Abstract
Objective
We aimed to look at the burden of disease caused by SARS-COV-2 reinfections and identified potential risk factors for disease severity.
Methods
We used national surveillance data to collect information on all SARS-CoV-2 primary infection and suspected reinfection cases between January 2020 until early May 2021. Reinfection cases were positive COVID-19 PCR or antigen test, 90 days after their first COVID-19 positive test. We collected information on case demographics, hospital and ICU admission, immunisation status and if individuals were at risk of complication for COVID-19.
Results
Deaths reported within 28 days of testing positive were 61% (95% confidence interval: 56% to 65%) lower in suspected COVID-19 reinfection than primary infection cases. In the unvaccinated cohort, reinfections were associated with 49% (37% to 58%) lower odds of hospital admission in cases aged 50 to 65 years in the population not identified at risk of complication for COVID-19, and 34% (17% to 48%) in those at risk. ICU admission at reinfection compared to primary infection decreased 76% (55% to 87%). Individuals at risk and those aged below 50 years, who received at least 1 dose of vaccine against COVID-19, were 62% (39% to 74%) and 58% (24% to 77%) less likely to get admitted to hospital at reinfection, respectively.
Conclusion
Prior SARS-CoV-2 infection was associated with lower odds of dying, and both prior infection and immunisation showed a protective effect against severe disease in selected populations. Older age, sex and underlying comorbidities appeared as principal risk factors for illness severity at reinfection.
Funding
PHE/UKHSA
Keywords: SARS-CoV-2, COVID-19, Reinfection, Primary infection, Disease severity, Immunisation
Introduction
The first documented case of confirmed SARS-Cov-2 reinfection was in Hong Kong in August 2020. Reinfection status was ascertained by comparison of genetic sequences from each episode.1 , 2 Since then, multiple accounts of SARS-Cov-2 reinfections have been reported worldwide.1, 2, 3, 4
Primary viral infections are the first exposure of a susceptible individual to the pathogen while reinfections are subsequent infections following a primary infection by the same pathogen; these may be due to reactivation of a latent virus (endogenous) or a new infection (exogenous). With exogenous reinfection, circulating antibody does not necessarily protect an individual against infection.5 Viral reinfections caused by parainfluenza, respiratory syncytial virus and other strains of coronavirus are not uncommon in children nor in healthy adults.6 , 7 , 8
Like with other viral reinfections, it is challenging to prove that individuals have been infected with SARS-CoV-2 on two separate occasions and to exclude persistent infection or viral reactivation.9 On an individual basis this may be done by looking at the Ct values of confirmatory PCR samples (denoting viral load) at each episode, an absence of symptoms between each episode, and by comparing the viral genomes collected for each episode of illness.9 This is more challenging on a population level. Serological markers indicative of reinfection (high IgG, low IgM) are often used to distinguish primary and re-infections for other viral illness but these are not yet well-defined for SARS-CoV-2.
European Centre for Disease Control (ECDC) defined a suspected COVID-19 reinfection case as: a positive Polymerase Chain Reaction (PCR) test or rapid antigen test (RAT) sample (also called Lateral Flow Devices (LFD) in the UK) 60 days or more after having previously tested positive through PCR testing, RAT/LFD testing or through serology (anti-spike IgG Ab) testing.10 In England an interval of 90 days between two consecutive samples has been applied in the SIREN study and to published population-level surveillance data on reinfection,11 to exclude most persistent infections which may arise in those with underlying immunodeficiency.
UK testing facilities have expanded with the COVID-19 pandemic enabling a 7-day average test number in excess of 100,000 since 28th June 2020.12 As of 27th September 2021, about 14.6% of SARS-CoV-2 samples have been sequenced in the UK.13 This has enabled the UK to monitor reinfections through positive test intervals complimented with sequencing in a proportion of possible reinfections.
Numbers of suspected and confirmed SARS-COV-2 reinfections are increasing as the pandemic continues and the population eligible to be reinfected (those with a first infection) rises.11 It is crucial to estimate the burden and impact of SARS-CoV-2 reinfection on the general population to inform modelling and adequate mitigating policies.
By utilising data from the COVID-19 surveillance systems in place in England, this study reports the incidence of possible SARS-COV-2 reinfections and estimates its severity relative to the first episode of infection while identifying potential risk factors.
Methods
Routine laboratory reports of COVID-19
PCR testing was available either through NHS testing of patients or health care workers, or through community testing as part of the government strategy to offer mass testing to the wider population using commercial partners. All positive PCR/LFD tests were reported to UK Health Security Agency's (UKHSA, formerly PHE) Second Generation Surveillance System (SGSS), using basic demographics (name, date of birth, sex, ethnicity (community testing only), geography and NHS number).
The SGSS dataset was used to obtain results for all positive SARS-CoV-2 tests between 27th January 2020 and 2nd May 2021.
Defining a reinfection
Primary or first infections were defined by the first SARS-CoV-2 positive PCR or LFD test result for an individual. A possible reinfection (referred in this paper as reinfection) was characterised by sequential positive PCR or LFD SARS-CoV-2 tests with a minimum interval of 90 days. To exclude likely persistent SARS-COV-2 infections, individuals could not test SARS-CoV-2 positive during this 90-day interval. People that test positive for SARS-CoV-2 through LFD testing have been advised to take a confirmatory PCR test from 29th March 2021 and those who subsequently test PCR negative within 3 days were removed from the data set. It is also advised that further testing should not be done within 90 days of a positive SARS-CoV-2 test result unless new symptoms arise.
34 individuals tested positive for COVID-19 on three separate occasions with a minimum interval of 90 days between each episode. These individuals were retained in the suspected reinfection dataset, but their third episode of illness was excluded from the analysis.
Hospitalisations
The Secondary Uses Service (SUS)14 collects NHS healthcare data about an individual that has received treatment or care in secondary care in England. SUS was used to identify reinfection cases that were admitted to hospital and/or ICU (Main speciality =192 or OPCS code E85, E89, X58 or X52 in any of the first 12 procedure codes) within 21 days of testing positive for SARS-CoV-2 for each of their two episodes of illness. Data was linked using patient NHS number, specimen date for either episode and/or a specimen identifier.
