Summary
Chitosan (CS) and graphene oxide (GO) nanocomposites have received wide attention in biomedical fields due to the synergistic effect between CS which has excellent biological characteristics and GO which owns great physicochemical, mechanical, and optical properties. Nanocomposites based on CS and GO can be fabricated into a variety of forms, such as nanoparticles, hydrogels, scaffolds, films, and nanofibers. Thanks to the ease of functionalization, the performance of these nanocomposites in different forms can be further improved by introducing other functional polymers, nanoparticles, or growth factors. With this background, the current review summarizes the latest developments of CS–GO nanocomposites in different forms and compositions in biomedical applications including drug and biomacromolecules delivery, wound healing, bone tissue engineering, and biosensors. Future improving directions and challenges for clinical practice are proposed as well.
Subject areas: Materials science, Biomaterials, Composite materials
Graphical abstract

Materials science; Biomaterials; Composite materials
Introduction
Chitosan (CS), originated from the deacetylation of chitin, is the only natural cationic polysaccharide due to the protonation of its lateral amino group under acid condition (Wang et al., 2013, 2020; Yan et al., 2017). CS is readily available because chitin widely exists in the exoskeleton of crustaceans and cephalopods (Peers et al., 2020; Rinaudo, 2006). With excellent physicochemical characteristics and biological properties like stimulus responsiveness, biocompatibility, biodegradability, antibacterial activity, readily film and hydrogel-forming capability, oxygen impermeability (Ahmed et al., 2020), CS has extensive use in drug delivery (Gooneh-Farahani et al., 2019), tissue engineering and regenerative medicine (Croisier and Jérôme, 2013), wound healing (Tylman et al., 2018), food industries (Paiva et al., 2020; Tien et al., 2021) and water filtration and purification (Croitoru et al., 2020; Jin et al., 2021), etc. Importantly, thanks to abundant amino and hydroxyl active groups on CS main chain, it is easy to modify CS through a variety of interactions such as covalent bonding, hydrogen bonding, and electrostatic interactions, thus conquering its defects of poor mechanical property and solubility (Croisier and Jérôme, 2013). The modification methods can be classified as chemical modification (Jayakumar et al., 2005), adding plasticizers (Thakhiew et al., 2015) or a new polymer network, and introducing nanoparticles including carbon-based nanoparticles, polymeric nanoparticles, and inorganic nanoparticles (Chen et al., 2020b; Liu et al., 2021b; Wahid et al., 2020). Among them, the combination with graphene oxide (GO) has attracted great attention.
GO, a type of highly oxidized and chemically modified graphene nanosheet material (Zhao et al., 2015), inherits some of the commendable physical, chemical, mechanical and conductive properties of graphene. Furthermore, benefiting from a huge number of oxygen-containing polar groups on its surface, for example, hydroxyl, epoxy, and carboxyl, GO has outstanding dispersibility in water and can interact physically or chemically with many polymers. So it is a great matrix or filler to obtain useful materials which have better mechanical and other functional properties (Han and Yan, 2014; Hu et al., 2014; Jing et al., 2017; Wahid et al., 2020). In addition, GO exhibits remarkable biological properties such as antibacterial activity (Akhavan and Ghaderi, 2010; Liu et al., 2011) and anticancer activity resulting from its photothermal performance (Gu et al., 2019; Liu et al., 2018a). For this reason, apart from being extensively exploited in a flexible transistor, solar cells, and sensors, GO also has great potential in diverse biomedical fields including drug delivery, tissue engineering, biosensing, and bioimaging (Georgakilas et al., 2016; Maiti et al., 2019; Zhao et al., 2017a). However, plenty of studies have indicated that GO did not exhibit obvious toxicity only at low doses (Chang et al., 2011; Liao et al., 2011; Wang et al., 2011), which undoubtedly limits its applications in humans (Bianco, 2013). Fortunately, the toxicity of GO can be eliminated by the addition of biocompatible polymer CS (Gurunathan and Kim, 2016; Liao et al., 2011).
Thanks to abundant functional groups that can interact with each other, CS and GO can readily form composites through covalent bonding, hydrogen bonding, or electrostatic interactions. These composites exhibit performance which overcomes limitations observed by the individual components (i.e., poor mechanical performance and low biocompatibility, respectively). Recent studies have shown that the synergistic effect between CS and GO generates hybrids with not only improved thermal stability, mechanical and optical properties (Chen et al., 2019; Cobos et al., 2017; Kumar and Koh, 2014; Zhang et al., 2018a) but also excellent in vitro and in vivo biocompatibility (López Tenorio et al., 2019; Valencia et al., 2021; Zhang et al., 2021), angiogenic and cell growth effect (Zhang et al., 2018b, 2021), antimicrobial properties (Grande et al., 2017; Khalil et al., 2020), electrical conductivity (Jiang et al., 2019) and adsorption capacities (Wu et al., 2020a; Yu et al., 2017b), etc. Therefore, nanocomposites containing CS and GO have been widely created for developing improved thermomechanical and antimicrobial properties of food packaging film (Ahmed et al., 2017; Grande et al., 2017), conductive three-dimensional scaffolds, coating, and nanofiber for tissue engineering (Arnaldi et al., 2020; Cao et al., 2017; Jiang et al., 2019; Karimi et al., 2019), nanoparticle and hydrogel with great loading capacity and releasing profile for drug delivery (Wang et al., 2018a; Zhao et al., 2017b), antibacterial film and hydrogel patch for wound healing (Najafabadi et al., 2020; Yang et al., 2019c), nanofiber and film with high load carrying capacity and electrochemical properties for biosensor (Fazial and Tan, 2021; Li et al., 2019), sponge and membrane with strong adsorption power for wastewater treatment (Bandara et al., 2019; Qi et al., 2018), and so on. In view of the broad uses of CS–GO nanocomposites in the past few years and the bright future, it is meaningful to summarize the latest advances convenient for further study and applications.
Although judging from the preliminary literature, CS–GO nanocomposites are superb absorbents for dyes and heavy metals which are the two most common water pollutants in daily life (Liu et al., 2021b), and excellent antimicrobial materials in the food industry, their strengths are still more reflected in biomedical science. Therefore, the focus of this review is to present the main direction of biomedical applications of CS–GO nanocomposites with specially made functionalities in recent years (Table 1). These nanocomposite materials are in a variety of forms including, but not limited to hydrogels, scaffolds and sponges, films, and nanoparticles. Meanwhile, this review also analyzes the key point to improve the properties and the using effects of fabricated CS–GO nanocomposite materials. Current limitations and challenges for clinical practice are proposed as well. There is no elaborate discussion about the effect of structure on the properties of nanocomposites due to the recent researches mainly focusing on composition rather than structure controlling. Nevertheless, we firmly believe that this review will provide a very clear understanding of the uses of these important CS–GO nanocomposites in biomedical science and enlighten the researchers in related areas.
Table 1.
Main biomedical applications of the CS–GO nanocomposites in different forms
| Main biomedical application(s) | Biomaterial type | Main advantage(s) | Main disadvantage(s) | Reference(s) |
|---|---|---|---|---|
| Drug and biomacromolecules delivery | Nanoparticle | High loading capacity | Low stability | Abbasian et al. (2018) and Zhao et al. (2018) |
| Hydrogel | Minimal invasiveness and multi-responsive release | Poor mechanical strength | Chen (2018), Lu et al. (2020) and Zhao et al. (2017b) | |
| Wound healing | Nanofibrous membrane | High specific area and porosity and gases permeability | Complex preparation process | Yang et al. (2018, 2019a) |
| Film | Impermeable to bacteria | Wound exudates accumulation | Chen et al. (2020a) and Xue et al. (2019) | |
| Hydrogel | High exudates absorption capacity | Poor mechanical stability and bacterial barrier | Wang et al. (2021) and Zhang et al. (2020) | |
| Bone tissue engineering | Scaffold | More like bone extracellular matrix and high cell attachment | Unchangeable shape and need implantation surgery | Vlasceanu et al. (2020) and Xue et al. (2018) |
| Hydrogel | Minimal invasiveness and can form any desired shapes | Poor mechanical strength | Liu et al. (2017a) and Yu et al. (2017a) | |
| Biosensor | Film, nanofiber | Low detection limit, excellent sensitivity, and good stability | Limited electron transfer | Fazial and Tan (2021) and Mehdizadeh et al. (2020) |
Drug and biomacromolecules delivery
GO has been widely employed in drug and biomacromolecules (e.g., proteins, peptides, or nucleic acids) delivery. Owing to its large specific surface area and π-conjugated structure, and significant surface activity deriving from abundant oxygen-containing functional groups, GO nanocarriers can load a mass of drugs (Wang et al., 2018a). CS, as a natural cationic polyelectrolyte, will be positively charged when encountering an acidic extracellular medium of solid tumors, which is very helpful for cellular uptake because nanocarriers with a positive charge are more attracted to negatively charged tumor cell membranes (Qiao et al., 2020; Yang et al., 2019b; Zhao et al., 2018). As a consequence, nanocomposites based on CS and GO are often used to efficiently immobilize or load antitumor drugs and biomacromolecules for controlled delivery and long-term sustained release. The current CS–GO delivery systems are mostly in the form of nanoparticles, followed by hydrogels.
CS–GO nanoparticles
In CS–GO nanoparticle delivery systems, GO often acts as the core which is the main force in absorbing the drugs or genes via π–π stacking interactions, hydrogen bonds, and van der Waals interactions. And CS basically plays a role as a building material in enhancing the biocompatibility, stability, aqueous dispersion and solubility, and pH-responsiveness of the carrier systems through modifying GO nanosheets. In most cases, CS is grafted to GO by forming covalent bonds (Emadi et al., 2017; Figueroa et al., 2020; Hasanzade and Raissi, 2019; Mahajan et al., 2019; Wang et al., 2018b), while sometimes electrostatic interactions are exploited as well (Zhang et al., 2017). Compared to GO, the CS-modified GO nanoparticles possess not only better biocompatibility and higher stability but also increased loading capacity and cellular uptake efficiency. In the meantime, sustained release of chemotherapeutic drugs or biomolecules can be realized (Emadi et al., 2017; Figueroa et al., 2020; Hasanzade and Raissi, 2019; Mahajan et al., 2019; Wang et al., 2018b; Zhang et al., 2017).
However, the effect of CS on the dispersity of GO is finite and the pure CS–GO nanoparticle delivery system lacks targeting function. These limitations have reduced the possibility of CS–GO nanoparticles to achieve clinical applications, thus starving for further improvements. At present, there are three main modification methods on CS–GO nanocomposites including improving water solubility of CS, adding targeting ligands, and introducing magnetic nanoparticles.
Improving water solubility of CS
The reason why CS can only dissolve in weak acid is owing to hydrogen bonds which signify its low solubility at neutral pH. Accordingly, in order to increase its hydrophilic nature, a common choice is to chemically modify CS through its amine and/or hydroxyl functional groups. The introduction of an active polymer segment can not only improve the solubility of CS–GO nanoparticles but also result in greatly enhanced binding with drug molecules, and improve the pH-responsive drug release profile.
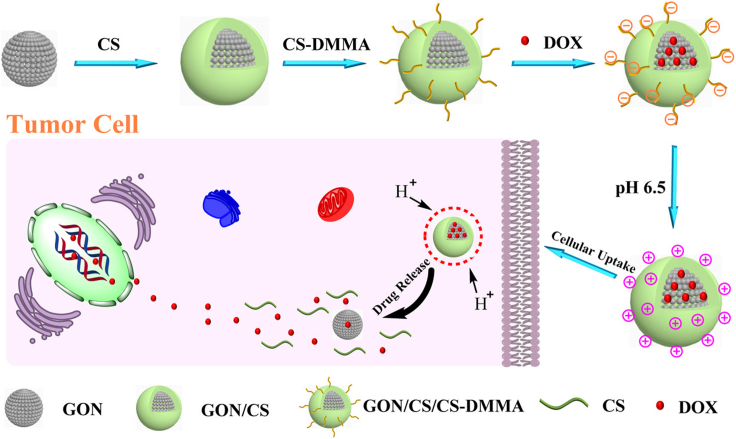
For instance, Abbasian et al. (2018) grafted poly(methacrylic acid) (PMAA) onto CS and then added GO nanosheets to formulate a CS-g-PMAA/GO nanoparticle to load doxorubicin hydrochloride (DOX) as a kind of model anticancer drug. The nanoparticle exhibited high drug loading and encapsulation efficiencies (93.8 and 78.6%, respectively). And the pH-responsive drug release was better realized due to the presence of the PMAA segment as a pH-responsive biopolymer. More interestingly, Zhao et al. (2018) successfully constructed a charge-reversible GO nanoparticle (GON)/CS/dimethylmaleic anhydride-modified CS (CS-DMMA) hybrid capable of responding to the slightly acidic microenvironment of solid tumors to efficiently deliver DOX as shown in Figure 1. The negative surface charge endowed itself with high stability and a prolonged circulation time during blood circulation (pH 7.4). And when at the tumor extracellular microenvironment (pH 6.5), the transition from positive charge to negative enhanced cellular uptake by tumor cells. This charge-reversible ability could be more deeply studied for efficient intracellular DOX delivery.
Figure 1.
Schematic illustration of the fabrication, charge reversal, and DOX release of the GON/CS/CS-DMMA by pH trigger
Reprinted with permission from Zhao et al. (2018) Copyright 2018 American Chemical Society. GON, graphene oxide nanoparticle; CS-DMMA, dimethylmaleic anhydride-modified chitosan; DOX, doxorubicin hydrochloride.
Furthermore, through making γ-polyglutamic acid (γ-PGA) covalently linked with chitosan oligosaccharide (CO) and then loaded into GO, the obtained GO-CO-γ-PGA nanoparticles could even be soluble in physiological solutions besides water (Liu et al., 2019). The nanoparticles were easily transferred into cells and showed low toxicity and excellent antitumor effect due to their good connection with DOX. In vitro experiments also certified the antitumor mechanisms induced by GO-CO-γ-PGA-DOX. But the effects of the delivery systems on animal xenograft models were not further studied, which is the common shortcoming of the other research findings mentioned above.
