Abstract
The purpose of this review is to summarize the available antidiabetic medicinal plants in the Kingdom of Saudi Arabia with its phytoconstituents and toxicological findings supporting by the latest literature. Required data about medicinal plants having antidiabetic activities and growing in the Kingdom of Saudi Arabia were searched/collected from the online databases including Wiley, Google, PubMed, Google Scholar, ScienceDirect, and Scopus. Keywords used in search are in vivo antidiabetic activities, flora of Saudi Arabia, active ingredients, toxicological evaluations, and medicinal plants. A total of 50 plant species belonging to 27 families were found in the flora of Saudi Arabia. Dominant family was found Lamiaceae with 5 species (highest) followed by Moraceae with 4 species. β-Amyrin, β-sitosterol, stigmasterol, oleanolic acid, ursolic acid, rutin, chlorogenic acid, quercetin, and kaempferol are the very common bioactive constituents of these selected plant species. This paper has presented a list of antidiabetic plants used in the treatment of diabetes mellitus. Bioactive antidiabetic phytoconstituents which showed that these plants have hypoglycemic effects and highly recommended for further pharmacological purposes and to isolate/identify antidiabetes mellitus (anti-DM) active agents also need to investigate the side effects of active ingredients.
1. Introduction
Medicinal plants are used for the treatment of different infections [1, 2]. These plants contributed as a source of inspiration for novel therapeutic compounds [3]. The medicinal value of plants is due to the presence of a wide variety of secondary metabolites including alkaloids, glycosides, tannins, volatile oil, and terpenoids [4, 5]. Medicinal plants and their extracts represent a rich source of crude medications that possess therapeutic properties. Indeed, the World Health Organization reports that various plant fractions and their dynamic constituents are utilized as traditional medicines by 80% of the world population [6]. Plants are the primary source for different pharmaceutical, perfumery, flavor, and cosmetics industries; the use of modern drugs dramatically resulted into resistant microorganisms toward different modern drugs; the researchers are now in search for alternate source of treatment of various disorders [7, 8]. For this purpose, the medicinal herbs are the best alternate to various drugs. Most of natural products possess interesting biological activities and medicinal potential. Various herbs, fruits, and grains have been found to have different important biological activities such as antioxidant, [9] antitumor, antimutagenic, antidiabetes, antianalgesic, [10] antidementia, inflammation inhibitory effect, [9] antitumor, [11] anticancer, [12] antimicrobial, antileishmanial, and antimalarial properties [13, 14]. The consumption of natural antioxidants will reduce risk of many diseases including cancer, cardiovascular disease, diabetes, and other diseases allied with aging [15]. For natural antioxidants, a larger number of medicinal herbs have been evaluated by applying laboratories' developed procedures. Plants derived substances, collectively called phytonutrients or phytochemicals, been recognized as good source of natural antioxidants [16, 17].
The Kingdom of Saudi Arabia is a huge arid land with an area of about 2,250,000 km2 covering the major part of the Arabian Peninsula, characterized by different ecosystems and diversity of plant species. The climate in Saudi Arabia differs greatly between the coast and the interior. High humidity coupled with more moderate temperatures is prevalent along the coast, whereas aridity and extreme temperatures characterize the interior. The flora of Saudi Arabia is one of the richest biodiversities in the Arabian Peninsula and comprises very important genetic resources of crops and medicinal plants. Saudi Arabia contains 97 trees, 564 shrubs, and about 1620 herbs, which cover, respectively, 4.25%, 24.73%, and 71.02% of higher plant diversity of the country [18].
Diabetes mellitus is one of the most prevalent diseases in endocrine gland system with an increasing incidence in human community [19]. Type I diabetes is caused by insulin secretion deficit, while type II diabetes is accompanied with progressive rate of insulin resistance in liver and peripheral tissues, reducing β-cell mass, and deficient insulin secretion [20, 21]. This disease brings about acute metabolic side effects including ketoacidosis, hyperosmolar coma accompanied with chronic disorders, and long term, adverse side effects such as retinopathy, renal failure, neuropathy, skin complications, as well as increasing cardiovascular complication risks [22, 23]. Also, common symptoms of diabetes are frequent urine, thirsty, and overeating [24]. Diabetes inflicts 100 million people yearly and is recognized as the seventh cause of death in the world [25]. It has been estimated that the number of diabetic people will increase from 150 million individuals in 2003 to 300 million by 2025 [26]. The essential and effective drugs for diabetes mellitus are insulin injection and hypoglycemic agents, but these compounds possess several adverse effects and have no effects on diabetes complications in long term. Therefore, it is important to find effective compounds with lower side effects in treating diabetes [27]. Medicinal plants are good sources as alternative or complementary treatments for this and other diseases [28–30]. Although various plants have been traditionally used throughout history to reduce blood glucose and improve diabetes complications, there is not enough scientific information about some of them. Herbal medicines are commonly prescribed throughout the world because of low side effects, availability, roughly low cost, and also its effectiveness [31, 32].
In Saudi Arabia, the number of people who suffer from DM increased from 890,000 in 2000 to a staggering projection of 2,523,000 in 2030. In 2011, Saudi Arabia reported a prevalence of DM at 30% of the total population, with a rate of 27.6% in women and 34.1% in men [33]. According to 2010 data from several sources (WHO, World Bank, UNESCO, CIA, and individual country databases), DM is the number three disease-related cause of death in Saudi Arabia [34].
In the present situation, herbal medicines' usage has significantly increased and published studies from developed countries emphasize that a paramount proportion of medicines supplied by them have herbal origins, so growing and producing the herbal medicines could be helpful to both economic development and community's health [35]. Keeping in mind the importance of medicinal plants, in the current review various medicinal plants used for antidiabetic treatment around the world, native to or cultivated in Saudi Arabia, are documented for the purpose to provide up-to-date insight on medicinal plant used for DM, so that researcher easily selects plant for bioscreening and active constituents' identification purposes. Therefore, we invite researchers' attention to carry out detailed ethnopharmacological and toxicological studies on unexplored antidiabetic plants in order to provide reliable knowledge to the patients and develop novel antidiabetic drugs.
2. Methods
Required data about medicinal plants having antidiabetic activities and growing in the Kingdom of Saudi Arabia were searched/collected from the online databases including Wiley, Google, PubMed, Google Scholar, ScienceDirect, and Scopus. Keywords used in search are in vivo antidiabetic activities, flora of Saudi Arabia, active ingredients, toxicological evaluations, and medicinal plants. Latest published data approximately in the last ten years with the key outcome of change in blood glucose level in animal model were included. One or two articles are selected as references for each plant's species on priority basis from the journals found in web of science and latest years.
3. Results
The names, families, used parts, location, and antidiabetic properties in animal model of the native/cultivated Saudi medicinal plants are summarized in Table 1. The active ingredients and toxicological effect of these plants in animal model are given in Table 2. A total of 50 plant species belong from 27 families were found in the flora of Saudi Arabia. Dominant family was found Lamiaceae with 5 species (highest) followed by Moraceae with 4 species.
Table 1.
Antidiabetic medicinal plants growing in Saudi Arabia.
| S. no. | Names of plants | Family | Part used | location | Antidiabetes Activities |
|---|---|---|---|---|---|
| 1. | Allium cepa | Liliaceae | Bulb | Central Saudi Arabia [36] | Ethanol extract of A. cepa in STZ-induced diabetic rats causes 66% decreased at 200 mg/kg after 24 h in blood glucose level [37]. 0.4 g/100gbw of A. cepa reduced 50% the fasting glucose levels of diabetic rats [38]. Similar results reported by other researchers [39]. |
| 2. | Anthemis herba-alba | Compositae/Asteraceae | Aerial parts | Farasan Island of Red Sea [40] | 72% plasma glucose levels decreased in albino mice by ethyl alcohol extract of Artemisia herba-alba [41] |
| 3. | Cichorium intybus | Asteraceae | Seeds | Qassim region [42] | C. intybus leaf powder, ethanol, aqueous seed extracts, and hexane extracts led to a decrease in blood glucose levels to near normal value. Hypoglycemic effects of C. intybus were observed in diabetic rats, and a dose of 125 mg of plant extract/kg body weight exhibited the most potent hypoglycemic effect [43–45] |
| 4. | Clitoria ternatea | Fabaceae | Aerial parts | Cultivated throughout Saudi Arabia [46] | The aqueous extract of Clitoria ternatea leaves and flower administered for 84 days to diabetic rats significantly decreased blood glucose [46–48] |
| 5. | Ficus carica | Moraceae | Leaves | Southwest of Saudi Arabia [49] | Different extracts and fractions of F. carica showed a clear hypoglycemic effect in diabetic rats. F. carica leaves exerted significant effect on carbohydrate metabolism enzymes with promising hypoglycemic and hypolipidemic activities in type 2 diabetic rats [50, 51] |
| 6. | Ficus benghalensis | Moraceae | Bark | Riyadh [52] | In streptozotocin-induced diabetic rats, bark aqueous extract, and an isolated compound, α-amyrin acetate exhibited antidiabetic activity by decreasing the blood glucose level and increasing the HDL level [53] |
| 7. | Ficus religiosa | Moraceae | Root bark, stem bark, aerial roots | Riyadh [52] | The aqueous extract of bark and ethanol extract of leaves and fruits had a promising antidiabetic effect in streptozotocin-induced diabetic rats by decreasing the blood glucose, serum triglyceride, and total cholesterol levels and increasing serum insulin, body weight, and glycogen content in the liver and skeletal muscle [53] |
| 8. | Ficus microcarpa | Moraceae | Leaves | Riyadh [52] | F. microcarpa leaves showed protective effect against alloxan-induced diabetic rats by reducing blood glucose, cholesterol and triglyceride levels, and increased insulin level [53] |
| 9. | Hypericum perforatum | Hypericaceae | Leaves | Western Saudi Arabia [54] | H. perforatum ethyl acetate extract possesses potent antihyperglycemic activity in STZ-induced diabetic rats [55]. |
| 10 | Anethum graveolens | Apiaceae | Seeds | Makka [56] | Different extracts and tablets of Anethum graveolens possess potent antihyperglycemic activity in alloxan-induced diabetic mice [57] |
| 11 | Cuminum cyminum L. | Apiaceae or Umbelliferae | Seeds | Makka [56] | Oral administration of cumin seeds crude ethanol extract and glibenclamide to diabetic rats significantly and progressively restored toward normal. Cumin seeds crude ethanol extract and glibenclamide reduced plasma glucose levels by 38.34 and 37.73%, respectively, compared with diabetic control [58]. Other studies also reported similar results [59]. |
| 12 | Marrubium vulgare | Lamiaceae | Whole plant | Widely distributed in Saudi Arabia [60] | M. vulgare extracts lower blood glucose level 30 to 60% in dose-dependent manner in streptozotocin-induced diabetic rats [60]. |
| 13 | Mentha longifolia | Lamiaceae | Whole plant | Madinah [61] | Remarkable antidiabetic, anticholinesterase, and antityrosinase effects were recorded for the mint oil [61, 62]. Still need to investigate in vivo antidiabetic potential. |
| 14 | Origanum syriacum | Lamiaceae | Leaves | Saudi Desert [63] | The whole plant extract of O. syriacum at 100 and 400 mg/kg significantly lowers glucose level in diabetic induced rats [64]. |
| 15 | Teucrium oliverianum | Lamiaceae | Aerial parts | Throughout Saudi Arabia [65,66] | Aqueous and ethanol extract of Teucrium oliverianum were tested for antidiabetic activity in alloxan-induced diabetic mice. Both extracts significantly reduced blood sugar levels [65] |
| 16 | Teucrium polium | Lamiaceae | Leaves | Madinah [67] | Infusion orally (64% decrease glucose level) and intraperitoneal of different extracts of T. polium caused significant reductions in blood glucose concentration in STZ hyperglycemic rats [68] |
| 17 | Achyranthes aspera | Amaranthaceae | Whole plant | Al Hada Road Taif [69] | The methanolic and ethanolic extract of A. aspera exhibited significant hypoglycemic activity in streptozotocin-induced diabetic rats [70] |
| 18 | Aerva lanata | Amaranthaceae | Leaves | Southwest region of Saudi Arabia [71, 72] | Extracts of Aerva lanata and glibenclamide were found to significantly (P < 0.01 and P < 0.05) reduce the blood glucose level and lipid profile in streptozotocin-induced diabetic rats [73] |
