Abstract
Objectives:
To describe varicella cases in Tshuapa Province of the Democratic Republic of the Congo identified during monkeypox surveillance.
Methods:
Demographic, clinical, and epidemiological data were collected from each suspected monkeypox case during 2009–2014. Samples were tested by PCR for both Orthopoxviruses and varicella-zoster virus (VZV); a subset of VZV positive samples were genotyped. We defined a varicella case as a rash illness with laboratory-confirmed VZV.
Results:
There were 366 varicella cases were identified; 66% were ≤19 years old. Most patients had non-typical varicella rash with lesions reported as the same size and stage of evolution (86%), deep and profound (91%), on palms of hands and/or soles of feet (86%), and not itchy (49%). Many had non-typical signs and symptoms, including lymphadenopathy (70%) and sensitivity to light (23%). A higher proportion of persons aged ≥20 years compared with persons aged ≤19 years had ≥50 lesions (79% versus 65%, p = 0.007) and were bedridden (15% versus 9%, p = 0.056). All VZV isolates genotyped from 79 varicella cases were clade 5. During the surveillance period, one possible VZV-related death occurred in a 7-year-old child.
Conclusions:
A large proportion of patients presented with non-typical varicella rash and clinical signs and symptoms, highlighting challenges identifying varicella in an area with endemic monkeypox. Continued surveillance and laboratory diagnosis will help in rapid identification and control of both monkeypox and varicella and improve our understanding of varicella epidemiology in Africa.
Keywords: Varicella, Varicella-zoster virus, Chickenpox, Democratic Republic of Congo, DRC, Africa, Epidemiology, Monkeypox
Introduction
Varicella is a highly communicable viral disease caused by varicella-zoster virus (VZV). There is an annual estimate of 140 million cases worldwide and most people become infected (or acquire the disease) during their lifetime [1]. Varicella typically presents as a febrile illness with a generalized itchy maculopapulovesicular rash that is self-limited, but can sometimes lead to severe disease—including death—mainly in infants, adults, pregnant women, and immunocompromised populations [2]. In developed countries, the rates of hospitalizations and deaths are estimated to be 3–6/1,000 cases and 3/100,000 cases, respectively [1]. The presence of lesions in various stages of evolution on a single site of the body at the same time, superficial appearance on the surface of the skin, and in a centripetal distribution on the body are characteristic of the varicella rash [3]. In countries that have introduced the varicella vaccine as part of their routine immunization program, mainly developed countries, disease burden has substantially decreased [1, 2, 4–7].
Few data are available on varicella epidemiology in Africa and most are limited to specific sub-populations, such as HIV-positive populations or refugees [8–16]. Varicella epidemiology in temperate climates usually differs from that in tropical climates; in temperate climates most children are infected by 10 years of age while children in tropical climates acquire varicella at older ages and a higher proportion of young adults remain susceptible [17–20]. The reasons for these differences are unknown, but hypotheses include environmental factors, individual behavior, population density, and immune factors influenced by malnutrition [17, 20, 21].
Monkeypox virus, a member of the genus Orthopoxvirus, causes a disease with clinical features that can resemble varicella. In humans, infection begins with a febrile prodrome followed by a rash illness often accompanied by lymphadenopathy. The lesions typically first appear as macular, then slowly change to papular, and then vesicular and pustular and are described as “deep-seated, well-circumscribed, and umbilicated.” In contrast to varicella, for which lesions appear in successive waves, lesions in monkeypox are all in the same stage of development (e.g., all vesicular) at a single site on the body. The distribution of monkeypox lesions tends to be centrifugal, and often includes the palms of hands and the soles of feet [22, 23]. Compared to varicella, monkeypox is usually more sporadic, has a lower secondary attack rate, involves more lesions with potentially up to thousands that can be painful, and has a higher mortality rate [12]. In areas where monkeypox is endemic, these two illnesses are often difficult to distinguish, particularly due to non-typical presentations of varicella [24].
Since 1981, majority of monkeypox cases are reported in Central Africa, with most cases occurring in the Congo Basin of the Democratic Republic of the Congo (DRC) [23]. In monkeypox-endemic areas, outbreaks may be associated with co-occurrence of varicella cases [24]. A monkeypox surveillance project in Tshuapa Province in DRC identified 383 (37%) cases with only VZV infection out of 1,025 suspected monkeypox cases during 2009–2014 [14], providing an opportunity to better understand the epidemiology of varicella in DRC.
Methods
DRC has a population of approximately 83 million persons [25]. Tshuapa Province (population estimated to be 1.7 million in 2017 [26]) is one of 26 provinces in DRC and is separated into 12 health zones. Since 2010, the U.S. Centers for Disease Control and Prevention (CDC) and the Kinshasa School of Public Health (KSPH) have supported enhanced monkeypox surveillance in Tshuapa Province [27]. A suspected case of monkeypox was defined as a vesicular or pustular eruption with deep-seated, firm pustules and at least one of the following, fever preceding the eruption, lymphadenopathy (inguinal, axillary, or cervical), and/or pustules or crusts on palms of hands or soles of feet [14]. Suspected monkeypox cases were identified in several ways: (1) an ill person with monkeypox-like symptoms presented to a clinic for care, (2) another person seeking care at the clinic reported a suspected monkeypox case from their community, or (3) a trained community health worker learned of a suspected monkeypox case. For the suspected cases that were investigated, a monkeypox-specific case investigation form was completed, and specimens were collected from at least two separate lesions (lesion fluid collected via swabs and/or crusts) or blood from a few cases earlier in the surveillance program. Photos were taken whenever possible. The case investigation form and specimens were sent to the Institut National de Recherche Biomédicale (INRB) in Kinshasa.
At INRB, DNA was extracted from one sample from each specimen type and first tested for Orthopoxvirus DNA by real-time PCR, and if negative, were tested by real-time PCR for VZV DNA [14]. DNA aliquots from samples tested at INRB and all other remaining specimens were sent to CDC, where laboratory testing for both Monkeypox virus (real-time PCR) and VZV (real-time PCR) was conducted. For a small proportion of cases (3%), samples were tested for VZV only at CDC. For VZV PCR testing at CDC, four different PCR protocols were performed on every sample, each of which can detect <10 copies/reaction [28–30]. VZV genotyping was also performed at CDC on a subset of VZV positive samples. A case was classified as laboratory-confirmed VZV if a specimen was VZV-positive by PCR at INRB or CDC. Patients with discordant VZV PCR results in different specimens (≥1 VZV-positive and ≥1 VZV-negative) were considered VZV positive. For this analysis, we defined a varicella case as both having laboratory-confirmed VZV and reported rash present [14]. All cases co-infected with monkeypox and VZV were excluded from this analysis.
