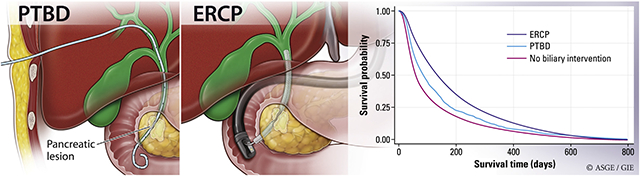
Abstract
Background and Aims:
Most patients with pancreatic cancer are diagnosed at a late stage and are not candidates for surgical resection. Many have jaundice requiring biliary drainage, which can be accomplished using ERCP or percutaneous transhepatic biliary drainage (PTBD). To date, no studies have evaluated the impact of ERCP or PTBD on survival among patients with unresectable pancreatic cancer. The aims of our study were to compare overall survival between patients with unresectable pancreatic cancer receiving ERCP with those receiving PTBD, to compare overall survival between patients who received a biliary intervention (ERCP or PTBD) versus those who received no biliary intervention, and to compare secondary outcomes, such as length of hospital stay and costs, between ERCP and PTBD.
Methods:
We conducted a retrospective cohort study using the Surveillance, Epidemiology, and End Results–Medicare database. Patients with known pancreatic cancer were included if they had a pancreatic head mass and/or evidence of biliary obstruction. We used a time-varying Cox proportional hazards model to estimate overall survival of patients receiving ERCP versus PTBD and overall survival among patients who received a biliary intervention versus no biliary drainage. Secondary outcomes included length of hospital stay, costs, and admissions within 30 days.
Results:
Of 14,808 patients with unresectable pancreatic cancer, 8898 patients (60.0%) underwent biliary drainage and 5910 patients (39.9%) received no biliary intervention. ERCP accounted for most biliary interventions (8271, 93.0%), whereas 623 patients (7.0%) underwent PTBD. In multivariable analysis, ERCP was associated with reduced mortality compared with PTBD (adjusted hazard ratio [aHR], .67; 95% confidence interval [CI], .60-.75). When ERCP or PTBD was compared with no biliary intervention, both procedures were associated with a survival benefit (aHR, .51 [95% CI, .49-.54] and .53 [95% CI, .48-.59], respectively). Compared with patients receiving PTBD, those who underwent ERCP had shorter mean length of hospital stay (7.0 ± 5.7 days vs 9.6 ± 6.6 days, respectively; P < .001) and lower hospital charges ($54,899.25 vs $75,246.00, P < .001) but no significant difference in hospitalization or 30-day readmissions.
Conclusions:
ERCP is associated with reduced mortality compared with PTBD in pancreatic cancer patients, highlighting the critical role of ERCP in the management of biliary obstruction from pancreatic cancer.
GRAPHICAL ABSTRACT
In the past decade, pancreatic cancer incidence has increased and is projected to become the second leading cause of cancer-related deaths by 2030.1-3 With a 5-year survival of only 9%, pancreatic cancer has one of the lowest survival rates among all cancers in the United States.1
By the time patients are symptomatic with jaundice, fatigue, and pain, the pancreatic cancer is typically at an advanced stage.1 When present, treating jaundice is a cornerstone in the management of patients with advanced pancreatic cancer, either through ERCP or percutaneous transhepatic biliary drainage (PTBD).4 Treating jaundice consists of the use of life-prolonging chemotherapy and is often associated with improvements in symptoms and quality of life.5,6 However, despite the widespread use of drainage procedures in patients with unresectable pancreatic cancer, no study has evaluated survival after ERCP and PTBD in this setting. The need for supporting data are particularly relevant in light of prior studies demonstrating ERCP in resectable pancreatic cancer leads to adverse events that may delay surgical treatment.7 The subsequent impact on mortality is less clear.8,9
Beyond concerns about adverse events related to ERCP, few data directly compare outcomes after ERCP with PTBD in pancreatic cancer patients.4,10,11 Although some studies suggest that PTBD has higher success rates and lower adverse events compared with ERCP for obstructive jaundice, these studies were primarily conducted in patients with cholangiocarcinoma and few with pancreatic cancer.12-16 To address these gaps in the literature, we were interested in the following 3 aims: to compare overall survival between unresectable pancreatic cancer patients receiving ERCP with those receiving PTBD, to compare overall survival between unresectable pancreatic cancer patients who received a biliary intervention (ERCP or PTBD) with those who received no biliary intervention, and to characterize secondary outcomes, such as length of hospital stay and costs, between ERCP and PTBD.
METHODS
Data sources
We conducted a retrospective cohort study using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.17 The SEER-Medicare database contains data on patient demographics, clinical characteristics, tumor location and staging, diagnostic and therapeutic treatments, and overall survival for Medicare patients diagnosed with cancer in SEER-defined geographic regions. The SEER program collects data from 17 cancer registries and represents roughly 27% of the population of the United States, whereas the Medicare database contains health insurance claims for approximately 97% of the population age ≥65 years.17 Institutional Review Board exemption was obtained to review previously collected data (HUM00128282).