| 19 | Alternanthera sessilis | Amaranthaceae | Whole plant | Hail region, Saudi Arabia [74] | In diabetic mice at doses of 50, 100, 200, and 400 mg per kg body weight, the extract reduced blood sugar levels by 22.9, 30.7, 45.4, and 46.1%, respectively, compared to control animals. By comparison, a standard antihyperglycemic drug, glibenclamide, when administered at a dose of 10 mg per kg body weight, reduced blood glucose level by 48.9% [75] |
| 20 | Carissa edulis | Apocynaceae | Leaves | Southern region of Saudi Arabia [76] | Oral administration of C. edulis extracts of the leaves significantly reduced the blood glucose level in STZ diabetic rats [77]. |
| 21 | Catharanthus roseus | Apocynaceae | Flower, leaves, stem, and root | Western Saudi Arabia [78] | C. roseus (100 mg/kg BW) lowered the glucose level more than metformin-treated group (100 mg/kg BW) in STZ-induced hyperglycemia rats. C. roseus 200 mg/kg dose was found to be more effective in reducing fasting blood glucose levels [79] |
| 22 | Rhazya stricta | Apocynaceae | Leaves, seeds | Middle and western region of Saudi Arabia [80] | Extracts Rhazya stricta lowered 37.9% blood glucose level in the streptozotocin-induced diabetic rats. Serum cholesterol and triglyceride levels were significantly (P < 0.05) reduced in the treated diabetic group compared to the untreated diabetic group [81] |
| 23 | Calotropis procera | Asclepiadaceae | Latex | Al-Kharj [82] | Different extracts of C. procera at dose of 250 mg/kg were orally administered as single dose per day to diabetes-induced rats for the period of 15 days significantly decreases blood glucose level to the level of standard drug glibenclamide [83] |
| 24 | Opuntia dillenii | Cactaceae | Fruit | Jazan Region [84] | Researcher observed the significant hypoglycemic activity of Opuntia dillenii extract in streptozotocin-induced diabetic mice and rabbits [85] |
| 25 | Opuntia ficus-indica | Cactaceae | Stem | Jazan Region [84] | Powder and water extract of O. ficus-indica significantly (in comparison with control group) returned blood glucose level to the initial level, 180 min after administration in STZ-induced diabetic rats [86]. Many studies confirmed the hypoglycemic activities of O. ficus-indica [87] |
| 26 | Capparis decidua | Capparaceae | Fruits, seeds | Jazan Region [84] | C. decidua extracts at dose level of 200 and 800 mg/kg significantly reduce sugar level (in a dose-dependent manner) compared to standard drug in STZ-induced diabetic and normal rats [88]. |
| 27 | Beta vulgaris | Chenopodiaceae | Root bark | North Hejaz and Eastern Najd region of Saudi Arabia [89] | Extract of B. vulgaris at does level 50, 100, and 200 mg/kg of significantly reduced sugar level and increased in insulin level (in a dose-dependent manner) in streptozotocin or alloxan-induced diabetic mice [90]. Other researchers also concluded similar finding in STZ-induced diabetic rats [91]. |
| 28 | Haloxylon salicornicum Bunge | Chenopodiaceae | Whole plant | Wadi-Hafr-Al-Batin, Saudi Arabia [92] | Ethanol extract (100 and 200 mg/kg of bw) of H. salicornicum (oral administration) exhibited persistent hypoglycemic effects in STZ-induced diabetic rats [93] |
| 29 | Evolvulus alsinoides | Convolvulaceae | Whole plant | Jazan Region [84] | E. alsinoides ethanol extract at dose level (150 mg/kg bw) in normal and streptozotocin-induced diabetic rats leads to hyperglycemia in experimental diabetic rats that decreased utilization of glucose by insulin-dependent pathways [94, 95] |
| 30 | Ipomea aquatica | Convolvulaceae | Whole plant | Jazan Region [96] | I. aquatica ethanol extract at dose level (10, 100, and 1000 µg/ml in streptozotocin-induced diabetic rats significantly (P < .05) exhibited the ability to enhance insulin-mediated glucose uptake into 3T3F442A adipocytes cells compared to insulin alone [97]. Another study confirmed that doses (200 mg/kg and 400 mg/kg) reduced blood glucose level, and it was statistically highly significant (P < 0.001) in comparison with control group [98]. |
| 31 | Citrullus colocynthis | Cucurbitaceae | Fruits | Jazan Region [84] | 1 ml/kg and 2 ml/kg of C. colocynthis extract (orally administered) stabilized animal body weight and ameliorated hyperglycemia in a dose- and time-dependent manner in alloxan-induced diabetic rats [99] |
| 32 | Citrullus lanatus | Cucurbitaceae | Seed | Wadi Lajab, Saudi Arabia [100] | C. lanatus seed extract (2, 4 g/kg) treatment significantly lowers glucose level which suggested that C. lanatus had antidiabetic property in STZ-induced diabetes mice [101]. Other researcher also concluded similar finding in STZ-induced diabetic rats [102] |
| 33 | Coccinia grandis | Cucurbitaceae | Whole plant | Jazan Region [84] | The C. grandis extract (0.75 mg/kg, orally) showed remarkable glycemic effect which confirmed antidiabetic potential in streptozotocin-induced diabetic rats [103]. |
| 34 | Jatropha curcas | Euphorbiaceae | Leaves | Jazan Region [84] | Ethanolic extract of J. curcas leaves at doses of (250 and 500 mg ml−1 bw by administered orally) reduced glucose level from 219.5 to 116.5 and 237 to 98.8, respectively, in alloxan-induced diabetic rats. The results were comparable to reduction in rats treated with the standard glibenclamide 232–94.5 at 600 μg kg−1 [104]. |
| 35 | Ricinus communis | Euphorbiaceae | Leaves | Jazan Region [84] | R. communis extracts at doses of 300 and 600 mg/kg/BW administered orally caused hyperglycemia in a dose-dependent manner in streptozotocin-induced diabetic rats [105]. |
| 36 | Ficus carica | Moraceae | Leaves | Jazan Region [84] | A review article focusing on antidiabetic potential of F. carica confirmed that different extracts and fractions of F. carica and different doses significantly reducing hyperglycemia in streptozotocin-induced diabetic rats compared to standard drug [106]. |
| 37 | Ficus sycomorus | Moraceae | Leaves | Jazan Region [84] | Alloxan-induced type 2 diabetic albino Wistar rats treated with 250, 500, and 1000 mg/kg (body weight) of the extract of F. sycomorus intraperitoneally reduced glucose level in diabetic rats almost to the normal as compared to diabetic control [107] |
| 38 | Sesamum indicum | Pedaliaceae | Seeds | Jazan Region [84] | Alloxan-induced diabetic rats treated with 5% and 10% of Sesamum indicum seed powder significantly decreased blood glucose and increased insulin levels as compared with the positive (diabetic) control group [108] |
| 39 | Plantago ovata | Plantaginaceae | Husk | Northern border region of Saudi Arabia [18] | In intravenous administration of alloxan-induced diabetic rabbits glucose level lowering effect observed (time dependent manner) with P. ovata husk extract of dose level (300 mg/kg, orally administered) [109] |
| 40 | Polygala erioptera | Polygalaceae | Aerial part | Jazan Region [84] | 0.7 g/kg of P. erioptera extract showed significant antidiabetic effect compared to standard drug metformin and glibenclamide in normal and alloxan-induced diabetic rats [110] |
| 41 | Polygonum aviculare L | Polygonaceae | Aerial parts | Taif Region [111, 112] | Many ethnopharmacological investigations reported its antidiabetic potential but still need to study its in vivo and in vitro antidiabetic potential [113, 114] |
| 42 | Ziziphus spina-christi | Rhamnaceae | Leaves | Eastern region of Saudi Arabia [84, 112] | The strongest (P < 0.001) antidiabetic activity (25.59 and 39.48% after 7 and 15 days, respectively) was found following treatment with dose level of 500 mg/kg of Z. spina-christi extract in streptozotocin-induced diabetes mice [115]. |
| 43 | Bacopa monnieri | Scrophulariaceae | Aerial parts | Jazan Region [84] | B. monnieri extract at dose level of 50, 100, 200, and 400 mg/kg significantly inhibited (33.3, 34.2, 42.1, and 44.2%, respectively) the increase in serum glucose concentration in a dose-dependent manner compared to standard drug [116]. |
| 44 | Lycium shawii | Solanaceae | Aerial parts | Taif Region [112] | The strongest (P < 0.001) antidiabetic activity of L. shawii extract of 250 and 500 mg/kg bw was found in a dose-dependent manner in streptozotocin-induced diabetes rats [117]. |
| 45 | Solanum nigrum | Solanaceae | Whole plant | Jazan Region [84] | S. nigrum extract was given orally in the dose level of 200 and 400 mg/kg/day (7 days) significantly lowering the blood glucose level in fasting compared to standard drug in alloxan-induced diabetic albino Wistar rats [118]. |
| 46 | Withania somnifera | Solanaceae | Leaves | Jazan Region [84] | W. somnifera extract oral administration at two doses (200 and 400 mg/kg) reduced the blood glucose level significantly (P < 0.001) in a dose‐dependent manner in streptozotocin-induced diabetes rats. Only WS treatment did not register any significant change in the blood glucose level when compared to citrate control rats [119]. Another study also confirmed similar results in alloxan-induced diabetic rats [120] |
| 47 | Lantana camara | Verbenaceae | Leaves | Jazan Region [84] | Literature survey showed that L. camara leaf extract oral administration (200, 250, and 500 mg/kg of bw) showed antidiabetic potential in alloxan-induced diabetic rats [121] |
| 48 | Peganum harmala | Zygophyllaceae | Seeds | Taif Region [112] | P. harmala seed extract at dose level of (30, 60, and 120 mg/kg, orally administered for four weeks) significantly decreases in blood glucose (in all doses, P < 0.001), in comparison with diabetic group [122]. |
| 49 | Tribulus terrestris | Zygophyllaceae | Stem, leaves | Jazan Region [84] Taif Region [112] |
T. terrestris extract at (2 g/kg body weight) produced protective effect in streptozotocin-induced diabetic rats by inhibiting oxidative stress [123]. T. terrestris L. extract (250 mg/kg of bw orally administered) significantly lowers glucose level to normal compared to standard drug in glucose-loaded normal rabbits [124] |
| 50 | Urtica dioica | Urticaceae | Leaves | Wild plant, Tanhat, Saudi Arabia [125] | Urtica dioica extract at 100 mg/kg (P < 0.01) and 200 mg/kg (P < 0.001) significantly decreased serum glucose fructose-induced insulin resistance rats [126]. The aqueous extract of U. dioica significantly (P < 0.001; 67.92%) reduced the blood glucose level at dose of 300 mg/kg, IP) in streptozotocin-induced diabetes rats [127] |
Table 2.
Active ingredients and toxicological evaluation of the medicinal plants given in Table 1.
| S. No | Names | Active ingredients | Toxicological evaluation |
|---|---|---|---|
| 1 | Allium cepa | Quercetin, N-acetylcysteine, alliuocide, cycloalliin, S-methyl-L-cysteine, S-propyl-L-cysteine, sulfoxide, dimethyl trisulfide, S-methyl-L-cysteine sulfoxide [128] | The animals tested were found healthy with no sign of toxicity up to the dose of 2 500 mg/kg. However, at 5 000 mg/kg, animals were weak and had intense extreme tachycardia and disorientation but no death was recorded. Thus, LD50 was more than 5 000 mg/kg [129]. |
| 2 | Anthemis herba alba | Guainalides, eudesmanolide, pseudogua inolides, xanthonolides, flavone, flavonol glycosides, hispidulin, cirsilineol, vicenin-2, schaftoside, isoschaftoside, 5′,4-dihydroxy-6,7,3-trimethoxyflavone, quercetin-3-rutinoside, patuletin 3-rutinoside, patuletin 3-glucoside [130] | The available toxicological investigations have shown generally that Anthemis herba-alba is free from toxic effects at the different doses used in the studies [130] |
| 3 | Cichorium intybus | Chicoric acid, inulin, cichoralexin, cichoriin, esculetin, isochlorogenic acid, chlorogenic acid, caffeic acid, dicaffeoylquinic acid, aesculin, arginine, histidine, isoleucine, leucine, lysine, methionine, cysteine, phenylalanine, tyrosine, threonine, valine, serine, glutamic acid, glycine, alanine, aspartic acid, and proline [44, 45] | There were no treatment-related toxic effects from chicory extract administered orally at 70, 350, or 1000 mg/kg/day. There were no observed adverse effects of chicory extract in these studies [45] |
| 4 | Clitoria ternatea | Kaempferol, quercetin, myricetin, taxaxerol, tannic acid, 3-monoglucoside, β-sitosterol, delphinidin-3,5-diglucoside, anthoxanthin glucoside, p-hydroxycinnamic acid, kaempferol 3-neohesperidoside, myricetin 3-rutinoside, hexacosanol [48] | Ethanolic extract of aerial parts and root of CT led to lethargy in mice at the doses of 1500 mg/kg and above, orally [31]. Ptosis was seen above 2000 mg/kg dose in mice. Through intraperitoneal route, 2900 mg/kg dose was lethal within 6 hr due to severe CNS depression [47]. |
| 5 | Ficus carica | Over 100 bioactive compounds have been identified in fig such as rutin, arabinose, chlorogenic acid, β-amyrins, syringic acid, β-carotenes, glycosides, β-sitosterols, and xanthotoxol [131] | The rats tested were found healthy with no sign of toxicity up to the dose of 5000, 5500, and 6000 mg/kg. However, at 5 000 mg/kg, animals were weak and had intense extreme tachycardia and disorientation but no death was recorded. Thus, LD50 was more than 6000 mg/kg [132] |
| 6 | Ficus benghalensis | Leucopelargonidin-3-0-α-L rhamnoside, eucodelphinidin, leucoanthocyanidia, leucoanthocyanin, α-amyrin acetate [53] | In acute toxicity studies, no mortality and signs of toxicity were observed at the dose of 2000 and 5000 mg/kg body weight for aqueous and ethanol extracts, respectively [53] |
| 7 | Ficus religiosa | Lupeol, β-sitosterol, β-sitosterol-d-glucoside, stigmasterol, lanosterol, campesterol, octacosanol, methyl oleonate, lupen-3-one, bergapten, and bergaptol [53] | Acute toxicity reported up to dose level 2000 mg/kg showed no mortality [53] |
