Abstract
Background
Data from a randomized controlled efficacy trial of an inactivated quadrivalent influenza vaccine in children 6–35 months of age were used to determine whether hemagglutination inhibition (HI) antibody titer against A/H1N1 and A/H3N2 is a statistical correlate of protection (CoP) for the risk of reverse-transcription polymerase chain reaction (RT-PCR)–confirmed influenza associated with the corresponding strain.
Methods
The Prentice criteria were used to statistically validate strain-specific HI antibody titer as a CoP. The probability of protection was identified using the Dunning model corresponding to a prespecified probability of protection at an individual level. The group-level protective threshold was identified using the Siber approach, leading to unbiased predicted vaccine efficacy (VE). A case-cohort subsample was used for this exploratory analysis.
Results
Prentice criteria confirmed that HI titer is a statistical CoP for RT-PCR–confirmed influenza. The Dunning model predicted a probability of protection of 49.7% against A/H1N1 influenza and 54.7% against A/H3N2 influenza at an HI antibody titer of 1:40 for the corresponding strain. Higher titers of 1:320 were associated with >80% probability of protection. The Siber method predicted VE of 61.0% at a threshold of 1:80 for A/H1N1 and 46.6% at 1:113 for A/H3N2.
Conclusions
The study validated HI antibody titer as a statistical CoP, by demonstrating that HI titer is correlated with clinical protection against RT-PCR–confirmed influenza associated with the corresponding influenza strain and is predictive of VE in children 6–35 months of age.
Clinical Trials Registration
Keywords: HI antibodies, correlate of protection, influenza, children
Strain-specific hemagglutination inhibition antibody titer is a statistical correlate of protection against RT-PCR–confirmed influenza associated with the corresponding influenza strain in children 6–35 months of age. The Dunning model predicted probability of protection against illness of 43.2%–60.2% at a titer of 1:40.
PLAIN LANGUAGE SUMMARY.
What is the context?
Influenza is a global public health concern, especially in young children. Evaluation of the concentration of antibodies in the blood can be linked to the level of protection against influenza disease in adults. However, only a few studies have addressed the correlation between influenza vaccine efficacy and the level of immune response in young children. We need to better understand this correlation and how to use the data to predict vaccine efficacy in children.
What is new?
Data from a large influenza vaccine efficacy trial in children 6−35 months of age were used to evaluate the statistical correlation between the level of immune response and the risk of influenza disease. The trial was the first study in this age group of an influenza vaccine that protects against four types of influenza virus (traditionally, influenza vaccines have protected against only three types). It was a large trial, done across five influenza seasons in different regions throughout the world. The data therefore provide an excellent basis from which to evaluate the correlation between immune response and risk of illness.
What is the impact?
Higher levels of immune response were found to correlate with progressively higher levels of protection. Our predicted level of protection against influenza illness in children is in line with traditionally accepted levels of immune response in adults. The level of immune response in children could help us to know what the efficacy of the influenza vaccine is likely to be. Our methodology for predicting efficacy could be a useful tool in vaccine development, to predict vaccine efficacy, and to guide vaccination policies and regulatory decisions.
Few randomized trials have evaluated the protective efficacy of influenza vaccines in young children, and those that have been reported describe variable estimates of vaccine efficacy (VE) [1–6]. Multiple challenges are associated with conducting randomized trials of influenza VE, including large sample sizes. Evaluation of influenza vaccines could be improved with a better understanding of the association between the immune response to vaccination and protective effect of the vaccine against influenza illness.
Identification of immune markers that correlate with protection against infection following vaccination (ie, an immune correlate of protection [CoP]) is important in vaccine development. A CoP can be mechanistic or statistical [7]. A mechanistic CoP is a causal agent of protection; it is mechanistically and causally responsible for protection [7]. A statistical CoP is an immune marker that is statistically correlated with VE; it may or may not be a mechanistic causal agent of protection [7].
Hemagglutination inhibition (HI) antibody titers are currently accepted as providing a measure of protection against influenza, and several studies have quantified the HI titer that corresponds to protection against illness [8–12]. An HI titer of 1:40 is often recognized as associated with a 50% reduction in the incidence of influenza illness in adults and is used as an immunologic CoP [9, 10]. However, children might have a different CoP than adults. We are aware of only 2 studies evaluating a CoP in children, both of inactivated trivalent influenza vaccines. One study reported that 22% protection against A/H3N2 influenza was associated with an HI antibody titer of 1:40 [13], while the other reported 48% protection against A/H1N1pdm09 influenza associated with an HI antibody titer of 1:40 [14].