Adding targeting ligands
Adding targeting ligand into the CS–GO nanocomposite is effective for endowing the drug and biomacromolecule vehicles with the ability of initiative targeting. Some ligands can only target specific tumor cells, while some can target all (Table 2). Therefore, with the help of different ligands, the CS–GO nanoparticle delivery systems will suit many application scenarios in anticancer therapy.
Table 2.
Targeting ligands for inhibition of the expression of receptors and their applicable diseases
| Type of targeting ligand | Type of receptor | Applicable diseases | Reference(s) |
|---|---|---|---|
| DsiRNA | VEGF gene | Colon cancer | Katas et al. (2017) |
| HA | CD44 | Various cancers | Liu et al. (2018b) |
| Galactose | ASGP-R | Hepatic carcinoma | Wang et al. (2018a) |
| FA | F-R | Various cancers | Anirudhan et al. (2020) and Hosseini et al. (2021) |
| NH2-AS1411 | C23 | Various cancers | Liu et al. (2021a) |
DsiRNA, Dicer-substrate small interfering RNA; VEGF, vascular endothelial growth factor; HA, hyaluronic acid; ASGP-R, the asialoglycoprotein receptor; FA, folic acid; F-R, folate receptor.
For example, to combat colon-related diseases, Katas et al. (2017) invented a chitosan–graphene oxide–Dicer-substrate small interfering RNA (CS–GO-DsiRNA-pectin) colonic-targeted drug delivery system via electrostatic interaction. The DsiRNA can target the vascular endothelial growth factor (VEGF) gene in colon cancer cells. And pectin, which will only be degraded by colonic microflora, was used to protect the CS–GO-DsiRNA nanocomposites from unnecessary degradation behavior. This nanocomposite was able to entrap a high amount of DsiRNA (entrapment efficiency of 92.6 ± 3.9%) with strong binding efficiency, selectively interfering with cell growth of colon cancer cell line (Caco-2 cells), and decrease VEGF level dramatically.
More recently, Anirudhan et al. (2020) and Hosseini et al. (2021) both used folic acid (FA) with an excessive affinity for the internal folate receptor to target cancers that are folate receptors. What is noteworthy is that, in the latter research, the combination of CS and GO by chemical interaction led to creating holes, which increased FA loading to 99.97% and release to 94% for 72 h. And after GO was modified to amine-functionalized GO and CS was conjugated with FA and grafted with itaconic acid and acrylic acid monomers, the DOX loading capacity was as high as 95.0% and the drug release rate at pH 5.3 was significantly higher than that under physiological conditions of pH 7.4 (Anirudhan et al., 2020).
Significantly, previously mentioned GO-CO-γ-PGA-DOX nanocomposite was brought in the targeting ability via adding the nucleic acid aptamer NH2-AS1411 (APT) of targeted nucleolin (C23) (Liu et al., 2021a). Cell experiments indicated that as C23 was overexpressed on the surface of HeLa cells but not on the surface of Beas-2B cells, APT-GO-CO-γ-PGA-DOX can target HeLa cells and exhibit increased toxicity to HeLa cells than to Beas-2B cells. The future needs to check out the toxicity and antitumor mechanism in vivo is emphasized.
In the above results, although they all proved the broad prospect of CS–GO nanocarriers in targeting tumor cells, the proof in the animal model to support clinical application was not shown.
The good news is that the safety and efficacy in vivo are under consideration in recent years. In 2018, Liu et al. (2018b) introduced hyaluronic acid (HA), the target ligand for CD44, into the CS–GO nanocomposite to load the anticancer drug SNX-2112. Not only had the nanocomposites displayed enormous drug loading efficiency, pH-triggered and sustained release but also stronger effects on the inhibition and killing of A549 cells, which indicated that HA could provide a targeting function. Above all, the use of GO-CS-HA/SNX-2112 in vivo caused no severe long-term injury. In the same year, CS was modified with galactose to form galactosylated chitosan (GC)/GO/DOX system for hepatic carcinoma therapy (Wang et al., 2018a). Cell uptake experiments and a cell proliferation analysis demonstrated that the nanoparticles had higher cytotoxicity for HepG2 and SMMC-7721 cells than CS/GO/DOX nanoparticles. During in vivo anti-tumor experiments, the GC/GO/DOX nanoparticles exhibited a higher fluorescence intensity in tumor cells and inhibited tumors better. In summary, there are great superiorities of the CS–GO nanoparticles in targeted tumor therapy.
Introducing magnetic nanoparticles
Introducing magnetic nanoparticles (MNPs) is also a superb idea because their superparamagnetic properties can improve the performance of CS–GO nanoparticle delivery systems, offer magnetic guidance thus achieve targeted delivery (Aliabadi et al., 2017; Huang et al., 2017), or enable other functions such as photothermal therapy or bioimaging (Aliabadi et al., 2017; Baktash et al., 2021; Zhang et al., 2019).
As an example, the Fe–GO–CS nanocomposite prepared by Rebekah et al. (2021) exhibited an outstanding protection role against enzymatic cleavage when soaked in trypsin solution for 30 min, 1, 3, and 6 h at 37°C, respectively. Selected protein BSA stayed close to Fe–GO–CS in 6 h. As it also shows excellent protein loading and releasing profile when compared with other nanocarriers reported so far, the Fe-GO-CS composite can be considered as a superior nanocarrier for in vivo protein delivery.
Some researchers added Fe3O4 to ensure the antitumor drugs were delivered to target sites. In 2017, Huang et al. (2017) prepared magnetic GO by chemical co-precipitation of Fe3O4 magnetic nanoparticles on GO nanosheets. As evidenced by the dramatic decrease of the IC50 value, magnetic GO would selectively deliver DOX to U87 cells at the magnetic targeting site. After 2 years, Zhang et al. (2019) confirmed that the Fe3O4 nanoparticle-modified GO nanosheets could be internalized by A549 cells with a magnetically targeted drug delivery profile. Furthermore, the system had an excellent photothermal ablation effect, which means it may be a proper option for targeted anticancer drug delivery and photothermal therapy.
Considering colloidal dispersions of superparamagnetic iron oxide nanoparticles (SPIONs) are known as a negative contrast in magnetic resonance imaging (MRI) (Aliabadi et al., 2017), Aliabadi et al. (2017) and Baktash et al. (2021) both have successfully developed the theranostic nanoplatforms combining bio-imaging with drug delivery by mounting SPIONs on the surface of CS-grafted GO. They also found that when the hybrid MNP/GO was grafted with high molecular weight CS at 6.0 g/dL, the systems would show optimal properties for theranostic applications. Cytotoxicity assays with healthy L929 cell lines revealed high biocompatibility of MNP/GO/CS nano-system.
Although magnetic CS–GO nanoparticles undoubtedly exhibited some advantages, the current biological studies have mainly focused on in vitro assays, which is not enough to affirm that these materials are completely harmless to humans. As a matter of fact, both high concentration and long circulation time are important factors in the toxicity of magnetic nanoparticles which could cause deposition in the liver, spleen, kidney, and other tissues and induce related diseases (Li et al., 2018; Zamay et al., 2020). Therefore, in the future, attention should be paid to strongly verify and improve the in vivo biocompatibility of the magnetic CS–GO nanoparticles.
CS–GO hydrogels
As mentioned earlier, GO and CS-based hydrogels have been reported for controlled drug delivery as well. In most cases, different from only acting as a drug absorbent in CS–GO nanoparticle, GO can also assume the role of the mechanical reinforcing agent which interconnects CS chains to form a tighter hydrogel network. For example, Chen (2018) used only GO rather than toxic organic crosslinkers as the crosslink centers. The ultimate prepared double network hydrogel exhibited the highest compressive strength of 0.792 MPa at the failure strain of 0.836 under optimal conditions. In the meantime, the higher amount of the GO nanosheets, the lower rate of initial burst release. The pH-sensitive GO-based drug delivery system was fabricated through dispersing GO in the CS hydrogel matrix and crosslinking with tripolyphosphate anions by Jafari et al. (2020) As the percentage of GO in hydrogel beads increased, drug release occurred less rapidly in a controlled manner.
CS and GO themselves have certain antimicrobial property, but their effects are not strong enough to kill most bacteria while delivering the drugs. As a result, it makes sense that someone takes advantage of other antibacterial materials for a strengthened resistance effect. In that case, the CS–GO hydrogel delivery system will be superior to other delivery systems because of its multifunctionality.
Rasoulzadehzali and Namazi (Rasoulzadehzali and Namazi, 2018) successfully designed a pH-sensitive nanocomposite hydrogel based on CS and GO-Ag nanohybrid particles to load antitumor drugs DOX. CS/GO-Ag hydrogel had a good resistance effect to both gram-negative and gram-positive bacteria and the effect was stronger as the amounts of GO-Ag nanohybrid particles increased, which determined that Ag endorsed the nanocomposite hydrogel delivery system with effective antimicrobial properties.
Omidi’s team (Omidi et al., 2020) constructed a hydrogel from the cross-linking of aminated-GO, CS, and cellulose nano-whisker for co-delivery of DOX and curcumin. Interestingly, antimicrobial investigations demonstrated that hydrogel components showed weak antibacterial activities while the hydrogel showed strong antimicrobial activity against gram-positive bacteria S. aureus, which indicates a synergic effect of components by the formation of hydrogel.
Moreover, sometimes CS–GO nanocomposite hydrogels can have a more complex drug stimulus release mechanism instead of single pH-responsive, which suggests their applications will not be limited to merely delivery anticancer drugs to tumor sites.
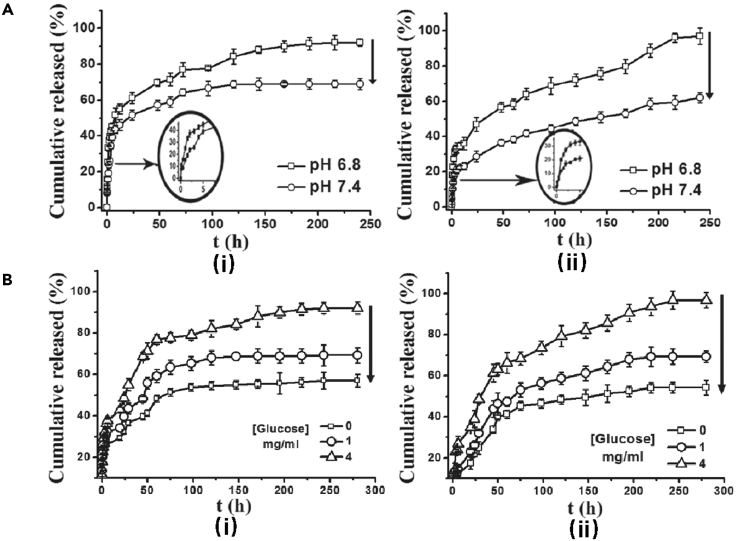
To realize tight control of hyperglycemia for diabetic patients, Zhao et al. (2017b) used GO as a nanocarrier for BSA and introduced GO-BSA into cationic chitosan (HTCC) thus gaining the ability to accommodate enzyme glucose oxidase (GOD), the glucose-sensitive receptor. It is illustrated in Figure 2 that HTCC/2.0wt%GO-BSA hydrogel exhibited not only pH-responsiveness but also a more distinct glucose-sensitivity and a much lower initial burst release compared with HTCC/BSA hydrogels.
Figure 2.
pH- and glucose-responsive release behavior of HTCC/GO-BSA hydrogels
(A) pH-responsive release behavior of HTCC/GO-BSA hydrogels in different pH conditions (pH 6.8 and 7.4) at 37°C (i-HTCC/BSA; ii-HTCC/2.0 wt%GO-BSA) (Zhao et al., 2017b). Copyright 2016 Elsevier B.V.
(B) Glucose-responsive release behavior of HTCC/GO-BSA hydrogels under different glucose concentrations: 0, 1, and 4 mg/mL at 37°C (i-HTCC/BSA; ii-HTCC/2.0 wt%GO-BSA). Reprinted with permission from Zhao et al. (2017b) Copyright 2016 Elsevier B.V.
Through the introduction of targeting ligand, CS–GO nanocomposite hydrogels can get the ability of targeting delivery as well. In the work of Lu et al. (2020), a multifunctional hydrogel drug/gene delivery system for the treatment of glioblastoma multiforme was fabricated by combining the ligand-mediated active targeting and the pH-triggered drug release features of GO. To make the hydrogel injectable, they adopted the thermo-responsive phase-transition characteristic of N-isopropylacrylamide (PNIPAM) which is grafted into CS. The combination of positively charged CS and negatively charged GO led to the controlled drug release and improved mechanical strength. A xenograft tumor model in nude mice demonstrated 40% tumor size compared with the untreated control group after 12 days through the intratumoral injection.
Overall, the CS–GO nanoparticles have shown superiorities in loading capacity while CS–GO hydrogels exhibit minimal invasiveness and multiple responsiveness. Further work is expected to be done in the future to broaden the application of these drug delivery systems to apply them to other related drugs besides DOX. For common clinical practice, more detailed biological studies for potential biotoxicity should be urgently carried out as well.
Wound healing
Bacterial infection in the wound area is a major incentive resulting in delayed wound healing (Jayakumar et al., 2010). Therefore, wound dressings consisting of antibacterial materials and further added antibiotic drugs received attention from a great many researchers. With excellent antibacterial ability, wound dressings can accelerate the wound healing process by eradicating pathogenic bacteria on the injury and keeping a sterile wound healing environment.
CS and GO both are good antibacterial materials thus nanocomposites based on CS and GO are widely used in wound healing. CS–GO nanocomposite wound dressings also have multiple advantages including adequate mechanical strength, anti-inflammation, and tissue adhesive properties. Moreover, they are mainly fabricated in the forms of nanofibrous membrane, film, and hydrogel.
