Abstract
Currently, the laboratory diagnosis of typhoid fever is dependent upon either the isolation of Salmonella enterica subsp. enterica serotype Typhi from a clinical sample or the detection of raised titers of agglutinating serum antibodies against the lipopolysaccharide (LPS) (O) or flagellum (H) antigens of serotype Typhi (the Widal test). In this study, the serum antibody responses to the LPS and flagellum antigens of serotype Typhi were investigated with individuals from a region of Vietnam in which typhoid is endemic, and their usefulness for the diagnosis of typhoid fever was evaluated. The antibody responses to both antigens were highly variable among individuals infected with serotype Typhi, and elevated antibody titers were also detected in a high proportion of serum samples from healthy subjects from the community. In-house enzyme-linked immunosorbent assays (ELISAs) for the detection of specific classes of anti-LPS and antiflagellum antibodies were compared with other serologically based tests for the diagnosis of typhoid fever (Widal TO and TH, anti-serotype Typhi immunoglobulin M [IgM] dipstick, and IDeaL TUBEX). At a specificity of ≥0.93, the sensitivities of the different tests were 0.75, 0.55, and 0.52 for the anti-LPS IgM, IgG, and IgA ELISAs, respectively; 0.28 for the antiflagellum IgG ELISA; 0.47 and 0.32 for the Widal TO and TH tests, respectively; and 0.77 for the anti-serotype Typhi IgM dipstick assay. The specificity of the IDeaL TUBEX was below 0.90 (sensitivity, 0.87; specificity, 0.76). The serological assays based on the detection of IgM antibodies against either serotype Typhi LPS (ELISA) or whole bacteria (dipstick) had a significantly higher sensitivity than the Widal TO test when used with a single acute-phase serum sample (P ≤ 0.007). These tests could be of use for the diagnosis of typhoid fever in patients who have clinical typhoid fever but are culture negative or in regions where bacterial culturing facilities are not available.
Salmonella enterica subsp. enterica serotype Typhi is the etiological agent of typhoid fever. Typhoid is an important cause of morbidity in many regions of the world (19), with an estimated 13 million cases occurring annually in Asia alone (B. Ivanhof, Abstr. Third Asia-Pacific Symp. Typhoid Fever Other Salmonelloses, abstr. S1-1, 1997). The diagnosis of typhoid fever on clinical grounds is difficult, as the presenting symptoms are diverse (30) and similar to those observed with other common febrile illnesses, such as malaria and nonsevere dengue fever. The isolation of serotype Typhi from blood remains the method of choice for the laboratory diagnosis (33). However, the availability of microbiological culturing facilities is often limited in regions in which typhoid is endemic, and blood cultures can be negative when patients have received prior antibiotic therapy. Bone marrow culturing has a higher sensitivity than blood culturing (6, 31) but is a more invasive procedure.
The Widal test, which detects agglutinating antibodies to lipopolysaccharide (LPS) (TO test) and flagella (TH test), was introduced over a century ago and is widely used for the serological diagnosis of typhoid fever (24). In the original format, the Widal test required acute- and convalescent-phase serum samples taken approximately 10 days apart. More recently, the test has been adapted for use with a single, acute-phase serum sample (2, 3, 13, 20, 21, 23, 25, 26). Enzyme-linked immunosorbent assays (ELISAs) have been considered an alternative approach for the diagnosis of typhoid fever. For the most part, these assays have been based on the detection of anti-LPS antibodies and have been reported to be more sensitive than the Widal TO test (7, 17, 27–29). More recently, ELISAs for the detection of antiflagellum antibodies have been developed (11, 14).
Typhoid fever is the major cause of community-acquired septicemia in southern Vietnam and many other areas in the developing world (8). In the southern provinces of Vietnam, microbiological culturing facilities are limited to a few centers, and the Widal test is widely used for the laboratory diagnosis of typhoid fever (21). In this study, we used antibody-class-specific ELISAs to describe the antibody responses to the LPS and flagellum antigens of serotype Typhi in typhoid patients and community members living in this area. We also report on the potential use of serotype Typhi anti-LPS and antiflagellum levels in serum for the diagnosis of typhoid fever.
MATERIALS AND METHODS
Patients and samples.
The serological studies were performed at the Centre for Tropical Diseases (CTD), Ho Chi Minh City, and the Dong Thap Provincial Hospital, Cao Lanh, Dong Thap Province, in the Mekong Delta region of southern Vietnam. All studies were approved by the scientific and ethical committees of the participating institutions, and informed consent was obtained from all participants or, in the case of children, from a parent or guardian. Serum samples were collected from children (<15 years) and adults with typhoid fever and also from hospital and community control subjects. These subjects were being recruited into either treatment or epidemiology studies of typhoid fever which have been reported elsewhere (16, 18, 32).
