Abstract
Background
Safe and effective vaccines are urgently needed to end the COVID-19 pandemic caused by SARS-CoV-2 infection. We aimed to assess the preliminary safety, tolerability, and immunogenicity of an mRNA vaccine ARCoV, which encodes the SARS-CoV-2 spike protein receptor-binding domain (RBD).
Methods
This single centre, double-blind, randomised, placebo-controlled, dose-escalation, phase 1 trial of ARCoV was conducted at Shulan (Hangzhou) hospital in Hangzhou, Zhejiang province, China. Healthy adults aged 18–59 years negative for SARS-CoV-2 infection were enrolled and randomly assigned using block randomisation to receive an intramuscular injection of vaccine or placebo. Vaccine doses were 5 μg, 10 μg, 15 μg, 20 μg, and 25 μg. The first six participants in each block were sentinels and along with the remaining 18 participants, were randomly assigned to groups (5:1). In block 1 sentinels were given the lowest vaccine dose and after a 4-day observation with confirmed safety analyses, the remaining 18 participants in the same dose group proceeded and sentinels in block 2 were given their first administration on a two-dose schedule, 28 days apart. All participants, investigators, and staff doing laboratory analyses were masked to treatment allocation. Humoral responses were assessed by measuring anti-SARS-CoV-2 RBD IgG using a standardised ELISA and neutralising antibodies using pseudovirus-based and live SARS-CoV-2 neutralisation assays. SARS-CoV-2 RBD-specific T-cell responses, including IFN-γ and IL-2 production, were assessed using an enzyme-linked immunospot (ELISpot) assay. The primary outcome for safety was incidence of adverse events or adverse reactions within 60 min, and at days 7, 14, and 28 after each vaccine dose. The secondary safety outcome was abnormal changes detected by laboratory tests at days 1, 4, 7, and 28 after each vaccine dose. For immunogenicity, the secondary outcome was humoral immune responses: titres of neutralising antibodies to live SARS-CoV-2, neutralising antibodies to pseudovirus, and RBD-specific IgG at baseline and 28 days after first vaccination and at days 7, 15, and 28 after second vaccination. The exploratory outcome was SARS-CoV-2-specific T-cell responses at 7 days after the first vaccination and at days 7 and 15 after the second vaccination. This trial is registered with www.chictr.org.cn (ChiCTR2000039212).
Findings
Between Oct 30 and Dec 2, 2020, 230 individuals were screened and 120 eligible participants were randomly assigned to receive five-dose levels of ARCoV or a placebo (20 per group). All participants received the first vaccination and 118 received the second dose. No serious adverse events were reported within 56 days after vaccination and the majority of adverse events were mild or moderate. Fever was the most common systemic adverse reaction (one [5%] of 20 in the 5 μg group, 13 [65%] of 20 in the 10 μg group, 17 [85%] of 20 in the 15 μg group, 19 [95%] of 20 in the 20 μg group, 16 [100%] of 16 in the 25 μg group; p<0·0001). The incidence of grade 3 systemic adverse events were none (0%) of 20 in the 5 μg group, three (15%) of 20 in the 10 μg group, six (30%) of 20 in the 15 μg group, seven (35%) of 20 in the 20 μg group, five (31%) of 16 in the 25 μg group, and none (0%) of 20 in the placebo group (p=0·0013). As expected, the majority of fever resolved in the first 2 days after vaccination for all groups. The incidence of solicited systemic adverse events was similar after administration of ARCoV as a first or second vaccination. Humoral immune responses including anti-RBD IgG and neutralising antibodies increased significantly 7 days after the second dose and peaked between 14 and 28 days thereafter. Specific T-cell response peaked between 7 and 14 days after full vaccination. 15 μg induced the highest titre of neutralising antibodies, which was about twofold more than the antibody titre of convalescent patients with COVID-19.
Interpretation
ARCoV was safe and well tolerated at all five doses. The acceptable safety profile, together with the induction of strong humoral and cellular immune responses, support further clinical testing of ARCoV at a large scale.
Funding
National Key Research and Development Project of China, Academy of Medical Sciences China, National Natural Science Foundation China, and Chinese Academy of Medical Sciences.
Research in context.
Evidence before this study
We searched PubMed for research articles up to May 8, 2021, using a combination of the search terms “COVID-19”, “SARS-CoV-2”, “vaccine”, and “trial”. No language or date restrictions were applied. We identified peer-reviewed publications of 28 clinical trials for 16 COVID-19 vaccines, with six phase 3 studies and 22 studies described as phase 1 or phase 2, or both. These 16 vaccines include five inactivated vaccines, four adenovirus-vectored vaccines, three mRNA-based vaccines, three protein subunit vaccines, and one DNA vaccine. 14 vaccines target either the whole virus or spike protein. The receptor-binding domain of the SARS-CoV-2 spike protein is targeted by two vaccines; one is the protein subunit vaccine ZF2001 and the other is the mRNA-based vaccine BNT162b1. The three mRNA-based vaccines are mRNA-1273, manufactured by Moderna, and BNT162b2 and BNT162b1, manufactured by BioNTech and Pfizer. The first two mRNA-based vaccines target the spike protein whereas the third targets the receptor binding domain of the spike protein.
Added value of this study
No vaccine-related serious adverse event was reported in the participants, and the incidence of solicited adverse event was similar after the first and second vaccinations. In this phase 1 trial, ARCoV exhibited safety, tolerability, and immunogenicity in healthy Chinese adults. A two-dose immunisation schedule (on days 0 and 28) with three of the five doses trialled (5 μg, 10 μg, 15 μg) elicited a time-dependent and dose-dependent neutralising antibody response (100% in the 15 μg group) and T-cell response (100% in the 15 μg group).
Implications of all the available evidence
These findings indicate that the receptor-binding domain-based mRNA vaccine ARCoV has an acceptable safety profile and induces a strong immune response. A large phase 3 trial at 15 μg is currently underway to assess the efficacy of ARCoV (NCT04847102).