| 8 | Ficus microcarpa | Polyphenols, organic acids, alkaloids, polysaccharides, megastigmanes, pheophytins, catechin, epicatechin, isovitexin, phenolic acids [53] | The oral administration of a single dose of 2000 mg/kg ethanol or methanol extract of leaves showed no mortality or behavioral alterations in the tested animals [53] |
| 9 | Hypericum perforatum | Quercitrin, rutin, hypericin, kaempferol, biapigenin, hyperforin [133] | Acute toxicity studies revealed the nontoxic nature of the H. perforatum [55] |
| 10 | Anethum graveolens | Carvone, α-phellandrene, limonene, dill ether, myristicin coumarins, flavonoids, phenolic acids, steroids [134] | The mice treated with AG of different doses of 1000, 2000, 3000, 4000, and 5000 mg/kg of body showed no toxicity [135] |
| 11 | Cuminum cyminum | Cuminaldehyde, limonene, α- and β-pinene, 1, 8-cineole, o-and p-cymene, α- and γ-terpinene, safranal, and linalool [58, 59] | The acute lethal toxicity test revealed that cumin crude extract was very safe [58] |
| 12 | Marrubium vulgare | Furanic labdane diterpenes, marrubenol, marrubiin, ladanein [60] | An acute toxicity study of M. vulgare (1 g/kg) extract orally administered at a dose of 1 g/kg body weight to the mice and treated mice showed tachycardia 1 h after intake of the infusion and loss of appetite 3 h after intake of the infusion. In another experiment, a single dose of 2000 mg/kg extract of M. vulgare for an acute toxicity study showed no toxicity [60]. |
| 13 | Mentha longifolia | Lucenin-1, lucenin-2, camphelinone, camphene, carveol, carvone, carvone oxide, limonene, linalool, menthatriene, menthofuran, menthol, menthone, myrcene, p-cymene, piperitenone, piperitone, sabinene, α-pinene, α-terpinene, α-terpineol, longifone, pulegone, longifoamide-A, longifoamide-B, longiside-A, longiside-B, eugenol, salvianolic acid, eriodictyol-7-rutinoside, apigenin-7-O-glucoside, hypolaetin, longitin, luteolin, etc. [136] | M. longifolia extract was safe, and no toxicity or mortality was observed in both the oral (3200 mg/kg) and intraperitoneal (1730 mg/kg) administration in rats. Fourteen days of oral administration of the essential oil (125, 250, 375, and 500 µL/kg) resulted in the reduction of red blood cells and lymphocytes and elevation of neutrophils and monocytes compared with normal animals [136]. |
| 14 | Origanum syriacum | Carvacrol, thymol, thymoquinone [137] | Not available |
| 15 | Teucrium oliverianum | 8-O-acetylharpagide, 12-O-methylteucrolin A, teucrolivin A, eupatorin, teucrolivin B, μ24(S)-stigmasta-5,22,25-trin-3β-ol [66] | Not available |
| 16 | Teucrium polium | Apigenin, luteolin, rutin, cirsiliol, cirsimaritin, salvigenin, and eupatorin in the roots, aerial parts, and inflorescences, teucardoside, b-sitosterol, stigmasterol, campesterol, brassicasterol, and clerosterol [68] | All rats treated with different concentrations of the total extract of TP were alive during the 14 days of observation. The animals did not show visible signs of acute toxicity. It suggested that the LD50 of the total extract was higher than 8 g/kg [138] |
| 17 | Achyranthes aspera | Aliphatic acid, betaine, achyranthine, β-ecdysterone, achyranthes saponins A, B, C, D, oleonolic acid, glycosides, triacontanol, E-sitosterol and spinasterol, triacontanol, hydroquinone, eugenol [70] | In acute oral toxicity studies, there was no increase or decrease in any of the parameters studied, in comparison with control animals [139] |
| 18 | Aerva lanata | Quercetin, betulin, aervine, ervoside, methylervine, aervine, lupeol, kaempferol, aervolanine, aervolanine, ervoside, methylaervine, persinosides A and B, tannic acid, lupeol acetate, benzoic acid, methyl grevillate [140] | The LD50 of the extract of AL for oral and IP acute toxicity tests were 22.62 g/kg and 0.432 g/kg, respectively. The extract produced apparent changes in body weights of both male and female rats and increased the weights of lung, brain, and pancreas of female rats while reducing the weight of testes in male rats. Hematological parameters were also altered [72] |
| 19 | Alternanthera sessilis | Stigmasterol, β-sitosterol, β-carotene, ricinoleic acid, myristic, palmitic, stearic, oleic, and linoleic acids, α-spiraterol, uronic acid, cyclo eucalenol, choline, oleanolic acid, lupeol [141] | The crude extract did not show any toxicity in mice even at the highest dose tested [75] |
| 20 | Carissa edulis | Β-Amyrin, (+)-carissone, 2α-carissanol, 6α-carissanol, dehydrocarissone, pinene, myrcene, limonene, sabanene, rutin, epicatechin gallate, carinol, lariciresinol, β-sitosterol, sitosterol glucoside, stigmasterol glucoside, scopoletin, isofraxidin, pinitol [77] | Lethal effects were not observed after the oral administration of the standardized ethanol extract at doses of 1600, 2900, and 5000 mg/kg. No behavioral changes were observed during the observation period. The oral LD50 of the extract was estimated to be greater than 5000 mg/kg. [142]. |
| 21 | Catharanthus roseus | Vinblastine, vincristine, vindesine, vindeline tabersonine, ajmalicine, vinceine, vineamine, raubasin, reserpine, catharanthine, rosindin [143] | No mortality, but dose level higher than 300 mg of C. roseus extract can produce signs of biochemical and histopathological toxicity in liver, kidney, and heart. It is recommended that lower doses than the studied ones should be used for treatment [144] |
| 22 | Rhazya stricta | Polyneuridine, stemmadenine, strictanol, rhazimine, rhazinilam, rhazimanine, sewarine, vallesiachotamine, tetrahydrosecamine [145] | Daily oral dosing of R. stricta extract (0.25 g/kg) for 42 days was not fatal to sheep [145]. |
| 23 | Calotropis procera | Calotropin, calotoxin, calactin, uscharin, voruscharin, uzarigenin, syriogenin, proceroside, calotropagenin, calotropain enzymes, α-amyrin, β-amyrin, lupeol, β-sitosterol, ursolic acid, calotropin, gigantin, giganteol [146] | 2000 mg/kg body weight in single oral administration of aqueous and hydroalcoholic extract did not cause any death after 72 h post-treatment in male and female mice. Daily administration of aqueous extract to male and female Wistar rats during 3 and 6 weeks at the dose of 20 mg/kg/day induced no mortality in either sex [147]. Whoever C. procera is a toxic plant that is avoided by grazing animals. Its latex is used by tribes to poison arrows used for hunting. If in contact with human eye, it could cause ocular toxicity, causing loss of vision and photophobia [146] |
| 24 | Opuntia dillenii | Betanin, betanidin, kaempferol, kaempferide, quercetin, isorhamnetin, β-sitosterol, C29-5β-sterols, taraxerol, friedelin, methyl linoleate, 7-oxositosterol, 6β-hydroxystigmast-4-ene-3-one, daucosterol, methyl eucomate, eucomic acid [85] | During the oral toxicity study of the crude drug in rats, given doses up to 50 ml/kg exhibited no symptoms of toxicity [85] |
| 25 | Opuntia ficus indica | Quercetin, isorhamnetin, kaempferol, luteolin, isorhamnetin, isorhamnetin glycosides, gallic acid, coumaric, narcissin, rutin, nicotiflorin, isoquercetin, ferulic acid [87]. | In vivo toxicity study suggests that the oral administration of Opuntia ficus indica extract at levels up to 2000 mg/kg/day does not cause adverse effects in male and female rats [148]. |
| 26 | Capparis decidua | n-Triacontane, n-pentacosane, β-carotene, n-triacontanol, kaempferol, quercetin, isodulcite, nanocosane, capric acid, glucocapparin, capparine, capparinine, cappariline, codonocarpin, β-sitosterol [88] | The oral administration of C. decidua extract (500, 1000, 2000, and 4000 mg/kg) did not provoke any gross behavioral changes or manifestations of toxic symptoms in male rats [149]. |
| 27 | Beta vulgaris | Betaine, betacyanins, betaxanthins, oxalic acid, and ascorbic acid [89] | In acute oral toxicity studies, the BVBF did not show any sign and symptoms of toxicity and mortality up to 2000 mg/kg dose, considered relatively safe [150] |
| 28 | Haloxylon salicorlicum Bunge | Kaempferol, quercetin, betaine chloride, piperidine, anabasine, aldotripiperideine, haloxine, halosaline, oxedrine, tyramine, N-methyltyramine, scopoletin, scopolin, umbelliferone, xanthotoxol, isooxyimperatorin, esculetin, β-sitosterol, ursolic acid, β-amyrin [93]. | H. salicorlicum extract at doses 0.1, 0.2, 0.3, 0.4, and 0.5 mL/kg orally administered in rats was safe and showed no mortality or adverse effect [92]. |
| 29 | Evolvulus alsinoides | β-Sitosterol, betaine, shankpushpin, evolvine, caffeic acid, 6-methoxy-7-O-β-glucopyranoside coumarin, 2-C-methyl erythritol, kaempferol-7-O-β-glucopyranoside, kaempferol-3-O-β-glucopyranoside, quercetin-3-O-β-glucopyranoside, scopoletin, scopolin [151] | The Evolvulus alsinoides extract did not cause any mortality up to a dose of 1500 mg/kg body weight and no behavioral, neurological, and autonomic profiles and was found to be safe [151]. |
| 30 | Ipomea aquatica | Caffeic acid, chlorogenic acid, quercetin glucoside, quercetin malonyl glucoside, quercetin diglucoside, catechin, isochlorogenic acid A, C, aspartic acid, glycine, alanine and leucine, 7-O-β-D-glucopyranosyldihydroquercetin-3-O-α-D-glucopyranoside [97, 98] | In acute toxicity studies, I. aquatica extract was found to be safe up to 2g to 5 g/kg in mice. No mortality or toxic symptoms were observed during the entire duration of the study [152, 153]. |
| 31 | Citrullus colocynthis | Cucurbitane, gallic acid, kaempferol, cucurbitacin A-E, I-L, chlorogenic acid, caffeic acid, colocynthoside A,B,C; choline, almitic acid, stearic acid, linoleic acid, oleic acids, catechin, myricetin, α-tocopherol, γ-tocopherol, β-carotene [153] | C. colocynthis plant is safe to use. Studies showed that lethal dose (LD50) to be 200 mg/kg, which indicate that the studied plant is not toxic when comparing the LD50 values of most bioactive pharmaceuticals currently used in therapeutics [154] |
| 32 | Citrullus lanatus | Lycopene, vitamin A, cucurbitacin E, citrulline arginine, glutamine and aspartic acid, pectin, vitamin b-complex and minerals [155, 156] | In acute toxicity study, there was no mortality observed up to the maximum dose level of 2000 mg/kg body weight of the extract after administered orally [102] |
| 33 | Coccinia grandis | Cephalandrol, β-sitosterol, cephalandrins A and B, β-amyrin acetate, lupeol, cucurbitacin B, taraxerone, taraxerol, β-carotene, lycopene, cryptoxanthin, xyloglucan, carotenoids, β-sitosterol, stigma-7-en-3-one, lupeol, β-amyrin, β-sitosterol, taraxerol [157] | The acute toxicity study indicated that treatment of C. grandis is safe up to 2 g/kg tested on animal [158]. |
| 34 | Jatropha curcas | Jatrophol, jatropha factor C1, C2, C3,C4, C5, C6, jatropholones A, B, palmarumycin CP1, JC1, JCV2, curcin, curcacycline A, curcain, β-amyrin, β-sitosterol, stigmasterol, friedelin, taraxasterol, diamide, pyrimidine-2,4-dione, nobiletin, tomentin [103] | Many researchers have confirmed that J. curcas is highly toxic for animal as well as human. All parts of J. curcas are toxic and toxic compound reported from this plant like lectins, curcin, phorbol esters, phytate, protease inhibitors [103] |
| 35 | Ricinus communis | Rutin, quercetin, gallic acid, ricin, ricin A, kaempferol-3-O-β rutinoside, gentistic acid, linolenic acid, α-pinene, α-thujone, stigmasterol, ricinine, β-sitosterol, lupeol, castor oil [159, 160] | R. communis extracts given by oral route were safe up to a dose of 2,000 mg/kg/BW and did not show any mortality and toxic effects in the behavior of the treated animals [104]. |
| 36 | Ficus carica | Ferulic acid, quercetin-3-O-glucoside, quercetin-3-O-rutinoside, psoralen, bergapten, coumarin, oleanolic acid, eugenol, angelicin, germacrene D, menthol, α-pinene, β-pinene [161] | Toxicity of 70% methanolic extract of Ficus carica leaves showed LD50 value of brine shrimp assay was 0.158 mg/ml [162] |
| 37 | Ficus sycomorus | Tannins, saponins, flavones, aglycones, anthraquinone glycosides, and flavonoid glycosides [53] | F. sycomorus methanol extract of stem bark is nontoxic up to the dose of 5000 mg/kg [53] |
| 38 | Sesamum indicum | Thelignans, sesamolin, sesamin, pinoresinol, lariciresinol, α-globulin, β-globulin, triacylglycerols, oleic, linoleic acids, sesamol, γ-tocopherol, 2-furfurylthiol, 2-phenylethylthiol, 2-methoxyphenol, 2-pentylpyridine, vitamin E, quinone, sesangolin [163, 164] | S. indicum ethanol extract is safe, up to dose level of 2000 mg/kg in acute toxicity studies in tested animals [165] |
| 39 | Plantago ovata | Hemicellulose,d-xylose, l-arabinose, d-glucose, d-galactose, and l-rhamnose, 5, 6,8-epiloganic acid, gardoside, plantamajoside [166] | 4,5 Gram seed husk one to four times a day soaked in 150 ml of warm water recommend by WHO. Studies confirmed its side effect like bloating, gas, and allergy. No mortality reported [167] |
| 40 | Polygala erioptera | Helioxanthin [168]. No literature available. Recommended for natural products, isolation, biological and toxicological evaluation. | No literature available. Recommended for pharmacological and toxicological evaluation. |
| 41 | Polygonum aviculare L | Protocatechuic acid, catechin, myricitrin, epicatechin-3-O-gallate, avicularin, quercetin, juglanin, kaempferol, myricetin 3-0-(3′-0-galloyl-rhamnopyranoside, cinaroside, liquiritin, rutin [169, 170] | No data available |
