Abstract
Type I interferons (IFNs) have broad and potent antiviral activity. We review the interplay between type I IFNs and SARS-CoV-2. Human cells infected with SARS-CoV-2 in vitro produce low levels of type I IFNs, and SARS-CoV-2 proteins can inhibit various steps in type I IFN production and response. Exogenous type I IFNs inhibit viral growth in vitro. In various animal species infected in vivo, type I IFN deficiencies underlie higher viral loads and more severe disease than in control animals. The early administration of exogenous type I IFNs improves infection control. In humans, inborn errors of, and auto-antibodies against type I IFNs underlie life-threatening COVID-19 pneumonia. Overall, type I IFNs are essential for host defense against SARS-CoV-2 in individual cells and whole organisms.
Current Opinion in Immunology 2022, 74:172–182
This review comes from a themed issue on Innate Immunity (2022)
Edited by Dusan Bogunovic and Zhijian James
For complete overview of the section, please refer to the article collection, “Innate Immunity (2022)”
Available online 25th January 2022
https://doi.org/10.1016/j.coi.2022.01.003
0952-7915/© 2022 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Humans have co-evolved with various viruses, with infinite interactions selecting both human and viral variants [1]. Most human-tropic viruses currently cause life-threatening disease in only a minority of infected individuals, but a few such viruses have caused major pandemics [2,3]. December 2019 saw the start of a pandemic of coronavirus disease 19 (COVID-19) [4], caused by a new virus, severe respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus rapidly spread worldwide, causing the deadliest pandemic of a viral respiratory disease since the ‘Spanish Flu’ in 1918. Most infected individuals are asymptomatic or have mild flu-like clinical manifestations, but about 20% suffer pneumonia. Non-hypoxemic ‘moderate’ pneumonia rarely requires hospitalization and is seen in about half these cases, whereas the other half experience hypoxemic pneumonia, typically requiring hospital admission for oxygen therapy. It is thought that between 400 million and 1 billion people have been infected. More than 5 million people have died from COVID-19 [5], with some estimates placing the true figure closer to 7–9 million. The estimated infection fatality rate (IFR) in unvaccinated individuals is about 1%. Age is the major epidemiological risk factor for hospitalization or death from pneumonia, the risk doubling with every five years of age from childhood onward [6,7••,8]. The risk is 10 000 times greater at the age of 85 years (10%) than at the age of five years (0.001%). Other epidemiological risk factors have been described, but with much lower odds ratios (ORs), almost always <2 [9,10••].
SARS-CoV-2 is an enveloped, positive-sense single-stranded RNA virus from the Coronaviridae family [11]. Seven coronaviruses are known to infect humans, four of which are endemic (2 alphaviruses: 229E and NL63; 2 betaviruses: OC43 and HKU1), the other three being epidemic or pandemic betacoronaviruses: SARS-CoV, MERS-CoV, and SARS-CoV-2. These last three viruses are the most virulent, with an IFR due to pulmonary disease at least 1000 times greater than that for the endemic coronaviruses. SARS-CoV-2 expresses four structural proteins — S (spike), E (envelope), M (membrane), and N (nucleocapsid) — 16 nonstructural proteins (Nsp1–16), and nine accessory proteins (ORF3a, 3b,6, 7a, 7b, 8, 9b, 9c and 10) [12•]. SARS-CoV-2 can enter many cell types, but can replicate in human cells only after entry involving interaction between its S protein and angiotensin-converting enzyme 2 (ACE2). ACE2 is the main facilitator of virus entry and is expressed principally by ciliated epithelial cells in the nasal cavity, type II pneumocytes and ciliated cells in the respiratory tract, together with cells in the small intestine, testis, kidney, heart muscle, colon, and thyroid gland. TMPRSS2 is a coreceptor of SARS-CoV-2 with a potential role in priming, through cleavage of the S protein; it is also expressed in respiratory cells [13, 14, 15]. Although the suspected natural hosts of SARS-CoV-2 include bats and pangolins [16], the origin of transmission from wildlife to humans is currently debated [17, 18, 19].
Type I interferons (IFNs) were described by Isaacs and Lindenman in 1956 [20], and act to fend off viruses in most, if not all jawed vertebrates [21,22•]. Their evolution attests to both strong negative selection across species and a surprising degree of diversity within each species [23]. There are 17 subtypes of type I IFNs in humans, encoded by 17 intron-less genes: 13 IFN-α subtypes, IFN-ω, IFN-β, IFN-ε, and IFN-κ, all of which bind to the same heterodimeric receptor (IFNAR1 and IFNAR2) [24]. IFN-β is induced by viruses in most cell types, and can then induce the other type I IFNs, whereas IFN-α and IFN-ω are produced principally by leukocytes [1,25]. IFN-ε, and IFN-κ are constitutively expressed but also induced in the female reproductive tract and skin, respectively [26,27]. Excessive type I IFN activity is deleterious in humans, as shown by type I interferonopathies [28]. Conversely, inborn deficiencies of type I IFN immunity underlie various viral illnesses, including adverse events following vaccination with live-attenuated viruses (measles-mumps-rubella and yellow fever), severe or recurrent VZV infection, severe influenza pneumonia, and herpes simplex virus encephalitis [29]. These observations suggest that the levels of type I IFNs must be tightly regulated, both at baseline and during viral infection. Many viral products inhibiting the induction of or response to human type I IFNs have been described [30, 31, 32, 33, 34, 35]. We review here the interplay between SARS-CoV-2 and type I IFNs, both in vitro and in vivo, in animals and in humans.
Sensing of SARS-CoV-2 and poor induction of type I IFNs in vitro
The infection of human cells with SARS-CoV-2 in vitro induces type I IFNs (Figure 1 ). These molecules are produced following the recognition of viral intermediates or their by-products by microbial sensors. Mitochondrial antiviral signaling protein (MAVS) is activated by the sensing of SARS-CoV-2 RNA in the cytosol of human lung epithelial cell lines (A549, Calu-3) by melanoma differentiation-associated gene 5 (MDA5) and RNA helicase retinoic acid-inducible gene I (RIG-I) [36,37•,38,39]. Endosomal receptors have also been implicated in this process in the same cells. Indeed, SARS-CoV-2 can activate IRF3 via TICAM1 following sensing by TLR3, and NF-κB via MyD88 after sensing by TLR7 [24,40]. TLR3 and TLR7 have been shown to sense SARS-CoV-2 in Calu-3/Medical Research Council cell strain 5 (MRC-5) [41•]. In addition, TLR7 and TLR8 may activate plasmacytoid dendritic (pDCs) cells following infection [42]. The activation of these three pathways leads to the activation of IFN-regulatory factors 3 and 7 (IRF3, IRF7) and NF-κB, resulting in the production of type I IFNs, by pDCs, for example [43]. These type I IFNs then induce the expression of interferon-stimulated genes (ISGs) [44, 45, 46]. Induction levels range from low to high, depending on the cells studied. Interestingly, other viruses, such as seasonal influenza A viruses (IAV) [47•] and Sendai virus (SeV) [30], induce type I IFNs more strongly than SARS-CoV-2. The three epidemic coronaviruses induce much lower levels of type I IFNs than common coronaviruses, which generally cause benign infections, suggesting that the capacity to diminish type I IFN induction is a key component of their virulence [30, 31, 32, 33].