CS–GO nanofibrous membranes
Electrospun nanofibrous membrane has many merits in wound healing including high specific surface area, porosity and permeability, wide ductility of size and shape, tunable mechanical properties, and most vitally, resemblance to extracellular matrix (ECM) facilitating cell adhesion, migration, and proliferation (Mahmoudi et al., 2017; Yang et al., 2018). Limitation factors of CS, include poor flexibility and solubility in most conventional solvents due to the existence of hydrogen bonds. To exert electrospinning technology on producing antibacterial CS–GO nanocomposite fibrous membranes, flexible, ductile, and biocompatible polymers such as polyvinyl alcohol (PVA), polyethylene oxide (PEO), polyglycolic acid (PGA), polycaprolactone (PCL), polyvinylpyrrolidone (PVP) are often blended with CS to make up for its lack of electrospinnability. With GO acting as antibacterial and mechanical reinforcing agent, nanofiber wound dressings have a promising future in wound healing.
Many kinds of CS–GO nanocomposite electrospun membranes have demonstrated the ability to promote wound healing. For instance, a temporary skin graft based on CS/PVP membranes containing GO nanosheets (up to 2%) was synthesized by electrospinning (Mahmoudi et al., 2017; Mahmoudi and Simchi, 2017). The physico-mechanical properties of the skin grafts are close to those of natural skin. In vitro and in vivo experiments showed improved cell viability and accelerated wound healing rate by 92% of fibrous mats in the presence of GO. Surprisingly, no scar was found on the treated skin of rat after 14 to 21 days post-operation. Similarly, Yang et al. (2020) coated GO on shell (CS)-core (PLLA) structured nanofibrous scaffolds for wound healing. The GO coating improved the surface roughness, hydrophilicity of CS/PLLA nanofibrous scaffolds without destroying the structure of nanofibers. The GO-coated CS/PLLA nanofibrous scaffolds greatly enhanced the antimicrobial activities against E. coli and S. aureus and promoted cell proliferation. In vivo experiments confirmed the good potential for wound healing as well.
CS/PVA/GO electrospun nanofibrous membranes also play an essential role in wound healing, which is supported, for instance, by the work presented in Yang et al. (2018, 2019a) and Liu et al. (2020).
The work of Yang et al. (2018) confirmed the good antibacterial activity of CS/PVA/GO membranes, which is an essential property for promoting wound healing. Figure 3A shows the preparation process of CS/PVA/GO composite nanofibrous membranes. Clearly seen from Figure 3B, highly uniform and smooth composite nanofibers were formed without the occurrence of bead defects and GO sheets partially embedded into nanofibers with the shape of spindle and spherical. Contact angle tests suggested that hydrophilicity was improved with increasing GO amount. Most importantly, good antibacterial activity of the prepared nanofibrous membranes was revealed against E. coli and S. aureus (Figure 3C).
Figure 3.
Preparation, morphology, and antibacterial activity of CS/PVA/GO composite nanofibrous membranes for wound healing
(A) Schematic illustration of the preparation of electrospun CS/PVA/GO composite nanofibrous membranes (Yang et al., 2018). Copyright 2017 Elsevier B.V.
(B) SEM image of composite nanofibrous membranes at weight ratio: CS:PVA:GO = 1:9:0.05(Yang et al., 2018). Copyright 2017 Elsevier B.V.
(C) Inhibition zones of composite nanofibrous membranes at weight ratio: CS:PVA:GO = 1:9:0.03. Reprinted with permission from Yang et al. (2018) Copyright 2017 Elsevier B.V.
Besides being used as biomaterials, another common use for CS/PVA/GO membranes is for carrying antibiotic drugs. Due to the high specific surface area and the existence of GO, CS/PVA electrospun membranes can carry a lot of drugs and release them in a controlled and sustained manner. Thus, several groups have been devoted to investigating the addition of antibiotic drugs to CS/PVA/GO electrospun membranes.
Yang et al. (2019a) further enhanced the antibacterial activity of the aforesaid CS/PVA-GO membranes by the addition of antibiotic drugs Ciprofloxacin (Cip) and Ciprofloxacin hydrochloride (CipHcl). GO and drugs were both loaded into nanofibers and partial drugs absorbed in GO nanosheets prevented burst release of drug at the initial stage. Furthermore, the addition of GO increased the distance between nanofibers, promoting the release of the drugs encapsulated into the nanofibers, raising the drug release ratio. Profiting from the load of antibiotic drugs, the membrane displayed significantly enhanced antibacterial activity against E. coli, S. aureus, and B. subtilis. Moreover, the membrane showed excellent cytocompatibility with Melanoma cells.
Liu et al. (2020) added a different antibiotic drug, allicin, to CS/PVA-GO nanofibers. Comparing to CS/PVA-GO composite loaded with Cip and CipHcl, although the drug release behavior and water contact angle test results were similar, antimicrobial activity results have shown a big difference. When facing the same bacteria, the radius of the inhibition zone of CS/PVA-GO nanofibers loaded with Cip and CipHcl was more than three times larger than that of CS/PVA-GO nanofibers loaded with allicin. The difference can be attributed to the amount and antimicrobial property of the drugs.
CS–GO films
Film is another popular material form in wound healing resulting from good oxygen permeability and forceful resistance to bacteria and fluids. CS–GO nanocomposite films are usually produced by wet casting and drying (typically using oven or infrared drying). Many results confirmed their promising future in wound healing.
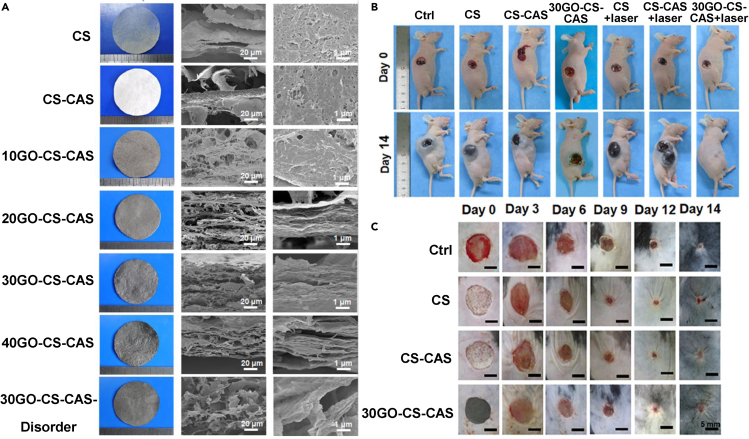
Particularly noteworthy is the research of Xue et al. (2019) Hierarchical and porous graphene oxide–chitosan–calcium silicate (GO-CS-CAS) film biomaterials emulating an orderly porous lamellar micron-scale structure and the “brick-and-mortar”-layered nanostructure were fabricated by combining vacuum filtration-assisted assembly and freeze-drying methods (Figure 4A). The hierarchical microstructure endowed the biomaterials with desired properties of tensile strength (∼10 MPa), breathability, and water absorption. Moreover, the film exhibited good in vitro photothermal antibacterial and antitumor effects. In vivo studies suggested that the GO-CS-CAS biomaterials had satisfactory photothermal antitumor efficacy and the capacity for skin wound healing (Figures 4B and 4C).
Figure 4.
Morphologies, in vivo photothermal antitumor, and wound healing efficiency of GO-CS-CAS film biomaterials
(A) Morphologies of hierarchical and porous GO-CS-CAS film biomaterials (Xue et al., 2019). Copyright 2019 Elsevier Ltd.
(B) In vivo photothermal antitumor efficiency of hierarchical GO-CS-CAS film biomaterials (Xue et al., 2019). Copyright 2019 Elsevier Ltd.
(C) In vivo skin wound healing efficiency of hierarchical GO-CS-CAS film biomaterials. Reprinted with permission from Xue et al. (2019) Copyright 2019 Elsevier Ltd.
On account of the limited effect of CS–GO nanocomposites themselves in promoting wound healing, there are some reports on consolidating the antibacterial activity by functionalizing GO. At the same time, loading some growth factors can also make a difference. The greatly improved effectiveness of the CS–GO nanocomposites as film wound dressings through introducing growth factors or other antibacterial agents can be certified as explained in the following examples.
Among various growth factors, the basic fibroblast growth factor (bFGF) has been reported to play a critical role in the wound healing process (Ayvazyan et al., 2011). Therefore, Liu et al. (2017b) constructed a collagen-CS composite film modified with the GO drug delivery system to carry bFGF. The release of bFGF continued for more than 28 d in vitro. Furthermore in vivo evaluation of wound healing indicated that the film could accelerate the wound healing process compared with the blank group. Polyhexamethylene guanidine (PHMG) is an efficient broad-spectrum antibacterial agent. In 2020, Chen et al. (2020a) incorporated PHMG-modified graphene oxide (mGO) into the CS/PVA matrix thus greatly improving antibacterial activity. The film with 0.5 wt% mGO sheets displayed the best wound healing properties, as manifested by the 50% higher antibacterial rate compared to GO and the wound healing rate of the mouse using this dressing was about 41% faster than the control group and 31% faster than the pure PVA/CS dressing. In turn, Venkataprasanna et al. (2020) incorporated CuO which had high antibacterial, antifungal, and bacteriostatic properties to fabricate CS/PVA/GO/CuO patch. The patch promoted wound healing by increasing cell proliferation, antimicrobial activities, and rapid initiation of inflammatory response. Additionally, the patch exhibited lower level of erythrocyte lysis, adding to its superiority.
Different from all the above film dressings regarding CS as matrix, Najafabadi and coworkers (Najafabadi et al., 2020) created a polyurethane nanocomposite film (PU/CS–GO) synthesized by incorporating CS modified GO nanosheets into PU matrices. The utilization of castor oil as low cost, renewable, and environmentally friendly polyol combined with the good dispersibility of CS–GO nanosheets, enabled the PU/CS–GO nanocomposite to become a powerful antibacterial wound dressing.
CS–GO hydrogels
As a variety of wound dressing, hydrogel dressings own unique superiorities like high exudates absorption capacity and ability to be easily removed from the wound area (Chen et al., 2020a). Therefore, CS–GO nanocomposite hydrogels are also constantly applied to wound healing. Researches of the hydrogels based on CS and GO in wound healing are focused on improving the injectability, water-absorption and water-retention capability, mechanical property, and antibacterial activity.
A low-temperature CS hydrogel was fabricated via a simple mixing method, under the synergistic effect of GO and β-glycerophosphate (GP) by Dai et al. (2017). It can form a gel under skin temperature (below 37°C) after adding 0.3 wt% GO, which allows the material to be injected and form a scaffold on the skin. In addition, the gelation time is shortened to 9 min. The controlled release of drugs from this hydrogel is also improved by the addition of GO. And benefiting from the great water-absorption and water-retention capabilities of amphipathic chitosan (carboxymethyl-hexanoyl chitosan), and great antibacterial activity against a variety of microorganisms (gram-negative and gram-positive bacteria and fungi), the chitosan–graphene oxide–cellulose nanocrystalline composite hydrogel is also expected to be applied in the field of medical coatings (Yang et al., 2019c).
Notably, the CS-CS covalently grafted GO (CS-CGO) hydrogel demonstrated an outstanding mechanical performance (Wang et al., 2021). The tensile stress and strain of CS-CGO composite hydrogel improved by 2.1 and 1.2 times, respectively, as compared to the CS–GO composite hydrogel. Meanwhile, the cytotoxicity tests indicated that the CS-CGO composite hydrogel possessed excellent biocompatibility and promoted the adhesion and proliferation of fibroblasts. In vivo evaluation on full-thickness excision, wounds showed that the CS-CGO composite hydrogel significantly accelerated wound healing, and the wound closure rate reached up to 92.2% after 21 days.
Equally noteworthy are works dealing with enhancing the antibacterial activity of CS–GO systems in wound healing.
Liang et al. (2019) constructed a hybrid CS hydrogel via simple electrostatic interaction embedded with GO modified with zinc oxide quantum dots (ZnO QDs). The antibacterial efficacy reached 98.90% and 99.50% against S. aureus and E. coli bacteria, respectively. The excellent performance stems from the combined effects of hyperthermia produced from the near-infrared irradiation of GO sheets, reactive oxygen species, the release of Zn2+ from ZnO QDs under acidic environment, and the bioactivity of hydrogel itself. The synergy of antibacterial nanoplatforms can be used for wound anti-inflammatory in vivo indicated by the wound healing results.
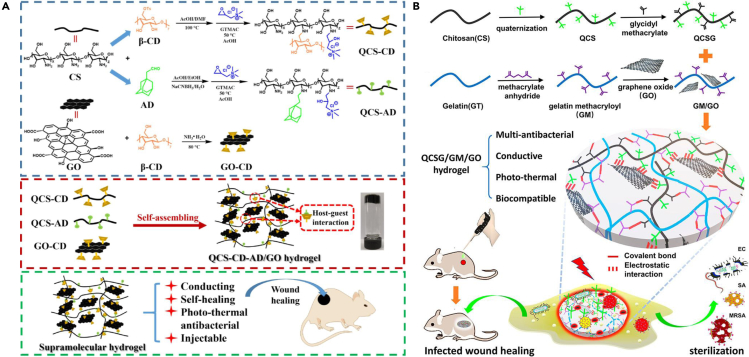
Except for functionalizing GO, some chemical modifications on CS can also be useful. Among these methods, introducing the quaternary ammonium group into CS can not only improve the solubility but also bring better antibacterial properties. Based on this, injectable antimicrobial hydrogels were created both by Zhang et al. (2020) and Liang et al. (2020) Schematic diagram of the synthesis and applications of the two hybrid hydrogels is illustrated in Figure 5. The injectable properties of the two hydrogels are, respectively, derived from the dynamic host-guest interaction between β-cyclodextrin (β-CD) and adamantane (AD) and the time-consuming in situ gelation process. Furthermore, the association of QCS and GO made them biomaterials with good antibacterial effects for infected wound healing.
Figure 5.
Design and fabrication of injectable antimicrobial hydrogels for wound healing
(A) Schematic illustration of QCS-CD-AD/GO supramolecular hydrogels preparation; Preparation scheme of QCS-CD, QCS-AD, and GO-CD, and QCS-CD-AD/GO supramolecular hydrogel; Characteristic of QCS-CD-AD/GO hydrogel and the application in wound healing. Reprinted with permission from Zhang et al. (2020) Copyright 2020 Elsevier B.V.