Introduction
COVID-19,1, 2 caused by SARS-CoV-2, has resulted in more than 155 million confirmed cases and more than 3·2 million mortalities since May 7, 2021.3 The global pandemic is ongoing and has placed substantial pressures on health-care systems, social stability, and the global economy. Further health consequences are anticipated. Safe and effective vaccines are urgently needed to control the COVID-19 pandemic. Encouragingly, 97 vaccine candidates have been assessed in clinical trials, of which 29 are ongoing phase 3 or 4 trials, and an additional 183 vaccine candidates are in preclinical studies according to WHO.4
mRNA-based prophylactic vaccines have emerged as a leading platform for SARS-CoV-2 protection and are being investigated in basic and clinical research5, 6 Preclinical studies have demonstrated that mRNA-based vaccines can induce potent and broad protective immune responses with an acceptable safety profile.7 Two mRNA vaccines developed by Moderna and Pfizer–BioNTech have been approved for emergency use, with more than 94% efficacy in phase 3 clinical trials. Both vaccines choose the full-length spike protein of SARS-CoV-2 as protective antigens. On the basis that the receptor binding domain (RBD) of SARS-CoV-2 spike protein had been identified to play a key role in helping coronaviruses to enter human cells (and the RBD can induce strong humoral and cellular immune responses when used as an antigen in various SARS-CoV and MERS-CoV studies),8, 9, 10 we developed a candidate mRNA vaccine (ARCoV) that encodes the RBD of SARS-CoV-211 as our antigen of selection. Preclinical studies showed immunisation with ARCoV in a two-dose schedule, eliciting robust neutralising antibodies against SARS-CoV-2 as well as T-helper-1-biased cellular responses in mice and non-human primates.11 Additionally, ARCoV was manufactured as a liquid formulation and can be stored at standard refrigerated condition (2–8°C), which is convenient for transportation and application. This phase 1 clinical trial aimed to assess the tolerability and safety profile of ARCoV in Chinese adults.
Methods
Study design and participants
In this single centre, double-blind, randomised, placebo-controlled, dose-escalation, phase 1 trial, participants were recruited from Shulan (Hangzhou) hospital in Hangzhou, China. Eligible participants were healthy adults aged 18–59 years. Key exclusion criteria included history of COVID vaccination; history of infection with SARS-CoV-2 or suspected cases; history of infection with SARS-CoV or MERS-CoV; history of travelling to high outbreak areas or regions outside of China; any history of serious adverse reactions to vaccines or drugs; abnormalities in health examination; severe diseases with atypical clinical manifestations; and pregnancy, lactation, or menstruating (appendix p 6).
Participants were recruited through advertisements and screened for SARS-CoV-2 infection with nucleic acid and serology tests (spike-RBD-specific IgG or IgM). All participants had a screening visit in which a full medical history and examination were taken in addition to blood and urine tests (treponema pallidum, HIV, hepatitis B and C serology, kidney and liver function tests, full blood count, urinary screen for blood, protein, and glucose, and a pregnancy test done in women of childbearing potential). Written informed consent was obtained from each participant before enrolment.
The protocol and informed consent were approved by the Clinical Trial Ethics Committee of Shulan (Hangzhou) hospital (YW2020-031-01). This study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
Randomisation and masking
The trial consisted of five blocks. Participants in each block were randomly assigned (5:1) to the ARCoV vaccine or placebo. Statisticians performed randomisation using SAS (version 9.4). The randomisation code was assigned to each participant in sequence in order of enrolment and then the participants received the investigational products labelled with the same code. The vaccine and placebo were indistinguishable in appearance. All participants, investigators, and staff doing laboratory analyses were masked to treatment allocation.
Procedures
ARCoV doses were 5 μg, 10 μg, 15 μg, 20 μg, or 25 μg total mRNA per 0·5 mL (co-developed by AMMS, Abogen Biosciences, and Walvax Biotech, and manufactured as a liquid formulation).11 The vaccine was administered in the deltoid muscle on day 0 and day 28. Placebo was saline solution (0·9% sodium chloride, Suzhou Abogen Biosciences).
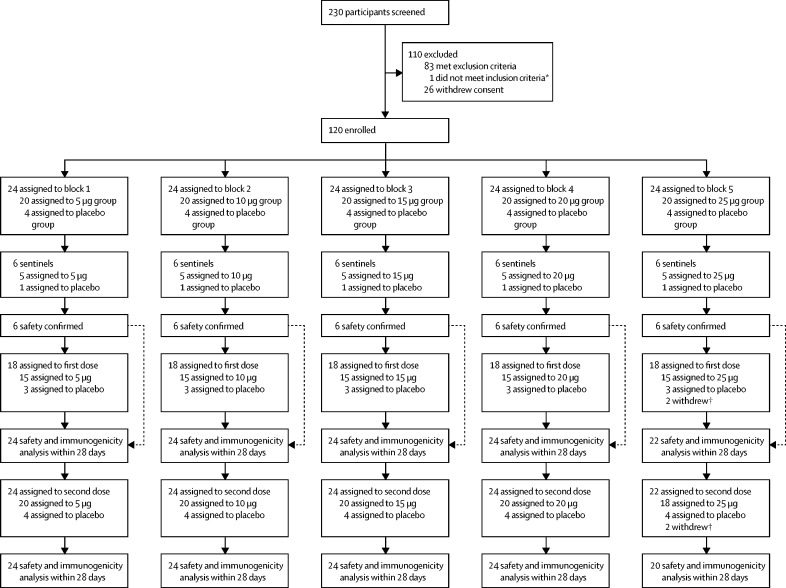
The trial was done in a dose-escalation manner. The first six participants in each block were sentinels and were randomly assigned (5:1) to receive the vaccine ARCoV or placebo, along with the remaining 18 participants. For example, in block 1, the six sentinels were given the lowest dose (5 μg) of vaccine and after a 4-day observation with confirmed safety analyses, the remaining 18 participants in the same dose group proceeded to receive 5 μg ARCoV or placebo, and six sentinels in block 2 were given their first administration (figure 1 ). The sentinels stayed in hospital for observation for at least 24 h after the first dose. The criteria that had to be met from the 4-day safety observation were no vaccine-related deaths or life-threatening serious adverse events; no fester, abscess, or necrosis at the inoculation site associated with vaccination; no severe acute allergic reactions within 24 h after vaccination; no systemic urticaria (defined as appearing in three or more sites of the body) within 72 h after vaccination; no fever above 40°C within 7 days after vaccination; no more than two participants receiving first dose with grade 3 or above adverse events that did not resolve within 48 h of vaccination. The same criteria applied to all participants.
Figure 1.
Trial profile
*Axillary temperature 37·3°C or higher. †Withdrew voluntarily in 25 μg group: three withdrew voluntarily and one received freeze-dried rabies vaccines for human use and tetanus vaccine due to cat scratch.