We describe demographic characteristics, clinical presentation, epidemiology, and geographical distribution of varicella cases identified during 2009–2014. Some suspected monkeypox cases were reported in 2009, before the start of the enhanced surveillance project. Differences in clinical and epidemiological characteristics of cases by age group (0–19 years vs. ≥20 years) were tested using Chi-square or Fisher’s exact test. P-values <0.05 were considered statistically significant. Varicella cases were mapped by health zone. All data were analyzed using SAS version 9.3 or Quantum GIS (QGIS) [31].
The surveillance was determined not to be human subjects research by a CDC human subjects advisor, therefore the Centers for Disease Control’s Institutional Review Board approval was not required.
Results
Epidemiology and Clinical Characteristics
During September 2009–February 2014, 383 laboratory-confirmed varicella cases were identified by monkeypox surveillance in Tshuapa Province [14]; 366 (96%) met the varicella case definition. Figure 1 shows the distribution of these cases by rash onset date. Two of the 12 health zones (Busanga and Djolu) contributed to almost half of the varicella cases (Figure 2).
Figure 1. Epidemiological curve of laboratory-confirmed varicella cases in Tshuapa Province, Democratic Republic of the Congo, September 2009–February 2014 (N = 357)abc.
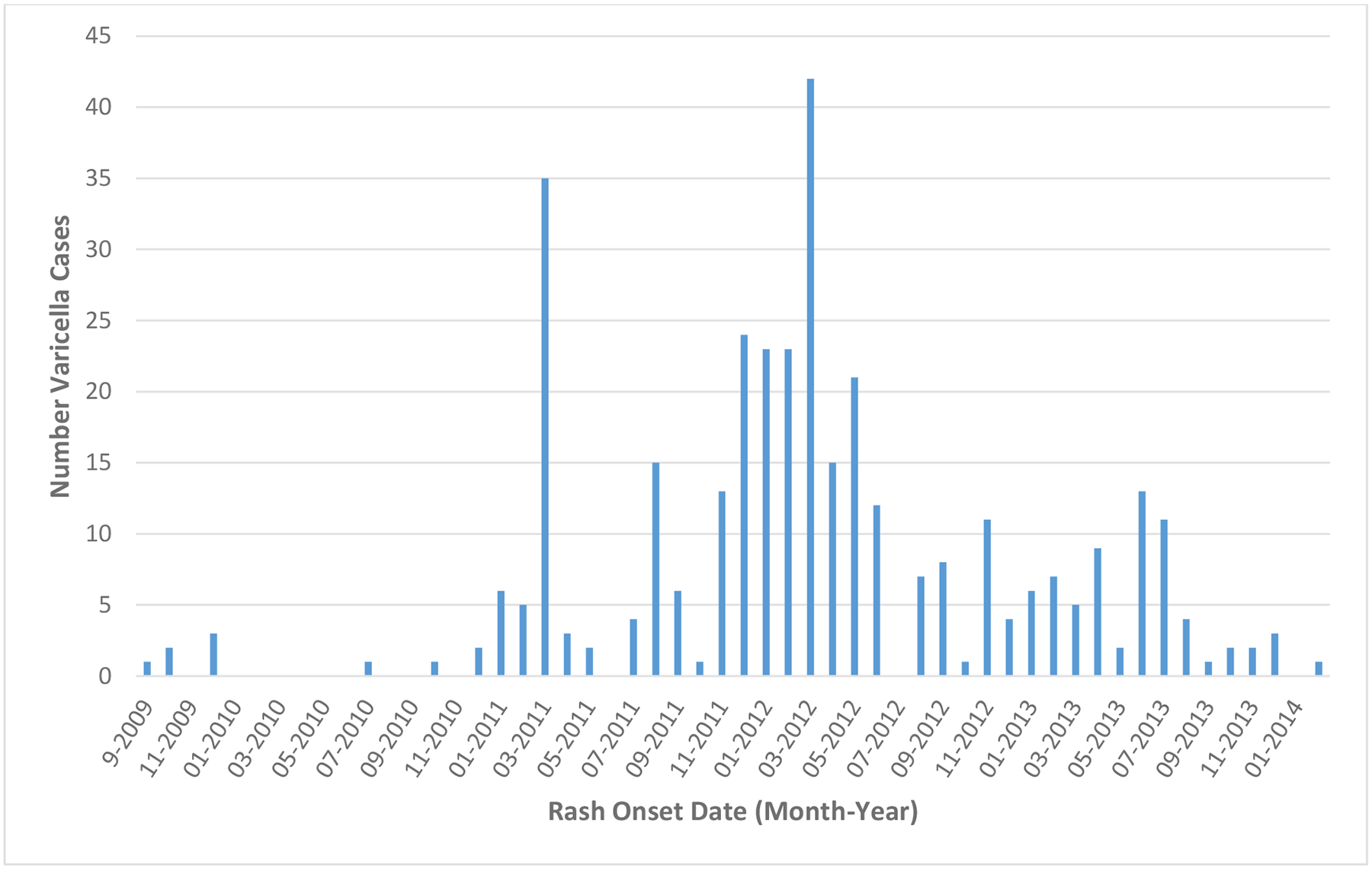
a 357 varicella cases had rash onset date available; 9 cases were missing information on rash onset date.
bThe peaks in February 2011 and February 2012 correspond to workshops on monkeypox surveillance that were conducted for public health officials in Tshuapa Province, Democratic Republic of the Congo during those months.
cThere were 6 varicella cases reported in 2009, before the start of the enhanced surveillance project.
Figure 2. Geographical distribution of laboratory-confirmed varicella cases in Tshuapa Province, Democratic Republic of the Congo by Health Zone, September 2009–February 2014 (n = 366)ab.
