Abstract
A new extended-release buprenorphine (XR), an FDA-indexed analgesic, has recently become available to the laboratory animal community. However, the effectiveness and dosing of XR has not been extensively evaluated for rats. We investigated XR’s effectiveness in attenuating postoperative hypersensitivity in a rat incisional pain model. We hypothesized that high dose of XR would attenuate mechanical and thermal hypersensitivity more effectively than the low dose of XR in this model. We performed 2 experiments. In experiment 1, male adult Sprague–Dawley rats (n = 31) were randomly assigned to 1 of the 4 treatment groups: 1) saline (saline, 0.9% NaCl, 5 mL/kg, SC, once); 2) sustained-release buprenorphine (Bup-SR; 1.2 mg/kg, SC, once), 3) low-dose extended-release buprenorphine (XR-Lo; 0.65 mg/kg, SC, once), and 4) high-dose extended-release buprenorphine (XR-Hi; 1.3 mg/kg, SC, once). After drug administration, a 1 cm skin incision was made on the plantar hind paw under anesthesia. Mechanical and thermal hypersensitivity were evaluated 1 d before surgery (D-1), 4 h after surgery (D0), and for 3 d after surgery (D1, D2, and D3). In experiment 2, plasma buprenorphine concentration (n = 39) was measured at D0, D1, D2, and D3. Clinical observations were recorded daily, and a gross necropsy was performed on D3. Mechanical and thermal hypersensitivity were measured for 3 d (D0-D3) in the saline group. Bup-SR, XR-Lo, and XR-Hi effectively attenuated mechanical hypersensitivity for D0-D3. Plasma buprenorphine concentrations remained above 1 ng/mL on D0 and D1 in all treatment groups. No abnormal clinical signs were noted, but injection site reactions were evident in the Bup-SR (71%), XR-Lo (75%), and XR-Hi (87%) groups. This study indicates that XR-Hi did not attenuate hypersensitivity more effectively than did XR-Lo in this model. XR 0.65 mg/kg is recommended to attenuate postoperative mechanical hypersensitivity for up to 72 h in rats in an incisional pain model.
Abbreviations: Bup-SR, sustained-release buprenorphine; XR, extended-release buprenorphine; XR-Hi, high-dose extended-release buprenorphine; XR-Lo, low-dose extended-release buprenorphine
Introduction
Providing effective postoperative analgesia is an important component of laboratory animal welfare, regulatory compliance and high-quality animal research.22 Many laboratory rodent surgical procedures require a prolonged post-procedural period of analgesia; however, repeat dosing of analgesics requires additional handling and injections, which can be associated with physical or psychologic stress.11,17 To minimize this stress, alternative dosing techniques including the use of gel-based oral compounds,34 medicated drinking water,23,30 and flavored tablets41 can be used to provide analgesia. However, to ensure accurate and consistent analgesia, sustained-release analgesics can be used to minimize the need for repeated dosing while maintaining therapeutic drug levels. Sustained-release buprenorphine (Bup-SR) is commonly used to provide postoperative analgesia in laboratory rodents because a single-dose provides analgesia for 2 to 3 d.8,16,34 Although sustained release buprenorphine is effective and convenient, it is not pharmaceutical grade. This feature can pose regulatory and compliance challenges that may limit its use.14
Recently, an extended-release formulation of buprenorphine (XR) became available to the laboratory research community. This new formulation of buprenorphine is pharmaceutical grade, c-GMP compliant, and FDA-indexed.14 XR contains buprenorphine hydrochloride encapsulated in a lipid solution that slows and sustains drug diffusion and release.3,15,31 XR is reported to provide effective plasma concentration levels in rats and mice for up to 3 d after injection.13 Recent work from our lab indicated that XR effectively attenuated postoperative hypersensitivity in male C57BL/6 mice in an incisional paw pain model for up to 2 d.32 However, the efficacy and dosing for rats has not been extensively evaluated. The aim of this study was to investigate XR’s effectiveness and whether it provides dose dependent attenuation of mechanical or thermal hypersensitivity when using a low dose (0.65 mg/kg, the manufacture’s recommended dose) or a higher dose (1.3 mg/kg, or twice the manufacture’s recommended dose) in a rat plantar incisional pain model. The plasma buprenorphine concentration of Bup-SR and the low and high dose of XR were also measured for comparison. We hypothesized that high dose of XR would attenuate mechanical and thermal hypersensitivity more effectively than the low dose of XR.
Materials and Methods
Rats.
