (3-5). The adverse mental health prognosis of children with PLEs, even beyond those who eventually develop schizophrenia, has inspired efforts to improve our understanding of PLE etiology to ultimately facilitate advances in prevention and treatment.
Building upon twin work documenting the moderate heritability of PLEs, genome-wide association studies (GWASs) have shown that PLEs are highly polygenic, much like other complex behavioral and biological phenotypes (6). Results from well-powered discovery GWASs may be projected onto individuals in an independent sample by averaging common variants weighted by GWAS effect size and number of risk alleles present across the genome to generate polygenic scores (PGS) that represent an individual’s genomic predisposition for the discovery GWAS phenotype (7). Initial evidence suggested null associations between schizophrenia PGS and adolescent PLEs using an initial discovery GWAS (cases n=9,394(8)) (9-11). In contrast, recent work leveraging results from a larger GWAS of schizophrenia (cases n=36,989 (12)) has generally linked schizophrenia PGS to PLEs during adolescence and adulthood (e.g., ages 15-19) (13) (although see (14)) and predicted psychosis conversion in individuals at risk for psychosis (15). Further, other work has found associations between later-life PLEs and both schizophrenia and mood disorder PGSs (6).
This study examined associations between childhood PLEs and several polygenic scores associated with risk for psychopathology (e.g., psychosis), including scores that may be putatively associated with PLEs through neural or behavior mechanisms (e.g., inflammation, birthweight). In addition to PLEs being related to schizophrenia risk, associations between PLEs and general psychopathology (6,16) suggest that genomic vulnerability to broad-spectrum psychopathology may confer risk for PLEs in childhood. Further, clues from epidemiological studies linking psychosis spectrum symptoms to low birthweight (17), inflammation (18), and reduced educational attainment (i.e., a measure of both cognitive functioning and non-cognitive factors, including risk taking and household income, considered risk factors for psychosis (19,20)) (21) raise the possibility that polygenic propensity for these phenotypes may correlate with PLEs independently or through their phenotypic expression. PGS scores for birthweight and inflammation may provide important insights regarding associations between genetic liability for early developmental environmental insults and PLEs. Finally, emerging evidence linking PLEs to lower global brain volume (22) as well as evidence of brain volume indirectly linking risk factors to PLEs in the ABCD Study® (23), provide a basis for the possibility that brain structure may indirectly link genomic risk to PLE expression (24).
This study examined data from non-Hispanic children of European ancestries (n=4,650; aged 9-10) who completed the baseline session of the ABCD Study®. We tested whether PLEs are associated with genomic liability to schizophrenia, psychiatric cross-disorder risk, PLEs, educational attainment (EDU), birthweight, and inflammation (i.e., c-reactive protein) in school-age children. As childhood PLEs represent potential harbingers of adult psychopathology, it is critical to understand whether, as expected, polygenic liability estimates derived from GWASs of adult phenotypes are associated with their expression in childhood, whether these associations with PGS vary according to PLE severity, and whether variability in brain structure and behavior indirectly link polygenic vulnerability to the expression of PLEs in children.
Methods
Participants
A sample of 11,875 individuals was obtained from the ABCD Study® (Data Release 2.0.1; see Acknowledgements), a large-scale ongoing longitudinal study of children recruited from 22 research sites across the United States (25). The ABCD Study aimed to explore factors associated with development of both healthy behaviors and mental health challenges, and aimed to utilize a multi-stage probability sample of eligible youth (Supplement for study-wide exclusion criteria, power analysis), selecting a stratified, probability sample of schools across the United States designed to capture demographic diversity (26-28). Participants who did not pass quality control metrics and those who were not of European ancestries were removed, leaving a final analytic sample of 4,650 (46.8% female; mean age=9.93±0.63 [range=9.00-10.92] years; Supplemental Figure 1 for sample overlap with European ancestry reference population). Additionally, a sample of individuals with African ancestries was used in exploratory analyses (n=1,201; see Supplement). Caregivers provided written informed consent and all children provided assent.
Measures
Psychotic-like Experiences.
Participants completed the Prodromal Questionnaire-Brief Child Version (PQ-BC), a 21-item questionnaire previously validated for use with school-age children using the ABCD Study® sample (5,29), which asks about the occurrence of PLEs (e.g., unusual, thought content, perceptual abnormalities) in the past month. All PQ-BC questions were read to participants by research assistants. The dimensional total score was used to measure PLEs. Total scores index the full dimension of PLEs, including more normative PLEs, although individuals endorsing at least one PLE generally show greater impairment than those endorsing no PLEs (Supplemental Table 1). To address the large number of 0 PLE values (44.6% of the sample) and obtain a more clinically-relevant PLE metric, we also formed 3 groups based upon PLE endorsement: Group 1 - reporting 0 PLEs (n=2,076), Group 2 - reporting ≥ 1 PLE but no significant distress associated with PLEs (n=1,601), Group 3 - reporting ≥ 1 PLE with significant distress (i.e., rating a PLE ≥3 on a five-point scale of distress; n=972; Table 1).
Table 1.