Participants were observed in the clinic for 60 min after the vaccination. Local and systemic reactions and all other events were recorded using a diary card during the 28-day follow-up. At days 1, 4, 7, and 28 after each dose of vaccination, all participants had blood and urine samples taken for blood biochemical, blood routine, blood coagulation, and urine routine tests. We graded adverse events and abnormal changes in laboratory tests according to the China National Medical Products Administration guidelines or the US Food and Drug Administration (FDA) toxicity grading scale (appendix pp 8–9). 28 days after the first vaccine dose and at days 7, 15, and 28 after the second dose, venous blood was collected from all participants for detecting IgG and neutralising antibodies. For comparison of the participants' humoral responses with those elicited by SARS-CoV-2 infection, 34 serum specimens from patients with COVID-19 were tested as reference samples (appendix p 7). At 7 days after the first dose and 7 days and 15 days after the second dose of vaccine, venous blood from the first 12 participants (ten vaccine group, two placebo group) of each group was collected for cellular immunoassay.
Outcomes
The primary endpoint for safety was incidence of adverse events or adverse reactions within 60 min, and at days 7, 14, and 28 after each vaccine dose. The secondary endpoint for safety was abnormal changes in laboratory tests at days 1, 4, 7, and 28 after each vaccine dose. For immunogenicity, the secondary endpoint was titres of neutralising antibodies to live SARS-CoV-2, neutralising antibodies to a pseudovirus, and RBD-specific IgG at baseline and 28 days after the first vaccination and at days 7, 15, and 28 after the second vaccination. The exploratory endpoint was T-cell responses at 7 days after the first vaccination and at days 7 and 15 after the second vaccination. Seroconversion was defined as a change from seronegative at the lower limit of quantification to seropositive, or a fourfold titre increase if the participant was seropositive at the lower limit of quantification. Regarding the ELISpot measured T-cell response, the results were expressed as the number of spot-forming cells per 1 000 000 cells, which was 50 or more and twice the negative control that was considered positive.
Statistical analysis
We assessed the safety endpoints in the safety population, which included all participants who received at least one vaccine dose. We analysed the number and proportion of participants with adverse reactions after vaccination and compared safety profiles across the dose groups. We assessed immunogenic endpoints in the per-protocol population, who completed their assigned two-dose vaccination schedule and with available antibodies results.
The sample size was not determined on the basis of statistical power calculations. The National Medical Products Administration of China recommended a minimum sample size of 20–30 participants for a pilot vaccine trial. Measurement data were expressed as mean (SD) or geometric mean and counting data or grade data were expressed as frequency. Statistical analysis of multiple mean was performed using unpaired t test or one-way ANOVA, and statistical analysis of categorical outcomes was done by Pearson χ2 test or Fisher's exact test. Hypothesis testing was two sided and significance was defined as p value less than 0·05.
An independent data and safety monitoring committee, consisting of an independent statistician, clinician, epidemiologist, and statistical expert, was established before the start of the trial. Safety data for 14 days and 28 days after the second vaccination was assessed and reviewed by the committee.
We used SAS (version 9.4) or GraphPad Prism (version 8.0) to analyse data. This trial is registered with Chictr.org.cn, ChiCTR2000039212.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article.
Results
Between Oct 30 and Dec 2, 2020, 230 individuals were screened and 120 were enrolled in the phase 1 trial (figure 1). There were 20 participants in each of the vaccine dose groups and the placebo group. All 120 participants received at least one dose of the vaccine or placebo and were included in the safety analysis. Four participants withdrew, none due to adverse events (two withdrew voluntarily after first vaccination, two withdrew after second vaccination; appendix p 15). 116 participants were eligible for immunogenic assessment.
The median age of participants was 27·0 years (IQR 22·0–34·8), and mean body-mass index was 24·2 kg/m2 (SD 3·1). Of 120 participants, 75 (62·5%) were male sex and 45 (37·5%) female sex. Overall male to female ratio was 5:3. The majority of participants (118 [98%]) were of the Han ethnic group. One participant with a history of hypertension was enrolled in the 5 μg dose group. Baseline demographic characteristics of the participants at enrolment were similar among the treatment groups in terms of median age, ethnic group, body-mass index, and medical history or pre-existing conditions (gender ratio of participants χ2=8·00, ν=1; table 1 ).
Table 1.
Baseline demographic characteristics in phase 1
| 5 μg group (n=20) | 10 μg group (n=20) | 15 μg group (n=20) | 20 μg group (n=20) | 25 μg group (n=20) | Placebo group*(n=20) | Total(n=120) | ||
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 15 (75%) | 12 (60%) | 14 (70%) | 8 (40%) | 11 (55%) | 15 (75%) | 75 (62.5) | |
| Female | 5 (25%) | 8 (40%) | 6 (30%) | 12 (60%) | 9 (45%) | 5 (25%) | 45 (37.5) | |
| Age, years | 30·5 (23·3–38·0) | 28·0 (24·3–38·8) | 24·0 (21·0–29·3) | 27·0 (22·3–39·0) | 26·5 (22·3–29·8) | 25·0 (22·0–30·0) | 27·0 (22·0–34·8) | |
| Ethnicity | ||||||||
| Han | 20 (100%) | 20 (100%) | 19 (95%) | 20 (100%) | 19 (95%) | 20 (100%) | 118 (98%) | |
| Others | 0 | 0 | 1 (5%) | 0 | 1 (5%) | 0 | 2 (2%) | |
| Body-mass index, kg/m2 | 24·3 (3·2) | 24·1 (3·1) | 24·8 (3·5) | 23·4 (2·8) | 23·7 (3·6) | 23·6 (2·8) | 24·2 (3·1) | |
| Medical history or existing disorder | ||||||||
| Hypertension | 1 (5) | 0 | 0 | 0 | 0 | 0 | 0 | |
Data are n (%), mean (SD), or median (IQR).
Participant data taken from all five blocks.