| 42 | Ziziphus spina-christi | Jujuboside B1, christinin A, christinin A1 and A2, lotoside II, catechin, epicatechin, kaempferol 3-O-(6-O-rhamnosyl-galactoside), quercetin 7-O-(6-O-rhamnosyl-glucoside), quercetin 3-O-glucoside, kaempferol 3-O-glucoside [171, 172] | Butanol and water extract of Ziziphus spina-christi up to 100 mg/kg and 5 g/kg, respectively, in animal model produced no functional or structural disturbances in liver and kidney and no hematological changes [173, 174] |
| 43 | Bacopa monnieri | Bromine, β-sitosterol, betulinic acid, stigmasterol nicotinine, herpestine, bacosides A, bacopasides (I, II, III, IV, V), pseudojujubogenin glycoside, saponins (A, B, C) [175] | B. monnieri extract at the dose of 5,000 mg/kg did not cause any side effects. Similarly doses of 30, 60, 300, and 1,500 mg/kg given for 270 days did not produce any toxicity in rats [176]. |
| 44 | Lycium shawii | Lyciumate, cyclopentapyrrolidine, imidazole, piperidine, nortropane, tropane, pyrrole, spermine, costunolide, catechin, lyciumaside, emodin, betulinic acid, β-sitosterol glucopyranoside, quercetin, gallic acid, rutin, ρ-coumaric acid, ferulic acid [177, 178] | Reported data revealed that LD50 of the L. shawii extract was greater than 2000 mg/kg b.w in animal model [179]. |
| 45 | Solanum nigrum | Chlorogenic acid, quercetin, naringenin, solasodine, solamargine, solasonine, α-solanigrine, β-solanigrine, ascorbic acid, nigrumnins I and II [180] | S. nigrum extract at a dose of 2000 mg/kg p.o. was safe and showed no changes/alteration in normal behavior in animal model. No mortality was observed [181] |
| 46 | Withania somnifera | Withanolides, withaferin, withaferin A, withanone, withanolide A, withanolide IV, withanolide V, withanolide D [182]. | LD50value of W. somnifera extract in rates was greater than 2000 mg/kg body weight. Compared to the control group in subacute toxicity study, administration of extract did not show any toxicologically significant treatment-related changes in clinical observations, and the toxicological studies revealed that the reasonable doses of W. somnifera are nontoxic and safe [182, 183] |
| 47 | Lantana camara | Eicosane, squalene, β-ionone, caryophyllene oxide, β-caryophyllene, hexanoic acid, tiglic acid, lantanilic acid, camaric acid, lantadene B, oleanolic acid, lantadene A, lantaninilic acid, lantoic acid, ursolic acid, betulinic acid [184] | L. camara extracts at dose level of 2000 mg/kg and 5000 mg.kg body weight in mice and rats, respectively, showed no significant toxic signs or mortality [185, 186] |
| 48 | Peganum harmala | Harmine, harmaline, harmalol, harman, vasicine and vasicinone, pegamine, acacetin 7-O-rhamnoside, 7-O-6″-O-glucosyl-2 ″-O-(3‴-acetylrhamnosyl) glucoside, 7-0-(2‴-0-rhamnosyl-2″-O-glucosylglucoside), peganone 1 and 2, p-cymene, limonene, eugenol, thujico acid, β-cubebene [187–189] | P. harmala different doses of different extracts in animal and human clinical studies confirmed that this plant showed side effect like intoxications, abdominal writhing, body tremors, and toxic at high does level causing paralysis, liver degeneration, euphoria, convulsions, nausea, vomiting, hypothermia. However, therapeutic doses have been reported to be safe in a rodent model [189] |
| 49 | Tribulus terrestris | Naringin, rutin, hyperoside, quercitrin, naringenin, quercetin, hesperetin, kaempferol, apigenin, pyrogallol, gallic acid, catechin, catechol, chlorogenic acid, caffeic acid, vanillic acid, ferulic acid, salicylic acid, ellagic acid, coumaric acid, cinnamic acid [190] | T. terrestris extract showed no mortality/or toxicity at a dose up to 1 g kg−1 of bw in mice [190] |
| 50 | Urtica dioica | β-Amyrin, β-sitosterol, stigmasterol, oleanolic acid, ursolic acid, quercetin, rutin, chlorogenic, and 2-O-caffeoyl malic acid expressed as caffeic acid, isoquercetin, kaempferol 3-O-rutinoside [191–193]. | U. dioica extracts up to dose level of 2000 mg/kg body weight in animal model showed no mortality or changes/alteration in normal behavior [127]. |
4. Discussion
The majority of the experiments confirmed the benefits of medicinal plants with hypoglycemic effects in the management of diabetes mellitus. From Table 1, it can be concluded that among the plants used for the treatment of diabetes, H. salicornicum, T. oliverianum, A. cepa, A. herba-alba, Teucrium polium, Sesamum indicum, Z. spina-christi, and U. dioica seem to be most common plants used to treat diabetes and are available everywhere in the world. The leaves were most commonly used plant part, and other parts (root, stem, bark, flower, seed, and whole plant) were also useful for curing. The most common diabetic model that was used was the streptozotocin and alloxan-induced diabetic mouse or rat as diabetic models. The most commonly involved active constituents are flavonoid, alkaloid, saponin, carbohydrate, vitamins, amino acid and its derivatives, phenol and its derivatives, and benzoic acid derivatives. The very common phytoconstituents, targeted metabolic pathways, and its structure are given in Table 3 [194, 195]. The native to or cultivated plant species of the kingdom given in Table 1 are selected from the published literature about ethnobotanical value and antidiabetic potential of medicinal plants around the world. The ethnobotanical information reports about 800 plants that may possess antidiabetic potential [196, 197]. Jeeva and Anlin also reported 177 plants belonging to 156 genera and 76 families used traditionally for antidiabetic treatment [198]. In the Middle East countries, there are 129 plant species still in use in traditional Arabic medicine. This indicates that the medicinal plant species require preservation as well as the ethnobotanical and ethnopharmacological knowledge. The preservation of the herbs is an essential requirement for maintaining traditional Arabic medicine as a medicinal and cultural resource [199]. The selected plant species H. salicornicum, T. oliverianum, A. cepa, A. herba-alba, Teucrium polium, Sesamum indicum, Z. spina-christi, and F. religiosa are the native Saudi medicinal plants traditionally used for the treatment of DM [200]. Similarly published data showed that 20 medicinal plants are traditionally used in Tabuk region of Saudi Arabia [201]. Anisotes trisulcus, Artemisia judaica, and Moringa peregrine are used in Al Khobah village, Saudi Arabia, for DM treatment [202]. O. europaea is used in Al Bahah region of KSA for DM treatment [203]. C. roseus, A. cepa, U. dioica, A. aspera, C. intybus, C. cyminum, F. bengalensis, C. colocynthis, and T. polium are the highly investigated medicinal plants for antidiabetic potential [204–206].
Table 3.
Selected antidiabetic phytoconstituents and its targeted metabolic pathway.
| Phytoconstituents | Targeted metabolic pathways | Structures |
|---|---|---|
| Catharanthine | Free radical; our body has a defense system containing several enzymes, which are catalase, superoxide dismutase, and glutathione-S transferases and reduced glutathione. Catharanthine activates these free radical scavenging enzymes and prevents our body from their adverse effects. |
|
| Harmine | Insulin secretion and β-cell regeneration |
|
| Betaine Achyranthine β-ecdysone |
Carbohydrate digestion and absorption |
|
| Apigenin | Cholesterol synthesis, glycogen synthesis |
|
| Betaine choline | Regeneration of pancreatic β cells and insulin secretion |
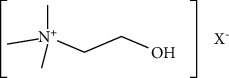
|
| Ferulic acid | Free radical scavenging activity, insulin secretion |
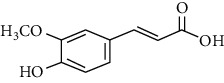
|
| Leucine, isoleucine, alanine | Insulin secretion |
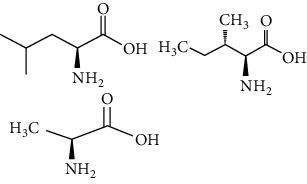
|
| Chlorogenic acid | Krebs cycle |
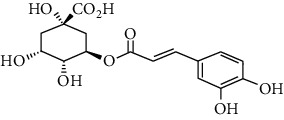
|
| Emodin, cinnamic acid | Insulin secretion |
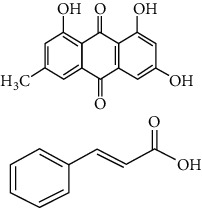
|
| Eugenol, α-pinene, limonene, p-cymene thujone | Insulin secretion, regeneration of pancreatic β cells |
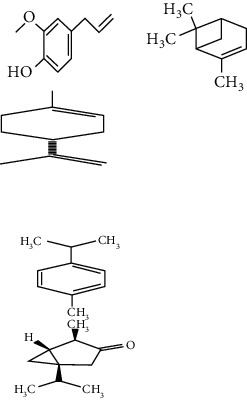
|
| Pectin | Glucose transport, carbohydrate metabolism, stabilizing agents |
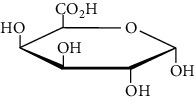
|
| Inulin | Glucose transport, carbohydrate digestion and absorption |
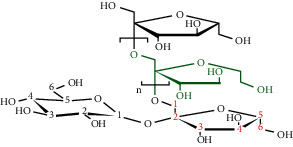
|
| Cucurbitacin B | Insulin secretion, glycogen synthesis |
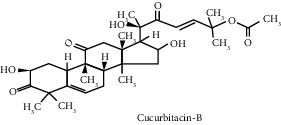
|
| Catechin Epicatechin |
Scavenging activity Insulinomematic |
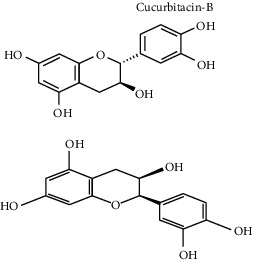
|
| Naringin | Glycogen synthesis, glycolysis, gluconeogenesis |
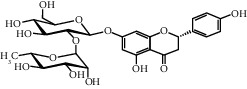
|
| Flavones | Insulin secretion |
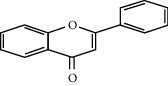
|
| Quercetin, quercitrin, apigenin, rutin, apigenin-7-O-glucoside | Insulin secretion |
|
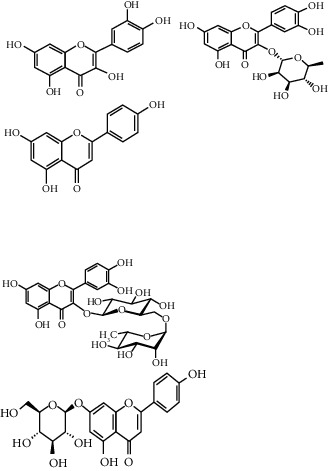
| Naringenin | Insulin secretion |
|
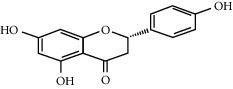
| α-Terpineol | Insulin secretion |
|
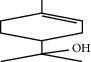
| Kaempferol, isorhamnetin, caffeic acid, p-coumaric acid | Free radical scavenging activity Carbohydrate digestion and absorption, insulin secretion |
|
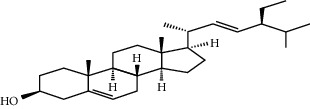
| Vitamin A, E | Free radical scavenging activity |
|
| Ellagic acid | Carbohydrate digestion and absorption, insulin secretion |
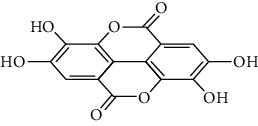
|
| Carvacrol, linalool | Insulin secretion, carbohydrate digestion and absorption |
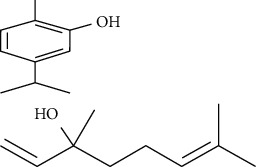
|
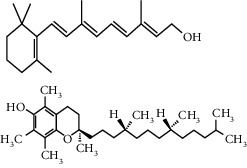
| Stigmasterol | Regeneration of pancreatic β cells, insulin secretion |
|
| Ursolic acid | Regeneration of pancreatic β cells and insulin secretion |
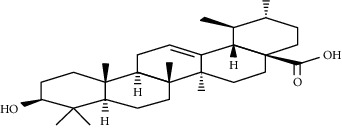
|
| β-Sitosterol | Insulin receptor (IR) and glucose transporter 4 |
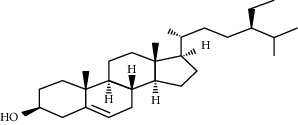
|
Desiring to contribute to the conservation priorities of traditional medicine knowledge of various medicinal plants native to or cultivated in Saudi Arabia and to make it easy and familiarized with disease treatment, the present compilation was conducted. According to the International Union for Conservation of Nature and the World Wildlife Fund, there about 15,000 medicinal plant species are threatened with extinction from overharvesting and habitat destruction and 20% of their wild resources have already been nearly exhausted with the increasing human population and plant consumption [207]. Each plant species lost due to extinction phenomena could represent not only the loss of healthcare saving cures for special diseases but also the loss of probable primary metabolite liker protein- or vitamin-rich foods [208]. Medicinal plants have been cited as a potential source of heavy metal toxicity to both man and animals. The most common heavy metals implicated in human toxicity include lead, mercury, arsenic, and cadmium, although aluminum and cobalt may also cause toxicity. From the study, the levels of these metals differed in the same plant collected from different geographical locations. A study conducted showed that the levels of lead in Cassia alata varied from 17.7 to 4.45 μg/g for the 5 collection sites. Similarly for Cassia occidentalis and Rauvolfia vomitoria, the level is varied between 7.85-4.35 and 9.25–1.55 μg/g, respectively. Similarly, that of aluminum varied between 105.53 and 23.3 for Rauvolfia vomitoria and 104.25–12.4 μg/g for Paullinia pinnata. The levels of heavy metals also varied for different plants collected from the same location. Uptake of metals by plants is influenced by a number of factors including metal concentrations in soils, cation exchange capacity, soil pH, organic matter content, types and varieties of plants, and plant age. However, the prevailing factor is the concentration of the metal in the soil and thus the existing environmental conditions [209]. Another study conducted on onion bulb showed that the concentrations of Cr in onion bulb and Fe in onion leaf were above the permissible level (2.3 mg/kg, 425.5 mg/kg) set by FAO/WHO at Mojo (4.87 mg/kg, 1090.40 mg/kg), Meki (4.13 mg/kg, 1836.47 mg/kg), and Ziway (3.33 mg/kg, 764.33 mg/kg), respectively. The results generally indicate that the consumption of these onion bulbs could be the health risk respective to Cr [210]. Therefore, it is suggested that the medicinal plant source for the treatment of diabetes must not be taken from heavy metal contaminated areas to avoid their uptake by the plants because migration of these contaminants into noncontaminated areas (or leaching through the soil and spreading of heavy metal contaminated sewage sludge) are a few examples of events contributing to contamination of the ecosystem.
5. Conclusion and Recommendations
The present review provides a picture of medicinal plants that have been studied as anti-DM drugs, which can be grown either in combination with other medicinal plants or alone as treatment for diabetes and drawbacks should be properly addressed so that medicinal plants can be effectively utilized as anti-DM drugs. Diabetes is a metabolic disorder which can be considered as a major cause of high economic loss which can in turn impede the development of nations. Moreover, uncontrolled diabetes leads to many chronic complications such as blindness, heart failure, and renal failure. In order to prevent this alarming health problem, the development of research into new hypoglycemic and potentially antidiabetic agents is of great interest. In conclusion, this paper has presented a list of anti-DM plants used in the treatment of diabetes mellitus. Bioactive antidiabetic phytoconstituents which showed that these plants have hypoglycemic effects and highly recommended for further pharmacological purposes and to isolate/identify anti-DM active agents also need to investigate the side effects of active ingredients.