Study sample
We identified patients diagnosed with pancreatic cancer from 2003 to 2013. Pancreatic cancer histology was based on International Classification of Diseases (ICD) for Oncology 3 codes (Supplementary Table 1, available online at www.giejournal.org). Patients with known pancreatic cancer were included if they had a pancreatic head mass and/or evidence of biliary obstruction. Patients were excluded if they had a history of other cancer, histology other than adenocarcinoma, diagnosis at the time of death or autopsy, age <66 years, no date of diagnosis, received ERCP or PTBD before pancreatic cancer diagnosis, received more than 1 biliary intervention on the same date, or received a Whipple, surgical bypass operation, or site-specific surgery, as determined by SEER, after their diagnosis (Supplementary Fig. 1, available online at www.giejournal.org). Patients were required to have continuous enrollment in Medicare Part A and B coverage, without concomitant enrollment in a health maintenance organization, for at least 12 months before their pancreatic cancer diagnosis and through death or up to 12 months after their diagnosis (Supplementary Fig. 1).
The Medical Provider Analysis and Review files, outpatient files, and carrier claims were used to identify diagnosis and procedure codes using ICD-9 codes, the American Common Procedure Terminology codes, and the healthcare common procedure codes (Supplementary Table 1). Patients were designated as having received ERCP or PTBD based on the first procedure they received after the date of diagnosis. If patients underwent more than 1 type of procedure after their diagnosis, they were designated by the first procedure that occurred after diagnosis.
Study variables and outcomes
Independent variables.
The independent variables in our study were ERCP, PTBD, or no biliary intervention.
Covariates of interest.
Our covariates of interest were age at the time of diagnosis; sex; SEER region; American Joint Committee on Cancer tumor stage, sixth edition; comorbidity by Charlson comorbidity index; presence of pruritus, cholangitis, jaundice, obstructive jaundice, abnormal liver function tests, obstruction bile duct, gastric outlet obstruction, jaundice, or biliary obstruction (composite of cholangitis, pruritus, jaundice, obstructive jaundice, obstruction bile duct, and abnormal liver function tests); and receipt of chemotherapy and/or radiation therapy. The development of these variables were all based on available data in SEER-Medicare.18 ICD-9 codes were used to identify obstructive biliary pathophysiology within 30 days of diagnosis and to identify patients treated with chemotherapy and/or radiation after their pancreatic cancer diagnosis (Supplementary Table 1).
Primary outcome.
The primary outcome was survival from time of pancreatic cancer diagnosis, with patients censored at time of death or last Medicare follow-up on December 31, 2015. The Cox proportional hazard model was used to compare survival between groups. For the primary analysis, patients who received ERCP were compared with those who received PTBD. We also evaluated survival among patients who received either biliary interventions, defined as ERCP or PTBD, as compared with those who received no biliary intervention.
Secondary outcomes.
We examined several secondary outcomes: length of hospital stay, total hospital charges if the initial procedure was performed during an inpatient hospital stay, total number of procedures, and readmissions and hospitalizations after either procedure. Length of stay was calculated as the number of days from admission to discharge if a patient had a claim for their initial ERCP or PTBD during an inpatient hospital stay. Total hospital charges for an inpatient hospital stay where the beneficiary received their initial biliary intervention included cost covered by Medicare and uncovered costs charged to the beneficiary. Total procedures were calculated as the number of procedures from diagnosis to death by number of person-years of follow-up. To avoid double counting of procedures in the Medical Provider Analysis and Review, outpatient claims, and carrier claims, we compared claims from all 3 files and removed any duplicate procedures that occurred ±1 day from the initial procedure. Readmission to the hospital was defined as a second admission to a hospital within 30 days from the admission in which the patient received their ERCP or PTBD. Hospitalization was defined as any patient who required an admission to the hospital within 30 days of their biliary intervention, regardless of location of the first procedure. For both readmission and hospitalization rates, patients were only included if they survived more than 30 days after their diagnosis.
Statistical analysis
To avoid an immortal time bias, we analyzed receipt of ERCP or PTBD as time-varying covariates. Immortal time bias can occur when treatment varies by time, because participants have to live long enough to receive treatment.19,20 Placing a disproportionate number of patients in the “untreated group” can artificially make a treatment group appear better in comparison. To account for the fact that receipt of treatment changes over time rather than treated as a static group, a time-varying Cox regression analysis was performed.19,20
Sensitivity analysis was performed using a propensity score with an inverse probability of treatment weighting approach (Appendix 1, available online at www.giejournal.org). Two groups were evaluated in our sensitivity analysis: patients who received an ERCP versus PTBD, and patients who received a biliary intervention versus no treatment. Each observation was weighted by the inverse of the probability of a patient receiving an ERCP, for group 1, or receiving any biliary intervention, for group 2. This created a pseudo-population in which the exposure to treatment was independent of measured confounders.21 Cox proportional hazards regression models adjusted for an inverse probability of treatment weighting were used to estimate survival among our 2 groups of interest.
To address the concern for confounding, a number of subgroup analyses were performed to compare survival between our 2 groups of interest: ERCP versus PTBD and biliary intervention (ERCP and PTBD) versus no biliary intervention. These subgroup analyses are shown in Supplementary Tables 2 and 3 (available online at www.giejournal.org).
The Student t test and χ2 test were used to compare continuous and categorical variables across our patient populations. Stepwise forward regression was used to identify covariates of interest included in our multivariable Cox model. A P < .05 was considered statistically significant. Analyses were conducted using Stata 15.0 (Stata Corp, College Station, Tex, USA).