Vaccination status
Reinfection cases were linked to records on the National Immunisation Management System (NIMS) using NHS number, date of birth, first name and surname. NIMS was commissioned in August 2020 and records influenza and COVID-19 vaccination details across England including immunisation date, vaccine batch and manufacturer and flags people at highest risk of complication for COVID-19. There are two categories for individuals at high risk: those clinically extremely vulnerable (CEV) as determined on the 26th November 2020 and high-risk individuals. High risk individuals were detected through a combination of factors including age, sex, body mass index (BMI) and a list of specific health conditions approved by the Chief Medical Officer (CMO) for England.15 It covered solid organ transplants, specific cancers, severe respiratory conditions, rare diseases, immunosuppressive therapies and congenital heart disease.16 CEV people are included in those identified at high risk but have health conditions exceeding an agreed threshold.
To be considered vaccinated, an interval of at least 14 days between the date of the first dose of vaccine and the SARS-CoV-2 positive test date was required to allow induction of an immune response. Individuals who received one dose of vaccine and went on to test SARS-CoV-2 positive within 14 days were therefore excluded from the analysis. Those who tested positive within 14 days of a second vaccine dose were categorised as having received a single dose.
Reinfections were separated into two groups that were analysed separately; the “healthy cohort” that were not flagged COVID-19 at risk nor CEV, and those categorised at-risk <65 years. Individuals were only flagged at risk until 65 years of age at which point age is considered a risk.
Timeline
First infections and reinfections confirmed between week 5 2020 and week 17 2021, ending 2nd May 2021 were included in the analysis with data on infections extracted on 11th May 2021. This was before the emergent Delta variant became dominant in England. By the end of April, the variant distribution in England was estimated to be 75% Alpha, and 25% Delta plus other variants17.
Deaths
COVID-19 deaths were identified using the UKHSA COVID-19 deaths data series. The data series uses data on laboratory confirmed cases to identify deaths amongst persons with COVID-19. The data series combines four sources, by checking vital status of SARS-CoV-2 laboratory reports to the NHS clinical spine, and triangulating death reports notified to NHS England by NHS trusts, deaths notified to local UKHSA health protection teams, and death registrations where COVID-19 is recorded on the death certificate provided by the Office for National Statistics (ONS). For SARS-CoV-2 first infections and reinfections, a death was considered COVID-19 related where the individual died within 28 days of testing positive for SARS-Cov-2. Such deaths were identified through linkage with ONS registered deaths as extracted on 2nd June 2021.
Statistical analysis
All statistical analyses were completed using STATA® 15.1. to produce odds ratios with 95% confidence intervals. Multivariable logistic regression was used to calculate the adjusted odd ratios to ascertain variables that were risk factors for reinfection compared to first infection, this includes age, sex, ethnicity and region of test (which is not included in the results for constant lack of statistical significance in the models). We also compared the odds of dying after a primary infection and after reinfection.
Data on hospital and ICU admission were used to model disease severity in the population that experienced a reinfection. The reinfection population was categorised into several groups. Firstly, the unvaccinated population was analysed. When looking at hospital admission, the population not identified at risk of complication for COVID-19 and the at-risk population were analysed separately. Within the population not at risk, the population aged above 50 were analysed separately to the population aged below 50 to account for the interaction between age and reinfection. It was not possible to do separate analyses for ICU admission because of the low number of admitted patients at reinfection.
The analysis was then restricted to second episodes of infection occurring between January and April 2021, when mass COVID-19 immunisation was being rolled out according to priority based on clinical risk (including age) and risk of exposure.18 Disease severity was analysed through hospital and ICU admission and vaccine status at time of reinfection was ascertained. For hospital admissions, as before, at-risk individuals and those not categorised at-risk were modelled separately.
A potential risk factor was included in our model if the likelihood ratio test p-value was <0.05. All multivariable logistic regression models were tested for the linear effect of age by comparing models with age as linear to models with age as categorical. If the model with age as categorical gave a significantly improved fit age was entered as a categorical variable. The interaction between age and episode of illness (re-infection status) was assessed and, this clearly indicated different effects of re-infection status by age we presented the results within the population aged 49 years old and younger and 50 years old and over for the non at-risk population separately.
The number of ICU admissions was low in the younger age groups; thus the populations were divided into working age population (20 to 64 years old) and older individuals (65 years old or more).
Role of the funding source
This study was funded by PHE which became part of UKHSA on 1st October 2021. The authors are all employed by UKHSA, and were responsible for the study design, data collection, data analysis, data interpretation, and writing of the report.
Ethical approval
PHE has legal permission, provided by Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002, to process patient confidential information for national surveillance of communicable diseases and as such, individual patient consent is not required. The reinfection surveillance work was reviewed by PHE ethics.
Results
Between 27th January 2020 and 2nd May 2021 there were 3860,054 first infections of SARS-Cov-2 in England with 13,960 COVID-19 reinfections; an overall reinfection rate of 3.62 per thousand first infections.
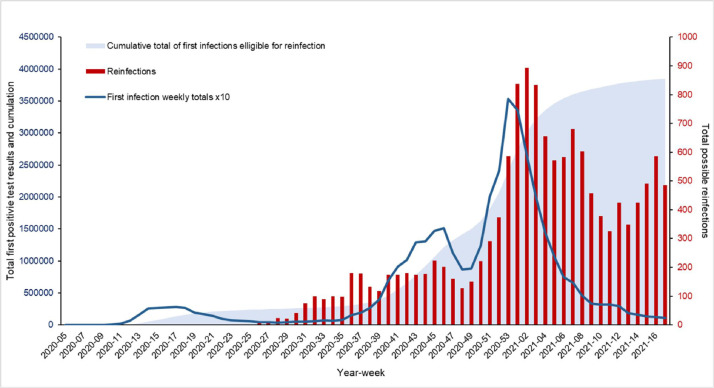
The first recorded SARS-COV-2 reinfection case was in early June 2020 (Week 24). Since then, as the cumulative total of individuals first infected and eligible for SARS-COV-2 reinfection has grown, the number of suspected reinfection cases has fluctuated, mirroring trends seen in incidence rates of first infection; including the second epidemic wave of COVID-19 in December 2020 and January 2021 (Fig. 1 ).
Fig. 1.
Weekly cumulative total of SARS-CoV-2 infection eligible for reinfection (within 90 days interval), weekly number of possible SARS-CoV-2 reinfection and primary infection (times 10) in all ages, England, January 2020 April 2021.