Three-mo-old male Sprague–Dawley rats (Rattus norvegicus; n = 70; weighing 283- 360g, Charles River, Wilmington, WA) were used for the study. Based on the vendor report, the rats were free of Theiler virus, reovirus type 3, Kilham rat virus, Sendai virus, murine adenovirus types 1 and 2, rat parvovirus, Toolan H1, Hantaan, pneumonia virus of mice, rat coronavirus, rat minute virus, lymphatic choriomeningitis virus, Mycoplasma pulmonis, and endo-and ectoparasites. Rats were housed in pairs in reusable conventional rat cages (Allentown, Allentown, NJ) with Sani-Chips bedding (Envigo-7090 Teklad Sani-Chips, Indianapolis, IN) and shredded paper as enrichment (Enviro-dri paper bedding, Shepherd Specialty Papers, Milford, NJ) on a 12:12h dark:light cycle, at 70 to 74 °F (21 to 23 °C), and 30% to 70% relative humidity. Rats were given a commercial diet (Teklad Global 18% Protein Rodent Diet 2018, Harlan Laboratories, Madison, WI) and reverse osmosis purified water ad libitum. Experiments were conducted with approval by the Administrative Panel for Laboratory Animal Care at Stanford University. All rats were treated in accordance with the Guide for the Care and Use of Laboratory Animals.22 Rats were acclimated in the facility for at least 3 d before the start of the experiment. All rats were weighed before surgery, and each day after the completion of thermal and mechanical hypersensitivity testing (at approximately 1000 h) from day -1 to day +3. At the conclusion of the study, rats that underwent surgery were euthanized by carbon dioxide asphyxiation, and gross necropsy was performed in consultation with a board-certified veterinary pathologist to detect any abnormality. The rats for the plasma collection group were euthanized by exsanguination with secondary bilateral thoracotomy.
Study design.
This study consisted of 2 experiments—Experiment 1: hypersensitivity testing and Experiment 2: plasma drug concentration analysis. For both experiments, Bup-SR, XR-Lo, and XR-Hi were administered using a 1ml Luer lock syringe and 22 G needle due to the viscosity of the drug; saline was administered using a 1 mL tuberculin syringe and 25 G needle. All treatments were administered once, subcutaneously, over the left shoulder approximately 5 min before the skin incision. Digital pressure was applied for about 5 s immediately after the injections to prevent the back flow of the drugs.
Experiment 1: hypersensitivity testing.
Rats (n = 31) underwent paw incisional surgery after being randomly assigned to receive one of the 4 treatments: saline (Saline; n = 8; 5 mL/kg SC; 0.9% NaCl, Hospira, Lake Forest, IL); sustained release buprenorphine (Bup-SR; = 7; 1.2 mg/kg SC; buprenorphine SR-LAB, 1 mg/mL, Zoopharm, Fort Collins, CO); low dose extended release buprenorphine (XR-Lo; n = 8; 0.65 mg/kg SC; 1.3 mg/mL, Fidelis, North Brunswick, NJ); high dose extended release buprenorphine (XR-Hi; n = 8; 1.3mg/kg SC, 1.3 mg/mL, Fidelis, North Brunswick, NJ). All rats in this experiment were tested for both mechanical and thermal hypersensitivity before euthanasia on 3 d after surgery.
Surgery.
Rats were placed in an induction chamber, and anesthesia was induced with 4% to 5% isoflurane in 100% oxygen with a delivery rate of 2 L/min. Anesthesia was maintained with 2% to 2.5% isoflurane and 100% oxygen with a delivery rate of 1 L/min using a nose cone. Sterile eye ointment was applied before surgery, and rats were kept warm using circulating warm-water blanket for the entire duration of the surgery. A single dose of Cefazolin (30 mg/kg SC; GlaxoSmithKline, Research Triangle Park, NC) and prewarmed 0.9% saline (5 mL/kg SC) were administered on the right shoulder of the rats prior to surgery.
The surgery was performed aseptically as required by IACUC Guidelines for Rodent Survival Surgery as previously described.6 Briefly, rats were placed in sternal recumbency and the plantar surface of the left (ipsilateral) hind paw was aseptically prepared by applying 3 alternative betadine swabs and alcohol wipes. Rats were then covered with a sterile drape. After the rat reached a surgical plane of anesthesia (as determined by a lack of withdrawal response to toe pinch), a 1 cm longitudinal skin incision was made using #15 blade. The incision began 0.5 cm below the heel and extended for 1 cm toward the digits. After incision, the plantaris muscle was bluntly dissected from the surrounding connective tissue and raised by inserting curved iris tissue forceps under the muscle. The muscle was incised 0.5 cm longitudinally without disturbing its attachment and another curved iris tissue forceps was inserted into the muscle incision for 5 s to cause tissue injury. Saline was then applied to the surgical area, and the extra fluid was collected using sterile gauze after the curved forceps were removed. The incision was closed using 2 horizontal mattress sutures with 4-0 silk suture. Rats were moved to the recovery station with a clean cage and heating blanket and monitored until they were fully recovered from anesthesia.
Behavioral testing.
Rats were acclimated to the veterinary facility for at least 3 d before the baseline tests. Before every behavioral test, rats were also acclimated to the testing environment for 15 min. Rats underwent behavioral testing of mechanical and thermal hypersensitivity on the day before surgery to acquire baseline data [(Day -1 (D-1)], and at 4 h after surgery on day-0 (D0), followed by 3 consecutive daily tests (D1, D2 and D3). Mechanical testing was always performed before thermal testing. Each day after the completion of the tests, test chambers were disinfected with Virkon-S 1% solution (Pharmacal Research Labs INC, Waterbury, CT) for 5 min and rinsed with tap water.
Mechanical hypersensitivity testing.
Rats were acclimated for a minimum of 15 min in the testing room before they were transferred to a clear plastic chamber (21 × 27 × 15 cm) on an elevated wire mesh platform for an additional 15 min of acclimation prior to testing. A calibrated Semmes-Weinstein von Frey filament (8 g) was applied to the plantar surface of both the ipsilateral (test) and contralateral (control) hind paws at random locations, avoiding the heel, pads and toes. For each trial, the same 8g filament was applied 10 times for 1 to 2 s with an interstimulus interval of approximately 5 s until a withdrawal response was observed. Mechanical hypersensitivity was defined as an increase in the frequency of paw withdrawals. Throughout the entire experiment, mechanical hypersensitivity testing was performed first, followed by thermal hypersensitivity testing.