Serum samples from typhoid patients and hospital control subjects were collected either before or within the first few days of treatment and processed within a few hours of collection. Serum samples from community control subjects were processed within 8 h of collection. Samples were stored at or below −20°C until assayed. Clinical details were recorded on a standard form. A diagnosis of typhoid fever was established by the isolation of serotype Typhi from either bone marrow or blood.
Antigens.
The LPS and flagellum antigens were purified from serotype Typhi vaccine strains BRD 985 (Vi negative) (22) and BRD 691 (4), respectively. LPS was purified using a modified aqueous phenol method (12). The preparation showed a typical ladder pattern on a silver-stained sodium dodecyl sulfate gel (data not shown) and contained <2% (wt/wt) protein, as determined by the Bradford assay (Bio-Rad, Hemel Hempstead, United Kingdom). Flagella were purified using the method of Ibrahim et al. (9). A large polypeptide band of 50 kDa and corresponding to the flagella was visible on a Coomassie blue-stained 10% sodium dodecyl sulfate gel (data not shown), and the LPS content was determined to be 1% (wt/wt) by the Limulus amebocyte lysate assay (Sigma, Poole, Dorset, United Kingdom).
ELISAs.
In-house indirect sandwich ELISAs were established to detect anti-LPS IgA, IgM, and IgG and antiflagellum IgG. All reagents used were purchased from Sigma unless stated otherwise. Immulon 1b flat-bottom 96-well microtiter plates (Dynex Technologies, Billinghurst, United Kingdom) were coated overnight at 4°C with 100 μl of either 1 μg of antigen/ml in coating buffer (0.1 M carbonate buffer [pH 9.4], antigen positive) or coating buffer alone (antigen negative). The plates were blocked for 1 h at 37°C with 200 μl of phosphate-buffered saline containing 1% bovine serum albumin (BSA). Sera were either assayed at a single dilution (1/1,000 for anti-LPS IgG, 1/500 for anti-LPS IgA, or 1/250 for anti-LPS IgM and antiflagellum IgG) or serially diluted (starting at a dilution of 1/50). Sera were diluted in phosphate-buffered saline containing 0.1% BSA and 0.05% Tween 20, 100 μl was applied to the appropriate wells, and the plates were incubated for 4 h at room temperature. Bound antibodies (IgA, IgG, or IgM) were detected using heavy-chain-specific goat antibodies directly conjugated to alkaline phosphatase. The latter were diluted (anti-IgG, 1/5,000; anti-IgA, 1/500; and anti-IgM, 1/2,500) in Tris-buffered saline containing 0.1% BSA and 0.05% Tween 20. One hundred microliters was added to each well, and the plates were incubated overnight at 4°C. One hundred microliters of p-nitrophenyl phosphate (1 mg/ml) was added to each well, and the plates were incubated at ambient temperature in the dark for 30 to 40 min. The absorbance at 405 nm (reference filter, 450 nm) was determined using an automated ELISA reader (Bio-Rad).
For sera assayed at a single dilution, antibody levels were expressed in optical density (OD) units. These were taken as the mean OD of three wells with antigen minus the OD of a single well without antigen. For the titration assays, sera were assayed in triplicate (two wells antigen positive and one well antigen negative), and the titer was taken as the highest dilution giving a net OD (mean OD of antigen-positive wells minus OD of antigen-negative well) of ≥0.3 (anti-LPS IgG) or ≥0.2 (all other antibodies). Six standards were included on each plate, and the OD or titer of the samples was adjusted accordingly. Blank wells with no sera were included to monitor background.
Widal test.
The Widal tube agglutination test (Sanofi Diagnostics Pasteur, Marnes la Coquette, France) was performed according to the manufacturer's instructions by a single operator blind to the patient's diagnosis. Briefly, serial tube dilutions of sera were made in 0.85% saline, starting at a dilution of 1/100. Standard preparations of Salmonella O and H antigens were added, and the tubes were incubated at 37°C for 1 h. The tubes were centrifuged for 5 min, and agglutination was determined by eye. The Widal TO or TH titer was taken as the highest dilution of serum with visible agglutination.
IDeaL TUBEX.
The IDeaL TUBEX (IDL Biotech AB, Borlange, Sweden) was performed according to the manufacturer's instructions. The test is a semiquantitative competitive agglutination test for the detection of anti-O9 antibodies. The test was performed by a single operator, and the results were interpreted by three members of the laboratory staff who had no knowledge of the samples being tested. Samples were graded as 0 to 10 according to the color of the reaction mixture at the end of the procedure. Those with a grade of >2 were considered positive.
Serotype Typhi IgM dipstick assay.