(B) Schematic diagram of the synthesis of QCSG, GM and the preparation of GM/GO mixture; Scheme of QCSG/GM/GO hydrogel’s network and applications in sterilization and wound healing. Reprinted with permission from Liang et al. (2020) Copyright 2020 American Chemical Society. QCS, quaternized chitosan; β-CD, β-cyclodextrin; AD, adamantane; QCSG, glycidyl methacrylate functionalized quaternized chitosan; EC, embryonal carcinoma cells; SA, Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
In summary, CS–GO nanocomposites in whatever forms all have a strong competitive edge in wound healing. Current studies mainly focus on improving their antibacterial effect, which is generally achieved through chemically modifying GO or CS alone or simultaneously, or introducing some antibiotic drugs. However, when introducing a new substance, it is necessary to clearly know whether it is safe, which undoubtedly increases the difficulty of applying to clinical application. Furthermore, the overuse of antibiotics can also build up bacterial resistance (Li et al., 2021). Considering the physical characteristics also influencing its antibacterial effect, improving the physical properties of the CS–GO nanocomposites (such as topological structure, etc.) is a convenient and advantageous research direction. Besides, it is also important that the degradation rate of the wound dressings match the speed of wound healing.
Bone tissue engineering
There is a big amount of evidence demonstrating that CS-based composites can stimulate regeneration, repair damaged tissues, accelerate patient recovery and reduce the costs (Solìs Moré et al., 2018). With the rapid development of GO, CS–GO nanocomposites are exceedingly promising systems possessing the potential for application in tissue engineering (Olad and Bakht Khosh Hagh, 2019; Ruiz et al., 2019; Wang et al., 2018b). After researching the literature of recent years, it is obvious that CS–GO nanocomposites may eventually be leveraged to the regeneration of diverse tissues such as bone (Kosowska et al., 2018; Prakash et al., 2020), skin (Pourjavadi et al., 2020), heart (Jiang et al., 2019; Saravanan et al., 2018a), nerve (Arnaldi et al., 2020) or other electroactive tissue (Jing et al., 2017). Among them, research in bone tissue engineering occupies a great part, while research in other tissues takes up a relatively small proportion.
The reason why CS–GO nanocomposites are mostly utilized for bone tissue engineering is that the molecular structure of CS is similar to glycosaminoglycan (a major component of bone extracellular matrix) (Liu et al., 2021b) and GO shows the ability to promote osteogenic differentiation when combined with CS (Francolini et al., 2019). The prepared CS–GO nanocomposites are normally 3D matrices (i.e., scaffolds and hydrogels) which promote new bone tissue ingrowth by mimicking native bone microarchitecture.
CS–GO scaffolds
Cryogel is generally freeze-dried hydrogel with supermacroporous structure, which is one kind of scaffold for biomedical applications. A three-dimensional porous scaffold is a support that allows invasion of cells from surrounding tissues and offers a suitable environment where the cells can migrate, proliferate, differentiate and mineralize (Hermenean et al., 2017). Damaged tissue is expected to regenerate from the scaffold. For speeding up the process of bone regeneration, placing a porous scaffold mimicking the bone structure in the injury site is the most common option. Profiting from good biocompatibility and osteo-conductivity, porous CS–GO nanocomposite scaffolds with different contents of GO were prepared for bone regeneration by the freeze-drying method in many studies.
First of all, Pandele et al. (2017) proved that the CS–GO scaffold was capable of mimicking the bone extracellular matrix and could have potential applications for bone tissue engineering on the basis of the physical and chemical features, high porosity, enzymatic degradation, and mineralization. Next, the works of Hermenean et al. (2017) and Dinescu et al. (2019) both exhibited that the CS–GO scaffold with 3 wt% GO revealed the highest levels of osteogenic markers in vitro and in vivo. The excellent biocompatibility of CS–GO scaffolds in vivo was confirmed by López Tenorio et al. (2019) and Valencia et al. (2018). The former results displayed that in all cases of implantation, both CS and CS–GO are degraded by a mixed inflammatory infiltrate, surrounded by a fibrous capsule compatible with a chronic inflammatory response, which is strong evidence of biocompatibility. In the latter, all CS–GO scaffolds behaved as biocompatible in the 30 days after implantation. Last but not least, Shamekhi et al. (2019) verified the higher GO percentage of CS–GO scaffolds, the more surface roughness, and stiffness which stimulated human articular chondrocyte proliferation particularly in the late times of culture period.
CS and GO are readily combined with other materials which can largely enhance the osteogenesis potential of CS–GO nanocomposite. Those materials can divide into other biopolymers complexing with CS, or nanoparticles grafted into GO nanosheets or both.
Complexing with other biopolymers
With the structure of the scaffold more like bone extracellular matrix (bECM), thereby improving cell attachment, spreading, and differentiation, CS is always complexed with other biopolymers which are important components of bECM such as collagen and its partially hydrolyzed product gelatin, hyaluronic acid (HA) and alginate.
In one study, composite scaffolds enriched with CS, gelatin (Gn), and GO were fabricated (Saravanan et al., 2017). The GO/CS/Gn scaffolds were cyto-friendly to rat osteoprogenitor cells, and they promoted the differentiation of mouse mesenchymal stem cells (mMSCs) into osteoblasts. Through regulating the concentration of GO, GO/CS/Gn scaffolds could initiate the healing of rat tibial bone defects as early as 2 weeks post-implantation with the deposition of collagen. In another, gelatins from mammalian strains are not safe because they are vehicles for viral outbreaks, Vlasceanu et al. switched to get gelatins from cold-water fish (Vlasceanu et al., 2020). The fish gelatin/CS/GO scaffold characterizations revealed a constant swelling degree and good resistance to enzyme degradation. In vitro biocompatibility assays demonstrated an overall beneficial interaction between preosteoblasts and the composites. Furthermore, it was confirmed that low and moderate (0.5 and 1 wt%) amounts of GO endowed superior cytocompatibility, viability, and minimum foreign body reaction by in vitro and in vivo assays.
The scaffolds obtained from complexing HA and agarose with CS by Rajan Unnithan et al. and Sivashankari and Prabaharan, respectively, were gratifying as well. They verified the ability of the two composites to be a favorable environment for cell attachment and proliferation (Rajan Unnithan et al., 2017; Sivashankari and Prabaharan, 2020). Interestingly, the GO/CS/HA scaffold loaded drug simvastatin offered a significant influence on osteogenesis and biomineralization, and possessed excellent biocompatibility. Noteworthy, the agrose/CS/GO composite scaffolds could be blood compatible as they presented ˂ 5% of hemolysis against the porcine RBCs.
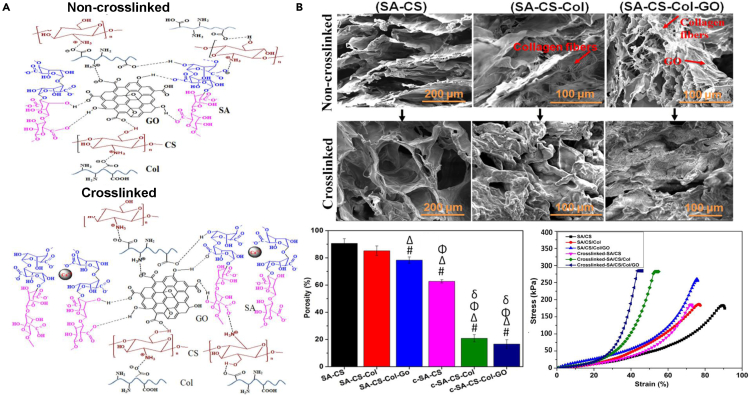
There are also GO scaffolds combining CS with sodium alginate (SA) and collagen (Col) (Kolanthai et al., 2018). Hydrogen bond was the main interaction between SA, CS, Col molecules, and GO particle in SA-CS-Col-GO scaffold while Ca2+ offered another cross-link between alginate chains of cross-linked SA-CS-Col-GO scaffold (c-SA-CS-Col-GO) (Figure 6A). In Figure 6B, SEM images indicated preserved porous structure with elongated Col fibrils of SA-CS-Col-GO scaffolds. Percentage porosity and compressive stress–strain curves proved both GO and Ca2+ incorporated into the SA-CS-Col matrix decreased the porosity and increased mechanical strength. In vitro biochemical studies displayed that mouse osteoblast cells grew and proliferated on the SA-CS-Col-GO scaffold better compared to SA-CS or SA-CS-Col composites. However, chemical cross-linking showed no effect on the osteogenic ability of osteoblasts.
Figure 6.
Design, structure, and performances of SA-CS-Col-GO scaffolds for engineering bone tissues
(A) Schematic representation of possible hydrogen bond formation between SA, CS, Col molecules, and GO particle of non-cross-linked SA-CS-Col-GO scaffold and cross-linked SA-CS-Col-GO scaffold (Kolanthai et al., 2018). Copyright 2018 American Chemical Society.
(B) SEM images showing a porous structure, percentage porosity, and typical compressive stress–strain curves of non-cross-linked SA-CS, SA-CS-Col, and SA-CS-Col-GO and cross-linked c-SA-CS, c-SA-CS-Col, and c-SA-CS-Col-GO scaffolds (Kolanthai et al., 2018). Copyright 2018 American Chemical Society.
Adding nanoparticles
It was mentioned earlier that the incorporation of GO into CS scaffold could increase the surface roughness and stiffness which can simulate the nanoscale extracellular matrix properties of tissue thus promoting osteoblast cell activity and proliferation. However, considering overdose will restrain the biosafety in vivo, the contents of GO need to be controlled under safety line, which limits the effect of the scaffolds, to a certain extent.
To further rough the surface of the CS–GO scaffold, many reports have introduced various inorganic nanomaterials into the scaffold. Among them, the nanohydroxyapatite (nHA) was mostly exploited benefiting from its highest similarity with the mineral component in natural bone (Yu et al., 2018). Moreover, it has good biocompatibility, osteoconductivity, and osteoinductivity. It was confirmed that CS–GO nanocomposite scaffold containing nHA was very popular and effective by subsequent publications.
For example, a nanocomposite scaffold with surface chemistry (oxygen-containing groups) and root-mean-square (Rq) roughness of 74.1 nm modifications were prepared by doping nHA, GO and carboxymethyl chitosan (CMC) by Yu and coworkers (Yu et al., 2018). The biocompatibility and osteoinductivity of the nanocomposite CMC/nHA/GO scaffold were confirmed in vitro and in vivo. The data demonstrated that improved surface chemistry and roughness would enhance osteoinductivity of CMC/nHA/GO, profiting from the β1 integrin interactions with the ECM and activated FAK-ERK signaling pathway to upregulate the expression of osteogenic special proteins.
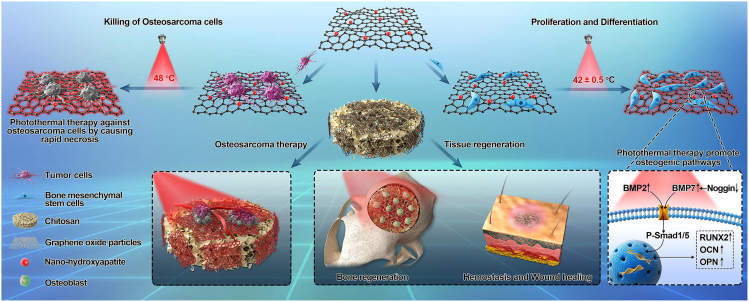
Similarly, Ma et al. (2020) explored the temperature-controlled multifunctionality of the nHA/GO/CS scaffold (Figure 7). Their preliminary results revealed that 30% of nHA in GO offers not only good biocompatibility but also a superior photothermal effect in eliminating residual human osteosarcoma cells (HOS) and promoting osteogenesis differentiation of human bone marrow mesenchymal stem cells (hBMSC). Under NIR irradiation, this nHA/GO/CS scaffold showed an excellent photothermal response in vitro and worked efficiently to kill osteosarcoma cells and facilitate tissue regeneration. Furthermore, this scaffold has a good hemostatic effect.
Figure 7.
Schematic diagram of nHA/GO/CS multifunctional scaffold
Reprinted with permission from Ma et al. (2020) Copyright 2019 Elsevier Ltd. BMP2, bone morphogenetic protein-2; BMP7, bone morphogenetic protein-7; P-Smad1/5, Phospho-Smad1/5; RUNX2, runt-related transcription factor 2; OCN, osteocalcin; OPN, osteopontin.
In another contribution about osteosarcoma-affected bone regeneration, anticancer drug cisplatin was loaded into nHA/GO/CS scaffold (Sumathra et al., 2018). The advantages of loading cisplatin were improved viability on MG63 osteoblast-like cells and enhanced cytotoxicity against cancer cells (A549). Meanwhile, the nHA/GO/CS/cisplatin composite was found to show enhanced proliferative, adhesive, and osteoinductive effects on the alkaline phosphatase (ALP) activity of osteoblast-like cells compared to nHA/GO/CS scaffold.
Considering the ability of strontium-substituted nHA (SrHA) to enhance osteoblast activity and differentiation and inhibit osteoclast production and proliferation, Wu et al. incorporated SrHA/GO nanoparticles into CS and QCS mixed solutions (Wu et al., 2020b). The compressive modulus of the CS/QCS/SrHA/GO scaffold reached 438.5 kPa, which was 4-fold higher than that of the CS/QCS scaffold. The CS/QCS/SrHA/GO scaffold also exhibited highest in vitro mineralization levels and ALP activity. In vivo rat skull repair indicated that the bone density of the CS/QCS/SrHA/GO scaffold in the 12th week was 0.83 mg cm−3, significantly higher than that in the blank group (∼0.35 mg cm−3).
Except for roughing scaffold surface to promote bone tissue regeneration, substitutes with excellent mechanical strength can also have big effects. In 2018, multilayered graphene oxide/chitosan/calcium silicate (GO/CS/CAS) biomaterial was successfully prepared via a bottom-up assembly approach by Xue et al. (2018). This biomaterial emulated the “brick and mortar” layered microstructure and the multilayered helical cylinder macrostructure. Final prepared material had high flexural strength (137.2 MPa), compressive strength (80.2 MPa), toughness (1.46 MJ/m3), and specific strength (124.7 MPa Mg−1 m−3), close to those of cortical bone. As a result of the contributions of bioactive chemical components (CS and GO) and the multilayered helical cylinder structure, the GO/CS/CAS biomaterials showed enhanced in vivo bone-forming ability.