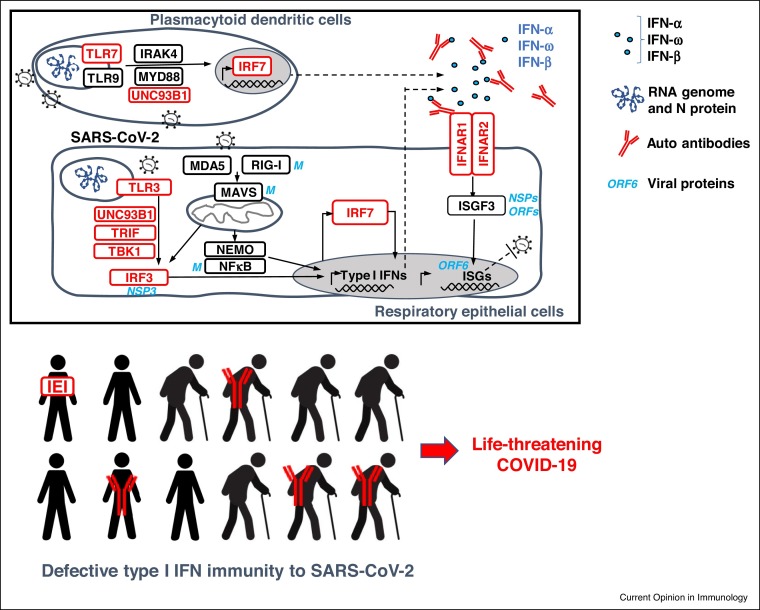
Figure 1.
Life-threatening COVID-19 can be caused in Humans by inborn errors of type I IFN immunity or autoantibodies neutralizing type I IFNs. Both mechanisms interfering with type I IFN immunity in respiratory epithelial cells and blood plasmacytoid dendritic cells.
SARS-CoV-2 can replicate in respiratory epithelial cells (REC), but not in pDC which is why the responsive pathway is only shown in REC, where anti-viral ISG will matter, while the self-amplification loop of type I IFN does probably also operate in pDCs. Monogenic inborn errors of type I IFN immunity are shown in red. Auto-Abs to type I IFNs are shown in red. SARS-CoV-2 proteins which can inhibit proteins of the type I IFN pathway are shown in blue. Type I IFNs are shown in blue circles. IFN: interferon; Auto-Ab: autoantibody, ISGs: interferon-stimulated genes.
Mechanisms of inhibition by SARS-CoV-2, of type I IFN induction and response
SARS-CoV-2 is a poor inducer of type I IFNs, not only because it inhibits core cellular functions, such as transcription and translation [48], but also because it specifically inhibits various molecules involved in type I IFN production. About half the 29 known viral proteins target human proteins in the type I IFN induction pathways, including IRF3, NEMO, TBK1, MAVS and RIG-I, and/or in the type I response pathway, including IFNB self-amplification [49, 50, 51, 52, 53]. The viral proteins involved include Nsp1, Nsp5, Nsp6, Nsp13, Nsp14, Nsp15, ORF3a, ORF3b, ORF6, ORF7a, ORF7b, ORF9b, M, and N. Several unbiased screens have shown that both type I IFN production and ISG induction can be inhibited by ORF6 [54]. The production of, and response to type I IFNs can also be inhibited by Nsp1, via inhibition of the association of IRF3 with the IFNB promoter, of protein translation [55, 56, 57], and via the blocking of mRNA access to ribosomes [58]. The nuclear translocation of ISGF3, the key transducer of the type I IFN response, can be inhibited by ORF3b [59]. One variant of SARS-CoV-2 displays an even stronger inhibitory effect, due to its production of a longer ORF3b. Interestingly, the cleavage of ISG15 from IRF3 is promoted by Nsp3, leading to an attenuated IFN response [60]. The induction of lower levels of endogenous IFN-β has also been reported for an ORF7b variant [55]. Nsp14 can shut down translation, by impairing the type I IFN-dependent induction of ISGs [61]. MERS-CoV and SARS-CoV have also developed approaches for evading type I IFNs [62]. Other molecules have been shown to inhibit the type I IFN pathway in different experimental settings (such as overexpression and knockout), further suggesting a major role of type I IFNs in the anti-SARS-CoV-2 response [22•].
High vulnerability to exogenous type I IFNs and key host factors
SARS-CoV-2 is highly sensitive to pretreatment with type I IFNs [50,63,64]. Exogenous type I IFN does not seem to be inhibited by the virus [55,65,66]. The impact of treatment with type I and type III IFNs on the intracellular growth of various viral strains has been evaluated. Concentrations of type I IFNs in the pg/mL range can inhibit SARS-CoV-2 replication in various cells [67••,68•,69•]. Recent variants, such as B.1, B.1.1.7 (alpha), and B.1.1351 (beta), can resist concentrations of type I and III IFNs 25–322 times higher than those effective against earlier strains, such as strains A and B [70]. The virus becoming more resistant to anti-viral mechanisms, such as type I IFNs, may in principle increase its fitness or its virulence. In addition to these studies of the impact of exogenous type I IFNs, genome-wide or proteome-wide screens have been performed to identify other host factors for which an absence leads to higher levels of SARS-CoV-2 replication, and host proteins capable of interacting with viral proteins [71•,72, 73, 74,75•]. Some of these factors interact with SARS-CoV-2 and other coronaviruses (for example, HMGB1 and proteins related to cholesterol metabolism), whereas others, such as Rab GTPase, interact specifically with SARS-CoV-2 [72,73]. These studies let to the identification of therapeutic options, including exogenous type I IFNs which could be tested and validated by future work.
Type I IFN immunity in animal models naturally susceptible to SARS-CoV-2
Studies have also been performed in animals in vivo, including species that are naturally susceptible to SARS-CoV-2 [50,76,77•]. In hamsters, SARS-CoV-2 infection triggers an ISG signature in the lungs [78, 79, 80]. In ferrets, the ISG signature triggered by SARS-CoV-2 and detected in respiratory swab samples is weaker than that observed after IAV infection [47•]. In non-human primates, SARS-CoV-2 infection triggers a stronger induction of type I IFNs in the lungs when comparing to IAV [81,82]. The interspecies variability of type I IFN induction may reflect differences between the animal species used, the viral inoculum [78], the age of the animals [83], and the stage of infection studied [50]. These animals are naturally susceptible to SARS-CoV-2 infection, but it is, nevertheless, difficult to extrapolate these findings to humans, because viral proteins may block animal and human proteins involved in type I IFN immunity differently. Interestingly, Stat2−/− (lacking both type I and III IFN responses) hamsters fail to control SARS-CoV-2 infection, whereas this infection is successfully controlled by Il-28r−/− (deficient for the type III IFN response only) animals [84]. Moreover, intranasal treatment with universal IFN-α within one to three days of infection can protect hamsters from severe disease [85,86].
Type I IFN immunity in mouse models
Mice are less physiologically and pathologically relevant in this context, as they are not naturally susceptible to SARS-CoV-2 infection. Transgenic mice expressing human ACE2 (hACE2) under the control of the cytokeratin 18 (KRT18) promoter predominantly in epithelial cells are susceptible to SARS-CoV-2 [87]. Type I IFN induction was detected in the lungs of SARS-CoV-2-infected K18-hACE2 mice, which developed severe lung damage [88,89]. Mice transduced intranasally with replication-defective adenoviruses encoding hACE2 can be infected at peak hACE2 expression [90,91]. In this model, SARS-CoV-2 infection is self-limited due to the transient nature of hACE2 expression. Nevertheless, two days after SARS-CoV-2 infection, an absence of type I IFN induction was observed in Ifnar1−/− and Irf3−/−Irf7−/− mice, although these mice did not display a poorer control of viral replication [91]. Consistently, mice lacking both type I and II IFN receptors (IFNR-DKO) infected with a SARS-CoV-2 strain genetically adapted to the mouse ACE2 (SARS-CoV-2 MA) display enhanced susceptibility to the virus [92]. Finally, the administration of the type III interferon IFN-λ1 decreased SARS-CoV-2 replication in wild-type mice; the effects of type I IFNs were not investigated [93]. Overall, endogenous and exogenous type I IFNs have been reported to exert antiviral activity against SARS-CoV-2 in most animal studied in vivo. Extrapolation of these findings to humans is however difficult.