The serotype Typhi IgM dipstick assay was provided by H. L. Smits, Department of Biomedical Research, Royal Tropical Institute, Amsterdam, The Netherlands. Briefly, serum samples were diluted (1/50) in the detecting reagent (containing dye-labeled, anti-human IgM antibodies). Nitrocellulose dipsticks coated with heat-inactivated serotype Typhi were immersed in the diluted serum and incubated at room temperature for 4 h. The strips were then washed and dried at room temperature. The sera were graded (0 to 4) according to the staining intensity of the colored band corresponding to the antigen. The test was performed by a single operator, and the results were interpreted by three members of the laboratory staff who had no knowledge of the samples being tested.
Statistics and calculations.
Statistical analyses were performed using the computer package SPSS for Windows (SPSS Benelux Inc., Gorinchem, The Netherlands). The Mann-Whitney U test was used for comparison of nonpaired samples, and the Wilcoxon sign rank test was used for comparison of paired samples. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the diagnostic tests were calculated using the following formulae: sensitivity is a/(a + c), specificity is d/(d + b), PPV is a/(a + b), and NPV is d/(d + c); in these formulae, a is test positive and true positive, b is test positive and true negative, c is test negative and true positive, and d is test negative and true negative, where true positive and true negative are culture (blood or bone marrow) positive or negative, respectively.
The sensitivity and specificity of the tests were compared using the McNemar test for paired samples.
RESULTS
Serum anti-LPS and anti-flagellum antigen antibody titers in culture-confirmed typhoid patients admitted to the CTD.
To initially assess the serum antibody responses to serotype Typhi LPS and flagellum antigens in patients infected with serotype Typhi, serum samples were collected from 160 patients with culture-confirmed typhoid fever and admitted to the CTD. Of these individuals, 84 were male and 76 were female. The median (interquartile range [IQR]) age of the group was 18 (11 to 26) years. The median (IQR) duration of illness (time from onset of symptoms to time of sample collection) was 12 (9 to 17) days. Clinical details for these patients have been reported elsewhere (18, 32).
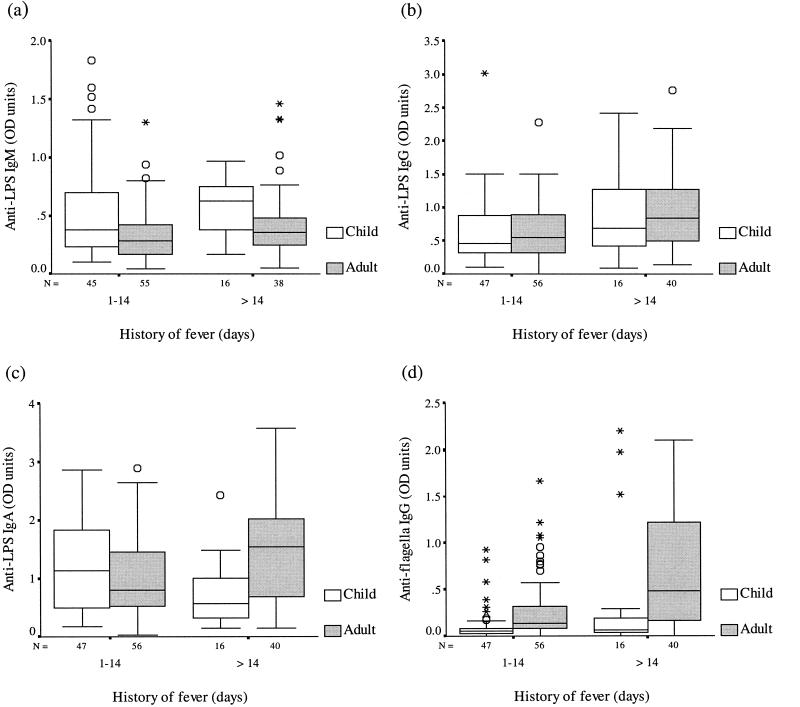
Serum antibody levels were determined by an ELISA using a single dilution of the patient's serum and were expressed as ELISA OD units. Patients were grouped and analyzed according to length of illness (less than or greater than 2 weeks) and age (children [<15 years old] versus adults). The serum antibody responses to the LPS and flagellum antigens of serotype Typhi observed in these typhoid patients were highly variable. Serum anti-LPS IgA, IgM, and IgG antibody levels were broadly similar for adults and children (Fig. 1a to c). No significant differences in the responses were seen with regard to length of illness, either for children or adults. Serum anti-flagellum IgG OD values (Fig. 1d) were generally higher for adults than for children, both for patients in the first 2 weeks of illness and for those who had been ill for longer than 14 days (P ≤ 0.005). Serum antibody levels against the flagellum antigen were higher in adult typhoid patients with a long history of illness (>2 weeks) than in those who had been ill for less than 2 weeks (P = 0.009). No significant difference in this response was found for children with different lengths of illness (P > 0.05).
FIG. 1.
