Abstract
Introduction
Vitamin D insufficiency is highly prevalent and is a negative predictor for survival in ischemic stroke patients. We evaluated the effect of a high dose of vitamin D3 on the Neuron-Specific Enolase (NSE) level, National Institute of Health Stroke Scale (NIHSS), and Barthel Index (BI) scoring system in moderate ischemic stroke patients.
Methods
This prospective, double-blind, randomized clinical trial (RCT) study was conducted from April 2020 to March 2021. Patients with moderate ischemic stroke (NIHSS 5 to 15) who had vitamin D deficiency (serum 25-OH vitamin D ≤30 ng/mL) were recruited and randomized into intervention and control groups. Subjects in the intervention group received a single dose, intramuscular (IM) injection of 600000 international unit (IU) vitamin D3, in addition to the standard treatment. NSE level and NIHSS were evaluated at baseline and 48 hours after the intervention. The BI was monitored three months after discharge.
Results
During the study period, 570 patients were assessed; finally, forty-one patients completed the study. Except for the age which was higher in the control group (p = 0.04), there were no statistically significant differences in other baseline characteristics between the two groups. After 48 hours, the NIHSS score was significantly lower in the intervention group (median 8 vs. 6.5, p = 0.008 in the control and intervention groups, respectively), but there was no significant difference in the NSE level (p = 0.80). Three months after discharge, the BI was significantly higher in the intervention group (median 8 vs. 9, p = 0.03 in the control and intervention groups, respectively).
Conclusions
Administration of a single 600000 IU of vitamin D3 could have neuroprotective effects in patients with moderate ischemic stroke, according to its significantly positive effects on functional clinical outcomes (NIHSS and BI), but this effect on the biomarker related to neural damage (NSE) was not significant.
1. Introduction
Vitamin D insufficiency is highly prevalent in acute ischemic stroke patients compared with the patients without stroke [1]. Also, there is an association between the severity of vitamin D deficiency and ischemic infarct volumes, functional outcomes, and stroke recurrence. Therefore, vitamin D deficiency could be considered as a negative predictor for survival in patients with ischemic stroke [1–4].
Several mechanisms have been proposed for the neuroprotective properties of vitamin D [5]. Vitamin D promotes the expression of insulin-like growth factor 1 (IGF-1) which has neuroprotection capabilities [6]. Also, it has been suggested that vitamin D has antithrombotic and vasodilatory effects which, therefore, improve the blood flow of neurons [7]. Vitamin D, as an antioxidant, with inhibition of reactive oxygen can prevent blood-brain barrier (BBB) dysfunction after an ischemic stroke [5, 8].
Vitamin D supplementation is suggested to reduce neurological, psychological, and musculoskeletal complications in stroke patients. Poststroke patients would benefit from the antidepressive and anticonvulsant effects of vitamin D [9, 10]. Also, vitamin D supplementation could improve muscle strength in poststroke patients with hemiplegia and improve their motor functions [11].
Nowadays, besides stroke severity and prognosis scales, serum biomarkers are investigated for diagnosis and outcome prediction in ischemic stroke patients [12]. Neuron-Specific Enolase (NSE), an enzyme released after neuronal damage, has been studied as a marker for brain injury including ischemic stroke [13], and NSE level correlates with a patient's clinical deficits and infarct volume [14, 15].
Most randomized controlled trials (RCTs) directly investigated the effects of the oral forms of vitamin D in stroke patients [5], while intramuscular (IM) injection of single high doses of vitamin D can increase serum 25-OH vitamin D level rapidly and safely and also could improve patients' balance performance [16]. Few studies examined the effect of single high doses of vitamin D on functional outcome scales and did not evaluate the serum biomarkers in patients with ischemic stroke [17, 18].
Therefore, with this knowledge gap in the background, we designed this study to evaluate the effect of a high dose of vitamin D3 on the NSE level as a neuromarker, National Institute of Health Stroke Scale (NIHSS), and Barthel Index (BI) scoring system as the functional outcomes in patients with moderate ischemic stroke.