Adverse events were graded according to the guidelines for vaccine clinical trials issued by the US FDA and the China National Medical Products Administration (Table 2, Table 3 ). 7 days after first or second vaccination, reactogenicity was partly dose-related (table 2). The incidence of solicited adverse events was 12 (60%) of 20 in the 5 μg group, 16 (80%) of 20 in the 10 μg group, 19 (95%) of 20 in the 15 μg group, 19 (95%) of 20 in the 20 μg group, and 16 (100%) of 16 in the 25 μg group, compared with one (5%) of 20 in the placebo group. The most common solicited adverse reactions reported were injection site pain, fever, headache, fatigue or malaise, injection site redness, muscle pain, injection site induration and itch, joint pain, diarrhoea, and chills (Table 2, Table 3). Adverse events after first or second vaccination were transient and managed with simple standard medication or care, or resolved spontaneously (appendix pp 16–18). Most reported adverse reactions were mild or moderate in severity and about 95% resolved in the first 2 days after first or second vaccinations: the remaining resolved within 5 days. Only one participant developed grade 3 pain in the injection site. Grade 3 fever was the only severe systemic adverse reaction associated with vaccination. No grade 3 fever was reported in the 5 μg group, whereas three (15%) of 20 participants reported grade 3 fever in the 10 μg group. This incidence increased with higher dosage (six [30%] of 20 participants in the 15 μg group, seven [35%] of 20 in the 20 μg group, and six [38%] of 20 in the 25 μg group). Participants who received the placebo had no adverse events except for one who had mild pain at the injection site. The incidence of solicited adverse events after first vaccination, for systemic adverse events and local adverse events, was similar to that after the second vaccination (appendix pp 11–12). For example, the incidence of grade 3 fever after the first vaccination was similar to that after the second vaccination (one [5%] of 20 participants in the 10 μg group, four [20%] of 20 in the 15 μg group, four [20%] of 20 in the 20 μg group, four [20%] of 20 in the 25 μg group vs two [10%] of 20, three [15%] of 20, five [25%] of 20, four [25%] of 16). For fatigue or malaise, headache, and redness compared with the first vaccination, the incidence slightly decreased after the second vaccination (appendix pp 11–12). Additionally, unsolicited adverse events related to vaccination were mild and moderate and mainly included infections (mostly urinary tract infections and upper respiratory tract infections), and kidney and urinary system diseases (mostly albuminuria). Unsolicited adverse events unrelated to vaccination mainly included infections (urinary tract infections, upper respiratory tract infections, nasopharyngitis, conjunctivitis, periodontitis, and otitis media), gastrointestinal disorders (toothache, nausea, diarrhoea, abdominal distension, oral ulcer, and vomit), and nervous system disorders (headache and hypoesthesia; appendix p 10).
Table 2.
Solicited adverse reactions for 7 days after first or second vaccinations, and unsolicited adverse reactions until day 56, graded by US Food and Drug Administration criteria in phase 1
| 5 μg group (n=20) | 10 μg group (n=20) | 15 μg group (n=20) | 20 μg group (n=20) | 25 μg group (n=16)* | Placebo group (n=20)† | p value | |
|---|---|---|---|---|---|---|---|
| Solicited adverse reactions | |||||||
| Any | 12 (60%) | 16 (80%) | 19 (95%) | 19 (95%) | 16 (100%) | 1 (5%) | <0·0001 |
| Grade 3 | 0 | 3 (15%) | 6 (30%) | 8 (40%) | 6 (38%) | 0 | 0·0005 |
| Injection site adverse reactions | |||||||
| Any | 8 (40%) | 14 (70%) | 16 (80%) | 19 (95%) | 14 (88%) | 1 (5%) | <0·0001 |
| Grade 3 | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 1·0 |
| Pain | 6 (30%) | 13 (65%) | 14 (70%) | 18 (90%) | 12 (75%) | 1 (5%) | <0·0001 |
| Redness | 3 (15%) | 4 (20%) | 9 (45%) | 8 (40%) | 9 (56%) | 0 | 0·0012 |
| Swelling | 1 (5%) | 0 | 2 (10%) | 3 (15%) | 5 (31%) | 0 | 0·046 |
| Induration | 0 | 1 (5%) | 3 (15%) | 6 (30%) | 6 (38%) | 0 | 0·0019 |
| Itch | 2 (10%) | 1 (5%) | 3 (15%) | 5 (25%) | 3 (19%) | 0 | 0·19 |
| Systemic adverse reactions | |||||||
| Any | 10 (50%) | 15 (75%) | 18 (90%) | 19 (95%) | 16 (100%) | 0 | <0·0001 |
| Grade 3 | 0 | 3 (15%) | 6 (30%) | 7 (35%) | 5 (31%) | 0 | 0·0013 |
| Fever | 1 (5%) | 13 (65%) | 17 (85%) | 19 (95%) | 16 (100%) | 0 | <0·0001 |
| Headache | 5 (25%) | 5 (25%) | 9 (45%) | 11 (55%) | 9 (56%) | 0 | 0·0004 |
| Fatigue or malaise | 8 (40%) | 8 (40%) | 7 (35%) | 6 (30%) | 9 (56%) | 0 | 0·0024 |
| Joint pain | 1 (5%) | 5 (25%) | 1 (5%) | 5 (25%) | 2 (13%) | 0 | 0·046 |
| Muscle pain | 3 (15%) | 8 (40%) | 3 (15%) | 5 (25%) | 5 (31%) | 0 | 0·022 |
| Chills | 0 | 3 (15%) | 0 | 7 (35%) | 2 (13%) | 0 | 0·0006 |
| Nausea | 3 (15%) | 2 (10%) | 2 (10%) | 1 (5%) | 2 (13%) | 0 | 0·60 |
| Diarrhoea | 1 (5%) | 2 (10%) | 4 (20%) | 2 (10%) | 2 (13%) | 0 | 0·40 |
| Vomiting | 1 (5%) | 0 | 1 (5%) | 1 (5%) | 2 (13%) | 0 | 0·38 |
| Unsolicited adverse reactions‡ | |||||||
| Any | 18 (90%) | 20 (100%) | 19 (95%) | 19 (95%) | 16 (100%) | 12 (60%) | 0·0003 |
| Fever | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 1·0 |
| Low lymphocyte count | 11 (55%) | 20 (100%) | 17 (85%) | 18 (90%) | 15 (94%) | 2 (10%) | <0·0001 |
| Grade 3 | 0 | 2 (10%) | 4 (20%) | 3 (15%) | 2 (13%) | 0 | 0·14 |
| CRP increased | 2 (10%) | 2 (10%) | 2 (10%) | 1 (5%) | 2 (13%) | 1 (5%) | 0·97 |
| Injection site discomfort | 1 (5%) | 0 | 0 | 0 | 0 | 0 | 1·0 |
| Neutrophil decreased | 0 | 2 (10%) | 2 (10%) | 5 (25%) | 2 (13%) | 0 | 0·065 |
| Cough | 0 | 0 | 1 (5%) | 2 (10%) | 0 | 0 | 0·43 |
| URI | 2 (10%) | 2 (10%) | 0 | 1 (5%) | 1 (6%) | 2 (10%) | 0·87 |
| UTI | 2 (10%) | 2 (10%) | 1 (5%) | 3 (15%) | 2 (13%) | 1 (5%) | 0·97 |
| Albuminuria | 0 | 3 (15%) | 2 (10%) | 2 (10%) | 1 (6%) | 1 (5%) | 0·72 |
Data are n (%). CRP=C-reactive protein. URI=upper respiratory tract infection. UTI=urinary tract infection.
Four participants withdrew.
Participant data taken from all five blocks.
Until day 56 related to investigational vaccine.
Table 3.