Autosomal inborn errors of type I IFN immunity in humans
Patients with inborn errors (IE) of type I IFN immunity affecting either IFN production or the response to IFNs have been shown to suffer from severe viral infections in childhood or early adulthood [29]. Interestingly, three inborn errors of immunity (IEI) — deficiencies of IRF7, IRF9 and TLR3 — have been shown to underlie life-threatening influenza pneumonia [94, 95, 96]. The COVID Human Genetic Effort (COVID-HGE) (www.covidhge.com) tested the hypothesis that patients with life-threatening forms of COVID-19 pneumonia might have IE of the three genes associated with influenza susceptibility and of 10 other related genes. None of the patients with selective deficiencies of the type III IFN pathway (IL10RB deficiency) have been reported to suffer from life-threatening viral infections, including COVID-19 pneumonia. Loss-of-function mutations were found in 23 patients with critical COVID-19 pneumonia (about 3%) [97••]. These patients included four unrelated previously healthy adults, aged 25–50 years, with autosomal recessive (AR) complete IRF7 or IFNAR1 deficiency. The other patients had heterozygous loss-of-function variants of genes that were known (n = 11) or hitherto unknown (n = 8) to underlie autosomal dominant (AD), partial deficiencies. Most (16/23) of these patients were less than 60 years old.
X-linked TLR7 deficiency in men
A chromosome-wide gene approach identified X-linked recessive (XR) TLR7 deficiency as another cause of life-threatening COVID-19 in 1.8% of unrelated men under 60 years old [98]; 16 (1.3% overall) of 1202 men carried biochemically deleterious hemizygous TLR7 mutations, whereas none of the 331 men with asymptomatic/mild COVID-19 carried such mutations. Three young children carried the genotype but presented only mild infection [99••]. The cumulative MAF of deleterious TLR7 variants reported in a public database (gnomAD) was 6.5 × 10−4 in men, and only half (4/8) the TLR7 variants reported by other groups were found to be deleterious [100, 101, 102, 103]. Interestingly, endosomal TLR7 had long been known to sense ssRNA [104], and its gene as known to be under strong negative selection [105], but its role in host defense in humans had remained unclear. These new studies demonstrate that pDCs, which constitutively express both TLR7 and IRF7, are crucial for host defense against SARS-CoV-2, due to their production of type I IFNs [46,106]. Remarkably, TLR3 is expressed by respiratory epithelial cells, whereas TLR7 is not [96,107]. Overall, TLR3-dependent and TLR7-dependent type I IFN immunity in PECs and pDCs, respectively, is crucial for defense against SARS-CoV-2.
Auto-Abs against type I IFNs underlie critical COVID-19
Auto-Abs against type I IFNs were first reported in the 1980s, in patients treated with type I IFNs and in patients with systemic lupus erythematosus (SLE) [108,109]. They were widely thought to have no impact on antiviral immunity. However, the COVID-HGE showed in 2020 that at least 10% of individuals with critical COVID-19 and without type I IFN inborn errors had auto-Abs neutralizing supraphysiological concentrations (10 ng/mL in plasma diluted 1/10) of IFN-α2 and/or IFN-ω [110••]. In addition, APS-1 patients, who display these auto-Abs from infancy onward, are at very high risk of developing severe or critical COVID-19 pneumonia [111••,112•]. These auto-Abs were present before infection in the few cases tested, suggesting they were not induced by the virus. Moreover, these auto-Abs to type I IFNs were not found in patients with asymptomatic or mild SARS-CoV-2 infection. These findings were later replicated in various regions [113, 114, 115, 116,117•,118•,119, 120, 121]. Longitudinal profiling studies of over 600,000 peripheral blood mononuclear cells from patients with or without auto-Abs neutralizing type I IFNs described in depth the consequences of these auto-Abs, reporting a lack of ISGs in myeloid cells [118•]. Unbiased auto-Ab screening assays also identified other auto-Abs triggered by the virus [117•,122].
Auto-Abs neutralizing lower concentrations of type I IFNs
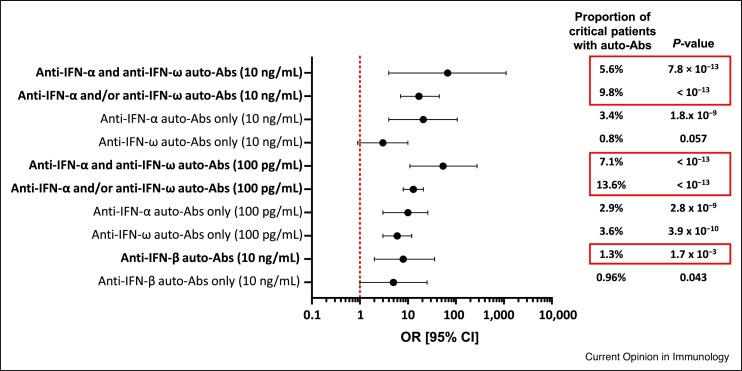
Physiological concentrations of type I IFNs in COVID-19 patients range between 1 and 100 pg/mL in plasma. At least 13.6% of the patients with critical COVID-19 had auto-Abs neutralizing 100 pg/mL IFN-α2 and/or IFN-ω, and this proportion was higher (20%) in the elderly and in individuals who died from COVID-19 [67••]. Auto-Abs neutralizing IFN-β were found in about 1% of patients with critical COVID-19 with no other anti-type I IFN auto-Abs. In addition, 6.8% of patients with severe (but not critical) COVID-19 carried neutralizing auto-Abs. We estimated the ORs by comparing the prevalence of auto-Abs in patients with critical disease with those in patients with asymptomatic or mild infection (Figure 2 ) [67••]. The highest ORs were those for patients with auto-Abs neutralizing both IFN-α2 and IFN-ω, at 10 ng/mL and 100 pg/mL (67, P < 7.8 × 10−13 for 10 ng/mL,and 54, P < 10−13 for 100 pg/mL; Figure 2). The ORs for auto-Abs against IFN-α2 and/or IFN-ω, or against IFN-ω only, were lower, but still highly significant. For auto-Abs against IFN-β only, the ORs for critical disease were lower, at 5 (P = 0.043). These observations suggest that deficiencies of single type I IFNs can underlie life-threatening COVID-19 pneumonia, albeit with incomplete penetrance.
Figure 2.
Odds Ratios for critical disease in SARS-CoV-2-infected individuals with auto-Abs against type I IFNs.
Odds ratios (OR) and P-values were estimated by means of Firth’s bias-corrected logistic regression.
Auto-Abs against type I IFNs in the uninfected general population
These auto-Abs were found to be present in about 0.3% of the few thousand individuals under the age of 65 years tested [110••,118•]. In a sample of more than 34 000 uninfected individuals aged 18–100 years, the prevalence of auto-Abs neutralizing 10 ng/mL (and 100 pg/mL) of IFN-α2 or IFN-ω increased significantly with age, with 0.17% (1.1%) of individuals positive for these antibodies under the age of 70 years, and more than 1.4% (4.4%) positive over the age of 70 years, consistent with the higher risk of life-threatening and lethal COVID-19 in the elderly population [67••]. Patients under the age of 18 years were not tested, and no tests were performed for auto-Abs neutralizing IFN-ε or IFN-κ. Conversely, auto-Abs neutralizing IFN-β were found to have the same prevalence in all age categories tested. These findings are related to the enigmatic presence of 17 individual type I IFNs in humans, all of which bind to the same receptors [23,24]. The 13 forms of IFN-α and IFN-ω are secreted by leukocytes and are very closely related biochemically and phylogenetically, whereas IFN-β, IFN-ε, and IFN-κ are more distantly related and have specific functions [26,27]. Interestingly, patients with severe COVID-19 and auto-Abs against type I IFNs displayed impaired ISG induction remain silent, until an emergent virus, such as SARS-CoV-2, reveals the deficiency with life-threatening pneumonia.