Data from a large, randomized efficacy trial of a inactivated quadrivalent influenza vaccine (IIV4) in children 6–35 months of age have been previously reported [15]. We have used data from this trial to determine whether HI titers induced by IIV4 correlate statistically with the risk of influenza illness and are predictive of VE. We focused on evaluating a statistical CoP because of the difficulty in demonstrating a mechanistic CoP in influenza.
METHODS
Patient Consent Statement
The trial was approved by independent ethics committees or institutional review boards of participating study centers, conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and regulatory requirements of participating countries. Parents or legally acceptable representatives of participating children provided written informed consent.
Study Design
This was a phase 3, observer-blind, randomized controlled study (NCT01439360) conducted in 13 countries in Europe, Central America, and Asia as described previously [15]. In brief, 12 018 children 6–35 months of age with no risk factors for influenza complications were recruited in 5 independent cohorts over 5 influenza season between 2011 and 2014 (Supplementary Table 1). Children were randomized 1:1 to receive IIV4 (Fluarix Quadrivalent, GSK, Dresden, Germany) or noninfluenza control vaccines (pneumococcal polysaccharide conjugate vaccine, hepatitis A vaccine, or varicella vaccine; control vaccines were allocated based on age and vaccine-priming status). Children were regarded as primed for influenza vaccination if they had previously received ≥2 doses of seasonal influenza vaccine separated by ≥28 days. All children <12 months of age were considered vaccine-unprimed. Vaccine-primed children received a single dose and vaccine-unprimed children received 2 doses; >99% of children were vaccine-unprimed. Vaccine composition is shown in Supplementary Table 1. The study lasted for 6–8 months for each participant; the study period included vaccination, surveillance for influenza illness, and monitoring for safety.
Active surveillance for influenza illness in an individual child was conducted from 14 days after final vaccination until the end of the influenza season (Supplementary Table 1). The surveillance period covered the peak of the influenza season in each country, based on available local epidemiologic data. Study periods and the end date of the surveillance period for each seasonal cohort are shown in Supplementary Table 1. Parents were asked to contact the study center if their child developed a temperature ≥38°C in combination with 1 or more of the following: cough, runny nose, nasal congestion or breathing difficulty, physician-diagnosed acute otitis media, or lower respiratory infection. A nasal swab was collected within 7 days of onset of each episode reported. Influenza A or B was confirmed by reverse-transcription polymerase chain reaction (RT-PCR) (Supplementary Methods). HI antibody titer was measured using standard methods (Supplementary Methods) at 28 days after the last vaccination and prevaccination from children in the immuno-subcohort (described below). For the CoP analysis, HI antibody titer was also measured in children not included in the immuno-subcohort who experienced an RT-PCR–confirmed influenza episode.
Study Objectives and Endpoints
The primary objectives of the study have been previously reported [15]. The objective of the present, prespecified, exploratory analysis was to identify an immunological surrogate endpoint that is statistically correlated with clinical protection against RT-PCR–confirmed influenza illness.
The clinical endpoints considered were the time starting from 14 days after completion of the vaccination course to the first occurrence of (1) RT-PCR–confirmed influenza illness associated with A/H1N1 and (2) RT-PCR–confirmed influenza illness associated with A/H3N2. The immunological surrogate endpoint considered was strain-specific HI antibody titer at 28 days after the last vaccine dose, that is, A/H1N1 HI titer in relation to A/H1N1-associated influenza illness and A/H3N2 titer in relation to A/H3N2-associated influenza illness.
Statistical Analysis of CoP
The statistical CoP was defined according to the Qin framework (level 1, statistical surrogate of protection), the basis for the World Health Organization (WHO) report on correlates of vaccine-induced protection [16, 17]. The analysis was done in 2 steps. First, statistical validity of strain-specific HI antibody titer as an immunological CoP for strain-specific RT-PCR–confirmed influenza was assessed. Second, the protective threshold was identified.
The Prentice criteria [18] were used to statistically validate HI antibody titer as a CoP for RT-PCR–confirmed influenza illness as follows: (1) show the effect of vaccination on RT-PCR–confirmed influenza illness (demonstrate IIV4 protection against RT-PCR–confirmed influenza illness); (2) show the effect of vaccination on HI antibody titer (demonstrate an increase in HI titer in the vaccinated group relative to control group); (3) show that HI antibody titer correlates with RT-PCR–confirmed influenza illness (demonstrate that HI antibody titers correlate with protection against RT-PCR–confirmed influenza illness); and (4) show that the full effect of vaccination on RT-PCR–confirmed influenza illness is captured by HI antibody titer (demonstrate that the probability of having RT-PCR–confirmed influenza illness is independent of treatment status given the level of the immune marker [HI titer]).