2. Methods
2.1. Settings
The present prospective, double-blind, randomized clinical trial (RCT) study was conducted in the neurology ward of Imam Hossein Medical Center, affiliated with Shahid Beheshti University of Medical Sciences (SBMU) in Tehran, Iran, from April 2020 to March 2021. This study has been approved by the Institutional Review Boards of the Ethics Committee of SBMU (IR.SBMU.PHARMACY.REC.1399.213). Also, the study protocol was registered, reviewed, and approved by the Iranian Registry of Clinical Trials (IRCT), with the registry number of IRCT20120703010178N24.
2.2. Study Population
Adult patients suffering from moderate ischemic stroke, NIHSS score 5 to 15, admitted to the neurology ward during the last 24 hours with vitamin D deficiency (serum 25-OH vitamin D ≤30 ng/mL) were included. Patients with a history of acute or chronic renal (creatinine clearance (CrCl) (<30 mL/min)/1.73 m2) and liver failure were excluded from the study.
2.3. Interventions
Written informed consent was obtained from each subject before enrollment in the study. Included patients were randomized into two groups of intervention and control. Randomization was done by simple randomization method, using series of random numbers generated by randomize (RND) command of Excel software. All patients were managed according to the standard treatment protocol based on the AHA/ASA (American Heart Association/American Stroke Association) guidelines [19]. The intervention group, in addition to the standard treatment, received a single dose, IM injection of 600000 international unit (IU) vitamin D3 (Daroupakhsh Co. Ltd., Tehran, Iran). Subjects in the intervention group were kept blinded to the study intervention.
2.4. Assessments
The baseline data consist of age, sex, serum 25-OH vitamin D level, and hospital length of stay and were recorded for all patients. To evaluate NSE level, venous blood samples were collected at baseline (NSE 0) and 48 hours after recruitment (NSE 1). The serum was separated by centrifuged (at 2000 rotations per minute (rpm) for 10 minutes) and immediately stored at -80 °C. NSE levels were measured by using human Enzyme-Linked Immunosorbent Assay (ELISA) kits (CanAg Diagnostics, Fujirebio, Japan) as instructed by the manufacturer.
As a criterion for the clinical evaluation, the severity of stroke was assessed by NIHSS at baseline (NIHSS 0) and 48 hours after admission (NIHSS 1) by a trained neurology resident who was blinded to the study. Patient's long-time outcome was assessed using the BI with a structured follow-up telephone interview three months after hospital discharge by a trained nurse who was kept blinded to the study groups.
2.5. Definition
The NIHSS is a standard stroke assessment scale and measures neurologic impairment using 15 items. NIHSS categorizes stroke as mild (scores 1-4), moderate (scores 5-15), moderate to severe (scores 16-20), and severe (scores higher than 20) [20].
The BI is the standard scale used to measure performance in daily living activities. The BI measures 10 basic aspects of self-care and physical dependency. A normal score is 20, and lower scores indicate an increasing disability. A BI higher than 12 corresponds to assisted independence, and a BI lower than 8 corresponds to severe dependency [21].
NSE is one of the biomarkers of the brain and vascular injury. The reported range in 95% of the healthy subject is <12.5 ng/mL [22]. The NSE level increases within 2-3 hours after onset of the stroke symptoms and then decrease until 12 hours, and the second increase is until day 5 [15].
2.6. Outcomes
The primary objective of this study was to evaluate the effect of high doses of vitamin D3 on the NSE level, as the biomarker of brain damage in patients with moderate ischemic stroke. The effects of this regimen on neurological functions, according to NIHSS and BI scores, were evaluated as secondary outcomes.
2.7. Sample Size
The sample size of the study was calculated with Minitab software using 2-samplet-test function considering type I error of 0.05 and power of 0.8. The NSE level was considered 5.03 ± 3.25 ng/mL in the intervention group and 10.04 ± 5.72 ng/mL in the control group [23]. The sample size was calculated as 17 in each group. Considering 20% lost to follow-up, we considered 20 patients in each group.