Type I IFNs and auto-Abs in the respiratory tract of patients
The impact of effective or defective type I IFN immunity has been studied in several tissues. First, like other coronaviruses, SARS-CoV-2 infects humans via the upper respiratory tract. The impact of IFNs in the upper respiratory tract was therefore assessed by RNA-seq in a cohort of 58 individuals, including 14 with mild COVID-19 and 21 with severe COVID-19 [123]. The patients with mild COVID-19 presented a robust type I IFN response, whereas those with severe COVID-19 did not. Conversely, impaired local intrinsic immunity to COVID-19 was associated with high levels of type I IFNs and low levels of type III IFNs (except for IFN-λ2) in the upper and lower respiratory tract, with low levels of ISG expression [124]. Auto-Abs against type I IFNs were found in one of eight patients tested with severe COVID-19, and were associated with impaired local type I IFN immunity [124]. Auto-Abs against type I IFNs were detected in tracheal aspirates and in nasal swabs from COVID-19 patients with circulating auto-Abs [69•,125]. Interestingly, patients with severe COVID-19 and auto-Abs against type I IFNs displayed impaired ISG induction in nasal mucosa [69•]; while the impact of these antibodies in the lower respiratory tract is unknown. Overall, these findings suggest a strong antiviral effect of type I IFNs on SARS-CoV-2 in the upper respiratory tract, which can be blocked by auto-Abs neutralizing these type I IFNs [126].
Conclusion
Since December 2019, more than 225 000 articles about ‘COVID-19’ have been listed on PubMed. Many more studies will be published. What has emerged from the study of COVID-19 is that the pathogenesis of the life-threatening pneumonia it causes revolves around deficiencies of type I IFN immunity [127•,128]. The identification of both patients with genetic deficiencies of type I IFNs and other patients with pre-existing auto-Abs neutralizing type I IFNs has shown that a single unifying mechanism can explain almost a fifth of critical cases. This proportion of mechanistically and causally explained cases is unprecedented among common human infectious diseases [129,130]. Along with the in vitro studies showing that SARS-CoV-2 can inhibit type I IFN induction in numerous ways, these findings indicate that a key virulence factor of SARS-CoV-2 — possibly even its principal virulence factor — is its ability to suppress the induction and action of type I IFNs [131]. These findings also suggest that the administration of exogenous type I IFNs (for example IFN-β) early in the course of infection (i.e. before the inflammatory phase) [132] might be beneficial in some patients, such as those with defects of type I IFN production, those with auto-Abs against IFN-α, IFN-ω but not IFN-β, and individuals at risk (such as elderly individuals). In infected and unvaccinated patients unresponsive to type I IFNs, mAbs neutralizing the virus should be administered promptly, as recently shown for an IRF9-deficient patient [133]. Patients with insufficient type I IFN immunity should be vaccinated as a matter of priority and their levels of protective Abs against SARS-CoV-2 monitored to determine the frequency with which vaccination boosters should be delivered, and patients hospitalized for breakthrough infections should also be studied for both Abs developed in response to the vaccine and type I IFN deficiencies.
Author contributions
P.B. and J.-L.C. wrote the manuscript, with the assistance of Q.Z., S.-Y.Z. and E.J., who edited the manuscript, tables and figures.
Conflict of interest statement
J.-L.C. reports being an inventor on patent application PCT/US2021/042741, filed 22 July 2021, submitted by The Rockefeller University, which covers the diagnosis of, susceptibility to, and treatment of viral disease and viral vaccines, including COVID-19 and vaccine-associated diseases. The other authors have no conflicts of interest to report.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank the patients and their families for placing their trust in us. We warmly thank the members of both branches of the Laboratory of Human Genetics of Infectious Diseases. We warmly thank Y. Nemirovskaya, M. Woollett, D. Liu, E. Williams, S. Boucherit, C. Rivalain, M. Chrabieh and L. Lorenzo for administrative assistance. Funding: The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the National Institutes of Health (NIH) (R01AI088364 and R01AI163029), the National Center for Advancing Translational Sciences (NCATS), NIH Clinical and Translational Science Award (CTSA) program (UL1 TR001866), a Fast Grant from Emergent Ventures, Mercatus Center at George Mason University, the Yale Center for Mendelian Genomics and the GSP Coordinating Center funded by the National Human Genome Research Institute (NHGRI) (UM1HG006504 and U24HG008956), the Yale High Performance Computing Center (S10OD018521), the Fisher Center for Alzheimer’s Research Foundation, the Meyer Foundation, the JPB Foundation, the French National Research Agency (ANR) under the ‘Investments for the Future’ program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the ANRS-COV05, ANR GENVIR (ANR-20-CE93-003), ANR GenMISC (ANR-21-COVR-0039) and ANR AABIFNCOV (ANR-20-CO11-0001) projects, the European Union’s Horizon 2020 research and innovation programme under grant agreement No 824110 (EASI-genomics), the Square Foundation, Grandir - Fonds de solidarité pour l’enfance, the Fondation du Souffle, the SCOR Corporate Foundation for Science, Institut National de la Santé et de la Recherche Médicale (INSERM), the French Ministry of Higher Education, Research, and Innovation (MESRI-COVID-19), REACTing-INSERM and the University of Paris. PB was supported by the French Foundation for Medical Research (FRM, EA20170638020). PB was supported by the MD-PhD program of the Imagine Institute (with the support of the Fondation Bettencourt-Schueller).
References
- 1.Hoffmann H.H., Schneider W.M., Rice C.M. Interferons and viruses: an evolutionary arms race of molecular interactions. Trends Immunol. 2015;36:124–138. doi: 10.1016/j.it.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theves C., Crubezy E., Biagini P. History of smallpox and its spread in human populations. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.PoH-0004-2014. [DOI] [PubMed] [Google Scholar]
- 3.Breitbart M., Rohwer F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005;13:278–284. doi: 10.1016/j.tim.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worldometer . vol 2021. 2021. (COVID-19 Coronavirus Pandemic). [Google Scholar]
- 6.Levin A.T., Hanage W.P., Owusu-Boaitey N., Cochran K.B., Walsh S.P., Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35:1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.O’Driscoll M., Ribeiro Dos Santos G., Wang L., Cummings D.A.T., Azman A.S., Paireau J., Fontanet A., Cauchemez S., Salje H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]; Study of data from 45 countries to estimate COVID-19 mortality. Main findings are that the risk of death doubles every five years from childhood onward, accounting for about a 10 000-fold greater risk at 85 years old (10%) than five years old (0.001%).
- 8.Bogunovic D., Merad M. Children and SARS-CoV-2. Cell Host Microbe. 2021;29:1040–1042. doi: 10.1016/j.chom.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhaskaran K., Bacon S., Evans S.J., Bates C.J., Rentsch C.T., MacKenna B., Tomlinson L., Walker A.J., Schultze A., Morton C.E., et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg Health Eur. 2021;6 doi: 10.1016/j.lanepe.2021.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study of over 17 million individuals assessing the risk factors of severe COVID-19 and COVID-19 death. Main findings include that age is the major epidemiological risk factor.