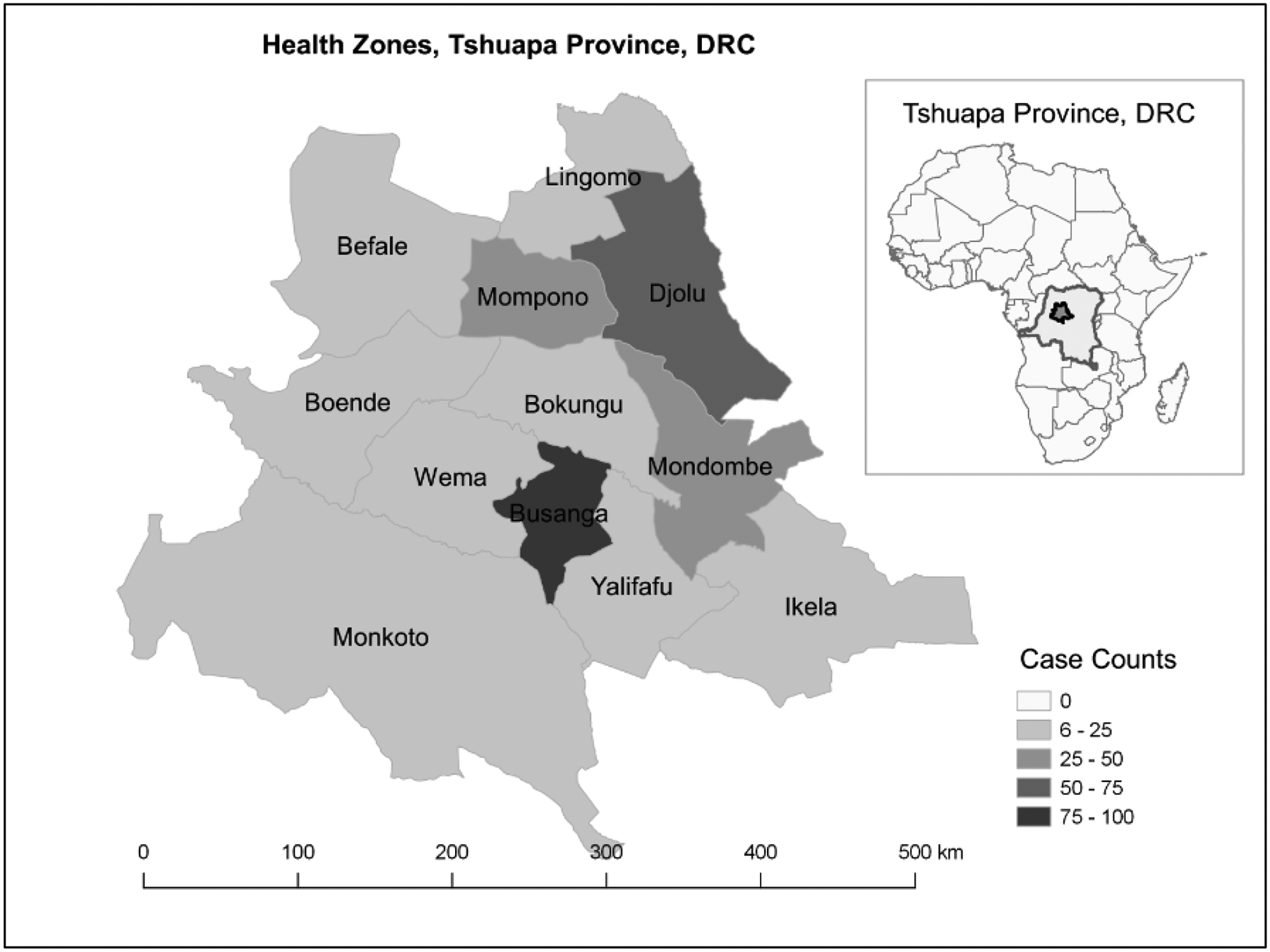
aThe distribution of varicella cases by health zone are as follows: Busanga (27%), Djolu (20%), Mondombe (13%), Mompono (9%), Wema (6%), Ikela (6%), Boende (4%), Befale (4%), Bokungu (4%), Lingomo (3%), Monkoto (2%), and Yalifafu (2%).
bThe higher concentration of varicella cases identified in Busanga and Djolu could be due to involvement of these two areas, as well as Ikela, in a special monkeypox research project during 2013–2015. Busanga is a smaller area in which most people live near the main road, which may make it easier to reach and investigate suspected cases.
The median age of varicella patients was 13 years (range: 1 month–86 years; interquartile: 7–27 years); 34% were aged ≥20 years. Fifty-four percent were male. Almost all patients had fever (99%), with no difference by age group. The majority (≥85%) had lesions reported as the same size and stage of evolution, and as deep-seated and firm (Table 1). Over 90% of varicella patients had lesions on the face, trunk, and extremities (arms or legs), and a large proportion also had lesions on the palms of hands and the soles of feet (84% and 69%, respectively; 86% with lesions on either palms or soles). By age group, the characteristics of the lesions and the rash location were similar with the exception of lesions that were the same size and stage of evolution; a higher proportion of persons aged 0–19 years than persons aged ≥20 years had lesions described as both the same size and stage of evolution (21% vs. 11%, p = 0.021).
Table 1.
Clinical and epidemiologic characteristics of patients with laboratory-confirmed varicella, Tshuapa Province, Democratic Republic of Congo, September 2009–February 2014 by age group (N = 366)
| All | Persons Aged 0–19a | Persons Aged ≥ 20a | p-valueb | |
|---|---|---|---|---|
| 366 | 242 | 124 | ||
| Variable | # (%) | # (%) | # (%) | |
| Patient status | ||||
| Alive | 362 (99.7) | 240 (99.6) | 122 (100.0) | 1.000c |
| Deadd | 1 (0.3) | 1 (0.4) | 0 (0.0) | |
| Fever present | ||||
| Yes | 356 (99) | 236 (99) | 120 (99) | 1.000c |
| No | 4 (1) | 3 (1) | 1 (1) | |
| Febrile prodrome | ||||
| Yes | 356 (98) | 235 (98) | 121 (99) | 0.668c |
| No | 6 (2) | 5 (2) | 1 (1) | |
| Lesions same stage of evolution | ||||
| Yes | 254 (85) | 170 (87) | 84 (82) | 0.312 |
| No | 44 (15) | 26 (13) | 18 (18) | |
| All lesions same size and stage of evolution | ||||
| Yes | 255 (86) | 174 (89) | 81 (79) | 0.021 |
| No | 42 (14) | 21 (11) | 21 (21) | |
| Lesions deep-seated and firm | ||||
| Yes | 273 (91) | 180 (92) | 93 (89) | 0.487 |
| No | 27 (9) | 16 (8) | 11 (11) | |
| Location of lesions | ||||
| Face | 356 (97) | 237 (98) | 119 (96) | 0.316c |
| Trunk | 346 (95) | 229 (95) | 117 (94) | 0.913 |
| Arm | 332 (91) | 214 (88) | 116 (94) | 0.120 |
| Legs | 119 (33) | 85 (35) | 34 (27) | 0.136 |
| Arm or Legs | 332 (91) | 216 (89) | 116 (94) | 0.181 |
| Palms of hands | 306 (84) | 202 (83) | 104 (84) | 0.922 |
| Soles of feet | 252 (69) | 168 (69) | 84 (68) | 0.743 |
| Genitals | 48 (13) | 32 (13) | 16 (13) | 0.932 |
| Number of lesions | ||||
| <50 lesions | 105 (30) | 81 (35) | 24 (21) | 0.007 |
| ≥50 lesions | 245 (70) | 152 (65) | 93 (79) | |