Sample Characteristicsa
| Variable (n) | No PLEs (n=2076) |
≥ 1 PLE but no Significant Distress (n=1,601) |
Endorsed ≥ 1 Significantly Distressing PLEs (n=972) |
Total Sample (N=4650) |
|---|---|---|---|---|
| Age in years (4650) | 9.960±0.627 | 9.933±0.627 | 9.882±0.613 | 9.934±0.625 |
| Sex, % female (4650) | 999 (48.1%) | 723 (45.2%) | 454 (46.7%) | 2176 (46.8%) |
| Scanner Type (4532) | ||||
| Siemens, % | 1431 (68.9%) | 977 (61.0%) | 576 (59.3%) | 2984 (64.2%) |
| Philips, % | 241 (11.6%) | 240 (15.0%) | 125 (12.9%) | 606 (13.0%) |
| General Electric, % | 354 (17.1%) | 340 (21.2%) | 247 (25.4%) | 942 (20.3%) |
| PLEs (4649) | ||||
| Total PLEs | 0±0 | 2.548±2.101 | 6.180±3.686 | 2.170±3.142 |
| Significantly Distressing PLEs | 0±0 | 0±0 | 2.158±1.470 | 0.451±1.105 |
| PGS (4650) | ||||
| Schizophrenia | 4.867x10−7±1.768x10−7 | 4.800x10−7±1.753x10−7 | 5.033x10−7±1.756x10−7 | 4.879x10−7±1.762x10−7 |
| EDU | −1.387x10−7±9.044x10−8 | −1.553x10−7±9.349x10−8 | −1.638x10−7±9.203x10−8 | −1.497x10−7±9.238x10−8 |
| PLE | −4.498x10−9±6.846x10−8 | −4.711x10−9±6.717x10−8 | 3.910x10−10±6.879x10−8 | −3.553x10−9±6.810x10−8 |
| Cross-disorder | 3.030x10−7±8.376x10−8 | 3.029x10−7±8.658x10−8 | 3.180x10−7±8.447x10−8 | 3.061x10−7±8.508x10−8 |
| Birth-weight | −3.611x10−9±8.670x10−8 | −3.612x10−9±8.536x10−8 | −7.710x10−9±8.250x10−8 | −4.478x10−9±8.537x10−8 |
| Inflammation | 2.355x10−8±1.135x10−7 | 2.722x10−8±1.113x10−7 | 3.132x10−8±1.127x10−7 | 2.641x10−8±1.126x10−7 |
| Global Brain Metrics | ||||
| Intracranial Volume (3914) | 1.550x106±1.270x105 | 1.550x106±1.313x105 | 1.534x106±1.344x105 | 1.547x106±1.302x105 |
| Total Cortical Volume (4520) | 6.147x105±5.143x104 | 6.122x105±5.218x104 | 6.070x105±5.328x104 | 6.122x105±5.216x104 |
| Total Subcortical Volume (4520) | 6.140x104±4.550x103 | 6.134x104±4.612x103 | 6.070x104±4.824x103 | 6.123x104±4.637x103 |
| Total Cortical Thickness (4520) | 2.801±0.089 | 2.798±0.092 | 2.794±0.089 | 2.798±0.090 |
| Total Surface Area (4520) | 1.907x105±1.662x104 | 1.902x105±1.648x104 | 1.885x105±1.655x104 | 1.901x105±1.658x104 |
| Proximal PGS Behaviors | ||||
| Cognitive Scores (4581) | 90.076±7.659 | 88.412±7.848 | 86.774±7.968 | 88.814±7.893 |
| General Psychopathology Scores (3120) | −0.064±0.868 | 0.056±0.867 | 0.292±0.938 | 0.050±0.892 |
| Birthweight in ounces (4546) | 114.275±23.502 | 113.244±22.946 | 111.077±24.362 | 113.258±23.518 |
Abbreviations: PLEs=psychotic-like experiences; PGS=polygenic scores; EDU=Educational Attainment.
Means ± standard deviations, unless otherwise noted.
Proximal EDU, Birth-weight, and Cross-Disorder PGS Behaviors.
Total cognition composite scores assessed using the National Institutes of Health Toolbox Cognitive Battery (30), caregiver-reported child birthweight (Supplement for birth complications and gestational age), and a general psychopathology factor (31) created using the Child Behavior Checklist (32), served as proximal behavioral measures of EDU, birthweight, and cross-disorder PGS, respectively.
Brain Structure.
T1- and T2-weighted structural scans (1mm isotropic) were acquired using 3T scanners (either Siemens, General Electric, or Phillips) with 32-channel head coils (Supplement). The following structural MRI metrics were examined: global: intracranial, total cortical, and total subcortical volume; total surface area; total cortical thickness; regional: 34 Desikan cortical regions for surface area, thickness, and volume, as well as 23 Freesurfer segmentation subcortical volume regions (33).
Polygenic Scores.
Summary statistics from the most well-powered “discovery” GWASs of schizophrenia (N=69,369 cases + 236,642 controls) (34), cross-disorder (N=232,964 cases [anorexia nervosa, attention-deficit/hyperactivity disorder, autism spectrum disorder, bipolar disorder, major depressive disorder, obsessive-compulsive disorder, schizophrenia, and Tourette’s syndrome] + 494,162 controls) (16), PLEs (N=127,966) (6), EDU (N=766,345; Supplement for executive functioning PGS; results generally consistent) (35), birthweight (N=321,223) (36), and inflammation (N=469,856; http://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=30710), were used to generate PGS (n=6) within the ABCD Study® dataset. Summary statistics from a schizophrenia GWAS study with individuals of African ancestries were used for our exploratory analyses (Supplement) (37).1 PGSs were generated using polygenic risk scores-continuous shrinkage (PRS-CS) (38), which uses a Bayesian regression framework to include all SNPs in PGS calculation by placing a continuous shrinkage prior on SNP effect sizes; simulation studies show that PRS-CS outperforms other PGS methods (38). Analyses using traditional p-value clumping and thresholding produced results consistent with PRS-CS (Supplemental Tables 2-3).
Statistical Analyses
Continuous predictor and outcome variables were Winsorized to ±3 SD to minimize the influence of extreme values. Analyses nested data with random intercepts for site (n=22) and family (n=3,874; siblings n=616). The following covariates were included in all analyses: age, sex (Supplemental Table 4 for results stratified by sex), genotyping batch, and the first ten ancestrally-informative principal components (described in the Supplement; Supplemental Table 5 for associations with PLEs). We used ComBat harmonization (https://github.com/ncullen93/neuroCombat), with age and sex added as biological covariates to the design matrix, to estimate and remove scanner model effects from MRI measures. Further, intracranial volume was included as an additional covariate in regional brain structure analyses. Financial adversity and highest parental/caregiver education were included as additional covariates in supplemental analyses (Supplement), as these variables are proxies of socioeconomic status (39) and therefore important predictors in risk for psychopathology and cognitive functioning.
First, we used hierarchical linear models (HLMs) to estimate associations between schizophrenia, PLE, cross-disorder, EDU, birth-weight, and inflammation PGS and each PLE metric (i.e., dimensional total score and binary significantly distressing PLEs) as outcomes. Benjamini-Hochberg False Discovery Rate (FDR) correction was used to account for the six PGSs tested.2 For cross-disorder, birth-weight, and EDU, post-hoc HLMs examined models including measured behaviors most proximal to the PGS, to examine the extent to which available measured outcomes proximal to PGSs accounted for these associations (e.g., the extent to which cognition accounted for the association between EDU PGS and PLEs).
Second, we estimated associations between reported PLEs and MRI-derived brain structure phenotypes. FDR was used to adjust for multiple testing of 5 global MRI metrics (e.g., total cortical thickness), as well as 102 regional metrics (i.e., 34 each for bilateral [i.e., averaged across hemispheres] cortical thickness, cortical surface area, and cortical volume), and 23 tests for bilateral subcortical volumes (e.g., hippocampal volume), for a total of 130 FDR corrections for each PLE metric. Any significant regional association with PLEs was followed up with post hoc testing for lateral (i.e., right, left) associations.
Third, we estimated associations between PGS and brain structure phenotypes associated with reported PLEs. Subsequently, we examined whether any brain structure and PGS proximal behavioral phenotypes (e.g., cognition) indirectly linked PGS to reported PLEs using a series of mediation analyses.