CS–GO hydrogels
Hydrogel is another porous 3D matrix widely employed in bone tissue engineering. Unlike the scaffolds made by the freeze-drying technique, the superiorities of hydrogels are due to their high water content, similarity to the natural ECM, minimal invasive properties, and ability to match irregular defects (Liu et al., 2017a).
The fabricated hydrogels based on natural biomaterial CS and multifunctional nanosheet GO are relatively common in bone tissue repair. At recent, injectability and mechanical performance of CS–GO nanocomposite hydrogels are still two major problems needing to be settled urgently. It is worth noting that numerous scientists have thought of many different modifications on CS–GO nanocomposite hydrogels, which made them promising candidates in bone tissue regeneration.
The works of Saravanan et al. and Amiryaghoubi et al. are noteworthy for the fabrication of injectable thermosensitive hydrogels based on CS and GO for bone regeneration. Saravanan et al. (2018b) ensured that the physicochemical properties (porous architecture, swelling property, protein adsorption ability, degradation rate, and exogenous biomineralization, etc.) of thermosensitive CS/GP hydrogel showed notable betterment after the addition of GO. The CS/GP/GO hydrogel was biocompatible to mMSCs and promoted osteogenic differentiation of mMSCs by upregulation of Runt-related transcription factor 2 (Runx2), Alkaline phosphatase (ALP), Type −1 collagen (COL-1), and osteocalcin (OC) under osteogenic conditions. Differently, Amiryaghoubi et al. (2020) fabricated thermosensitive injectable hydrogel containing poly (N-isopropylacrylamide) (PNIPAAm)-based copolymer/GO composite with a different feed ratio to CS. The hydrogel could also enhance the deposition of calcium and the activity of ALP, upregulating the expression of the Runx2 and OC in the human dental pulp stem cells (hDPSCs) cultivated in both the normal and osteogenic media.
Another way to make injectable hydrogels is to form the gel in situ. As an example, Lee et al. (2020) designed an in situ forming glycol chitosan/oxidized hyaluronic acid injectable hydrogel containing GO. The hydrogel matrix exhibited robust mechanical properties and stability. Meaningfully, both in vitro and in vivo results exhibited that the GO incorporated injectable hydrogel possessed little toxicity and excellent osteogenic activity.
The publication from Yu et al. (2017a) dealing with synthesizing high-strength composite graphene hydrogel is especially significant. They made GO, nHA, and CS self-assemble into a 3D hydrogel with the assistance of crosslinking agent genipin for CS and reducing agent sodium ascorbate for GO simultaneously. The dense and oriented microstructure of the resulted hydrogel endowed it with high mechanical strength, high fixing capacity of HA, and high porosity, which meets the requirement of bone materials.
In general, the major problem of the CS–GO nanocomposite hydrogels in bone tissue engineering is that they cannot have strong mechanical performance and injectable property at the same time, which greatly reduces the potential for their application. As for CS–GO nanocomposite scaffolds, many researchers have convincingly verified their excellent osteogenic inductivity through in vivo experiments. Therefore, in addition to further enhancing osteogenic properties, researchers should focus on conducting more detailed biological studies on potential biotoxicity for clinical applications as well.
Biosensor
The optical and electrical properties of nanocomposites based on CS and GO have been determined and analyzed in detail (Dhayal et al., 2021). With the help of strong interactions between biomacromolecules and CS–GO nanocomposites, the last but equally important potential application domain of nanocomposites based on CS and GO is their use as biosensors.
Biosensors are analytical devices that can detect biological substances and convert their concentration into an electrical signal. In these sensors, to enhance electron transfer rate, biological sensitive materials (including enzymes, cells, nucleic acids, antibodies, antigens, protein, tissue, and so on) are generally absorbed with CS–GO nanocomposites as identification elements. The complex is usually immobilized into an electrode as film or nanofiber.
There are a variety of biosensors used in biomedical situations containing CS–GO nanocomposites. The common advantages are that they all display a low detection limit, a wide detection range, high selectivity, excellent sensitivity, and good stability.
Biosensor systems are effective tools for determining blood glucose, which is confirmed by Li et al. (2019), Kafi et al. (2020), Mehdizadeh et al. (2020), and Zhang (2021).
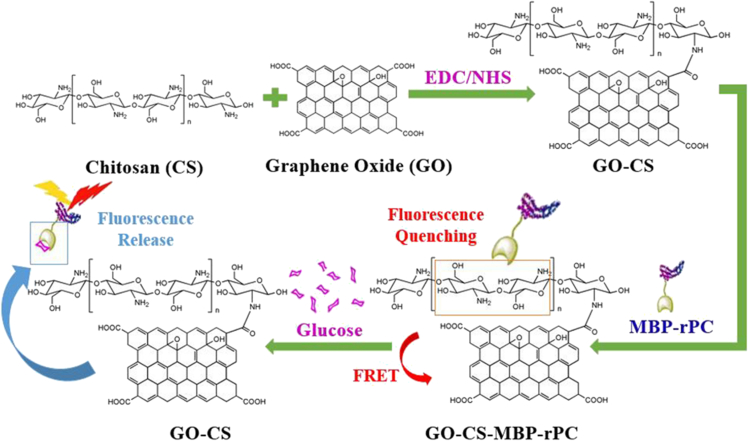
The first mentioned finding comes from Li et al. (2019). Specifically, the CS modified GO was used to detect glucose based on fluorescence resonance energy transfer (FRET) in this work. The schematic diagram of glucose detection with biosensor was presented (Figure 8). The maltose-binding protein (MBP) was labeled by the α-subunit of recombinant phycocyanin (rPC) which emits far-red fluorescence. Glucose was sensitively and selectively detected with this biosensor, with a linear detection range of 0.1–1 mg glucose/mL. The limit of detection for glucose was ∼0.05 mg/mL.
Figure 8.
Schematic diagram of glucose detection with biosensor based on MBP-rPC and GO-CS
Reprinted with permission from Li et al. (2019) Copyright 2018 The Institution of Engineering and Technology. MBP, maltose-binding protein; rPC, recombinant phycocyanin; FRET, fluorescence resonance energy transfer.
In another contribution, an array of CS–GO-based ultra-thin biosensors with gold (Au)-based microgap (60 μm) electrode were fabricated by Kafi et al. (2020). The sensor patch had been evaluated for label-free monitoring by immobilizing the CS–GO surface with human dermal fibroblast (HDF) cells. The device was capable of monitoring HDF cell growth period and cellular malfunctions at hyperglycaemic condition by analyzing and quantifying the cyclic voltammetry redox peak intensities and linear swipe voltammetry signal shifts.
In turn, Mehdizadeh et al. and Zhang fixed glucose oxidase (GOD)/CS/GO into a glassy carbon electrode (GCE) for detecting glucose. Mehdizadeh et al. modified GCE with GOD/CS/GO electrospinning nanofibers (Mehdizadeh et al., 2020). However, Zhang coated GCE with ferrocenecarboxylic acid-CS–GO/GOD nanocomposite precursor solution and dried it at room temperature (Zhang, 2021). Both modifications helped efficiently catalyze the oxidation of glucose and facile direct electron transfer for GOD. The developed electrodes exhibited superior performance for glucose detection, making them promising candidates in detecting the blood glucose level.
Besides, there are numerous achievements with respect to biosensors containing CS–GO hybrid nanocomposites used for clinical diagnosis as well.
For instance, in 2020, Xu fabricated a simple and sensitive DNA biosensor for the detection of E. coli O157:H7 based on GO/CS nanocomposites modified GCE (Xu, 2017). It was proved that electron transfer rate and biocompatibility were markedly enhanced due to the addition of GO and CS on the electrode surface. Under optimum conditions, the ssDNA/GO/CS/GCE biosensor based on electrochemical impedance spectroscopy showed a wider detection range (1.0 × 10−14–1.0 × 10−8 M) and lower detection limit (3.584 × 10−15 M) for target DNA as compared with previously reported biosensors. All results revealed that the GO/CS modified electrode possessed excellent performance for detecting E. coli O157:H7.
In the same year, a specific biosensor based on the detection of glycated hemoglobin (HbA1c) proteolytic digestion product, fructosyl valyl histidine (Fru-ValHis) was presented (Shahbazmohammadi et al., 2020). A recombinant engineered fructosyl peptide oxidase (FPOX) enzyme with improved specificity was immobilized on the electrode surface modified by CS, GO, and gold nanoparticles (AuNPs). Most importantly, the biosensor showed high sensitivity and selectivity for analyzing of Fru-ValHis in real blood samples, thus could be effectively applied for HbA1c determination in medical management.
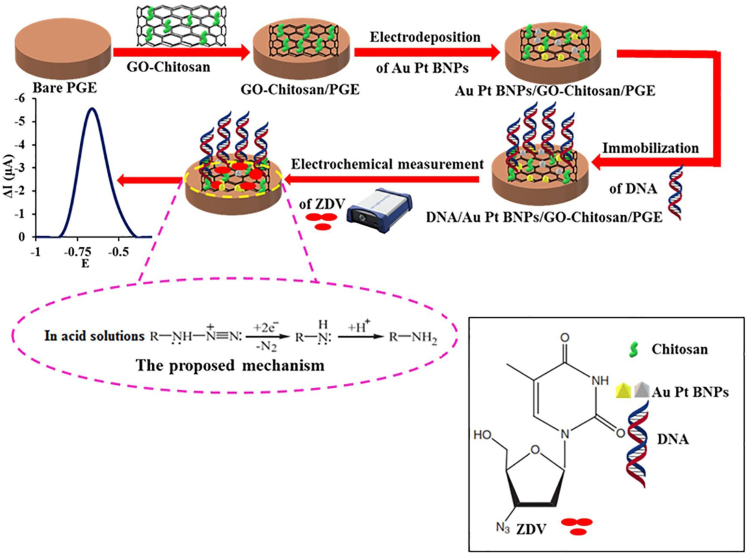
The study of Akbari Hasanjani and Zarei was also interesting. A modified pencil graphite electrode was made using deoxyribonucleic acid/Au-Pt bimetallic nanoparticles/graphene oxide–chitosan (DNA/Au-Pt BNPs/GO-CS/PGE) for the determination of zidovudine (ZDV), a type of drug broadly employed for the treatment of AIDS (Figure 9) (Akbari Hasanjani and Zarei, 2021). The modified electrode demonstrated a good electrocatalytic activity to the ZDV because of the effect of DNA and the synergetic effects of Au-Pt BNPs and GO-CS. In optimum conditions, the proposed sensor displayed a wide linear dynamic range from 0.01 pM to 10.0 nM, a detection limit of 0.003 pM, high selectivity, and well reproducibility. Furthermore, the modified electrode was successfully utilized to determine ZDV in human serum samples.
Figure 9.
A schematic of the construction of the DNA/Au-Pt BNPs/GO-CS/PGE electrochemical biosensor for ZDV detection
Reprinted with permission from Akbari Hasanjani and Zarei (2021) Copyright 2021 Elsevier B.V. PGE, pencil graphite electrode; Au-Pt BNPs, Au-Pt bimetallic nanoparticles; ZDV, zidovudine.
Although CS–GO nanocomposites have good biocompatibility and electrical properties, their ability to enhance electron transfer between electrodes is limited. In view of the excellent electrical conductivity of graphene, directly introducing graphene into CS–GO nanocomposites or partial reduction of GO could be considered in the future.
Conclusion and perspectives
Combining CS and GO can complement each other and thus successfully prepare nanocomposites with excellent mechanical performance, bioactivity, and biocompatibility. Therefore, there are increasing numbers of scientific publications into the use of nanocomposites made of CS and GO in various kind fields, mostly in biomaterials.
Owing to the large specific surface area and lots of amino and oxygen-containing functional groups from CS–GO hybrid systems, they are promising candidates for drug and biomacromolecule (including protein, peptide, nucleic acid, and other) delivery. Through interacting with targeting ligand or magnetic nanoparticles, the fabricated delivery systems can also be targeted while having high drug loading capacity and sustained release rate. Moreover, they are also excellent platforms for use as biosensors characterized by high sensitivity and selectivity, which generates from their ability to enhance electron transfer when immobilized into electrodes. The problem is that CS–GO nanocomposites have limited electron transfer compared to other reported systems.
In addition, CS–GO nanocomposite 3D materials can offer a suitable environment for cell migration, proliferation, differentiation. They all show great osteoinductive activity as well. Therefore, CS–GO nanocomposites play an extremely important role in tissue engineering, especially bone regeneration. Besides, it is believed that CS–GO systems with high antibacterial activity against gram-negative and gram-positive bacteria occupy a key position among wound dressings. However, the current related researches mainly focused on utilizing the functions of the two bioactive component itself, neglecting the same significance of designing unique micro and nano-topographies. Special surface topography can make a big breakthrough in bone-forming ability or antibacterial activity (Rostami et al., 2021; Xue et al., 2018). In wound healing, the hemostasis effect of the dressing is also an important element easily be ignored.
Overall, future attention should be more paid to develop CS–GO nanocomposite materials with topographic features. Simultaneously, integrating several different properties (such as anti-bacteria, hemostasis as well as promoting tissue regeneration) is also promising. It should be noted that enhancing the electron transfer ability of CS–GO nanocomposite is still difficulty to overcome when applied to biosensors. Furthermore, in order to implement the nanocomposites based on CS and GO for common clinical practice, detailed biological studies for potential biotoxicity should be carried out as well. Equally worrying is where the CS–GO nanocomposites finally go after being implanted into the body. Moreover, it is best to make the degradation rate of the prepared biomaterials match the time of tissue regeneration. We steadily believe the CS–GO nanocomposites will possess greater performance and wider biomedical applications.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51873187), Science Fund for Distinguished Young Scholars of Zhejiang Province (No. LR20E030004), National Basic Research Program of China (No. 2018YFC1004803 and 2017YFE0117700), and Fundamental Research Funds for the Central Universities (No. 2020QNA4048).