The first Prentice criterion was evaluated using a Cox model; the vaccine was considered to have a significant effect on protection against RT-PCR–confirmed influenza illness if the P value was < .05. The second criterion was evaluated using a linear model with the immunogenicity endpoint as a dependent variable and the vaccine received as an independent variable; the vaccine was considered to have a significant effect on HI antibody titer if the P value was <.05. The third criterion was evaluated using a Cox model for case-cohort design; HI antibody titer was considered to have a significant effect on time to first occurrence of RT-PCR–confirmed influenza illness if the P value was <.05. The fourth criterion was evaluated using a Cox model for case-cohort design; RT-PCR–confirmed influenza illness was considered to be independent of vaccination status if the P value associated with vaccination was >.05, and RT-PCR–confirmed influenza illness was considered dependent on the HI antibody titer if the P value associated with HI antibody titer was <.05. In a case-cohort design, samples are not random and specific modeling to obtain unbiased estimates is required. The standard deviation for Prentice criteria 3 and 4 was therefore estimated using the method proposed by Barlow [19] to account for the case-cohort approach used in the analysis.
The proportion of the VE (treatment effect, PE) explained by HI titer was evaluated using the Freedman method [20]. The PE based on observed data from the clinical trial was calculated, as well as the mean, median, 2.5th percentile, and 97.5th percentile of the PE using a resampling technique (bootstrap method with unrestricted random sampling).
Following validation of HI titer as a potential immunologic CoP, the protective threshold was identified using 2 methodologies: the Dunning model and the Siber approach [21, 22]. The Dunning method provides predicted probabilities of protection with respect to various antibody titer thresholds at an individual level (Dunning curve) [21]. The inverse probability weighting technique was used to fit the Dunning model to account for the effect of case-cohort sampling [23]. The Siber approach identifies a threshold by using the proportion of vaccinated and unvaccinated individuals with HI antibody titer below specified thresholds to estimate VE [22]. This method was adapted for case-cohort sampling and identified the threshold as the HI titer that provides a derived VE value (described here as predicted VE) equal to the VE observed based on the clinical outcome, leading to unbiased predicted VE (group-level threshold).
The analyses were not adjusted for covariates in order to keep the model simple and general for easy interpretation.
Analysis Sets
The analysis was based on a per-protocol cohort for CoP (PP-CoP), defined as all vaccinated children who met inclusion and exclusion criteria, complied with the protocol, started the influenza surveillance period, and did not have RT-PCR–confirmed influenza illness before the postvaccination blood sample was taken (Supplementary Figure 1). The study included an immuno-subcohort from whom prevaccination and postvaccination blood samples were taken for assessment of immunogenicity. The immuno-subcohort comprised a predefined number of children from each influenza season: approximately 400 children from the IIV4 group and 200 children from the control group in the first 2 seasonal cohorts, approximately 150 children in the third seasonal cohort (approximately equal numbers from both vaccine groups), and up to 50 children from each participating country in the fourth and fifth seasonal cohorts (approximately equal numbers from both vaccine groups).
A case-cohort subsampling method was used for analysis because of the logistical constraints in analyzing all samples from all participants. We considered that this approach would be unlikely to influence outcomes. The case-cohort subsample consisted of 3 further subgroups, as shown in Supplementary Figure 1: (1) children from the immuno-subcohort (described above) who were positive for RT-PCR–confirmed influenza; (2) children from the immuno-subcohort who were negative for RT-PCR–confirmed influenza; and (3) children from a non–immuno-subcohort (ie, all children not included in the immuno-subcohort) who were positive for RT-PCR–confirmed influenza. Since 2 types of influenza cases were evaluated (RT-PCR–confirmed A/H1N1 and A/H3N2), the number of children selected from the non–immuno-subcohort for the case-cohort subsample varied according to the associated strain as shown in Supplementary Figure 1. As mentioned above, HI testing was performed for all participants in the immuno-subcohort; additional HI testing was performed for participants in the non–immuno-subcohort who were positive for RT-PCR–confirmed influenza and included in the case-cohort subsample.
The first Prentice criterion was evaluated in the PP-CoP cohort, the second in the immuno-subcohort, and the third and fourth in the case-cohort subsample. The Dunning model and Siber approach were applied in the case-cohort subsample. The immune response parameters were also evaluated in the case-cohort subsample.
RESULTS
A total of 12 018 children were vaccinated, of whom 11 047 were included in the PP-CoP (Supplementary Figure 1). In the PP-CoP, mean age at first vaccination was 21.9 months (standard deviation, 8.0 months) and 48.9% were female. Most children were of Southeast Asian (29.1%), White European (22.3%), Central/South Asian (18.3%), or other (mainly Hispanic) (27.7%) ancestry. A total of 1.8% of children in the IIV4 group and 1.9% in the control group had a history of influenza vaccination in ≥1 previous season.