Default settings were used to conduct HLMs for total PLEs (lmer) and hierarchical logistic regressions for significantly distressing PLEs (glmer) using the lme4 package (34). MuMIn was used to calculate pseudo-R-squared, with results comparing pseudo-R2 for models with and without the predictor of interest (e.g., PRS score) converted to a percentage to create a %ΔR2. The lavaan package (40) was used to conduct mediation analyses, with models incorporating clustering and bootstrapping commands.
Results
Polygenic Scores and Psychotic-like Experiences
In our sample (n=4,650), 55.33% (n=2,573) of children endorsed at least one reported PLE and 20.90% (n=972) endorsed at least one significantly distressing PLE. Total PLEs were associated with higher cross-disorder and lower EDU PGS (all ∣βs∣>0.045, all ps<0.002, pFDRs<0.006; %ΔR2s>0.20%), but not schizophrenia, PLE, or birthweight PGS (all ∣βs∣≤0.023, all ps>0.11); After correction for multiple testing, there was a trend for a positive association between Total PLEs and inflammation PGS (β=0.031, p=.03, pFDR=0.06, %ΔR2=0.092%; Table 2; Figure 1a).
Table 2.
Associations between PLEs and both PGS and Structural Neural Metricsa
| Metric | Total PLEs | Significant Distress Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b [95% CI] | β | t | p | p FDR | %ΔR2 | OR [95% CI] | β | Z | p | p FDR | %ΔR2 | |
| PGS | ||||||||||||
| Schizophrenia | 4.17x105 [−9.35x104,9.27x105] | 0.023 | 1.598 | .11 | .17 | 0.052% | 1.130 [1.050,1.220] | 0.043 | 3.166 | .002 | .01 | 0.216% |
| Educational Attainment | −2.76x106 [3.74x106,−1.79x106] | −0.081 | −5.561 | 2.85x10−8 | 1.71x10−7 | 0.660% | 0.825 [0.766,0.890] | −0.072 | −4.988 | 6.09x10−7 | 3.65x10−6 | 0.587% |
| PLEs | 6.21x105 [−6.93x105,1.94x106] | 0.013 | 0.925 | .36 | .36 | 0.019% | 1.090 [1.010,1.170] | 0.032 | 2.259 | .02 | .03 | 0.120% |
| Cross-disorder | 1.67x106 [6.18x105,2.73x10] | 0.045 | 3.099 | .002 | .006 | 0.202% | 1.190 [1.110,1.290] | 0.066 | 4.600 | 4.23x10−6 | 1.27x10−5 | 0.505% |
| Birth weight | −5.17x105 [−1.57x106,5.40x105] | −0.014 | −0.96 | .34 | .36 | 0.021% | 0.954 [0.885,1.030] | −0.016 | −1.23 | .22 | .22 | 0.037% |
| Inflammation | 8.62x105 [6.54x104,1.66x106] | 0.031 | 2.12 | .03 | .06 | 0.092% | 1.050 [0.973,1.130] | 0.018 | 1.228 | .22 | .22 | 0.036% |
| Neural Metrics | ||||||||||||
| Global Metrics | ||||||||||||
| Intracranial Volume | −1.56x10−6 [−2.36x10−6,−7.59x10−7] | −0.065 | −3.815 | 1.38x10−4 | 5.98 x10−3 | 0.332% | 0.841 [0.770,0.919] | −0.063 | −3.851 | 1.18E-04 | 5.11x10−3 | 0.371% |
| Total Cortical Volume | −4.12 x10−6 [−6.06x10−6, −2.18x10−6] | −0.068 | −4.169 | 3.12x10−5 | 4.06 x10−3 | 0.388% | 0.845 [0.776,0.920] | −0.062 | −3.898 | 9.72E-05 | 5.11x10−3 | 0.370% |
| Total Subcortical Volume | −3.93x10−5 [−6.09x10−5, −1.76x10−5] | −0.058 | −3.555 | 3.82x10−4 | 1.24 x10−2 | 0.291% | 0.823 [0.756,0.895] | −0.071 | −4.519 | 6.21E-06 | 8.07x10−4 | 0.503% |
| Total Cortical Thickness | −0.908 [−1.918,0.105] | −0.026 | −1.760 | .08 | .42 | 0.085% | 0.936 [0.868,1.009] | −0.024 | −1.723 | .08 | .50 | 0.079% |
| Total Surface Area | −1.22x10−5 [−1.85x10−5, −5.97x10−6] | −0.065 | −3.824 | 1.33x10−4 | 5.98 x10−3 | 0.330% | 0.847 [0.777,0.924] | −0.061 | −3.727 | 1.94E-04 | 6.31x10−3 | 0.343% |
| Regional Metrics | ||||||||||||
| Average Inferior Parietal Thickness | −1.270 [−2.040, −0.492] | −0.048 | −3.206 | 1.35x10−3 | .04 | 0.226% | 0.926 [0.853,1.006] | −0.027 | −1.810 | .07 | .50 | 0.078% |
| Right Inferior Parietal Thickness | −1.150 [−1.880, −0.432] | −0.047 | −3.131 | 1.76x10−3 | 0.214% | 0.927 [0.854,1.007] | −0.027 | −1.798 | .07 | 0.077% | ||
Abbreviations: PLEs=psychotic-like experiences; PGS=polygenic scores; b=unstandardized beta coefficient; 95% CI=95% confidence interval; β=standardized beta coefficient; t=t-test test statistic; p=p−value; pFDR=False Discovery Rate-corrected p; %ΔR2=percentage change in marginal proportion of variance explained, calculated as the difference in pseudo-R2 between the current model versus a model excluding the predictor of interest, converted to a percentage; OR=odds ratio; Z=Z statistic.
pFDR<.05 models are in bold. Tests are two-tailed. All PGS (i.e., schizophrenia, educational attainment, cross-disorder, PLE) results remained consistent with accounting for financial adversity and parental education.
Figure 1.