Thermal hypersensitivity testing.
Rats were placed individually in a clear plastic chamber (23 × 13 × 13 cm) with 15 min for acclimation before being tested on an elevated glass platform. The average temperature of the glass platform was 31.5 °C. Thermal stimuli were produced by a radiant heat generated from a 50-W light bulb (Plantar Analgesia Meter, IITC Life Science, Woodland Hills, CA) that was focused on the middle of the plantar surface of each hind paw. 20 s was used as a maximal threshold exposure time to prevent tissue injury. Each paw was tested 4 times, with a minimum of 4 min between each trial. The right (contralateral) hind paw was used as a control for every left (ipsilateral) hind paw of a particular rat. Withdrawal latency was determined by taking the average of the last 3 trials. Thermal hypersensitivity was defined as a significant decrease in paw withdrawal latency after the onset of focal thermal stimuli.
Clinical observations and gross pathology.
Body weight was recorded daily, and rats were observed for abnormal behaviors, such as sedation, hyperactivity, greasy fur, or skin rection throughout the experiment. At the end of the study on D3, rats were euthanized by CO2 inhalation and gross necropsy was performed.
Experiment 2: plasma drug concentration analysis.
To analyze plasma drug concentration, a total of 39 rats were randomly assigned to the following treatment groups: Saline; 5 mL/kg SC (n = 3); XR-Hi 1.3 mg/kg SC (n = 12); XR-Lo 0.65 mg/kg SC (n = 12); Bup-SR 1.2 mg/kg SC (n = 12). For drug administration, rats were anesthetized with 4% to 5% of isoflurane and 100% oxygen with a flow rate of 2 L/min in an induction chamber; 2% to 2.5% of isoflurane and 100% oxygen with the flow rate of 1 L/min with a nose cone were used to maintain the anesthesia. The rats recovered in a warm recovery cage as described in experiment 1. For blood collection, the rats were anesthetized as described for drug administration. After a surgical plane of anesthesia was confirmed by toe pinch, whole blood was collected by cardiac puncture using heparinized 10 mL syringes and 19 G needles h. The first blood collection was performed at 4 h after drug injection and was repeated every 24 h for 3 d (3 rats daily per group except for the saline group). For the saline group, blood collection performed only at 4 h after injection.
Euthanasia was performed by exsanguination of rats, with secondary bilateral thoracotomy performed using a #11 blade while rats remained anesthetized. Blood samples were transferred to 10 mL lithium heparin tubes and were centrifuged at 1455 x g for 20 min. The plasma was separated into 5 mL cryogenic tubes and stored at -80 °C until shipment for drug concentration analyses. About 3 mL of plasma from each rat was shipped overnight on dry ice to the McWhorter School of Pharmacy, Pharmaceutical Sciences Research Institute (Samford University, Birmingham, AL) for measurement of plasma buprenorphine concentrations by using liquid chromatography-tandem mass spectrometry (HPLC MS/MS).
Buprenorphine standard spiking solutions were prepared in 50:50 DI water: acetonitrile to provide concentrations ranging from 0.2 to 200 ng/mL. An internal standard (50 ng/mL terfenadine) was added to both the plasma samples and standards (100 μL). Acetonitrile (1 mL) was added to precipitate the plasma proteins, and the mixture was vortexed and centrifuged. The organic layer was transferred to a clean test tube and evaporated to dryness under nitrogen in a water bath set at 50 °C. The samples were reconstituted in dilution solvent and analyzed by HPLC MS/MS. Matrix matched standards and QC samples were prepared using blank control plasma.
Statistical analysis.
Mean withdrawal responses were analyzed by using 2-way repeated measures ANOVA with Bonferroni correction for multiple comparisons (R Development Core Team, 2015) to determine significance of differences in withdrawal responses by and between group and over time. Data are expressed as mean ± SEM. A P value of less than 0.05 was considered significant.
Results
Body weight.
The starting body weights of rats in this study was 330 ± 3.9 g. Body weights in all groups did not significantly differ throughout the study as compared with their D-1 values (Figure 1).
Figure 1.

Daily body weight (mean ± SEM) of Saline, Bup-SR, XR-Lo, and XR-Hi treatment groups. Surgery was performed on day 0 as indicated by the arrowhead.
Experiment 1: Mechanical hypersensitivity.