2.8. Statistical Analysis
All statistical analyses were performed, using SPSS for Windows (version 21.0; SPSS Inc., Chicago, IL, USA). Quantitative data were tested for normality of distributions by the Kolmogorov–Smirnov test. The data are presented as mean ± standard deviation (SD) or median (percentile, Q1, Q3) for normal and nonnormal distribution, respectively. Two groups were compared by unpaired Student's t-test and Mann–Whitney U test for normal and nonnormal distribution data, respectively. Qualitative data were analyzed by the chi-squared test. A p value of <0.05 was considered significant.
3. Results
3.1. Baseline Data
During the study period, 570 patients were assessed according to the eligibility criteria, and forty-five patients with moderate ischemic stroke were randomized into two groups of this study. Finally, forty-one patients completed the study, whereas 20 (48.7%) of them were in the intervention group (Figure 1). The baseline data of the two groups of the study are shown in Table 1. Except for the age, which was higher in the control group (t (28.64) = 2.145, p = 0.04), there was no statistically significant difference in other baseline characteristics between the two groups.
Figure 1.
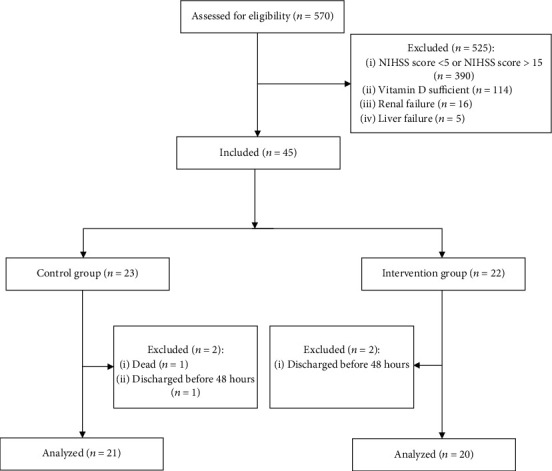
Consort chart of the study.
Table 1.
Baseline data of two groups of the study.
| Intervention group | Control group | p valuea | ||
|---|---|---|---|---|
| Age (year) (mean ± SD) | 60.05 ± 7.75 | 64.24 ± 4.12 | 0.04 | |
| Sex (N (%)) | Male | 14 | 14 | 0.81 |
| Female | 6 | 7 | ||
| 25-OH vitamin D (ng/mL) (mean ± SD) | 23.20 ± 4.15 | 23.14 ± 4.29 | 0.96 | |
| Hospital length of stay (day) (mean ± SD) | 2.05 ± 0.75 | 1.95 ± 0.65 | 0.80 | |
aUnpaired Student's t-test and chi-squared test based on the data.
3.2. Serum NSE Levels
The median (Q1, Q3) of the serum NSE levels in the baseline (NSE 0) was 16.42 ng/mL (15.92, 18.01) and 16.04 ng/mL (15.67, 17.08) in the control and intervention groups, respectively. There was no statistically significant difference in comparison with NSE 0 between the two groups (U(NControl group = 21, NIntervention group = 19) = 161.0, z = −1.043, p = 0.29). Also, 48 hours after the intervention, there was no statistically significant difference in NSE 1 between the two arms of the study (6.74 ng/mL (5.93, 7.38) vs. 6.42 ng/mL (6.11, 9.87) in the control and intervention groups, respectively; U(NControl group = 17, NIntervention group = 16) = 129.0, z = −252, p = 0.80) (Table 2).
Table 2.
Assessment data during study days.
| Intervention group | Control group | p valuea | ||
|---|---|---|---|---|
| NSE (ng/mL) (median (Q1, Q3)) | Baseline | 16.04 (15.67, 17.08) | 16.42 (15.92, 18.01) | 0.29 |
| 48 h after intervention | 6.42 (6.11, 9.87) | 6.74 (5.93, 7.38) | 0.80 | |
| Differences | -9.98 (-11.60, -9.20) | -9.64 (-11.31, -8.06) | 0.48 | |
|
| ||||
| NIHSS (median (Q1, Q3)) | Baseline | 8 (6, 8) | 8 (8, 9) | 0.07 |
| 48 h after intervention | 6.5 (5.5, 7) | 8 (7, 8) | 0.008 | |
aMann–Whitney U test.