Author contributions
Z.W. proposed the topic of the review. W.F. investigated the literature and prepared the manuscript. Z.W. revised the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- Abbasian M., Roudi M.-M., Mahmoodzadeh F., Eskandani M., Jaymand M. Chitosan-grafted-poly(methacrylic acid)/graphene oxide nanocomposite as a pH-responsive de novo cancer chemotherapy nanosystem. Int. J. Biol. Macromol. 2018;118:1871–1879. doi: 10.1016/j.ijbiomac.2018.07.036. [DOI] [PubMed] [Google Scholar]
- Ahmed J., Mulla M., Arfat Y.A., Thai T L.A. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocolloids. 2017;71:141–148. doi: 10.1016/j.foodhyd.2017.05.013. [DOI] [Google Scholar]
- Ahmed J., Mulla M., Maniruzzaman M. Rheological and dielectric behavior of 3D-printable chitosan/graphene oxide hydrogels. ACS Biomater. Sci. Eng. 2020;6:88–99. doi: 10.1021/acsbiomaterials.9b00201. [DOI] [PubMed] [Google Scholar]
- Akbari Hasanjani H.R., Zarei K. DNA/Au-Pt bimetallic nanoparticles/graphene oxide-chitosan composites modified pencil graphite electrode used as an electrochemical biosensor for sub-picomolar detection of anti-HIV drug zidovudine. Microchem. J. 2021;164 doi: 10.1016/j.microc.2021.106005. [DOI] [Google Scholar]
- Akhavan O., Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4:5731–5736. doi: 10.1021/nn101390x. [DOI] [PubMed] [Google Scholar]
- Aliabadi M., Shagholani H., Yunessnia Lehi A. Synthesis of a novel biocompatible nanocomposite of graphene oxide and magnetic nanoparticles for drug delivery. Int. J. Biol. Macromol. 2017;98:287–291. doi: 10.1016/j.ijbiomac.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Amiryaghoubi N., Noroozi Pesyan N., Fathi M., Omidi Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020;162:1338–1357. doi: 10.1016/j.ijbiomac.2020.06.138. [DOI] [PubMed] [Google Scholar]
- Anirudhan T.S., Chithra Sekhar V., Athira V.S. Graphene oxide based functionalized chitosan polyelectrolyte nanocomposite for targeted and pH responsive drug delivery. Int. J. Biol. Macromol. 2020;150:468–479. doi: 10.1016/j.ijbiomac.2020.02.053. [DOI] [PubMed] [Google Scholar]
- Arnaldi P., Carosio F., Di Lisa D., Muzzi L., Monticelli O., Pastorino L. Assembly of chitosan-graphite oxide nanoplatelets core shell microparticles for advanced 3D scaffolds supporting neuronal networks growth. Colloids Surf. B Biointerfaces. 2020;196:111295. doi: 10.1016/j.colsurfb.2020.111295. [DOI] [PubMed] [Google Scholar]
- Ayvazyan A., Morimoto N., Kanda N., Takemoto S., Kawai K., Sakamoto Y., Taira T., Suzuki S. Collagen-gelatin scaffold impregnated with bFGF accelerates palatal wound healing of palatal mucosa in dogs. J. Surg. Res. 2011;171:e247–e257. doi: 10.1016/j.jss.2011.06.059. [DOI] [PubMed] [Google Scholar]
- Baktash M.S., Zarrabi A., Avazverdi E., Reis N.M. Development and optimization of a new hybrid chitosan-grafted graphene oxide/magnetic nanoparticle system for theranostic applications. J. Mol. Liquids. 2021;322:114515. doi: 10.1016/j.molliq.2020.114515. [DOI] [Google Scholar]
- Bandara P.C., Nadres E.T., Rodrigues D.F. Use of response surface methodology to develop and optimize the composition of a chitosan–polyethyleneimine–graphene oxide nanocomposite membrane coating to more effectively remove Cr(VI) and Cu(II) from water. ACS Appl. Mater. Interfaces. 2019;11:17784–17795. doi: 10.1021/acsami.9b03601. [DOI] [PubMed] [Google Scholar]
- Bianco A. Graphene: safe or toxic? The two faces of the medal. Angew. Chem. Int. Ed. 2013;52:4986–4997. doi: 10.1002/anie.201209099. [DOI] [PubMed] [Google Scholar]
- Cao L., Zhang F., Wang Q., Wu X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C. 2017;79:697–701. doi: 10.1016/j.msec.2017.05.056. [DOI] [PubMed] [Google Scholar]
- Chang Y., Yang S.T., Liu J.H., Dong E., Wang Y., Cao A., Liu Y., Wang H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011;200:201–210. doi: 10.1016/j.toxlet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Chen L., Jiang H., Li Y., Zimba B.L., Yu X., Chen C., Xiong G., Wu Q. Influences on mechanical properties of chitosan nanofibrous membranes induced by incorporating graphene oxide nanosheets. Mater. Res. Express. 2019;6:075404. doi: 10.1088/2053-1591/ab1555. [DOI] [Google Scholar]
- Chen S., Wang H., Jian Z., Fei G., Qian W., Luo G., Wang Z., Xia H. Novel poly(vinyl alcohol)/chitosan/modified graphene oxide biocomposite for wound dressing application. Macromol. Biosci. 2020;20:1900385. doi: 10.1002/mabi.201900385. [DOI] [PubMed] [Google Scholar]
- Chen Y. A novel dual responsive nanocomposite double network hydrogel with good mechanical property. IOP Conf. Ser. Earth Environ. Sci. 2018;186:012042. [Google Scholar]
- Chen Y., Du M., Yu J., Rao L., Chen X., Chen Z. Nanobiohybrids: a synergistic integration of bacteria and nanomaterials in cancer therapy. BIO Integration. 2020;1:25–36. doi: 10.15212/bioi-2020-0008. [DOI] [Google Scholar]
- Cobos M., González B., Fernández M.J., Fernández M.D. Chitosan-graphene oxide nanocomposites: effect of graphene oxide nanosheets and glycerol plasticizer on thermal and mechanical properties. J. Appl. Polym. Sci. 2017;134:45092. doi: 10.1002/app.45092. [DOI] [Google Scholar]
- Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013;49:780–792. doi: 10.1016/j.eurpolymj.2012.12.009. [DOI] [Google Scholar]
- Croitoru A.-M., Ficai A., Ficai D., Trusca R., Dolete G., Andronescu E., Turculet S.C. Chitosan/graphene oxide nanocomposite membranes as adsorbents with applications in water purification. Materials. 2020;13:1687. doi: 10.3390/ma13071687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Lu Q., Quan Q., Mo R., Zhou C., Hong P., Li C. Novel low temperature (<37 °C) chitosan hydrogel fabrication under the synergistic effect of graphene oxide. New J. Chem. 2017;41:671–676. doi: 10.1039/c6nj03509d. [DOI] [Google Scholar]
- Dhayal V., Hashmi S.Z., Kumar U., Choudhary B.L., Dalela S., Dolia S.N., Alvi P.A. Optical and electrical properties of biocompatible and novel (CS–GO) polymer nanocomposites. Opt. Quan. Electron. 2021;53:1–13. doi: 10.1007/s11082-020-02723-9. [DOI] [Google Scholar]
- Dinescu S., Ionita M., Ignat S.-R., Costache M., Hermenean A. Graphene oxide enhances chitosan-based 3D scaffold properties for bone tissue engineering. Int. J. Mol. Sci. 2019;20:5077. doi: 10.3390/ijms20205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi F., Amini A., Gholami A., Ghasemi Y. Functionalized graphene oxide with chitosan for protein nanocarriers to protect against enzymatic cleavage and retain collagenase activity. Sci. Rep. 2017;7:42258. doi: 10.1038/srep42258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazial F.F., Tan L.L. Phenylalanine-responsive fluorescent biosensor based on graphene oxide-chitosan nanocomposites catalytic film for non-destructive fish freshness grading. Food Control. 2021;125:107995. doi: 10.1016/j.foodcont.2021.107995. [DOI] [Google Scholar]
- Figueroa T., Aguayo C., Fernandez K. Design and characterization of chitosan-graphene oxide nanocomposites for the delivery of proanthocyanidins. Int. J. Nanomed. 2020;15:1229–1238. doi: 10.2147/ijn.s240305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francolini I., Perugini E., Silvestro I., Lopreiato M., Scotto D’Abusco A., Valentini F., Placidi E., Arciprete F., Martinelli A., Piozzi A. Graphene oxide oxygen content affects physical and biological properties of scaffolds based on chitosan/graphene oxide conjugates. Materials. 2019;12:1142. doi: 10.3390/ma12071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakilas V., Tiwari J.N., Kemp K.C., Perman J.A., Bourlinos A.B., Kim K.S., Zboril R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016;116:5464–5519. doi: 10.1021/acs.chemrev.5b00620. [DOI] [PubMed] [Google Scholar]
- Gooneh-Farahani S., Naimi-Jamal M.R., Naghib S.M. Stimuli-responsive graphene-incorporated multifunctional chitosan for drug delivery applications: a review. Expert Opin. Drug Deliv. 2019;16:79–99. doi: 10.1080/17425247.2019.1556257. [DOI] [PubMed] [Google Scholar]
- Grande C.D., Mangadlao J., Fan J., De Leon A., Delgado-Ospina J., Rojas J.G., Rodrigues D.F., Advincula R. Chitosan cross-linked graphene oxide nanocomposite films with antimicrobial activity for application in food industry. Macromol. Symp. 2017;374 doi: 10.1002/masy.201600114. 1600114. [DOI] [Google Scholar]
- Gu Z., Zhu S., Yan L., Zhao F., Zhao Y. Graphene-based smart platforms for combined cancer therapy. Adv. Mater. 2019;31 doi: 10.1002/adma.201800662. 1800662. [DOI] [PubMed] [Google Scholar]
- Gurunathan S., Kim J.-H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016;11:1927. doi: 10.2147/ijn.s105264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D., Yan L. Supramolecular hydrogel of chitosan in the presence of graphene oxide nanosheets as 2D cross-linkers. ACS Sustain. Chem. Eng. 2014;2:296–300. doi: 10.1021/sc400352a. [DOI] [Google Scholar]
- Hasanzade Z., Raissi H. Assessment of the chitosan-functionalized graphene oxide as a carrier for loading thioguanine, an antitumor drug and effect of urea on adsorption process: combination of DFT computational and molecular dynamics simulation studies. J. Biomol. Struct. Dyn. 2019;37:2487–2497. doi: 10.1080/07391102.2018.1496140. [DOI] [PubMed] [Google Scholar]
- Hermenean A., Codreanu A., Herman H., Balta C., Rosu M., Mihali C.V., Ivan A., Dinescu S., Ionita M., Costache M. Chitosan-graphene oxide 3D scaffolds as promising tools for bone regeneration in critical-size mouse calvarial defects. Sci. Rep. 2017;7:16641. doi: 10.1038/s41598-017-16599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S.M., Mazinani S., Abdouss M., Kalhor H., Kalantari K., Amiri I.S., Ramezani Z. Designing chitosan nanoparticles embedded into graphene oxide as a drug delivery system. Polym. Bull. 2021;11:1927. doi: 10.1007/s00289-020-03506-8. [DOI] [Google Scholar]
- Hu K., Kulkarni D.D., Choi I., Tsukruk V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014;39:1934–1972. doi: 10.1016/j.progpolymsci.2014.03.001. [DOI] [Google Scholar]
- Huang Y.-S., Lu Y.-J., Chen J.-P. Magnetic graphene oxide as a carrier for targeted delivery of chemotherapy drugs in cancer therapy. J. Magnetism Magn. Mater. 2017;427:34–40. doi: 10.1016/j.jmmm.2016.10.042. [DOI] [Google Scholar]
- Jafari Z., Rad A.S., Baharfar R., Asghari S., Esfahani M.R. Synthesis and application of chitosan/tripolyphosphate/graphene oxide hydrogel as a new drug delivery system for Sumatriptan Succinate. J. Mol. Liquids. 2020;315:113835. doi: 10.1016/j.molliq.2020.113835. [DOI] [Google Scholar]
- Jayakumar R., Menon D., Manzoor K., Nair S.V., Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr. Polym. 2010;82:227–232. doi: 10.1016/j.carbpol.2010.04.074. [DOI] [Google Scholar]
- Jayakumar R., Prabaharan M., Reis R.L., Mano J.F. Graft copolymerized chitosan—present status and applications. Carbohydr. Polym. 2005;62:142–158. doi: 10.1016/j.carbpol.2005.07.017. [DOI] [Google Scholar]
- Jiang L., Chen D., Wang Z., Zhang Z., Xia Y., Xue H., Liu Y. Preparation of an electrically conductive graphene oxide/chitosan scaffold for cardiac tissue engineering. Appl. Biochem. Biotechnol. 2019;188:952–964. doi: 10.1007/s12010-019-02967-6. [DOI] [PubMed] [Google Scholar]
- Jin T., Liu T., Lam E., Moores A. Chitin and chitosan on the nanoscale. Nanoscale Horizons. 2021;6:505–554. doi: 10.1039/d0nh00696c. [DOI] [PubMed] [Google Scholar]
- Jing X., Mi H.-Y., Napiwocki B.N., Peng X.-F., Turng L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon. 2017;125:557–570. doi: 10.1016/j.carbon.2017.09.071. [DOI] [Google Scholar]
- Kafi M.A., Paul A., Vilouras A., Hosseini E.S., Dahiya R.S. Chitosan-graphene oxide-based ultra-thin and flexible sensor for diabetic wound monitoring. IEEE Sensors J. 2020;20:6794–6801. doi: 10.1109/jsen.2019.2928807. [DOI] [Google Scholar]
- Karimi N., Kharaziha M., Raeissi K. Electrophoretic deposition of chitosan reinforced graphene oxide-hydroxyapatite on the anodized titanium to improve biological and electrochemical characteristics. Mater. Sci. Eng. C. 2019;98:140–152. doi: 10.1016/j.msec.2018.12.136. [DOI] [PubMed] [Google Scholar]