Solicited adverse reactions for 14 days after first or second vaccinations, and unsolicited adverse reactions until day 56, graded by National Medical Products Administration criteria in phase 1
| 5 μg group (n=20) | 10 μg group (n=20) | 15 μg group (n=20) | 20 μg group (n=20) | 25 μg group (n=16)* | Placebo group (n=20)† | p value | |
|---|---|---|---|---|---|---|---|
| Solicited adverse reactions | |||||||
| Any | 13 (65%) | 19 (95%) | 20 (100%) | 19 (95%) | 16 (100%) | 2 (10%) | <0·0001 |
| Grade 3 | 0 | 7 (35%) | 12 (60%) | 15 (75%) | 14 (88%) | 0 | <0·0001 |
| Injection site adverse reactions | |||||||
| Any | 8 (40%) | 14 (70%) | 16 (80%) | 19 (95%) | 14 (88%) | 1 (5%) | <0·0001 |
| Grade 3 | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 1·0 |
| Pain | 6 (30%) | 13 (65%) | 14 (70%) | 18 (90%) | 12 (75%) | 1 (5%) | <0·0001 |
| Redness | 3 (15%) | 4 (20%) | 9 (45%) | 8 (40%) | 9 (56%) | 0 | 0·0012 |
| Swelling | 1 (5%) | 0 | 2 (10%) | 3 (15%) | 5 (31%) | 0 | 0·046 |
| Induration | 0 | 1 (5%) | 3 (15%) | 6 (30%) | 6 (38%) | 0 | 0·0019 |
| Itch | 2 (10%) | 1 (5%) | 3 (15%) | 5 (25%) | 3 (19%) | 0 | 0·19 |
| Systemic adverse reactions | |||||||
| Any | 11 (55%) | 19 (95%) | 19 (95%) | 19 (95%) | 16 (100%) | 1 (5%) | <0·0001 |
| Grade 3 | 0 | 7 (35%) | 12 (60%) | 15 (75%) | 14 (88%) | 0 | <0·0001 |
| Fever | 7 (35%) | 17 (85%) | 18 (90%) | 19 (95%) | 16 (100%) | 1 (5%) | <0·0001 |
| Headache | 5 (25%) | 5 (25%) | 9 (45%) | 11 (55%) | 9 (56%) | 0 | 0·0004 |
| Fatigue or malaise | 8 (40%) | 8 (40%) | 7 (35%) | 6 (30%) | 9 (56%) | 0 | 0·0024 |
| Joint pain | 1 (5%) | 5 (25%) | 1 (5%) | 5 (25%) | 2 (13%) | 0 | 0·046 |
| Muscle pain | 3 (15%) | 8 (40%) | 3 (15%) | 5 (25%) | 5 (31%) | 0 | 0·022 |
| Chills | 0 | 3 (15%) | 0 | 7 (35%) | 2 (13%) | 0 | 0·0006 |
| Nausea | 3 (15%) | 2 (10%) | 2 (10%) | 1 (5%) | 2 (13%) | 0 | 0·60 |
| Diarrhoea | 1 (5%) | 3 (15%) | 4 (20%) | 2 (10%) | 2 (13%) | 0 | 0·35 |
| Vomiting | 1 (5%) | 0 | 1 (5%) | 1 (5%) | 2 (13%) | 0 | 0·38 |
| Unsolicited adverse reactions‡ | |||||||
| Any | 18 (90%) | 20 (100%) | 19 (95%) | 19 (95%) | 16 (100%) | 12 (60%) | 0·0003 |
| Fever | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 1·0 |
| Low lymphocyte count | 11 (55%) | 20 (100%) | 17 (85%) | 18 (90%) | 15 (94%) | 2 (10%) | <0·0001 |
| Grade 3 | 0 | 2 (10%) | 4 (20%) | 3 (15%) | 2 (13%) | 0 | 0·14 |
| CRP increased | 2 (10%) | 2 (10%) | 2 (10%) | 1 (5%) | 2 (13%) | 1 (5%) | 0·97 |
| Injection site discomfort | 1 (5%) | 0 | 0 | 0 | 0 | 0 | 1·0 |
| Neutrophil decreased | 0 | 2 (10%) | 2 (10%) | 5 (25%) | 2 (13%) | 0 | 0·065 |
| Cough | 0 | 0 | 1 (5%) | 2 (10%) | 0 | 0 | 0·43 |
| URI | 2 (10%) | 2 (10%) | 0 | 1 (5%) | 1 (6%) | 2 (10%) | 0·87 |
| UTI | 2 (10%) | 2 (10%) | 1 (5%) | 3 (15%) | 2 (13%) | 1 (5%) | 0·97 |
| Albuminuria | 0 | 3 (15%) | 2 (10%) | 2 (10%) | 1 (6%) | 1 (5%) | 0·72 |
Data are n (%). CRP=C-reactive protein. URI=upper respiratory tract infection. UTI=urinary tract infection.
Four participants withdrew.
Participant data taken from all five blocks.
Until day 56 related to investigational vaccine.
During the safety observation, most participants in each vaccination group reported at least one unsolicited adverse reaction. The most common abnormalities were transient decreases in lymphocyte and neutrophil counts; and increases in C-reactive protein concentrations, urinary tract infections, and albuminuria in vaccine participants. Lymphocyte count on day 1 after first or second vaccination significantly reduced in all vaccine groups compared with the placebo group then recovered back to baseline 4 days after first or second vaccination. Additionally, on day 1 after first vaccination, lymphocyte counts increased significantly in the placebo group, which was still within normal clinical range (appendix p 13). All laboratory abnormalities were self-limited and resolved in a short period without clinical symptoms. Results were consistent with lymphocyte counts12 and concentrations of C-reactive protein13, 14 reported as pharmacodynamics markers after administration of mRNA vaccines against influenza and rabies.