The decrement of serum NSE levels were -9.64 ng/mL (-11.31, -8.06) vs. -9.98 ng/mL (-11.60, -9.20) in the control and intervention arms of the study, which did not revealed a statistically significant difference (U(NControl group = 17, NIntervention group = 15) = 109.0, z = −0.699, p = 0.48) (Table 2).
3.3. Neurological Function Assessment Scales
As shown in Table 2, the baseline NIHSS (NIHSS 0) did not show statistically significant difference between the two groups (median (Q1, Q3) was 8 [8, 9] and 8 [6, 8] in the control and intervention groups, respectively; U(NControl group = 21, NIntervention group = 20) = 145.5, z = −1.760, p = 0.07), but 48 hours after the intervention, NIHSS 1 was significantly lower in the intervention arm of the study (median (Q1, Q3) was 8 [7, 8] and 6.5 (5.5, 7) in the control and intervention groups, respectively; U(NControl group = 21, NIntervention group = 20) = 110.5, z = −2.666, p = 0.008) (Table 2).
All patients were monitored after three months from hospital discharge, and the BI was calculated and recorded by a trained nurse. The analysis showed that the BI was significantly higher in the intervention group of the study (median (Q1, Q3) was 8 (7.5, 8.5) and 9 [8, 9] in the control and intervention groups, respectively; U(NControl group = 16, NIntervention group = 17) = 81, z = −2.098, p = 0.03).
4. Discussion
The current study revealed that a single high-dose vitamin D3 injection could significantly improve the neurological function of patients with moderate ischemic stroke, evaluated by the NIHSS and BI as the standard tools for evaluation of neurological function in patients with stroke. This effect was not detected on NSE as a biomarker of neurological damage.
Several observational studies showed worsening stroke severity, based on the NIHSS, and poor poststroke functional outcomes, assessed by the modified Rankin Scale (mRS) or BI scores at the discharge and/or 3-month poststroke, in patients with vitamin D deficiency [24–27].
Most studies examined the low doses of vitamin D on stroke-related comorbidities and complications such as neuromuscular disorders, osteoporosis, falls, and fractures in ischemic stroke patients [11, 28–30]. Improved muscle strength, reduction in falls, and decreased risk of hip fractures were obtained in poststroke patients with the intake of 700-1000 IU/day of vitamin D [28, 29].
The use of IM injection of single high-dose vitamin D in patients with ischemic stroke can be an appropriate treatment option in patients with poor compliance to daily oral vitamin D supplementation and could increase serum 25-OH vitamin D level rapidly and safely [16]. A few studies have examined the effect of high doses of vitamin D on the stroke severity and functional outcome scales of patients with ischemic stroke [17, 18, 31]. In accordance with our findings, Narasimhan et al. and Sari et al. revealed that a single dose, IM injection of 300000 to 600000 IU of vitamin D could improve functional outcomes of patients with ischemic stroke including Scandinavian Stroke Scale (SSS), mRS, NIHSS, and BI besides their balance [17, 18], whereas Rezaei et al. showed a single dose of 300000 IU IM vitamin D had no favorable effects on NIHSS score [31].
To the best of our knowledge, the exact mechanism of vitamin D in the improvement of neurological function of patients with ischemic stroke is not completely understood. NSE is a biomarker for acute ischemic stroke, and it is proven that serum concentration of NSE is correlated with the volume of infarcted tissue [13, 15]. A study that evaluated a high dose of vitamin D on the serum biomarkers such as NSE has not been conducted, but in the current study, we evaluated the NSE serum levels in moderated ischemic stroke patients but did not find any significant difference between the two arms of the study regarding this biomarker.
Serum NSE level increases during the first 24 hours after the stroke. After a decrease in its level, again, the NSE level rises on the 5th day of poststroke [15]. The limitation of this study was that we only evaluated serum NSE levels at the baseline (first 24 hours) and 48 hours after the stroke. Generally, the patients with moderate ischemic stress discharge from the hospital before 5 days, so we were not able to take a blood sample on the 5th day of the poststroke. So, we could not follow the second peak of NSE. For the future studies, we recommended the sequence evaluation of serum NSE levels until day 5 after the onset of the ischemic stroke.