- Katas H., Mohd Amin M.C.I., Moideen N., Ng L.Y., Megat Baharudin P.A.A. Cell growth inhibition effect of DsiRNA vectorised by pectin-coated chitosan-graphene oxide nanocomposites as potential therapy for colon cancer. J. Nanomater. 2017;2017:1–12. doi: 10.1155/2017/4298218. [DOI] [Google Scholar]
- Khalil W.F., El-Sayyad G.S., El Rouby W.M.A., Sadek M.A., Farghali A.A., El-Batal A.I. Graphene oxide-based nanocomposites (GO-chitosan and GO-EDTA) for outstanding antimicrobial potential against some Candida species and pathogenic bacteria. Int. J. Biol. Macromol. 2020;164:1370–1383. doi: 10.1016/j.ijbiomac.2020.07.205. [DOI] [PubMed] [Google Scholar]
- Kolanthai E., Sindu P.A., Khajuria D.K., Veerla S.C., Kuppuswamy D., Catalani L.H., Mahapatra D.R. Graphene oxide—a tool for the preparation of chemically crosslinking free alginate–chitosan–collagen scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces. 2018;10:12441–12452. doi: 10.1021/acsami.8b00699. [DOI] [PubMed] [Google Scholar]
- Kosowska K., Domalik-Pyzik P., Nocuń M., Chłopek J. Chitosan and graphene oxide/reduced graphene oxide hybrid nanocomposites – evaluation of physicochemical properties. Mater. Chem. Phys. 2018;216:28–36. doi: 10.1016/j.matchemphys.2018.05.076. [DOI] [Google Scholar]
- Kumar S., Koh J. Physiochemical and optical properties of chitosan based graphene oxide bionanocomposite. Int. J. Biol. Macromol. 2014;70:559–564. doi: 10.1016/j.ijbiomac.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Nah H., Heo D.N., Kim K.-H., Seok J.M., Heo M., Moon H.-J., Lee D., Lee J.S., An S.Y., et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an in situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon. 2020;168:264–277. doi: 10.1016/j.carbon.2020.05.022. [DOI] [Google Scholar]
- Li J., Cha R., Zhang Y., Guo H., Long K., Gao P., Wang X., Zhou F., Jiang X. Iron oxide nanoparticles for targeted imaging of liver tumors with ultralow hepatotoxicity. J. Mater. Chem. B. 2018;6:6413–6423. doi: 10.1039/c8tb01657g. [DOI] [PubMed] [Google Scholar]
- Li P., Liu S., Yang X., Du S., Tang W., Cao W., Zhou J., Gong X., Xing X. Low-drug resistance carbon quantum dots decorated injectable self-healing hydrogel with potent antibiofilm property and cutaneous wound healing. Chem. Eng. J. 2021;403:126387. doi: 10.1016/j.cej.2020.126387. [DOI] [Google Scholar]
- Li W., Jiang T., Pu Y., Jiao X., Tan W., Qin S. Glucose biosensor using fluorescence quenching with chitosan-modified graphene oxide. Micro Nano Lett. 2019;14:344–348. doi: 10.1049/mnl.2018.5269. [DOI] [Google Scholar]
- Liang Y., Chen B., Li M., He J., Yin Z., Guo B. Injectable Antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromolecules. 2020;21:1841–1852. doi: 10.1021/acs.biomac.9b01732. [DOI] [PubMed] [Google Scholar]
- Liang Y., Wang M., Zhang Z., Ren G., Liu Y., Wu S., Shen J. Facile synthesis of ZnO QDs@GO-CS hydrogel for synergetic antibacterial applications and enhanced wound healing. Chem. Eng. J. 2019;378:122043. doi: 10.1016/j.cej.2019.122043. [DOI] [Google Scholar]
- Liao K.-H., Lin Y.-S., Macosko C.W., Haynes C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces. 2011;3:2607–2615. doi: 10.1021/am200428v. [DOI] [PubMed] [Google Scholar]
- Liu B., Che C., Liu J., Si M., Gong Z., Li Y., Zhang J., Yang G. Fabrication and antitumor mechanism of a nanoparticle drug delivery system: graphene oxide/chitosan oligosaccharide/γ -polyglutamic acid composites for anticancer drug delivery. ChemistrySelect. 2019;4:12491–12502. doi: 10.1002/slct.201903145. [DOI] [Google Scholar]
- Liu B., Yang W., Che C., Liu J., Si M., Gong Z., Gao R., Yang G. A targeted nano drug delivery system of AS1411 functionalized graphene oxide based composites. ChemistryOpen. 2021;10:408. doi: 10.1002/open.202000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Dong J., Zhang T., Peng Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control Release. 2018;286:64–73. doi: 10.1016/j.jconrel.2018.07.034. [DOI] [PubMed] [Google Scholar]
- Liu M., Zeng X., Ma C., Yi H., Ali Z., Mou X., Li S., Deng Y., He N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zeng T.H., Hofmann M., Burcombe E., Wei J., Jiang R., Kong J., Chen Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano. 2011;5:6971–6980. doi: 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- Liu T., Dan W., Dan N., Liu X., Liu X., Peng X. A novel grapheme oxide-modified collagen-chitosan bio-film for controlled growth factor release in wound healing applications. Mater. Sci. Eng. C. 2017;77:202–211. doi: 10.1016/j.msec.2017.03.256. [DOI] [PubMed] [Google Scholar]
- Liu X., Cheng X., Wang F., Feng L., Wang Y., Zheng Y., Guo R. Targeted delivery of SNX-2112 by polysaccharide-modified graphene oxide nanocomposites for treatment of lung cancer. Carbohydr. Polym. 2018;185:85–95. doi: 10.1016/j.carbpol.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Liu X., Wu Y., Zhao X., Wang Z. Fabrication and applications of bioactive chitosan-based organic-inorganic hybrid materials: a review. Carbohydr. Polym. 2021;267:118179. doi: 10.1016/j.carbpol.2021.118179. [DOI] [PubMed] [Google Scholar]
- Liu Y., Song R., Zhang X., Zhang D. Enhanced antimicrobial activity and pH-responsive sustained release of chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane loading with allicin. Int. J. Biol. Macromol. 2020;161:1405–1413. doi: 10.1016/j.ijbiomac.2020.08.051. [DOI] [PubMed] [Google Scholar]
- López Tenorio D., Valencia C., Valencia C., Zuluaga F., Valencia M., Mina J., Grande Tovar C. Evaluation of the biocompatibility of CS-graphene oxide compounds in vivo. Int. J. Mol. Sci. 2019;20:1572. doi: 10.3390/ijms20071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.-J., Lan Y.-H., Chuang C.-C., Lu W.-T., Chan L.-Y., Hsu P.-W., Chen J.-P. Injectable thermo-sensitive chitosan hydrogel containing CPT-11-loaded EGFR-targeted graphene oxide and SLP2 shRNA for localized drug/gene delivery in glioblastoma therapy. Int. J. Mol. Sci. 2020;21:7111. doi: 10.3390/ijms21197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Feng X., Liang H., Wang K., Song Y., Tan L., Wang B., Luo R., Liao Z., Li G., et al. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today. 2020;36:48–62. doi: 10.1016/j.mattod.2019.12.005. [DOI] [Google Scholar]
- Mahajan C.R., Joshi L.B., Varma U., Naik J.B., Chaudhari V.R., Mishra S. Sustainable drug delivery of famotidine using chitosan-functionalized graphene oxide as nanocarrier. Glob. Chall. 2019;3:1900002. doi: 10.1002/gch2.201900002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi N., Eslahi N., Mehdipour A., Mohammadi M., Akbari M., Samadikuchaksaraei A., Simchi A. Temporary skin grafts based on hybrid graphene oxide-natural biopolymer nanofibers as effective wound healing substitutes: pre-clinical and pathological studies in animal models. J. Mater. Sci. Mater. Med. 2017;28:73. doi: 10.1007/s10856-017-5874-y. [DOI] [PubMed] [Google Scholar]
- Mahmoudi N., Simchi A. On the biological performance of graphene oxide-modified chitosan/polyvinyl pyrrolidone nanocomposite membranes: in vitro and in vivo effects of graphene oxide. Mater. Sci. Eng. C. 2017;70:121–131. doi: 10.1016/j.msec.2016.08.063. [DOI] [PubMed] [Google Scholar]
- Maiti D., Tong X., Mou X., Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front. Pharmacol. 2019;9:1401. doi: 10.3389/fphar.2018.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdizadeh B., Maleknia L., Amirabadi A., Shabani M. Glucose sensing by a glassy carbon electrode modified with glucose oxidase/chitosan/graphene oxide nanofibers. Diamond Relat. Mater. 2020;109:108073. doi: 10.1016/j.diamond.2020.108073. [DOI] [Google Scholar]
- Najafabadi S.A.A., Mohammadi A., Kharazi A.Z. Polyurethane nanocomposite impregnated with chitosan-modified graphene oxide as a potential antibacterial wound dressing. Mater. Sci. Eng. C. 2020;115:110899. doi: 10.1016/j.msec.2020.110899. [DOI] [PubMed] [Google Scholar]
- Olad A., Bakht Khosh Hagh H. Graphene oxide and amin-modified graphene oxide incorporated chitosan-gelatin scaffolds as promising materials for tissue engineering. Composites B Eng. 2019;162:692–702. doi: 10.1016/j.compositesb.2019.01.040. [DOI] [Google Scholar]
- Omidi S., Pirhayati M., Kakanejadifard A. Co-delivery of doxorubicin and curcumin by a pH-sensitive, injectable, and in situ hydrogel composed of chitosan, graphene, and cellulose nanowhisker. Carbohydr. Polym. 2020;231:115745. doi: 10.1016/j.carbpol.2019.115745. [DOI] [PubMed] [Google Scholar]
- Paiva C.A., Vilvert J.C., Menezes F.L.G., Leite R.H.D.L., Santos F.K.G., Medeiros J.F., Aroucha E.M.M. Extended shelf life of melons using chitosan and graphene oxide-based biodegradable bags. J. Food Process. Preserv. 2020;44:e14871. doi: 10.1111/jfpp.14871. [DOI] [Google Scholar]
- Pandele A.M., Ionita M., Lungu A., Vasile E., Zaharia C., Iovu H. Porous chitosan/graphene oxide biocomposites for tissue engineering. Polym. Composites. 2017;38:363–370. doi: 10.1002/pc.23594. [DOI] [Google Scholar]
- Peers S., Montembault A., Ladavière C. Chitosan hydrogels for sustained drug delivery. J. Control. Release. 2020;326:150–163. doi: 10.1016/j.jconrel.2020.06.012. [DOI] [PubMed] [Google Scholar]
- Pourjavadi A., Mazaheri Tehrani Z., Salami H., Seidi F., Motamedi A., Amanzadi A., Zayerzadeh E., Shabanian M. Both tough and soft double network hydrogel nanocomposite based on O-carboxymethyl chitosan/poly(vinyl alcohol) and graphene oxide: a promising alternative for tissue engineering. Polym. Eng. Sci. 2020;60:889–899. doi: 10.1002/pen.25297. [DOI] [Google Scholar]
- Prakash J., Prema D., Venkataprasanna K.S., Balagangadharan K., Selvamurugan N., Venkatasubbu G.D. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 2020;154:62–71. doi: 10.1016/j.ijbiomac.2020.03.095. [DOI] [PubMed] [Google Scholar]
- Qi C., Zhao L., Lin Y., Wu D. Graphene oxide/chitosan sponge as a novel filtering material for the removal of dye from water. J. Colloid Interface Sci. 2018;517:18–27. doi: 10.1016/j.jcis.2018.01.089. [DOI] [PubMed] [Google Scholar]
- Qiao F., Ke J., Liu Y., Pei B., Hu Q., Tang B.Z., Wang Z. Cationic quaternized chitosan bioconjugates with aggregation-induced emission features for cell imaging. Carbohydr. Polym. 2020;230:115614. doi: 10.1016/j.carbpol.2019.115614. [DOI] [PubMed] [Google Scholar]
- Rajan Unnithan A., Ramachandra Kurup Sasikala A., Park C.H., Kim C.S. A unique scaffold for bone tissue engineering: an osteogenic combination of graphene oxide–hyaluronic acid–chitosan with simvastatin. J. Ind. Eng. Chem. 2017;46:182–191. doi: 10.1016/j.jiec.2016.10.029. [DOI] [Google Scholar]
- Rasoulzadehzali M., Namazi H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018;116:54–63. doi: 10.1016/j.ijbiomac.2018.04.140. [DOI] [PubMed] [Google Scholar]
- Rebekah A., Sivaselvam S., Viswanathan C., Prabhu D., Gautam R., Ponpandian N. Magnetic nanoparticle-decorated graphene oxide-chitosan composite as an efficient nanocarrier for protein delivery. Colloids Surf. A Physicochem. Eng. Aspects. 2021;610:125913. doi: 10.1016/j.colsurfa.2020.125913. [DOI] [Google Scholar]
- Rinaudo M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- Rostami S., Puza F., Ucak M., Ozgur E., Gul O., Ercan U.K., Garipcan B. Bifunctional sharkskin mimicked chitosan/graphene oxide membranes: reduced biofilm formation and improved cytocompatibility. Appl. Surf. Sci. 2021;544:148828. doi: 10.1016/j.apsusc.2020.148828. [DOI] [Google Scholar]
- Ruiz S., Tamayo J.A., Ospina J.D., Navia Porras D.P., Valencia Zapata M.E., Hernandez J.H.M., Valencia C.H., Zuluaga F., Grande Tovar C.D. Antimicrobial films based on nanocomposites of chitosan/poly(vinyl alcohol)/graphene oxide for biomedical applications. Biomolecules. 2019;9:109. doi: 10.3390/biom9030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan S., Chawla A., Vairamani M., Sastry T.P., Subramanian K.S., Selvamurugan N. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2017;104:1975–1985. doi: 10.1016/j.ijbiomac.2017.01.034. [DOI] [PubMed] [Google Scholar]
- Saravanan S., Sareen N., Abu-El-Rub E., Ashour H., Sequiera G.L., Ammar H.I., Gopinath V., Shamaa A.A., Sayed S.S.E., Moudgil M., et al. Graphene oxide-gold nanosheets containing chitosan scaffold improves ventricular contractility and function after implantation into infarcted heart. Sci. Rep. 2018;8:15069. doi: 10.1038/s41598-018-33144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan S., Vimalraj S., Anuradha D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed. Pharmacother. 2018;107:908–917. doi: 10.1016/j.biopha.2018.08.072. [DOI] [PubMed] [Google Scholar]