- 11.Weiss S.R. Forty years with coronaviruses. J Exp Med. 2020;217 doi: 10.1084/jem.20200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.V’Kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive review of coronaviruses and mechanisms involved in viral entrance and replication.
- 13.Scialo F., Daniele A., Amato F., Pastore L., Matera M.G., Cazzola M., Castaldo G., Bianco A. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198:867–877. doi: 10.1007/s00408-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Helden J., Butler C.D., Achaz G., Canard B., Casane D., Claverie J.M., Colombo F., Courtier V., Ebright R.H., Graner F., et al. An appeal for an objective, open, and transparent scientific debate about the origin of SARS-CoV-2. Lancet. 2021:1402–1404. doi: 10.1016/S0140-6736(21)02019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z., Jin Q., Wu G., Lu J., Li M., Guo D., Lan K., Feng L., Qian Z., Ren L., et al. SARS-CoV-2’s origin should be investigated worldwide for pandemic prevention. Lancet. 2021:1299–1303. doi: 10.1016/S0140-6736(21)02020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 21.Shields L.E., Jennings J., Liu Q., Lee J., Ma W., Blecha F., Miller L.C., Sang Y. Cross-species genome-wide analysis reveals molecular and functional diversity of the unconventional interferon-omega subtype. Front Immunol. 2019;10:1431. doi: 10.3389/fimmu.2019.01431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Kim Y.M., Shin E.C. Type I and III interferon responses in SARS-CoV-2 infection. Exp Mol Med. 2021;53:750–760. doi: 10.1038/s12276-021-00592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive review of type I and III interferons and SARS-CoV-2. The authors detail the known type I IFN inhibitory mechanisms by SARS-CoV-2.
- 23.Manry J., Laval G., Patin E., Fornarino S., Itan Y., Fumagalli M., Sironi M., Tichit M., Bouchier C., Casanova J.L., et al. Evolutionary genetic dissection of human interferons. J Exp Med. 2011;208:2747–2759. doi: 10.1084/jem.20111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazear H.M., Schoggins J.W., Diamond M.S. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asselin-Paturel C., Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marks Z.R.C., Campbell N., deWeerd N.A., Lim S.S., Gearing L.J., Bourke N.M., Hertzog P.J. Properties and functions of the novel type I interferon epsilon. Semin Immunol. 2019;43 doi: 10.1016/j.smim.2019.101328. [DOI] [PubMed] [Google Scholar]
- 27.LaFleur D.W., Nardelli B., Tsareva T., Mather D., Feng P., Semenuk M., Taylor K., Buergin M., Chinchilla D., Roshke V., et al. Interferon-kappa, a novel type I interferon expressed in human keratinocytes. J Biol Chem. 2001;276:39765–39771. doi: 10.1074/jbc.M102502200. [DOI] [PubMed] [Google Scholar]
- 28.Crow Y.J., Stetson D.B. The type I interferonopathies: 10 years on. Nat Rev Immunol. 2021:1–13. doi: 10.1038/s41577-021-00633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyts I., Casanova J.L. Viral infections in humans and mice with genetic deficiencies of the type I IFN response pathway. Eur J Immunol. 2021:1039–1061. doi: 10.1002/eji.202048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesev E.V., LeDesma R.A., Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol. 2019;4:914–924. doi: 10.1038/s41564-019-0421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park A., Iwasaki A. Type I and type III interferons - induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia H., Cao Z., Xie X., Zhang X., Chen J.Y., Wang H., Menachery V.D., Rajsbaum R., Shi P.Y. Evasion of type I interferon by SARS-CoV-2. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Sastre A. Ten strategies of interferon evasion by viruses. Cell Host Microbe. 2017;22:176–184. doi: 10.1016/j.chom.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kikkert M. Innate immune evasion by human respiratory RNA viruses. J Innate Immun. 2020;12:4–20. doi: 10.1159/000503030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampaio N.G., Chauveau L., Hertzog J., Bridgeman A., Fowler G., Moonen J.P., Dupont M., Russell R.A., Noerenberg M., Rehwinkel J. The RNA sensor MDA5 detects SARS-CoV-2 infection. Sci Rep. 2021;11:13638. doi: 10.1038/s41598-021-92940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Yamada T., Sato S., Sotoyama Y., Orba Y., Sawa H., Yamauchi H., Sasaki M., Takaoka A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat Immunol. 2021;22:820–828. doi: 10.1038/s41590-021-00942-0. [DOI] [PubMed] [Google Scholar]; Report that RIG-I can restrict SARS-CoV-2 infection in a type I and III IFN-dependent manner.
- 38.Nilsson-Payant B.E., Uhl S., Grimont A., Doane A.S., Cohen P., Patel R.S., Higgins C.A., Acklin J.A., Bram Y., Chandar V., et al. The NF-kappaB transcriptional footprint is essential for SARS-CoV-2 replication. J Virol. 2021:23–28. doi: 10.1128/JVI.01257-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorne L.G., Reuschl A.K., Zuliani-Alvarez L., Whelan M.V.X., Turner J., Noursadeghi M., Jolly C., Towers G.J. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021;40 doi: 10.15252/embj.2021107826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNab F., Mayer-Barber K., Sher A., Wack A., O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Bortolotti D., Gentili V., Rizzo S., Schiuma G., Beltrami S., Strazzabosco G., Fernandez M., Caccuri F., Caruso A., Rizzo R. TLR3 and TLR7 RNA sensor activation during SARS-CoV-2 infection. Microorganisms. 2021;9 doi: 10.3390/microorganisms9091820. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report that TLR3 and TLR7 are important in Calu-3/MRC-5 cells to induce type I IFNs.
- 42.Salvi V., Nguyen H.O., Sozio F., Schioppa T., Gaudenzi C., Laffranchi M., Scapini P., Passari M., Barbazza I., Tiberio L., et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onodi F., Bonnet-Madin L., Meertens L., Karpf L., Poirot J., Zhang S.Y., Picard C., Puel A., Jouanguy E., Zhang Q., et al. SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4. J Exp Med. 2021;218 doi: 10.1084/jem.20201387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greene T.T., Zuniga E.I. Type I interferon induction and exhaustion during viral infection: plasmacytoid dendritic cells and emerging COVID-19 findings. Viruses. 2021;13 doi: 10.3390/v13091839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang M., Kolehmainen P., Kakkola L., Maljanen S., Melen K., Smura T., Julkunen I., Osterlund P. SARS-CoV-2 isolates show impaired replication in human immune cells but differential ability to replicate and induce innate immunity in lung epithelial cells. Microbiol Spectr. 2021;9 doi: 10.1128/spectrum.00774-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Severa M., Diotti R.A., Etna M.P., Rizzo F., Fiore S., Ricci D., Iannetta M., Sinigaglia A., Lodi A., Mancini N., et al. Differential plasmacytoid dendritic cell phenotype and type I interferon response in asymptomatic and severe COVID-19 infection. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e1039. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of patients with COVID-19, low type I and III IFN production and high chemokine and IL-6 expression.