5. Conclusion
In the conclusion, administration of a single 600000 IU of vitamin D3 could have neuroprotective effects in patients with moderate ischemic stroke, according to its significant positive effects on functional clinical outcomes (NIHSS and BI), but this effect on the biomarker related to neural damage (NSE) was not significant.
Data Availability
The data that support the findings of this study are available upon a reasonable request from the corresponding author, RH. The data are not publicly available due to the containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Wajda J., Świat M., Owczarek A. J., Brzozowska A., Olszanecka-Glinianowicz M., Chudek J. Severity of vitamin D deficiency predicts mortality in ischemic stroke patients. Disease Markers . 2019;2019:10. doi: 10.1155/2019/3652894.3652894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji W., Zhou H., Wang S., Cheng L., Fang Y. Low serum levels of 25-hydroxyvitamin D are associated with stroke recurrence and poor functional outcomes in patients with ischemic stroke. The Journal of Nutrition, Health & Aging. . 2017;21(8):892–896. doi: 10.1007/s12603-016-0846-3. [DOI] [PubMed] [Google Scholar]
- 3.Yalbuzdag S. A., Sarifakioglu B., Afsar S. I., et al. Is 25 (OH) D associated with cognitive impairment and functional improvement in stroke? A retrospective clinical study. Journal of Stroke and Cerebrovascular Diseases . 2015;24(7):1479–1486. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Feng C., Tang N., Huang H., Zhang G., Qi X., Shi F. 25-Hydroxy vitamin D level is associated with total MRI burden of cerebral small vessel disease in ischemic stroke patients. The International Journal of Neuroscience . 2019;129(1):49–54. doi: 10.1080/00207454.2018.1503182. [DOI] [PubMed] [Google Scholar]
- 5.Yarlagadda K., Ma N., Doré S. Vitamin D and stroke: effects on incidence, severity, and outcome and the potential benefits of supplementation. Frontiers in Neurology . 2020;11 doi: 10.3389/fneur.2020.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balden R., Selvamani A., Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology . 2012;153(5):2420–2435. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu W.-X., He D.-R. Low vitamin D levels are associated with the development of deep venous thromboembolic events in patients with ischemic stroke. Clinical and Applied Thrombosis/Hemostasis . 2018;24(9_suppl):69S–75S. doi: 10.1177/1076029618786574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Won S., Sayeed I., Peterson B. L., Wali B., Kahn J. S., Stein D. G. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PloS One. . 2015;10(3, article e0122821) doi: 10.1371/journal.pone.0122821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tombini M., Palermo A., Assenza G., et al. Calcium metabolism serum markers in adult patients with epilepsy and the effect of vitamin D supplementation on seizure control. Seizure . 2018;58:75–81. doi: 10.1016/j.seizure.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Casseb G. A. S., Kaster M. P., Rodrigues A. L. S. Potential role of vitamin D for the management of depression and anxiety. CNS Drugs . 2019;33(7):619–637. doi: 10.1007/s40263-019-00640-4. [DOI] [PubMed] [Google Scholar]
- 11.Gunton J. E., Girgis C. M. Vitamin D and muscle. Bone Reports. . 2018;8:163–167. doi: 10.1016/j.bonr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makris K., Haliassos A., Chondrogianni M., Tsivgoulis G. Blood biomarkers in ischemic stroke: potential role and challenges in clinical practice and research. Critical Reviews in Clinical Laboratory Sciences . 2018;55(5):294–328. doi: 10.1080/10408363.2018.1461190. [DOI] [PubMed] [Google Scholar]
- 13.Anand N., Stead L. G. Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovascular Diseases . 2005;20(4):213–219. doi: 10.1159/000087701. [DOI] [PubMed] [Google Scholar]
- 14.Tokshilykova A. B., Sarkulova Z. N., Kabdrakhmanova G. B., Utepkaliyeva A. P., Tleuova A. S., Satenov Z. K. Neuron-specific markers and their correlation with neurological scales in patients with acute neuropathologies. Journal of Molecular Neuroscience . 2020;70(8):1267–1273. doi: 10.1007/s12031-020-01536-5. [DOI] [PubMed] [Google Scholar]