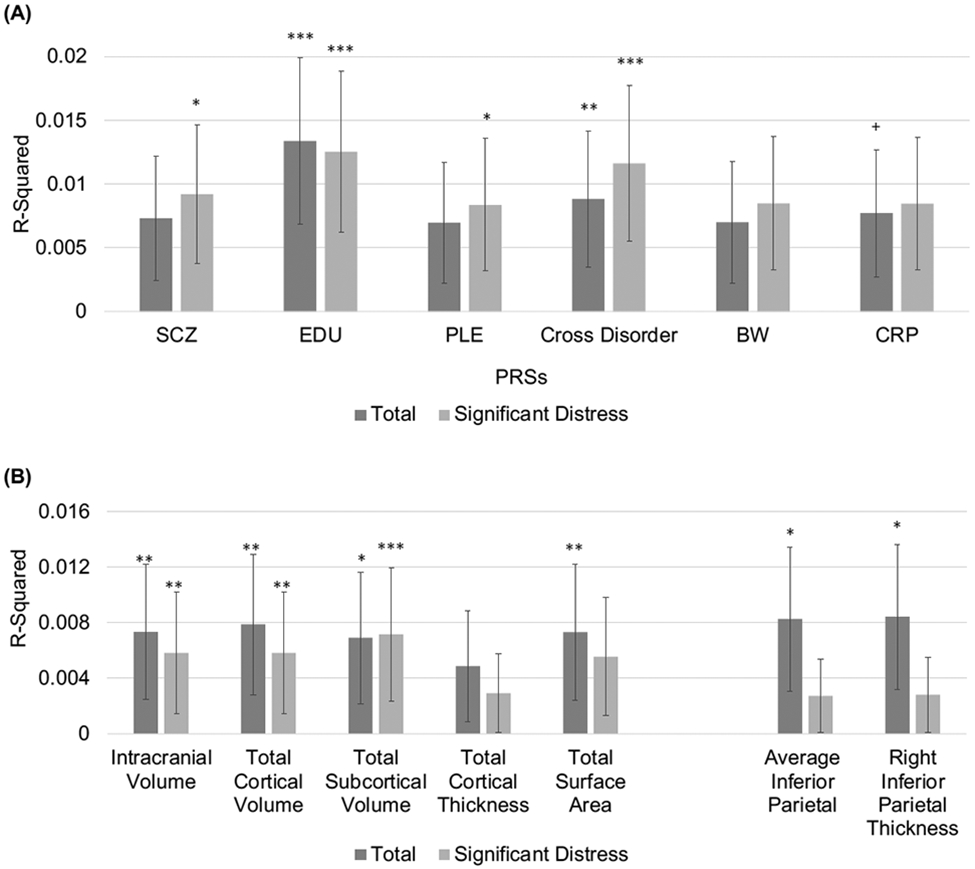
Proportion of variance explained (R-squared) by (A) each of the different PGS scores (SCZ=Schizophrenia; EDU =Educational Attainment; PLE=Psychotic-like experiences; CROSS=Cross Disorder; BW= Birth weight; CRP = Inflammation) for both Total and Significantly Distressing PLEs, and (B) each of the significant global (intracranial volume, total cortical volume, total subcortical volume, surface area) and regional (inferior parietal thickness) MRI metrics for both Total and Significantly Distressing PLEs. Error bars represent the 95% confidence interval, * pFDR<0.05, ** pFDR<0.01, *** pFDR<0.001.
Endorsement of Significantly Distressing PLEs was associated with higher schizophrenia, PLE, and cross-disorder PGS as well as lower EDU PGS (all ∣βs∣>0.032, all ps≤0.02, pFDRs<0.03; %ΔR2s≥0.12%; Table 2; Figure 1a), but not birthweight or inflammation PGS (all ∣βs∣≤0.018, all ps>0.22). All associations remained similar when accounting for financial adversity and parental/caregiver education (Supplemental Table 6), or when computing PGS using a traditional clustering and thresholding approach (Supplemental Tables 2-3; Supplement for executive functioning PGS results).
Comparing PLE Groups (i.e., no PLEs, PLEs without significant distress, significantly distressing PLEs) generally revealed a pattern of results suggesting a gradient of severity (Supplement; Table 1); those reporting significantly distressing PLEs showed the greatest divergence from those without PLEs on PGSs, brain structure, and behavior, while those reporting PLEs not associated with significant distress were intermediary between these two groups (Supplemental Table 1).
Consideration of Proximal PGS Behaviors
Measured cognition was negatively associated with Total PLEs and endorsement of Significantly Distressing PLEs, and positively associated with EDU PGS (βs≥−0.131, all ps<2.00x10−16, %ΔR2s≥1.55%; Supplemental Table 7; Supplemental Figures 2-3). Cognitive performance accounted for 32.3-32.8% of the association between EDU PGS and PLE metrics (Supplemental Table 8), though associations between EDU PGS and reported PLE metrics remained, even when considering cognition (∣βs∣>0.047, ps<0.002; %ΔR2s≥0.58%; Supplemental Table 9). The general psychopathology factor was positively associated with Total PLEs and endorsement of Significantly Distressing PLEs, as well as cross-disorder PGS (all βs≥0.055, all ps≤0.002, %ΔR2s>0.29%; Supplemental Table 7; Supplemental Figures 2-3). General psychopathology accounted for 11.3-17.8% of the association between cross-disorder PGS and reported PLEs, though associations between cross-disorder PGS with reported PLEs remained, even when considering general psychopathology (Total: β=0.034, p=0.048, ΔR2=0.24%; Significantly Distressing PLEs: β=0.056, p=0.002, %ΔR2=0.72%; Supplemental Table 9). Although PLEs were not significantly associated with birth-weight PGS (Table 1), reported birthweight was associated with PLEs and birth-weight PGS (∣βs∣≥0.047, all ps≤.003, %ΔR2s>0.25%; Supplemental Table 7; Supplemental Figure 2).
Psychotic-like Experiences and Brain Structure
Global Metrics.
Greater reported PLEs (both Total and endorsement of Significantly Distressing PLEs) were associated with lower intracranial volume, total cortical volume, total subcortical volume, and total surface area (all ∣βs∣>0.058, all ps<3.82x10−4, all pFDRs<1.24x10−2; all %ΔR2s>0.29%; Table 2; Figure 1b), but not cortical thickness (all ∣βs∣<0.026, all ps>0.08). Regional Metrics. When examining individual structural MRI regions for volume, surface area, and thickness, greater Total PLEs were associated with lower bilateral (pFDR=0.04) and right inferior parietal cortical thickness (both ∣βs∣>0.047, both ps<1.76x10−3, both %ΔR2s>0.21%; Table 2; Figure 1b). No other individual structural MRI regions passed FDR correction (see Supplemental Tables 10-13).
Mediation Analyses
Educational Attainment Polygenic Scores.