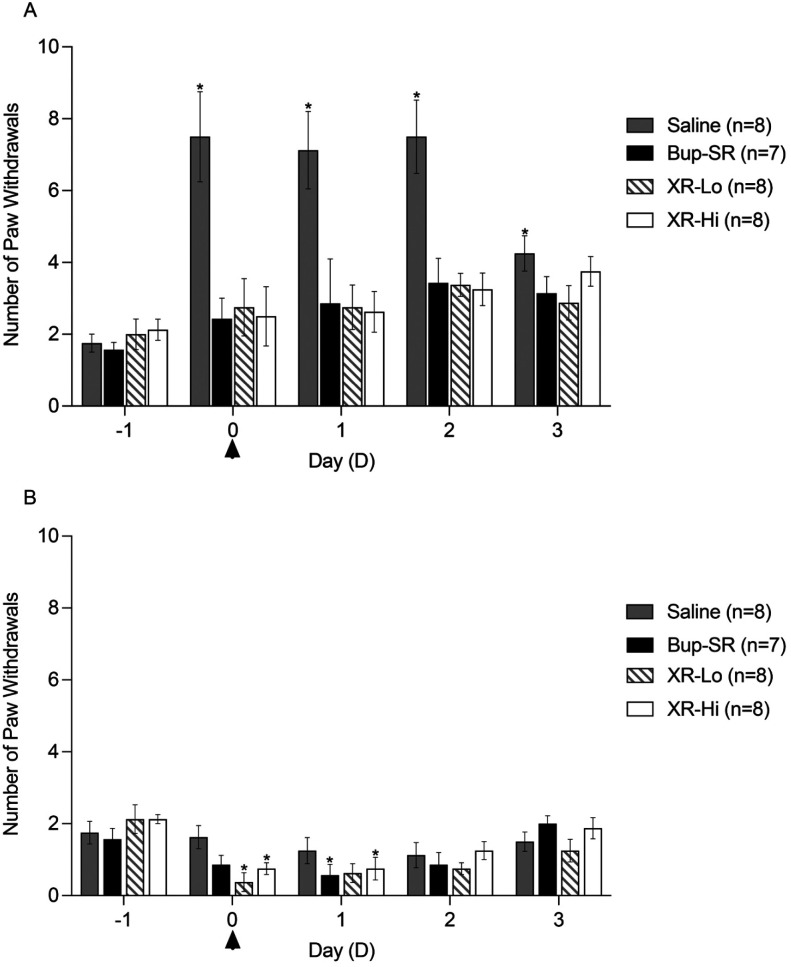
Responses to mechanical stimuli in ipsilateral hind paws (Figure 2A) were not statistically different across all treatment groups prior to treatment (D-1). In the saline group, mechanical hypersensitivity significantly increased on D0, D1, D2, and D3 as compared with the response on D-1. Mechanical hypersensitivity of the Bup-SR, XR-Lo and XR-Hi groups on D0, D1, D2, and D3 did not statistically differ as compared with the D-1 values. In contralateral hind paws, mechanical hypersensitivity (Figure 2B) was not different among treatment groups on D-1. Contralateral mechanical hypersensitivity was not significantly different from the baseline (D-1) in saline group at any timepoint throughout the study. Contralateral hypersensitivity was significantly decreased in Bup-SR on D1, XR-Lo on D0, and XR-Hi on D0 and D1 as compared with D-1.
Figure 2.
A) Number of paw withdrawals of ipsilateral hind paw in rats. B) Number of paw withdrawals of contralateral hind paw in rats. Surgery was performed on D0 as indicated by the arrowhead. Data presented as number of paw withdrawals (mean ± SEM). *, Value significantly different (P < 0.05) when compared with baseline (D-1) value of the same treatment group; #, value is significantly (P < 0.05) different from that for other buprenorphine groups at the same time point.
Thermal hypersensitivity.
Thermal latencies in ipsilateral hind paws (Figure 3A) were not significantly different among treatment groups at D-1. In the saline group, thermal latencies were significantly lower on D0, D1, D2, and D3 as compared with D-1. When compared with their baseline values (D-1), thermal latencies were significantly lower on D0, D1, D2, and D3 in all groups. Thermal latencies in contralateral hind paws (Figure 3B) in all treatment groups on D0, D1, D2, and D3 were not significantly different from their baseline values (D-1).
Figure 3.
A) Paw withdrawal latencies of ipsilateral hind paw in rats. B) Paw withdrawal latencies of contralateral hind paw in rats. Surgery was performed on D0 as indicated by the arrowhead. Data presented as thermal latencies in seconds (mean± SEM). *, Value significantly different (P < 0.05) when compared with baseline (D-1) value of the same treatment group; #, value is significantly (P < 0.05) different from that for other buprenorphine groups at the same time point.
Clinical observations and gross pathology.
No abnormal behaviors, except for mild sedation in Bup-SR, XR-Lo and XR-Hi groups, were noted in all rats throughout the study period. On D0, greasy fur was observed near the injection site in 43% of the Bup-SR group, 62% of the XR-Lo group, and 25% of the XR-Hi group. No signs of skin irritation or inflammation and hair loss were observed. All rats underwent necropsy at the end of the study (D3). The necropsies were performed by individuals who were blind to the treatments given to the rats. No gross lesions were observed in saline groups. However, a marked increase in adherence of skin to the muscle (as compared with the saline group) and subcutaneous nodules of varying size were observed at the injection site (left shoulder) of the rats given Bup-SR (71%; 5 of 7 rats) (nodule size of approximately 2.5 to 3 cm), XR-Lo (75%; 6 of 8 rats) (nodule size of approximately 0.6 to 1.1cm) and XR-Hi (87%; 7 of 8 rats) (nodule size of approximately 1 to 1.5 cm). The lumps contained an oily substance that leaked out when the lumps were opened.
Experiment 2: plasma buprenorphine concentration.