Acknowledgments
The authors wish to thank Research Center College of Pharmacy at King Saud University, Riyadh, Saudi Arabia for their financial support and for providing free access to digital library and laboratory.
Data Availability
This is a review article. All data are taken from published research papers and available online.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
All three authors contributed equally.
References
- 1.Mahesh B., Satish S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World Journal of Agricultural Sciences . 2008;4:839–843. [Google Scholar]
- 2.van Wyk B.-E., van Staden J. A review of ethnobotanical research in southern Africa. South African Journal of Botany . 2002;68(1):1–13. doi: 10.1016/s0254-6299(15)30433-6. [DOI] [Google Scholar]
- 3.Bhojane P., Damle S., Thite A., Dabholkar V. Anti-microbial effects of some leafy vegetables—a comparative analysis. International Research Journal of Biological Sciences . 2014;3:26–32. [Google Scholar]
- 4.Talib W., Mahasneh A. Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules . 2010;15(3):1811–1824. doi: 10.3390/molecules15031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman H., Khan I., Hussain A., et al. Glycyrrhiza glabra HPLC fractions: identification of Aldehydo Isoophiopogonone and Liquirtigenin having activity against multidrug resistant bacteria. BMC Complementary and Alternative Medicine . 2018;18(1):p. 140. doi: 10.1186/s12906-018-2207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahat A. A., Ullah R., Alqahtani A. S., Alsaid M. S., Husseiny H. A., Meanazel O. T. R. Hepatoprotective effect of Eriobotrya japonica leaf extract and its various fractions against carbon tetra chloride induced hepatotoxicity in rats. Evidence-based Complementary and Alternative Medicine . 2018;2018:8. doi: 10.1155/2018/3782768.3782768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali N. A. A., Wurster M., Arnold N., Lindequist U., Wessjohan L. Chemical composition of the essential oil of Teucrium yemense Deflers. Records of Natural Products . 2012;2(2):p. 25. [Google Scholar]
- 8.Livermore D. M. Has the era of untreatable infections arrived? Journal of Antimicrobial Chemotherapy . 2009;64(1):29–36. doi: 10.1093/jac/dkp255. [DOI] [PubMed] [Google Scholar]
- 9.Mohd Azman N., Gallego M., Segovia F., Abdullah S., Shaarani S., Almajano Pablos M. Study of the properties of bearberry leaf extract as a natural antioxidant in model foods. Antioxidants . 2016;5(2):p. 11. doi: 10.3390/antiox5020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radhakrishnan R., Zakaria M. N. M., Islam M. W., et al. Analgesic and anti-inflammatory activities of Teucrium stocksianum. Pharmaceutical Biology . 2001;39(6):455–459. doi: 10.1076/phbi.39.6.455.5885. [DOI] [Google Scholar]
- 11.Kanwal S., Ullah N., Haq I. U., Afzal I., Mirza B. Antioxidant, antitumor activities and phytochemical investigation of Hedera nepalensis K. Koch, an important medicinal plant from Pakistan. Pakistan Journal of Botany . 2011;43(8):85–89. [Google Scholar]
- 12.Madhuri S., Pandey G. Some anticancer medicinal plants of foreign origin. Current Science . 2009;96(6):779–783. [Google Scholar]
- 13.Gauniyal P., Teotia U. V. S. Antimicrobial activity and phytochemical medicinal plants against oral microorganism. International Journal of Pharmaceutical and Medicinal Research . 2014;2(1):21–27. [Google Scholar]
- 14.Goren A. C., Bilsel G., Bilsel M., Demir H., Kocabaş E. E. Analysis of essential oil of Coridothymus capitatus (L.) and its antibacterial and antifungal activity. Zeitschrift fur Naturforschung. C, Journal of biosciences . 2003;58(9-10):687–690. doi: 10.1515/znc-2003-9-1016. [DOI] [PubMed] [Google Scholar]
- 15.Afolayan A. J. Extracts from the shoots of Arctotis arctotoides inhibit the growth of bacteria and fungi. Pharmaceutical Biology . 2003;41(1):22–25. doi: 10.1076/phbi.41.1.22.14692. [DOI] [Google Scholar]
- 16.Veerapur V. P., Prabhakar K. R., Parihar V. K., et al. Ficus racemosaStem bark extract: a potent antioxidant and a probable natural radioprotector. Evidence-based Complementary and Alternative Medicine . 2009;6(3):317–324. doi: 10.1093/ecam/nem119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. The American journal of clinical nutrition . 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 18.Osman A. K., Al-Ghamdi F., Bawadekji A. Floristic diversity and vegetation analysis of wadi arar: a typical desert wadi of the northern border region of Saudi Arabia. Saudi Journal of Biological Sciences . 2014;21(6):554–565. doi: 10.1016/j.sjbs.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morey S. C. ADA recommends a lower fasting glucose value in the diagnosis of diabetes mellitus. American Family Physician . 1997;56(8):2128–2130. [PubMed] [Google Scholar]
- 20.Srinivasan K., Ramarao P. Animal models in type 2 diabetes research: an overview. Indian Journal of Medical Research . 2007;125(3):451–472. [PubMed] [Google Scholar]
- 21.Kazemi S., Asgary S., Moshtaghian J., Rafieian M., Adelnia A., Shamsi F. Liver-protective effects of hydroalcoholic extract of Allium hirtifolium Boiss. In rats with alloxan-induced diabetes mellitus. ARYA atherosclerosis . 2010;6(1):11–15. [PMC free article] [PubMed] [Google Scholar]
- 22.Gleckman R., Morr J. Diabetes-related foot infections. Contemporary Internal Medicine . 1994;6(8):57–64. [PubMed] [Google Scholar]
- 23.Behradmanesh S., Derees F., Rafieian-kopaei M. Effect of Salvia officinalis on diabetic patients. Journal of Renal Injury Prevention . 2013;2(2):51–54. doi: 10.12861/jrip.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meigs J. B. Epidemiology of the metabolic syndrome. American Journal of Managed Care . 2002;8(11):S283–S292. [PubMed] [Google Scholar]
- 25.Boulton A. J., Vileikyte L., Ragnarson-Tennvall G., Apelqvist J. The global burden of diabetic foot disease. The Lancet . 2005;366(9498):1719–1724. doi: 10.1016/s0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 26.Williams R. H., Larsen P. R. Textbook of Endocrinology . 10th. Philadelphia, PA, USA: Saunders; 2003. [Google Scholar]
- 27.Swanston-Flatt S. K., Day C., Bailey C. J., Flatt P. R. Evaluation of traditional plant treatments for diabetes: studies in streptozotocin diabetic mice. Acta Diabetologica Latina . 1989;26(1):51–55. doi: 10.1007/bf02581196. [DOI] [PubMed] [Google Scholar]
- 28.Nasri H., Rafieian-Kopaei M. Herbal medicine and diabetic kidney disease. Journal of Nephropharmacology . 2013;2(1):1–2. [PMC free article] [PubMed] [Google Scholar]
- 29.Eddouks M., Chattopadhyay D., De Feo V., Cho W. C.-s. Medicinal plants in the prevention and treatment of chronic diseases 2013. Evidence-based Complementary and Alternative Medicine . 2014;2014:3. doi: 10.1155/2014/180981.180981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamila F., Mostafa E. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. Journal of Ethnopharmacology . 2014;154(1):76–87. doi: 10.1016/j.jep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Venkatesh S., Reddy G. D., Reddy B. M., Ramesh M., Rao A. V. N. A. Antihyperglycemic activity of Caralluma attenuata. Fitoterapia . 2003;74(3):274–279. doi: 10.1016/s0367-326x(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 32.Nasri H., Shirzad H. Toxicity and safety of medicinal plants. Journal of Herbmed Pharmacology . 2013;2(2):21–22. [Google Scholar]
- 33.El-Bab M., Shawky N., Al-Sisi A., Akhtar M. Retinopathy and risk factors in diabetic patients from Al-Madinah Al-Munawarah in the Kingdom of Saudi Arabia. Clinical Ophthalmology . 2012;6(1):269–276. doi: 10.2147/opth.s27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alqahtani S. N., Omar Alkholy S., Pontes Ferreira M. Antidiabetic and anticancer potential of native medicinal plants from Saudi Arabia. Polyphenols in Human Health and Disease . 2014;1:119–132. [Google Scholar]
- 35.Behradmanesh M., Ahmadi M., Rafieian-kopaei M. Effect of glycogol on blood glucose level of patients with type II diabetes. Iranian Journal of Endocrinology and Metabolism . 2012;14(2):163–168. [Google Scholar]
- 36.Abdulrahman M. Al-moshileh effects of planting date and irrigation water level on onion (Allium cepa L.) production under central Saudi arabian conditions. Scientific Journal of King Faisal University (Basic and Applied Sciences) . 2007;8(1):75–85. [Google Scholar]
- 37.Baragob A. E., Al-Wabel N. A., Ahmed N. A., Babiker M. F., Abdalkarim A. S., Elboshra M. I. Study to investigate the pancreatic regeneration and evaluation of the antidiabetic and antihyperlipidemic potential of aerial parts of Allium cepa. Biochemistry and Biotechnology Research . 2015;3:19–29. [Google Scholar]
- 38.Ojieh A. E., Adegor E. C., Okolo A. C., Ewhre O. L., Njoku I. P., Onyekpe C. U. Hypoglycaemic and hypolipidaemic effect of Allium cepa in streptozotocin-induced diabetes. International Journal of Scientific Engineering and Research . 2015;6:23–29. [Google Scholar]
- 39.Imad M., Eldin T., Ahmed E. M., Abd Elwahab H. M. Preliminary study of the clinical hypoglycemic effects of Allium cepa (red onion) in type 1 and type 2 diabetic patients. Environmental Health Insights . 2010;4:71–77. doi: 10.4137/ehi.s5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammed A., Syed S., Syed M. Y., Ali A. D. Use of herbal extract from Artemisia herba-alba (Shih) in pharmaceutical preparations for dental hygiene. Saudi Pharmaceutical Journal: the Official Publication of the Saudi Pharmaceutical Society . 2018;26:822–828. doi: 10.1016/j.jsps.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awad N. E., Seida A. A., El-Khayat Z., Shaffie N., Ahmed M., Abd El-Aziz Hypoglycemic activity of Artemisia herba-alba (asso.) used in Egyptian traditional medicine as hypoglycemic remedy. Journal of Applied Pharmaceutical Science . 2012;2(3):30–39. [Google Scholar]
- 42.Pérez-López E., Omar A. F., Al-Jamhan K. M., Dumonceaux T. J. Molecular identification and characterization of the new 16SrIX-J and cpn60 UT IX-J phytoplasma subgroup associated with chicory bushy stunt disease in Saudi Arabia. International Journal of Systematic and Evolutionary Microbiology . 2018;68(2):518–522. doi: 10.1099/ijsem.0.002530. [DOI] [PubMed] [Google Scholar]
- 43.Street R. A., Sidana J., Prinsloo G. Cichorium intybus: traditional uses, phytochemistry, pharmacology, and toxicology. Evidence-Based Complementary and Alternative Medicine . 2013;2013:13. doi: 10.1155/2013/579319.579319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanj D., Raafat K., El-Lakany A., Baydoun S., Aboul-Ela M. Phytochemical compounds of cichorium intybus by exploring its antioxidant and antidiabetic activities. Pharmacognosy Journal . 2019;11(2):248–257. doi: 10.5530/pj.2019.11.39. [DOI] [Google Scholar]
- 45.Das S., Vasudeva N., Sharma S. Cichorium intybus: a concise report on its ethnomedicinal, botanical, and phytopharmacological aspects. Drug Design, Development and Therapy . 2016;7:1–12. [Google Scholar]
- 46.Al-Snafi A. E. Pharmacological importance of Clitoria ternatea—a review. IOSR Journal of Pharmacy . 2016;6(3):68–83. [Google Scholar]
- 47.Gollen B., Mehla J., Gupta P. Clitoria ternatea linn: a herb with potential pharmacological activities: future prospects as therapeutic herbal medicine. Journal of Reports in Pharmaceutical Sciences . 2018;3(141):1–8. [Google Scholar]
- 48.Lijon M. B., Meghla N. S., Jahedi E., Rahman M. A., Hossain I. Phytochemistry and pharmacological activities of Clitoria ternatea. International Journal of Natural and Social Sciences . 2017;4(1):1–10. [Google Scholar]
- 49.Khemira H., Mars M. Fig production in subtropical south-western Saudi Arabia. Acta Horticulturae . 2017;1173(1173):169–172. doi: 10.17660/actahortic.2017.1173.28. [DOI] [Google Scholar]
- 50.Khan K. Y., Khan M. A., Ahmad M., et al. Hypoglycemic potential of genus Ficus L.: a review of ten years of plant based medicine used to cure diabetes (2000–2010) Journal of Applied Pharmaceutical Science . 2011;1(6):223–227. [Google Scholar]
- 51.Stephen Irudayaraj S., Christudas S., Antony S., Duraipandiyan V., Abdullah A.-D. N., Ignacimuthu A.-D. Protective effects of Ficus carica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and β-cells in type 2 diabetic rats. Pharmaceutical Biology . 2017;55(1):1074–1081. doi: 10.1080/13880209.2017.1279671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Juhany L. I., Abdullah A., Al-Harby Status and diversity of ornamental plants in king Saud university campus at Riyadh, Saudi Arabia American-eurasian. Journal of Agriculture and Environmental Sciences . 2013;13(4):471–478. [Google Scholar]