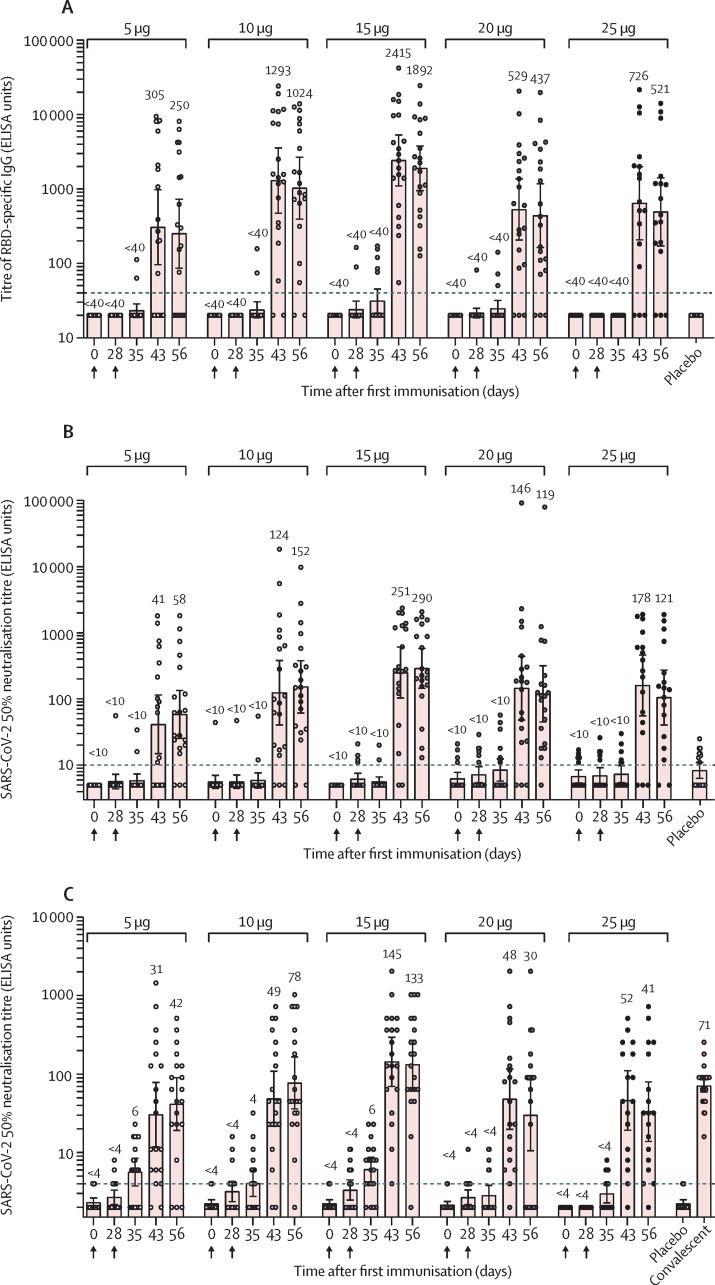
Before vaccination, no participants had detectable serum IgG that recognised the RBD of the SARS-CoV-2 spike protein through ELISA (figure 2A ). 28 days after the first vaccination, only a few participants in the 15 μg and 20 μg groups had detectable concentrations of RBD-specific IgG. 15 days after the second vaccination, RBD-specific IgG antibodies were detected in most participants and the seroconversion rates were 13 (65%) of 20 participants in the 5 μg group, 18 (90%) of 20 in the 10 μg group, 20 (100%) of 20 in the 15 μg group, 17 (85%) of 20 in the 20 μg group, and 13 (81%) of 16 in the 25 μg group. The peak IgG antibody titre was observed in the 15 μg group 15 days after the second vaccination and the geometric mean titre was 2414·8 ELISA units. Geometric mean titres were 305 ELISA units in the 5 μg vaccine group and 1293 units in the 10 μg vaccine group 15 days after the second vaccination, and 529 ELISA units in the 20 μg vaccine group and 726 ELISA units in the 25 μg vaccine group, which were lower than that in the 15 μg group.
Figure 2.
ARCoV antibody and neutralisation responses in phase 1
Geometric mean titres of the receptor binding domain-specific IgG by ELISA (A), neutralising antibodies to a pseudovirus (B), and live SARS-CoV-2 (C). Serum samples of the participants were collected at baseline (day 0), before the second dose (day 28), and after the second dose (days 35, 43, 56). Dots represent a serum sample. Bars represent geometric mean titre (SD). Numbers above dots show the geometric mean titre of the group. Dashed line indicates the lower limit of quantification.
Serum samples were also assessed for neutralising antibodies against SARS-CoV-2 using a pseudovirus-based assay.15 After first immunisation only a few participants developed low titres of neutralising antibodies, whereas after the second immunisation most participants developed high titres of anti-SARS-CoV-2 neutralising antibodies (figure 2B). 28 days after the second vaccination, seroconversion rates increased to 17 (85%) of 20 participants in the 5 μg group, 18 (90%) of 20 in the 10 μg group, 20 (100%) of 20 in the 15 μg group, 19 (95%) of 20 in the 20 μg group, and 14 (88%) of 16 in the 25 μg group. In the 5 μg, 10 μg, and 15 μg groups, geometric mean titres of the pseudovirus 50% neutralising antibodies significantly increased by day 15 and peaked at day 28 after the second vaccination, whereas in the 20 μg and 25 μg groups, titres peaked at day 15 then declined slightly at day 28 after second vaccination (figure 2B). The peak titre of neutralising antibodies was detected in the 15 μg group, and geometric mean titres reached 250·9 and 289·7 ELISA units at days 15 and 28 after second vaccination, respectively.
Consistent with pseudovirus neutralising titres, ARCoV induced high concentrations of neutralising antibodies against live SARS-CoV-2 (figure 2C). 15 days after second vaccination, seroconversion rates for the live SARS-CoV-2 neutralising antibody were 16 (80%) of 20 in the 5 μg group, 18 (90%) of 20 in the 10 μg group, 19 (95%) of 20 in the 15 μg group, 19 (95%) of 20 in the 20 μg group, and 15 (94%) of 16 in the 25 μg group. After second vaccination, geometric mean titres of the live SARS-CoV-2 neutralising antibody in all vaccine groups rapidly increased (figure 2C). Peak titre of neutralising antibodies against live SARS-CoV-2 was detected in the 15 μg group 15 days after second vaccination and the geometric mean titre was about twofold higher than that of a panel of patients recovering from COVID-19 (figure 2C). As expected, the correlation of RBD-specific IgG antibody and neutralising antibodies to pseudovirus and live SARS-CoV-2 was strong in the vaccinated participants by days 43 and 56 (r>0·85, p<0·0001; appendix p 19).