On D0 (4 h after drug administration) (Figure 4), plasma concentrations were 3.1 ± 1.0 ng/mL in Bup-SR, 1.8 ± 0.1 ng/mL in XR-Lo, and 1.7 ± 0.1 ng/mL in XR-Hi; these values were not statistically different. The highest plasma concentration of Bup-SR was observed on D0 and significantly fell on D1, D2, and D3 (1.0 ± 0.4, 0.8 ± 0.2, 0.5 ± 0.1 ng/mL respectively). In XR-Lo group, plasma values on D1 (1.4 ± 0.2 ng/mL), D2 (0.8 ± 0.2 ng/mL), and D3 (0.7 ± 0.1 ng/mL) were not statistically different from D0. Values for XR-Hi rats did not differ significantly throughout the study (D1, 3.2 ± 0.5 ng/mL; D2, 1.9 ± 0.5 ng/mL, and D3, 0.7 ± 0.0 ng/mL) as compared with D0 values (D0, 1.7 ± 0.1).
Figure 4.

Plasma concentration (ng/mL, mean ± SEM) in rats treated with either Bup-SR, XR-Lo or XR-Hi was determined at each time point (n = 3/group/timepoint). Samples were analyzed on D0 (4 h), D1 (24 h), D2 (48 h), and D3 (72 h) after administration. *, Value significantly different (P < 0.05) when compared with D0 value of the same treatment group.
Discussion
This is the first study demonstrating that extended-release buprenorphine, dosed at 0.65 mg/kg, effectively attenuates mechanical hypersensitivity in a rat paw incisional pain model for at least 3 d. XR-Hi did not attenuate hypersensitivity more effectively than XR-Lo. Buprenorphine plasma concentration for all treatment groups (Bup-SR, XR-Lo, and XR-Hi) was maintained at or above the clinically effective plasma concentration for 1 d. We recommend the use of XR at a dose of 0.65 mg/kg for minor surgical procedures in rats as an alternative for 1.2 mg/kg of Bup-SR.
The aim of this study was to evaluate the effectiveness and dose dependency of the newly marketed, lipid bound extended-release buprenorphine (XR) using mechanical and thermal hypersensitivity testing in rats used as a paw incisional pain model. Our lab has extensive experience with the incisional pain model in rats,8,26,29,32,34,41 which produces mild to moderate pain that is reproducible and quantifiable.6 With our previous studies, mechanical hypersensitivity in the control group lasted from 1 to 4 d,8,26,29,32,34,41 while thermal hypersensitivity lasted for 3 to 4 d.26,29,34,41 In this current study, mechanical and thermal hypersensitivity in the saline-treated group was observed as early as 4 h and lasted for 3 d.
Sustained analgesia is a technical refinement and an important component of effective postoperative management in both laboratory and companion animals. Sustained release buprenorphine (Bup-SR) is widely used for pain management in laboratory rodents as it provides a sustained period of analgesia without the need for repeated dosing and restraint. Past research has indicated that Bup-SR provides effective analgesia for 2 to 3 d in a rat tibial defect model.16 The current study found that one injection of Bup-SR at 1.2 mg/kg SC effectively attenuated mechanical, but not thermal hypersensitivity, for 3 d in a rat incisional pain model. Previous work showed that Bup-SR provided effective attenuation of mechanical hypersensitivity (von Frey monofilament) for 2 to 3 d34,41 but reports were mixed with regard to thermal hypersensitivity (including effective attenuation for 4 to 5 d16,34 and no attenuation seen41). Previous research has indicated that attenuation of thermal hypersensitivity may require a higher dose of buprenorphine than is needed for attenuation of mechanical hypersensitivity.39 For example, a study that used the paw incisional pain model found that the dose of morphine needed to attenuate thermal hypersensitivity (1.8 mg/kg SC) was higher than that needed to attenuate mechanical hypersensitivity (1.5 mg/kg SC).39 We suspect that the dose of Bup-SR needed to attenuate thermal hypersensitivity in the paw incisional pain model is higher than what was used in this current study.
Lipid bound extended-release buprenorphine (XR) is a new FDA-indexed formulation of long-lasting buprenorphine. In this study, we evaluated 2 doses of XR, 0.65 mg/kg (the manufacturer’s recommended dose for rats) and 1.3 mg/kg (twice the manufacture’s recommended dose), to identify dose dependent mechanical and thermal hypersensitivity attenuation. We found that both doses (0.65 and 1.3 mg/kg) effectively attenuated mechanical hypersensitivity with an onset as early as 4 h and lasting up to 3 d (D0, D1, D2 and D3). This is similar to the results of a recently published study by our group in which we found that low (3.25 mg/kg) and high (6.5 mg/kg) doses of XR effectively attenuated mechanical hypersensitivity in male C57BL/6 mice in an incisional pain model.32 The onset of XR may be earlier than 4 h after surgery, but we decided to use this time point to eliminate the possibility of interference from residual effects of the inhaled anesthetic. Similar to the outcome for Bup-SR, we found that neither dose of XR attenuated thermal hypersensitivity, which we suspect may mean that a higher dose of buprenorphine is necessary to attenuate thermal hypersensitivity.