Since April 2021, the weekly number of SARS-COV-2 reinfections has risen, likely due to both increasing numbers of first infections eligible for reinfection and escalating rates of COVID-19 in the community likely due to the emergence of the more infectious Delta variant.
Risk factors for suspected SARS-CoV-2 reinfection and deaths after primary infection or reinfection
Male and female distribution was similar in first infections (women=53%) whilst the proportion of females with reinfection was higher (67%). The median age at first episode was lower at 39 years (Interquartile Range (IQR): 25–55) than for COVID-19 reinfection at 48 years (IQR: 31–65).
Men were 42% less likely to experience a SARS-CoV-2 reinfection compared to women (adjusted OR (aOR)=0.58, 95% CI: 0.56–0.60) (Table 1 ). Individuals under 20 years old had significantly lower odds of becoming a reinfection than 20 to 29-year-olds: aOR=0.49 (95% CI: 0.45–0.53). The odds of reinfection rose overall with each increase in age category, however confidence intervals overlapped between some age groups (Table 1).There was a marked increase in the risk of reinfection in the oldest age categories: 70 to 79 years aOR=1.73 (95% CI: 1.60–1.87) and, 80 plus years old aOR= 3.19 (95% CI: 3.01–3.39) (Table 1).
Table 1.
SARS-CoV-2 primary infection and possible reinfection by sex, age, ethnicity in the population crude and adjusted odds ratio, January 2020 until April 2021, England.
| COVID-19 infections | ||||||
|---|---|---|---|---|---|---|
| Variable | Category | Primary infections n(%) | Reinfections n(%) | Crude OR(95% CI) | Adjusted* OR(95% CI) | LRT p-value |
| Sex n = 3845,990 | Women | 2050,929 (53.52) | 9316 (67.28) | 1 | 1 | |
| Men | 1781,214 (46.48) | 4531 (32.72) | 0.56 (0.54–0.58) | 0.58 (0.56–0.60) | p<0.0001 | |
| Age group (Years) n = 3865,600 | <20 | 587,044 (15.24) | 866 (6.20) | 0.48 (0.44–0.52) | 0.49 (0.45–0.53) | |
| 20 to 29 | 705,245 (18.31) | 2178 (15.61) | 1 | 1 | ||
| 30 to 39 | 687,201 (17.84) | 2151 (15.41) | 1.01 (0.95–1.08) | 1.02 (0.96–1.08) | ||
| 40 to 49 | 599,209 (15.56) | 2070 (014.83) | 1.12 (1.05–1.19) | 1.12 (1.06–1.19) | ||
| 50 to 59 | 573,819 (14.90) | 2326 (16.67) | 1.31 (1.24–1.39) | 1.33 (1.25–1.41) | ||
| 60 to 69 | 307,054 (7.97) | 1250 (8.96) | 1.32 (1.23–1.41) | 1.35 (1.26–1.45) | ||
| 70 to 79 | 173,946 (4.52) | 902 (6.46) | 1.68 (1.56–1.82) | 1.73 (1.60–1.87) | ||
| 80+ | 218,125 5.66) | 2214 (15.86) | 3.29 (3.10–3.49) | 3.19 (3.01–3.39) | ||
| Ethnicity n = 3016,037 | Asian | 453,519 (15.10) | 1648 (12.39) | 0.79 (0.75–0.83) | 0.59 | |
| Black | 131,234 (4.37) | 576 (4.33) | 0.95 (0.87–1.03) | |||
| Mixed | 71,850 (2.39) | 202 (1.52) | 0.61 (0.53–0.70) | |||
| Other | 56,741 (1.89) | 282 (2.12) | 1.07 (0.95–1.21) | |||
| White | 2289,391 (76.24) | 10,594 (79.64) | 1 | |||
Odd ratios adjusted for all variable in table provided the p-value for the likelihood ratio test is <0.05.
Death within 28 days testing positive for COVID-19
Overall death rate in this dataset was 2863 per 100,000 first infections and 2851 per 100,000 reinfections.
Men were at an elevated risk of dying from COVID-19 when compared to women aOR=1.83 (95% CI: 1.81–1.86). Age and sex were the strongest confounders of the association between infection episode and death, with crude OR= 1.0 (95% CI: 0.90–1.10) showing no significant difference for death within 28 days of testing positive for COVID-19 in reinfection compared to primary infection cases. After adjusting, there was a 61% decline for deaths reported within 28 days (aOR=0.39, 95% CI: 0.35–0.44) of reinfection compared to deaths from first infection (Table 2 ).
Table 2.
Alive and death status 28 days after testing positive for SARS-CoV-2 by sex, age, ethnicity and episode of infection in the population crude and adjusted odds ratio, January 2020 until April 2021, England.
| COVID-19 deaths 28 days after testing positive | ||||||
|---|---|---|---|---|---|---|
| Variable | Category | Alive n(%) | Deceased n(%) | Crude OR(95% CI) | Adjusted OR(95% CI) | LRT p-value |
| Sex n = 3845,990 | Women | 2011,881 (97.65) | 48,364 (2.35) | 1 | 1 | |
| Men | 1726,349 (96.67) | 59,396 (3.33) | 1.43 (1.41–1.45) | 1.83 (1.81–1.86) | p<0.0001 | |
| Age group (Years) n = 3865,600 | <20 | 587,841 (99.99) | 69 (0.01) | 0.48 (0.36–0.63) | 0.47 (0.36–0.62) | p<0.0001 |
| 20 to 29 | 707,250 (99.98) | 173 (0.02) | 1 | 1 | ||
| 30 to 39 | 688,816 (99.92) | 536 (0.08) | 3.18 (2.68–3.78) | 3.18 (2.67–3.77) | ||
| 40 to 49 | 599,655 (99.73) | 1624 (0.27) | 11.07 (9.46–12.95) | 11.04 (9.43–12.92) | ||
| 50 to 59 | 571,075 (99.12) | 5070 (0.88) | 36.29 (31.18–42.25) | 36.15 (31.05–42.08) | ||
| 60 to 69 | 296, 811 (96.27) | 11,493 (3.73) | 158 (136–184) | 155 (133–180) | ||
| 70 to 79 | 149,456 (85.48) | 25,392 (14.52) | 695 (592–815) | 685 (590–797) | ||
| 80+ | 156,867 (71.19) | 63,472 (28.81) | 1654 (1395–1962) | 1785 (1536–2073) | ||
| Ethnicity n = 3016,037 | Asian | 453,693 (99.68) | 1474 (0.32) | 0.46 (0.43–0.48) | 0.15 | |
| Black | 131,447 (99.72) | 363 (0.28) | 0.39 (0.35–0.43) | |||
| Mixed | 71,997 (99.92) | 55 (0.08) | 0.11 (0.08–0.14) | |||
| Other | 56,926 (99.83) | 97 (0.17) | 0.24 (0.20–0.29) | |||
| White | 2283,810 (99.30) | 16,175 (0.70) | 1 | |||
| Episode n = 3874,014 | Primary infection | 3752,595 (97.22) | 107,459 (2.78) | 1 | 1 | |
| Reinfection | 13,573 (97.23) | 387 (2.77) | 1.0 (0.90- 1.10) | 0.39 (0.35–0.44) | p<0.0001 | |
*Odd ratios adjusted for all variable in table provided the p-value for the likelihood ratio test is <0.05.