- 48.Banerjee A.K., Blanco M.R., Bruce E.A., Honson D.D., Chen L.M., Chow A., Bhat P., Ollikainen N., Quinodoz S.A., Loney C., et al. SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell. 2020;183:1325–1339. doi: 10.1016/j.cell.2020.10.004. e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palermo E., Di Carlo D., Sgarbanti M., Hiscott J. Type I interferons in COVID-19 pathogenesis. Biology. 2021;10 doi: 10.3390/biology10090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowery S.A., Sariol A., Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: implications for COVID-19. Cell Host Microbe. 2021;29:1052–1062. doi: 10.1016/j.chom.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mariano G., Farthing R.J., Lale-Farjat S.L.M., Bergeron J.R.C. Structural characterization of SARS-CoV-2: where we are, and where we need to be. Front Mol Biosci. 2020;7 doi: 10.3389/fmolb.2020.605236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen D.Y., Khan N., Close B.J., Goel R.K., Blum B., Tavares A.H., Kenney D., Conway H.L., Ewoldt J.K., Chitalia V.C., et al. SARS-CoV-2 disrupts proximal elements in the JAK-STAT pathway. J Virol. 2021;95 doi: 10.1128/JVI.00862-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miorin L., Kehrer T., Sanchez-Aparicio M.T., Zhang K., Cohen P., Patel R.S., Cupic A., Makio T., Mei M., Moreno E., et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc Natl Acad Sci U S A. 2020;117:28344–28354. doi: 10.1073/pnas.2016650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palermo E., Di Carlo D., Sgarbanti M., Hiscott J. Type I interferons in COVID-19 pathogenesis. Biology (Basel) 2021;10 doi: 10.3390/biology10090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shemesh M., Aktepe T.E., Deerain J.M., McAuley J.L., Audsley M.D., David C.T., Purcell D.F.J., Urin V., Hartmann R., Moseley G.W., et al. SARS-CoV-2 suppresses IFNbeta production mediated by NSP1, 5, 6, 15, ORF6 and ORF7b but does not suppress the effects of added interferon. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar A., Ishida R., Strilets T., Cole J., Lopez-Orozco J., Fayad N., Felix-Lopez A., Elaish M., Evseev D., Magor K.E., et al. SARS-CoV-2 nonstructural protein 1 inhibits the interferon response by causing depletion of key host signaling factors. J Virol. 2021;95 doi: 10.1128/JVI.00266-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin J.W., Tang C., Wei H.C., Du B., Chen C., Wang M., Zhou Y., Yu M.X., Cheng L., Kuivanen S., et al. Genomic monitoring of SARS-CoV-2 uncovers an Nsp1 deletion variant that modulates type I interferon response. Cell Host Microbe. 2021;29:489–502. doi: 10.1016/j.chom.2021.01.015. e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thoms M., Buschauer R., Ameismeier M., Koepke L., Denk T., Hirschenberger M., Kratzat H., Hayn M., Mackens-Kiani T., Cheng J., et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369:1249–1255. doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konno Y., Kimura I., Uriu K., Fukushi M., Irie T., Koyanagi Y., Sauter D., Gifford R.J., Consortium U.-C., Nakagawa S., et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A.R., Tascher G., et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu J.C., Laurent-Rolle M., Pawlak J.B., Wilen C.B., Cresswell P. Translational shutdown and evasion of the innate immune response by SARS-CoV-2 NSP14 protein. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2101161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lokugamage K.G., Hage A., de Vries M., Valero-Jimenez A.M., Schindewolf C., Dittmann M., Rajsbaum R., Menachery V.D. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol. 2020;94 doi: 10.1128/JVI.01410-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alfi O., Yakirevitch A., Wald O., Wandel O., Izhar U., Oiknine-Djian E., Nevo Y., Elgavish S., Dagan E., Madgar O., et al. Human nasal and lung tissues infected ex vivo with SARS-CoV-2 provide insights into differential tissue-specific and virus-specific innate immune responses in the upper and lower respiratory tract. J Virol. 2021;95 doi: 10.1128/JVI.00130-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Felgenhauer U., Schoen A., Gad H.H., Hartmann R., Schaubmar A.R., Failing K., Drosten C., Weber F. Inhibition of SARS-CoV-2 by type I and type III interferons. J Biol Chem. 2020;295:13958–13964. doi: 10.1074/jbc.AC120.013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanderheiden A., Ralfs P., Chirkova T., Upadhyay A.A., Zimmerman M.G., Bedoya S., Aoued H., Tharp G.M., Pellegrini K.L., Manfredi C., et al. Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol. 2020;94 doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Bastard P., Gervais A., Le Voyer T., Rosain J., Philippot Q., Manry J., Michailidis E., Hoffmann H.H., Eto S., Garcia-Prat M., et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report that auto-Abs neutralizing low concentrations of type I IFNs can account for about 15% of life-threatening cases. The prevalence of these auto-Abs increases with age in the uninfected population.
- 68•.Bastard P., Michailidis E., Hoffmann H.H., Chbihi M., Le Voyer T., Rosain J., Philippot Q., Seeleuthner Y., Gervais A., Materna M., et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J Exp Med. 2021;218 doi: 10.1084/jem.20202486. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report that auto-Abs to type I IFNs can underlie severe adverse reactions to the yellow-fever live attenuated virus vaccine.
- 69•.Lopez J., Mommert M., Mouton W., Pizzorno A., Brengel-Pesce K., Mezidi M., Villard M., Lina B., Richard J.C., Fassier J.B., et al. Early nasal type I IFN immunity against SARS-CoV-2 is compromised in patients with autoantibodies against type I IFNs. J Exp Med. 2021;218 doi: 10.1084/jem.20211211. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report that auto-Abs to type I IFNs are present and functional in the upper respiratory tract.
- 70.Guo K., Barrett B.S., Mickens K.L., Hasenkrug K.J., Santiago M.L. Interferon resistance of emerging SARS-CoV-2 variants. bioRxiv. 2021 doi: 10.1073/pnas.2203760119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Daniloski Z., Jordan T.X., Wessels H.H., Hoagland D.A., Kasela S., Legut M., Maniatis S., Mimitou E.P., Lu L., Geller E., et al. Identification of required host factors for SARS-CoV-2 infection in human cells. Cell. 2021;184:92–105. doi: 10.1016/j.cell.2020.10.030. e116. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of CRISPR screens identifying cellular factors important for SARS-CoV-2 entry.
- 72.Gordon D.E., Hiatt J., Bouhaddou M., Rezelj V.V., Ulferts S., Braberg H., Jureka A.S., Obernier K., Guo J.Z., Batra J., et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370 doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffmann H.H., Sanchez-Rivera F.J., Schneider W.M., Luna J.M., Soto-Feliciano Y.M., Ashbrook A.W., Le Pen J., Leal A.A., Ricardo-Lax I., Michailidis E., et al. Functional interrogation of a SARS-CoV-2 host protein interactome identifies unique and shared coronavirus host factors. Cell Host Microbe. 2021;29:267–280. doi: 10.1016/j.chom.2020.12.009. e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider W.M., Luna J.M., Hoffmann H.H., Sanchez-Rivera F.J., Leal A.A., Ashbrook A.W., Le Pen J., Ricardo-Lax I., Michailidis E., Peace A., et al. Genome-scale identification of SARS-CoV-2 and pan-coronavirus host factor networks. Cell. 2021;184:120–132. doi: 10.1016/j.cell.2020.12.006. e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Wang R., Simoneau C.R., Kulsuptrakul J., Bouhaddou M., Travisano K.A., Hayashi J.M., Carlson-Stevermer J., Zengel J.R., Richards C.M., Fozouni P., et al. Genetic screens identify host factors for SARS-CoV-2 and common cold coronaviruses. Cell. 2021;184:106–119. doi: 10.1016/j.cell.2020.12.004. e114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of CRISPR screens identifying cellular factors important for SARS-CoV-2 entry.
- 76.de Vries R.D., Rockx B., Haagmans B.L., Herfst S., Koopmans M.P., de Swart R.L. Animal models of SARS-CoV-2 transmission. Curr Opin Virol. 2021;50:8–16. doi: 10.1016/j.coviro.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.Munoz-Fontela C., Dowling W.E., Funnell S.G.P., Gsell P.S., Riveros-Balta A.X., Albrecht R.A., Andersen H., Baric R.S., Carroll M.W., Cavaleri M., et al. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of animal models of SARS-CoV-2 infection.