- 15.Najmi E., Bahbah E., Negida A., Afifi A., Baratloo A. Diagnostic value of serum neuron-specific enolase level in patients with acute ischemic stroke; a systematic review and meta-analysis. International Clinical Neuroscience Journal . 2019;6(2):36–41. doi: 10.15171/icnj.2019.08. [DOI] [Google Scholar]
- 16.Tellioglu A., Basaran S., Guzel R., Seydaoglu G. Efficacy and safety of high dose intramuscular or oral cholecalciferol in vitamin D deficient/insufficient elderly. Maturitas . 2012;72(4):332–338. doi: 10.1016/j.maturitas.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Narasimhan S., Balasubramanian P. Role of vitamin D in the outcome of ischemic stroke-a randomized controlled trial. Journal of Clinical and Diagnostic Research: JCDR . 2017;11(2):p. CC06. doi: 10.7860/JCDR/2017/24299.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sari A., Durmus B., Karaman C. A., Ogut E., Aktas I. A randomized, double-blind study to assess if vitamin D treatment affects the outcomes of rehabilitation and balance in hemiplegic patients. Journal of Physical Therapy Science . 2018;30(6):874–878. doi: 10.1589/jpts.30.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powers W. J., Rabinstein A. A., Ackerson T., et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke . 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 20.Brott T., Adams H. P., Jr., Olinger C. P., et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke . 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 21.Quinn T. J., Langhorne P., Stott D. J. Barthel index for stroke Trials. Stroke . 2011;42(4):1146–1151. doi: 10.1161/STROKEAHA.110.598540. [DOI] [PubMed] [Google Scholar]
- 22.Casmiro M., Maitan S., de Pasquale F., Cova V., Scarpa E., Vignatelli L. Cerebrospinal fluid and serum neuron-specific enolase concentrations in a normal population. European Journal of Neurology . 2005;12(5):369–374. doi: 10.1111/j.1468-1331.2004.01021.x. [DOI] [PubMed] [Google Scholar]
- 23.Mokhtari M., Nayeb-Aghaei H., Kouchek M., et al. Effect of memantine on serum levels of neuron-specific enolase and on the Glasgow coma scale in patients with moderate traumatic brain injury. The Journal of Clinical Pharmacology. . 2018;58(1):42–47. doi: 10.1002/jcph.980. [DOI] [PubMed] [Google Scholar]
- 24.Turetsky A., Goddeau R. P., Henninger N. Low serum vitamin D is independently associated with larger lesion volumes after ischemic stroke. Journal of Stroke and Cerebrovascular Diseases . 2015;24(7):1555–1563. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Zhang B., Wang Y., Zhong Y., Liao S., Lu Z. Serum 25-hydroxyvitamin D deficiency predicts poor outcome among acute ischemic stroke patients without hypertension. Neurochemistry International . 2018;118:91–95. doi: 10.1016/j.neuint.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Park K.-Y., Chung P.-W., Kim Y. B., et al. Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovascular Diseases . 2015;40(1-2):73–80. doi: 10.1159/000434691. [DOI] [PubMed] [Google Scholar]
- 27.Lelli D., Pérez Bazan L. M., Calle Egusquiza A., et al. 25 (OH) vitamin D and functional outcomes in older adults admitted to rehabilitation units: the safari study. Osteoporosis International . 2019;30(4):887–895. doi: 10.1007/s00198-019-04845-7. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y., Iwamoto J., Kanoko T., Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovascular Diseases . 2005;20(3):p. 187. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 29.Bischoff-Ferrari H. A., Dawson-Hughes B., Staehelin H. B., et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ . 2009;339(oct01 1):p. b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karasu A. U., Karataş G. K. Effect of vitamin D supplementation on lower extremity motor function and ambulation in stroke patients. Turkish Journal of Medical Sciences. . 2021;51(3):1413–1419. doi: 10.3906/sag-2010-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rezaei O., Ramezani M., Roozbeh M., et al. Does vitamin D administration play a role in outcome of patients with acute ischemic stroke? A randomized controlled trial. Current Journal of Neurology . 2021;20:8–14. doi: 10.18502/cjn.v20i1.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon a reasonable request from the corresponding author, RH. The data are not publicly available due to the containing information that could compromise the privacy of research participants.