Serum anti-LPS IgM (a), anti-LPS IgG (b), anti-LPS IgA (c), and antiflagellum IgG ELISA OD values for children (≤14 years) and adults (>14 years) with typhoid fever. Solid line, median; box, quartile; bar, range; circles, outliers; asterisks, extremes.
Evaluation of serological tests for the diagnosis of typhoid fever.
Based on the results of the above study, an evaluation of a panel of serological tests for the diagnosis of typhoid fever was undertaken. Sera were collected from patients with culture-confirmed typhoid fever and hospital control subjects admitted to Dong Thap Provincial Hospital. The hospital control was the next febrile patient of the same age group (within 5 years) and sex and admitted to the hospital with a diagnosis other than typhoid fever. Sera also were collected from healthy community control subjects. These were persons who resided in the household adjacent to the homes of the typhoid patients and who were of the same age group (within 5 years) and sex as the typhoid patients.
Sixty-five subjects were recruited into each of the three study groups. The median (IQR) age of the typhoid patients was 7 (5 to 14) years, compared to 6 (5 to 15) years for the hospital controls and 8 (5 to 13.5) years for the community controls. The median (IQR) duration of illness for the typhoid patients was 8 (5 to 14) days, compared to 2 (1 to 4) days for the hospital controls (P ≤ 0.001). The diagnoses for the hospital controls are provided in Table 1. Further details for these subjects are reported elsewhere (16).
TABLE 1.
Diagnoses for hospital controls
| Diagnosis | No. of patients |
|---|---|
| Pneumonia | 14 |
| Dengue fever | 13 |
| Abdominal discomfort or diarrhea | 9 |
| Viral infection, febrile convulsions, or pyrexia of unknown origin (POU) | 6 |
| Liver disease | 4 |
| Pharyngitis or tonsillitis | 3 |
| Kidney disease | 3 |
| Measles | 2 |
| Allergy | 2 |
| Joint infections | 3 |
| Other | 6 |
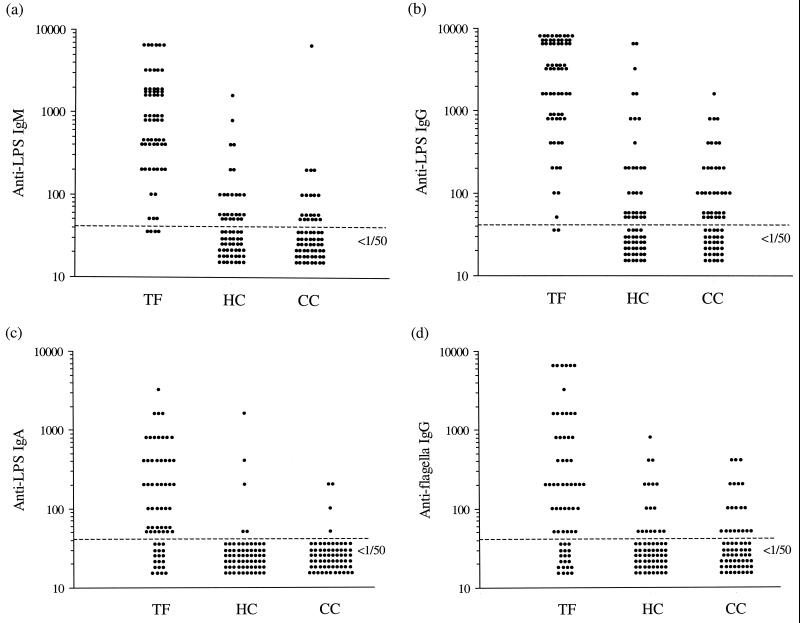
Serum anti-LPS (IgG, IgA, and IgM) and antiflagellum (IgG) ELISA antibody titers were evaluated for use in a diagnostic test (Fig. 2). As with the above study, the antibody responses to the two antigens were found to be highly variable. Antibody titers were generally higher in sera from typhoid patients than in sera from control subjects (P ≤ 0.001), but no significant differences were found between the two control groups (P > 0.05). Overall, raised (i.e., ≥1/50) anti-LPS and antiflagellum IgG titers were seen in a number of sera from hospital and community control subjects (66 of 130 [51%] and 37 of 130 [28%], respectively). Within the two control groups, the frequencies of these responses increased with age for both anti-LPS IgG and antiflagellum IgG (Table 2). Raised anti-LPS IgM titers were also common (26 to 36%), whereas raised anti-LPS IgA titers were seen in only a low proportion (4 to 6%) of the control sera tested. These results show that, in a region in which serotype Typhi is endemic, elevated anti-serotype Typhi LPS and flagellum antibody titers are common among persons not infected with serotype Typhi.
FIG. 2.
Serum anti-LPS IgM (a), anti-LPS IgG (b), anti-LPS IgA (c), and antiflagellum IgG (d) titers for patients with typhoid fever (TF) and age- and sex-matched hospital control (HC) and community control (CC) subjects.