RESULTS
Patient characteristics
Of 14,808 patients with unresectable pancreatic adenocarcinoma who fulfilled our inclusion and exclusion criteria (Supplementary Fig. 1), 8898 patients received biliary drainage (ERCP, 8271 [93.0%]; PTBD, 627 [7.0%]). The median time from diagnosis to ERCP was 17 days (interquartile range, 9-26) and to PTBD was 21 days (interquartile range, 11-32). When comparing ERCP with PTBD, there were no statistically significant differences by sex, comorbidity, location of pancreatic tumor, or SEER region (Table 1). Compared with ERCP, patients who received PTBD were more likely to have stage IV disease (PTBD 35.3% vs ERCP 32.0%, P < .001) and gastric outlet obstruction (PTBD 7.2% vs ERCP 2.3%, P < .001) (Table 1).
TABLE 1.
Characteristics of patients with unresectable pancreatic cancer
| ERCP vs PTBD vs none |
|||||
|---|---|---|---|---|---|
| All patients (n = 14,808) |
ERCP (n = 8271) |
PTBD (n = 627) |
No intervention (n = 5910) |
P value* | |
| Sex | .65 | ||||
| Male | 6166 (41.6) | 3419 (41.3) | 268 (42.7) | 2479 (41.9) | |
| Female | 8642 (58.4) | 4852 (58.7) | 359 (57.3) | 3431 (58.1) | |
| Age at diagnosis | <.001 | ||||
| 66-70 y | 2545 (17.2) | 1436 (17.4) | 151 (24.1) | 958 (16.2) | |
| 71-75 y | 2728 (18.4) | 1573 (19.0) | 119 (19.0) | 1036 (17.5) | |
| 76-80 y | 3003 (20.3) | 1730 (20.9) | 126 (20.1) | 1147 (19.4) | |
| 81-85 y | 2982 (20.1) | 1711 (20.7) | 113 (18.0) | 1157 (19.6) | |
| 86-90 y | 2244 (15.2) | 1221 (14.8) | 74 (11.8) | 949 (16.1) | |
| >90 y | 1307 (8.9) | 600 (7.3) | 44 (7.0) | 663 (11.2) | |
| Race | .08 | ||||
| White | 11,948 (80.7) | 6695 (81.0) | 472 (75.3) | 4781 (80.9) | |
| Black | 1591 (10.7) | 855 (10.3) | 87 (13.9) | 649 (11.0) | |
| Asian | 512 (3.5) | 295 (3.6) | 27 (4.3) | 190 (3.2) | |
| Hispanic | 326 (2.2) | 186 (2.3) | 17 (2.7) | 123 (2.1) | |
| Other | 431 (2.9) | 240 (2.9) | 24 (3.8) | 167 (2.8) | |
| American Joint Committee on Cancer stage | <.001 | ||||
| I | 1129 (7.6) | 731 (8.8) | 36 (5.7) | 362 (6.1) | |
| II | 2417 (16.3) | 1809 (219) | 126 (20.1) | 482 (8.2) | |
| III | 1104 (7.5) | 698 (8.4) | 57 (9.1) | 349 (5.9) | |
| IV | 6033 (40.7) | 2650 (32.0) | 221 (35.3) | 3162 (53.5) | |
| Unknown | 4125 (27.9) | 2383 (28.8) | 187 (29.8) | 1555 (26.3) | |
| Location of tumor | <.001 | ||||
| Head of pancreas | 11,147 (75.3) | 6527 (78.9) | 483 (77.0) | 4137 (70.0) | |
| Body/tail | 940 (6.4) | 269 (3.3) | 31 (4.9) | 640 (10.8) | |
| Unknown | 2721 (18.4) | 1475 (17.8) | 113 (18.0) | 1133 (19.2) | |
| SEER location | <.001 | ||||
| Northeast | 3045 (20.6) | 1746 (21.3) | 120 (20.1) | 1155 (19.5) | |
| Southeast | 3508 (23.7) | 1911 (23.1) | 140 (22.3) | 1457 (24.7) | |
| Midwest | 1895 (12.8) | 1006 (12.2) | 85 (13.6) | 804 (13.6) | |
| West | 6360 (43.0) | 3590 (43.4) | 276 (44.0) | 2494 (42.2) | |
| Charlson comorbidity index | <.001 | ||||
| 0 | 3437 (23.2) | 2011 (24.3) | 145 (23.1) | 1281 (21.7) | |
| 1 | 3389 (22.9) | 1969 (23.8) | 136 (21.7) | 1284 (21.7) | |
| 2 | 7982 (53.9) | 4291 (51.9) | 346 (55.2) | 3345 (56.6) | |
| Cholangitis | 983 (6.6) | 788 (9.5) | 80 (12.8) | 115 (2.0) | <.001 |
| Pruritus | 653 (4.4) | 516 (6.2) | 32 (5.1) | 105 (1.8) | <.001 |
| Jaundice | 6831 (46.1) | 5390 (65.2) | 358 (57.1) | 1083 (18.3) | <.001 |
| Obstructive jaundice | 7705 (52.0) | 5990 (72.4) | 419 (66.8) | 1296 (21.9) | <.001 |
| Obstruction, bile duct | 7264 (49.1) | 6267 (75.8) | 428 (68.3) | 569 (9.6) | <.001 |
| Abnormal liver function tests | 2983 (20.1) | 1801 (21.8) | 112 (17.9) | 1070 (18.1) | <.001 |
| Obstruction† | 10,957 (74.0) | 7509 (90.8) | 534 (85.2) | 2914 (49.3) | <.001 |
| Gastric outlet obstruction | 352 (2.4) | 187 (2.3) | 45 (7.2) | 120 (2.0) | <.001 |
| Receipt of chemoradiation | 5987 (40.4) | 3895 (39.9) | 250 (47.1) | 1842 (31.2) | <.001 |
Values are n (%).