| Presence of following symptoms | ||||
| Chills or sweats | 291 (80) | 199 (82) | 92 (74) | 0.095 |
| Fatigue | 275 (75) | 176 (73) | 99 (80) | 0.238 |
| Headache | 270 (74) | 171 (71) | 99 (80) | 0.121 |
| Muscle pain (myalgia) | 200 (55) | 120 (50) | 80 (65) | 0.018 |
| Lesions that itch | 187 (51) | 126 (52) | 61 (49) | 0.737 |
| Sore throat when swallowing | 179 (49) | 123 (51) | 56 (45) | 0.336 |
| Cough | 130 (36) | 97 (40) | 33 (27) | 0.010 |
| Oral ulcers | 127 (35) | 94 (39) | 33 (27) | 0.019 |
| Sensitivity to light | 84 (23) | 51 (21) | 33 (27) | 0.233 |
| Vomiting/nausea | 56 (15) | 35 (14) | 21 (17) | 0.461 |
| Conjunctivitis | 43 (12) | 27 (11) | 16 (13) | 0.634 |
| Lymphadenopathy | ||||
| Inguinal | 137 (37) | 82 (34) | 55 (44) | 0.046 |
| Axillary | 163 (45) | 101 (42) | 62 (50) | 0.169 |
| Cervical | 189 (52) | 125 (52) | 64 (52) | 0.885 |
| Patient bedridden | 40 (11) | 21 (9) | 19 (15) | 0.056 |
| Type of interaction with a contact that had similar symptoms | ||||
| Family contact/live together | 148 (66) | 109 (72) | 39 (54) | 0.018 |
| Prepared food together | 47 (21) | 27 (18) | 20 (28) | 0.062 |
| Shared a bed/slept together | 46 (21) | 31 (21) | 15 (21) | 0.845 |
| Took care of this sick person | 40 (18) | 19 (13) | 21 (29) | 0.001 |
| Friend at school or play | 13 (6) | 11 (7) | 2 (3) | 0.354c |
| Met at the market | 2 (1) | 0 (0) | 2 (3) | 0.098c |
| Hunted together | 6 (3) | 1 (1) | 5 (7) | 0.012 c |
| Friend/social acquaintance | 14 (6) | 6 (4) | 8 (11) | 0.040 c |
Note, significant p-values are bolded
The age group distributions are as follows: < 1 (4%), 1–4 (14%), 5–9 (21%), 10–14 (16%), 15–19 (11%), 20–29 (14%), 30–39 (10%), 40–49 (4%), and ≥50 (6%) year-olds.
Comparison of clinical and epidemiologic characteristics of varicella cases by age group; comparisons are of varicella cases in persons aged 0–19 years versus persons aged ≥20 years.
Fisher’s exact test used.
This was a death that occurred in a 7 year-old with laboratory-confirmed varicella-zoster virus (VZV) infection though the actual cause of death is unknown.
A statistically higher proportion of older persons (aged ≥20 years) had ≥50 lesions compared with younger persons (aged 0–19 years) (79% versus 65%, p = 0.007) (Table 1). Figure 3 shows an example of two adult cases, one in a 38-year-old female with 350 lesions (Figure 3A) and one in a 61-year-old female with 140 lesions (Figure 3B). Among persons aged 0–19 years and ≥20 years, respectively, the most common other symptoms included chills or sweats, 82% and 74%; fatigue 73% and 80%; and headache, 71% and 80%. A number of non-typical varicella symptoms were reported, which included lymphadenopathy in 70% of patients, sensitivity to light in 23% of patients, and a non-itchy rash in 48% of patients, with no statistically significant difference by age group.
Figure 3. Photos of two laboratory-confirmed varicella patients identified as part of monkeypox surveillancea.
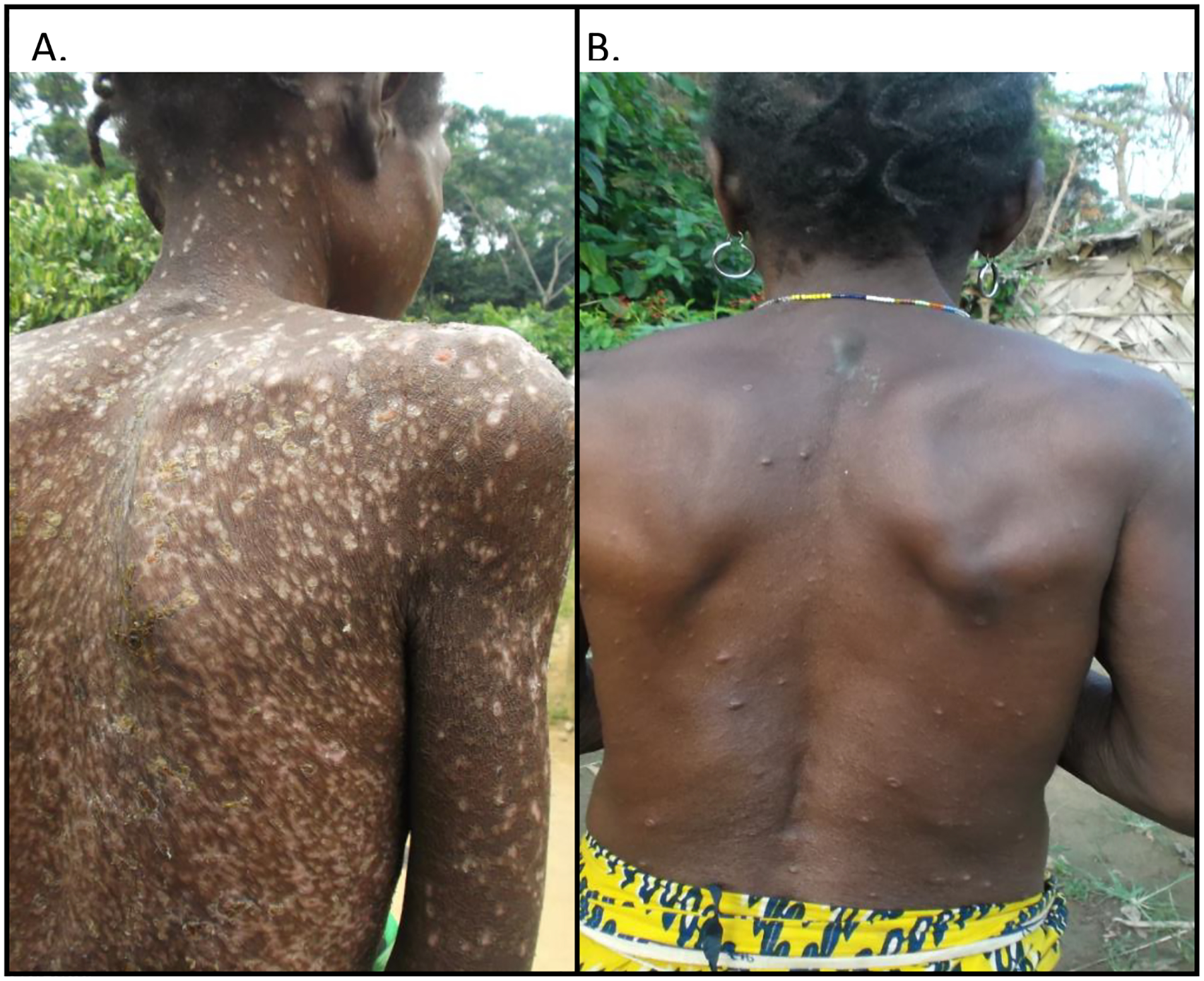
a Image 3A: 38-year-old female patient with desquamation of crusts from an extensive disseminated rash. Image 3B: 61-year-old female patient with active lesions.