TABLE 2.
Number (percentage) of control subjects with raised serum IgG titers (≥1/50) by age
| Control group | Age (yr) | n | No. (%) with raised IgG titer for:
|
|
|---|---|---|---|---|
| LPS | Flagella | |||
| Hospital | 2–5 | 28 | 9 (32) | 4 (14) |
| 6–10 | 19 | 10 (53) | 4 (21) | |
| >10 | 18 | 12 (67) | 9 (50) | |
| Community | 2–5 | 19 | 5 (28) | 4 (21) |
| 6–10 | 24 | 13 (54) | 5 (21) | |
| >10 | 22 | 17 (77) | 11 (50) | |
Serological tests (i.e., the Widal test) are often used to differentiate between patients with typhoid fever and those with other febrile illnesses. Therefore, a panel of serologically based typhoid diagnostic tests, including the in-house anti-LPS and antiflagellum antibody-class-specific ELISAs, were evaluated alongside the Widal TO and TH tests using sera from the typhoid patients and the hospital control subjects. The sensitivity, specificity, PPV, and NPV of the various serological tests are given in Table 3. Several of the tests had a high specificity (≥0.95), although the sensitivity was low (≤0.77). The anti-LPS IgM ELISA (titer, ≥1/400) and the IgM dipstick assay (grade, ≥1+) had a higher diagnostic sensitivity than the Widal TO test (titer, ≥1/400) at a specificity of ≥0.93 (P < 0.001). Both tests based on the detection of antiflagellum antibodies (antiflagellum IgG ELISA and the Widal TH test) were highly specific (0.98) but lacked sensitivity (0.28 to 0.32).
TABLE 3.
Sensitivity, specificity, PPV, and NPV of serological tests for the diagnosis of typhoid fevera
| Test | Titer or grade | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV |
|---|---|---|---|---|---|
| LPS IgMb | ≥1/50 | 0.94 (0.88–1.00) | 0.60 (0.48–0.72) | 0.70 | 0.91 |
| LPS IgMb | ≥1/400 | 0.75 (0.64–0.86)g | 0.94 (0.88–1.00) | 0.92 | 0.79 |
| LPS IgMb | ≥1,600 | 0.40 (0.28–0.52) | 0.98 (0.95–1.00) | 0.96 | 0.62 |
| LPS IgGb | ≥1/200 | 0.92 (0.85–0.99) | 0.77 (0.67–0.87) | 0.80 | 0.91 |
| LPS IgGb | ≥1/1,600 | 0.68 (0.57–0.79) | 0.92 (0.85–0.98) | 0.90 | 0.74 |
| LPS IgGb | ≥1/3,200 | 0.55 (0.43–0.67) | 0.95 (0.90–1.00) | 0.92 | 0.68 |
| LPS IgAb | ≥1/100 | 0.52 (0.39–0.63) | 0.95 (0.90–1.00) | 0.92 | 0.67 |
| LPS IgAb | ≥1/400 | 0.29 (0.18–0.40) | 0.97 (0.93–1.00) | 0.90 | 0.58 |
| LPS IgM or IgGb | ≥1/200 or ≥1/400h | 0.98 (0.95–1.00) | 0.83 (0.73–0.92) | 0.85 | 0.98 |
| Flagellum IgGb | ≥1/200 | 0.49 (0.37–0.61) | 0.89 (0.81–0.97) | 0.82 | 0.64 |
| Flagellum IgGb | ≥1/800 | 0.28 (0.17–0.39) | 0.98 (0.95–1.00) | 0.95 | 0.58 |
| Widal TOc | ≥1/100 | 0.92 (0.85–0.99) | 0.57 (0.44–0.70) | 0.70 | 0.87 |
| Widal TOc | ≥1/200 | 0.72 (0.61–0.83) | 0.85 (0.76–0.94) | 0.83 | 0.75 |
| Widal TOc | ≥1/400 | 0.47 (0.35–0.59) | 0.93 (0.87–0.99) | 0.88 | 0.64 |
| Widal THd | ≥1/100 | 0.60 (0.47–0.72) | 0.90 (0.83–0.97) | 0.84 | 0.70 |
| Widal THd | ≥1/200 | 0.32 (0.20–0.43) | 0.98 (0.95–1.00) | 0.95 | 0.60 |
| TUBEXe | 0.87 (0.66–0.87) | 0.76 (0.63–0.89) | 0.77 | 0.84 | |
| LPS dipstickf | ≥1+ | 0.77 (0.66–0.87)g | 0.95 (0.90–1.00) | 0.94 | 0.80 |
| LPS dipstickf | ≥2+ | 0.48 (0.36–0.60) | 0.98 (0.95–1.00) | 0.99 | 0.65 |
The values were calculated using data from culture-positive typhoid fever patients and age- and sex-matched hospital control subjects. CI, confidence interval.