Bup-SR provides sustained buprenorphine plasma concentration levels that remain above the effective concentration of 1 ng/mL9,19 for 48 to 72 h.16 XR also provides sustained buprenorphine plasma concentration levels, but its plasma concentration in rats has not been extensively investigated. In the current study, on D0 (4 h after drug administration) the highest buprenorphine plasma concentration was achieved with Bup-SR (3.1 ± 1 ng/mL) which significantly decreased on D1, D2, and D3 (1.0 ± 0.4, 0.8 ± 0.2, and 0.5 ± 0.1 ng/mL, respectively). On D0, XR-Lo and XR-Hi also reached the clinically effective plasma concentrations (1.8 ± 0.1 ng/mL and 1.7 ± 0.1 ng/mL, respectively) but lower than the level of Bup-SR on D0 (3.1 ± 1 ng/mL). For D1-D3, the plasma buprenorphine concentration of XR-Lo remained steady. On D2, the XR-Lo group had the lowest clinically effective plasma level of buprenorphine (0.8 ± 0.2 ng/mL) which still attenuated mechanical hypersensitivity. These results confirm that a buprenorphine plasma concentration approximately 1 ng/mL is sufficient to effectively attenuate mechanical hypersensitivity for rats in an incisional pain model, and that the doses of XR-Lo, XR-Hi, and Bup-SR we tested provide effective attenuation of mechanical hypersensitivity for postoperative incisional pain. Thermal hypersensitivity attenuation was not achieved by Bup-SR, XR-Lo or XR-Hi at any of the study time points, even when the plasma level of buprenorphine was above 3 ng/mL (Bup-SR group on D0 and XR-Hi group on D1). This confirms that the plasma concentration of buprenorphine required to attenuate thermal hypersensitivity for this incisional pain model in rats is higher than the reported clinically effective concentration of 1 ng/mL. Plasma concentration of Bup-SR and XR differ in part because of the carrier vehicle used (including solvent type and polymer concentration).33 Bup-SR is a polymeric formulation that contains a water-insoluble, biodegradable polymer encapsulating buprenorphine and a biocompatible organic solvent.40 After Bup-SR administration, buprenorphine is released through erosion of the polymer, hydrolysis and drug diffusion.12,25,32 Conversely, for XR, buprenorphine is lipid-bound and suspended in medium chain fatty acid triglyceride (MCT) oil that is degraded overtime with lipase and esterase activity.15,31 These differences in formulation may explain the different plasma concentrations and delayed release of buprenorphine from XR as compared with Bup-SR.
Buprenorphine has been associated with reduced body weight,4,5,8 weight gain,2 and reduced food and water consumption.24 Body weight is a simple measurement of postoperative wellbeing that can also reflect adverse effects of the drug provided.5 In this current study, we found no significant impact of the drug treatment on food and water consumption (by observation) or weight. Other side effects that have been observed after buprenorphine administration include respiratory depression,1,18,20 gastrointestinal tract motility problems,10,21 pica,37 rebound hyperalgesia,36 dose-dependent cardiovascular depression28 and sedation.38 In our study, we did not observe any of these side effects, except for mild sedation after administration of either Bup-SR or XR. Bup-SR (1.2 mg/kg) was reported to cause mild sedation in rats.8,27,34 Therefore, a significant decrease of contralateral paw withdrawal could have been mostly due to sedative effect of Bup-SR (D1), XR-Lo (D0) and XR-Hi (D0 and D1). Previous studies have reported skin lesions, ulcerations, and scabbing at the administration site of Bup-SR in rats and mice.7,16 In the current study, we did not observe erythema, ulceration, or irritation at the site of administration of Bup-SR, XR-Lo or XR-Hi. However, on D0, greasiness was observed in rats in the Bup-SR (42.9%), XR-Lo (62.5%), and XR-Hi (25%,) groups. Similarly, our previous study in mice also reported greasiness after administration of Bup-SR and XR.32 To try to minimize leakage and greasiness, we used a 22 G needle and applied gentle pressure on skin fold (5 to 10 s) after injection. The manufacturer’s product insert for XR states that oily skin or greasiness may be observed after administration. Although not clinically evident, gross necropsy revealed subcutaneous nodules at the injection sites of the Bup-SR (71%), XR-Lo (75%), and XR-Hi (87%) groups. The nodules in the Bup-SR group extended beyond the injection site (approximately 2.5 to 3 cm), whereas in the XR groups, they were smaller and encapsulated (approximately 1 to 1.5 cm). Our study team did not observe these nodules in mice that received Bup-SR or XR,32 but they have been observed previously in Sprague–Dawley rats given meloxicam SR (4 mg/kg SC once; Zoopharm, Wildlife Pharmaceutical)35 and may be attributed to the polymer matrix used in the sustained release formulations.
From a practical standpoint, cost may have a significant impact on the choice of analgesic agents used for rodent surgery. In a 300 g rat, the current cost of one dose of Bup-SR (1.2 mg/kg SC) is 8.28 USD, XR (0.65 mg/kg SC once) is 18.75 USD, and XR (1.3 mg/kg SC once) is 37.50 USD. The cost of using XR will be a limiting consideration for some studies. However, XR is currently the only pharmaceutical grade, cGMP-compliant, FDA-indexed extended-release (more than 24 h) buprenorphine analgesic available in North America. The extended-release technology offers the benefit of reduced labor time (as compared with buprenorphine HCl, which should be administered twice daily) and is a welfare improvement for the rodents used for survival surgical procedures.