- Shahbazmohammadi H., Sardari S., Omidinia E. An amperometric biosensor for specific detection of glycated hemoglobin based on recombinant engineered fructosyl peptide oxidase. Int. J. Biol. Macromol. 2020;142:855–865. doi: 10.1016/j.ijbiomac.2019.10.025. [DOI] [PubMed] [Google Scholar]
- Shamekhi M.A., Mirzadeh H., Mahdavi H., Rabiee A., Mohebbi-Kalhori D., Baghaban Eslaminejad M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019;127:396–405. doi: 10.1016/j.ijbiomac.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Sivashankari P.R., Prabaharan M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020;146:222–231. doi: 10.1016/j.ijbiomac.2019.12.219. [DOI] [PubMed] [Google Scholar]
- Solìs Moré Y., Panella G., Fioravanti G., Perrozzi F., Passacantando M., Giansanti F., Ardini M., Ottaviano L., Cimini A., Peniche C., Ippoliti R. Biocompatibility of composites based on chitosan, apatite, and graphene oxide for tissue applications. J. Biomed. Mater. Res. A. 2018;106:1585–1594. doi: 10.1002/jbm.a.36361. [DOI] [PubMed] [Google Scholar]
- Sumathra M., Sadasivuni K.K., Kumar S.S., Rajan M. Cisplatin-loaded graphene oxide/chitosan/hydroxyapatite composite as a promising tool for osteosarcoma-affected bone regeneration. ACS Omega. 2018;3:14620–14633. doi: 10.1021/acsomega.8b02090. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thakhiew W., Champahom M., Devahastin S., Soponronnarit S. Improvement of mechanical properties of chitosan-based films via physical treatment of film-forming solution. J. Food Eng. 2015;158:66–72. doi: 10.1016/j.jfoodeng.2015.02.027. [DOI] [Google Scholar]
- Tien N.D., Lyngstadaas S.P., Mano J.F., Blaker J.J., Haugen H.J. Recent developments in chitosan-based micro/nanofibers for sustainable food packaging, smart textiles, cosmeceuticals, and biomedical applications. Molecules. 2021;26:2683. doi: 10.3390/molecules26092683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylman M., Pieklarz K., Owczarz P., Maniukiewicz W., Modrzejewska Z. Structure of chitosan thermosensitive gels containing graphene oxide. J. Mol. Struct. 2018;1161:530–535. doi: 10.1016/j.molstruc.2018.02.065. [DOI] [Google Scholar]
- Valencia A.M., Valencia C.H., Zuluaga F., Grande-Tovar C.D. Dataset on in-vitro study of chitosan-graphene oxide films for regenerative medicine. Data Brief. 2021;39:107472. doi: 10.1016/j.dib.2021.107472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia C., Valencia C., Zuluaga F., Valencia M., Mina J., Grande-Tovar C. Synthesis and application of scaffolds of chitosan-graphene oxide by the freeze-drying method for tissue regeneration. Molecules. 2018;23:2651. doi: 10.3390/molecules23102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataprasanna K.S., Prakash J., Vignesh S., Bharath G., Venkatesan M., Banat F., Sahabudeen S., Ramachandran S., Devanand Venkatasubbu G. Fabrication of Chitosan/PVA/GO/CuO patch for potential wound healing application. Int. J. Biol. Macromol. 2020;143:744–762. doi: 10.1016/j.ijbiomac.2019.10.029. [DOI] [PubMed] [Google Scholar]
- Vlasceanu G.M., elaru A., Dinescu S., Balta C., Herman H., Gharbia S., Hermenean A., Ionita M., Costache M. Comprehensive appraisal of graphene–oxide ratio in porous biopolymer hybrids targeting bone-tissue regeneration. Nanomaterials. 2020;10:1444. doi: 10.3390/nano10081444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid F., Zhao X.-J., Jia S.-R., Bai H., Zhong C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: current state and perspectives. Composites Part B Eng. 2020;200:108208. doi: 10.1016/j.compositesb.2020.108208. [DOI] [Google Scholar]
- Wang C., Zhang Z., Chen B., Gu L., Li Y., Yu S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J. Colloid Interface Sci. 2018;516:332–341. doi: 10.1016/j.jcis.2018.01.073. [DOI] [PubMed] [Google Scholar]
- Wang K., Ruan J., Song H., Zhang J., Wo Y., Guo S., Cui D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011;6:1–8. doi: 10.1007/s11671-010-9751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu S., Yu W. Functionalized graphene oxide-reinforced chitosan hydrogel as biomimetic dressing for wound healing. Macromol. Biosci. 2021;21:2000432. doi: 10.1002/mabi.202000432. [DOI] [PubMed] [Google Scholar]
- Wang Y., Nie J., Fang W., Yang L., Hu Q., Wang Z., Sun J.Z., Tang B.Z. Sugar-based aggregation-induced emission luminogens: design, structures, and applications. Chem. Rev. 2020;120:4534–4577. doi: 10.1021/acs.chemrev.9b00814. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou Y., Wang X., Liu Y., Wen S., Zheng X., Wang P. Cytocompatibility and in vivo biodegradation of graphene-modified chitosan 3D porous scaffold. Mater. Lett. 2018;220:1–4. doi: 10.1016/j.matlet.2018.02.074. [DOI] [Google Scholar]
- Wang Z., Chen S., Lam J.W.Y., Qin W., Kwok R.T.K., Xie N., Hu Q., Tang B.Z. Long-term fluorescent cellular tracing by the aggregates of AIE bioconjugates. J. Am. Chem. Soc. 2013;135:8238–8245. doi: 10.1021/ja312581r. [DOI] [PubMed] [Google Scholar]
- Wu K., Liu X., Li Z., Jiao Y., Zhou C. Fabrication of chitosan/graphene oxide composite aerogel microspheres with high bilirubin removal performance. Mater. Sci. Eng. C. 2020;106:110162. doi: 10.1016/j.msec.2019.110162. [DOI] [PubMed] [Google Scholar]
- Wu T., Li B., Wang W., Chen L., Li Z., Wang M., Zha Z., Lin Z., Xia H., Zhang T. Strontium-substituted hydroxyapatite grown on graphene oxide nanosheet-reinforced chitosan scaffold to promote bone regeneration. Biomater. Sci. 2020;8:4603–4615. doi: 10.1039/d0bm00523a. [DOI] [PubMed] [Google Scholar]
- Xu S. Electrochemical DNA biosensor based on graphene oxide- chitosan hybrid nanocomposites for detection of Escherichia coli O157:H7. Int. J. Electrochem. Sci. 2017;12:3443–3458. doi: 10.20964/2017.04.16. [DOI] [Google Scholar]
- Xue J., Feng C., Xia L., Zhai D., Ma B., Wang X., Fang B., Chang J., Wu C. Assembly preparation of multilayered biomaterials with high mechanical strength and bone-forming bioactivity. Chem. Mater. 2018;30:4646–4657. doi: 10.1021/acs.chemmater.8b01272. [DOI] [Google Scholar]
- Xue J., Wang X., Wang E., Li T., Chang J., Wu C. Bioinspired multifunctional biomaterials with hierarchical microstructure for wound dressing. Acta Biomater. 2019;100:270–279. doi: 10.1016/j.actbio.2019.10.012. [DOI] [PubMed] [Google Scholar]
- Yan H., Jiang L., Xu X., Li Y., Shen Y., Zhu S. Ultrastrong composite film of Chitosan and silica-coated graphene oxide sheets. Int. J. Biol. Macromol. 2017;104:936–943. doi: 10.1016/j.ijbiomac.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Yang S., Zhang X., Zhang D. Electrospun chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane with Ciprofloxacin antibiotic drug for potential WoundDressing application. Int. J. Mol. Sci. 2019;20:4395. doi: 10.3390/ijms20184395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Yan Z., Lian Y., Wang J., Zhang K. Graphene oxide coated shell-core structured chitosan/PLLA nanofibrous scaffolds for wound dressing. J. Biomater. Sci. Polym. Ed. 2020;31:622–641. doi: 10.1080/09205063.2019.1706149. [DOI] [PubMed] [Google Scholar]
- Yang L., Fang W., Ye Y., Wang Z., Hu Q., Tang B.Z. Redox-responsive fluorescent AIE bioconjugate with aggregation enhanced retention features for targeted imaging reinforcement and selective suppression of cancer cells. Mater. Chem. Front. 2019;3:1335–1340. doi: 10.1039/c9qm00216b. [DOI] [Google Scholar]
- Yang M.-C., Tseng Y.-Q., Liu K.-H., Cheng Y.-W., Chen W.-T., Chen W.-T., Hsiao C.-W., Yung M.-C., Hsu C.-C., Liu T.-Y. Preparation of amphiphilic chitosan–graphene oxide–cellulose nanocrystalline composite hydrogels and their biocompatibility and antibacterial properties. Appl. Sci. 2019;9:3051. doi: 10.3390/app9153051. [DOI] [Google Scholar]
- Yang S., Lei P., Shan Y., Zhang D. Preparation and characterization of antibacterial electrospun chitosan/poly (vinyl alcohol)/graphene oxide composite nanofibrous membrane. Appl. Surf. Sci. 2018;435:832–840. doi: 10.1016/j.apsusc.2017.11.191. [DOI] [Google Scholar]
- Yu P., Bao R.-Y., Shi X.-J., Yang W., Yang M.-B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017;155:507–515. doi: 10.1016/j.carbpol.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Yu R., Shi Y., Yang D., Liu Y., Qu J., Yu Z.-Z. Graphene oxide/chitosan aerogel microspheres with honeycomb-cobweb and radially oriented microchannel structures for broad-spectrum and rapid adsorption of water contaminants. ACS Appl. Mater. Interface. 2017;9:21809–21819. doi: 10.1021/acsami.7b04655. [DOI] [PubMed] [Google Scholar]
- Yu Z., Xiao C., Huang Y., Chen M., Wei W., Yang X., Zhou H., Bi X., Lu L., Ruan J., Fan X. Enhanced bioactivity and osteoinductivity of carboxymethyl chitosan/nanohydroxyapatite/graphene oxide nanocomposites. RSC Adv. 2018;8:17860–17877. doi: 10.1039/c8ra00383a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamay G.S., Zamay T.N., Lukyanenko K.A., Kichkailo A.S. Aptamers increase biocompatibility and reduce the toxicity of magnetic nanoparticles used in biomedicine. Biomedicines. 2020;8:59. doi: 10.3390/biomedicines8030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., He J., Shi M., Liang Y., Guo B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020;400:125994. doi: 10.1016/j.cej.2020.125994. [DOI] [Google Scholar]
- Zhang F., Xie M., Zhao Y., Zhang Y., Yang M., Yang N., Deng T., Zhang M., Xie J. Chitosan and dextran stabilized GO-iron oxide nanosheets with high dispersibility for chemotherapy and photothermal ablation. Ceramics Int. 2019;45:5996–6003. doi: 10.1016/j.ceramint.2018.12.070. [DOI] [Google Scholar]
- Zhang H., Yan T., Xu S., Feng S., Huang D., Fujita M., Gao X.-D. Graphene oxide-chitosan nanocomposites for intracellular delivery of immunostimulatory CpG oligodeoxynucleotides. Mater. Sci. Eng. C. 2017;73:144–151. doi: 10.1016/j.msec.2016.12.072. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li X., Shi C., Ran G., Peng Y., Zeng S., He Y. Biocompatibility and angiogenic effect of chitosan/graphene oxide hydrogel scaffolds on EPCs. Stem Cells Int. 2021;2021:1–17. doi: 10.1155/2021/5594370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang Y., Yang X. Structure and properties of graphene oxide - chitosan composite membrane prepared by the plasma acid solvent. J. Fiber Sci. Technol. 2018;74:143–149. doi: 10.2115/fiberst.2018-0020. [DOI] [Google Scholar]
- Zhang S., Ma B., Wang S., Duan J., Qiu J., Li D., Sang Y., Ge S., Liu H. Mass-production of fluorescent chitosan/graphene oxide hybrid microspheres for in vitro 3D expansion of human umbilical cord mesenchymal stem cells. Chem. Eng. J. 2018;331:675–684. doi: 10.1016/j.cej.2017.09.014. [DOI] [Google Scholar]
- Zhang X. Implantable glucose biosensor with enhancing electron transfer - nanocomposite functional layers. Int. J. Electrochem. Sci. 2021:151061. doi: 10.20964/2021.01.77. [DOI] [Google Scholar]
- Zhao H., Ding R., Zhao X., Li Y., Qu L., Pei H., Yildirimer L., Wu Z., Zhang W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today. 2017;22:1302–1317. doi: 10.1016/j.drudis.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Zhao H., Jiao T., Zhang L., Zhou J., Zhang Q., Peng Q., Yan X. Preparation and adsorption capacity evaluation of graphene oxide-chitosan composite hydrogels. Sci. China Mater. 2015;58:811–818. doi: 10.1007/s40843-015-0090-x. [DOI] [Google Scholar]
- Zhao X., Wei Z., Zhao Z., Miao Y., Qiu Y., Yang W., Jia X., Liu Z., Hou H. Design and development of graphene oxide nanoparticle/chitosan hybrids showing pH-sensitive surface charge-reversible ability for efficient intracellular doxorubicin delivery. ACS Appl. Mater. Interface. 2018;10:6608–6617. doi: 10.1021/acsami.7b16910. [DOI] [PubMed] [Google Scholar]
- Zhao X., Zou X., Ye L. Controlled pH- and glucose-responsive drug release behavior of cationic chitosan based nano-composite hydrogels by using graphene oxide as drug nanocarrier. J. Ind. Eng. Chem. 2017;49:36–45. doi: 10.1016/j.jiec.2016.12.023. [DOI] [Google Scholar]