RT-PCR–Confirmed Influenza Associated With A/H1N1
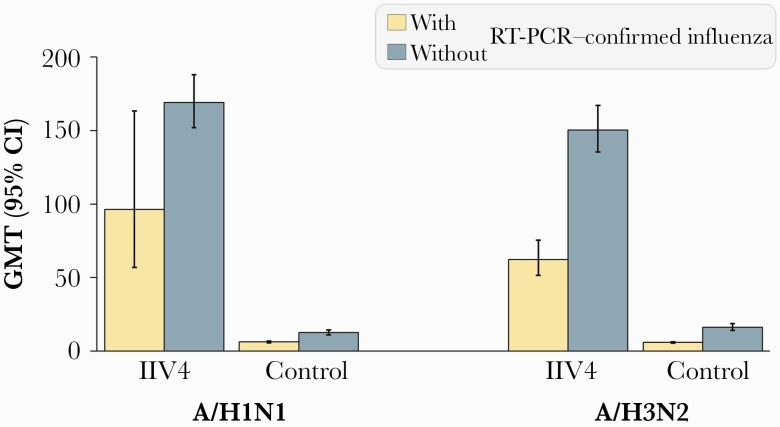
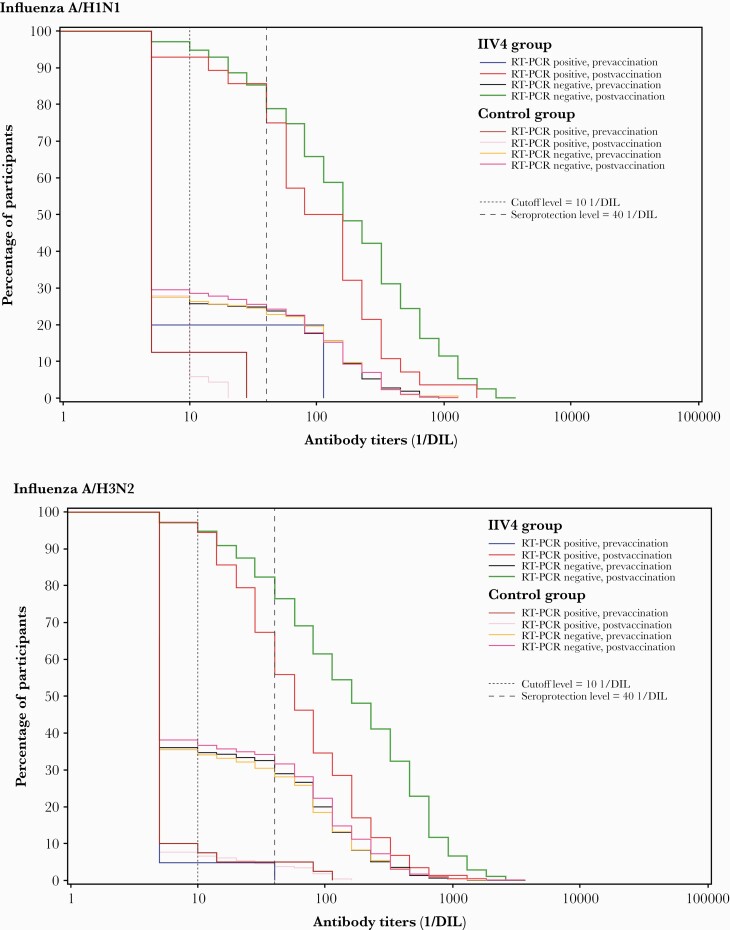
Of 800 children in the case-cohort subsample who received IIV4, 28 experienced RT-PCR–confirmed A/H1N1 influenza illness; of 655 children in the control group, 71 experienced RT-PCR–confirmed A/H1N1 influenza illness (Supplementary Figure 1). Geometric mean titers (GMTs) for HI antibodies against A/H1N1 were higher in children who did not experience RT-PCR–confirmed A/H1N1 influenza illness in both the IIV4 and control groups (Figure 1; Figure 2).
Figure 1.
Hemagglutination inhibition antibody titers in children with or without reverse-transcription polymerase chain reaction–confirmed influenza illness at 28 days after final vaccination (per-protocol correlate of protection cohort). Abbreviations: CI, confidence interval; GMT, geometric mean titer; IIV4, inactivated quadrivalent influenza vaccine; RT-PCR, reverse-transcription polymerase chain reaction.
Figure 2.