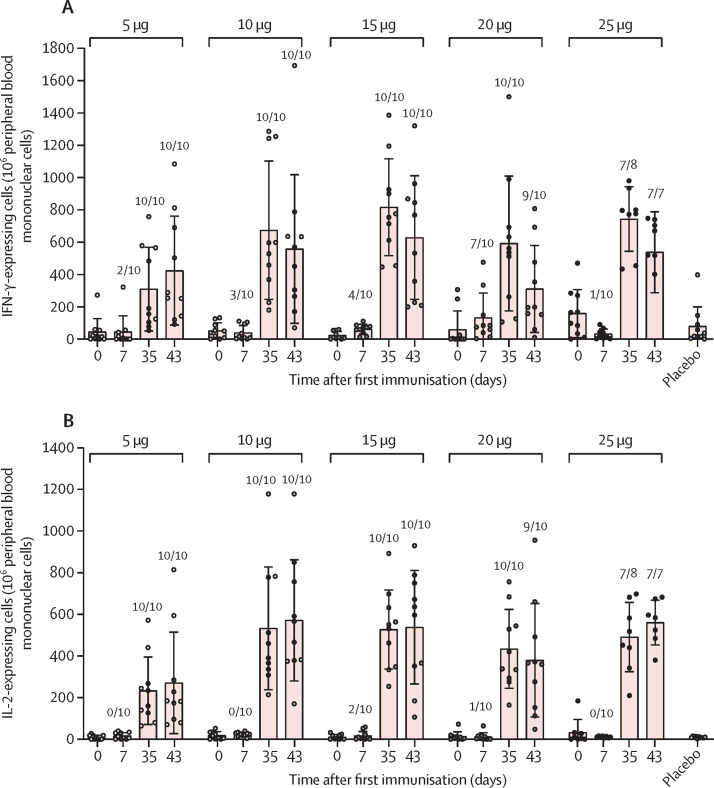
Finally, we performed ELISpot assays against the SARS-CoV-2 RBD using peripheral blood mononuclear cells isolated from the participants in the phase 1 trial. SARS-CoV-2-specific T-cell responses were measured by detecting the production of IFN-γ and IL-2 in response to SARS-CoV-2 RBD targeting peptide pool stimulation (figure 3A, B ). Following first vaccination, only a small proportion of participants in each vaccine group was positive with IFN-γ-expressing cells. However, after second vaccination all participants in the 5 μg, 10 μg, 15 μg, and 20 μg groups were positive for IFN-γ-expressing cells and only one participant in the 25 μg group was negative (figure 3A). Similarly, all participants, except one in the 25 μg group, were positive with IL-2-expressing cells at day 7 after second vaccination (figure 3B). Mean values for IFN-γ and IL-2-expressing cells were maintained 15 days after the second vaccination. These results suggested that ARCoV elicited strong SARS-CoV-2-specific T-cell responses.
Figure 3.
ARCoV-induced specific T-cell responses in phase 1
Blood samples of participants were collected on days 0 (baseline), 7, 35, and 43. The number of IFN-γ-expressing cells (A) and IL-2-expressing cells (B) from peripheral blood mononuclear cells were measured by ELISpot. Dots represent a blood sample. Bars represent mean (SD). Ratio of positive samples for each group are shown above the dots.
Discussion
In this phase 1 clinical trial, ARCoV, a SARS-CoV-2 mRNA vaccine encoding the RBD of spike protein, demonstrated an acceptable tolerability and safety profile in Chinese adults. No serious adverse event occurred during this trial. The primary grade 3 systemic adverse event was fever, which showed an obvious dose-dependent response. When adverse events were graded according to the guidelines for vaccine clinical trials issued by National Medical Products Administration, China, about 50% of participants in the 15 μg, 20 μg, and 25 μg groups after first vaccination and 88% of participants in the 25 μg group after the second vaccination had grade 3 fever. However, when adverse events were graded according to the guidelines for vaccine clinical trials issued by the US FDA, 30% of participants had grade 3 fever in the 15 μg group of ARCoV. Thus, the incidence of grade 3 fever of ARCoV was similar to the trial results of another RBD-based mRNA vaccine, BNT162b1.16 The incidence of grade 3 fever of BNT162b1 varied significantly in the same 30 μg group in adults aged 18–55 years, with none (0%) of 12 in the USA,17 one (8%) of 12 in Germany,18 and four (17%) of 24 in China,16 suggesting varying tolerability in different populations. Additionally, the incidence of grade 3 fever of the two spike protein-based mRNA vaccines, mRNA-127319, 20 and BNT162b2,17, 21 also varied between the phase 1 and 3 trials. The safety profile of ARCoV in large-scale populations is under investigation. Previous studies17, 18, 19 showed that solicited systemic adverse events were more frequent and more severe at higher doses, which were consistent with our data. Meanwhile, several studies17, 18, 19 have reported more frequent and severe solicited systemic adverse events following the second vaccination than we found. However, this finding was not observed with ARCoV: the incidence of adverse events were similar after first and second vaccination.
ARCoV was immunogenic because participants in all groups had strong humoral and cellular immunity after the second vaccination. Across the 5 μg, 10 μg, and 15 μg groups, RBD-specific IgG, neutralising antibodies to pseudovirus or live SARS-CoV-2, and secretion of IFN-γ and IL-2 cytokines were induced in a dose-dependent manner. For neutralising antibodies to live SARS-CoV-2, the geometric mean titres in the 15 μg group were significantly higher than those in the 5 μg and 10 μg groups at day 15 after the second vaccination. Notably, the second vaccination with 15 μg of ARCoV elicited neutralising geometric mean titres about two times greater than that from the convalescent sera from COVID-19 patients, showing promising efficacy against COVID-19.22 The immunogenicity of ARCoV was dose-dependent from 5 μg to 15 μg, but increasing the antigen dose from 20 μg to 25 μg did not improve immunogenicity, which was similar to BNT162b1 and BNT162b2.17 The underlying mechanism for this unusual phenotype remains to be determined and could partly be associated with the imbalance of host innate and adaptive immune responses.23
Cellular immune responses are protective in SARS-CoV-2 infection and T-helper-1-biased responses might reduce the risk of vaccine-associated antibody-dependent enhancement of virus replication or other adverse reactions.24 The SARS-CoV-2 RBD was identified as the key antigen to induce a strong T-helper-1-biased cellular response.11 In phase 1 trials, mRNA-based vaccines (ARCoV, BNT162b1)16 and adenovirus-vector vaccines (ChAdOx1,25 Ad5 vectored COVID-19 vaccine)26 induced a strong T-helper-1-cell response in most participants. A primary goal of vaccination is to induce long-term immunity. In humans, T cells contribute to the resolution of SARS-CoV infection and can form a long-lasting memory response to SARS-CoV up to 11 years post-infection in recovered patients.27 For SARS-CoV-2, increasing evidence indicates that T cells play a major role in the resolution of COVID-19,28, 29 and SARS-CoV-2-specific memory T cells were detected in convalescent patients, even in exposed individuals who are seronegative.30, 31 Among cytokine profiles of spike-responsive memory T cells, production was dominated by the expression of IL-2 and IFN-γ that were highly expressed by CCR6− subsets.32, 33 However, presence of SARS-CoV-2-specific T cells in vaccinated participants is a promising sign that vaccination might give rise to immunity; but whether these T cells provide long-term protection remains to be tested.