Our findings indicate that a single dose of XR (0.65 mg/kg, SC, once) provides at least 3 d of attenuation of postoperative mechanical hypersensitivity in a rat incisional pain model. This result has a potential to enhance postoperative care compliance because it confirms that the FDA-indexed extended-release opioid, XR, provides effective postoperative analgesia in this model. Additional studies evaluating the efficacy and duration of XR using other surgical models and pain testing modalities are warranted.
Acknowledgments
We would like to thank Marlon Pailano for his outstanding care of our rats and his support during the experiment, Janis Atuk-Jones for her assistance in formatting and editing the manuscript, Dr. Michael Klukinov for his technical assistance, Mingyun Zhang, Rhea Matsuura, Ebony Webber, Dr. Kaela Navarro, Dr. Jose G Vilches-Moure, Elias Godoy for their help during the study period, and Dr. Gregory Gorman at Samford University for the plasma analysis. The study was supported by the Stanford Department of Comparative Medicine.
References
- 1. Angel C, Glovak ZT, Alami W, Mihalko S, Price J, Jiang Y, Baghdoyan HA, Lydic R. 2018. Buprenorphine depresses respiratory variability in obese mice with altered leptin signaling. Anesthesiology 128: 984– 991. 10.1097/ALN.0000000000002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baykara S, Alban K. 2019. The effects of buprenorphine/naloxone maintenance treatment on sexual dysfunction, sleep and weight in opioid use disorder patients. Psychiatry Res 272: 450– 453. 10.1016/j.psychres.2018.12.153. [DOI] [PubMed] [Google Scholar]
- 3. Bethune CR, Bernards CM, Bui-Nguyen T, Shen DD, Ho RJY. 2001. The role of drug-lipid interactions on the disposition of liposome-formulated opioid analgesics in vitro and in vivo. Anesth Analg 93: 928– 933. 10.1097/00000539-200110000-00026. [DOI] [PubMed] [Google Scholar]
- 4. Bourque SL, Adams MA, Nakatsu K, Winterborn A. 2010. Comparison of buprenorphine and meloxicam for postsurgical analgesia in rats: Effects on body weight, locomotor activity, and hemodynamic parameters. J Am Assoc Lab Anim Sci 49: 617– 622. [PMC free article] [PubMed] [Google Scholar]
- 5. Brennan MP, Sinusas AJ, Horvath TL, Collins JG, Harding MJ. 2009. Correlation between body weight changes and postoperative pain in rats treated with meloxicam or buprenorphine. Lab Anim (NY) 38: 87– 93. 10.1038/laban0309-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brennan TJ, Vandermeulen EP, Gebhart GF. 1996. Characterization of a rat model of incisional pain. Pain 64: 493– 502. 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 7. Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51: 815– 819. [PMC free article] [PubMed] [Google Scholar]
- 8. Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 53: 193– 197. [PMC free article] [PubMed] [Google Scholar]
- 9. Clark TS, Clark DD, Hoyt RF. 2014. Pharmacokinetic comparison of sustained-release and standard buprenorphine in mice. J Am Assoc Lab Anim Sci 53: 387– 391. [PMC free article] [PubMed] [Google Scholar]
- 10. Cowan A, Doxey JC, Harry EJR. 1977. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol 60: 547– 554. 10.1111/j.1476-5381.1977.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deutsch-Feldman M, Picetti R, Seip-Cammack K, Zhou Y, Kreek MJ. 2015. Effects of handling and vehicle injections on adrenocorticotropic and corticosterone concentrations in Sprague-Dawley compared with Lewis rats. J Am Assoc Lab Anim Sci 54: 35– 39. [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn RL. Inventor; Dunn Research & Consulting, LLC A. 2012. Low viscosity liquid polymeric delivery system. US patent US 8,187,640 B22012.
- 13. Fidelis Pharmaceuticals North Brunswick N. [Internet]. 2021. Ethiqa XR (buprenorphine extended-release injectable suspension) 1.3 mg/mL. [Cited 4 May 2021]. Available at: https://ethiqaxr.com/efficacy-and-safety/
- 14. Fidelis Pharmaceuticals North Brunswick N. [Internet]. 2021. FDA-indexed, extended-release pain management. 2021. [Cited 4 May 2021]. Available from: https://www.fda.gov/media/87139/download
- 15.Fidelis Pharmaceuticals North Brunswick NJ. [Internet]. 2021. Ethiqa XR (buprenorphine extended-release injectable suspension) 1.3 mg/mL. [Cited 19 March 2021]. Available from: www.EthiqaXR.com
- 16. Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50: 198– 204. [PMC free article] [PubMed] [Google Scholar]
- 17. Grandin T. 1997. Assessment of Stress during Handling and Transport. J Anim Sci 75: 249– 257. 10.2527/1997.751249x. [DOI] [PubMed] [Google Scholar]
- 18. Greer JJ, Carter JE, al-Zubaidy Z. 1995. Opioid depression of respiration in neonatal rats. J Physiol 485: 845– 855. 10.1113/jphysiol.1995.sp020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 41: 337– 343. 10.1038/laban.152. [DOI] [PubMed] [Google Scholar]
- 20. Gueye PN. 2002. Buprenorphine and Midazolam Act in Combination to Depress Respiration in Rats. Toxicol Sci 65: 107– 114. 10.1093/toxsci/65.1.107. [DOI] [PubMed] [Google Scholar]