Risk factors for SARS-CoV-2 infection severity in the refection case population
During the study period, in England, 10.31% of all SARS-COV-2 primary infection cases were admitted to hospital.12 Over the same period 2552 of 13,636 (13.71%) individuals with reinfection had a recorded hospital admission at one or both episodes; 1747 (12.80%) were admitted during their first episode of illness and 1413 (10.37%) during their second.
Unvaccinated population
Of the 13,636 reinfections, immunisation records were linked for 10,299 individuals (75.5%) using the NIMS database. Of these, 9228 (84%) had not received any dose of COVID-19 vaccine before primary infection and, 5858 (53%) before reinfection.
Admission to hospital within 21 days of testing positive
Between January 2020 and April 2021, hospital admission in the COVID-19 not at-risk population showed no statistically significant difference, in those aged below 50-years, between primary infection and reinfection (aOR=1.09 (95% CI:0.81–1.46) while, in the population aged over 50 years old, there was a 49% (37% to 58%) decline in odds of hospital admission during reinfection (Table 3 ).
Table 3.
Hospital admission during SARS-CoV-2 infection in unvaccinated population by sex, age, ethnicity, and months of test in 1) <50 years 2) 50 years and above individuals not identified at risk of complication for COVID-19 and 3) individuals identified at risk, January 2021 until April 2021, England.
| Hospital admission in people not identified at risk of complication for COVID-19 | ||||||
|---|---|---|---|---|---|---|
| Variable | Category | Not admitted n(%) | Hospital admission n(%) | Crude OR(95% CI) | Adjusted* OR(95% CI) | LRT p-value |
| under 50 years | ||||||
| Episode n = 3535 | Primary infection | 3428 (97.00) | 106 (3.00) | 1 | 1 | |
| reinfection | 2554 (96.82) | 84 (3.18) | 1.06 (0.80–1.42) | 1.09 (0.81–1.46) | ||
| Age group (Years) n = 6173 | <20 | 474 (97.53) | 12 (2.47) | 1.00 (0.53–1.90) | 1.20 (1.03–1.41) | 0.01 |
| 20 to 29 | 2020 (97.54) | 51 (2.46) | 1 | |||
| 30 to 39 | 1853 (96.81) | 61 (3.19) | 1.30 (0.89–1.90) | |||
| 40 to 49 | 1636 (96.12) | 66 (3.88) | 1.60 (1.10–2.32) | |||
| Sex n = 6144 | Women | 4176 (96.89) | 134 (3.11) | 1 | 1 | 0.70 |
| Men | 1781 (97.11) | 53 (2.89) | 0.93 (0.67–1.28) | 0.94 (0.68–1.30) | ||
| Ethnicity n = 5687 | Asian | 916 (96.12) | 37 (3.88) | 1.28 (0.88–1.86) | 0.69 | |
| Black | 239 (97.55) | 6 (2.45) | 0.80 (0.35–1.83) | |||
| Mixed | 109 (95.61) | 5 (4.39) | 1.46 (0.58–3.63) | |||
| Other | 173 (96.11) | 7 (3.89) | 1.29 (0.59–2.79) | |||
| White | 4067 (96.95) | 128 (3.05) | 1 | |||
| 50 years and older | ||||||
| Episode n = 4806 | Primary infection | 2593 (83.92) | 497 (16.08) | 1 | 1 | |
| reinfection | 1566 (91.26) | 150 (8.74) | 0.50 (0.41–0.61) | 0.51 (0.42–0.63) | ||
| Age group (Years) n = 4808 | 50 to 59 | 1554 (95.99) | 65 (4.01) | 1 | 1 | p<0.0001 |
| 60 to 69 | 682 (86.66) | 105 (13.34) | 3.68 (2.65–5.10) | 3.36 (2.41–4.68) | ||
| 70 to 79 | 432 (68.14) | 202 (31.86) | 11.18 (8.08–15.47) | 9.45 (6.90–12.95) | ||
| 80+ | 1493 (84.45) | 275 (15.55) | 4.40 (3.31–5.85) | 4.33 (3.23–5.79) | ||
| Sex n = 4801 | Women | 2978 (90.00) | 331 (10.00) | 1 | 1 | p<0.0001 |
| Men | 1177 (78.89) | 315 (21.11) | 2.41 (2.03–2.86) | 2.03 (1.70–2.44) | ||
| Ethnicity n = 4553 | Asian | 248 (85.22) | 43 (14.78) | 1.07 (0.77–1.50) | 1.72 (1.19–2.48) | 0.02 |
| Black | 90 (92.78) | 7 (7.22) | 0.48 (0.22–1.05) | 0.66 (0.30–1.47) | ||
| Mixed | 33 (97.06) | 1 (2.94) | 0.19 (0.03–1.38) | 0.36 (0.05–2.66) | ||
| Other | 49 (81.67) | 11 (18.33) | 1.39 (0.72–2.69) | 1.52 (0.75–3.06) | ||
| White | 3505 (86.10) | 566 (13.90) | 1 | |||
| Hospital admission in people identified at risk of complication for COVID-19 | ||||||
| Episode n = 3088 | Primary infection | 1665 (85.47) | 283 (14.53) | 1 | 1 | |
| reinfection | 1024 (89.82) | 116 (10.18) | 0.67 (0.53–0.84) | 0.66 (0.52–0.83) | ||
| Age group (Years) n = 3088 | <20 | 42 (89.36) | 5 (10.64) | 1.36 (0.50–3.70) | 1.17 (1.07–1.28) | p<0.0001 |
| 20 to 29 | 343 (91.96) | 30 (8.04) | 1 | |||
| 30 to 39 | 465 (88.74) | 59 (11.26) | 1.45 (0.91–2.30) | |||
| 40 to 49 | 604 (89.75) | 69 (10.25) | 1.31 (0.83–2.05) | |||
| 50 to 59 | 881 (84.55) | 161 (15.45) | 2.09 (1.38–3.15) | |||
| 60 to 65 | 354 (82.52) | 75 (17.48) | 2.42 (1.54–3.81) | |||
| Sex n = 3076 | Women | 1927 (90.81) | 195 (9.19) | 1 | 1 | p<0.0001 |
| Men | 751 (78.72) | 203 (21.28) | 2.67 (2.15–3.32) | 2.40 (1.92–2.99) | ||
| Ethnicity n = 2970 | Asian | 354 (85.51) | 60 (14.49) | 1.18 (0.87–1.59) | 0.81 | |
| Black | 150 (86.21) | 24 (13.79) | 1.11 (0.71–1.75) | |||
| Mixed | 58 (90.63) | 6 (9.38) | 0.72 (0.31–1.69) | |||
| Other | 52 (72.22) | 20 (27.78) | 2.68 (1.57–4.56) | |||
| White | 1964 (87.44) | 282 (12.56) | 1 | |||
| CEV n = 3088 | No | 2495 (89.46) | 294 (10.54) | 1 | 1 | p<0.0001 |
| Yes | 194 (64.88) | 105 (35.12) | 4.59 (3.50–6.03) | 3.96 (3.01–5.22) | ||
Odd ratios adjusted for all variable in table provided the p-value for the likelihood ratio test is <0.05.