Overall, 11% of patients were bedridden, an indication of disease severity: 15% of 0–19 years-olds and 9% of ≥20 years-olds (p = 0.056). One death possibly attributable to VZV occurred in a 7-year-old male. The patient had fever, then rash two days after fever onset, and died two days after rash developed. He had ≥500 lesions; the rash was not described as itchy. At presentation, the patient had “profound cough” and was thought to have pneumonia. Other signs and symptoms included lymphadenopathy, sensitivity to light, lesions in the mouth, vomiting/nausea, and dehydration. No additional clinical or laboratory information to distinguish between varicella and disseminated herpes zoster was available. The cause of death was unknown.
Laboratory Findings
Among the 366 varicella patients, specimens from 313 patients were VZV positive at both INRB and CDC; specimens from 21 tested positive only at INRB; and specimens from 32 tested positive only at CDC (Figure 4). Specimens from 79 (23%) of 345 patients tested at CDC were genotyped and all were VZV clade 5.
Figure 4. Flow diagram of varicella-zoster virus (VZV) laboratory results from specimens tested at Institut National de Recherche Biomedicale (INRB) and the Centers for Disease Control and Prevention (CDC).
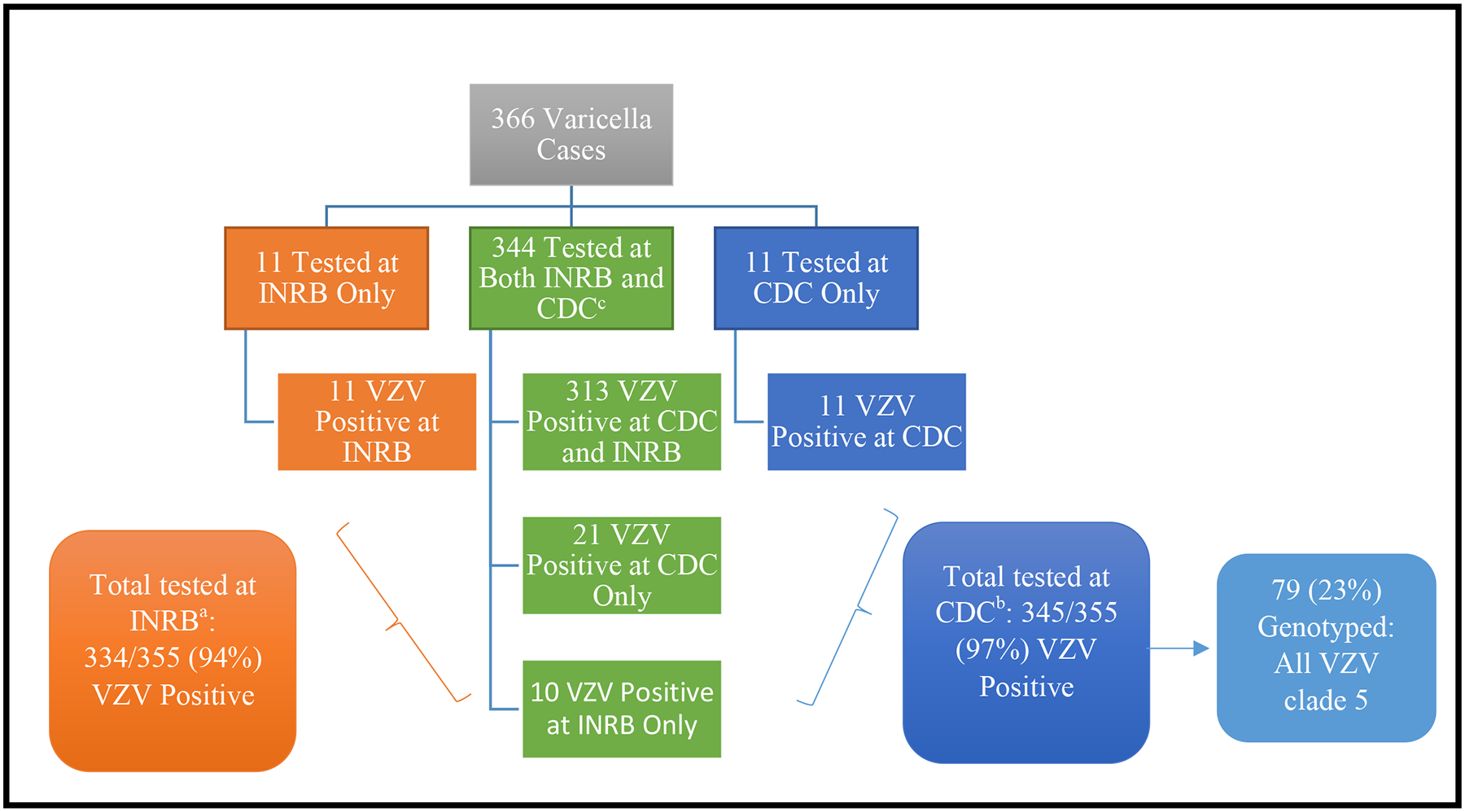
aA total of 476 specimens from 355 varicella patients were submitted to INRB for testing; 263 specimens (55%) were vesicular fluid, 192 (40%) were crusts, and 21 (4%) were blood specimens. Of these 355 patients, 237 (67%) had 1 specimen tested for VZV and 118 (33%) had ≥3 specimens tested. After testing at INRB, 334 (94%) of the 355 case-patients had ≥1 VZV positive specimen by PCR.