EDU PGS was positively associated with all brain structure phenotypes linked to reported PLEs (i.e., intracranial volume, total cortical volume, total subcortical volume, total cortical surface area; all βs>0.069, all ps<4.28x10−7; Table 3), except inferior parietal cortical thickness (all ∣βs∣<0.027, all ps>0.08). A series of individual mediational models examined whether each brain structure phenotype associated with both EDU PGS and PLEs (i.e., intracranial volume, total cortical volume, total subcortical volume, total cortical surface area) indirectly linked EDU PGS to Total PLEs alongside cognitive performance in parallel. There was evidence consistent with all volume metrics partially mediating the association between EDU PGS and Total PLEs (all indirect effect [path a*b] bias-corrected 95% confidence intervals [CI] within −0.012 to −0.001; proportion mediated: 3.33%-8.79%; Figure 2a-c). Specifically, evidence was consistent with lower educational attainment PGS being associated with reduced volumes, which were, in turn, associated with higher PLEs. There was no evidence consistent with total surface area indirectly linking EDU PGS to Total PLEs (indirect effect 95% CI: −0.004 to 0.002).
Table 3.
Associations between PGS and Structural Neural Metricsa
| Metric | Educational Attainment PGS | Cross-disorder PGS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| b [95% CI] | β | t | p | %ΔR2 | b [95% CI] | β | t | p | %ΔR2 | |
| Global Structural Metrics | ||||||||||
| Intracranial Volume | 1.27x1011 [9.10x1010, 1.63x1011] | 0.090 | 6.908 | 5.61x10−12 | 0.819% | 2.21x1010 [−1.69x1010, 6.10x1010] | 0.014 | 1.112 | .27 | 0.010% |
| Total Cortical Volume | 5.49x1010 [4.01x1010, 6.99x1010] | 0.097 | 7.219 | 6.14x10−13 | 0.949% | 1.45x1010 [−1.68x109, 3.06x1010] | 0.024 | 1.759 | .08 | 0.040% |
| Total Subcortical Volume | 3.45x109 [2.12x109, 4.79x109] | 0.069 | 5.064 | 4.28 x10−7 | 0.460% | 4.70x108 [−9.79x108, 1.91x109] | 0.009 | 0.637 | .52 | 0.000% |
| Total Cortical Thickness | −1.71x103 [−3.05x104, 2.72 x104] | −0.002 | −0.116 | .91 | 0.000% | −1.42x104 [−4.54x104, 1.70x104] | −0.013 | −0.889 | .37 | 0.017% |
| Total Surface Area | 1.72x1010 [1.26x1010, 2.19x1010] | 0.096 | 7.343 | 2.48 x10−13 | 0.923% | 8.15x109 [3.16x109, 1.31x1010] | 0.042 | 3.207 | .001 | 0.142% |
| Regional Metrics | ||||||||||
| Average Inferior Parietal Thickness | −1.56x104 [−5.36x104, 2.23x104] | −0.012 | −0.806 | .42 | 0.013% | −5.73x103 [−4.68x104, 3.54x104] | −0.004 | −0.273 | .79 | 0.001% |
| Right Inferior Parietal Thickness | −3.68x104 [−7.73x104, 3.73x103] | −0.027 | −1.776 | .08 | 0.068% | −1.40x104 [−5.79x104, 2.99x104] | −0.009 | −0.626 | .53 | 0.008% |
Abbreviations: PLEs=psychotic-like experiences; PGS=polygenic scores; b=unstandardized beta coefficient; 95% CI=95% confidence interval; β=standardized beta coefficient; t=t-test test statistic; p=p−value; %ΔR2=percentage change in marginal proportion of variance explained, calculated as the difference in pseudo-R2 between the current model versus a model excluding the predictor of interest, converted to a percentage; OR=odds ratio; Z=Z statistic.
pFDR<.05 models are in bold. Tests are two-tailed.
Figure 2.
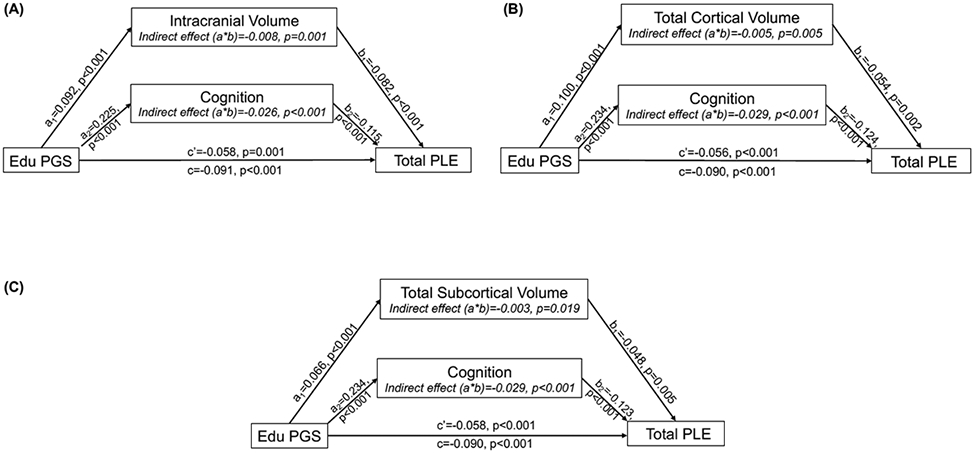
Depiction of a series of parallel mediation models, examining evidence for each neural metric ((A) intracranial, (B) cortical volume, and (C) subcortical volume) indirectly linking Educational Attainment PGS (EDU PGS) and total psychotic-like experiences (Total PLEs) alongside cognitive performance in parallel. Covariates (i.e., age, sex, genotyping batch, and the first ten ancestrally-informative PCs) were included in all models. Each parallel mediation model depiction includes unstandardized regression coefficients, showing the association between EDU PGS, each neural metric and cognition, and Total PLEs.
There was also evidence consistent with cognition uniquely partially mediating the association between EDU PGS and Total PLEs in each model (all within 95% CI: −0.038 to −0.020; proportion mediated: 28.57-32.22%; Figure 2). Specifically, lower cognitive performance indirectly linked lower EDU PGS to greater PLEs.
For all models, similar results were found when endorsement of Significantly Distressing PLEs was the outcome instead of Total PLEs (Supplement; Supplemental Figure 4). Mediation models remained consistent when accounting for financial adversity (Supplement). A single parallel mediation model with all neural metrics entered as simultaneous parallel mediators is reported in the Supplement.
Other Polygenic Scores.
Schizophrenia, PLE, birthweight, and inflammation PGS were not associated with global or regional brain structure measures associated with reported PLEs (Supplemental Table 14). Although total surface area was associated with cross-disorder PGS (β=0.042, p=0.001, %ΔR2=0.14; Table 3), there was not strong evidence consistent with total surface area mediating associations between cross-disorder PGS and reported PLEs (95% CI: −0.005 to 0.000; Supplement).
Exploratory Analyses: African-American Ancestral subsample.
There was no association between schizophrenia PGS and PLEs among individuals with African ancestry (Supplement).