In the population at risk of COVID-19 complication (Table 3), there was an estimated 34% decrease in hospital admission following a reinfection compared to a primary infection (aOR=0.66, 95% CI:0.52–0.83). The odds of hospital admission in the COVID-19 at-risk group increased by 17% per increase in age group (aOR= 1.17, 95% CI:1.07–1.28).
Ethnicity did not appear to have a clear association with hospital admission once adjusted for other variables and was therefore not included in the final model for either population.
ICU admission
There was only one ICU admission in a person aged under 20 years at their reinfection episode. This was a young child with a severe congenital disorder. Because of the disparity in ICU admission across age categories, age groups were combined to compare the working age population (20 to 64 years) to the older population (65 years old and over).
Adjusted odd ratios for the association between ICU admission and disease episode showed the protective effect of primary infection with a 76% reduction in ICU admission during a SARS-CoV-2 reinfection (aOR= 0.24, 95% CI: 0.13–0.45) (Supplemental Table 1).
During a period of vaccination roll-out
Immunisation of frontline healthcare workers, those with specified underlying health conditions and those aged 80 years and older started from 8th December 2020. The program then moved in stages to offer vaccines to gradually younger age groups.19 This analysis was therefore restricted to reinfection cases during the period 1st January 2021 until 30th April 2021. Table 4 summarises three models that looked at risk of hospital admission in COVID not at-risk and at-risk populations over that period by vaccination status and other factors.
Table 4.
Hospital admission during SARS-CoV-2 reinfection by vaccine status, sex, age, ethnicity, and months of test in 1) <50 years 2) 50 years and above individuals not identified at risk of complication for COVID-19 and 3) individuals identified at risk, January 2021 until April 2021, England.
| Hospital admission in people not identified at risk of complication for COVID-19 | ||||||
|---|---|---|---|---|---|---|
| Variable | Category | Not admitted n(%) | Hospital admission n(%) | OR(95% CI) | Adjusted* OR(95% CI) | LRT p-value |
| under 50 years | ||||||
| Vaccine status n = 2362 | Not vaccinated | 1650 (96.32) | 63 (3.68) | 1 | 1 | |
| At least 1 dose of vaccine | 634 (97.69) | 15 (2.31) | 0.62 (0.35–1.10) | 0.42 (0.23–0.76) | ||
| sex n = 2613 | Women | 1769 (96.40) | 66 (3.60) | 1 | 1 | 0.01 |
| Men | 760 (97.69) | 18 (2.31) | 0.63 (0.37–1.08) | 0.44 (0.24–0.80) | ||
| Age group (Years) n = 2616 | <20 | 206 (97.63) | 5 (2.37) | 0.82 (0.31–2.16) | 1.15 (0.90–1.47) | 0.35 |
| 20 to 29 | 835 (96.87) | 27 (3.13) | 1 | |||
| 30 to 39 | 779 (96.77) | 26 (3.23) | 1.03 (0.60–1.78) | |||
| 40 to 49 | 712 (96.48) | 26 (3.52) | 1.13 (0.65–1.95) | |||
| Ethnicity n = 2410 | Asian | 384 (96.97) | 12 (3.03) | 0.89 (0.48–1.67) | 0.94 | |
| Black | 95 (96.94) | 3 (3.06) | 0.90 (0.28–2.92) | |||
| Mixed | 38 (90.48) | 4 (9.52) | 3.00 (1.04–8.69) | |||
| Other | 71 (97.26) | 2 (2.74) | 0.80 (0.19–3.35) | |||
| White | 1740 (96.61) | 61 (3.39) | 1 | |||
| Months n = 2616 | January | 1025 (98.09) | 20 (1.91) | 1 | 1.50 (1.22–1.84) | 0.0001 |
| February | 703 (96.30) | 27 (3.70) | 1.97 (1.09–3.54) | |||
| March | 441 (96.71) | 15 (3.29) | 1.74 (0.88–3.44) | |||
| April | 363 (94.29) | 22 (5.71) | 3.11 (1.67–5.78) | |||
| 50 years and older | ||||||
| Vaccine status n = 1954 | Not vaccinated | 588 (88.02) | 80 (11.98) | 1 | 1 | |
| At least 1 dose of vaccine | 1058 (82.27) | 228 (17.73) | 1.58 (1.20–2.08) | 0.72 (0.49–1.05) | ||
| sex n = 2336 | Women | 1412 (89.99) | 157 (10.01) | 1 | 1 | p<0.0001 |
| Men | 581 (75.75) | 186 (24.25) | 2.88 (2.27–3.65) | 2.45 (1.89–3.18) | ||
| Age group (Years) n = 2339 | 50 to 59 | 649 (95.58) | 30 (4.42) | 1 | 1 | p<0.0001 |