- 53.Deepa P., Sowndhararajan K., Kim S., Park S. J. A role of Ficus species in the management of diabetes mellitus: a review. Journal of Ethnopharmacology . 2018;215:210–232. doi: 10.1016/j.jep.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 54.Scotti F., Löbel K., Booker A., Heinrich M. St. John’s wort (Hypericum perforatum) products—how variable is the primary material? Frontiers of Plant Science . 2019;9:p. 1973. doi: 10.3389/fpls.2018.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arokiyaraj S., Balamurugan R., Augustian P. Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin-induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine . 2011;1(5):386–390. doi: 10.1016/S2221-1691(11)60085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alqethami A., Hawkins J. A., Teixidor-Toneu I. Medicinal plants used by women in Mecca: urban, Muslim and gendered knowledge. Journal of Ethnobiology and Ethnomedicine . 2017;13(62):62–24. doi: 10.1186/s13002-017-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodarzi M. T., Khodadadi I., Tavilani H., Abbasi Oshaghi E. The role of Anethum graveolens L. (Dill) in the management of diabetes. Journal of Tropical Medicine . 2016;2016:11. doi: 10.1155/2016/1098916.1098916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohamed D. A., Mohamed Hamed I., Aly Fouda K. Antioxidant and anti-diabetic effects of cumin seeds crude ethanol. Extract Journal of Biological Sciences . 2018;18(5):251–259. [Google Scholar]
- 59.Singh R. P., Gangadharappa H. V., Mruthunjaya K. Cuminum cyminum—a popular spice: an updated review. Pharmacognosy Journal . 2017;9(3):292–301. doi: 10.5530/pj.2017.3.51. [DOI] [Google Scholar]
- 60.Lodhi S., Vadnere G., Sharma V. K., Rageeb Usman M. Marrubium vulgare L.: a review on phytochemical and pharmacological aspects. Journal of Intercultural Ethnopharmacology . 2017;6(4):429–452. doi: 10.5455/jice.20170713060840. [DOI] [Google Scholar]
- 61.Murad H. A. S., Abdallah H. M., Ali S. S. Mentha longifolia protects against acetic-acid induced colitis in rats. Journal of Ethnopharmacology . 2016;190:354–361. doi: 10.1016/j.jep.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 62.Asgharia B., Zenginb G., Babak Bahadoric M., Abbas-Mohammadid M. Leila Dinparast Amylase, glucosidase, tyrosinase, and cholinesterases inhibitory, antioxidant effects, and GC-MS analysis of wild mint (Mentha longifolia var. calliantha) essential oil: a natural remedy. European Journal of Integrative Medicine . 2018;22:44–49. doi: 10.1016/j.eujim.2018.08.004. [DOI] [Google Scholar]
- 63.Eldesouky Zain M., Amani Shafeek Awaad, Al-Outhman M. R. Reham mostafa el-meligy antimicrobial activities of Saudi arabian desert plants. Phytopharmacology . 2012;2(1):106–113. [Google Scholar]
- 64.Rasheed A.-A. A., Musbah A.-Q. T., Sulaiman A. M., Saed S. M., Mohammad B. A. Short-term feeding effects of origanum syriacum crude extract on blood constituents in rats. International Journal of Research in Ayurveda and Pharmacy . 2017;8(1):118–120. [Google Scholar]
- 65.Fatima N. A Review on Teucrium oliveranum, a plant found abundantly in Saudi Arabia. Science International . 2016;28(2):1229–1231. [Google Scholar]
- 66.Shahat A. A., Alsaid M. S., Khan J. A., Higgins M., Dinkova-Kostova A. T. Chemical constituents and NAD(P)H:quinone oxidoreductase 1 (NQO1) inducer activity of Teucrium oliverianum Ging. ex Benth. Indian Journal of Traditional Knowledge . 2016;15(2):232–236. [Google Scholar]
- 67.Hossam M., Abdallah G. A. M., Farag M. A., Alshali K. Z., Alsherif E. A., Ross S. A. Volatile oil profile of some lamiaceous plants growing in Saudi Arabia and their biological activities. Zeitschrift für Naturforschung C . 2017;72(1-2):35–41. doi: 10.1515/znc-2015-0234. [DOI] [PubMed] [Google Scholar]
- 68.Bahramikia S., Yazdanparast R. phytochemistry and medicinal properties of Teucrium polium L. (Lamiaceae) Phytotherapy Research . 2012;26(11):1581–1593. doi: 10.1002/ptr.4617. [DOI] [PubMed] [Google Scholar]
- 69.Abdallah H. M., Abdel-Naimc A. B., Ashourd O. M., Shehata I. A., Abdel-Sattar E. A. Anti-infl ammatory activity of selected plants from Saudi Arabia Z. Naturforscher . 2014;69c:1–9. doi: 10.5560/znc.2012-0168. [DOI] [PubMed] [Google Scholar]
- 70.Vijayaraj R., Vidhya R. Biological activity of Achyranthes aspera linn.—a review. Asian Journal of Biochemical and Pharmaceutical Research . 2016;69(1):2231–2560. [Google Scholar]
- 71.Rahman M. A., Jaber S. M., Al-Said M. S., Al-Yahya M. A. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia . 2004;75:149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 72.Omotoso K. S., Aigbe F. R., Salako O. A., Chijioke M. C., Adeyem O. O. Toxicological evaluation of the aqueous whole plant extract of Aerva lanata (l.) Juss. ex Schult (Amaranthaceae) Journal of Ethnopharmacology . 2017;208:174–184. doi: 10.1016/j.jep.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 73.Rajesh R., Chitra K., Paarakh P. M. Anti hyperglycemic and antihyperlipidemic activity of aerial parts of Aerva lanata Linn Juss in streptozotocin induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine . 2012;2(2):S924–S929. doi: 10.1016/s2221-1691(12)60338-4. [DOI] [Google Scholar]
- 74.El-Ghanim W. M., Hassan L. M., Galal T. M., Badr A. Floristic composition and vegetation analysis in Hail region north of central Saudi Arabia. Saudi Journal of Biological Sciences . 2010;17(2):119–128. doi: 10.1016/j.sjbs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hossain A. I., Faisal M., Rahman S., Jahan R., Rahmatullah M. A preliminary evaluation of antihyperglycemic and analgesic activity of Alternanthera sessilis aerial parts. BMC Complementary and Alternative Medicine . 2014;14(169):1–5. doi: 10.1186/1472-6882-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Youssef H., Hassan W. Chemical constituents of carissa edulis vahl. Arabian Journal of Chemistry . 2017;10:109–113. [Google Scholar]
- 77.Kaunda J. S., Zhang Y.-J. The genus carissa: an ethnopharmacological, phytochemical and pharmacological review. Natural Products and Bioprospecting . 2017;7:181–199. doi: 10.1007/s13659-017-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elbeshehy E. K. F., AL-Zahrani H. S. M., Aldhebiani A. Y., Elbeaino T. Viruses infecting periwinkle (catharanthus roseus L.) in western Saudi Arabia phytopathologia mediterranea. 2017;56(3):479–485. [Google Scholar]
- 79.Al-Shaqha W. M., Khan M., Salam N., Azzi A., Chaudhary A. A. Anti-diabetic potential of Catharanthus roseus Linn. and its effect on the glucose transport gene (GLUT-2 and GLUT-4) in streptozotocin induced diabetic wistar rats. BMC Complementary and Alternative Medicine . 2015;15(379):1–8. doi: 10.1186/s12906-015-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bukhari N. A., Al-Otaibi R. A., Ibhrahim M. M. Phytochemical and taxonomic evaluation of Rhazya stricta in Saudi Arabia. Saudi Journal of Biological Sciences . 2017;24(7):1513–1521. doi: 10.1016/j.sjbs.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menon N., Sparks J., Omoruyi F. Hypoglycemic and hypocholesterolemic activities of the aqueous preparation of Kalanchoe pinnata leaves in streptozotocin-induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine . 2015;5(1):3–9. doi: 10.1016/s2221-1691(15)30162-3. [DOI] [Google Scholar]
- 82.Awaad A. A., Alkanhal H. F., El-Meligy R. M., et al. Anti-ulcerative colitis activity of Calotropis procera Linn. Saudi Pharmaceutical Journal . 2018;26(1):75–78. doi: 10.1016/j.jsps.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhagour K., Arya D., Gupta R. S. A review: antihyperglycemic plant medicines in management of diabetes. Acupuncture and Related Therapies . 2016;4(4):7–16. doi: 10.1016/j.arthe.2016.11.001. [DOI] [Google Scholar]
- 84.Alfarhan A. H. Al-Turki T. A., Basahy A. Y. Flora of jizan region. 2005 Final Report Volume-1.
- 85.Sharma C., Rani S., Kumar B., Kumar A., Raj V. Plant opuntia dillenii: a review on its traditional uses, phytochemical and pharmacological properties. EC Pharmaceutical Science . 2015;1(1):29–43. [Google Scholar]
- 86.Hwang S. H., Kang I. J., Lim S. S. Antidiabetic effect of fresh nopal (Opuntia ficus-indica) in low-dose streptozotocin-induced diabetic rats fed a high-fat diet. Evidence-based Complementary and Alternative Medicine . 2017;2017:8. doi: 10.1155/2017/4380721.4380721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El-Mostafa K., El Kharrassi Y., Badreddine A., et al. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules . 2014;19(9):14879–14901. doi: 10.3390/molecules190914879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gull T., Anwar F., Sultana B., Alcayde M. A. C., Nouman W. Capparis species: a potential source of bioactives and high-value components: a review. Industrial Crops and Products . 2015;67:81–96. doi: 10.1016/j.indcrop.2014.12.059. [DOI] [Google Scholar]
- 89.Al-Asmari A. K., Al-Elaiwi A. M., Athar M. T., Tariq M., Al Eid A., Al-Asmary S. M. A review of hepatoprotective plants used in Saudi traditional medicine. Evidence-based Complementary and Alternative Medicine . 2014;2014:22. doi: 10.1155/2014/890842.890842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kabir A. U., Samad M. B., Ahmed A., et al. Aqueous fraction of Beta vulgaris ameliorates hyperglycemia in diabetic mice due to enhanced glucose stimulated insulin secretion, mediated by acetylcholine and GLP-1, and elevated glucose uptake via increased membrane bound GLUT4 transporters. PLoS One . 2015;10(2) doi: 10.1371/journal.pone.0116546.e0116546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gezginci-Oktayoglu S., Sacan O., Bolkent S., et al. Chard (Beta vulgaris L. var. cicla) extract ameliorates hyperglycemia by increasing GLUT2 through Akt2 and antioxidant defense in the liver of rats. Acta Histochemica . 2014;116(1):32–39. doi: 10.1016/j.acthis.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 92.Ullah R., Alsaid M. S., Shahat A. A., et al. Antioxidant and hepatoprotective effects of methanolic extracts of Zilla spinosa and Hammada elegans against carbon tetrachlorideinduced hepatotoxicity in rats. Open Chemistry . 2018;16(1):133–140. doi: 10.1515/chem-2018-0021. [DOI] [Google Scholar]
- 93.Singh J. P., Rathore V. S., Roy M. M. Notes about Haloxylon salicornicum (Moq.) Bunge ex Boiss., a promising shrub for arid regions. Genetic Resources and Crop Evolution . 2015;62(3):451–463. doi: 10.1007/s10722-014-0212-4. [DOI] [Google Scholar]
- 94.Duraisamy G., Ganesan R., Manokaran K., Kanakasabapathi D., Chandrasekar U. Protective effect of the whole plant extract of Evolvulus alsinoides on glycoprotein alterations in streptozotocin induced diabetic rats. Journal of Acute Disease . 2013;2(2):148–150. doi: 10.1016/s2221-6189(13)60116-x. [DOI] [Google Scholar]
- 95.Sundaramurthi P., Packiam K. K. A review on pharmacognosy and pharmacology of Evolvulus alsinoides (l.) L. International Research Journal of Pharmacy . 2017;8(7):1–4. doi: 10.7897/2230-8407.087110. [DOI] [Google Scholar]
- 96.Al-Sodany Y. M. A new record to the flora of Saudi Arabia: Ipomoea carneaJacq., Convolvulaceae. World Journal of Research and Review . 2016;3(4):25–30. [Google Scholar]
- 97.Sajak A. A., Mediani A., Dom N. S., et al. Effect of Ipomoea aquatica ethanolic extract in streptozotocin (STZ) induced diabetic rats via1H NMR-based metabolomics approach. Phytomedicine . 2017;36:201–209. doi: 10.1016/j.phymed.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 98.Hamid K., Ullah M. O., Sultana S., et al. Evaluation of the leaves of Ipomoea aquatica for its hypoglycemic and antioxidant activity. Journal of Pharmaceutical Sciences and Research . 2011;3(7):1330–1333. [Google Scholar]
- 99.Amin A., Tahir M., Lone K. P. Effect of Citrullus colocynthis aqueous seed extract on beta cell regeneration and intra-islet vasculature in alloxan induced diabetic male albino rats. Journal of Pakistan Medical Association . 2017;67(5):715–721. [PubMed] [Google Scholar]
- 100.Masrahi Y. S., Remesh M., Sayed O. H. Enumeration of the flora of wadi lajab Saudi Arabia. Ecology Environment and Conservation . 2017;23(1):115–123. [Google Scholar]
- 101.Wang F., Li H., Zhao H., et al. Antidiabetic activity and chemical composition of sanbai melon seed oil. Evidence-based Complementary and Alternative Medicine . 2018;2018:14. doi: 10.1155/2018/5434156.5434156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Varghese S., Narmadha R., Gomathi D., Kalaiselvi M., Devaki K. Evaluation of hypoglycemic effect of ethanolic seed extracts of Citrullus lanatus. Journal of Phytopharmacology . 2013;2:31–40. doi: 10.1016/s2221-6189(13)60111-0. [DOI] [Google Scholar]
- 103.Attanayake A. P., Jayatilaka K. A., Pathirana C., Mudduwa L. K. Antihyperglycemic activity of Coccinia grandis (L.) Voigt in streptozotocin induced diabetic rats. Indian journal of traditonal knowledge . 2015;14(3):376–381. [Google Scholar]
- 104.Abdelgadir H. A., Van Staden J. Ethnobotany, ethnopharmacology and toxicity of Jatropha curcas L.(Euphorbiaceae): a review. South African Journal of Botany . 2013;88:204–218. doi: 10.1016/j.sajb.2013.07.021. [DOI] [Google Scholar]
- 105.Gad-Elkareem M. A., Abdelgadir E. H., Badawy O. M., Kadri A. Potential antidiabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ . 2019;7:1–15. doi: 10.7717/peerj.6441.e6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan K. Y., Khan M. A., Ahmad M., et al. Hypoglycemic potential of genus Ficus L.: a review of ten years of plant based medicine used to cure diabetes (2000–2010) Journal of Applied Pharmaceutical Science . 2011;1(6):223–227. [Google Scholar]