- 78.Cantwell A.M., Singh H., Platt M., Yu Y., Lin Y.H., Ikeno Y., Hubbard G., Xiang Y., Gonzalez-Juarbe N., Dube P.H. Kinetic multi-omic analysis of responses to SARS-CoV-2 infection in a model of severe COVID-19. J Virol. 2021:1010–1021. doi: 10.1128/JVI.01010-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Francis M.E., Goncin U., Kroeker A., Swan C., Ralph R., Lu Y., Etzioni A.L., Falzarano D., Gerdts V., Machtaler S., et al. SARS-CoV-2 infection in the Syrian hamster model causes inflammation as well as type I interferon dysregulation in both respiratory and non-respiratory tissues including the heart and kidney. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nouailles G., Wyler E., Pennitz P., Postmus D., Vladimirova D., Kazmierski J., Pott F., Dietert K., Muelleder M., Farztdinov V., et al. Temporal omics analysis in Syrian hamsters unravel cellular effector responses to moderate COVID-19. Nat Commun. 2021;12 doi: 10.1038/s41467-021-25030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh D.K., Singh B., Ganatra S.R., Gazi M., Cole J., Thippeshappa R., Alfson K.J., Clemmons E., Gonzalez O., Escobedo R., et al. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat Microbiol. 2021;6:73–86. doi: 10.1038/s41564-020-00841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L., Tostanoski L.H., Yu J., Maliga Z., Nekorchuk M., et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Selvaraj P., Lien C.Z., Liu S., Stauft C.B., Nunez I.A., Hernandez M., Nimako E., Ortega M.A., Starost M.F., Dennis J.U., et al. SARS-CoV-2 infection induces protective immunity and limits transmission in Syrian hamsters. Life Sci Alliance. 2021;4 doi: 10.26508/lsa.202000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boudewijns R., Thibaut H.J., Kaptein S.J.F., Li R., Vergote V., Seldeslachts L., Van Weyenbergh J., De Keyzer C., Bervoets L., Sharma S., et al. STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bessiere P., Wasniewski M., Picard-Meyer E., Servat A., Figueroa T., Foret-Lucas C., Coggon A., Lesellier S., Boue F., Cebron N., et al. Intranasal type I interferon treatment is beneficial only when administered before clinical signs onset in the SARS-CoV-2 hamster model. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoagland D.A., Moller R., Uhl S.A., Oishi K., Frere J., Golynker I., Horiuchi S., Panis M., Blanco-Melo D., Sachs D., et al. Leveraging the antiviral type I interferon system as a first line of defense against SARS-CoV-2 pathogenicity. Immunity. 2021;54:557–570. doi: 10.1016/j.immuni.2021.01.017. e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 88.Oladunni F.S., Park J.G., Pino P.A., Gonzalez O., Akhter A., Allue-Guardia A., Olmo-Fontanez A., Gautam S., Garcia-Vilanova A., Ye C., et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng J., Wong L.R., Li K., Verma A.K., Ortiz M.E., Wohlford-Lenane C., Leidinger M.R., Knudson C.M., Meyerholz D.K., McCray P.B., Jr., et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2021;589:603–607. doi: 10.1038/s41586-020-2943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hassan A.O., Case J.B., Winkler E.S., Thackray L.B., Kafai N.M., Bailey A.L., McCune B.T., Fox J.M., Chen R.E., Alsoussi W.B., et al. A SARS-CoV-2 infection model in mice demonstrates protection by neutralizing antibodies. Cell. 2020;182:744–753. doi: 10.1016/j.cell.2020.06.011. e744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Israelow B., Song E., Mao T., Lu P., Meir A., Liu F., Alfajaro M.M., Wei J., Dong H., Homer R.J., et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J Exp Med. 2020;217 doi: 10.1084/jem.20201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leist S.R., Dinnon K.H., 3rd, Schafer A., Tse L.V., Okuda K., Hou Y.J., West A., Edwards C.E., Sanders W., Fritch E.J., Mouse-Adapted A., et al. SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell. 2020;183:1070–1085. doi: 10.1016/j.cell.2020.09.050. e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dinnon K.H., 3rd, Leist S.R., Schafer A., Edwards C.E., Martinez D.R., Montgomery S.A., West A., Yount B.L., Jr., Hou Y.J., Adams L.E., et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586:560–566. doi: 10.1038/s41586-020-2708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ciancanelli M.J., Huang S.X., Luthra P., Garner H., Itan Y., Volpi S., Lafaille F.G., Trouillet C., Schmolke M., Albrecht R.A., et al. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–453. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hernandez N., Melki I., Jing H., Habib T., Huang S.S.Y., Danielson J., Kula T., Drutman S., Belkaya S., Rattina V., et al. Life-threatening influenza pneumonitis in a child with inherited IRF9 deficiency. J Exp Med. 2018;215:2567–2585. doi: 10.1084/jem.20180628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lim H.K., Huang S.X.L., Chen J., Kerner G., Gilliaux O., Bastard P., Dobbs K., Hernandez N., Goudin N., Hasek M.L., et al. Severe influenza pneumonitis in children with inherited TLR3 deficiency. J Exp Med. 2019;216:2038–2056. doi: 10.1084/jem.20181621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97••.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report that inborn errors of type I IFN immunity can underlie life-threatening COVID-19 pneumonia. Surprisingly, four unrelated adults had complete IFNAR1 or IRF7 deficiency.
- 98.Zhang S.Y., Zhang Q., Casanova J.L., Su H.C. Severe COVID-19 in the young and healthy: monogenic inborn errors of immunity? Nat Rev Immunol. 2020;20:455–456. doi: 10.1038/s41577-020-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99••.Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., Zhang P., Meertens L., Bolze A., Materna M., et al. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report that X-linked recessive TLR7 deficiency impairs production of type I IFNs by pDCs and underlies at least 1% of cases of life-threatening COVID-19 pneumonia in men.
- 100.Zhang Q., Cobat A., Bastard P., Notarangelo L.D., Su H.C., Abel L., Casanova J.L. Association of rare predicted loss-of-function variants of influenza-related type I IFN genes with critical COVID-19 pneumonia. J Clin Invest. 2021;131 doi: 10.1172/JCI152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van der Made C.I., Simons A., Schuurs-Hoeijmakers J., van den Heuvel G., Mantere T., Kersten S., van Deuren R.C., Steehouwer M., van Reijmersdal S.V., Jaeger M., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fallerini C., Daga S., Mantovani S., Benetti E., Picchiotti N., Francisci D., Paciosi F., Schiaroli E., Baldassarri M., Fava F., et al. Association of toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. eLife. 2021;10 doi: 10.7554/eLife.67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pessoa N.L., Bentes A.A., de Carvalho A.L., de Souza Silva T.B., Alves P.A., de Sousa Reis E.V., Rodrigues T.A., Kroon E.G., Campos M.A. Case report: hepatitis in a child infected with SARS-CoV-2 presenting toll-like receptor 7 Gln11Leu single nucleotide polymorphism. Virol J. 2021;18 doi: 10.1186/s12985-021-01656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang K., Puel A., Zhang S., Eidenschenk C., Ku C.L., Casrouge A., Picard C., von Bernuth H., Senechal B., Plancoulaine S., et al. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barreiro L.B., Ben-Ali M., Quach H., Laval G., Patin E., Pickrell J.K., Bouchier C., Tichit M., Neyrolles O., Gicquel B., et al. Evolutionary dynamics of human toll-like receptors and their different contributions to host defense. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu C., Martins A.J., Lau W.W., Rachmaninoff N., Chen J., Imberti L., Mostaghimi D., Fink D.L., Burbelo P.D., Dobbs K., et al. Time-resolved systems immunology reveals a late juncture linked to fatal COVID-19. Cell. 2021;184:1836–1857. doi: 10.1016/j.cell.2021.02.018. e1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Maglorius Renkilaraj M.R.L., Zhang P., Meertens L., Bolze A., Materna M., et al. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vallbracht A., Treuner J., Flehmig B., Joester K.E., Niethammer D. Interferon-neutralizing antibodies in a patient treated with human fibroblast interferon. Nature. 1981;289:496–497. doi: 10.1038/289496a0. [DOI] [PubMed] [Google Scholar]
- 109.Panem S., Check I.J., Henriksen D., Vilcek J. Antibodies to alpha-interferon in a patient with systemic lupus erythematosus. J Immunol. 1982;129:1–3. [PubMed] [Google Scholar]
- 110••.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Beziat V., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report that auto-Abs neutralizing high concentrations of type I IFNs underlie at least 10% of cases of life-threatening COVID-19 pneumonia.