| 60 to 69 | 345 (86.68) | 53 (13.32) | 3.32 (2.07–5.33) | 2.94 (1.76–4.91) | ||
| 70 to 79 | 266 (68.21) | 124 (31.79) | 10.08 (6.38–15.93) | 9.98 (6.23–15.96) | ||
| 80+ | 736 (84.40) | 136 (15.60) | 4.00 (2.64–6.06) | 4.38 (2.77–6.91) | ||
| Ethnicity n = 2221 | Asian | 107 (82.95) | 22 (17.05) | 1.15 (0.71–1.84) | 0.38 | |
| Black | 43 (93.48) | 3 (6.52) | 0.39 (0.12–1.26) | |||
| Mixed | 9 (81.82) | 2 (18.18) | 1.24 (0.27–5.76) | |||
| Other | 21 (91.30) | 2 (8.70) | 0.53 (0.12–2.28) | |||
| White | 1706 (84.79) | 306 (15.21) | 1 | |||
| Months n = 2339 | January | 773 (90.94) | 77 (9.06) | 1 | 1.45 (1.25–1.69) | p<0.0001 |
| February | 546 (86.80) | 83 (13.20) | 1.53 (1.10–2.12) | |||
| March | 338 (78.42) | 93 (21.58) | 2.76 (1.98–3.85) | |||
| April | 339 (79.02) | 90 (20.98) | 2.67 (1.91–3.73) | |||
| Hospital admission in people identified at risk of complication for COVID-19 | ||||||
| Vaccine status n = 1226 | Not vaccinated | 524 (88.22) | 70 (11.78) | 1 | 1 | |
| At least 1 dose of vaccine | 565 (89.40) | 67 (10.60) | 0.89 (0.62–1.27) | 0.38 (0.24–0.61) | ||
| sex n = 1397 | Women | 887 (91.07) | 87 (8.93) | 1 | 1 | 0.02 |
| Men | 358 (84.63) | 65 (15.37) | 1.85 (1.31–2.62) | 1.31 (0.89–1.93) | ||
| Age group (Years) n = 2339 | <20 | 17 (85.00) | 3 (15.00) | 2.34 (0.59–9.20) | 1.18 (1.02–1.38) | 0.03 |
| 20 to 29 | 159 (92.98) | 12 (7.02) | 1 | |||
| 30 to 39 | 201 (89.73) | 23 (10.27) | 1.52 (0.73–3.15) | |||
| 40 to 49 | 273 (92.23) | 23 (7.77) | 1.12 (0.54–2.31) | |||
| 50 to 59 | 422 (87.92) | 58 (12.08) | 1.82 (0.95–3.49) | |||
| 60 to 65 | 175 (84.13) | 33 (15.87) | 2.50 (1.24–5.04) | |||
| Ethnicity n = 1344 | Asian | 162 (90.00) | 18 (10.00) | 0.91 (0.54–1.54) | 0.97 | |
| Black | 69 (88.46) | 9 (11.54) | 1.07 (0.52–2.21) | |||
| Mixed | 25 (92.59) | 2 (7.41) | 0.66 (0.15–2.81) | |||
| Other | 22 (81.48) | 5 (18.52) | 1.87 (0.69–5.03) | |||
| White | 920 (89.15) | 112 (10.85) | 1 | |||
| Months n = 1399 | January | 439 (93.40) | 31 (6.60) | 1 | 1 | p<0.0001 |
| February | 376 (89.74) | 43 (10.26) | 1.62 (1.00–2.63) | 2.18 (1.25–3.80) | ||
| March | 234 (85.09) | 41 (14.91) | 2.48 (1.51–4.08) | 3.89 (2.11–7.15) | ||
| April | 198 (84.26) | 37 (15.74) | 2.65 (1.59–4.41) | 4.88 (2.56–9.29) | ||
| CEV n = 1399 | No | 1154 (91.44) | 108 (8.56) | 1 | 1 | p<0.0001 |
| Yes | 93 (67.88) | 44 (32.12) | 5.06 (3.32–7.70) | 4.97 (3.16–7.81) | ||
Odd ratios adjusted for all variable in table provided the p-value for the likelihood ratio test is <0.05.
Forty-six percent of the 5567 individuals had received at least one dose of vaccine before their second infection episode. In those under 50-years-old and not at-risk of complication, COVID-19 immunisation had a protective effect against hospital admission with a 58% reduction in hospital admission amongst individuals who received at least one vaccine dose (aOR=0.42, 95% CI: 0.23–0.76) (Table 4). A similar effect was observed in those at-risk (aOR=0.38, 95% CI:0.24–0.61) with age being the single strongest confounder of this association. However, there was no significant reduction in hospital admission in the population aged 50 or older and not identified at-risk (aOR=0.72, 95% CI: 0.49–1.05) but we note that confidence intervals overlap with the reduction observed in the younger age group and in the at-risk group. Older age was associated with increased odds of hospital admissions in both the at-risk group and in those not at-risk aged 50 and over (p-value=0.026 and <0.0001, respectively), however, this association was not observed in those not at-risk aged under 50 years (p-value=0.35; Table 4).
ICU admission was similarly modelled for all cases of possible reinfection that arose in the January to April period. Increased odds of ICU admission were associated with males (aOR=5.11, 95% CI:1.34–19.46) and there was no significant difference in ICU admission amongst the vaccinated and unvaccinated populations at reinfection (Supplemental Table 2).