- 107.Adoum O. A., Micheal B. O., Mohammad I. S. Phytochemicals and hypoglycaemic effect of methanol stem-bark extract of Ficus sycomorus Linn (Moraceae) on alloxan induced diabetic Wistar albino rats. African Journal of Biotechnology . 2012;11(17):4095–4097. doi: 10.5897/ajb11.2115. [DOI] [Google Scholar]
- 108.Ibrahiem T. A. Beneficial effects of diet supplementation with Nigella sativa (Black Seed) and sesame seeds in Alloxan-Diabetic Rats. International Journal of Current Microbiology and Applied Sciences . 2016;5:411–423. doi: 10.20546/ijcmas.2016.501.041. [DOI] [Google Scholar]
- 109.Diez R., García J. J., Diez M. J., Sierra M., Sahagun A. M., Fernandez N. Influence of Plantago ovata husk (dietary fiber) on the bioavailability and other pharmacokinetic parameters of metformin in diabetic rabbits. BMC Complementary and Alternative Medicine . 2017;17(1):p. 298. doi: 10.1186/s12906-017-1809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sammaiah G., Srivastava R. S. Hypoglycemic activity of Polygala erioptera (Whole plant) in normal and alloxan induced diabetic rats. Asian Journal of Chemistry . 2008;20(1):107–112. [Google Scholar]
- 111.Al-Hazimi H. M., Haque S. N. A new naphthoquinone from Polygonum aviculare. Natural Product Letters . 2002;16(2):115–118. doi: 10.1080/10575630290020019. [DOI] [PubMed] [Google Scholar]
- 112.Al-Sodany Y. M., Salih A. B., Mosallam H. A. Medicinal plants in Saudi Arabia: I. Sarrwat mountains at taif, KSA. Academic Journal of Plant Sciences . 2013;6:134–145. [Google Scholar]
- 113.Baharvand-Ahmadi B., Bahmani M., Tajeddini P., Naghdi N., Rafieian-Kopaei M. An ethno-medicinal study of medicinal plants used for the treatment of diabetes. Journal of nephropathology . 2015;5(1):44–50. doi: 10.15171/jnp.2016.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bahmani M., Zargaran A., Rafieian-Kopaei M., Saki K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pacific journal of tropical medicine . 2014;7:S348–S354. doi: 10.1016/s1995-7645(14)60257-1. [DOI] [PubMed] [Google Scholar]
- 115.Al-Ghamdi A. A., Shahat A. A. Antioxidant, hypoglycemic and anti-diabetic activities of Ziziphus spina-christi (L) Willd (Rhamnacae) leaf extract. Tropical Journal of Pharmaceutical Research . 2017;16(11):2601–2610. [Google Scholar]
- 116.Inin T., Mohsina M., Mohammed R. Bacopa monnieri: an evaluation of antihyperglycemic and antinociceptive potential of methanolic extract of whole plants. Pakistan journal of pharmaceutical sciences . 2015;28(6):2135–2139. [PubMed] [Google Scholar]
- 117.Sher H., Alyemeni M. N. Evaluation of anti-diabetic activity and toxic potential of Lycium shawii in animal models. Journal of Medicinal Plants Research . 2011;5(15):3387–3395. [Google Scholar]
- 118.Umamageswari M. S., Karthikeyan T. M., Maniyar Y. A. Antidiabetic activity of aqueous extract of Solanum nigrum linn berries in alloxan induced diabetic wistar albino rats. Journal of Clinical and Diagnostic Research: Journal of Clinical and Diagnostic Research . 2017;11(7):FC16–FC19. doi: 10.7860/JCDR/2017/26563.10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anwer T., Sharma M., Pillai K. K., Iqbal M. Effect of withania somnifera on insulin sensitivity in non‐insulin‐dependent diabetes mellitus rats. Basic and Clinical Pharmacology and Toxicology . 2008;102(6):498–503. doi: 10.1111/j.1742-7843.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 120.Udayakumar R., Kasthurirengan S., Mariashibu T. S., et al. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. International Journal of Molecular Sciences . 2009;10(5):2367–2382. doi: 10.3390/ijms10052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saxena M., Saxena J., Khare S. A brief review on: therapeutical values of Lantana camara plant. International Journal of Pharmacy & Life Sciences . 2012;3(3):1551–1554. [Google Scholar]
- 122.Komeili G., Hashemi M., Bameri-Niafar M. Evaluation of antidiabetic and antihyperlipidemic effects of peganum harmala seeds in diabetic rats. Cholesterol . 2016;2016:6. doi: 10.1155/2016/7389864.7389864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chhatre S., Nesari T., Somani G., Kanchan D., Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacognosy Reviews . 2014;8(15):p. 45. doi: 10.4103/0973-7847.125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.El-Shaibany A., Molham A. H., Al-Tahami B., Al-Massarani S. Anti-hyperglycaemic activity of Tribulus terrestris L aerial part extract in glucose-loaded normal rabbits. Tropical Journal of Pharmaceutical Research . 2015;14(12):2263–2268. doi: 10.4314/tjpr.v14i8.17. [DOI] [Google Scholar]
- 125.Shahat A. A., Ibrahim A. Y., Ezzeldin E., Alsaid M. S. Acetylcholinesterase inhibition and antioxidant activity of some medicinal plants for treating neuro degenarative disease. African Journal of Traditional, Complementary and Alternative Medicines . 2015;12(3):97–103. [Google Scholar]
- 126.Ahangarpour A., Mohammadian M., Dianat M. Antidiabetic effect of hydroalcholic Urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iranian Journal of Medical Sciences . 2012;37(3):181–186. [PMC free article] [PubMed] [Google Scholar]
- 127.Dar S. A., Ganai F. A., Yousuf A. R., Balkhi M. U., Bhat T. M., Sharma P. Pharmacological and toxicological evaluation of Urtica dioica. Pharmaceutical Biology . 2013;51(2):170–180. doi: 10.3109/13880209.2012.715172. [DOI] [PubMed] [Google Scholar]
- 128.Marrelli M., Amodeo V., Statti G., Conforti F. Biological properties and bioactive components of Allium cepa L.: focus on potential benefits in the treatment of obesity and related comorbidities. Molecules . 2019;24(119):1–18. doi: 10.3390/molecules24010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.BashirLawal O., Shittu K., Oibiokpa F. I., Mohammed H., Umar S. I., Haruna G. M. Antimicrobial evaluation, acute and sub-acute toxicity studies of Allium sativum. Journal of Acute Disease . 2016;5(4):296–301. doi: 10.1016/j.joad.2016.05.002. [DOI] [Google Scholar]
- 130.Moufid A., Eddouks M. Artemisia herba alba: A popular plant with potential medicinal propertie. Pakistan Journal of Biological Sciences . 2012;15(24):1152–1159. doi: 10.3923/pjbs.2012.1152.1159. [DOI] [PubMed] [Google Scholar]
- 131.Gani G., Fatima T., Qadri T., Beenish N. J., Bashir O. Phytochemistry and pharmacological activities of fig (Ficus carica) A review nternational Journal of Research in Pharmacy and Pharmaceutical Sciences . 2018;3(2):80–82. [Google Scholar]
- 132.Odo G. E., Agwu J. E., Newze N., et al. Toxicity and effects of fig (Ficus carica) leaf aqueous extract on haematology and some biochemical indices of wistar albino rats (Rattus norvegicus) Journal of Medicinal Plants Research . 2016;10(22):298–305. [Google Scholar]
- 133.Oliveira A. I., Pinho C., Sarmento B., Dias A. C. P. Neuroprotective activity of Hypericum perforatum and its major components. Frontiers of Plant Science . 2016;7:p. 1004. doi: 10.3389/fpls.2016.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jana S., Shekhawat G. S. Anethum graveolens: an Indian traditional medicinal herb and spice. Pharmacognosy Reviews . 2010;4(8):179–184. doi: 10.3923/rjmp.2010.206.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swati N. D., Dipak K. P. Acute and sub chronic toxicoloigcal evaluation of anethum graveolens seed extract. Journal of Global bioseciences . 2016;5(6):4213–4220. [Google Scholar]
- 136.Farzaei M. H., Bahramsoltani R., Ali G., Farzaei F. Fariba najafi pharmacological activity of mentha longifolia and its phytoconstituents. Journal of Traditional Chinese Medicine . 2017;37(5):710–720. doi: 10.1016/s0254-6272(17)30327-8. [DOI] [PubMed] [Google Scholar]
- 137.Lukas B., Schmiderer C., Franz C., Novak J. Composition of essential oil compounds from different Syrian populations of origanum syriacum L. (Lamiaceae) Journal of Agricultural and Food Chemistry . 2009;57:1362–1365. doi: 10.1021/jf802963h. [DOI] [PubMed] [Google Scholar]
- 138.Rasekh H. R., Yazdanpanah H., Hosseinzadeh L. Narges bazmohammadi and mohammad kamalinejad acute and subchronic toxicity of Teucrium polium total extract in rats. Iranian Journal of Pharmaceutical Research . 2005;4:245–249. [Google Scholar]
- 139.Barua C. C., Talukdar A., Ara Begum S., et al. In vivo wound-healing efficacy and antioxidant activity of Achyranthes aspera in experimental burns. Pharmaceutical Biology . 2012;50(7):892–899. doi: 10.3109/13880209.2011.642885. [DOI] [PubMed] [Google Scholar]
- 140.Dinnimath B. M., Jalalpure S. S., Patil U. K. Antiurolithiatic activity of natural constituents isolated from Aerva lanata. Journal of Ayurveda and Integrative Medicine . 2017;8:226–233. doi: 10.1016/j.jaim.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sivakumar R., Sunmathi D. Phytochemical screening and antimicrobial activity of ethanolic leaf extract of alternanthera sessilis (l.) r.br. ex dc and alternanthera philoxeroides (MART.) griseb. European Journal of Pharmaceutical and Medical Research . 2016;3(3):409–412. [Google Scholar]
- 142.Ya’u J., Chindo B. A., Yaro A. H., Okhale S. E., Anuka J. A., Hussaini I. M. Safety assessment of the standardized extract of Carissa edulis root bark in rats. Journal of Ethnopharmacology . 2013;147(3):653–661. doi: 10.1016/j.jep.2013.03.064. [DOI] [PubMed] [Google Scholar]
- 143.Das S., Sharangi A. B. Madagascar periwinkle (Catharanthus roseus L.): diverse medicinal and therapeutic benefits to humankind. Journal of Pharmacognosy and Phytochemistry . 2017;6(5):1695–1701. [Google Scholar]
- 144.Vutukuri V. R., Das M. C., Reddy M., Prabodh S., Sunethri P. Evaluation of acute oral toxicity of ethanol leaves extract of Catharanthus roseus in wistar albino rats. Journal of Clinical and Diagnostic Research . 2017;11(3):FF01–FF04. doi: 10.7860/JCDR/2017/24937.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gilani S. A., Kikuchi A., Khan Shinwari Z., Khattak Z. I., Kazuo N. Watanabe phytochemical, pharmacological and ethnobotanical studies of rhazya stricta decne. Phytotherapy Research . 2007;21:301–307. doi: 10.1002/ptr.2064. [DOI] [PubMed] [Google Scholar]
- 146.Parihar G., Balekar N. Calotropis procera: a phytochemical and pharmacological review. Thai Journal of Pharmaceutical Sciences . 2016;40(3) [Google Scholar]
- 147.Ouedraogo G. G., Ouedraogo M., Lamien-Sanou A., Lompo M., Goumbri-Lompo O. M., Guissou P. I. Acute and subchronic toxicity studies of roots barks extracts of “calotropis procera” (ait.) R. Br used in the treatment of sickle cell disease in Burkina Faso. British Journal of Pharmacology and Toxicology . 2013;4(5):194–200. doi: 10.19026/bjpt.4.5401. [DOI] [Google Scholar]
- 148.Han E. H., Lim M. K., Lee S. H., Rahman M. M., Lim Y. H. An oral toxicity test in rats and a genotoxicity study of extracts from the stems of Opuntia ficus-indica var. saboten. BMC Complementary and Alternative Medicine . 2019;19(1):p. 31. doi: 10.1186/s12906-019-2442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dhakad P. K., Sharma P. K., Kumar S. Effect of hydroalcoholic extract of Capparis decidua (forssk.) edgew on sexual behavior of male rats. Advances in Pharmacology and Pharmacy . 2018;6(2):50–56. doi: 10.13189/app.2018.060203. [DOI] [Google Scholar]
- 150.Jain N. K., Singhai A. K. Protective role of Beta vulgaris L. leaves extract and fractions on ethanol-mediated hepatic toxicity. Acta Poloniae Pharmaceutica . 2012;69(5):945–950. [PubMed] [Google Scholar]
- 151.Chander T. R., Reddy Y. N. Evaluation of hepatoprotective and antihepatotoxic activity of ethanolic extract of evolvulus alsinoides linn on CCL4 induced rats. Asian Journal of Pharmacology and Toxicology . 2014;2(4):1–6. [Google Scholar]
- 152.Alkiyumi S. S., Abdullah M. A., Alrashdi A. S., Salama S. M., Abdelwahab S. I., Hadi A. H. Ipomoea aquatica extract shows protective action against thioacetamide-induced hepatotoxicity. Molecules . 2012;17(5):6146–6155. doi: 10.3390/molecules17056146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khan M. J., Saini V., Bhati V. S., Karchuli M. S., Kasturi M. B. Anxiolytic activity of Ipomoea aquatica leaves. European Journal of Experimental Biology . 2011;1:63–70. [Google Scholar]
- 154.Hussain A. I., Rathore H. A., Sattar M. Z., Chatha S. A., Sarker S. D., Gilani A. H. Citrullus colocynthis (L.) Schrad (bitter apple fruit): a review of its phytochemistry, pharmacology, traditional uses and nutritional potential. Journal of Ethnopharmacology . 2014;155(1):54–66. doi: 10.1016/j.jep.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 155.Rajasree R. S., Sibi P. I., Francis F., William H. Phytochemicals of Cucurbitaceae family—a review. International Journal of Pharmacognosy and Phytochemical Research . 2016;8:113–123. [Google Scholar]
- 156.Deshmukh C. D., Jain A., Tambe M. S. Phytochemical and pharmacological profile of Citrullus lanatus (THUNB) The Biologist . 2015;3(2):483–488. doi: 10.17812/blj2015.32.18. [DOI] [Google Scholar]