This study has several limitations. First, data interpretation is based on a small sample size and more data from phase 2 and 3 trials will provide further data to evaluate the safety and efficacy of ARCoV. Second, the trial was restricted to Chinese adult participants aged 18–59 years, and trials in older adults are taking place. Furthermore, the long-term safety and tolerability of ARCoV and persistence of the elicited immune responses is yet to be assessed; these aspects are being investigated strictly according to the study protocol.
In conclusion, this study confirmed the safety, tolerability, and immunogenicity profile of the SARS-CoV-2 mRNA vaccine, ARCoV. ARCoV has an excellent stability profile that can be stored and transported under refrigerated conditions, which is of great convenience for vaccine application to the public. A multi-regional phase 3 clinical trial is currently underway to test the efficacy of ARCoV.
Data sharing
The individual participant data that underlie the results reported in this Article will be shared after deidentification (text, tables, figures, and appendices). Individual participant data will be available beginning 3 months and ending 1 year after publication. Supporting clinical documents, including study protocol, statistical analysis plan, and the informed consent form, will be available immediately following publication for at least 1 year. Researchers who provide a scientifically sound proposal will be allowed access to individual participant data. Proposals should be directed to gaohainv@163.com, qincf@bmi.ac.cn, bo.ying@abogenbio.com, or ljli@zju.edu.cn. These proposals will be reviewed and approved by the funder, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.
Declaration of interests
C-FQ and BY are co-inventors on pending patent applications related to the ARCoV mRNA vaccine. LW is an employee of Suzhou Abogen Biosciences. S-YY and ZH are employees of Walvax. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was supported by the National Key Research and Development Project of China (2020YFC0842200, 2020YFA0707801), the Special Grant from AMS (JK2020NC002), the National Natural Science Foundation China (number 82041044), and Tsinghua University Spring Breeze Fund (2020Z99CFG008). C-FQ was supported by the National Science Fund for Distinguished Young Scholar (number 81925025), and the Innovative Research Group (number 81621005) from the National Natural Science Foundation China, and the Innovation Fund for Medical Sciences (number 2019-I2M-5-049) from the Chinese Academy of Medical Sciences. We thank all participants in the trial and members of the data and safety monitoring board: Jielai Xia (Air Force Medical University), Xuanyi Wang (Fudan University), Dongliang Yang (Tongji Medical College, Huazhong University of Science and Technology), Fuchun Zhang (The Eighth People's Hospital of Guangzhou), and Panyong Mao (Department of Infectious Disease Medicine, Fifth Medical Center, PLA General Hospital). The members have expertise in infectious disease prevention and treatment, vaccine and drug development, epidemiology, and statistics.
Contributors
L-JL and G-LC are the principal investigators of this trial. C-FQ, BY, ZH, and LW initiated, designed, and manufactured the vaccine candidate. ZH and S-YY designed the trial and study protocol. H-NG, G-PS, X-HD, NL, JS, Y-HG, Z-WS, K-QW, M-FZ, C-GP, and QJ contributed to operating the trial and participant safety. X-FL, Y-QD, HZ, N-NZ, and Y-FZ contributed to laboratory testing. M-LC, C-FQ, X-FL, HZ, Y-QD, S-YY, S-CC, Y-HL, D-HZ, X-HW, LN, H-HS, and CD contributed to data analysis and manuscript writing. All authors reviewed all the data and approved the final version of the manuscript. All authors reviewed and verified the data in the study, had full access to the data in the study, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Eng JMed. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Weekly epidemiological update on COVID-19. Dec 21, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---21-december-2021
- 4.WHO Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draftlandscape-of-covid-19-candidate-vaccines
- 5.Alameh MG, Weissman D, Pardi N. Messenger RNA-based vaccines against infectious diseases. Curr Top Microbiol Immunol. 2020 doi: 10.1007/82_2020_202. published online April 17. [DOI] [PubMed] [Google Scholar]
- 6.Mascola JR, Fauci AS. Novel vaccine technologies for the 21st century. Nat Rev Immunol. 2020;20:87–88. doi: 10.1038/s41577-019-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Tther. 2019;27:757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai W, Zhang X, Drelich A, et al. A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res. 2020;30:932–935. doi: 10.1038/s41422-020-0387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zang J, Gu C, Zhou B, et al. Immunisation with the receptor-binding domain of SARS-CoV-2 elicits antibodies cross-neutralising SARS-CoV-2 and SARS-CoV without antibody-dependent enhancement. Cell Dis. 2020;6:61. doi: 10.1038/s41421-020-00199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang NN, Li XF, Deng YQ, et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271. doi: 10.1016/j.cell.2020.07.024. 83.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 13.Tsai MY, Hanson NQ, Straka RJ, et al. Effect of influenza vaccine on markers of inflammation and lipid profile. J Lab Clin Med. 2005;145:323–327. doi: 10.1016/j.lab.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Doener F, Hong HS, Meyer I, et al. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine. 2019;37:1819–1826. doi: 10.1016/j.vaccine.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Nie J, Li Q, Wu J, et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Hui A, Zhang X, et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomised, placebo-controlled, double-blind phase 1 study. Nat Med. 2021;27:1062–1070. doi: 10.1038/s41591-021-01330-9. [DOI] [PubMed] [Google Scholar]
- 17.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. New Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 19.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. New Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. New Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 23.Linares-Fernandez S, Lacroix C, Exposito JY, Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 25.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng OW, Chia A, Tan AT, et al. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34:2008–2014. doi: 10.1016/j.vaccine.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canete PF, Vinuesa CG. COVID-19 makes B cells forget, but T cells remember. Cell. 2020;183:13–15. doi: 10.1016/j.cell.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158. doi: 10.1016/j.cell.2020.08.017. 68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren X, Wen W, Fan X, et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184:1895. doi: 10.1016/j.cell.2021.01.053. 913.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodda LB, Netland J, Shehata L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169. doi: 10.1016/j.cell.2020.11.029. 83.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual participant data that underlie the results reported in this Article will be shared after deidentification (text, tables, figures, and appendices). Individual participant data will be available beginning 3 months and ending 1 year after publication. Supporting clinical documents, including study protocol, statistical analysis plan, and the informed consent form, will be available immediately following publication for at least 1 year. Researchers who provide a scientifically sound proposal will be allowed access to individual participant data. Proposals should be directed to gaohainv@163.com, qincf@bmi.ac.cn, bo.ying@abogenbio.com, or ljli@zju.edu.cn. These proposals will be reviewed and approved by the funder, investigator, and collaborators on the basis of scientific merit. To gain access, data requesters will need to sign a data access agreement.