bThere were 294 DNA specimens extracted at INRB. An additional 412 skin lesion specimens (59% from vesicles) from 355 patients were sent to the CDC National VZV Laboratory. Among these 355 patients, 106 (30%) had 1 specimen tested and 249 (70%) had ≥3 specimens tested. After testing at CDC, 345 (97%) of the 355 patients with specimens sent to CDC had ≥1 VZV positive specimen by PCR.
cAmong the 344 specimens tested at both INRB and CDC, there were 91% (313) with concordant results, and 9% (31) with discordant results. Reasons for the discordant results could be due to differences in assay sensitivity, different specimens tested from the same patient, or potential false positive results if a high number of PCR cycles for amplification were used.
Discussion
We describe varicella cases identified as part of monkeypox surveillance in DRC. A high proportion of patients reported non-typical varicella rash presentations as well as other clinical signs and symptoms not commonly associated with varicella, including lymphadenopathy, conjunctivitis, and sensitivity to light. Genotyping identified only VZV clade 5. With information on nearly 400 varicella cases with detailed clinical and laboratory information, our report is a useful source of information in a region with little clinical and epidemiological data on varicella.
Varicella does not typically present with lesions that are all in the same stage of development, deep-seated and firm, or not pruritic. It is also uncommon for the rash to present on the palms of hands and soles of feet. Lesions on the palms of hands and soles of feet have been previously reported for varicella but in a very limited number of patients, 5 from Africa and 2 from Europe [11, 32–34]. The reason for the high proportion of non-typical clinical presentation of varicella in our study is unknown. The underlying health and comorbidities of patients described are unknown, but could have a role in affecting the epidemiology and clinical characteristics of varicella in this population. Although we were unable to collect information on HIV prevalence in this study, one seroprevalence study in DRC found a prevalence of 1–4% and another study based on a serological survey of pregnant women, including data from 2 zones in Tshuapa (Boende, and Ikela), found a prevalence of 2–3% [[35, 36]]. Also, since these varicella cases were identified as part of monkeypox surveillance, it is possible that surveillance staff might have inaccurately documented signs and symptoms which are more commonly associated with monkeypox. This bias is unlikely to fully explain our findings; moreover, the accompanying pictures support the validity of the clinical reports. Another possibility is that these non-typical presentations of varicella may occur more commonly than previously thought in DRC or other African regions.
Our identification of only VZV clade 5 viruses was not unexpected, and is consistent with a previous study that found only clade 5 viruses among 34 varicella isolates from other African nations (Tanzania, Morocco, and Chad) [37]. Further, among the 181 African VZV isolates genotyped at CDC thus far, which included specimens from this study [CDC unpublished data], only a single isolate belonged to a clade other than 5: a European clade 3 virus from a patient that was part of a family cluster of varicella cases in the Republic of Congo, which also had non-typical varicella presentations [11]. This suggests that clades other than clade 5 could be circulating in central Africa and studies of African nations with extensive histories of European colonization may reveal that European clades (clades 1 and 3) are also established on the continent. Conversely, clade 5 has also been found outside Africa (United States, France, and Spain) and can now be regarded as endemic in these countries [37].
The epidemiology of varicella is commonly observed to be different in tropical compared with temperate climates, though the reasons for these differences are unknown. Africa has varying climates, but most of the areas are tropical or subtropical [10]. Primary VZV infection tends to occur at older ages in tropical climates [12, 17, 19, 21, 38]. VZV seroprevalence estimates from several tropical countries almost uniformly confirm higher adult susceptibility compared with adults in temperate countries [38–43]. In temperate climates such as in the United States, prior to the vaccine program, varicella incidence was highest in 5–9-year-olds and VZV seroprevalence was 66% in 4–5-year-olds [44]. In seroprevalence studies of varicella in DRC and Kenya, 8–24% of children were found to be seropositive [8, 9]. However, other studies have reported high VZV seropositivity (~80%) in children from tropical climates and VZV epidemiology similar to temperate climates [41, 43, 45]. In our study the median age of patients with varicella was 13 years and a third of the cases were in adults aged ≥20 years, indicating susceptibility among adolescents and adults. Older age at VZV infection is concerning because varicella tends to be more severe as was seen in the case in the 38 year-olds shown in Figure 3 [21]. We documented that adults aged ≥20 years were more likely to have ≥50 lesions and were almost twice as likely to be bedridden than younger patients. However, severe varicella can occur in children: we identified one death in a 7-year-old child possibly attributable to VZV.
A literature review of papers published on varicella in Africa during a 41-year period (1974–2015) found only 20 articles 13 countries (24% of the countries in Africa) [10]. Though we were able to collect data on varicella through this monkeypox surveillance platform, distinguishing between varicella and monkeypox in areas where both diseases are endemic—such as in DRC—can be challenging. As part of monkeypox surveillance in DRC in 1981–1986 (then known as Zaire), 4% of 895 cases with clinical diagnoses of varicella or “atypical varicella” were later laboratory confirmed to be monkeypox, and 23% of 373 suspected monkeypox cases were not laboratory confirmed as monkeypox (no VZV testing was mentioned) [24]. We found that clinical features alone could not be used to distinguish between varicella and monkeypox cases, as many of the varicella cases described in this paper had non-typical clinical presentation; the clinical information should be paired with laboratory testing. Two of the most commonly cited signs as pathognomonic for varicella—presence of lesions in different stages of evolution and absence of lesions on the palms and soles—would not differentiate varicella from monkeypox in our study, as 85% of varicella patients had lesions in the same stage of evolution and 86% had lesions on the palms or soles. Previous studies have identified some laboratory-confirmed monkeypox cases with lesions in different stages of development [14, 24]. Two studies suggested that clinical features that may help distinguish varicella from monkeypox include lymphadenopathy, febrile prodrome, and slower maturation of skin lesions (i.e., no lesions observed crusting by 48 hours) [24] or presence of a febrile prodrome combined with 7–8 monkeypox-related signs and symptoms [14]. The mechanisms underlying common co-circulation of these two viruses in outbreak settings remains unknown. One hypothesis is that monkeypox infection may trigger VZV reactivation in a person harboring a latent VZV infection, resulting in symptomatic herpes zoster and transmission of VZV. This speculation will need to be confirmed by further studies.