PTBD, Percutaneous transhepatic biliary drainage; SEER, Surveillance, Epidemiology, and End Results.
P values represent comparisons made across all 3 groups of patients (ERCP, PTBD, and no biliary intervention).
Composite of cholangitis, pruritus, jaundice, obstructive jaundice, obstruction bile duct, and abnormal liver function tests.
A total of 5987 patients (40.4%) received chemotherapy or radiation after their diagnosis of pancreatic cancer. A total of 47.1% of patients who underwent an ERCP received chemoradiation, as compared with 39.9% of PTBD patients and 31.2% of patients who received no intervention (Table 1).
Survival among patients who received ERCP or PTBD
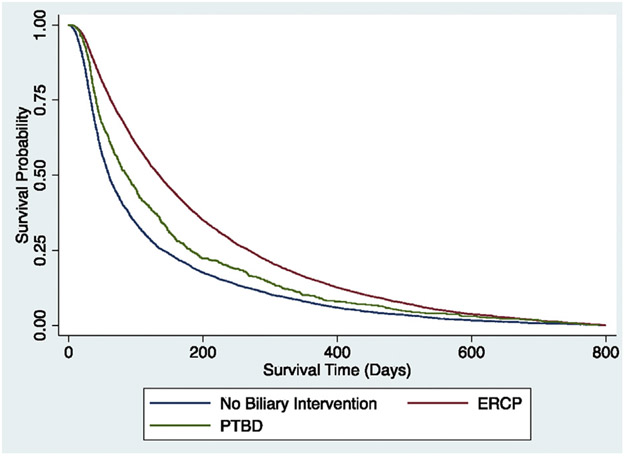
Patients receiving ERCP had a mean survival time of 7.4 months as compared with 5.8 months for those receiving PTBD (P < .001). In a time-varying multivariable analysis, ERCP was associated with reduced mortality compared with PTBD (adjusted hazard ratio [aHR], .67; 95% confidence interval [CI], .60-.75) (Table 2, Fig. 1). Receipt of other therapies, including chemotherapy and/or radiation, was also associated with reduced mortality (aHR, .401; 95% CI, −.39 to .43), whereas those who presented with obstructive jaundice (aHR, 1.18; 95% CI, 1.11-1.25), age over 70, and American Joint Committee on Cancer stages II to IV had worse survival (Table 2). Further, patients in the Northeast, Midwest, and West had reduced mortality compared with patients in the Southeast (Table 2).
TABLE 2.
Predictors of survival time in unresectable pancreatic cancer patients who received ERCP or PTBD (n = 8898)
| Hazard ratio |
95% Confidence interval |
P value | |
|---|---|---|---|
| Treatment | |||
| PTBD | Ref | ||
| ERCP | .67 | .60-.75 | <.001 |
| Receipt of chemoradiation | .41 | .39-.43 | <.001 |
| Charlson comorbidity index | |||
| 0 | Ref | ||
| 1 | 1.07 | 1.00-1.13 | .04 |
| 2 | 1.20 | 1.14-1.26 | <.001 |
| Pruritus | .76 | .69-.83 | <.001 |
| Cholangitis | 1.07 | .99-1.14 | .08 |
| Jaundice | .96 | .92-1.01 | .13 |
| Obstruction, bile duct | 1.03 | .97-1.09 | .39 |
| Obstructive jaundice | 1.18 | 1.11-1.25 | <.001 |
| Abnormal liver function tests | .90 | .85-.95 | <.001 |
| Obstruction* | 1.23 | 1.12-1.36 | <.001 |
| Gastric outlet obstruction | 1.40 | 1.203-1.60 | <.001 |
| Age at diagnosis | |||
| 65-70 y | Ref | ||
| 70-75 y | 1.07 | 1.00-1.15 | .05 |
| 75-80 y | 1.12 | 1.05-1.20 | .01 |
| 80-85 y | 1.10 | 1.02-1.18 | .01 |
| 85-90 y | 1.19 | 1.10-1.29 | <.001 |
| >90 y | 1.29 | 1.17-1.42 | <.001 |
| Gender | |||
| Male | Ref | ||
| Female | .96 | .92-1.01 | .09 |
| SEER region | |||
| Southeast | Ref | ||
| Northeast | .91 | .85-.97 | .003 |
| Midwest | .90 | .83-.97 | .005 |
| West | .85 | .81-.90 | <.001 |
| American Joint Committee on Cancer stage | |||
| I | Ref | ||
| II | 1.23 | 1.13-1.34 | <.001 |
| III | 1.21 | 1.09-1.35 | <.001 |
| IV | 2.10 | 1.93-2.8 | <.001 |
| Unknown | 1.21 | 1.11-1.31 | <.001 |
Cox survival model was used to predict time to death. Patients were censored when they died or at the last Medicare follow-up, defined as December 31, 2015. ERCP was compared with PTBD, ERCP, and PTBD were included as a time-varying covariate.
PTBD, Percutaneous transhepatic biliary drainage; SEER, Surveillance, Epidemiology, and End Results.