Reverse cumulative distribution curves for hemagglutination inhibition (HI) antibody titers in children with or without reverse-transcription polymerase chain reaction (RT-PCR)–confirmed influenza illness at 28 days after final vaccination (per-protocol correlate of protection cohort). The green line shows postvaccination HI titers in inactivated quadrivalent influenza vaccine (IIV4)–vaccinated children who did not experience RT-PCR–confirmed influenza illness. The red line shows postvaccination HI titers in IIV4-vaccinated children with RT-PCR–confirmed influenza illness. The bright pink line shows postvaccination HI titers in unvaccinated (control) children who did not experience RT-PCR–confirmed influenza illness. The pale pink line shows postvaccination HI titers in unvaccinated (control) children with RT-PCR–confirmed influenza illness. The other lines show prevaccination HI titers. The dotted lines show the assay cutoff level (titer ≥10 1/dilution [DIL]) and the level defined in the study protocol as seroprotective (titer ≥40 1/DIL).
All 4 Prentice criteria for validation of A/H1N1 HI antibody titer as a statistical CoP for RT-PCR–confirmed A/H1N1 influenza illness were met (Supplementary Table 2). Vaccination was inversely related to occurrence of RT-PCR–confirmed influenza illness (hazard ratio [HR] estimate, –0.941; P < .0001) and directly related to HI antibody titer (log10 of the geometric mean ratio, 1.129; P < .0001) (Supplementary Table 2). HI titers were inversely related to occurrence of RT-PCR–confirmed influenza illness (HR estimate, –0.928; P < .0001). For the fourth Prentice criterion, the effect of vaccination became nonsignificant after controlling for HI antibody titer (HR estimate, –0.235; P = .3455), while the effect of titer remained significant (HR estimate, –0.823; P < .0001); this indicates that HI antibody titer explains most of the effect on occurrence of RT-PCR–confirmed influenza illness.
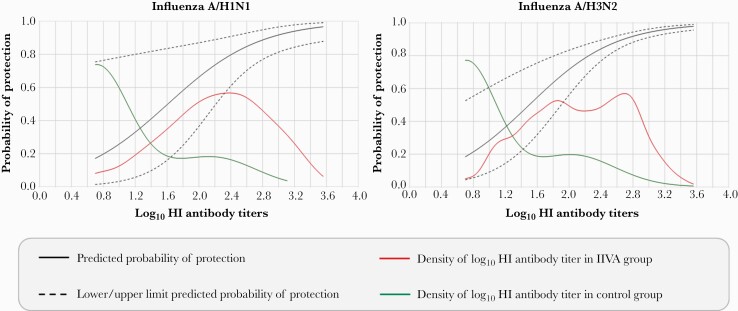
Using the Freedman method, the PE based on observed data from the clinical trial was 75.0% (Table 1). Based on the resampling (bootstrap) method, mean PE was 79.2% and median was 75.5% (Table 1). The probability of protection at different antibody thresholds was estimated by the Dunning regression model; the probability of protection against RT-PCR–confirmed A/H1N1 influenza illness ranged from 49.7% at a threshold of 1:40 to 88.8% at a threshold of 1:640 (Table 2; Figure 3). Using the Siber method, predicted VE against RT-PCR–confirmed A/H1N1 influenza illness was 61.0%, corresponding to an HI antibody titer of 1:80.
Table 1.
Proportion of the Treatment Effect Explained by Log10 Hemagglutination Inhibition Titer as the Correlate of Protection Using the Freedman Method
| Influenza Illness Endpoint | Proportion of Vaccination Effect | ||||
|---|---|---|---|---|---|
| Observed From Clinical Trial | Estimated From Resampling (Bootstrap) Method | ||||
| Mean | Median | 2.5th Percentile | 97.5th Percentile | ||
| RT-PCR–confirmed influenza illness: A/H1N1 | 0.750 | 0.792 | 0.755 | 0.367 | 1.479 |
| RT-PCR–confirmed influenza illness: A/H3N2 | 1.461 | 1.503 | 1.462 | 1.020 | 2.180 |
Per-protocol correlate of protection. Hemagglutination inhibition titer was measured at 28 days after last vaccination. Mean, median, 2.5th percentile, and 97.5th percentile of the proportion of the treatment effect were calculated using the bootstrap method with unrestricted random sampling. The parameter calculated can be >100% using this technique, but in this case, it is considered to be 100%.
Abbreviation: RT-PCR, reverse-transcription polymerase chain reaction.
Table 2.