Our study has several limitations. Since varicella cases were ascertained in the context of monkeypox surveillance, the epidemiologic and clinical findings may not be generalizable to all varicella cases in DRC, Africa, or tropical regions. It is likely that varicella cases were missed, especially cases with more typical varicella clinical presentation. The proportion of varicella cases among all investigations declined with time as surveillance staff became more experienced with monkeypox surveillance. Some of the epidemiological data (i.e., distribution of cases over time and by health zone) may reflect surveillance practices, rather than the epidemiology of varicella in DRC. Some of the collected clinical variables were subjective and based on the interviewer’s assessment, including the characteristics (e.g., deep-seated, whether lesions were in the same stage of evolution) and number of lesions, though some other clinical variables may have been more objective, such as the location of lesions (e.g., lesions on the palms and soles), and lymphadenopathy. Febrile prodrome is non-specific and could be potentially related to other illnesses that are endemic to this region, including malaria and diarrheal disease. Information on the HIV-status and underlying health of these patients was not collected. We were also unable to distinguish between varicella and herpes zoster cases (reactivation of VZV); cases in older adults may be more likely to be herpes zoster. If sera specimens were available for VZV avidity testing [12], this could have helped to distinguish between varicella and herpes zoster cases. Lastly, it is possible that some patients had co-infections with varicella and monkeypox that were not identified if both viruses were not present in the lesion specimens collected for testing. However, this is unlikely given that the majority of cases had > 1 lesion sampled and tested for both monkeypox and VZV. As it is possible that not all lesions would contain both viruses in co-infected cases, future studies may consider including serologic testing for monkeypox and VZV.
A vaccine against varicella is available; the effectiveness is 81% for 1-dose and 92% for 2-doses [46]. Africa has many competing public health priorities and routine varicella vaccination is not currently being considered in any African country [6, 10]. The estimated global burden of varicella is considerably lower than that of other vaccine-preventable diseases such as measles, pertussis, rotavirus, or invasive pneumococcal disease. Based on two small studies, varicella mortality has been found to be 20–50 times higher in developing countries versus developed countries, with an estimated case fatality rate of 50–100/100,000 in Guinea Bissau and India [1, 47, 48], potentially due to the high proportion of the population with HIV/AIDS and malnutrition; sub-optimal healthcare infrastructure; and challenges with access to care [49]. Nonetheless, as other vaccine-preventable diseases are controlled, varicella may represent an important preventable disease burden, with significant healthcare and societal costs. WHO recommends varicella vaccination be considered in countries where varicella is an important public health burden and vaccination coverage can be maintained at ≥80%. Countries with a high average age (≥15 years of age) of infection could consider vaccination of susceptible adolescents and adults [3, 10, 21]. Before a varicella vaccination program can be considered, adequate surveillance system would be needed to determine the burden of varicella for the region.
Information from this monkeypox surveillance contributes to the scarce published data on varicella in Africa. We document challenges with identifying varicella based on clinical symptoms alone and in the context of monkeypox surveillance. The non-typical symptoms in the varicella cases in this population were reported in a small number of other described cases [11]. It is possible that these non-typical symptoms are common in varicella cases in this population; without varicella surveillance data, it is unknown how varicella typically presents in this population. Continued surveillance and laboratory diagnosis will be helpful for rapid identification and control of both monkeypox and varicella, refining the case definition for varicella, and improving our understanding of the epidemiology of varicella in an region endemic for these two diseases and in Africa.
Acknowledgements
We would like to thank Mary Ann Hall, MPH, for her editorial review of the manuscript.
Funding Details: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, US Department of Health and Human Services.
Conflicts of interest:
None
References
- 1.Seward JF, Marin M; On bahlf of the VZV Sage Working Group. Varicella Disease Burden and Varicella Vaccines. Available at: https://www.scribd.com/document/365929945/2-SAGE-April-VZV-Seward-Varicella. Accessed 12 April 2018.
- 2.Gershon A, Takahashi M, and Seward JF, Varicella vaccine, in Vaccines, Plotkin S, Orenstein W, and Offit P, Editors. 2008, Saunders Elsevier. p. 915–958. [Google Scholar]
- 3.WHO. World Health Organization, SAGE working group on varicella and herpes zoster vaccines. Background paper on varicella vaccine. Available at http://www.who.int/immunization/sage/meetings/2014/april/1_SAGE_varicella_background_paper_FINAL.pdf, last accessed April 20, 2016. 2014. [Google Scholar]
- 4.Hirose M, et al. , The impact of varicella vaccination on varicella-related hospitalization rates: global data review. Rev Paul Pediatr, 2016. 34(3): p. 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spoulou V, et al. , Implementing Universal Varicella Vaccination in Europe: The Path Forward. Pediatr Infect Dis J, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Varela FH, Pinto LA, and Scotta MC, Global impact of varicella vaccination programs. Hum Vaccin Immunother, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wutzler P, et al. , Varicella vaccination - the global experience. Expert Rev Vaccines, 2017. 16(8): p. 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Admani B, Macharia WM, and Were F, Seroprevalence of varicella zoster antibodies among children with malnutrition, malignancies and HIV infection. East Afr Med J, 2008. 85(10): p. 480–6. [DOI] [PubMed] [Google Scholar]