Typhoid fever patients, n = 65; hospital control subjects, n = 65.
Typhoid fever patients, n = 62; hospital control subjects, n = 60.
Typhoid fever patients, n = 60; hospital control subjects, n = 63.
Typhoid fever patients, n = 64; hospital control subjects, n = 63.
Typhoid fever patients, n = 64; hospital control subjects, n = 63. Hemolysed samples (typhoid fever patients, n = 19; hospital control subjects, n = 25) could not be assayed.
For a comparison against the Widal TO test titer of ≥1/400, the P value was ≤0.007.
Titers for IgM and IgG, respectively.
DISCUSSION
This report describes serum antibody titers to the flagellum and LPS antigens of serotype Typhi in patients with typhoid fever and control subjects and evaluates the potential of serological approaches in the diagnosis of typhoid fever. In southern Vietnam, blood culture facilities are available in very few centers, and there is a need for a rapid, affordable diagnostic test for typhoid fever. There have been numerous reports on the single Widal test but no consensus as to its diagnostic value in regions in which typhoid is endemic (1, 3, 15, 20, 23, 25, 26). The results reported here show that the Widal TO test lacks either sensitivity or specificity in southern Vietnam, supporting the findings of a previous study from this region (21). Although the anti-LPS IgM ELISA (titers, ≥1/400) was more sensitive than the Widal TO test (titers, ≥1/400) in detecting culture-confirmed typhoid cases, none of the assays for the detection of anti-LPS antibodies (ELISAs and IDeaL TUBEX) had both high sensitivity and specificity (i.e., ≥0.95). The IgM dipstick assay (≥1+), which detects IgM antibodies against whole-cell serotype Typhi, performed as well as the anti-LPS IgM ELISA and was more sensitive than the Widal TO test (titers, ≥1/400). In agreement with previous studies (11, 14), the antiflagellum IgG ELISA and the Widal TH test were found to be highly specific but lacked sensitivity. In the present study, this result was probably due to the young age and/or the relatively short length of illness (52 of 65 ill for <14 days) of the typhoid patients. The sensitivity of an antiflagellum IgG diagnostic test might be increased if the test were used only for adults, particularly those with a long history of fever. However, this strategy would clearly limit the usefulness of such a test.
It could be argued that the raised serum anti-LPS and antiflagellum antibody titers seen in the sera of some of the hospital controls was due to current or recent infection with serotype Typhi. However, this possibility is unlikely, as all the hospital control patients had an alternative diagnosis (16). Furthermore, elevated anti-LPS and antiflagellum antibody titers were found in similar proportions of sera from healthy control subjects, indicating that background immunity to the two antigens can be high in this region of typhoid endemicity. The frequency of raised IgG titers (i.e., >1/50) increased with age, in agreement with previous studies showing that the prevalence of raised Widal TO and TH titers increases with age in regions of typhoid endemicity (5, 15). Titers of antibodies to the LPS and flagellum antigens can be raised following exposure to either serotype Typhi or another microorganism sharing common antigens (15), and this fact presumably contributes to the lack of specificity seen with serological diagnostic tests in regions of typhoid endemicity.
The results presented here show that ELISAs or dipstick tests for the detection of IgM antibodies to serotype Typhi LPS or whole bacteria perform better than the Widal TO test, as has been reported previously (7, 17, 28). Thus, a simple, inexpensive, rapid solid-phase immunoassay based on the detection of anti-LPS IgM or the prototype anti-serotype Typhi IgM dipstick test should be of greater diagnostic use than the Widal TO test for patients with suspected typhoid fever but who are blood culture negative or in areas where culturing facilities are not available. Similarly, Bhutta and Mansurali (1) recently reported that the Typhidot and Typhidot-M tests, which detect antibodies against a 50-kDa serotype Typhi outer membrane protein first described by Ismail et al. (10), had higher sensitivity (0.85 to 0.94) and specificity (0.77 to 0.89) than the Widal test (0.63 and 0.81, respectively). However, none of the serological diagnostic tests described in either this study or earlier studies have been shown to have both high sensitivity and high specificity (>0.95) for the diagnosis of typhoid fever in regions of typhoid endemicity when used with a single, acute-phase serum sample. Further studies to evaluate the sensitivity and specificity of these tests with paired serum samples taken 3 to 4 days apart are warranted.
ACKNOWLEDGMENTS
We acknowledge the assistance of the following persons: N. T. T. Quyen, P. T. Doan, Henck L. Smits, Helen Lee, and Christine Luxemburger. We thank the officials of the Centre for Tropical Diseases and Dong Thap Provincial Hospital for facilitating these studies.
This project was supported by the Wellcome Trust of the United Kingdom and the European Union (grant IC18CT998081).