Composite of cholangitis, pruritus, jaundice, obstructive jaundice, obstruction bile duct, and abnormal liver function tests.
Figure 1.
Kaplan-Meier survival curve comparing ERCP, PTBD, and no biliary intervention. PTBD, Percutaneous transhepatic biliary drainage.
Characteristics and survival among patients who received no biliary intervention
We identified 5910 patients (39.9%) who did not have biliary drainage. Among the 5910 patients, 4137 (70%) had a pancreas head mass (2996 [50.7%] without obstruction and 1141 [19.3%] with obstruction), and the remaining 1773 patients (30%) had evidence of biliary obstruction without a pancreas head mass. Patients who had no biliary intervention were more likely to be age ≥80 years (no biliary intervention 46.9% vs biliary intervention 42.3%, P < .001) and to have stage IV disease (no biliary intervention 53.5% vs biliary intervention 32.3%, P < .001) (Table 1). Evidence of biliary obstruction was less common in patients who did not undergo a biliary intervention (49.3% vs 90.4%, P < .001) (Table 1). Most patients without a biliary intervention had a pancreatic head mass (4137; 70.0%) (Table 1). In multivariable analysis, we found receipt of either ERCP (aHR,.51; 95% CI, .49-.54) or PTBD (aHR, .53; 95% CI, .48-.59) was independently associated with reduced mortality compared with no biliary intervention (Table 3, Fig. 1).
TABLE 3.
Predictors of survival time in unresectable pancreatic cancer patients who received ERCP, PTBD, or no biliary intervention (n = 14,808)
| Hazard ratio |
95% Confidence interval |
P value | |
|---|---|---|---|
| Treatment | |||
| No biliary intervention | Ref | ||
| ERCP | .51 | .49-.54 | <.001 |
| PTBD | .53 | .48-.59 | <.001 |
| Receipt of chemoradiation | .41 | .40-.43 | <.001 |
| Charlson comorbidity index | |||
| 0 | Ref | ||
| 1 | 1.09 | 1.04-1.15 | <.001 |
| 2 | 1.17 | 1.12-1.22 | <.001 |
| Pruritus | .78 | .72-.85 | <.001 |
| Cholangitis | 1.10 | 1.03-1.18 | .004 |
| Jaundice | .89 | .85-.93 | <.001 |
| Obstruction, bile duct | .96 | .92-1.01 | .11 |
| Obstructive jaundice | 1.38 | 1.32-1.45 | <.001 |
| Abnormal liver function tests | .94 | .90-.98 | .007 |
| Obstruction* | 1.39 | 1.32-1.47 | <.001 |
| Gastric outlet obstruction | 1.35 | 1.21-1.50 | <.001 |
| Age at diagnosis | |||
| 65-70 y | Ref | ||
| 70-75 y | 1.05 | .99-1.10 | .11 |
| 75-80 y | 1.13 | 1.08-1.20 | <.001 |
| 80-85 y | 1.14 | 1.07-1.20 | <.001 |
| 85-90 y | 1.22 | 1.15-1.30 | <.001 |
| >90 y | 1.29 | 1.20-1.39 | <.001 |
| Gender | |||
| Male | Ref | ||
| Female | .97 | .94-1.01 | .12 |
| SEER region | |||
| Southeast | |||
| Northeast | .95 | .91-1.00 | .06 |
| Midwest | .95 | .90-1.00 | .07 |
| West | .88 | .85-.92 | <.001 |
| American Joint Committee on Cancer stage | |||
| I | Ref | ||
| II | 1.27 | 1.18-1.37 | <.001 |
| III | 1.35 | 1.23-1.47 | <.001 |
| IV | 2.27 | 2.12-2.43 | <.001 |
| Unknown | 1.38 | 1.29-1.48 | <.001 |
Cox survival model was used to predict time to death. Patients were censored when they died or at the last Medicare follow-up, defined as December 31, 2015. ERCP and PTBD were compared with those who received no biliary intervention. ERCP, PTBD, and no biliary intervention were included as a time-varying covariate.
PTBD, Percutaneous transhepatic biliary drainage; SEER, Surveillance, Epidemiology, and End Results.
Composite of cholangitis, pruritus, jaundice, obstructive jaundice, obstruction bile duct, and abnormal liver function tests.
Secondary outcomes
ERCP patients had shorter mean length of hospital stay (7.0 ± 5.7 days vs 9.6 ± 6.6 days, P < .001) and lower inpatient hospital charges ($54,899.25 vs $75,246.00, P < .001) compared with patients receiving PTBD (Table 4). There was no statistically significant difference in 30-day readmission rate (ERCP, 7.0%; PTBD, 7.1%; P = .96) or hospitalization rate (ERCP, 17.9%; PTBD, 18.7%; P = .60) after either biliary intervention (Table 4).
TABLE 4.
Secondary outcomes among patients with unresectable pancreatic cancer who received ERCP or PTBD (n = 8829)
| ERCP (n = 8205) |
PTBD (n = 624) |
P value |
|
|---|---|---|---|
| Length of stay, days | 7.0 ± 5.6 | 9.6 ± 6.6 | <.001 |
| Inpatient hospital charges,* U.S.$ | 54,899.25 | 75,246.69 | <.001 |
| 30-day readmission rate† | 575 (7.0) | 44 (7.1) | .93 |
| 30-day hospitalization rate‡ | 1468 (17.9) | 116 (18.7) | .60 |
| Procedure count | |||
| 1 | 4042 (49.3) | 288 (46.2) | |
| >1 | 4163 (50.7) | 336 (53.8) | |
Values are mean ± standard deviation or n (%).