- 9.Doshi RH, et al. , Low Varicella Zoster Virus Seroprevalence Among Young Children in the Democratic Republic of the Congo. Pediatr Infect Dis J, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussey H, et al. , Varicella zoster virus-associated morbidity and mortality in Africa - a systematic review. BMC Infect Dis, 2017. 17(1): p. 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macneil A, et al. , Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin Infect Dis, 2009. 48(1): p. e6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacNeil A, et al. , Monkeypox or varicella? Lessons from a rash outbreak investigation in the Republic of the Congo. Am J Trop Med Hyg, 2009. 80(4): p. 503–7. [PubMed] [Google Scholar]
- 13.Nysse LJ, et al. , Seroprevalence of antibody to varicella among Somali refugees. Mayo Clin Proc, 2007. 82(2): p. 175–80. [DOI] [PubMed] [Google Scholar]
- 14.Osadebe L, et al. , Enhancing case definitions for surveillance of human monkeypox in the Democratic Republic of Congo. PLoS Negl Trop Dis, 2017. 11(9): p. e0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas EJ, et al. , Use of vaccination in a large outbreak of primary varicella in a detention setting for African immigrants. Int Health, 2014. 6(3): p. 203–7. [DOI] [PubMed] [Google Scholar]
- 16.Lesens O, et al. , Varicella outbreak in Sudanese refugees from Calais. J Travel Med, 2016. 23(5). [DOI] [PubMed] [Google Scholar]
- 17.Lee BW, Review of varicella zoster seroepidemiology in India and Southeast Asia. Trop Med Int Health, 1998. 3(11): p. 886–90. [DOI] [PubMed] [Google Scholar]
- 18.Nichols RA, et al. , Household size is critical to varicella-zoster virus transmission in the tropics despite lower viral infectivity. Epidemics, 2011. 3(1): p. 12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauerbrei A, Differences in varicella-zoster virus seroepidemiology between temperate and tropical regions. Indian J Med Sci, 2007. 61(3): p. 123–4. [PubMed] [Google Scholar]
- 20.Sengupta N and Breuer J, A global persepctive of the epidemiology and burden of varicella-zoster virus. Curr Pediatr Rev, 2009. 5: p. 207–228. [Google Scholar]
- 21.Heininger U and Seward JF, Varicella. Lancet, 2006. 368(9544): p. 1365–76. [DOI] [PubMed] [Google Scholar]
- 22.Arvin AM and Gilden D, Varicella-Zoster Virus, in Fields Virology. 2013. p. 2016–2057. [Google Scholar]
- 23.McCollum AM and Damon IK, Human monkeypox. Clin Infect Dis, 2014. 58(2): p. 260–7. [DOI] [PubMed] [Google Scholar]
- 24.Jezek Z, et al. , Human monkeypox: confusion with chickenpox. Acta Trop, 1988. 45(4): p. 297–307. [PubMed] [Google Scholar]
- 25.Nations United. DESA Population Division. World Population Prospects 2017. Available at: https://esa.un.org/unpd/wpp/. Accessed 12 April 2018. [Google Scholar]
- 26.Rose AN, et al. , LandScan 2017. [digital raster data](https://landscan.ornl.gov/). Accessed 22 Aug 2018. [Google Scholar]
- 27.Bass J, et al. , Enhancing health care worker ability to detect and care for patients with monkeypox in the Democratic Republic of the Congo. Int Health, 2013. 5(4): p. 237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loparev VN, et al. , Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J Virol, 2004. 78(15): p. 8349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen NJ, et al. , Revisiting the genotyping scheme for varicella-zoster viruses based on whole-genome comparisons. J Gen Virol, 2017. 98(6): p. 1434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng HF, et al. , Herpes zoster caused by vaccine-strain varicella zoster virus in an immunocompetent recipient of zoster vaccine. Clin Infect Dis, 2014. 58(8): p. 1125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.QGIS Development Team (YEAR). QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org. [Google Scholar]
- 32.Auvin S, et al. , Atypical varicella with palm and sole involvement. Int J Dermatol, 2002. 41(12): p. 903–5. [DOI] [PubMed] [Google Scholar]
- 33.Kipps A and Becker WB, The diagnosis of atypical varicella. S Afr Med J, 1971. 45(30): p. 839–40. [PubMed] [Google Scholar]
- 34.Nagore E, et al. , Atypical involvement of the palms and soles in a varicella infection. Acta Derm Venereol, 1999. 79(4): p. 322. [DOI] [PubMed] [Google Scholar]
- 35.Democratic Republic of Congo Ministry of Health. Integrated Behavioral Surveillance Survey and Seroprevalence in Democratic Republic of Congo. March 2014. [Google Scholar]
- 36.Democratic Republic of Congo Ministry of Public Health. National programme to fight HIV/AIDS and STI. Epidemiological Report on HIV/AIDS Surveillance in Pregnant Women Frequenting the Serivces of EIC-2013. September 2014. [Google Scholar]
- 37.Schmidt-Chanasit J and Sauerbrei A, Evolution and world-wide distribution of varicella-zoster virus clades. Infect Genet Evol, 2011. 11(1): p. 1–10. [DOI] [PubMed] [Google Scholar]
- 38.Rimoin AW, et al. , Endemic human monkeypox, Democratic Republic of Congo, 2001–2004. Emerg Infect Dis, 2007. 13(6): p. 934–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garnett GP, et al. , The age of infection with varicella-zoster virus in St Lucia, West Indies. Epidemiol Infect, 1993. 110(2): p. 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lolekha S, et al. , Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am J Trop Med Hyg, 2001. 64(3–4): p. 131–6. [DOI] [PubMed] [Google Scholar]
- 41.Mahamud A, et al. , Varicella zoster virus in American Samoa: seroprevalence and predictive value of varicella disease history in elementary and college students. Epidemiol Infect, 2014. 142(5): p. 1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maretic Z and Cooray MP, COMPARISONS BETWEEN CHICKENPOX IN A TROPICAL AND A EUROPEAN COUNTRY. J Trop Med Hyg, 1963. 66: p. 311–5. [PubMed] [Google Scholar]
- 43.Masuet-Aumatell C, et al. , Seroprevalence of varicella-zoster virus infection in children from Cochabamba: tropical or temperate pattern? Trop Med Int Health, 2013. 18(3): p. 296–302. [DOI] [PubMed] [Google Scholar]
- 44.Wharton M, The epidemiology of varicella-zoster virus infections. Infect Dis Clin North Am, 1996. 10(3): p. 571–81. [DOI] [PubMed] [Google Scholar]
- 45.Poulsen A, et al. , Varicella zoster in Guinea-Bissau: intensity of exposure and severity of infection. Pediatr Infect Dis J, 2005. 24(2): p. 102–7. [DOI] [PubMed] [Google Scholar]
- 46.Marin M M. M, Kambhampati A, Jeram SM, Seward JF., Global Varicella Vaccine Effectiveness: A Meta-analysis. Pediatrics published online: Feb 16, 2016. doi: 10.1542. 2016 [DOI] [PubMed] [Google Scholar]
- 47.Jezek Z, et al. , Fever with rash surveillance in India. Indian J Public Health, 1978. 22(1): p. 120–6. [PubMed] [Google Scholar]
- 48.Poulsen A, et al. , Growth, morbidity and mortality after chickenpox infection in young children in Guinea-Bissau. J Infect, 2005. 51(4): p. 307–13. [DOI] [PubMed] [Google Scholar]
- 49.Boyers LN, et al. , Global mortality from conditions with skin manifestations. J Am Acad Dermatol, 2014. 71(6): p. 1137–1143.e17. [DOI] [PubMed] [Google Scholar]