- 111••.Bastard P., Orlova E., Sozaeva L., Lévy R., James A., Schmitt M.M., Ochoa S., Kareva M., Rodina Y., Gervais A., et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J Exp Med. 2021;218 doi: 10.1084/jem.20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of an international cohort of APS-1 patients, harboring auto-Abs to type I IFNs since early childhood, most of whom developed life-threatening COVID-19 pneumonia.
- 112•.Meisel C., Akbil B., Meyer T., Lankes E., Corman V.M., Staudacher O., Unterwalder N., Kolsch U., Drosten C., Mall M.A., et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J Clin Invest. 2021;131 doi: 10.1172/JCI150867. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of four German APS-1 patients, who did not suffer from critical COVID-19 pneumonia.
- 113.Koning R., Bastard P., Casanova J.L., Brouwer M.C., van de Beek D., with the Amsterdam UMCC-BI Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 2021:704–706. doi: 10.1007/s00134-021-06392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Troya J., Bastard P., Planas-Serra L., Ryan P., Ruiz M., de Carranza M., Torres J., Martinez A., Abel L., Casanova J.L., et al. Neutralizing autoantibodies to type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in Madrid, Spain. J Clin Immunol. 2021:914–922. doi: 10.1007/s10875-021-01036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vazquez S.E., Bastard P., Kelly K., Gervais A., Norris P.J., Dumont L.J., Casanova J.L., Anderson M.S., DeRisi J.L. Neutralizing autoantibodies to type I interferons in COVID-19 convalescent donor plasma. J Clin Immunol. 2021:1169–1171. doi: 10.1007/s10875-021-01060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goncalves D., Mezidi M., Bastard P., Perret M., Saker K., Fabien N., Pescarmona R., Lombard R., Walzer T., Casanova J.-L., et al. Antibodies against type-I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin Transl Immunol. 2021 doi: 10.1002/cti2.1327. e collection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117•.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]; Report a large auto-Ab screening that identified various targets in patients with severe COVID-19.
- 118•.van der Wijst M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., Lee D.S., Greenland J.R., Sun Y., Perez R., et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci Transl Med. 2021 doi: 10.1126/scitranslmed.abh2624. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report that auto-Abs to type I IFNs impair type I IFN signaling in leukocytes at the single cell level in vivo.
- 119.Acosta-Ampudia Y., Monsalve D.M., Rojas M., Rodriguez Y., Gallo J.E., Salazar-Uribe J.C., Santander M.J., Cala M.P., Zapata W., Zapata M.I., et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J Autoimmun. 2021;118 doi: 10.1016/j.jaut.2021.102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Solanich X., Rigo-Bonnin R., Gumucio V.D., Bastard P., Rosain J., Philippot Q., Perez-Fernandez X.L., Fuset-Cabanes M.P., Gordillo-Benitez M.A., Suarez-Cuartin G., et al. Pre-existing autoantibodies neutralizing high concentrations of type I interferons in almost 10% of COVID-19 patients admitted to intensive care in Barcelona. J Clin Immunol. 2021:1733–1744. doi: 10.1007/s10875-021-01136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chauvineau-Grenier A., Bastard P., Servajean A., Gervais A., Rosain J., Jouanguy E., Cobat A., Casanova J.L., Rossi B. Autoantibodies neutralizing type I interferons in 20% of COVID-19 deaths in a French hospital. J Clin Immunol. 2022:1–12. doi: 10.1007/s10875-021-01203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang S.E., Feng A., Meng W., Apostolidis S.A., Mack E., Artandi M., Barman L., Bennett K., Chakraborty S., Chang I., et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12 doi: 10.1038/s41467-021-25509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sposito B., Broggi A., Pandolfi L., Crotta S., Clementi N., Ferrarese R., Sisti S., Criscuolo E., Spreafico R., Long J.M., et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell. 2021;184:4953–4968. doi: 10.1016/j.cell.2021.08.016. e4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ziegler C.G.K., Miao V.N., Owings A.H., Navia A.W., Tang Y., Bromley J.D., Lotfy P., Sloan M., Laird H., Williams H.B., et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell. 2021;184:4713–4733. doi: 10.1016/j.cell.2021.07.023. e4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Prost N., Bastard P., Arrestier R., Fourati S., Mahevas M., Burrel S., Dorgham K., Gorochov G., Tandjaoui-Lambiotte Y., Azzaoui I., et al. Plasma exchange to rescue patients with autoantibodies against type I interferons and life-threatening COVID-19 pneumonia. J Clin Immunol. 2021:536–544. doi: 10.1007/s10875-021-00994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hatton C.F., Botting R.A., Duenas M.E., Haq I.J., Verdon B., Thompson B.J., Spegarova J.S., Gothe F., Stephenson E., Gardner A.I., et al. Delayed induction of type I and III interferons mediates nasal epithelial cell permissiveness to SARS-CoV-2. Nat Commun. 2021;12 doi: 10.1038/s41467-021-27318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127•.Carvalho T., Krammer F., Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. 2021;21:245–256. doi: 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of the immunological milestones since the onset of the COVID-19 pandemic.
- 128.Stertz S., Hale B.G. Interferon system deficiencies exacerbating severe pandemic virus infections. Trends Microbiol. 2021;29:973–982. doi: 10.1016/j.tim.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Casanova J.L., Abel L. The human genetic determinism of life-threatening infectious diseases: genetic heterogeneity and physiological homogeneity? Hum Genet. 2020;139:681–694. doi: 10.1007/s00439-020-02184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Casanova J.L., Abel L. Lethal infectious diseases as inborn errors of immunity: toward a synthesis of the germ and genetic theories. Annu Rev Pathol. 2021;16:23–50. doi: 10.1146/annurev-pathol-031920-101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galani I.E., Andreakos E. Impaired innate antiviral defenses in COVID-19: causes, consequences and therapeutic opportunities. Semin Immunol. 2021;55 doi: 10.1016/j.smim.2021.101522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Q., Bastard P., Bolze A., Jouanguy E., Zhang S.Y., Effort C.H.G., Cobat A., Notarangelo L.D., Su H.C., Abel L., et al. Life-threatening COVID-19: defective interferons unleash excessive inflammation. Medicine (NY) 2020;1:14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Levy R., Zhang P., Bastard P., Dorgham K., Melki I., Hadchouel A., Hartoularos G.C., Neven B., Castelle M., Roy C., et al. Monoclonal antibody-mediated neutralization of SARS-CoV-2 in an IRF9-deficient child. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2114390118. [DOI] [PMC free article] [PubMed] [Google Scholar]