Discussion
Here, we show that psychotic-like experiences (PLEs) in middle childhood (n=4,650) are associated with GWAS-derived polygenic scores (%ΔR2s=0.120-0.660%) as well as putative intermediary neural and behavioral phenotypes that may partially underlie these associations (all %ΔR2s=0.21-2.85%). Genomic liability for broad-spectrum psychopathology (cross-disorder PGS) and educational attainment (EDU PGS) were associated with both PLEs measures; however, schizophrenia and late-life PLE PGS were only significantly associated with the presence of distressing PLEs. Consistent with these findings, group contrasts revealed that SCZ PGS among those experiencing significantly distressing PLEs was significantly higher than those reporting PLEs without significant distress (Supplemental Table 1). One possible explanation is that PLEs may portend broad psychopathology vulnerability (1), while significantly distressing PLEs may more specifically foreshadow psychosis risk (41). Finally, reported PLEs were associated with lower global (e.g., intracranial volume) and regional (i.e., inferior parietal thickness was associated with broad PLEs) brain structure metrics (Figure 1b), with evidence that lower volume (i.e., intracranial volume, total cortical and total subcortical) may indirectly link EDU PGS to reported PLEs alongside cognition (Figure 2). Collectively, these results show that polygenic scores derived from adult GWASs can generalize to indices of risk among children and provide incremental predictive usefulness beyond measured proximal phenotypes.
Polygenic Propensity for Education Attainment
Polygenic scores for lower educational attainment was the most robust PGS predictor of reported PLEs.3 These findings suggest that prior reports linking EDU PGS to severe psychosis among clinical patients (42) may generalize to earlier markers of psychosis spectrum symptoms during middle childhood. That cognition accounted for a large portion (28.57-32.22% of variance) of the association between EDU PGS and reported PLEs aligns with evidence that premorbid cognition prospectively predicts PLEs and psychopathology, including schizophrenia (21), and suggests that such vulnerability may be partially genomic in origin. However, other work has failed to find a strong genetic correlation between later-life PLEs and intelligence (6); it is possible that genomic associations between PLEs and cognition may differ across the life course and be more correlated during childhood with divergence in later life (e.g., PLEs related to cognitive decline and/or dementia (43)). Notably, executive functioning PGS generally showed similar patterns compared with models including EDU PGS, although executive functioning PGS showed weaker effects with neural metrics (Supplement). In addition to PGS, it is also likely that there were a number of additional influences on cognitive performance that were not included in this study, including additional pathophysiological factors (e.g., functional connectivity) and environmental influences (e.g., exposure to toxins).
Supportive of neurodevelopmental models of psychosis spectrum disorders positing that brain differences underlie cognition-related vulnerability for schizophrenia, we found evidence consistent with brain volume accounting for a portion of the association between educational attainment PGS and reported PLEs (3.22-8.79%; Supplemental Table 7; Figure 2). However, given that neural metrics are likely less proximal to EDU PGS than cognition, it is unsurprising that associations for cognition were larger than associations for brain structure (44). Associations between reported PLEs and global volume reductions are consistent with previous research examining volumetric alterations (22). That lower global volume may indirectly link EDU PGS and reported PLEs is consistent with the notion that genomic liability for lower cognitive functioning may be associated with altered neural maturational processes, which may contribute to the development or maintenance of psychosis spectrum symptoms (45).
General Psychopathology, Schizophrenia, and PLE Polygenic Risk
Genomic liability to general psychopathology (i.e., cross-disorder PGS) was associated with broadly defined (i.e., total) and severe (i.e., significantly distressing) PLEs, while polygenic scores for schizophrenia and PLEs were only associated with severe PLEs. These findings align with evidence that polygenic and phenotypic psychopathology associations may be non-specific in middle childhood and potentially become increasingly specific with increased severity (e.g., with more clinically significant PLEs) and/or maturation (e.g., in adolescence) (46).
We did not find strong evidence of associations between broadly defined PLEs and polygenic risk for later life PLEs, although there were associations between later life PLEs PGS and more severe PLEs. Alongside evidence of similar effect size estimates across both PLE measures within PGS, it is plausible that with better powered discovery GWASs and target samples, PLEs defined broadly and with greater severity will show similar relationships.
Genomic Propensity for Birth Weight and Inflammation
Reported birthweight was negatively associated with PLEs during middle childhood, consistent with research in young adults (47), and positively associated with birthweight PGS; however, birthweight PGS were not associated with reported PLEs. Together, this raises the intriguing possibility that environmental factors associated with lower birthweight, as opposed to genetic predisposition to low birthweight, may underlie the association between lower birthweight and PLEs (48).
There were nominally significant associations between inflammation PGS and total reported PLEs, although this was trend-level after adjusting for multiple testing. Inflammation has been widely associated with psychopathology, with emerging evidence suggesting that inflammation-driven variation in neurodevelopment (i.e., neural pruning during puberty) may play a prominent role in the etiology of schizophrenia (18). It is possible that associations between inflammation PGS and the expression of PLEs may increase following periods of heightened neural development (e.g., adolescence) and/or in interaction with other factors (e.g., infection) that will require larger samples to address.
Limitations
It is important to consider limitations of this study while interpreting these findings. First, the generalizability of these findings is limited, as we restricted most analyses to individuals of European ancestries due to the sample compositions of the discovery GWASs and evidence that polygenic risk does not translate across ancestries (49). Due to this exclusion and exclusion for quality control reasons, a number of participants (n=7,225) were not included in analyses. Excluded participants showed higher scores than included participants on a number of measures (Supplemental Table 15), and therefore, if anything, would have contributed to the clinical severity of this sample. Second, and consistent with expectations from the ABCD Study® (28), which uses a heterogeneous sample, and from prior PGS studies (44), the effects reported are generally small (for associations with reported PLEs, ∣βs∣<0.09, %ΔR2<0.7%) (50). Third, prevalence rates of PLEs (e.g., 55.3% for total PLEs) were higher than some previous estimates (i.e., ~10%) (1), although consistent with others (51). This high rate of endorsement may reflect over-endorsement or transient phenomena related to assessing PLEs in middle childhood that may have diluted the magnitude of associations found in the current study. Total PLEs are unlikely useful as a clinical indicator, although may be useful as a measure of the dimension of PLEs, including developmentally normative experiences and trait-relevant phenomena (e.g., oddness). Regardless, the PQ-BC, including Total scores, has been validated for use with children as young as age 9 (4), and there is evidence that only people with high trait levels of PLEs typically endorse PQ-BC items (52). Fourth, while the non-experimental and cross-sectional nature of our data does not preclude conducting mediation analyses (53), they should not be interpreted by themselves to imply causation. However, these analyses provide some empirical evidence consistent with putative gene-brain-behavior mechanisms underlying childhood PLE risk. Fifth, it is important to note that the GWASs have differential power to detect effects (e.g., the EDU GWAS was based on 766,345 individuals, whereas the PLEs GWAS was based on 127,966 individuals). These differences in power present challenges for interpreting across different PGSs. As “discovery” GWASs continue to grow, it will be critical to acquire additional GWASs datasets across development, including in childhood, to examine genetic correlations for the same phenotype across ages as well as differential associations with psychopathology and structural neural metrics. Future studies using the ABCD Study dataset should further examine the validity of the PGS scores including validity in other populations, and also examine potential clinically applicable thresholds (54). Finally, future research should begin to examine associations between PLEs, PGS, and other factors previously found to be associated with PLEs in the ABCD Study® (e.g., environmental toxins) (23).