Probability of Protection Against Reverse-Transcription Polymerase Chain Reaction–Confirmed Influenza Illness According to Various Thresholds of Log10 Hemagglutination Inhibition Titer Predicted by the Dunning Regression Model
| Influenza Illness Endpoint | Probability of Protection at Stated Threshold | ||||
|---|---|---|---|---|---|
| 1:40 | 1:80 | 1:160 | 1:320 | 1:640 | |
| RT-PCR–confirmed influenza illness: A/H1N1 | 49.7 | 62.5 | 73.7 | 82.5 | 88.8 |
| RT-PCR–confirmed influenza illness: A/H3N2 | 54.7 | 67.9 | 78.7 | 86.6 | 91.8 |
Per-protocol correlate of protection. Hemagglutination inhibition titer was measured at 28 days after last vaccination.
Abbreviation: RT-PCR, reverse-transcription polymerase chain reaction.
Figure 3.
Probability of protection against reverse-transcription polymerase chain reaction–confirmed influenza illness according to log10 hemagglutination inhibition (HI) titer predicted by the Dunning model (per-protocol correlate of protection; HI titer measured at 28 days after last vaccination). The density of log10 HI antibody titer lines show the distribution of postvaccination HI titers in study participants (the proportion of children with titers of the shown level). The graphs begin at a log10 HI antibody titer of 0.7, the assay cutoff level. As expected, a few children in the inactivated quadrivalent influenza vaccine (IIV4) group had very low or very high titers, but most had titers in the mid-range. In contrast, most children in the control group had low HI antibody titers.
RT-PCR–Confirmed Influenza Associated With A/H3N2
A total of 903 children who received IIV4 were included in the case-cohort subsample, of whom 147 experienced RT-PCR–confirmed A/H3N2 influenza illness; of 817 children in the control group, 270 experienced RT-PCR–confirmed A/H3N2 influenza illness (Supplementary Figure 1). GMTs for HI antibodies against A/H3N2 were higher in children who did not experience RT-PCR–confirmed A/H3N2 influenza illness in both study groups (Figure 1; Figure 2).
Three of the 4 Prentice criteria for validation of A/H3N2 HI antibody titer as a statistical CoP for RT-PCR–confirmed A/H3N2 influenza illness were met (Supplementary Table 2). The P value for the fourth criterion evaluating the effect of vaccination after controlling for HI antibody titer was borderline (P = .0388). According to the Freedman method, the effect of vaccination was fully explained by HI antibody titer (PE >100%; Table 1). Using the Dunning model, protection ranged from 54.7% at a threshold of 1:40 to 91.8% at a threshold of 1:640 (Table 2; Figure 3). The predicted VE using the Siber method was 46.6%, corresponding to an HI antibody titer of 1:113.
Discussion
An immunological marker that provides an accurate predictor of influenza VE will be a useful tool in vaccine development, to predict VE in different settings and guide vaccination policies and regulatory decisions [7, 16]. Two paradigms apply to evaluation of a vaccine CoP, the causal agent paradigm and the predictor of protection paradigm. The causal agent paradigm describes an immunological marker that mechanistically causes VE against a clinical endpoint [24], while the prediction paradigm describes a marker that reliably predicts VE [16]. Both are useful, but are assessed using different approaches.
In the present analysis, we adopted the Qin framework, which evaluates an immune marker as a predictor of VE (ie, not as a mechanistic agent) [16]. The Qin framework provides the basis for the WHO report on correlates of vaccine-induced protection [17]. We applied level 1 of the Qin framework, statistical CoP, which applies to a defined population of vaccinees. In this scenario, the CoP is predictive of VE only in the same setting as the trial, and cannot be considered for other populations and settings. Within the Qin framework, we applied the Prentice criteria to establish whether HI antibody titers are a statistical CoP. Once HI antibody titers were confirmed as a CoP, we used 2 approaches to identify the protective threshold, the Dunning curve and the Siber approach. The Dunning threshold is the HI antibody titer corresponding to a prespecified probability of protection (eg, 50%) at an individual level. The Siber threshold is the HI antibody titer leading to unbiased predicted VE (group-level threshold).
The Prentice criteria confirmed that HI antibody titers against A/H1N1 and A/H3N2 at 28 days after last vaccination are statistical CoPs for RT-PCR–confirmed influenza illness associated with the corresponding strain. Using the Freedman method, the PE indicated that most of the vaccination effect was explained by HI titer (75.0%–100% based on observed data from the clinical trial).
An HI antibody titer of 1:40 is traditionally considered to provide 50% reduction in the risk of acquiring influenza infection in adults [10]. In our study, the Dunning model predicted a probability of protection of 49.7% for influenza illness associated with A/H1N1 or 54.7% for influenza illness associated with A/H3N2 at an HI antibody titer of 1:40 for the corresponding influenza strain. The Dunning regression curve has substantial variability, which means that estimated thresholds also have substantial variability. The curve showed that increasing HI titers are associated with progressively higher levels of protection, which has also been observed in another study [9]. The model predicted a probability of protection of >80% at a strain-specific HI antibody titer of 1:320 for influenza illness associated with either A/H1N1 or A/H3N2. According to the Siber method, an A/H1N1 HI antibody titer of 1:80 corresponded to VE of 61.0% against A/H1N1 influenza illness and an A/H3N2 HI antibody titer of 1:113 corresponded to VE of 46.6% against A/H3N2 influenza illness.