Discussion
COVID-19 reinfection cases in the population closely followed trends observed in primary infections cases, but overall, SARS-CoV-2 reinfections in England were uncommon in the period studied at 3.62 per 1000 primary infections. Repeat infections are a common occurrence with other coronaviruses at a 12-month interval after the last exposure when the presence of antibodies after primary infection does not always provide protection against subsequent infections and/or disease. 20
When compared to primary infection, COVID-19 reinfection cases had a 61% (56% to 65%) reduced risk of death within 28 days of testing positive for SARS-CoV-2. In the population classified at-risk, both prior infection and immunisation showed a protective effect against hospital admission with 34% (17% to 48%) reduction in odds comparing both episodes of illness and 62% (39% to 76%) reduction at reinfection when comparing vaccine status which aligns with the protective effect against infection and severity found in other vaccine effectiveness and reinfection studies. Vaccine effectiveness in a population of health care workers showed a 70% (55% to 85%) protective effect against infection 21 days after one dose of Pfizer-Biotech BNT162b2 and 85% (74% to 96%) 7 days after the second dose.21 Further evidence of protection against severe disease and death in symptomatic reinfections was also described in those aged 80 years and older after one dose of BNT162b2 with a 43% (33% to 52%) reduced risk of hospital admission within 14 days and a 51% (37% to 62%) reduced risk of death within 21 days of a positive test.22 A large population study on COVID-19 reinfection demonstrated that primary infection offered 81% (75% to 85%) protection against repeat infection.23
When looking at both episodes of illness, in the unvaccinated population not identified at-risk for COVID-19 complications, prior infection was associated with a 49% (37% to 58%) reduction in hospital admission within 21 days of testing positive in the older population while the results were not significant in the younger age cohort as the confidence intervals overlapped (aOR=0.72, 95% CI: 0.49–1.05).
Older age (55+) has been described as a major risk factor for COVID-19 infection, reinfection, and severe disease.23, 24, 25 A study from Qatar showed that immunisation with BNT162b2 or mRNA-1273 vaccines following primary infection provided a 85% (95% CI 82–88) reduction in breakthrough reinfection,26 therefore the population with a reinfection described in our study was comprised of a subset of individuals that, despite the protection against subsequent infection and severe disease granted by prior infection and vaccination, went on to experience a statistically uncommon second bout of serious illness with SARS-CoV-2.
Within the younger cohort not identified at-risk of COVID-19 complications, prior infection did not appear to offer substantial protection against severe disease but, having received at least one dose of vaccine conferred a 58% (24% to 77%) reduction in hospital admission at reinfection. Younger individuals without comorbidities are already at a lower risk of suffering from severe COVID-19,27 and the identification of severe disease cases at refection suggests that not all risk factors for severe COVID-19 have been identified in this specific population.
It is difficult to establish a clear pattern of disease severity during reinfection when multiple case series report varying experiences in clinical symptoms with milder, worse or similar severity of disease during reinfection.28, 29, 30 This highlights the heterogeneous nature of the reinfection case population..
Severe COVID-19 can be explained by 1) SARS-CoV-2 virulence allowing it to evade the innate immune response, replicate extensively, and generate an hyperactive adaptative immune response 2) the host inflammatory response including immune-cell hyperactivations and elevated levels of circulating cytokines that lead to a cytokine storm.31 Furthermore, it has been suggested that the detection of neutralising antibodies does not correlate to protection against disease severity but rather is associated with the quality and potency of action of those antibodies.32 It is clear than a multitude of factors play a part in the pathogenesis resulting in asymptomatic, mild and severe COVID-19, for which known and unknown risk factors unaccounted for in this analysis might have confounded the results.
In the cohort of people at-risk of COVID-19 complications, individuals with underlying comorbidities, a risk factor for severe COVID-19 at reinfection,33 that were flagged for CEV had five times increased odds of hospital admission when compared to at-risk individuals not-CEV.
Identifying reinfections relies on identification of primary infection and subsequent testing which is more likely to happen in healthcare settings were people are routinely tested. In England, 76.7% of the NHS workforce is female and women represents 58% of carers across the UK.34 , 35 Thus whilst men were 42% (40% to 44%) less likely to become a reinfection case, we cannot rule out that the high proportion of reinfection amongst females could be in part due to a selection bias arising from higher testing in healthcare workers.
On the other hand, unvaccinated men aged 50 years or older not identified at-risk of COVID-19 complications were almost twice more likely to be admitted to hospital than women. Men also had increased risk of ICU admission and of death within 28 days of testing positive in the overall population. Differences in outcome for SARS-CoV-2 infection have been reported between sexes since the beginning of the pandemic, with higher mortality and severe morbidity observed in men possibly explained by hormonal and/or genetic differences between sexes.36 , 37, 38, 39
Strength and limitations
This study's strength lies in the availability of surveillance data since the beginning of the pandemic and the real-time ascertainment of suspected reinfection cases.
There are nonetheless limitations as the identification of a reinfection case is dependent on the ability to detect primary symptomatic and asymptomatic infection. During the first wave of the epidemic, in March 2020, SARS-CoV-2 testing was limited to people attending hospital, people in care homes and health care workers thus considerably reducing the potential to detect primary infection in the community.
Healthcare workers regularly testing for COVID-19 and older people closely monitored in care homes might be overrepresented in our data. Because suffering reinfection entails surviving primary infection, the people most at risk of dying when first infected will be less represented in the reinfection population, this includes older individuals, people categorised as CEV and COVID-19 at-risk and men. The relatively low number of ICU admissions in our analysis makes the interpretation of the results more difficult and should be reviewed with caution.
We acknowledge that SARS-CoV-2 reinfection cases in this study are suspected cases as genetic sequencing of a specimen at each episode is limited. As of 30 September 2021, they were 361 confirmed SARS-CoV-2 reinfections for which reinfection specimens were genetically distinct from that sequenced during primary infection.40
We did not assess for the changes in SARS-CoV-2 variant dominance over time but we purposefully covered the period including the emergence of the wildtype SARS-CoV-2 variant and the Alpha variant while restricting the study period before the dominance of the Delta variant across England.
Multiple mechanisms including the host immune response, associated health issues, and environmental factors are involved in the pathogenesis of SARS-CoV-2 in those suffering from severe COVID-19 making it a highly heterogenous group of individuals.31 Ascertaining the severity of illness in those with reinfections is further complicated by such heterogeneity.
Nonetheless our study provides a first glance at the profile of reinfection cases of COVID-19 in England and emphasises the variability in COVID-19 outcomes across different risk and age groups.
Conclusion
Community incidence rates of primary infection cases were closely linked to increase of reinfection cases. Prior infection and immunisation were associated with lower odds of severe disease in selected populations, highlighting the heterogeneity of the reinfection cohort. SARS-CoV-2 reinfections were associated with lower odds of dying. Older age, sex and underlying comorbidities arose as principal risk factors for illness severity at reinfection. Future research should try to identify predictive factors of disease severity in the population not yet considered at risk.