REFERENCES
- 1.Bhutta Z A, Mansurali N. Rapid serologic diagnosis of pediatric typhoid fever in an endemic area: a prospective comparative evaluation of two dot-enzyme immunoassays and the Widal test. Am J Trop Med Hyg. 1999;61:654–657. doi: 10.4269/ajtmh.1999.61.654. [DOI] [PubMed] [Google Scholar]
- 2.Brodie J. Antibodies and the Aberdeen typhoid outbreak of 1964. I. The Widal reaction. J Hyg Camb. 1977;79:161–180. doi: 10.1017/s0022172400052979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck R L, Escamilla J, Sangalang R P, Cabanban A B, Santiago L T, Ranoa C P, Cross J H. Diagnostic value of a single, pre-treatment Widal test in suspected enteric fever cases in the Philippines. Trans R Soc Trop Med Hyg. 1987;81:871–873. doi: 10.1016/0035-9203(87)90056-3. [DOI] [PubMed] [Google Scholar]
- 4.Chatfield S N, Fairweather N, Charles I, Pickard D, Levine M, Hone D, Posada M, Strugnell R A, Dougan G. Construction of a genetically defined Salmonella typhi Ty2 aroA, aroC mutant for the engineering of a candidate oral typhoid-tetanus vaccine. Vaccine. 1992;10:53–60. doi: 10.1016/0264-410x(92)90420-o. [DOI] [PubMed] [Google Scholar]
- 5.Cherian T, Sridharan G, Mohandas V, John T J. Prevalence of Salmonella typhi O and H antibodies in the serum of infants and preschool children. Indian Pediatr. 1990;27:293–294. [PubMed] [Google Scholar]
- 6.Farooqui B J, Khurshid M, Ashfaq M K, Khan M A. Comparative yield of Salmonella typhi from blood and bone marrow cultures in patients with fever of unknown origin. J Clin Pathol. 1991;44:258–259. doi: 10.1136/jcp.44.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschl A, Stanek G, Rotter M, Niemetz A H, Diridl G. Comparison of the ELISA (lipopolysaccharide) and Widal reactions (O antigen) in the diagnosis of Salmonella infections. Zentralbl Bakteriol Mikrobiol Hyg Ser A. 1983;255:247–257. [PubMed] [Google Scholar]
- 8.Hoa N T, Diep T S, Wain J, Parry C M, Hien T T, Smith M D, Walsh A L, White N J. Community-acquired septicaemia in southern Vietnam: the importance of multidrug-resistant Salmonella typhi. Trans R Soc Trop Med Hyg. 1998;92:503–508. doi: 10.1016/s0035-9203(98)90891-4. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim G F, Fleet G H, Lyons M J, Walker R A. Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol. 1985;22:1040–1044. doi: 10.1128/jcm.22.6.1040-1044.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail A, Hai O K, Kader Z A. Demonstration of an antigenic protein specific for Salmonella typhi. Biochem Biophys Res Commun. 1991;181:301–305. doi: 10.1016/s0006-291x(05)81417-2. [DOI] [PubMed] [Google Scholar]
- 11.Jesudason M V, Sridharan G, Arulselvan R, Babu P G, John T J. Diagnosis of typhoid fever by the detection of anti-LPS and anti-flagellin antibodies by ELISA. Indian J Med Res. 1998;107:204–207. [PubMed] [Google Scholar]
- 12.Johnson B, Perry M. Improved techniques for the preparation of bacterial lipopolysaccharide. Can J Microbiol. 1976;22:29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- 13.Koeleman J G, Regensburg D F, van Katwijk F, MacLaren D M. Retrospective study to determine the diagnostic value of the Widal test in a non-endemic country. Eur J Clin Microbiol Infect Dis. 1992;11:167–170. doi: 10.1007/BF01967071. [DOI] [PubMed] [Google Scholar]
- 14.Korbsrisate S, Thanomsakyuth A, Banchuin N, McKay S, Hossain M, Sarasombath S. Characterization of a phase 1-d epitope on Salmonella typhi flagellin and its role in the serodiagnosis of typhoid fever. Asian Pac J Allergy Immunol. 1999;17:31–39. [PubMed] [Google Scholar]
- 15.Levine M M, Grados O, Gilman R H, Woodward W E, Solis Plaza R, Waldman W. Diagnostic value of the Widal test in areas endemic for typhoid fever. Am J Trop Med Hyg. 1978;27:795–800. doi: 10.4269/ajtmh.1978.27.795. [DOI] [PubMed] [Google Scholar]
- 16.Luxemburger, C., C. M. Duc, M. L. Lanh, J. Wain, T. T. Hien, J. Simpson, L. H. Kam, N. T. T. Thuy, N. J. White, and J. J. Farrar. Risk factors for typhoid fever in the Mekong Delta, southern Vietnam: a case control study. Trans. R. Soc. Trop. Med. Hyg., in press. [DOI] [PubMed]