In a trial of inactivated trivalent influenza vaccine in children 6–72 months of age, the CoP against RT-PCR–confirmed influenza illness associated with A/H3N2 at 50 days after the first vaccine dose (approximately 3 weeks after the second dose) was evaluated using Prentice criteria and the Dunning model [13]. In contrast to our data, the study showed that an HI antibody titer of 1:40 was associated with only a probability of 22% protection against A/H3N2 influenza illness [13]. A 50% and 80% reduction in the risk of influenza illness was associated with HI antibody titers of 1:110 and 1:330, respectively [13]. In a trial of an inactivated trivalent influenza vaccine in children 6–17 years of age, a strain-specific HI antibody titer of 1:40 was associated with 48% and 55% protection against RT-PCR–confirmed infection with A/H1N1pdm09 and B/Victoria, respectively [14]. Results from different studies might vary depending on the population, the vaccine, and degree of vaccine matching in the season studied. In our study, VE was very high, despite poor vaccine matching in some seasons. Our study was conducted in very young children, almost all of whom were vaccine-naive. The high VE probably reflects the broad immune response normally achieved in such children, as previously discussed [15]. Overall, studies in children have concluded that HI antibody titers offer a useful CoP in children, indicating that this methodology could be a useful tool in influenza vaccine development, to predict VE in different settings, and to guide vaccination policies and regulatory decisions. In our study and the other 2 studies described [13, 14], higher strain-specific HI titers were associated with greater protection against influenza illness.
A limitation of our study is that we did not explore a CoP for influenza B lineages. Very few cases of RT-PCR–confirmed influenza associated with B/Victoria were identified in the trial (69 of 1049 confirmed influenza cases [7%]) [15]. Assay stability monitoring performed after the end of the clinical part of the study but before the CoP analysis identified a weakness in the performance of the assay for the archived B/Yamagata seasonal strain. These 2 factors prevented analysis of a CoP for this strain. We also did not explore a CoP using microneutralization or neuraminidase inhibition assays because the HI assay is more standardized. However, it should be noted that interlaboratory variation in HI assays does exist and CoP values derived from different assays might not be directly comparable.
The 5 independent study cohorts, 5 different influenza seasons, and the 3 geographical areas (Asia, Central America, and Europe/Mediterranean) covered by the study were potential confounders or effect modifiers, but we did not adjust the analysis to account for these. However, inclusion of geographically diverse cohorts across several seasons will alleviate some variation in VE related to vaccine matching. In addition, it should be noted that multiple immune responses interact to provide protection against infection, including CD4+ responses, which are key to development of B cells, antibody production, and production of cytokines; however, these factors might be more applicable to adults than to children. Further research into the role of cellular immunity as a CoP in children is warranted.
In conclusion, our study validated HI antibody titer as a statistical CoP, by demonstrating that HI titer is inversely correlated with the risk of experiencing RT-PCR–confirmed influenza illness associated with the corresponding influenza strain and is predictive of VE in children 6–35 months of age. At an HI titer of 1:40, the Dunning model predicted a probability of protection against influenza illness of 49.7% for A/H1N1 and 54.7% for A/H3N2, which is in line with a 50% reduction in the risk of infection traditionally associated with this antibody titer. Higher titers of 1:320 were associated with >80% probability of protection.
Supplementary Material
Notes
Author contributions. All authors participated in the design, implementation or analysis, and interpretation of the study, as well as the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Acknowledgments. The authors are indebted to the participating study volunteers and their parents, clinicians, nurses, and laboratory technicians at the study sites. The authors also thank the sponsor’s project staff for their support and contributions throughout the study and/or manuscript development, especially Pascale Paindavoine. Finally, the authors thank the Business & Decision Life Sciences platform (on behalf of GSK) for editorial assistance and manuscript coordination. Mary L. Greenacre (An Sgriobhadair, UK, on behalf of GSK) provided medical writing services, and Bruno Dumont (Business & Decision Life Sciences, on behalf of GSK) coordinated the manuscript development and editorial support. Fluarix Quadrivalent is a trademark owned by or licensed to the GSK group of companies.