- 157.Pekamwar S., Kalyankar T., Kokate S. Pharmacological activities of Coccinia grandis. Journal of Applied Pharmaceutical Science . 2013;3:114–119. [Google Scholar]
- 158.Attanayake A. P., Jayatilaka K. A., Pathirana C., Mudduwa L. K. Efficacy and toxicological evaluation of Coccinia grandis (Cucurbitaceae) extract in male Wistar rats. Asian Pacific Journal of Tropical Disease . 2013;3(6):460–466. doi: 10.1016/s2222-1808(13)60101-2. [DOI] [Google Scholar]
- 159.Khan Marwat S., Khan E. A., Baloch M. S., et al. Ricinus cmmunis: ethnomedicinal uses and pharmacological activities. Pakistan Journal of Pharmaceutical Sciences . 2017;30(5):1815–1827. [PubMed] [Google Scholar]
- 160.Abdul W. M., Hajrah N. H., Sabir J. S., et al. Therapeutic role of Ricinus communis L. and its bioactive compounds in disease prevention and treatment. Asian Pacific Journal of Tropical Medicine . 2018;11(3):177–185. doi: 10.4103/1995-7645.228431. [DOI] [Google Scholar]
- 161.Mawa S., Husain K., Jantan I. Ficus carica L.(Moraceae): phytochemistry, traditional uses and biological activities. Evidence-based Complementary and Alternative Medicine . 2013;2013:8. doi: 10.1155/2013/974256.974256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Allahyari S., Delazar A., Najafi M. Evaluation of general toxicity, anti-oxidant activity and effects of Ficus carica leaves extract on ischemia/reperfusion injuries in isolated heart of rat. Advanced Pharmaceutical Bulletin . 2014;4(2):577–582. doi: 10.5681/apb.2014.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Raeisi-Dehkordi H., Mohammadi M., Moghtaderi F., Salehi-Abargouei A. Do sesame seed and its products affect body weight and composition? A systematic review and meta-analysis of controlled clinical trials. Journal of Functional Foods . 2018;49:324–332. doi: 10.1016/j.jff.2018.08.036. [DOI] [Google Scholar]
- 164.Miraj S., Kiani S. Bioactivity of Sesamum indicum: a review study. Der Pharmacia Lettre . 2016;8(6):328–334. [Google Scholar]
- 165.Ruckmani A., Meti V., Vijayashree R., et al. Anti-rheumatoid activity of ethanolic extract of Sesamum indicum seed extract in Freund’s complete adjuvant induced arthritis in Wistar albino rats. Journal of Traditional and Complementary Medicine . 2018;8(3):377–386. doi: 10.1016/j.jtcme.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tewari D., Anjum N. Tripathi phytochemistry and pharmacology of plantago ovata: a natural source of laxative medicine. World Journal of Pharmaceutical Research . 2014;3(9):361–372. [Google Scholar]
- 167.Haddadian K., Haddadian K., Zahmatkash M. A review of Plantago plant. Indian Journal of Tradational knowledge . 2014;13(4):681–685. [Google Scholar]
- 168.Sammaiah G., Narsaiah N., Dayakar G., et al. Constituents and cytotoxicity of polygala erioptera. International Journal of Chemical Sciences . 2008;6(2):643–646. [Google Scholar]
- 169.Shin H., Chung H., Park B., Lee K. Y. Identification of antioxidative constituents from Polygonum aviculare using LC-MS coupled with DPPH assay. Natural Product Sciences . 2016;22(1):64–69. doi: 10.20307/nps.2016.22.1.64. [DOI] [Google Scholar]
- 170.Shen B. B., Yang Y. P., Yasamin S., et al. Analysis of the phytochemistry and bioactivity of the genus polygonum of polygonaceae. Digital Chinese Medicine . 2018;1(1):19–36. doi: 10.1016/s2589-3777(19)30005-9. [DOI] [Google Scholar]
- 171.Bozicevic A., De Mieri M., Di Benedetto A., Gafner F., Hamburger M. Dammarane-type saponins from leaves of Ziziphus spina-christi. Phytochemistry . 2017;138:134–144. doi: 10.1016/j.phytochem.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 172.Karar M. E., Quiet L., Rezk A., et al. Phenolic profile and in vitro assessment of cytotoxicity and antibacterial activity of Ziziphus spina-christi Leaf Extracts. Medicinal Chemistry . 2016;6(3):143–156. doi: 10.4172/2161-0444.1000339. [DOI] [Google Scholar]
- 173.Abdel-Zaher A. O., Salim S. Y., Assaf M. H., Abdel-Hady R. H. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. Journal of Ethnopharmacology . 2005;101(1–3):129–138. doi: 10.1016/j.jep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 174.Sireeratawong S., Vannasiri S., Nanna U., Singhalak T., Jaijoy K. Evaluation of acute and chronic toxicities of the water extract from Ziziphus attopensis pierre. ISRN pharmacology . 2012;2012:7. doi: 10.5402/2012/789651.789651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Al-Snafi A. E. The pharmacology of Bacopa monniera. A review. International Journal of Pharma Sciences and Research . 2013;4(12):154–159. [Google Scholar]
- 176.Sireeratawong S., Jaijoy K., Khonsung P., Lertprasertsuk N., Ingkaninan K. Acute and chronic toxicities of Bacopa monnieri extract in Sprague-Dawley rats. BMC Complementary and Alternative Medicine . 2016;16(1):1–10. doi: 10.1186/s12906-016-1236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rehman N. U., Hussain H., Al-Riyami S. A., Green I. R., Al-Harrasi A. Chemical constituents isolated from lycium shawii and their chemotaxonomic significance. Records of Natural Products . 2018;12(4):380–384. doi: 10.25135/rnp.34.17.09.160. [DOI] [Google Scholar]
- 178.Ahmed F. A., Abdallah N. M., Ezz M. K., El-Azab M. M. Seasonal variations and identification of biologically active constituents of lycium shawii plant roem. & shult. (family solanaceae) International Journal of Innovative Science, Engineering & Technology . 2017;4(11):2348–7968. [Google Scholar]
- 179.Saleem U., Amin S., Ahmad B., Azeem H., Anwar F., Mary S. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicology Reports . 2017;4:580–585. doi: 10.1016/j.toxrep.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saleem T. M., Chetty C. M., Ramkanth S., et al. Solanum nigrum Linn.—a review. Pharmacognosy Reviews . 2009;3(6):342–345. [Google Scholar]
- 181.Patel A., Biswas S., Shoja M. H., Ramalingayya G. V., Nandakumar K. Protective effects of aqueous extract of Solanum nigrum Linn. leaves in rat models of oral mucositis. The Scientific World Journal . 2014;2014:10. doi: 10.1155/2014/345939.345939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rai M., Jogee P. S., Agarkar G., Santos C. A. Anticancer activities of Withania somnifera: current research, formulations, and future perspectives. Pharmaceutical Biology . 2016;54(2):189–197. doi: 10.3109/13880209.2015.1027778. [DOI] [PubMed] [Google Scholar]
- 183.Patel S. B., Rao N. J., Hingorani L. L. Safety assessment of Withania somnifera extract standardized for Withaferin A: acute and sub-acute toxicity study. Journal of Ayurveda and Integrative Medicine . 2016;7(1):30–37. doi: 10.1016/j.jaim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Delgado-Altamirano R., López-Palma R. I., Monzote L., et al. Chemical constituents with leishmanicidal activity from a pink-yellow cultivar of Lantana camara var. aculeata (L.) collected in Central Mexico. International Journal of Molecular Sciences . 2019;20(4):1–17. doi: 10.3390/ijms20040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pour B. M., Latha L. Y., Sasidharan S. Cytotoxicity and oral acute toxicity studies of Lantana camara leaf extract. Molecules . 2011;16(5):3663–3674. doi: 10.3390/molecules16053663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jawonisi I., Adoga G. Acute toxicity, haematological and histopathological assessment of the ethanolic extract of Lantana camara leaf. Journal of Pharmacy & Bioresources . 2017;14(1):45–50. doi: 10.4314/jpb.v14i1.6. [DOI] [Google Scholar]
- 187.Moradi M. T., Karimi A., Fotouhi F., Kheiri S., Torabi A. In vitro and in vivo effects of Peganum harmala L. seeds extract against influenza A virus. Avicenna Journal of Phytomedicine . 2017;7(6):519–530. [PMC free article] [PubMed] [Google Scholar]
- 188.Shang X., Guo X., Li B., et al. Microwave-assisted extraction of three bioactive alkaloids from Peganum harmala L. and their acaricidal activity against Psoroptes cuniculi in vitro. Journal of Ethnopharmacology . 2016;192:350–361. doi: 10.1016/j.jep.2016.07.057. [DOI] [PubMed] [Google Scholar]
- 189.Majid A. A review study of the chemical constituents and therapeutic effects of Peganum harmala L. Global Journal of Pure and Applied Chemistry Research . 2018;6(2):12–19. [Google Scholar]
- 190.Ammar N. M., EL-Hawary S. S., Mohamed D. A., Afifi M. S., Ghanem D. M., Awad G. Phytochemical and biological studies of Tribulus terrestris L. growing in Egypt. International Journal of Pharmacology . 2018;14(2):248–259. [Google Scholar]
- 191.Ullah R., Hussain I., Ahmad S. Diocanol; one new phenol derivative isolated and characterized from Urtica dioica. Arabian Journal of Chemistry . 2017;10:S1284–S1286. doi: 10.1016/j.arabjc.2013.03.009. [DOI] [Google Scholar]
- 192.Bourgeois C., Leclerc É. A., Corbin C., et al. Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications. Comptes Rendus Chimie . 2016;19(9):1090–1100. doi: 10.1016/j.crci.2016.03.019. [DOI] [Google Scholar]
- 193.Vajic U. J., Grujic-Milanovic J., Miloradovic Z., et al. Urtica dioica L. leaf extract modulates blood pressure and oxidative stress in spontaneously hypertensive rats. Phytomedicine . 2018;46:39–45. doi: 10.1016/j.phymed.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 194.Bharti S. K., Krishnan S., Kumar A., Kumar A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Therapeutic Advances in Endocrinology and Metabolism . 2018;9(3):81–100. doi: 10.1177/2042018818755019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ponnulakshmi R., Shyamaladevi B., Vijayalakshmi P., Selvaraj J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicology Mechanisms and Methods . 2019;29(4):276–290. doi: 10.1080/15376516.2018.1545815. [DOI] [PubMed] [Google Scholar]
- 196.Arumugam G., Manjula P., Paari N. A review: anti diabetic medicinal plants used for diabetes mellitus. Journal of Acute Disease . 2013;2(3):196–200. doi: 10.1016/s2221-6189(13)60126-2. [DOI] [Google Scholar]
- 197.Jaya P. P. Herbal medicine for diabetes mellitus. A Review International Journal of Phytopharmacy . 2013;3(1):1–22. [Google Scholar]
- 198.Jeeva S., Anlin S. Y. A review of antidiabetic potential of ethnomedicinal plants. Medicinal & Aromatic Plants . 2014;3(4):1–8. [Google Scholar]
- 199.Azaizeh H., Fulder S., Khalil K., Said O. Ethnobotanical knowledge of local Arab practitioners in the Middle Eastern region. Fitoterapia . 2003;74(1-2):98–108. doi: 10.1016/s0367-326x(02)00285-x. [DOI] [PubMed] [Google Scholar]
- 200.El-Ghazali G. E., Al-Khalifa K. S., Saleem G. A., Abdallah E. M. Traditional medicinal plants indigenous to Al-Rass province, Saudi Arabia. Journal of Medicinal Plants Research . 2010;4(24):2680–2683. [Google Scholar]
- 201.Amal M. F., Masarrat M. M. Migahid ethnobotanical survey of plants used in the treatment of diabetes mellitus in Tabuk region. Saudi Arabia International Journal of Current Microbiology and Applied Sciences . 2016;5(6):258–270. [Google Scholar]
- 202.Abdel-Kader M. S., Hazazi A. M., Elmakki O. A., Alqasoumi S. I. A survey on traditional plants used in Al Khobah village. Saudi Pharmaceutical Journal . 2018;26(6):817–821. doi: 10.1016/j.jsps.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ali N. A., Al Sokari S. S., Gushash A., Anwar S., Al-Karani K., Al-Khulaidi A. Ethnopharmacological survey of medicinal plants in Albaha region, Saudi Arabia. Pharmacognosy Research . 2017;9(4):p. 401. doi: 10.4103/pr.pr_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Marlesa R. J., Farnsworth N. R. Antidiabetic plants and their active constituents. Phytomedicine . 1995;2(2):137–189. doi: 10.1016/s0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 205.Hasani-Ranjbar S., Larijani B., Abdollahi M. A systematic review of Iranian medicinal plants useful in diabetes mellitus. Archives of Medical Science . 2008;4(3):285–292. [Google Scholar]
- 206.Kavishankar G. B., Lakshmidevi N., Murthy S. M., Prakash H. S., Niranjana S. R. Diabetes and medicinal plants—a review. International Journal of Pharma Bio Sciences . 2011;2(3):65–80. [Google Scholar]
- 207.Chen S. L., Yu H., Luo H. M., Wu Q., Li C. F., Steinmetz A. Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chinese Medicine . 2016;11:p. 37. doi: 10.1186/s13020-016-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Robertson E. Medicinal Plants at Risk. Nature’s Pharmacy, Our Treasure Chest: Why We Must Conserve Our Natural Heritage . Tucson, AZ, USA: Cent. Biol. Divers.; 2008. [Google Scholar]
- 209.Annan K., Dickson R. A., Amponsah I. K., Nooni I. K. The heavy metal contents of some selected medicinal plants sampled from different geographical locations. Pharmacognosy Research . 2013;5(2):p. 103. doi: 10.4103/0974-8490.110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bedassa M., Abebaw A., Desalegn T. Assessment of selected heavy metals in onion bulb and onion leaf (Allium cepa L.), in selected areas of central rift valley of Oromia region Ethiopia. Journal of Horticulture and Forestry . 2017;4:p. 217. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article. All data are taken from published research papers and available online.