- 17.Nardiello S, Pizzella T, Russo M, Galanti B. Serodiagnosis of typhoid fever by enzyme-linked immunosorbent assay determination of anti-Salmonella typhi lipopolysaccharide antibodies. J Clin Microbiol. 1984;20:718–721. doi: 10.1128/jcm.20.4.718-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen T C, Solomon T, Mai X T, Nguyen T L, Nguyen T T, Wain J, To S D, Smith M D, Day N P, Le T P, Parry C, White N J. Short courses of ofloxacin for the treatment of enteric fever. Trans R Soc Trop Med Hyg. 1997;91:347–349. doi: 10.1016/s0035-9203(97)90102-4. [DOI] [PubMed] [Google Scholar]
- 19.Pang T, Bhutta Z A, Finlay B B, Altwegg M. Typhoid fever and other salmonellosis: a continuing challenge. Trends Microbiol. 1995;3:253–255. doi: 10.1016/s0966-842x(00)88937-4. [DOI] [PubMed] [Google Scholar]
- 20.Pang T, Puthucheary S D. Significance and value of the Widal test in the diagnosis of typhoid fever in an endemic area. J Clin Pathol. 1983;36:471–475. doi: 10.1136/jcp.36.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parry C M, Hoa N T, Diep T S, Wain J, Chinh N T, Vinh H, Hien T T, White N J, Farrar J J. Value of a single-tube Widal test in diagnosis of typhoid fever in Vietnam. J Clin Microbiol. 1999;37:2882–2886. doi: 10.1128/jcm.37.9.2882-2886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickard D, Li J, Roberts M, Maskell D, Hone D, Levine M, Dougan G, Chatfield S. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect Immun. 1994;62:3984–3993. doi: 10.1128/iai.62.9.3984-3993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha S K, Ruhulamin M, Hanif M, Islam M, Khan W A. Interpretation of the Widal test in the diagnosis of typhoid fever in Bangladeshi children. Ann Trop Paediatr. 1996;16:75–78. doi: 10.1080/02724936.1996.11747807. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder S A. Interpretation of serologic tests for typhoid fever. JAMA. 1968;206:839–840. [PubMed] [Google Scholar]
- 25.Sen R, Saxena S. A critical assessment of the conventional Widal test in the diagnosis of tyhoid and paratyphoid fevers. Indian J Med Res. 1969;57:1813–1819. [PubMed] [Google Scholar]
- 26.Senewiratne B, Senewiratne K. Reassessment of the Widal test in the diagnosis of typhoid. Gastroenterology. 1977;73:233–236. [PubMed] [Google Scholar]
- 27.Shaheen H I, Girgis N I, Rodier G R, Kamal K A. Evaluation of the response of human humoral antibodies to Salmonella typhi lipopolysaccharide in an area of endemic typhoid fever. Clin Infect Dis. 1995;21:1012–1013. doi: 10.1093/clinids/21.4.1012. [DOI] [PubMed] [Google Scholar]
- 28.Sippel J, Bukhtiari N, Awan M B, Krieg R, Duncan J F, Karamat K A, Malik I A, Igbal L M, Legters L. Indirect immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays (ELISAs) and IgM capture ELISA for detection of antibodies to lipopolysaccharide in adult typhoid fever patients in Pakistan. J Clin Microbiol. 1989;27:1298–1302. doi: 10.1128/jcm.27.6.1298-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sippel J E, Hanafy H M, Diab A S, Prato C, Arroyo R. Serodiagnosis of typhoid fever in paediatric patients by anti-LPS ELISA. Trans R Soc Trop Med Hyg. 1987;81:1022–1026. doi: 10.1016/0035-9203(87)90386-5. [DOI] [PubMed] [Google Scholar]
- 30.Stuart B M, Pullen R L. Typhoid fever; clinical analysis of three hundred and sixty cases. Arch Intern Med. 1946;78:629–661. doi: 10.1001/archinte.1946.00220060002001. [DOI] [PubMed] [Google Scholar]
- 31.Vallenas C, Hernandez H, Kay B, Black R, Gotuzzo E. Efficacy of bone marrow, blood, stool and duodenal content cultures for bacteriologic confirmation of typhoid fever in children. Pediatr Infect Dis. 1985;4:496–498. doi: 10.1097/00006454-198509000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Vinh H, Wain J, Vo T N, Cao N N, Mai T C, Bethell D, Nguyen T T, Tu S D, Nguyen M D, White N J. Two or three days of ofloxacin treatment for uncomplicated multidrug-resistant typhoid fever in children. Antimicrob Agents Chemother. 1996;40:958–961. doi: 10.1128/aac.40.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wain J, Diep T S, Ho V A, Walsh A M, Nguyen T T, Parry C M, White N J. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol. 1998;36:1683–1687. doi: 10.1128/jcm.36.6.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]