Data sharing statement. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Financial support. GlaxoSmithKline Biologicals SA funded this study and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Potential conflicts of interest. Jasur Danier, Andrea Callegaro, Jyoti Soni, Damien Friel, Wenji Pu, Valerie Vantomme and Jerome Wilson are employed by the GSK group of companies. Jasur Danier, Andrea Callegaro, Damien Friel, Wenji Pu, Anne Schuind and Jerome Wilson hold shares from the GSK group of companies. Pope Kosalaraska reports payments to his institution from the GSK group of companies for the conduct of this study. Ghassan Dbaibo reports payments to his institution from the GSK group of companies for the conduct of this study, and payments to his institution from Pfizer outside the submitted work. Bruce L. Innis and Anne Schuind were employed by the GSK group of companies at the time of the study. Jasur Danier, Andrea Callegaro, Jyoti Soni, Damien Friel, Wenji Pu, Valerie Vantomme, Anne Schuind, Jerome Wilson, Pope Kosalaraska, Ghassan Dbaibo and Bruce L. Innis declare no other financial or non-financial relationships or activities. Alfoso Carmona, Luis Rivera and Khalequ Zaman declare no financial or non-financial relationships or activities and no conflict of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hurwitz ES, Haber M, Chang A, et al. . Studies of the 1996-1997 inactivated influenza vaccine among children attending day care: immunologic response, protection against infection, and clinical effectiveness. J Infect Dis 2000; 182:1218–21. [DOI] [PubMed] [Google Scholar]
- 2. Jansen AG, Sanders EA, Hoes AW, et al. . Effects of influenza plus pneumococcal conjugate vaccination versus influenza vaccination alone in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled trial. J Pediatr 2008; 153:764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vesikari T, Knuf M, Wutzler P, et al. . Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011; 365:1406–16. [DOI] [PubMed] [Google Scholar]
- 4. Hoberman A, Greenberg DP, Paradise JL, et al. . Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003; 290:1608–16. [DOI] [PubMed] [Google Scholar]
- 5. Rolfes MA, Goswami D, Sharmeen AT, et al. . Efficacy of trivalent influenza vaccine against laboratory-confirmed influenza among young children in a randomized trial in Bangladesh. Vaccine 2017; 35:6967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maeda T, Shintani Y, Nakano K, et al. . Failure of inactivated influenza A vaccine to protect healthy children aged 6-24 months. Pediatr Int 2004; 46:122–5. [DOI] [PubMed] [Google Scholar]
- 7. Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012; 54:1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunning AJ, DiazGranados CA, Voloshen T, et al. . Correlates of protection against influenza in the elderly: results from an influenza vaccine efficacy trial. Clin Vaccine Immunol 2016; 23:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coudeville L, Bailleux F, Riche B, et al. . Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a Bayesian random-effects model. BMC Med Res Methodol 2010; 10:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Jong JC, Palache AM, Beyer WE, et al. . Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003; 115:63–73. [PubMed] [Google Scholar]
- 12. Gilbert PB, Fong Y, Juraska M, et al. . HAI and NAI titer correlates of inactivated and live attenuated influenza vaccine efficacy. BMC Infect Dis 2019; 19:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Black S, Nicolay U, Vesikari T, et al. . Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081–5. [DOI] [PubMed] [Google Scholar]
- 14. Ng S, Fang VJ, Ip DK, et al. . Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J Infect Dis 2013; 208:1320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Claeys C, Zaman K, Dbaibo G, et al. ; Flu4VEC Study Group. Prevention of vaccine-matched and mismatched influenza in children aged 6-35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health 2018; 2:338–49. [DOI] [PubMed] [Google Scholar]
- 16. Qin L, Gilbert PB, Corey L, et al. . A framework for assessing immunological correlates of protection in vaccine trials. J Infect Dis 2007; 196:1304–12. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. Correlates of vaccine-induced protection: methods and implications. WHO/IVB/13.01. 2013. https://apps.who.int/iris/bitstream/handle/10665/84288/WHO_IVB_13.01_eng.pdf.
- 18. Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989; 8:431–40. [DOI] [PubMed] [Google Scholar]
- 19. Barlow WE. Robust variance estimation for the case-cohort design. Biometrics 1994; 50:1064–72. [PubMed] [Google Scholar]
- 20. Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med 1992; 11:167–78. [DOI] [PubMed] [Google Scholar]
- 21. Dunning AJ. A model for immunological correlates of protection. Stat Med 2006; 25:1485–97. [DOI] [PubMed] [Google Scholar]
- 22. Siber GR, Chang I, Baker S, et al. . Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 2007; 25:3816–26. [DOI] [PubMed] [Google Scholar]
- 23. Robins J, Rotnitzky A, Zhao L. Estimation of regression coefficients when some regressors are not always observed. J Am Stat Assoc 1994; 89:846–66. [Google Scholar]
- 24. Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47:401–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.