PTBD, Percutaneous transhepatic biliary drainage.
Cost covered by Medicare and uncovered costs charged to beneficiary.
Second admission to a hospital within 30 days from an admission in which the patient received their ERCP or PTBD.
Any patient who required an admission to the hospital within 30 days of their biliary intervention.
ERCP patients underwent 2.0 ± 1.60 procedures over 5361.9 person-years of follow-up compared with 2.19 ± 1.77 procedures during 320.6 person-years of follow-up for PTBD (P = .004). Nearly half of ERCP patients (4042, 48.9%) received only 1 procedure (Table 4). Of the 4163 with more than 1 ERCP, 2565 patients (61.6%) received endoscopic interventions only with a mean number of 2.6 ± 1.2 procedures. However, 1598 patients (38.4%) received an initial ERCP and eventually required a PTBD, with a mean number of 3.5 ± 2.3 procedures. Similarly, nearly half of PTBD patients (288, 45.9%) received only 1 PTBD (Table 4). Of the 336 who received more than 1 procedure, 202 (60.1%) received repeat percutaneous interventions only and 134 (39.9%) eventually switched to an ERCP during the course of their disease.
Sensitivity and subgroup analyses
The results from our propensity score weighted analysis were unchanged from our time-varying Cox analysis, which showed that ERCP was associated with reduced mortality as compared with PTBD (aHR, .68; 95% CI, .60-.76). Further, receiving a biliary intervention was associated with reduced mortality as compared with those who did not receive a biliary intervention (aHR, .41; 95% CI, .35-.46).
Given the concern for confounding, a number of sensitivity analyses were performed to evaluate the effect of ERCP and PTBD on survival among unresectable patients with pancreatic cancer. Between the groups of interest (ERCP vs PTBD and biliary intervention vs no biliary intervention), our results were consistent with the primary analysis (Supplementary Tables 2 and 3). The only exception was among patients without biliary obstruction in whom ERCP was not associated with reduced mortality compared with PTBD, potentially because of a reduced sample size (n = 855) (Supplementary Tables 2 and 3).
DISCUSSION
To the best of our knowledge, this is the first population-based study evaluating the association between receipt of ERCP and PTBD and mortality among patients with unresectable pancreatic cancer complicated by biliary obstruction. Prior studies have evaluated the impact of preoperative ERCP among patients with resectable disease7,8; however, there has been a lack of studies focusing on the role of biliary decompression among pancreatic cancer patients who never receive curative surgery, which accounts for the large majority of pancreatic cancer patients. We found almost 40% of patients received no biliary intervention, despite having a pancreatic head mass and/or evidence of biliary obstruction. The clinical significance of this finding was highlighted by the significant survival benefit associated with receipt of ERCP or PTBD compared with no biliary intervention. Among those who underwent a biliary intervention, ERCP was associated with a significant survival benefit compared with PTBD. Further, we found patients who underwent ERCP had fewer days in the hospital and lower cost of inpatient hospitalization than PTBD.
Our study highlights that almost 40% of patients received no biliary intervention despite having either a pancreatic head mass or evidence of biliary obstruction. When compared with those patients who received no biliary intervention, PTBD and ERCP showed an increase in survival, again underscoring the importance of biliary decompression. Patients who received biliary decompression could have an increased survival as compared with those with no intervention because they are more likely to receive life-prolonging chemotherapy.4 However, it is unclear why such a large percentage of patients never undergo a biliary intervention. It is possible that these patients never required biliary drainage despite having a head of pancreas mass or billing claims for biliary obstruction. Other potential factors, such as patient preference, lack of local expertise in oncology and biliary procedures, clinical decompensation because of advanced cancer, or lack of perceived clinical necessity for biliary drainage, may all play a role. In addition, some literature states that the role of biliary drainage may be questionable if a patient’s life expectancy is believed to be less than 3 months.22 Although the risk of adverse events from either procedure is not insignificant, our results suggest that not undergoing any biliary drainage may be a harmful strategy in terms of survival. Moreover, patients may prefer to undertake the risk of drainage if it provides them with more time with their loved ones. Further research is needed to understand the values and preferences of this group of patients.
National Comprehensive Cancer Network guidelines state that ERCP is preferred among patients with pancreatic cancer who may be receiving neoadjuvant or adjuvant chemotherapy, with PTBD reserved if ERCP is not possible.23 However, prior studies have shown that as many as 10% to 20% of patients may initially receive PTBD instead of ERCP for biliary decompression.24 In our study, only some patients underwent PTBD (7.0%); however, this may still be clinically significant given the observed difference in mortality between patients who underwent ERCP versus PTBD. This survival benefit may be due to a higher risk of adverse events associated with PTBD, including cholangitis and hemobilia.4 We were able to show differences in length of hospitalization but did not have granular data on adverse events to identify reasons for observed survival differences. Although it is unclear what factors play a role in the decision between ERCP or PTBD among clinicians, our prior research has shown that regional variations and racial disparities in care are potential factors.18 Although our sensitivity analysis tried to account for differences in decision-making between ERCP or PTBD, future research should focus on delineating clinical variables that may lead a clinician toward referring an obstructed pancreatic cancer patient for PTBD over ERCP.