CRediT authorship contribution statement
Anna A. Mensah: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Joanne Lacy: Data curation, Writing – review & editing. Julia Stowe: Data curation, Writing – review & editing. Giulia Seghezzo: Data curation, Writing – review & editing. Ruchira Sachdeva: Data curation, Writing – review & editing. Ruth Simmons: Data curation, Writing – review & editing. Antoaneta Bukasa: Data curation, Writing – review & editing. Shennae O'Boyle: Data curation, Writing – review & editing. Nick Andrews: Conceptualization, Methodology, Supervision, Writing – review & editing. Mary Ramsay: . Helen Campbell: Conceptualization, Methodology, Supervision, Writing – review & editing. Kevin Brown: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
We declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.01.012.
Appendix. Supplementary materials
References
- 1.Wang J., Kaperak C., Sato T., Sakuraba A. COVID-19 reinfection: a rapid systematic review of case reports and case series. J Investig Med. 2021;69:1253–1255. doi: 10.1136/jim-2021-001853. [DOI] [PubMed] [Google Scholar]
- 2.Adrielle Dos Santos L., Filho P.G.G., Silva A.M.F., et al. Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J Infect. 2021;82:399–406. doi: 10.1016/j.jinf.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanif M., Haider M.A., Ali M.J., Naz S., Sundas F. Reinfection of COVID-19 in Pakistan: a first case report. Cureus. 2020;12:e11176. doi: 10.7759/cureus.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West J., Everden S., Nikitas N. A case of COVID-19 reinfection in the UK. Clin Med. 2021;21:e52–e53. doi: 10.7861/clinmed.2020-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang T.W. Recurrent viral infection (reinfection) N Engl J Med. 1971;284:765–773. doi: 10.1056/NEJM197104082841406. [DOI] [PubMed] [Google Scholar]
- 6.Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 7.Hall C.B., Long C.E., Schnabel K.C. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792–796. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs D., Flowers D., Clarke J.R., Valman H.B., MacNaughton M.R. Epidemiology of coronavirus respiratory infections. Arch Dis Child. 1983;58:500–503. doi: 10.1136/adc.58.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raveendran A.V. COVID-19 re-infection: diagnostic challenges and proposed diagnostic criteria. Diabetes Metab Syndr. 2021;15:645–648. doi: 10.1016/j.dsx.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Control ECfDPa Reinfection with SARS-CoV-2: implementation of a surveillance case definition within the EU/EEA. Stockholm. 2021 [Google Scholar]
- 11.2021. National flu and COVID-19 Surveillance Reports 2021.https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports at. [Google Scholar]
- 12.UK Coronavirus Dashboard. 2021. (Accessed 1 September 2021, at https://coronavirus.data.gov.uk/details/testing.)
- 13.Consortium C-GU . 2021. Summary Report: COG-UK Geographic Coverage of SARS-CoV-2 Sample Sequencing. 27 September. Report No.: 41. [Google Scholar]
- 14.Digital N. Secondary Uses Service (SUS).
- 15.COVID-19 Population Risk Assessment. 2021. (Accessed 20 October 2021, at https://digital.nhs.uk/coronavirus/risk-assessment/population.)
- 16.Shielded Patient List: Guidance for General Practice . 2021. https://digital.nhs.uk/coronavirus/shielded-patient-list/guidance-for-general-practice.) (Accessed 20 October 2021, at. [Google Scholar]
- 17.Variants of Concern (VOC) and Under Investigation (VUI) and any Other Variant by Weeks and Days . 2021. http://sars2.cvr.gla.ac.uk/cog-uk/.) (Accessed 20 October 2021, at. [Google Scholar]
- 18.Joint Committee on Vaccination and Immunisation: Advice on Priority Groups for COVID-19 Vaccination, 30 December 2020 . 2021. at https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020.) [Google Scholar]
- 19.Agency UHS . The Green Book. 2021. Immunisation against infectious disease. 16 September 2021 ed:13-5. [Google Scholar]
- 20.Edridge A.W.D., Kaczorowska J., Hoste A.C.R., et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 21.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen C.H., Michlmayr D., Gubbels S.M., Molbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang X., Li S., Yu H., et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12:12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallo Marin B., Aghagoli G., Lavine K., et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31:1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326:1930–1939. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lusignan S., Dorward J., Correa A., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brouqui P., Colson P., Melenotte C., et al. COVID-19 re-infection. Eur J Clin Invest. 2021;51:e13537. doi: 10.1111/eci.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta V., Bhoyar R.C., Jain A., et al. Asymptomatic reinfection in 2 healthcare workers from india with genetically distinct severe acute respiratory syndrome coronavirus 2. Clin Infect Dis. 2021;73 doi: 10.1093/cid/ciaa1451. e2823-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvaraj V., Herman K., Severe Dapaah-Afriyie K. Symptomatic reinfection in a patient with COVID-19. R I Med J (2013) 2020;103:24–26. [PubMed] [Google Scholar]
- 31.Domingo P., Mur I., Pomar V., Corominas H., Casademont J., de Benito N. The four horsemen of a viral Apocalypse: the pathogenesis of SARS-CoV-2 infection (COVID-19) EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Beltran W.F., Lam E.C., Astudillo M.G., et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184 doi: 10.1016/j.cell.2020.12.015. 476–88 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fakhroo A., AlKhatib H.A., Al Thani A.A., Yassine H.M. Reinfections in COVID-19 patients: impact of virus genetic variability and host immunity. Vaccines. 2021;9 doi: 10.3390/vaccines9101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Facts & Figures . 2021. https://www.carersuk.org/news-and-campaigns/press-releases/facts-and-figures.) (Accessed 10/12/2021, 2021, at. [Google Scholar]
- 35.NHS Celebrates the Vital Role Hundreds of Thousands of Women Have Played in the Pandemic . 2021. at https://www.england.nhs.uk/2021/03/nhs-celebrates-the-vital-role-hundreds-of-thousands-of-women-have-played-in-the-pandemic/) [Google Scholar]
- 36.Jin J.M., Bai P., He W., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozenberg S., Vandromme J., Martin C. Are we equal in adversity? Does Covid-19 affect women and men differently? Maturitas. 2020;138:62–68. doi: 10.1016/j.maturitas.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bienvenu L.A., Noonan J., Wang X., Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116:2197–2206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solanich X., Vargas-Parra G., van der Made C.I., et al. Genetic screening for TLR7 variants in young and previously healthy men with severe COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agency UHS . 2021. Weekly national Influenza and COVID-19 Surveillance Report. 28 October. Report No.: 43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.