Conclusions
Broadly, GWAS-based polygenic scores for psychopathology and EDU generated from adult samples are associated with indices of PLEs during middle childhood. Polygenic propensity to EDU was the most robust predictor of reported PLEs, and there was evidence that these associations may be partially mediated by cognitive performance and brain structure. Polygenic score associations mirrored phenotypic evidence that broadly defined PLEs are associated with broad-spectrum psychopathology risk while more severe PLEs may index psychosis liability. Taken together, this study documents that GWAS-derived polygenic scores index psychopathology and psychosis vulnerability in children. PGSs may support the identification of putative intermediate biological and behavioral mechanisms through which genomic risk for psychopathology emerges. Although there are a number of important ethical considerations with regards to using PGS for prediction purposes, including concerns about early identification efforts and the potential for exacerbating health disparities that must be addressed prior to clinical use (55), more severe PLEs may be important early indicators of psychosis liability. Therefore, future research should begin to examine whether severe PLEs can be utilized as markers for further assessment and potential intervention.
Supplementary Material
Acknowledgments
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 11,500 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041025, U01DA041028, U01DA041048, U01DA041089, U01DA041093, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI 10.15154/1460410.
The authors thank Drs. Michelini and Kotov for the creation of the general psychopathology scores. The authors also thank Dr. Tim Bigdeli for access to summary statistics for the schizophrenia GWAS in individuals of African ancestry. A version of this manuscript is available via preprint on medRxiv: https://www.medrxiv.org/content/10.1101/2020.07.14.20153551v1
Funding/Support:
This work was supported by National Institute of Health grants U01 DA041120 (DMB), K23MH121792, L30MH120574 (NRK), MH109532, DA032573 (AA), F32 AA027435 (ECJ), T32-DA007261 (ASH), R01-AG045231, R01-HD083614, R01-AG052564, R21-AA027827, R01-DA046224 (RB).
Footnotes
Conflict of Interest Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
As we are unaware of any other GWAS of African ancestry for the GWAS phenotypes investigated here, no other PGS were generated for this sample. Due to the relatively small sample of other ancestries in the ABCD dataset, we did not explore PGS in any other ancestries.
Given the strong correlation (r=.66) between PLE total score and PLE significant distress group, we did not adjust for multiple testing for these 2 phenotypes.
Additionally, associations between between EDU PGS and PLEs remained even when accounting for anhedonia (Supplement).
References
- 1.Healy C, Brannigan R, Dooley N, Coughlan H, Clarke M, Kelleher I, et al. (2019): Childhood and adolescent psychotic experiences and risk of mental disorder: a systematic review and meta-analysis. Psychol Med. 49:1589–1599. [DOI] [PubMed] [Google Scholar]
- 2.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H (2000): Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 57:1053–1058. [DOI] [PubMed] [Google Scholar]
- 3.Karcher NR, O'Brien KJ, Kandala S, Barch DM (2019): Resting-State Functional Connectivity and Psychotic-like Experiences in Childhood: Results From the Adolescent Brain Cognitive Development Study. Biol Psychiatry. 86:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karcher NR, Barch DM, Avenevoli S, Savill M, Huber RS, Simon TJ, et al. (2018): Assessment of the Prodromal Questionnaire-Brief Child Version for Measurement of Self-reported Psychoticlike Experiences in Childhood. JAMA Psychiatry. 75:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karcher NR, Loewy RL, Savill M, Avenevoli S, Huber RS, Simon TJ, et al. (2020): Replication of Associations with Psychotic-like Experiences in Middle Childhood from the Adolescent Brain Cognitive Development (ABCD) study. Schizophrenia Bulletin Open. 1:sgaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legge SE, Jones HJ, Kendall KM, Pardiñas AF, Menzies G, Bracher-Smith M, et al. (2019): Association of Genetic Liability to Psychotic Experiences With Neuropsychotic Disorders and Traits. JAMA Psychiatry. 76:1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdan R, Baranger DA, Agrawal A (2018): Polygenic risk scores in clinical psychology: bridging genomic risk to individual differences. Annual review of clinical psychology. 14:119–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(2011): Genome-wide association study identifies five new schizophrenia loci. Nature genetics. 43:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M, et al. (2016): Phenotypic Manifestation of Genetic Risk for Schizophrenia During Adolescence in the General Population. JAMA Psychiatry. 73:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieradzka D, Power RA, Freeman D, Cardno AG, McGuire P, Plomin R, et al. (2014): Are genetic risk factors for psychosis also associated with dimension-specific psychotic experiences in adolescence? PLoS One. 9:e94398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zammit S, Hamshere M, Dwyer S, Georgiva L, Timpson N, Moskvina V, et al. (2014): A population-based study of genetic variation and psychotic experiences in adolescents. Schizophr Bull. 40:1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature. 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pain O, Dudbridge F, Cardno AG, Freeman D, Lu Y, Lundstrom S, et al. (2018): Genome-wide analysis of adolescent psychotic-like experiences shows genetic overlap with psychiatric disorders. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 177:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor JH, Asabere N, Calkins ME, Moore TM, Tang SX, Xavier RM, et al. (2019): Characteristics of youth with reported family history of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins DO, Olde Loohuis L, Barbee J, Ford J, Jeffries CD, Addington J, et al. (2020): Polygenic Risk Score Contribution to Psychosis Prediction in a Target Population of Persons at Clinical High Risk. Am J Psychiatry. 177:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee PH, Anttila V, Won H, Feng Y-CA, Rosenthal J, Zhu Z, et al. (2019): Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 179:1469–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abel KM, Wicks S, Susser ES, Dalman C, Pedersen MG, Mortensen PB, et al. (2010): Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry. 67:923–930. [DOI] [PubMed] [Google Scholar]
- 18.Kroken RA, Sommer IE, Steen VM, Dieset I, Johnsen E (2018): Constructing the Immune Signature of Schizophrenia for Clinical Use and Research; An Integrative Review Translating Descriptives Into Diagnostics. Frontiers in psychiatry. 9:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gage SH, Hickman M, Zammit S (2016): Association Between Cannabis and Psychosis: Epidemiologic Evidence. Biol Psychiatry. 79:549–556. [DOI] [PubMed] [Google Scholar]