Because Medicare reimbursements continue to evolve, it is vital to also evaluate secondary outcomes of any biliary intervention, including total length of hospital stay, hospitalization costs, and readmission rates. Our study found that patients who undergo ERCP as their initial biliary intervention spend fewer days in the hospital as compared with PTBD patients. This is reflected in a lower cost to Medicare, with an almost $20,000 difference among ERCP and PTBD patients hospitalized for their initial biliary intervention. Our study also evaluated hospital readmission and hospitalization rates within 30 days of the index procedure. Interestingly, there was no statistically significant difference in readmission or hospitalization rates among ERCP and PTBD patients, with nearly 20% of patients hospitalized within 30 days of their inpatient or outpatient procedure. As Medicare cuts payments to hospitals, it is becoming increasingly important to critically evaluate the need for an inpatient hospitalization for biliary decompression and the reason for hospitalizations among these patients.
Our study has some important limitations. First, there are inherent limitations to using insurance claims data. Although linkage to Medicare claims data increases the reliability of the findings, we cannot exclude the possibility for residual confounding variables that are unaccounted for in our multivariable analysis. To help mitigate the risk of confounding that may occur with a multivariable analysis and to try to estimate the effect of the decision between ERCP and PTBD among patients with varying comorbidities, an inverse probability of treatment weighted propensity score analysis was performed. The propensity score weighted analysis confirmed the findings from the time-varying multivariable Cox analysis among both of our patient populations. Further, we performed a number of sensitivity analyses in an attempt to evaluate the precision of our findings; our sensitivity analyses were largely unchanged among all our analyses. Second, given the lack of granular data on laboratory abnormalities, clinical stability, or comorbidities, it is unclear whether the lack of a biliary intervention in almost 40% of our cohort was appropriate. It is possible that patients who did not undergo biliary intervention were more ill, partly explaining the worse survival observed in this group of patients. The lack of granular data prevents us from knowing whether patients refused treatment or how many patients had failed biliary cannulation that led to PTBD. We also did not look at receipt of palliative care or race in our study, but it is important to note that these have been shown to impact mortality and may affect the generalizability of our study.25,26 In addition, prior research has found that black pancreatic cancer patients are less likely to receive ERCP and more likely to receive PTBD as compared with whites.18,27 This disparity in receipt of ERCP may contribute to overall differences in mortality. Third, although we used a compilation of ICD-9 codes to identify patients who may be clinically obstructed after their diagnosis with pancreatic cancer, there is the possibility of either miscoding within the insurance data or missing ICD-9 codes that may help to capture more patients who may be clinically obstructed. Fourth, there are variations in time to treatment, defined as time to either ERCP or PTBD, that may lead to an immortal time bias because patients would have to survive long enough to receive treatment.19 To account for this bias, a time-varying Cox analysis was performed, which has been shown to provide more consistent unbiased results than a landmark analysis.19,28 Furthermore, we performed a propensity score weighted Cox regression and multiple sensitivity analyses to try to evaluate for a survival bias. However, we cannot eliminate the possibility of unaccounted for time to event bias in our study. Last, our study had several strict inclusion and exclusion criteria that may limit the generalizability of our results.
In summary, biliary drainage appears to be associated with improved survival in patients with unresectable pancreatic cancer. Among biliary interventions, ERCP is associated with significantly increased survival, shorter length of stay, and decreased inpatient costs compared with PTBD. Overall, these data suggest that ERCP should be the initial attempted drainage procedure for patients with unresectable pancreatic cancer and biliary obstruction and that careful consideration should be given before placement of PTBD in unresectable pancreatic cancer patients.
Supplementary Material
Abbreviations:
- ICD
International Classification of Diseases
- PTBD
percutaneous transhepatic biliary drainage
- SEER
Surveillance, Epidemiology, and End Results
APPENDIX
Appendix A: Propensity Score Development
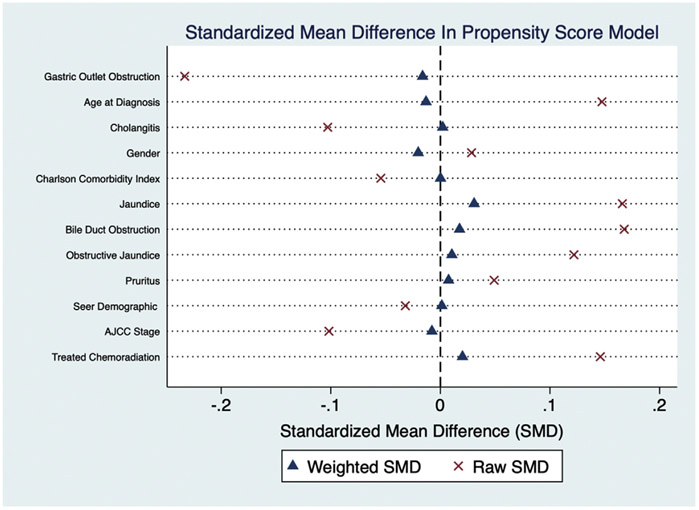
The propensity score model was developed using the guidelines published in Gastrointestinal Endoscopy (doi:10.1016/j.gie.2019.04.236). First, we chose covariates we believed would affect the risk for the outcome and not those related to the treatment only. The covariates included in our propensity score model were: age at diagnosis, jaundice, cholangitis, gastric outlet obstruction, pruritus, obstructive jaundice, obstruction of the bile duct, Charlson comorbidity index, treatment with chemotherapy or radiation, gender, SEER demographic, and AJCC stage. We next assessed the balance of our risk factors through the standardized mean difference using values >0.1 as a strong risk factor that our covariates are unbalanced. The dot plot below show the raw and weighted SMD. The propensity score developed for our inverse probability of treatment weighted Cox proportional hazards regression were well balanced.