- 21. Healy JR, Tonkin JL, Kamarec SR, Saludes MA, Ibrahim Y, Matsumoto RR, Wimsatt JH. 2014. Evaluation of an improved sustained-release buprenorphine formulation for use in mice. AJVR 75: 619- 625 [DOI] [PubMed] [Google Scholar]
- 22. Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 23. Jessen L, Christensen S, Bjerrum OJ. 2007. The antinociceptive efficacy of buprenorphine administered through the drinking water of rats. Lab Anim 41: 185– 196. 10.1258/002367707780378131. [DOI] [PubMed] [Google Scholar]
- 24. Jirkof P. 2017. Side effects of pain and analgesia in animal experimentation. Lab Anim (NY) 46: 123– 128. 10.1038/laban.1216. [DOI] [PubMed] [Google Scholar]
- 25. Kamaly N, Yameen B, Wu J, Farokhzad OC. 2016. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem Rev 116: 2602– 2663. 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang SC, Jampachaisri K, Seymour TL, Felt SA, Pacharinsak C. 2017. Use of liposomal bupivacaine for postoperative analgesia in an incisional pain model in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 56: 63– 68. [PMC free article] [PubMed] [Google Scholar]
- 27. Leach MC, Forrester AR, Flecknell PA, Leach M. 2010. Influence of preferred foodstuffs on the antinociceptive effects of orally administered buprenorphine in laboratory rats. Lab Anim 44: 54– 58. 10.1258/la.2009.009029. [DOI] [PubMed] [Google Scholar]
- 28. Martinez EA, Hartsfield SM, Melendez LD, Matthews NS, Slater MR. 1997. Cardiovascular effects of buprenorphine in anesthetized dogs. Am J Vet Res 58: 1280– 1284. [PubMed] [Google Scholar]
- 29. McKeon GP, Pacharinsak C, Long CT, Howard AM, Jampachaisri K, Yeomans DC, Felt SA. 2011. Analgesic effects of tramadol, tramadol-gabapentin, and buprenorphine in an incisional model of pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 50: 192– 197. [PMC free article] [PubMed] [Google Scholar]
- 30. Mickley GA, Hoxha Z, Biada JM, Kenmuir CL, Bacik SE. 2006. Acetaminophen self-administered in the drinking water increases the pain threshold of rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 45: 48– 54. [PubMed] [Google Scholar]
- 31. Mishra DK, Dhote V, Bhatnagar P, Mishra PK. 2012. Engineering solid lipid nanoparticles for improved drug delivery: Promises and challenges of translational research. Drug Deliv Transl Res 2: 238– 253. 10.1007/s13346-012-0088-9. [DOI] [PubMed] [Google Scholar]
- 32. Navarro K, Jampachaisri K, Huss M, Pacharinsak C. 2021. Lipid bound extended release buprenorphine (high and low doses) and sustained release buprenorphine effectively attenuate post-operative hypersensitivity in an incisional pain model in mice (Mus musculus). Animal Model Exp Med 4( 2): 129– 137. 10.1002/ame2.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Packhaeuser CB, Schnieders J, Oster CG, Kissel T. 2004. In situ forming parenteral drug delivery systems: An overview. Eur J Pharm Biopharm 58: 445– 455. 10.1016/j.ejpb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 34. Seymour TL, Adams SC, Felt SA, Jampachaisri K, Yeomans DC, Pacharinsak C. 2016. Postoperative analgesia due to sustained-release buprenorphine, sustained-release meloxicam, and carprofen gel in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 55: 300– 305. [PMC free article] [PubMed] [Google Scholar]
- 35. Stewart LA, Imai DM, Beckett L, Li Y, Lloyd KC, Grimsrud KN. 2020. Injection-site Reactions to Sustained-release Meloxicam in Sprague-Dawley Rats. J Am Assoc Lab Anim Sci 59: 726– 731. 10.30802/AALAS-JAALAS-20-000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stewart LSA, Martin WJ. 2003. Evaluation of postoperative analgesia in a rat model of incisional pain. Contemp Top Lab Anim Sci 42: 28– 34. [PubMed] [Google Scholar]
- 37. Thompson AC, Kristal MB, Sallaj A, Acheson A, Martin LBE, Martin T. 2004. Analgesic efficacy of orally administered buprenorphine in rats: Methodologic considerations. Comp Med 54: 293– 300. [PubMed] [Google Scholar]
- 38. Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. 1994. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther 55: 569– 580. 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 39. Zhu CZ, Nikkel AL, Martino B, Bitner RS, Decker MW, Honore P. 2006. Dissociation between post-surgical pain behaviors and spinal Fos-like immunoreactivity in the rat. Eur J Pharmacol 531: 108– 117. 10.1016/j.ejphar.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 40. Zoopharm Pharmaceuticals Swedesboro N. [Internet]. 2021. Veterinary Compounding Pharmacy | ZooPharm. SR(TM) formulation characteristics. [Cited 2021 March 19]. Available from: https://www.zoopharm.com/medication/buprenorphine-sr-lab-ciii-5ml-10ml-vial-1mg-ml/
- 41. Zude BP, Jampachaisri K, Pacharinsak C. 2020. Use of Flavored Tablets of Gabapentin and Carprofen to Attenuate Postoperative Hypersensitivity in an Incisional Pain Model in Rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 59: 163– 169. 10.30802/AALAS-JAALAS-19-000093. [DOI] [PMC free article] [PubMed] [Google Scholar]