- 20.Socrates A, Maxwell J, Glanville KP, Di Forti M, Murray RM, Vassos E, et al. (2021): Investigating the effects of genetic risk of schizophrenia on behavioural traits. NPJ schizophrenia. 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheffield JM, Karcher NR, Barch DM (2018): Cognitive Deficits in Psychotic Disorders: A Lifespan Perspective. Neuropsychology review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satterthwaite TD, Wolf DH, Calkins ME, Vandekar SN, Erus G, Ruparel K, et al. (2016): Structural Brain Abnormalities in Youth With Psychosis Spectrum Symptoms. JAMA Psychiatry. 73:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karcher NR, Schiffman JE, Barch DM (2021): Environmental Risk Factors and Psychotic-Like Symptoms in Children Aged 9-10. J Am Acad Child Adolesc Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alloza C, Blesa-Cábez M, Bastin ME, Madole JW, Buchanan CR, Janssen J, et al. (2020): Psychotic-like experiences, polygenic risk scores for schizophrenia, and structural properties of the salience, default mode, and central-executive networks in healthy participants from UK Biobank. Translational psychiatry. 10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, et al. Special Issue: Adolescent Brain and Cognitive Development Demographic, Physical and Mental Health Assessments in the Adolescent Brain and Cognitive Development Study: Rationale and Description. Developmental Cognitive Neuroscience.In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Compton WM, Dowling GJ, Garavan H (2019): Ensuring the Best Use of Data: The Adolescent Brain Cognitive Development Study. JAMA pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, et al. (2018): Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci. 32:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karcher NR, Barch DM (2020): The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology.1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karcher NR, Barch DM, Avenevoli S, Savill M, Huber RS, Simon TJ, et al. (2018): Assessment of the Prodromal Questionnaire-Brief Child Version for Measurement of Self-reported Psychoticlike Experiences in Childhood. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. (2013): Cognition assessment using the NIH Toolbox. Neurology. 80:S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelini G, Barch DM, Tian Y, Watson D, Klein DN, Kotov R (2019): Delineating and validating higher-order dimensions of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study. Translational psychiatry. 9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achenbach TM (2009): The Achenbach System of Emprically Based Assessment (ASEBA): Development, Findings, Theory and Applications. Burlington, VT: University of Vermont Research Center for Children, Youth, and Families. [Google Scholar]
- 33.Hagler DJ Jr, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, et al. (2019): Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage. 202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ripke S, Walters JT, O’Donovan MC (2020): Mapping genomic loci prioritises genes and implicates synaptic biology in schizophrenia. medRxiv.2020.2009.2012.20192922. [Google Scholar]
- 35.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. (2018): Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature genetics. 50:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freathy RM, Mook-Kanamori DO, Sovio U, Prokopenko I, Timpson NJ, Berry DJ, et al. (2010): Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nature genetics. 42:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigdeli TB, Genovese G, Georgakopoulos P, Meyers JL, Peterson RE, Iyegbe CO, et al. (2020): Contributions of common genetic variants to risk of schizophrenia among individuals of African and Latino ancestry. Molecular Psychiatry. 25:2455–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge T, Chen C-Y, Ni Y, Feng Y-CA, Smoller JW (2019): Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nature communications. 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farah MJ (2017): The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron. 96:56–71. [DOI] [PubMed] [Google Scholar]
- 40.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K (2014): Mediation: R package for causal mediation analysis. [Google Scholar]
- 41.Zammit S, Kounali D, Cannon M, David AS, Gunnell D, Heron J, et al. (2013): Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am J Psychiatry. 170:742–750. [DOI] [PubMed] [Google Scholar]
- 42.Dwyer DB, Kalman JL, Budde M, Kambeitz J, Ruef A, Antonucci LA, et al. (2020): An Investigation of Psychosis Subgroups With Prognostic Validation and Exploration of Genetic Underpinnings: The PsyCourse Study. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connors MH, Ames D, Woodward M, Brodaty H (2018): Psychosis and Clinical Outcomes in Alzheimer Disease: A Longitudinal Study. Am J Geriatr Psychiatry. 26:304–313. [DOI] [PubMed] [Google Scholar]
- 44.Bogdan R, Baranger DAA, Agrawal A (2018): Polygenic Risk Scores in Clinical Psychology: Bridging Genomic Risk to Individual Differences. Annual review of clinical psychology. 14:119–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS (2011): Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 36:316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riglin L, Thapar AK, Leppert B, Martin J, Richards A, Anney R, et al. (2019): Using Genetics to Examine a General Liability to Childhood Psychopathology. Behav Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drakesmith M, Dutt A, Fonville L, Zammit S, Reichenberg A, Evans CJ, et al. (2016): Mediation of Developmental Risk Factors for Psychosis by White Matter Microstructure in Young Adults With Psychotic Experiences. JAMA Psychiatry. 73:396–406. [DOI] [PubMed] [Google Scholar]
- 48.Fineberg AM, Ellman LM, Buka S, Yolken R, Cannon TD (2012): Decreased Birth Weight in Psychosis: Influence of Prenatal Exposure to Serologically Determined Influenza and Hypoxia. Schizophrenia Bulletin. 39:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ (2019): Clinical use of current polygenic risk scores may exacerbate health disparities. Nature genetics. 51:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dick AS, Watts AL, Heeringa S, Lopez DA, Bartsch H, Fan CC, et al. (2020): Meaningful Effects in the Adolescent Brain Cognitive Development Study. bioRxiv.2020.2009.2001.276451. [Google Scholar]
- 51.Laurens KR, Hodgins S, Taylor E, Murray R (2011): Is earlier intervention for schizophrenia possible? Identifying antecedents of schizophrenia in children aged 9–12 years. Schizophrenia: The final frontier.19–32. [Google Scholar]
- 52.Karcher NR, Perino MT, Barch DM (2020): An item response theory analysis of the Prodromal Questionnaire-Brief Child Version: Developing a screening form that informs understanding of self-reported psychotic-like experiences in childhood. J Abnorm Psychol. 129:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayes AF (2017): Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications. [Google Scholar]
- 54.Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. (2018): Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nature genetics. 50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palk AC, Dalvie S, de Vries J, Martin AR, Stein DJ (2019): Potential use of clinical polygenic risk scores in psychiatry – ethical implications and communicating high polygenic risk. Philosophy, Ethics, and Humanities in Medicine. 14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