Appendix 1.
Footnotes
DISCLOSURE: The following author disclosed financial relationships: B. J. Elmunzer: Consultant for Takeda Pharmaceuticals. All other authors disclosed no financial relationships. Anna Tavakkoli’s research support for this study was provided by an American Society for Gastrointestinal Endoscopy Endoscopic Research Award.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 3.Gores GJ, Lieberman D. Good news-bad news: current status of GI cancers. Gastroenterology 2016;151:13–6. [DOI] [PubMed] [Google Scholar]
- 4.Inamdar S, Slattery E, Bhalla R, et al. Comparison of adverse events for endoscopic vs percutaneous biliary drainage in the treatment of malignant biliary tract obstruction in an inpatient national cohort. JAMA Oncol 2016;2:112–7. [DOI] [PubMed] [Google Scholar]
- 5.Artifon ELA, Sakai P, Cunha JEM, et al. Surgery or endoscopy for palliation of biliary obstruction due to metastatic pancreatic cancer. Am J Gastroenterol 2006;101:2031–7. [DOI] [PubMed] [Google Scholar]
- 6.Floyd J, Mirza I, Sachs B, et al. Hepatotoxicity of chemotherapy. Semin Oncol 2006;33:50–67. [DOI] [PubMed] [Google Scholar]
- 7.van der Gaag NA, Rauws EAJ, van Eijck CHJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010;362:129–37. [DOI] [PubMed] [Google Scholar]
- 8.Rustgi SD, Amin S, Yang A, et al. Preoperative endoscopic retrograde cholangiopancreatography is not associated with increased pancreatic cancer mortality. Clin Gastroenterol Hepatol 2019;17:1580–6. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Gurusamy KS, Lin H, et al. Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev 2008:CD005444. [DOI] [PubMed] [Google Scholar]
- 10.Smith AC, Dowsett JF, Russell RC, et al. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet 1994;344:1655–60. [DOI] [PubMed] [Google Scholar]
- 11.Speer AG, Cotton PB, Russell RC, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet 1987;2:57–62. [DOI] [PubMed] [Google Scholar]
- 12.Murakami Y, Uemura K, Hashimoto Y, et al. Does preoperative biliary drainage compromise the long-term survival of patients with pancreatic head carcinoma? J Surg Oncol 2015;111:270–6. [DOI] [PubMed] [Google Scholar]
- 13.Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg 2010;14:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hameed A, Pang T, Chiou J, et al. Percutaneous vs. endoscopic preoperative biliary drainage in hilar cholangiocarcinoma—a systematic review and meta-analysis. HPB (Oxford) 2016;18:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sewnath ME, Karsten TM, Prins MH, et al. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg 2002;236:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kariya CM, Wach MM, Ruff SM, et al. Postbiliary drainage rates of cholangitis are impacted by procedural technique for patients with supra-ampullary cholangiocarcinoma: a SEER-Medicare analysis. J Surg Oncol 2019;120:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40 IV-3–18. [DOI] [PubMed] [Google Scholar]
- 18.Tavakkoli A, Singal AG, Waljee AK, et al. Regional and racial variations in the utilization of endoscopic retrograde cholangiopancreatography among pancreatic cancer patients in the United States. Cancer Med 2019;8:3420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt RW, Hutcheon JA, Suissa S. Immortal time bias in epidemiology. Curr Epidemiol Rep 2019;6:23–7. [Google Scholar]
- 20.Kollman C Survival analysis and the immortal time bias. JAMA Ophthalmol 2018;136:1314–5. [DOI] [PubMed] [Google Scholar]
- 21.Hernán MA, Robins JM. Causal inference: what if. Boca Raton: Chapman & Hall/CRC; 2020. [Google Scholar]
- 22.Gregory A, Coté SS. Endoscopic palliation of pancreatic cancer. Cancer J 2012;18:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. Pancreatic Cancer (Version 1.2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed February 1, 2020.
- 24.Dorcaratto D, Hogan NM, Muñoz E, et al. Is percutaneous transhepatic biliary drainage better than endoscopic drainage in the management of jaundiced patients awaiting pancreaticoduodenectomy? A systematic review and meta-analysis. J Vasc Interv Radiol 2018;29:676–87. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan DR, Chan B, Lapidus JA, et al. Association of early palliative care use with survival and place of death among patients with advanced lung cancer receiving care in the Veterans Health Administration. JAMA Oncol 2019;5:1702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michael N, Beale G, O'Callaghan C, et al. Timing of palliative care referral and aggressive cancer care toward the end-of-life in pancreatic cancer: a retrospective, single-center observational study. BMC Palliat Care 2019;18:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rustgi SD, Amin SP, Kim MK, et al. Age, socioeconomic features, and clinical factors predict receipt of endoscopic retrograde cholangiopancreatography in pancreatic cancer. World J Gastrointest Endosc 2019;11:133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones M, Fowler R. Immortal time bias in observational studies of time-to-event outcomes. J Crit Care 2016;36:195–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.